Abstract
The roles of the extracellular domain of type II TGF-β receptor (TBRII-ECD) in physiological processes ranging from development to cancer to wound healing render it an attractive target for exploration with chemical tools. For such applications, large amounts of active soluble protein are needed, but the yields of TBRII-ECD we obtained with current folding protocols were variable. To expedite the identification of alternative folding conditions, we developed an on-plate screen. This assay indicated that effective folding additives included the non-detergent sulfobetaine-201 (NDSB-201). Although NDSB-201 can facilitate protein folding, the mode by which it does so is poorly understood. We postulated that specific interactions between NDSB-201 and TBRII-ECD might be responsible. Analysis by X-ray crystallography indicates that the TBRII-ECD possesses a binding pocket for NDSB-201. The pyridinium group of the additive stacks with a phenylalanine side chain in the binding site. The ability of NDSB-201 to occupy a pocket on the protein provides a molecular mechanism for the additive’s ability to minimize TBRII-ECD aggregation and stabilize the folded state. NDSB-201 also accelerates TBRII-ECD crystallization, suggesting it may serve as a useful crystallization additive for proteins refolded with it. Our results also suggest there is a site on TBRII-ECD that could be targeted by small-molecule modulators.
Keywords: Non-detergent sulfobetaine 201, Transforming growth factor β, Type II transforming growth factor β, receptor, Protein folding, Pharmacological chaperone
Introduction
Transforming growth factor beta (TGF-β)2 signaling controls cell proliferation, differentiation, and tissue remodeling [1]. There are three main TGF-β members – TGF-β1, TGF-β2, and TGF-β3. TGF-β1 and TGF-β3 are 25 kDa homodimeric proteins that form a multimeric assembly with the type II TGF-β receptors (TBRII) [2]. The resulting complexes bind to type I receptors (TBRI) and promote their phosphorylation. This activated TGF-β/TBRI/TBRII kinase complex then mediates SMAD protein phosphorylation, which leads to the translocation of phospho-SMAD proteins into the nucleus where they regulate the expression of numerous genes [3]. The outcome of TGF-β responses depends upon the concentration of TGF-β, cell age, state of differentiation, presence of other growth factors, and the cellular environment [4]. Therefore, spatial and temporal control of TGF-β signaling is crucial for directing cell differentiation or to aid wound healing. Alternatively, dysregulation of TGF-β signaling has been implicated in several diseases, including proliferation of cancer cells and vascularization of tumors [5].
Because of the myriad roles of TGF-β signaling, chemical tools that modulate TGF-β signaling are valuable. An inhibitor of the kinase activity of the TBRI, SB-431542, blocks migration and vascular endothelial growth factor secretion, processes which are crucial for cancer growth and metastasis [6]. Exogenous application of TGF-β3 to wounds reduces scarring [7]. These diverse signaling outcomes led us to seek synthetic ligands for the extracellular domains of TBR that are orthogonal to TGF-β. Our goal was to immobilize such ligands to control TGF-β signaling, such that the pathway could be activated locally, as would be desirable for promoting wound healing.
We identified two peptide ligands (LTGKNFPMFHRN and MHRMPSFLPTTL) that can bind both the extracellular domains (ECDs) of TBRI and TBRII to pre-organize the receptors on the cell surface [8]. This preorganization increases the sensitivity of peptide-bound cells to minute concentrations (pM) of soluble TGF-β and allows cell differentiation in a spatially-controlled manner [9,10]. To exploit the potential of these peptides and design next-generation probes of TBR function, a molecular level knowledge of their binding mode is needed. To pursue the required structural and biophysical studies, large quantities of TBRs are required. In our hands, the previously reported expression and purification protocols resulted in variable yields of functional TBRII-ECD [11-13]. The variability resulted from the folding step; therefore, we sought to develop an on-plate screen to optimize folding.
We employed thioredoxin-fused TBRII-ECD (Trx-TBRII-ECD) in a folding screen that surveyed a variety of conditions. Non-detergent sulfobetaine 201 (NDSB-201) was found to be an effective additive, with a yield of active TBRII-ECD up to threefold higher than previously observed. NDSB-201 also facilitates the folding of urea-denatured, protease-resistant untagged TBRII-ECD (TBRII-ECD-PR) in solution. To understand the molecular basis for the ability of NDSB-201 to promote folding, we used X-ray crystallography. The data reveal that TBRII has an NDSB-201 binding site. These findings suggest that NDSB-201 acts as a pharmacological chaperone [14-16] not only by preventing aggregation of folding intermediates but also by binding and stabilizing the folded state[14-18].
