Abstract
Quantum dots (Qdots) are semiconductor nanoparticles with size-tunable fluorescence capabilities with diverse applications. Qdots typically contain cadmium or other heavy metals, hence raising concerns of their potential toxicity, especially in occupational settings where inhalation of nanomaterials may increase the risk of lung disease. Accordingly, we assessed the effects of tri-n-octylphosphine oxide, poly(maleic anhydride-alt-1-tetradecene) (TOPO-PMAT) coated CdSe/ZnS Qdots on mouse lung epithelial cells and macrophages. Mouse tracheal epithelial cells (MTEC), grown as organotypic cultures, bone marrow-derived macrophages (BMDM), and primary alveolar macrophages (AM) were derived from C57BL/6J or A/J mice and treated with TOPO-PMAT CdSe/ZnS Qdots (10–160 nM) for up to 24 h. Cadmium analysis showed that Qdots remained in the apical compartment of MTEC cultures, whereas they were avidly internalized by AM and BMDM, which did not differ between strains. In MTEC, Qdots selectively induced expression (mRNA and protein) of neutrophil chemokines CXCL1 and CXCL2 but only low to no detectable levels of other factors assessed. In contrast, 4 h exposure to Qdots markedly increased expression of CXCL1, IL6, IL12, and other pro-inflammatory factors in BMDM. Higher inflammatory response was seen in C57BL/6J than in A/J BMDM. Similar expression responses were observed in AM, although overall levels were less robust than in BMDM. MTEC from A/J mice were more sensitive to Qdot pro-inflammatory effects while macrophages from C57BL/6J mice were more sensitive. These findings suggest that patterns of Qdot-induced pulmonary inflammation are likely to be cell type specific and genetic background dependent.
Keywords: engineered nanomaterial, pulmonary inflammation, in vitro toxicity
Introduction
Production and use of engineered nanomaterials is increasing for many applications, including industrial, medical, and cosmetic purposes (Committee for Review of the Federal Strategy to Address Environmental, 2009). Quantum dots (Qdots) are semiconductor nanoparticles that usually contain heavy metal cores typically composed of cadmium selenide (CdSe) or cadmium telluride (CdTe) encased in another semiconductor of higher band gap, such as CdS or ZnS (McConnachie et al., 2012). Qdots range in size from 2–12 nm and have many desirable physico-chemical properties such as size-tunable emission with spectrally narrow fluorescence light upon excitation (Hu and Gao, 2010), high photostability, and large Stokes shifts leading to broad absorption profiles (Clapp et al., 2005, Pinaud et al., 2006, Zrazhevskiy and Gao, 2009). Thus, Qdots hold much potential for use in biomedical imaging, drug delivery, detection of disease, and other applications (Rosenthal et al., 2011). The increased use of these particles requires consideration of possible detrimental effects on human health due to occupational or consumer exposures.
Of the three potential routes of entry for nanoparticles into the body—inhalation, oral ingestion, and skin absorption—the airway epithelium and alveolar macrophages are the most important target cells for airborne exposures (Li et al., 2010). In a rat study tracking the distribution of inhaled gold nanoparticles (AuNPs), the lungs, not surprisingly, had about a 10-fold greater concentration of nanoparticles at 5 and 15 days of exposure compared to other organs (Yu et al., 2007). In addition, uptake of inhaled nanoparticles could lead to systemic delivery to other organs. Indeed, Cd was detected in lung-associated lymph nodes and kidneys in rats exposed via intratracheal instillation to functionalized CdSe Qdots (Roberts et al., 2013). Thus, understanding the response of resident cells within the lung that would first interact with inhaled nanoparticles – i.e. airway epithelium and macrophages – will be important for predicting adverse health outcomes.
Although inhalation of nanoparticles stimulates pro-inflammatory responses in the lung, it is not known which resident cell type responds to the exposure (Brown et al., 2001). For the current study, we assessed viability and the pro-inflammatory response of airway epithelial cells and alveolar macrophages (AM) to TOPO-PMAT coated CdSe/ZnS Qdots (hereafter referred to as ‘Qdots’). To mirror epithelial cells that contact inhaled particles, we established organotypic cultures of mouse tracheal epithelial cells (MTEC) grown at an air-liquid interface (ALI). Under such conditions, primary tracheal epithelial cells differentiate into a complete, polarized mucociliary epithelium that mirrors the cellular composition of the intact tissue (You et al., 2002). In addition to the resident cells, we assessed the response of bone marrow derived macrophages (BMDM), used as a model of infiltrating macrophages. Furthermore, to assess potential strain differences, we conducted these studies with ALI MTEC, AM, and BMDM from C57BL/6J and A/J mice.
Our findings show that BMDM were more responsive to Qdots than AM and that this response was more robust in C57BL/6J macrophages than in A/J macrophages. In contrast, A/J MTEC were more sensitive to Qdot pro-inflammatory stimulation than C57BL/6J MTEC, and overall, the epithelial response was much less than that of macrophages. Our findings indicate that Qdots induce pulmonary inflammation primarily by affecting gene expression in both resident and potentially recruited subpopulations of macrophages, as well as in the airway epithelium. Furthermore, our results demonstrate that the degree of these responses is mouse strain-dependent.
Materials and Methods
Cell Culture
C57BL/6J and A/J male mice (8–12 weeks) were obtained from Jackson Laboratory (Bar Harbor, ME). All mouse studies were approved by the Institutional Animal Care and Use Committee at the University of Washington. Tracheas were excised from euthanized mice, and air-liquid interface mouse tracheal epithelial cell (MTEC) cultures were grown and differentiated in transwells as described (Kassim et al., 2007). Bone marrow derived macrophages (BMDM) were cultured and differentiated in CSF-1-containing medium for 7 days as described (Manicone et al., 2009). Alveolar macrophages (AM) were isolated from bronchoalveolar lavage as described (Johnston et al., 2012) and pooled from 7 mice. We reported that macrophages comprise >95% of cells in bronchoalveolar lavage from naïve mice (Johnston et al., 2012). All experiments were done at least 3 times with cells from different mice or pools of mice.
