Abstract
Aims
To evaluate safety and efficacy of buprenorphine implants (BI) versus placebo implants (PI) for the treatment of opioid dependence. A secondary aim compared BI to open-label sublingual buprenorphine/naloxone tablets (BNX).
Design
Randomized, double-blind, placebo-controlled trial. Subjects received either 4 buprenorphine implants (80 mg/implant) (n=114), 4 placebo implants (n=54), or open-label BNX (12–16 mg/d) (n=119).
Setting
20 addiction treatment centers.
Participants
Adult outpatients (ages 18 to 65) with DSM-IV-TR opioid dependence.
Measurements
The primary efficacy endpoint was the percent of urine samples negative for opioids collected from weeks 1 to 24, examined as a cumulative distribution function (CDF).
Findings
The BI CDF was significantly different from placebo (P<.0001). Mean (95% CI) proportions of urines negative for opioids were: BI: 31.2% (25.3, 37.1) and PI: 13.4% (8.3, 18.6). BI subjects had a higher study completion rate relative to placebo (64% vs. 26%, P<.0001), lower clinician-rated (P<.0001) and patient-rated (P<.0001) withdrawal, lower patient-ratings of craving (P<.0001), and better subjects’ (P=.031) and clinicians’ (P=.022) global ratings of improvement. BI also resulted in significantly lower cocaine use (P=.0016). Minor implant-site reactions were comparable in the buprenorphine (27.2% [31/114]) and placebo groups (25.9% [14/54]). BI were non-inferior to BNX on percent urines negative for opioids [mean (95% CI): 33.5 (27.3, 39.6); CI for the difference of proportions, (−10.7, 6.2)].
Conclusions
Compared with placebo, buprenorphine implants result in significantly less frequent opioid use, and are non-inferior to sublingual buprenorphine/naloxone tablets.
INTRODUCTION
Based on substantial efficacy data, international guidelines specify sublingual buprenorphine and methadone as first-line treatments of opioid dependence [1]. Buprenorphine can be prescribed in office-based physician practice [1]. However, rates of misuse, abuse, and diversion of various forms of sublingual buprenorphine are increasing in the US [2,3,4,5].
An implantable formulation of buprenorphine was developed to address problems with adherence, diversion, and non-medical use. The implant is a polymeric ethylene vinyl acetate and buprenorphine matrix that, following an initial pulse release, delivers a constant, low medication level over 6 months.
A 6-month placebo-controlled, multicenter study established the efficacy of the buprenorphine implant in the treatment of opioid-dependent subjects, though the comparative efficacy of the implant to the standard sublingual buprenorphine was questioned [6,7]. The primary objective of the current randomized double-blind clinical trial was to confirm the efficacy of buprenorphine implants (BI) relative to placebo implants (PI) over 24 weeks of treatment for opioid dependence. A secondary objective was to establish the non-inferiority of BI relative to BNX over weeks 1 to 24.
METHODS
Participants
Twenty addiction treatment centers in the U.S. recruited subjects between April 2010 and September 2011. Institutional Review Boards approved the study at each site, and written informed consent was obtained from all participants.
Men and non-pregnant women (aged 18 to 65) met DSM-IV diagnosis of current opioid dependence as determined by the Mini-International-Neuropsychiatric-Interview [8]. Individuals were excluded if they had AIDS, a clinically-low platelet count, substance dependence on other than opioids or nicotine, received methadone or buprenorphine for opioid dependence within 90 days, current diagnosis of chronic pain requiring opioid analgesics; or currently using non-prescribed benzodiazepines. Subjects were also excluded with aspartate aminotransferase levels ≥3× upper limit of normal, alanine aminotransferase levels ≥3× upper limit of normal, total bilirubin ≥1.5× upper limit of normal, and/or creatinine ≥1.5× upper limit of normal.
Study Intervention and Randomization
An open-label induction phase evaluated whether buprenorphine could be safely administered to eligible subjects. Subjects who achieved a target dose of 12–16 mg/day BNX for at least 3 consecutive days were eligible to be randomized. Those who, at the end of the induction phase, reported significant opioid withdrawal symptoms, defined as >12 on the Clinical Opiate Withdrawal Scale (COWS) [9], or significant opioid cravings, defined as >20 mm on a 100 mm opioid craving Visual Analog Scale (VAS), were excluded. Final determination for study enrollment was made by site Investigators.
Subjects were randomized (stratified by gender) in a 2:1:2 ratio to 4 BI (80mg each), 4 PI, or open-label BNX (12–16mg/d-QD), using a computer-generated randomization scheme. An independent group of statisticians and programmers were responsible for the randomization scheme, the results of which were entered into an interactive voice response system (IVRS) program. Once enrollment began, clinical site staff would either 1) dial into the system by phone or 2) visit a web page to enter basic information about the subject, and receive the treatment assignment (open-label BNX, or the code number corresponding to a blinded implant kit).