Materials and methods
Expression of thioredoxin-fused TBRII (20–136) (Trχ-TBRII-ECD)
The sequence encoding the extracellular domain of TBRII (20–136) was amplified from a TBRII construct (encoding amino acids 15–136) [12] and cloned into the KpnI and NcoI sites of pET32b. Upstream of the multiple cloning sites is a thioredoxin coding sequence, so the resulting construct encodes a fusion protein. The plasmid was transformed into the Tuner (DE3) strain (Novagen), a BL21 (DE3) derivative with a lactose permease deletion allowing uniform induction by isopropyl β-thiogalactopyranose (IPTG). Cells were grown in Terrific Broth (Research Products International Corp.) at 37 °C to OD600 of 0.6. Gene expression was induced by addition of 0.1 mM IPTG and the cells were incubated for 14 h at 15 °C. Bacteria were harvested by centrifugation and lysed (25 g wet pellet) via sonication in 50 mL of 50 mM sodium phosphate (pH 7.4), 300 mM NaCl, 0.1 mg/mL lysozyme, 2.5 mM benzamidine, 1 mM phenylmethyl-sulfonyl fluoride (PMSF), and 1 tablet/100 mL SIGMAFAST™ Protease Inhibitor Cocktail Tablet (EDTA-Free) (Sigma–Aldrich). Lysate was clarified by centrifugation at 100,000 ×g for 1 h and filtration through a 0.45 μm syringe filter.
Folding assay
An on-plate screen for protein folding was implemented. Clarified lysate (200 μL) containing Trx-TBRII-ECD was added to each well of a Ni–NTA HisSorb plate (Qiagen). After 2 h at room temperature, the plate was washed 3 times for 5 min each with 200 μL of 50 mM sodium phosphate (pH 7.4), 300 mM NaCl, and 25 mM imidazole. Buffered solutions were screened for their ability to support folding (200 μL) by adding them to each well and holding the samples at 4 °C for 40 h. The plate was then washed with phosphate-buffered saline (PBS) with 0.05% Tween 20 (PBST). The relative amount of correctly folded TBRII-ECD was measured directly on the plate using a previously described protocol that monitors TGF-β1 interaction with the receptor [13]. Briefly, the plate was blocked with 200 μL of 5% BSA in PBST at 37 °C for 1.5 h. TGF-β1 (5 ng/mL, 100 μL, Cell Signaling Technology) was added. After 2 h at room temperature (rt), a solution of 100 μL (100 ng/mL) of biotinylated anti-TGF-β1 (BAF240, R&D Systems) was added, and the mixture was allowed to stand at rt for 1 h. After washing, 100 μL (200 ng/mL) of streptavidin-HRP (Jackson Immunoresearch) was added. After 30 min, functional complex formation was monitored via HRP activity by addition of the colorimetric substrate 1-Step Ultra TMB (100 μL, Pierce). The reaction was quenched with addition of 100 μL of 2 M sulfuric acid. Absorbance at 450 nm was measured to quantify the amount of correctly folded TBRII-ECD.
Production of TBRII (26–136) Q26A K97T (TBRII-ECD-PR)
The gene encoding untagged TBRII (26–136) containing two substitutions, Q26A and K97T (derived from Trx-TBRII-ECD by site-directed mutagenesis) that should confer protease resistance, was ligated into a pET24a plasmid between the NdeI and BamHI sites. The resulting construct was transformed into Tuner (DE3) cells. The bacteria were grown in Terrific Broth supplemented with 50 μg/mL kanamycin and 0.2% glucose until OD600 reached 0.6. Gene expression was induced with 1 mM IPTG, and the mixture was cultured for 6–8 h. Cells were collected by centrifugation and kept frozen at −80 °C until use.