Quantum Dots
Amphiphilic polymer-coated TOPO-PMAT CdSe/ZnS Qdots were synthesized as described (Bagalkot and Gao, 2011, Pellegrino, 2004). MTEC were plated at 150,000 cells/well and macrophages at 250,000 cells/well in 24-well dishes. Qdots were diluted in culture media (10–160 nM final concentrations), vortexed gently for an even particle suspension, and filtered through 0.22-μm syringe filters. Qdots solutions were added in volumes of 150 μl for MTEC and 500 μl for macrophage cultures. As a positive agonist of pro-inflammatory responses, cells were treated with 10 ng/ml of E. coli LPS (List Biologicals, Campbell, CA) for 24 h for MTEC and 4 h for macrophages, in volumes of medium equivalent to those used for Qdots.
Cellular Uptake/Association
To quantify uptake, cells were exposed to increasing concentrations of Qdots for 24 h. Cells were then washed and lysed in cell lytic buffer (Sigma, St Louis, MO) for 10 min at 4°C. Lysates were centrifuged at 50,000 x g for 1 h at 4°C to pellet internalized Qdots. Pellets were resuspended in water and assayed for cadmium levels using inductively coupled plasma-mass spectroscopy (ICP-MS; Agilent 7500ce, Agilent, Inc., Santa Clara, CA) following EPA Method 6020A.
To visualize Qdot uptake, cells were seeded onto glass chamber slides, allowed to adhere for 24 h, exposed to Qdots for 24 h, fixed with cold methanol for 10 min, and washed 3 times with PBS. Slides were mounted with ProLong Gold anti-fade reagent containing 4′, 6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA). Images were collected using a 490ex/525em LP dichroic cube with a Nuance multispectral camera (Caliper Life Sciences, Hopkinton, MA) mounted on an Optiphot microscope (Nikon, Melville, NY). Differential interference contrast (DIC) images were collected simultaneously with fluorescent digital images.
Cell Viability
Cell viability was assessed by release of lactate dehydrogenase (LDH) (Cayman Chemical Company, Ann Arbor, MI). After a 24-h exposure to Qdots, 100 μl of medium from each cell sample was transferred to a 96-well plate containing 100 μl/well of LDH reaction solution. Cells exposed to Triton-X 100 were used as a positive control. After incubation at room temperature for 30 min, absorbance was read at 490 nm. Values were compared to the Triton-X 100 samples and expressed as percent viability.
Gene Expression
Total RNA was isolated using a Qiagen RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany). RNA concentrations were measured using a Nano-Drop-Photometer (NanoDrop ND100 PeqLab, City, Germany). For real-time reverse-transcription PCR (qRT-PCR), 250 ng total RNA was reverse transcribed with a High-Capacity cDNA Archive kit (ABI-Applied Biosystems, Foster City, CA). TaqMan FAM dye-labeled probes for target genes (Cxcl1, Il6, Il12b, Hmox1, Gclm, and Gapdh) were obtained from ABI. PCR reactions were performed according to the manufacturer’s protocol with an ABI HT7900 Real-Time PCR System using TaqMan reagents for 40 cycles. The threshold cycle (Ct) for each probe set/cDNA was obtained from triplicate samples and averaged, and the ΔCt was the calculated difference between the average Ct for the target gene and the average Ct for Gapdh cDNA as a control for total starting RNA quantity. The ΔΔCt was calculated from average ΔCt at a given time point minus the average ΔCt of untreated cells. The data are expressed as relative quantification (RQ) or fold change, which is calculated as 2−ΔΔCt, and indicates the fold difference in gene expression relative to untreated cells.
Cytokine/Chemokine Protein Assay
Conditioned media from primary epithelial and macrophage cultures were collected 24 h after treatment, centrifuged to remove cells and debris, and stored at −80°C. The Millipore Milliplex MAP Mouse Cytokine/Chemokine Magnetic Bead Panel (EMD Millipore, Billerica, MA) was used to measure levels of the following proteins in undiluted samples: MCP-1, CXCL1 (KC), IL-1β, CXCL2 (MIP-2), IL-6, TNFα, IFNγ, IL-10, IL-12 (p40), and MIP-1α. Reagents were prepared according to manufacturer instructions, and cytokine standards were prepared with macrophage medium as the matrix solution. The assay was performed according to manufacturer instructions, using 25 μl of each sample, and fluorescence intensity was read on the Luminex Bio-Plex 200 system (Luminex Corporation, Austin, TX).
Statistics
Statistical analysis was performed using 2-way ANOVA with Bonferroni post-test where appropriate. Data are presented as mean ± SEM, with p < 0.05 considered statistically significant. Prism 5 (GraphPad Software, Inc., La Jolla, CA) was used for all statistical analyses.
Results
Qdots
The preparation and characterization of TOPO-PMAT encapsulated Qdots used in these studies has been described in detail (McConnachie et al., 2012). In brief, the particles have a hydrodynamic diameter of 12.7 ± 0.5 nm and a core diameter 6.8 ± 0.5 nm, with a peak fluorescence emission at 610 nm.