Implants were inserted in the subdermal space (2–3 mm below the skin) in the inner, upper side of the non-dominant arm by a physician. Implanting physicians were from various medical specialties with prior surgical training who received standardized training in implant insertion and removal from the study sponsor. All implants were removed at 6 months or upon early discontinuation. Subjects and study staff, with the exception of the implanting clinicians, were blinded to the buprenorphine or placebo implants.
During the 24-week study, all subjects could receive supplemental sublingual buprenorphine/naloxone for opiate withdrawal and cravings, in 2 mg/d increments as clinically indicated. Supplemental buprenorphine/naloxone tablets [(i.e., rescue medication (RM)] were generally administered under observation and dosing was directed by protocol specific parameters, excepting a maximum of 3 days of take-home dosing provided over weekends/holidays.
Subjects in BI and PI could receive one additional implant. BNX subjects could receive a dose increase of 2–4 mg/d (arriving at a fixed dose not to exceed 16 mg/d) if they required ≥3 days/week of any RM for 2 consecutive weeks, or ≥8 days of RM over 4 consecutive weeks. Subjects were considered a treatment failure (and withdrawn) if they met criteria for a second dose increase.
Manual-guided individual drug counseling sessions [10] were provided by experienced counselors twice weekly during weeks 1–12, and then weekly for the subsequent 12 weeks.
Temperature-verified urine samples were collected three times per week. Another sample was provided if a sample was outside a valid temperature range. A second sample outside the temperature range constituted a ‘missing' sample. A central lab conducted testing of urine samples for opioids and cocaine, and study staff and subjects remained blind to results. Subjects who failed to provide nine consecutive urines were designated as non-compliant and withdrawn. The double-blind for urine results was maintained throughout the study, and subjects’ participation in the study was independent of urine testing results.
Efficacy Assessments
The primary efficacy endpoint was the percent of urines that were negative for opioids from weeks 1 to 24, expressed as a Cumulative Distribution Function (CDF). As requested by the FDA prior to breaking the blind, an additional primary efficacy endpoint combined participants’ self-reported opioid use with their urine sample analyses. Secondary efficacy endpoints were: the percent of urines that were negative for opioids during weeks 1 to 16 and during weeks 17 to 24.
Additional secondary measures included the proportion of study completers, patient-report and clinician-report withdrawal scales, a craving scale, and patient and clinician improvement ratings. The Subjective Opiate Withdrawal Scale (SOWS) measured patient-reported withdrawal symptoms [11]. Clinician reports of withdrawal symptoms were assessed with the COWS. Opioid craving was measured using a 100mm VAS (0=no cravings, 100=maximum craving experienced). SOWS, COWS, and VAS were obtained at weeks 1, 4, 8, 12, 16, 20, and 24. Self-report illicit drug use was obtained weeks 1, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, and 24. Clinician-rated Clinical Global Impressions-Severity (CGI-S) (of opioid dependence) and Improvement scales (CGI-I) [12] were obtained at weeks 16 and 24 (and at baseline for the CGI-S). Hypothesis testing for primary and secondary efficacy endpoints was conducted using a fixed-sequence testing procedure.
Safety and Pharmacokinetic Assessments
Vital signs, laboratory tests (hematology, liver function tests, coagulation, pregnancy test), and ECGs were obtained at regular study visits. Clinical staff inspected the surgical implant location at each visit for evidence of any adverse event (AE) or unplanned removal. Levels of plasma buprenorphine were obtained from blood samples taken at baseline and monthly thereafter.
Statistical Analyses
The denominator for the primary efficacy endpoint was all possible urine samples that could have been collected from implantation through week 24 (72 urine samples per subject). Missed samples were counted as opioid-positive. When a subject discontinued or was withdrawn from the study, urine samples from that point onward were considered positive.
Using all randomized subjects who received any treatment, the primary statistical analyses used the non-parametric Wilcoxon rank-sum test, adjusted for gender and site using the van Elteren method [13], to compare BI and PI on: (1) cumulative distribution functions (CDFs) of the percentages of urine samples negative for opioids over 24 weeks, and (2) CDFs of the percentages of urine sample negative for opioids with imputation based on self-report of opioid use. Secondary analyses compared BI and PI on percent negative urines from weeks 1 to 16 (48 urines), and separately, over weeks 17 to 24 (24 urines).
Hypothesis testing for the primary and secondary hypotheses was conducted using a fixed-sequence testing procedure to reduce Type I error risk. The two primary hypotheses were tested using a 5% alpha level. Only if the null hypothesis was rejected for both primary analyses, testing proceeded to the secondary analyses (comparison of CDFs for weeks 1 to 16, and 17 to 24, for the two implant groups). Then, the two implant groups were sequentially compared on secondary endpoints (as ordered above).
Analyses of variance examined implant group differences in mean percent of urine samples negative for opioids separately over weeks 1 to 24, weeks 1 to 16, and weeks 17 to 24, with gender and site as covariates. Analyses of the COWS, SOWS, and opioid craving VAS were conducted with a mixed-effects repeated-measures analysis of covariance using all available assessments. Terms were included for treatment, week, treatment-by-week, gender, and site, baseline as a covariate, and subject as a random effect. An auto-regressive (AR1) correlation structure was specified. Endpoint patient and clinician-rated CGI-Improvement scales were analyzed as categorical variables using a CMH test stratified by gender and site.