Folding and purification of TBRII-ECD-PR
Production of TBRII-ECD-PR afforded inclusion bodies, which were isolated using a procedure similar to a protocol for production of TGF-β3 described previously [19]. Briefly, bacterial pellets were resuspended in 100 mM Tris (pH 8.0), 10 mM EDTA, 0.1 mg/mL lysozyme, 2.5 mM benzamidine, and 1 mM PMSF. The mixture was sonicated, and the insoluble material was isolated by centrifugation. The pellet was resuspended in 100 mM Tris (pH 8.0), 10 mM EDTA, and 1% Triton X-100 and sonicated again. After centrifugation, the pellet that contained the inclusion bodies was resuspended by sonication in 100 mM Tris (pH 8.0), 10 mM EDTA, and 1 M NaCl. The resulting suspension of washed inclusion bodies was pelleted. This pellet was solubilized in 20 mM Tris (pH 8.0), 20 mM Bis-Tris, 100 mM DTT and 8 M urea at room temperature using a homogenizer. Insoluble materials were removed by centrifugation and the pH of the supernatant was adjusted to 6.0. After passage through a 0.22 μm filter, the solution was loaded onto an anion exchange column (HiTrap Q FF, GE Healthcare) equilibrated with 20 mM Bis-Tris pH 6.0 and 8 M urea. TBRII-ECD-PR was eluted using a linear gradient of 0–300 mM NaCl. Fractions containing the desired protein were identified after analysis by SDS–PAGE. The protein concentration of the eluents was estimated by UV absorbance at 280 nm using an extinction coefficient of 8480 M− cm−1[20]. The protein was dispensed as 50 mg aliquots and kept at −80 °C until use.
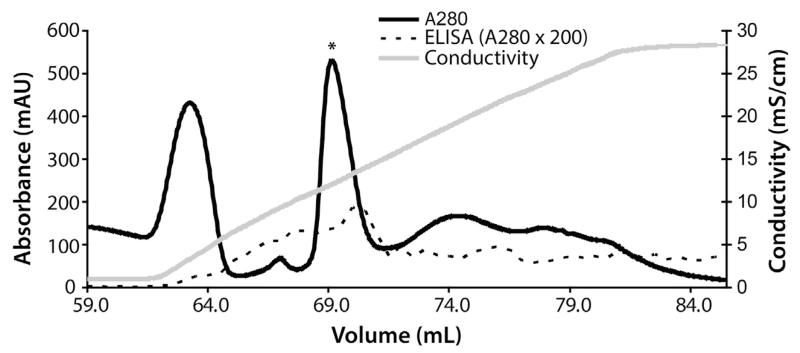
To initiate folding, the protein solution was added dropwise to a chilled solution of 75 mM Tris (pH 8.0), 1 M NDSB-201, 2 mM reduced glutathione (GSH), and 0.5 mM oxidized glutathione (GSSG). The protein concentration was kept at approximately 0.1 mg/mL. After stirring for 2 days at 4 °C, the solution was concentrated using an Amicon stirred cell (3 kDa cut-off) under nitrogen pressure, and the concentrate was dialyzed extensively against 20 mM Bis-Tris (pH 6.0). Refolded protein was captured with an anion exchange column (HiTrap Q HP, GE Healthcare) equilibrated with 20 mM Bis-Tris (pH 6.0). Protein was eluted with a linear gradient of 0–300 mM NaCl (Fig. 1). Fractions containing properly folded TBRII-ECD-PR were identified using an ELISA protocol similar to that employed in the aforementioned folding assay. For this ELISA, TBRII-ECD-PR was absorbed directly onto a Nunc MaxiSorp ELISA plate (Thermo Scientific). We found that this format could also be used for a functional binding assay. Active fractions (i.e., those with functional protein) were pooled and dialyzed against 50 mM sodium phosphate (pH 7.0). Ammonium sulfate was added to 1 M, and the resulting solution was subjected to hydrophobic interaction chromatography (HiTrap Butyl HP, GE Healthcare) to capture minor impurities. Active TBRII-ECD-PR did not bind the column under these conditions. The TBRII-ECD-PR-containing solution (flow through) was purified further on a Superdex 75 16/60 column using 20 mM Tris (pH 7.4), 300 mM NaCl as a running buffer.
Fig. 1.
Representative ion-exchange chromatogram of the refolded TBRII-ECD-PR. The peak containing active TBRII-ECD-PR is marked with an asterisk.