Uptake and Association with Cells
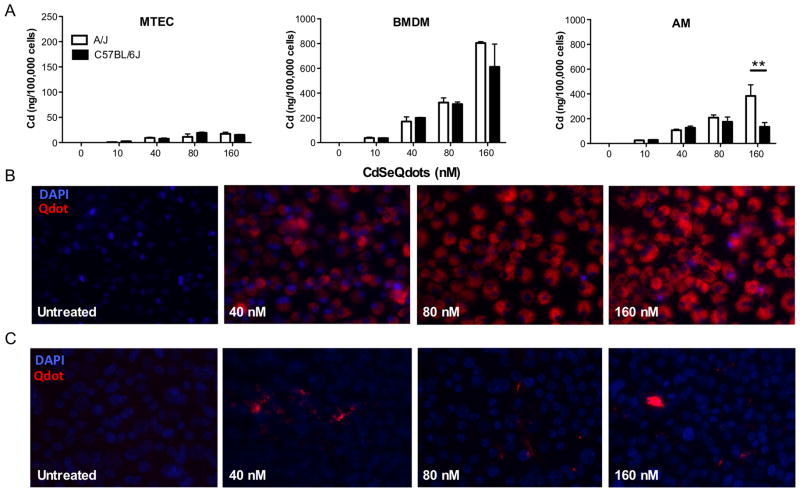
To evaluate the ability of cells to interact with and internalize Qdots, we used ICP-MS to quantify cadmium levels in cell lysates of MTEC, BMDM, and AM 24 h after exposure to Qdots. The association of Qdots with the different cell types increased in a dose dependent manner from 10 nM to 160 nM (Figure 1A). We did, however, observe marked differences in the degree of association and internalization between epithelial cells and the two types of macrophages. About 2-fold more particles were associated with BMDM than with AM, whereas 10 to 20-fold fewer particles were associated with MTEC on a per cell basis compared to macrophages (Fig. 1A). Other than a strain difference in AM at the highest dose (p < 0.01), the ability of Qdots to associate with cells was not influenced by genetic strain. Using Nuance Spectral Imaging (Figure 1B), we observed a dose-dependent increase in internalized Cd levels in macrophages that correlated with Qdot levels in the cell layer. In contrast, we did not detect intracellular fluorescence in MTEC, but we did observe aggregation of particles at the apical surface of the epithelial cell layer (Figure 1C).
Figure 1.
Association of CdSe Qdots with epithelial cells and macrophages. (A) Mouse tracheal epithelial cell (MTEC) organotypic cultures and alveolar (AM) and bone marrow-derived macrophages (BMDM) from A/J and C57BL/6J mice were exposed to the indicated concentrations of Qdots for 24 h. Samples were then processed for Cd analysis by ICP-MS. Cd levels are presented as nanograms cadmium per 105 cells. For all cell types, a significant increase (p < 0.0001) above baseline was seen at all doses. Cd levels did not vary among strains, except in A/J AM at 160 nM, where the cells had significantly more Cd than did C57BL/6J AM (p<0.01 by 2-way ANOVA with Bonferroni post-test.) Data are mean ± SEM. (B) BMDM were seeded onto CC2-treated chamber slides and exposed to the indicated concentrations of Qdots for 24 h. Cells were then fixed and imaged as described under Methods. (C) MTEC were seeded onto transwells and exposed to indicated concentrations of Qdots for 24 h. Cells were fixed and imaged as described under Methods.
Because the lung is the principal portal for systemic absorption of inhaled substances, we measured the potential for Qdots to cross an airway epithelial layer. For this, we added Qdots to the apical surface of established MTEC cultures and 24 h later measured fluorescence in the basal medium. To determine the signal range, we measured fluorescence in ALI medium containing no Qdots (0 nM) or 160 nM Qdots (positive control) (Figure 2). However, we observed no increase in fluorescence above background levels (Figure 2). These data indicate that Qdots did not appreciably cross the MTEC cell layer.
Figure 2.
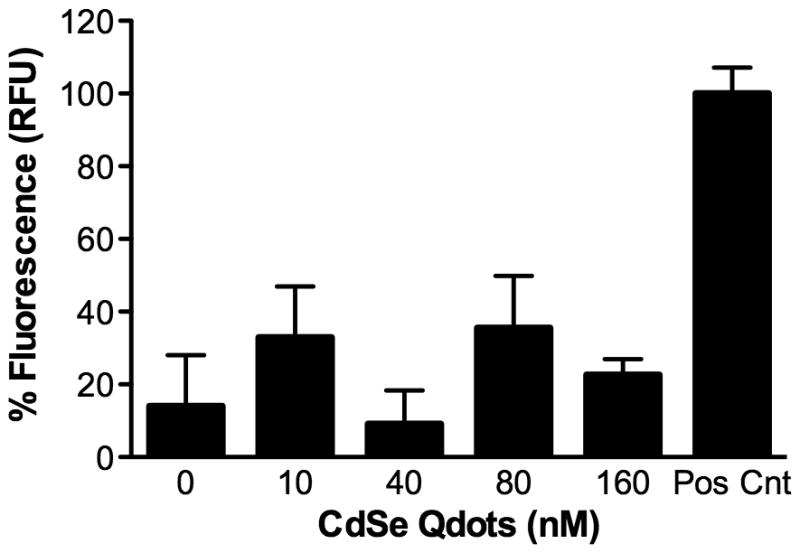
Lack of trans-epithelial transfer of Qdots. Qdots in 150 μl medium were added to the apical chamber of MTEC cultures derived from cells from C57BL/6J mice. Trans-epithelial Qdot transfer was assessed 24 h later by measuring fluorescence in the basal compartment medium, which did not differ significantly at any doses from control (0 nM). The positive control was 160 nM Qdots added to ALI culture medium. Data are the mean ± SEM.
Cytotoxicity
Previous findings demonstrated a relationship between cytotoxicity of Qdots with differences in their surface chemistry and core/shell material (Clift et al., 2011). To evaluate the cytotoxic effects of Qdots, we measured LDH released from treated cells. In all three cell types, Qdots induced minimal to no LDH release (Figure 3); however, at 160 nM Qdots were cytotoxic to A/J BMDM. These data indicate that at the concentrations and exposure times used, Qdot interaction with and uptake by cells largely produced no measurable cytotoxicity.