Because of recent reports of the potential efficacy of buprenorphine on cocaine use patterns in subjects with both opioid and cocaine dependence [14], we compared BI and PI on CDFs of the percentages of urine samples negative for cocaine over 24 weeks.
Sample size for the BI vs. PI comparison was calculated using 80% power to detect a shift of 20% (deemed clinically relevant) between groups on the distributions of the percent of urines negative for opioids over 24 weeks (alpha=.05; 2-sided). Approximately 150 subjects were required, assuming the 2:1 randomization scheme, a common standard deviation of 30%, and an attrition rate of approximately 40%.
Open-label comparison with BNX
For the non-blinded open-label comparison study (i.e., BI v BNX), the non-inferiority comparison of BI to BNX was conducted by calculating the 95% confidence interval for the mean difference between the proportions of urine samples negative for opioids in the two groups. Non-inferiority would be demonstrated if the lower bound of the confidence interval was greater than −15%. This margin was based on input from clinical experts and previous studies showing a difference between sublingual buprenorphine and placebo in the range of 30–40% [15, 16]. Thus, the 15% margin is smaller than the smallest effect that BNX can be reliably expected to have, and also insures that the efficacy of BI would be within a clinically relevant range of BNX. Sample size determination for the non-inferiority test of difference in percentages of urine samples negative for opioids over 24 weeks for BI compared to BNX was based on a fixed 15% margin of inferiority, a 1-sided significance of 0.025, 80% power, a 1:1 randomization ratio, an assumption of 50% of urines negative for opioids for BNX, and a common standard deviation of 30%, yielding approximately 66 subjects per treatment group.
RESULTS
Subject Characteristics and Disposition
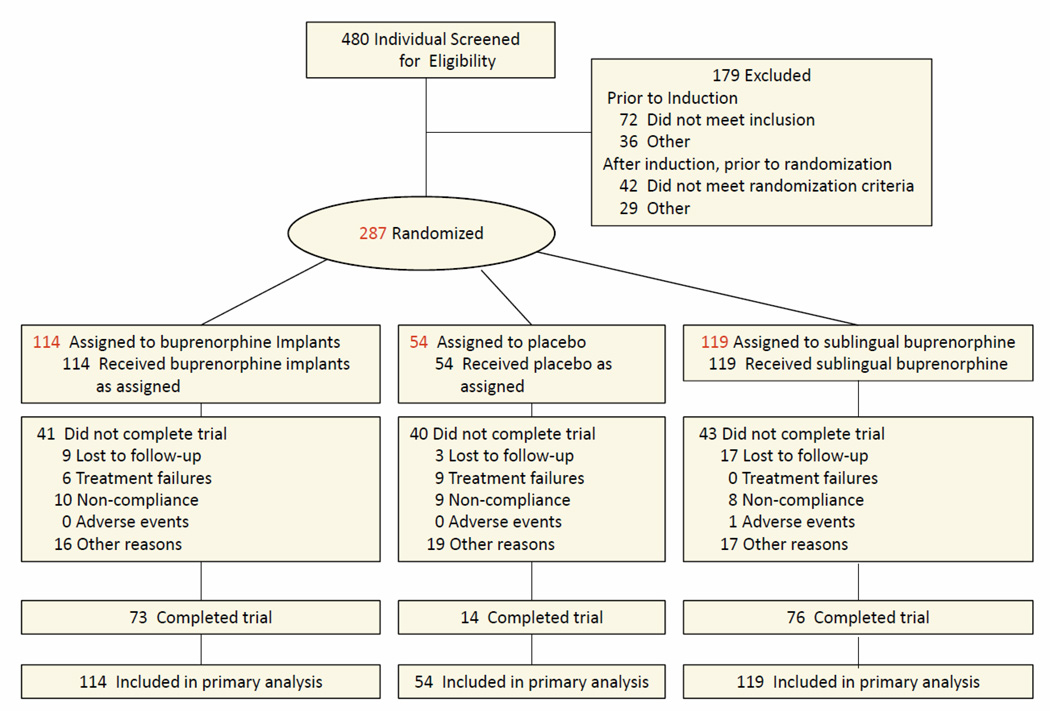
There were 480 subjects screened for the study (Figure 1). Prior to induction, 108 of those screened did not meet inclusion/exclusion criteria. Of the remaining 372 that entered the induction phase, 71 did not complete the induction phase within 16 days of screening or did not receive a fixed dose of 12 to 16 mg/day sublingual buprenorphine/naloxone for at least 3 consecutive days during induction, 14 were randomized and withdrew before receiving treatment, and 287 were randomized and received treatment. Following randomization, 6 (5.3%) subjects in BI, 9 (16.7%) in PI, and no subjects in the BNX, met the definition of treatment failure.
Figure 1.
Flow Diagram of Participants Through the Trial
The treatment groups did not differ on available baseline characteristics (Table 1).
Table 1.