Crystallization and structural analysis of TBRII-ECD-PR
Purified TBRII-ECD-PR was dialyzed extensively against water and concentrated to 20 mg/mL. The protein concentration of the final solution was determined by UV absorbance at 280 nm using an extinction coefficient of 9230 M−1 cm−1[20]. The resulting sample was subjected to previously reported crystallization conditions (100 mM sodium citrate pH 5.0, 30% PEG 2000) supplemented with 50 mM NDSB-201 [21]. Crystals appeared and grew to full size in 2–3 days. This time scale is accelerated compared with that reported previously (and consistent with our experience) of 7–14 days for conditions in which NDSB-201 is absent [21]. The crystals were cryoprotected using paratone, then vitrified and stored in liquid nitrogen. Single crystal diffraction data were collected at beamline 21-ID-D (LS-CAT) at the Advanced Photon Source, Argonne National Laboratory. Data reduction and scaling were performed with HKL2000 [22] (Table 1). Molecular replacement was performed with PHENIX AutoMR [23,24] using a previously reported TBRII structure (PDB ID 1M9Z) [25] as a search model. NDSB-201 coordinates and restraints were generated using eLBOW [26]. Coot [27] and PHENIX.refine [28], respectively, were used for model fitting and refinement (Table 1). The model was validated using MolProbity [29]. Figures depicting the protein structure were generated with PyMOL [30].
Table 1.
Crystallographic statistics.
| Data collection statistics | |
| Wavelength (Å) | 0.97919 |
| Resolution range (Å)* | 22.1–1.50 (1.55–1.50) |
| Space group | P 212121 |
| Unit cell (Å) | 33.6, 40.5, 75.8 |
| Total reflections | 104,863 (10,083) |
| Unique reflections | 17,064 (1,653) |
| Multiplicity | 6.1 (6.1) |
| Completeness (%) | 99.5 (98.5) |
| Mean I/σ (I) | 18.7 (3.9) |
| Wilson B-factor (Å2) | 17.9 |
| R-merge | 0.054 (0.419) |
| R-meas | 0.060 (0.458) |
| R-pim | 0.024 (0.182) |
| Refinement statistics | |
| Resolution range (Å) | 21.4–1.50 (1.55–1.50) |
| R-factor | 0.171 (0.194) |
| R-free (10%) | 0.206 (0.242) |
| Number of atoms | 1006 |
| Protein | 890 |
| Ligand | 26 |
| Water | 90 |
| Protein residues | 102 |
| RMSD (bonds, Å) | 0.007 |
| RMSD (angles, °) | 1.18 |
| Ramachandran favored (%) | 98.0 |
| Ramachandran outliers (%) | 0.0 |
| Average B-factor (Å2) | 22.4 |
| Protein | 20.8 |
| Ligand | 36.3 |
| Solvent | 34.6 |
Statistics for the highest-resolution shell are shown in parentheses. The coordinate and structure factors are deposited at the Protein Data Bank under accession code 4P7U.
R-merge = .
R-meas = .
R-pim = .
Refinement R-factor = .