Figure 3.
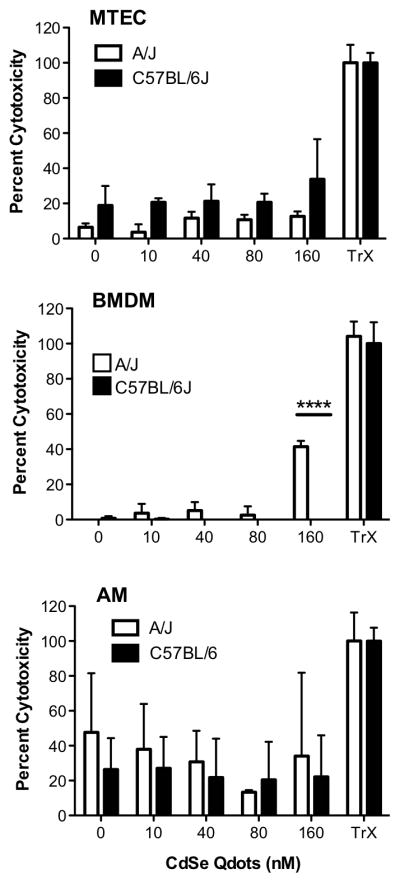
Qdots induced minimal to no cytotoxicity. MTEC, AM, and BMDM from A/J and C57BL/6J mice exposed to the indicated concentrations of Qdots for 24 h, and LDH levels were measured in culture medium. There were no significant dose-dependent increases or strain differences of LDH release from any cell type (p > 0.14 to 0.34) with one exception: BMDM from A/J mice had significant increase of LDH release at 160 nM (p < 0.0001).
Pro-Inflammatory Responses
Although the TOPO-PMAT Qdots did not induce cytotoxicity, the ability of macrophages to take up the Qdots and the association of the particles with the surface of epithelial cells may lead to potential non-lethal adverse effects, such as induction of an inflammatory response. Thus, we assessed the expression of selective, key pro-inflammatory genes in response to Qdot exposure. For our studies, we assessed both mRNA and protein levels for CXCL1/KC, an acute phase neutrophil chemokine, and IL-6 and IL-12, both pro-inflammatory cytokines that promote Th1 immune responses, as well as the levels of several other factors associated with inflammation.
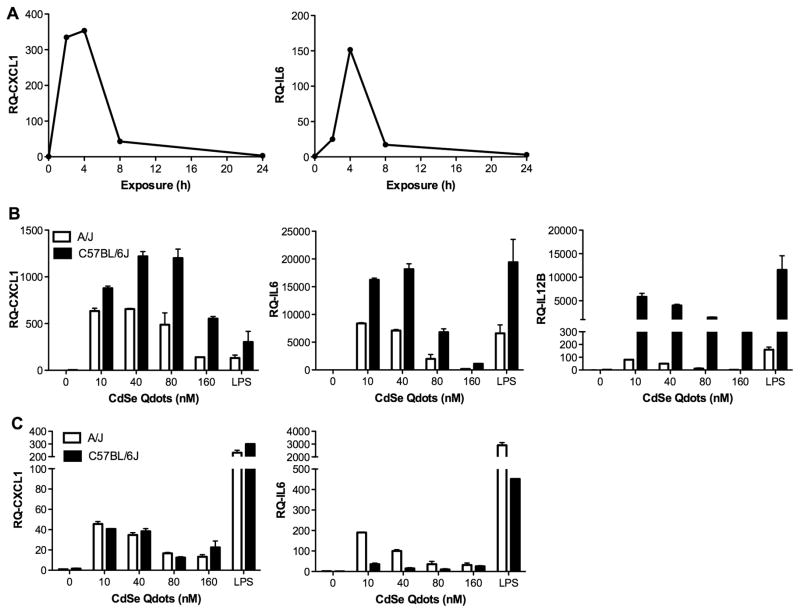
We found that after a 24 h exposure to MTEC, Qdots induced a dose-dependent increase in the mRNA levels for Cxcl1 and, to a lesser extent, for Il6 (Figure 4). In addition, we observed that the Qdot-mediated induction of these mRNAs was more robust in A/J MTEC than in C57BL/6J cultures, even though the LPS-mediated response was, as expected, more pronounced in the C57BL/6J cultures (Figure 4). Whereas the Ct values for Cxcl1 mRNA in MTEC ranged from 25–31, the Il6 mRNA Ct values were 34–36, indicating very low levels of expression. Consistent with the mRNA data, we did not detect IL-6 protein in MTEC conditioned medium (data not shown), but we did observe Qdot-mediated release of CXCL1 protein that was greater in A/J than C57BL/6J epithelial cultures (Supplement Figure 1A). In addition, Qdots stimulated a marked secretion of CXCL2 (MIP-2), another acute phase neutrophil chemokine, which did not differ markedly between strains (Supplement Figure 1A). In contrast, Qdots did not stimulate release of detectable levels of other chemokines/cytokines from MTEC, i.e., MCP-1, IL-1β, TNFα, IFNγ, IL-10, IL-12 (p40), and MIP-1α (data not shown). Overall, these data suggest that Qdots stimulate a neutrophil-specific recruitment response in airway epithelial cells.
Figure 4.