Baseline Characteristics of Subjects
| Characteristic | Buprenorphine Implant Group n=114 |
Placebo Implant Group n=54 |
Sublingual Buprenorphine n=119 |
|---|---|---|---|
| Age, mean (SD), y | 36.4 (11.0) | 35.2 (10.3) | 35.3 (10.9) |
| Male, No. (%) | 72 (63.2) | 31 (57.4) | 72 (60.5) |
| Race, N (%) | |||
| White | 95 (83.3) | 45 (83.3) | 97 (81.5) |
| Black | 14 (12.3) | 7 (13.0) | 16 (13.4) |
| Other | 5 (4.4) | 2 (3.8) | 6 (5.0) |
| Hispanic Ethnicity, N(%) | 24 (21.1) | 11 (20.4) | 17 (14.3) |
| Primary opioid of abuse, N (%) | |||
| Heroin | 76 (66.7) | 28 (51.9) | 75 (63.0) |
| Prescription pain med. | 38 (33.3) | 26 (48.1) | 43 (36.1) |
| Other | 0 | 0 | 1 (0.8) |
| Diagnosis of opioid dependence for > 5 years, N (%) | 29 (25.4) | 12 (22.2) | 37 (31.1) |
| Previous treatment for opioid Dependence, N (%) | 63 (55.3) | 31 (57.4) | 68 (57.1) |
Efficacy
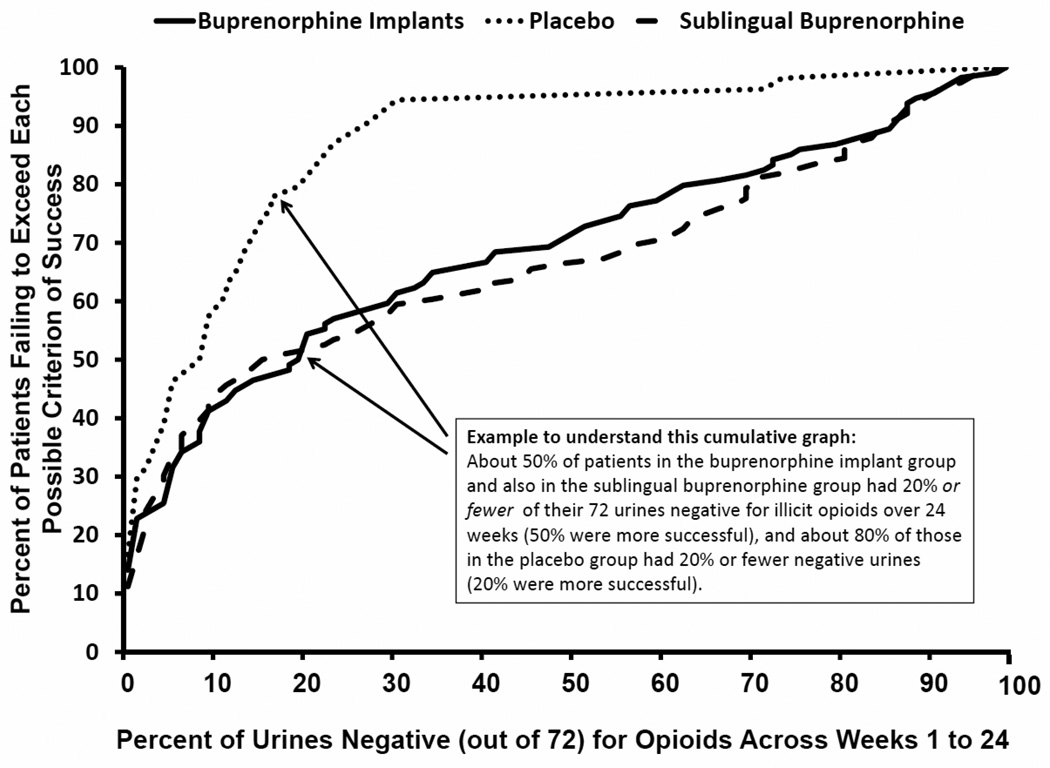
The primary endpoint, comparing the two implant groups on the CDF of percent opioid-negative urines from week 1 to week 24, revealed a significant difference between BI and PI (P<.0001) (Figure 2). The second primary endpoint analysis (i.e., urines with imputation based on self-report) comparing the two implant groups was also statistically significant (P<.0001). Unadjusted mean (95% CI) proportions of urines negative for opioids, without and with imputation based on self-report, were 31.2% (25.3, 37.1) and 31.0% (25.1, 36.8) for BI, 13.4% (8.3, 18.6) and 12.8% (7.1, 17.9) for PI, and 33.5% (27.3, 39.6) and 33.1% (27.0, 39.2) for the BNX, respectively. At all points on the CDF, BI was superior to PI, and the effect sizes were moderate to strong. Using some example clinical cut-points, patients assigned to BI were more likely to have at least 50% of urines negative for opioids (BI: 27% versus PI: 6%; Number Needed to Treat (NNT)=5), and were significantly more likely to have 30% or greater urines negative for opioids (BI: 42% versus PI: 7%; NNT=3). Patients assigned to PI were significantly more likely to have fewer than 5% of their urines negative for opioid use (PI: 43% versus BI: 27%; NNT=7).