Results
Folding screen for the extracellular domain of TBRII
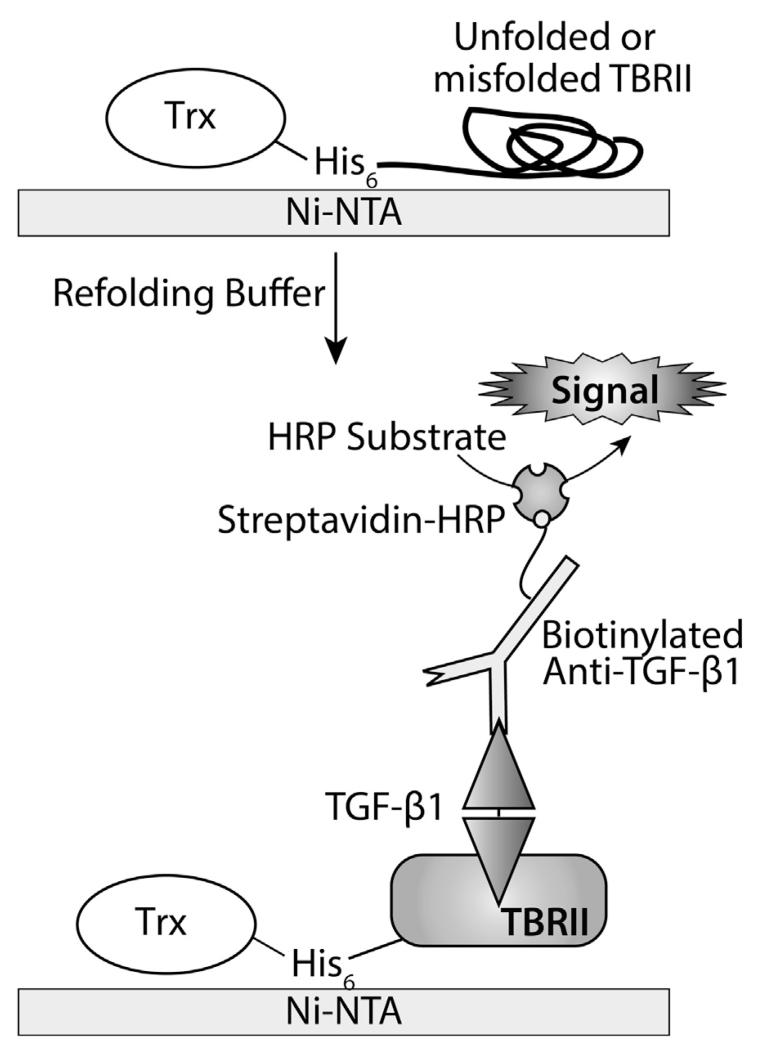
Because TBRII engagement by TGF-β precedes signaling, the receptor is an attractive target for chemical tools and therapeutics that modulate TGF-β activity. An efficient protocol to produce active TBRII-ECD is therefore an enabling technology. Our attempts to follow reported protocols for folding TBRIII-ECD from inclusion bodies [11,12,31] afforded highly variable yields of active protein; often less than 1 mg of protein was recovered from 50 mg of solubilized inclusion bodies. Although a thioredoxin fusion enhanced the solubility of chicken Trx-TBRII-ECD [32,33], production of the corresponding human Trx-TBRII-ECD fusion resulted in soluble but nonfunctional protein that failed to bind TGF-β1. A protocol for folding Trx-TBRII-ECD on Ni–NTA agarose was previously reported [13]; however, it employs a high concentration of arginine at pH 8.0, which interferes with protein binding to Ni–NTA agarose [34]. Still, we were inspired by this heterogenous folding strategy [13] to examine on-plate folding as a means of rapidly identifying improved conditions. For this assay, soluble Trx-TBRII-ECD was immobilized onto a Ni–NTA-coated 96-well plate (Fig. 2), and different conditions were tested. Each well was treated with an amalgam of the background buffer, one additive, and one redox couple; this mixture constituted a “folding buffer”. In total, 81 conditions were screened. Because TBRII-ECD contains multiple disulfide bonds, solutions that varied in redox potential were tested. To facilitate disulfide exchange, the pH was held constant at 8.0, which is similar to the pKa of cysteine thiol. The additives examined were selected on the basis of literature precedents[35-38] (Table 2). To quantify the efficiency of folding, a functional assay was employed that involved monitoring the amount of TGF-β1 bound as detected by a TGF-β1-specific antibody (Fig. 3).
Fig. 2.
Schematic depicting the on-plate refolding assay and ELISA scheme utilized to detect correctly folded Trx-TBRII-ECD.
Table 2.
Composition of buffers used in the on-plate refolding screen.
| Buffer | Additives | Redox buffer |
|---|---|---|
| 75 mM Tris pH 8.0 | Sorbitol (0.5, 1, 2 M) | 2 mM GSH + 0.5 mM GSSG |
| Urea (1,2, 4 M) | 2 mM GSH + 0.2 mM GSSG | |
| NaCl (100, 250 mM) | 2 mM GSH + 0.05 mM GSSG | |
| PEG 3350 (0.05%, 0.1%, 0.5%) | ||
| PEG 6000 (0.05%, 0.1%, 0.5%) | ||
| MgCl2 + CaCl2 (1 mM each) | ||
| β-Cyclodextrin (5, 10 mM) | ||
| β-Cyclodextrin (5, 10 mM) | ||
| NDSB-195 (0.5, 1 M) | ||
| NDSB-201 (0.5, 1 M) | ||
| NDSB-221 (0.5, 1 M) | ||
| NDSB-256 (0.5, 1 M) |
Fig. 3.