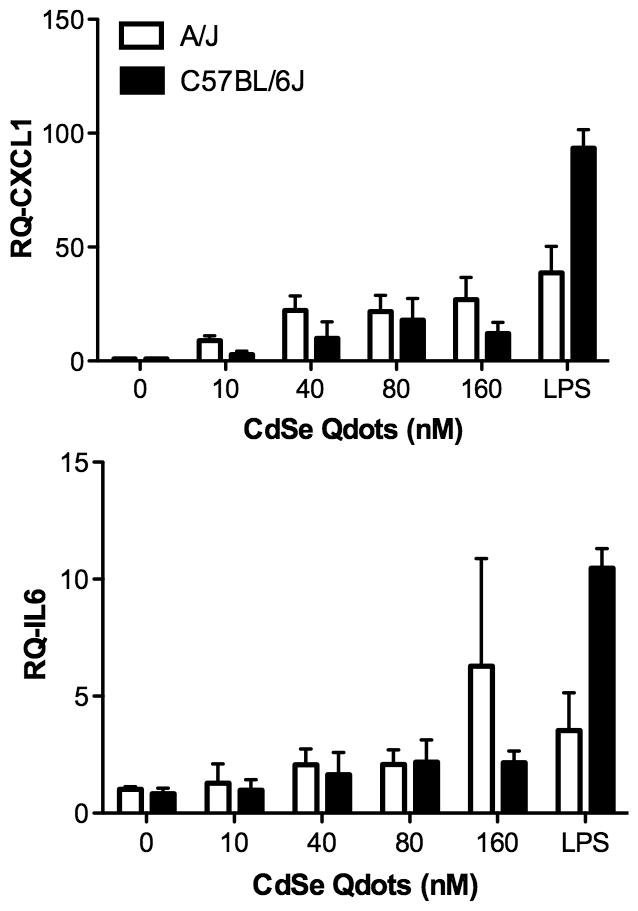
Pro-inflammatory response of epithelial cells. MTEC from A/J and C57BL/6J mice were exposed at their apical surface with 25 μl containing the indicated concentrations of Qdots for 24 h. Total RNA was isolated, and qRT-PCR was performed to quantify mRNA levels for Cxcl1 and Il6. A dose-dependent increase in Cxcl1 mRNA expression was seen (p = 0.0094) but not for Il6 mRNA (p = 0.2660).
Because macrophages have a rapid and robust response to inflammatory stimuli, we conducted a time-course study with A/J BMDM to determine the time of the peak response to Qdot exposure. BMDM cells were exposed to 40 nM Qdots and assessed at 0, 2, 4, 8 and 24 h. The maximal stimulation of Cxcl1 and Il6 mRNA expression occurred early, peaking at 2–4 h post-exposure (Figure 5A). Consequently, we used a 4-h exposure to assess macrophage gene expression responses in subsequent experiments.
Figure 5.
Cxcl1 and Il6 expression by BMDM peaks early after exposure. (A) BMDM from 1 A/J mouse were treated with 40 nM Qdots for 0–24 h. Total RNA was isolated, and mRNAs for Cxcl1 and Il6 were measured by qRT-PCR. (B) BMDM from A/J and C57BL/6J mice were exposed to the indicated concentrations of Qdot for 4 h. Total RNA was isolated, and mRNAs for Cxcl1, Il6 and Il12b were measured by qRT-PCR. All mRNA levels were significantly elevated from 0-nM control levels (p < 0.0001), and the response for all mRNAs was significantly greater for C57BL/6 BMDM than A/J BMDM (p < 0.0001). (C) AM from A/J and C57BL/6J mice were exposed to the indicated concentrations of Qdot for 4 h. Dose-dependent responses were significant for both Cxcl1 and Il6 (p < 0.0001), and a strain-specific response was significant only for Il6 (p < 0.0001).
For BMDM from both mouse strains, we found Qdots induced a potent increase in expression of Cxcl1, Il6 and Il12b (Figure 5B). The increase in pro-inflammatory transcripts by macrophage was much greater than that observed in the MTEC. In addition, we found that lower concentrations of Qdots (10–40 nM) stimulated them more than higher concentrations (80–160 nM). We also observed marked differences in the inflammatory gene expression response of BMDM from the two mouse strains. In contrast to ALI MTEC, in which the A/J cells showed a more robust pro-inflammatory response, we found that BMDM from C57BL/6J mice were more responsive than BMDM from A/J mice, and these patterns were largely reflected in the levels of proteins released by the treated BMDMs (Supplement Figure 1B). A noticeable difference between the mRNA and protein data was that whereas Cxcl1 mRNA was induced more robustly in C57BL/6J macrophages than in A/J BMDM (Figure 5B), we did not detect CXCL1 released from C57BL/6J macrophages (Supplement Figure 1B), suggesting strain-specific posttranscriptional regulation of chemokine production. We also observed Q-dot-mediated induction or stimulation in the release of IL-1β, MIP-1α, IL-10, and MCP-1 from BMDM that did not differ between strains (data not shown). For both strains, the magnitude of the LPS-induced mRNA responses was similar to that stimulated by Qdots.
To begin to explore the mechanism behind these strain differences, we compared the expression levels of mRNAs for heme-oxygenase-1 (Hmox1) and glutamate-cysteine ligase modifier subunit (Gclm), which we reported is induced by Qdot exposure in vivo (McConnachie et al., 2013a). Qdots stimulated the expression of both mRNAs in BMDM (Supplement Figure 2). Whereas Gclm mRNA levels were equivalent between C57BL/6J and A/J BMDM, Hmox1 mRNA levels were more robustly stimulated in A/J macrophages, a response that may contribute to the attenuated chemokine expression in these cells.
A more robust pro-inflammatory response at lower doses of Qdots than higher doses was also seen for AM (Figure 5C), but the magnitude of increase was lower than that observed for BMDM. Additionally, in contrast to BMDM, Qdots were markedly less potent than LPS at stimulating a pro-inflammatory response in AM. A/J AM were more sensitive to Qdots than AM from C57BL/6J mice, and these differences were mirrored in the higher levels of CXCL1, IL-6, and in particular, IL-1β released by these macrophages (Supplement Figure 1C). We also found that Qdot-induced release of CXCL2 (MIP-2), TNFα, MIP-1α, and IL-10 from AM but not MCP-1 or IL-12 (p40) (data not shown).