Figure 2.
Cumulative Distribution Functions of Percentage of Urine Samples Negative for Opioids
According to the fixed sequential analytic plan, significant results obtained on the primary endpoint permitted the secondary analyses. Comparison of BI and PI on CDFs of percent opioid-negative urines during the first 16 weeks of treatment showed statistically significant differences (P<.0001). The percent of urines negative for opioids also statistically differed between BI and PI for weeks 17 to 24 (P=.0002). The 95% confidence interval around the mean difference between BI and SB in the proportions of urine samples that were negative for illicit opioids over 24 weeks of treatment was [−10.7, 6.2]. The lower bound of this interval was greater than −15, meeting the pre-specified criterion of non-inferiority of BI relative to BNX.
Significant differences between BI and PI were also evident on the secondary efficacy measures (Table 2). Differences in adjusted mean urines negative for opioids were statistically significant for the full 24 week period (P<.0001), weeks 1 to 16 (P<.0001), and weeks 17 to 24 (P<.0001). Treatment was completed at week 24 by 64.0% (73/114) of those in BI compared to 25.9% (14/54) (P=.0002) in PI.
Table 2.
Descriptive Statistics on Secondary Efficacy Measures
| Buprenorphine Implant n=114 |
Placebo Implant n=54 |
Sublingual Buprenorphine n=119 |
P-Value Buprenorphine Implants vs. Placebo |
P-Value Buprenorphine Implant vs. Sublingual Buprenorphine |
|
|---|---|---|---|---|---|
| Urines Negative for Opioids, Weeks 1 to 24, meana | 36.0 | 14.4 | 35.1 | <.0001 | .81 |
| Urines Negative for Opioids, Weeks 1 to 16, meana | 39.6 | 17.9 | 37.8 | <.0001 | .65 |
| Urines Negative for Opioids, Weeks 17 to 24, meana | 28.9 | 7.2 | 29.6 | <.0001 | .86 |
| Proportion of Study Completers, N (%) | 73 (64.0) | 14 (25.9) | 76 (63.9) | .0002 | .62 |
| Clinical Opiate Withdrawal Scale (COWS) over 24 weeks, meanb | 2.49 | 4.52 | 1.71 | <.0001 | .0005 |
| Subjective Opiate Withdrawal Scale (SOWS) over 24 weeks, meanb | 5.30 | 8.42 | 2.83 | <.0001 | .0006 |
| VAS-opioid craving over 24 weeks, meanb | 10.2 | 21.8 | 7.1 | <.0001 | .054 |
| Patient Rated CGI-Improvement at week 24 (or endpoint),c N (%) | .031 | .30 | |||
| Very much improved | 47 (41.2) | 14 (25.9) | 57 (47.9) | ||
| Much improved | 35 (30.7) | 18 (33.3) | 29 (24.4) | ||
| Minimally improved | 10 (8.8) | 9 (16.7) | 9 (7.6) | ||
| No change | 3 (2.6) | 5 (9.3) | 0 | ||
| Minimally worse | 1 (0.9) | 1 (1.9) | 1 (0.8) | ||
| Much worse | 0 | 1 (1.9) | 0 | ||
| Very much worse | 0 | 0 | 0 | ||
| Clinician Rated CGI-Improvement at week 24 (or endpoint),c N (%) | .022 | .99 | |||
| Very much improved | 57 (50.0) | 12 (22.2) | 58 (48.7) | ||
| Much improved | 17 (14.9) | 8 (14.8) | 22 (18.5) | ||
| Minimally improved | 15 (13.2) | 11 (20.4) | 9 (7.6) | ||
| No change | 6 (5.3) | 10 (18.5) | 3 (2.5) | ||
| Minimally worse | 0 | 4 (7.4) | 1 (0.8) | ||
| Much worse | 0 | 1 (1.9) | 2 (1.7) | ||
| Very much worse | 0 | 1 (1.9) | 0 |
Significance tests for urine data based on analyses of variance with treatment, gender, and site in the model. Adjusted means presented.
Significance tests for COWS, SOWS, and VAS based on mixed-effects repeated measures analysis of variance using scores from week 1, 4, 8, 12, 16, 20, and 24, with treatment, week, treatment by week, baseline scores, gender, and site in the model.
Significance tests for Patient and Clinician CGI-Improvement scales based on CMH test stratified on gender and site. The COWS scale potentially ranges from 0 to 48, with 5–12 = mild, 13–24 = moderate, 25–36 = moderately severe, more than 36 = severe withdrawal. The SOWS scores potentially range from 0 to 64, with each of 16 questions rated on intensity of withdrawal on a 0 (not at all) to 4 (extremely) scale. The VAS scale ranges from 0 (no craving) to 100 mm (maximum experienced).