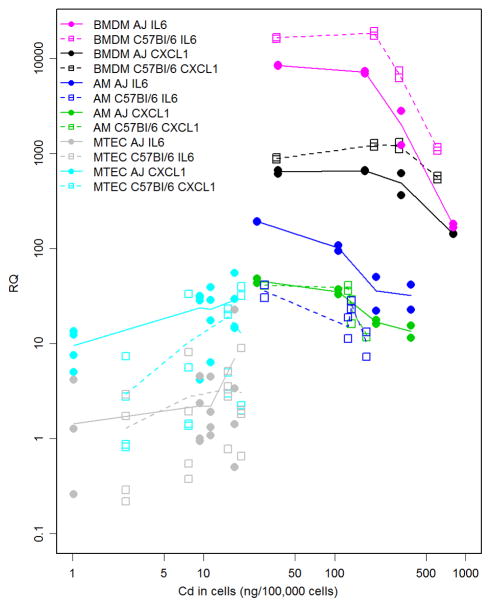
We also assessed the relationship between the RQ for Cxcl1 and Il6 and Qdot dose among cell types and strains (Figure 6). RQs for Cxcl1 and Il6 revealed statistically significant dose responses (p<0.05) by one-way analysis of variance for both types of macrophages, i.e. BMDM and AM, from both A/J and C57BL/6J mice. In contrast, MTEC were only statistically significant for Cxcl1 in A/J mice. In addition, the dose response patterns differed between macrophages and epithelial cells. Whereas the response (i.e. RQ) by macrophages peaked at low to moderate doses of Qdots and declined at higher doses, the MTEC response was linear and, at least for Il6, did not change with dose. BMDMs from C57BL/6J mice responded more robustly than BMDMs from A/J mice. In contrast, AM from A/J mice had a much greater Il6 response than did their C57BL/6J counterparts. The Cxcl1 response did not differ between AM of either strain, and we detected no significant difference between the responses of MTEC cultures for this cytokine.
Figure 6.
Response of Cxcl1 and Il6 as a function of the measured cadmium uptake dose of Qdots. BMDM, AM, and ALI MTEC were derived from A/J and C57BL/6J mice and the relationship between magnitude of gene expression response and cadmium uptake was plotted in relation to strain of cell origin.
Discussion
Rapid advances in nanoparticle development and engineering have brought forth many novel applications in biomedicine. With this progress comes a need for understanding potential nanoparticle-related impacts on health and safety, particularly through environmental or occupational exposure to the respiratory tract, as the lung is a main route of particle entry into the body (Li et al., 2010). Because of their high water solubility and exceptional stability, the TOPO-PMAT-coated CdSe/ZnS Qdot particles used in our studies are useful for biological imaging. Furthermore, the carboxyl functional groups present in this coating facilitate ligand attachment. Similarly modified Qdots have shown promise for tumor targeting in vivo using antibodies directed against surface receptors on cancer cells (Gao et al., 2004, Yang et al., 2009) and for siRNA delivery (Qi and Gao, 2008). Earlier work from our group demonstrated that immortalized human macrophage and lung epithelial cell lines internalized Qdots (McConnachie et al., 2012). Nagy et al. demonstrated charge- and functionalization-dependent effects of Qdots on primary normal human bronchial epithelial cells, further supporting the importance of the epithelium in response to Qdot exposure (Nagy et al., 2012). Thus, to further evaluate Qdot effects on lung cells, and to develop the basis for in vivo studies in mouse models of lung exposure, we used primary mouse lung airway epithelial cells differentiated into a mucociliary epithelium (MTEC) and two sources of primary macrophages, lung alveolar macrophages (AM) and bone marrow derived macrophages (BMDM).
Our findings indicate that Qdots were not overtly cytotoxic to lung tracheal epithelial cells or either type of macrophage, an observation consistent with our earlier assessment of the cytotoxicity of these TOPO-PMAT coated CdSe/ZnS Qdots in other cell lines (McConnachie et al., 2013b). Macrophages took up Qdots far more avidly than the epithelial cells, an observation consistent with their role as phagocytic cells. Although not overtly cytotoxic, we did observe that Qdots were internalized and stimulated pro-inflammatory responses in both cells types. These results are consistent with other groups studying the correlation between Qdot uptake and other harmful effects such as generation of reactive oxygen species (Soenen et al., 2012) with consequent changes in antioxidant gene expression (McConnachie et al., 2013b, McConnachie et al., 2013a). Other studies found a dose-dependent increase of TNFα expression by lung cell lines treated with iron-platinum nanoparticles embedded in a poly (methylacrylic acid) polymer shell (Lehmann et al., 2010).
Given that macrophages are professional phagocytes, the capacity of macrophages to readily take up Qdots is not surprising. Using various nanomaterials such as single and multi-walled carbon nanotubes, several studies have shown the greater capacity of macrophage cell lines to take up particles compared to epithelial cell lines (Shvedova et al., 2009). A recent study demonstrated that functionalized Qdots stimulate endocytosis and pro-inflammatory responses in immortalized human epithelial and macrophage cell lines (Zhang et al., 2013). In contrast to macrophages, MTEC did not efficiently take up Qdots. The mucous glycocalyx and beating cilia on the apical surface of these cultures likely hinder Qdots from reaching the cell membrane. In vivo, the airway epithelial surface may function to protect airway epithelial cells from Qdot exposure in their physiological environment.
MTEC from A/J mice were more sensitive than those from C57BL/6J mice. In contrast, BMDM from C57BL/6J mice were more sensitive to Qdot-induced pro-inflammatory effects than were BMDM from A/J mice. AM from both strains were less responsive to Qdot exposure than were BMDM. In addition, we found that the BMDM response occurred early and was transient. These data indicate that strain-dependent genetic differences among specific cell types may define the airway response to inhaled nanomaterials. Similarly, lung inflammatory responses differ between A/J and C57BL/6J mice in models of cigarette smoke exposure (Zhang et al., 2013), LPS (Alm et al., 2010), toxic injury (Zeidler-Erdely et al., 2010, Paun et al., 2013), and more. In these models, C57 mice tend to show a more profound pro-inflammatory response and enhanced susceptibility, findings that are largely consistent with our data with Qdots.
The patterns of dose-responses in Figure 6 provide insight into the complexity of the dose response for inflammation. These assays all showed very little cytotoxicity as measured by LDH. However, increased expression of CXCL1 and Il6 mRNAs were observed and these signals are important for understanding low-dose exposures to Qdots. The rapid rise in mRNA expression between controls and the lowest Qdot dose for the BMDM and AM indicates that additional studies are needed to characterize low dose exposures—in particular for the AM where the maximum expression response occurs at the lowest dose. The differences between A/J and C57BL/6J show that inflammation responses are dependent on genetic background.
The complexity of the patterns in Figure 6 also relate to the differences in the measured doses between cell types indicating that the degree of uptake from the Qdots is important and that these responses need to be interpreted in terms of the in vitro disposition and kinetics of Qdots. For example, comparing the Cxcl1 response among cell types shows that BMDM had a 15–30 fold greater mRNA response and 2–40 fold greater cadmium uptake than AM and ALI MTEC. AM and ALI MTEC shows similar high values of Cxcl1 mRNA expression response but 20-fold higher measured cadmium uptake doses for the AM. For Il6 we see a somewhat simpler pattern with the mRNA response and measured cadmium uptake doses for BMDM being higher than for AM which in turn is higher than ALI MTEC suggesting that interpretation of inflammatory signals may be somewhat easier for this cytokine.
Our studies suggest that both airway epithelial cells and AM are sentinels of inhaled nanoparticles. One response to such exposures is the secretion of factors that function primarily to promote the influx of other inflammatory leukocytes, and for the epithelial cells, our data suggest that their response – i.e., selective increase of neutrophil chemokines, CXCL1/2 – is directed more toward promoting an acute neutrophil influx than other leukocytes. Our data also indicate that macrophages respond more robustly than do epithelial cells. Considering that macrophages showed a much greater capacity to take up these nanoparticles and because they are established key effector cells of inflammation, their more potent response is not unexpected.
Our findings that BMDM–which reflect infiltrated macrophages–responded more potently to Qdot exposure than did resident AM suggests persistent airway exposure to these nanomaterials could lead to enhanced and prolonged inflammation. An initial exposure would stimulate resident AM and, to a lesser extent, airway epithelial cells, to release factors that would promote the recruitment of cells from the circulation. If nanomaterial exposure continues, the infiltrated macrophages could mount a much more vigorous inflammatory response with potentially deleterious consequences. Of course, in vivo exposure studies will be needed to test this hypothesis.
Supplementary Material
Acknowledgments
The authors thank Jianbo Yu and Russell Dills in the UW Environmental Health Laboratory for technical support with cadmium analysis, Collin White for assistance in fluorescence imaging of Qdot uptake, Timothy Birkland and Brian Johnson for help with the multiplex assays, and Maura Newell for technical support with the mRNA assays.
Footnotes
Declaration of Interest
The authors have no financial or consulting interests that impacts the work presented. This work was supported by NIH grants U19ES019545, P30ES07033, HL089455, and DK089507 and by the University of Washington Cystic Fibrosis Foundation Research and Development Program.
References
- Alm AS, Li K, Yang D, Andersson R, Lu Y, Wang X. Varying susceptibility of pulmonary cytokine production to lipopolysaccharide in mice. Cytokine. 2010;49:256–263. doi: 10.1016/j.cyto.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Bagalkot V, Gao X. siRNA-aptamer chimeras on nanoparticles: preserving targeting functionality for effective gene silencing. ACS Nano. 2011;5:8131–8139. doi: 10.1021/nn202772p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Wilson MR, Macnee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175:191–199. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- Clapp AR, Medintz IL, Uyeda HT, Fisher BR, Goldman ER, Bawendi MG, Mattoussi H. Quantum dot-based multiplexed fluorescence resonance energy transfer. J Am Chem Soc. 2005;127:18212–18221. doi: 10.1021/ja054630i. [DOI] [PubMed] [Google Scholar]
- Clift MJ, Brandenberger C, Rothen-Rutishauser B, Brown DM, Stone V. The uptake and intracellular fate of a series of different surface coated quantum dots in vitro. Toxicology. 2011;286:58–68. doi: 10.1016/j.tox.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Committee for Review of the Federal Strategy to Address Environmental, H., And Safety Research Needs for Engineered Nanoscale Materials, Committee on Toxicology, National Research Council. Review of Federal Strategy for Nanotechnology-Related Environmental, Health, and Safety Research. 2009. [Google Scholar]
- Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- Hu X, Gao X. Silica-polymer dual layer-encapsulated quantum dots with remarkable stability. ACS Nano. 2010;4:6080–6086. doi: 10.1021/nn1017044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LK, Rims CR, Gill SE, Mcguire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassim SY, Gharib SA, Mecham BH, Birkland TP, Parks WC, Mcguire JK. Individual matrix metalloproteinases control distinct transcriptional responses in airway epithelial cells infected with Pseudomonas aeruginosa. Infect Immun. 2007;75:5640–5650. doi: 10.1128/IAI.00799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann AD, Parak WJ, Zhang F, Ali Z, Rocker C, Nienhaus GU, Gehr P, Rothen-Rutishauser B. Fluorescent-magnetic hybrid nanoparticles induce a dose-dependent increase in proinflammatory response in lung cells in vitro correlated with intracellular localization. Small. 2010;6:753–762. doi: 10.1002/smll.200901770. [DOI] [PubMed] [Google Scholar]