For the COWS (P<.0001), SOWS (P<.0001), and opioid craving VAS (P<.0001) scales, there were higher mean scores (reflecting more withdrawal symptoms and craving) for PI compared with BI across 24 weeks of treatment. At Week 24, there were significant differences on the patient-rated (P=.031) and clinician-rated (P=.022) CGI-Improvement scales, favoring BI over PI. A post-hoc analysis compared the proportion of patients in each group who achieved “responder” status defined as at least 4 weeks of continuous abstinence. For the PI group, only patients who received no RM were included (n=69). Of these patients, 29% percent of BI participants met this responder criterion compared to 4% in PI patients (Cochran-Mantel-Haenszel test, adjusted for gender and site, p=.0029), and 29% in BNX.
A post-hoc analysis of the percent cocaine-negative urines across weeks 1 to 24 indicated a significant difference between BI and PI (P=.0016). For patients randomized to BI, the mean percent urines negative for cocaine use across 24 weeks, assuming missing values as positive, was significantly different from PI (50.2% versus 32.0%) and not significantly different from BNX (55.1%).
Outcome data from the open-label BNX group, including urine toxicology results, patient reported cravings, and physician ratings of patient cravings are shown in Table 2. The exploratory analyses comparing BI and BNX revealed no significant differences on the percent of treatment completers, mean percent of urine samples negative for opioids separately over weeks 1 to 24, weeks 1 to 16, and weeks 17 to 24, or CGI-Improvement scales. BI, compared to BNX, showed greater withdrawal symptoms on the COWS (P=.0005) and SOWS (P=.0006). VAS scale craving scores were not significantly different for BI compared to BNX.
Treatment Exposure
The median (mean; range) number of weeks of exposure to implants (before removal) was 25.0 (26.9; 4–60) for BI and 15.5 (18.4: 1–56) for PI. Additional implants were received by 21.9% (25/114) patients randomized to BI and 38.8% (21/54) of patients randomized to PI. Of the 114 patients randomized to BI, 89 (78%), received 4 implants. The majority of BI patients (n=69/114; 61%) took no RM. Twenty patients (22.5%) required the following amounts of RM over the 24 week study: mean days used per week = 0.10; mean mgs per week = 0.91. Patients requiring RM in amounts that exceeded the pre-specified threshold were required to receive a fifth implant. In the PI group, 38.8% (21/54) received an additional implant. In the open-label BNX treatment arm, the median (mean; range) exposure was 25.0 (20.7; 1–65) weeks.
Safety
In the BI group, 67.5% (77/114) of patients had at least 1 AE, compared to 61.1% (33/54) for PI and 71.4% (85/119) for BNX. The events were mild in severity and were unrelated to study intervention. AEs were further described as occurring at the implant location or not. Among non-implant site AEs with incidence ≥ 5% (Table 3), headache was most common in BI (13.2%) and BNX (16.0%); insomnia (14.8%) was most common in PI. There were no significant differences between groups on any AEs. Implant-site reactions were evident for 27.2% (31/114) in BI and 25.9% (14/54) in PI, most commonly hematomas (8[7.0%] and 6[11.1%], respectively) and pain (6 (5.3%) and 5 (9.3%), respectively) (NS). There was no evidence of unscheduled implant removal or attempted removal.
Table 3.
Non-Injection Site Treatment Emergent Adverse Events Across 24 Weeks with Incidence ≥5% in One or More Groups
| Event | Buprenorphine Implants n=114 |
Placebo Implants n=54) |
Sublingual Buprenorphine n=119 |
|---|---|---|---|
| Headache | 15 (13.2) | 5 (9.3) | 19 (16.0) |
| Upper respiratory Infection | 10 (8.8) | 4 (7.4) | 11 (9.2) |
| Depression | 10 (8.8) | 3 (5.6) | 4 (3.4) |
| Insomnia | 9 (7.9) | 8 (14.8) | 16 (13.4) |
| Sore throat | 8 (7.0) | 1 (1.9) | 4 (3.4) |
| Nausea | 7 (6.1) | 1 (1.9) | 8 (6.7) |
| Vomiting | 7 (6.1) | 1 (1.9) | 5 (4.2) |
| Nasopharyngitis | 6 (5.3) | 3 (5.6) | 12 (10.1) |
| Back pain | 6 (5.3) | 3 (5.6) | 7 (5.9) |
| Limb abscess | 3 (2.6) | 4 (7.4) | 5 (4.2) |
| Hyperhidrosis | 3 (2.6) | 3 (5.6) | 2 (1.7) |
| Anxiety | 2 (1.8) | 3 (5.6) | 7 (5.9) |
| Diarrhea | 2 (1.8) | 3 (5.6) | 2 (1.7) |
| Any “severe” event | 9 (7.9) | 3 (5.6) | 14 (11.8) |
| Any “serious” event | 6 (5.3)a | 3 (5.6) | 7 (5.9) |
Umbilical hernia, pneumonia (n=2), breast cancer, hypotension, tooth abscess.
Serious adverse events (SAEs) occurred in 16 patients. In BI, 5.3% (6/114) experienced SAEs, compared to 5.6% (3/54) in PI and 5.9% (7/119) in BNX. In BI, these were: umbilical hernia, pneumonia (n=2), breast cancer, hypotension, and tooth abscess. None were judged as related to treatment. SAEs resulted in 5 hospitalizations in BI (5/114;4.4%), 2 in PI (2/54;3.7%), and 6 in BNX (6/119;5.0%). There was one death in the study, which occurred in BNX (accidental overdose), three days following early study discontinuation initiated by the subject.