- Li JJ, Muralikrishnan S, Ng CT, Yung LY, Bay BH. Nanoparticle-induced pulmonary toxicity. Exp Biol Med. 2010;235:1025–1033. doi: 10.1258/ebm.2010.010021. [DOI] [PubMed] [Google Scholar]
- Manicone AM, Birkland TP, Lin M, Betsuyaku T, Van Rooijen N, Lohi J, Keski-Oja J, Wang Y, Skerrett SJ, Parks WC. Epilysin (MMP-28) restrains early macrophage recruitment in Pseudomonas aeruginosa pneumonia. J Immunol. 2009;182:3866–3876. doi: 10.4049/jimmunol.0713949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcconnachie LA, Botta D, White CC, Weldy CS, Wilkerson HW, Yu J, Dills R, Yu X, Griffith WC, Faustman EM, Farin FM, Gill SE, Parks WC, Hu X, Gao X, Eaton DL, Kavanagh TJ. The glutathione synthesis gene Gclm modulates amphiphilic polymer-coated CdSe/ZnS quantum dot-induced lung inflammation in mice. PLoS One. 2013a;8:e64165. doi: 10.1371/journal.pone.0064165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcconnachie LA, White CC, Botta D, Zadworny ME, Cox DP, Beyer RP, Hu X, Eaton DL, Gao X, Kavanagh TJ. Heme oxygenase expression as a biomarker of exposure to amphiphilic polymer-coated CdSe/ZnS quantum dots. Nanotoxicology. 2012 doi: 10.3109/17435390.2011.648224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcconnachie LA, White CC, Botta D, Zadworny ME, Cox DP, Beyer RP, Hu X, Eaton DL, Gao X, Kavanagh TJ. Heme oxygenase expression as a biomarker of exposure to amphiphilic polymer-coated CdSe/ZnS quantum dots. Nanotoxicology. 2013b;7:181–191. doi: 10.3109/17435390.2011.648224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Steinbruck A, Gao J, Doggett N, Hollingsworth JA, Iyer R. Comprehensive analysis of the effects of CdSe quantum dot size, surface charge, and functionalization on primary human lung cells. ACS Nano. 2012;6:4748–4762. doi: 10.1021/nn204886b. [DOI] [PubMed] [Google Scholar]
- Paun A, Lemay AM, Tomko TG, Haston CK. Association analysis reveals genetic variation altering bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol. 2013;48:330–336. doi: 10.1165/rcmb.2012-0078OC. [DOI] [PubMed] [Google Scholar]
- Pellegrino T. Hydrophobic Nanocrystals Coated with Amphiphilic Polyer Shell: A General Route to Water Soluable Nanocrystals. Nano Lett. 2004;4:703–707. [Google Scholar]
- Pinaud F, Michalet X, Bentolila LA, Tsay JM, Doose S, Li JJ, Iyer G, Weiss S. Advances in fluorescence imaging with quantum dot bio-probes. Biomaterials. 2006;27:1679–1687. doi: 10.1016/j.biomaterials.2005.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Gao X. Quantum dot-amphipol nanocomplex for intracellular delivery and real-time imaging of siRNA. ACS Nano. 2008;2:1403–1410. doi: 10.1021/nn800280r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JR, Antonini JM, Porter DW, Chapman RS, Scabilloni JF, Young SH, Schwegler-Berry D, Castranova V, Mercer RR. Lung toxicity and biodistribution of Cd/Se-ZnS quantum dots with different surface functional groups after pulmonary exposure in rats. Part Fibre Toxicol. 2013;10:5. doi: 10.1186/1743-8977-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal SJ, Chang JC, Kovtun O, Mcbride JR, Tomlinson ID. Biocompatible quantum dots for biological applications. Chem Biol. 2011;18:10–24. doi: 10.1016/j.chembiol.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Porter D, Schulte P, Kagan VE, Fadeel B, Castranova V. Mechanisms of pulmonary toxicity and medical applications of carbon nanotubes: Two faces of Janus? Pharmacol Ther. 2009;121:192–204. doi: 10.1016/j.pharmthera.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Soenen SJ, Demeester J, De Smedt SC, Braeckmans K. The cytotoxic effects of polymer-coated quantum dots and restrictions for live cell applications. Biomaterials. 2012;33:4882–4888. doi: 10.1016/j.biomaterials.2012.03.042. [DOI] [PubMed] [Google Scholar]
- Yang L, Mao H, Wang YA, Cao Z, Peng X, Wang X, Duan H, Ni C, Yuan Q, Adams G, Smith MQ, Wood WC, Gao X, Nie S. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small. 2009;5:235–243. doi: 10.1002/smll.200800714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1315–1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- Yu LE, Lanry Yung LY, Ong CN, Tan YL, Balasubramaniam KS, Hartono D, Shui G, Wenk MR, Ong WY. Translocation and effects of gold nanoparticles after inhalation exposure in rats. Nanotoxicology. 2007;1:235–242. [Google Scholar]
- Zeidler-Erdely PC, Kashon ML, Li S, Antonini JM. Response of the mouse lung transcriptome to welding fume: effects of stainless and mild steel fumes on lung gene expression in A/J and C57BL/6J mice. Respir Res. 2010;11:70. doi: 10.1186/1465-9921-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pan H, Zhang P, Gao N, Lin Y, Luo Z, Li P, Wang C, Liu L, Pang D, Cai L, Ma Y. Functionalized quantum dots induce proinflammatory responses in vitro: the role of terminal functional group-associated endocytic pathways. Nanoscale. 2013;5:5919–5929. doi: 10.1039/c3nr01653f. [DOI] [PubMed] [Google Scholar]
- Zrazhevskiy P, Gao X. Multifunctional quantum dots for personalized medicine. Nano Today. 2009;4:414–428. doi: 10.1016/j.nantod.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.