DISCUSSION
This study confirms the 24-week efficacy of BI relative to PI demonstrated in the previous trial [6]. Statistically significant differences were observed on both primary endpoints and all secondary endpoints. Effect sizes for primary and secondary endpoints were moderate to strong (NNTs<10), and were comparable or superior to effect sizes reported for frequently-prescribed psychiatric medications, including second-generation antipsychotic [17] and ADHD medications [18]. In addition, BI was found to be not inferior to BNX when comparing BI, provided on a double-blind basis, versus BNX provided as an open-label treatment. Lack of blinding is often associated with inflated treatment effects [19]. The 24-week retention rate for active treatment (BI and open-label BNX: 64%) was significantly higher than placebo (26%) and was within the range observed in other clinical studies of buprenorphine. Previous open-label and single-blind studies have reported completion rates ranging from 38% [20] to 78% [21]. Office-based treatment with buprenorphine/naloxone has reported retention rates of 55% %) [16].
It is unclear whether greater withdrawal symptoms found in BI, compared to BNX, relate to lower blood levels of buprenorphine; what is clear is that the difference in withdrawal symptoms does not translate into more drug use (compared to placebo). Notably, the absolute levels of withdrawal symptoms were very low for all three groups.
Diversion and misuse of sublingual buprenorphine is a significant clinical and societal concern. There was no evidence of attempted removal of the implants in the current, or previous [6], trials. Supplemental sublingual buprenorphine was used by 39.5% of subjects in the BI group, primarily by those who ultimately required an implant dose increase. The pattern of AEs for BI and PI was similar to that found previously with minor implant-site reactions commonly occurring.
Regarding cocaine use, earlier post hoc findings [14] were replicated suggesting a beneficial effect of BI in reducing cocaine use in opioid-dependent subjects. It is unknown whether this effect is an indirect result of buprenorphine treatment of opioid dependence or is a direct pharmacological effect. The potential utility of implantable buprenorphine in cocaine-use disorders, at least among opioid-dependent individuals, deserves further attention.
Although safety and efficacy of BI was confirmed in two randomized clinical trials, areas for further discussion remain. How many patients and physicians in the community would prefer buprenorphine implants instead of available forms of the medicine is unknown and was not measured in the study. Physicians may be reluctant to prescribe implants when other formulations are available. In the current and previous trials, physicians across a variety of specialties (e.g., psychiatry, obstetrics, family medicine) safely performed the procedure with training. It is possible that clinicians would identify certain patients more suitable for either a sublingual form of the medicine or the implant. For example, clinicians could decide to recommend implants for patients with young children in the home, patients with a pattern of inconsistent adherence to prescriptions or patients who may repeatedly misplace sublingual formats. Balancing individual patient and physician concerns with the burgeoning need to minimize harm resulting from opioid abuse and diversion [3,4,5] is a question for clinicians, future clinical research and for public health policy.
A limitation of the non-inferiority component of the study was that the comparison with BNX was un-blinded. Another limitation was use of rescue sublingual buprenorphine/naloxone across all groups, making it difficult to compare outcome and retention results to previous studies that did not use rescue medication. In addition, the generalization of these findings is uncertain for individuals who are also dependent on other substances, recently received methadone or buprenorphine, or have chronic pain that requires opioid analgesics.
In summary, buprenorphine implants compared with placebo implants resulted in significantly less opioid use over 24 weeks, replicating the efficacy observed in a previous randomized clinical trial. Buprenorphine implants were also found to be non-inferior to sublingual buprenorphine in regard to the proportion of urines negative for opioids over 24 weeks of treatment for opioid dependence.
Acknowledgments
We thank the following study investigators: George Bigelow, Ph.D., Eduardo Cifuentes, M.D., William Dickerson, D.O., David Flaherty, D.O., Valentin Isacescu, M.D., Saleem Ishaque, M.D., Nishant Kumar, D.O., Joseph Kwentus, M.D., Azfar Malik, M.D., Scott Segal, M.D., Michael Sheehan, M.D., Eugene Somoza, M,D., Ph.D., Amit Vijapura, M.D.
We also thank Edward Schweizer, M.D. of Paladin Consulting Group (Princeton, NJ), who provided assistance with the drafting of the manuscript, and Titan Pharmaceuticals employees Scott Henley, M.B.A, and Janice Yen, B.S., for their work on the conduct of the study, data management, statistical analyses, and editorial input. We also thank Jackie Johnson, Ph.D., Assistant Professor of Psychiatry at the University of North Carolina, Chapel Hill, who performed an independent statistical review of all analyses and conclusions presented in the manuscript, Ben Vaughn, Ph.D., Rho International, Chapel Hill, NC for statistical support, and Martin Mumenthaler, Ph.D., Consulting Assistant Professor at Stanford University, School of Medicine, Department of Psychiatry, Palo Alto, CA for editorial assistance.
Declaration of Interest
This study was funded by a grant from the National Institute on Drug Abuse under the American Recovery and Reinvestment Act and by Titan Pharmaceuticals.
Dr. Rosenthal has received grant funds (to his institution) from Titan and travel support from Titan.
Dr. Ling has received unrestricted education grants and/or served as a consultant to Reckitt/Benckiser and research support from Reckitt/Benckiser, Hythiam Inc, US World Med, Titan, and DemeRx.
Dr. Casadonte has received travel support from Titan.
Dr. Vocci has served as a consultant to Reckitt/Benckiser, Roxane Laboratories, DemeRx, Teva Pharmaceutical Industries Ltd., Purdue Pharma, and US World Meds, and has received grant funds from Titan and Friends Research Institute.
Dr. Bailey has received research support from the National Institute on Drug Abuse and Titan and Alkermes, and has received travel support to meetings from Titan.
Dr. Kampman has received grant funds (to his institution) from Titan and travel support for meetings from Titan.
Dr. Patkar has received research support from Forest Pharmaceuticals, Janssen, Pfizer, Titan, Shire, Sunovion; has been a consultant/ Advisory Board member of Gilead, Dey Pharmaceuticals, Avanir Pharma, and on Speakers Bureau of Alkermes, Bristol Myers Squibb, Dey Pharmaceuticals, and Sunovion.
Dr. Chavoustie has received consulting and review activity fees from Titan.
Dr. Blasey has received a consulting fee from Titan Pharmaceuticals.
Dr. Sigmon has received grant funds (to her institution) from Titan.
Dr. Beebe is an employee of Titan Pharmaceuticals.
Footnotes
Trial Registration: clinicaltrials.gov identifier: NCT01114308
REFERENCES
- 1.Soyka M, Kranzler HR, Van den Brink W, Krystal J, Möller HJ, Kasper S and the WFSBP Task Force for Treatment Guidelines on Substance Use Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of substance use and related disorders, part 2: opioid dependence. The World Journal of Biological Psychiatry. 2011;12:160–187. doi: 10.3109/15622975.2011.561872. [DOI] [PubMed] [Google Scholar]
- 2.Luty J, O’Gara C, Sessay M. Is methadone too dangerous for opiate addiction? BMJ. 2005;331:1352–1353. doi: 10.1136/bmj.331.7529.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Buprenorphine prescribing practices and exposures reported to a poison center--Utah, 2002–2011. MMWR Morb Mortal Wkly Rep. 2012;61(49):997–1001. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):7059. [PubMed] [Google Scholar]
- 5.Johanson CE, Arfken CL, di Menza S, Schuster CR. Diversion and abuse of buprenorphine: findings from national surveys of treatment patients and physicians. Drug Alcohol Depend. 2012;120(1–3):190–195. doi: 10.1016/j.drugalcdep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Ling W, Casadone P, Bigelow G, Kampman KM, Patkar A, Bailey GL, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010;304:1576–1583. doi: 10.1001/jama.2010.1427. [DOI] [PubMed] [Google Scholar]
- 7.Basu D, Kumar V. Buprenorphine implants and opioid dependence. JAMA. 2011;305:253–254. doi: 10.1001/jama.2010.1989. [DOI] [PubMed] [Google Scholar]
- 8.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM–IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 9.Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 10.Mercer D, Woody GE. Individual drug counseling. Rockville, MD: National Institute on Drug Abuse; 1999. NIH Publication Number 99-4380. [Google Scholar]
- 11.Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 12.Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: National Institute of Mental Health, US Dept of Health, Education, and Welfare; 1976. pp. 76–338. [Google Scholar]
- 13.Van Elteren P. On the combination of independent two sample tests of Wilcoxon. Bull InstInt Stat. 1960;37:351–361. [Google Scholar]
- 14.Montoya ID, Gorelick DA, Preston KL, Schroeder JR, Umbricht A, Cheskin LJ, et al. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther. 2004;75:34–48. doi: 10.1016/j.clpt.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend. 1995;40:17–25. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- 16.Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. New Engl J Med. 2003;349:949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 17.Citrome L. Show me the evidence: using number needed to treat. South Med J. 2007;100(9):881–884. doi: 10.1097/SMJ.0b013e3180f63246. [DOI] [PubMed] [Google Scholar]
- 18.McGough JJ, Faraone S. Estimating the size of treatment effects: moving beyond p values. Psychiatry (Edgmont) 2009;6(10):21–29. [PMC free article] [PubMed] [Google Scholar]
- 19.Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: Meta-epidemiological study. BMJ. 2008;336:601–605. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer G, Gombas W, Eder H, Jagsch R, Peternell A, Stühlinger G, et al. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction. 1999;94:1337–1347. doi: 10.1046/j.1360-0443.1999.94913376.x. [DOI] [PubMed] [Google Scholar]
- 21.Kakko J, Grönbladh L, Svanborg KD, Joachim von Wachenfeldt J, Rück C, Rawlings B, Nilsson LH, et al. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry. 2007;164:797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]