Preliminary screen for refolding conditions of Trx-TBRII-ECD. Relative amount of properly folded TBRII was measured by absorbance reading of the ELISA product.
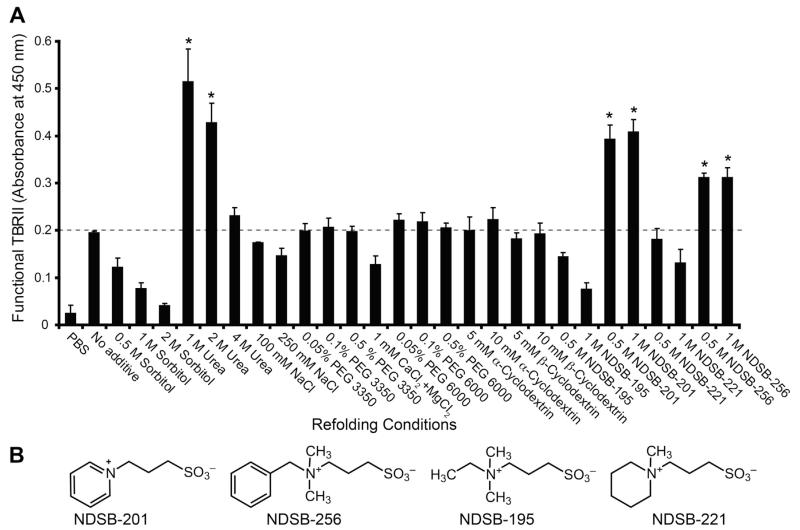
A single experiment folding screen indicated that urea, NDSB-201, or NDSB-256 each can serve as an effective additive (Fig. 3). Compared to the efficiency of folding obtained using PBS, the use of a redox buffer at pH 8.0 improves the yield of folded Trx-TBRII-ECD. We therefore held the redox couple constant (2 mM GSH + 0.5 mM GSSG) and repeated the refolding experiment in triplicate (Fig. 4). Using the level of folding obtained in redox buffer alone as a baseline, the influence of additives was compared. When either urea, NDSB-201, or NDSB-256 was added, a high level of active Trx-TBRII-ECD was obtained.
Fig. 4.
(A) Application of the Trx-TBRII-ECD refolding screen using a constant redox buffer: 2 mM GSH + 0.5 mM GSSG. Error bars represent standard deviations of triplicate measurements. Dashed line indicates the level of active Trx-TBRII-ECD when no additive is present, setting the baseline for effective additives. (B) Structures of the NDSB additives tested.
Although urea was effective, we focused on the NDSBs. NDSBs can promote protein folding but their mechanism of action is poorly understood. We reasoned that their effects on TBRII-ECD might be leveraged to provide insight into the mechanism of NDSB-aided folding. Moreover, we postulated that these additives could bind TBRII-ECD. This hypothesis arose because all NDSBs are zwitterionic but not all NDSBs improved folding in our assay, and these two observations suggest that the effects of the NDSBs on folding are unlikely to arise solely from bulk solution effects. As NDSB-201 was the most cost-effective of the useful additives ( ~$0.4/g vs ~$13/g), it was used in larger scale solution phase folding experiments of the untagged TBRII-ECD-PR construct that had been used previously for structure determination [25]. Our optimized conditions consisted of 75 mM Tris (pH 8.0), 1 M NDSB-201, 2 mM reduced glutathione (GSH), and 0.5 mM oxidized glutathione (GSSG) at 4 °C for 40 h. This protocol yields 8–13 mg of purified TBRII-ECD-PR from 50 mg of urea-solubilized protein. This protocol was successful for either Trx-TBRII-ECD or TBRII-ECD-PR, suggesting that the conditions identified in the on-plate folding assay were also effective for folding in solution. Because the protease-resistant variants of TBRII-ECD-PR undergo folding, we conclude these mutations do not impact folding significantly.
Crystallographic analysis of TBRII–NDSB-201 complex
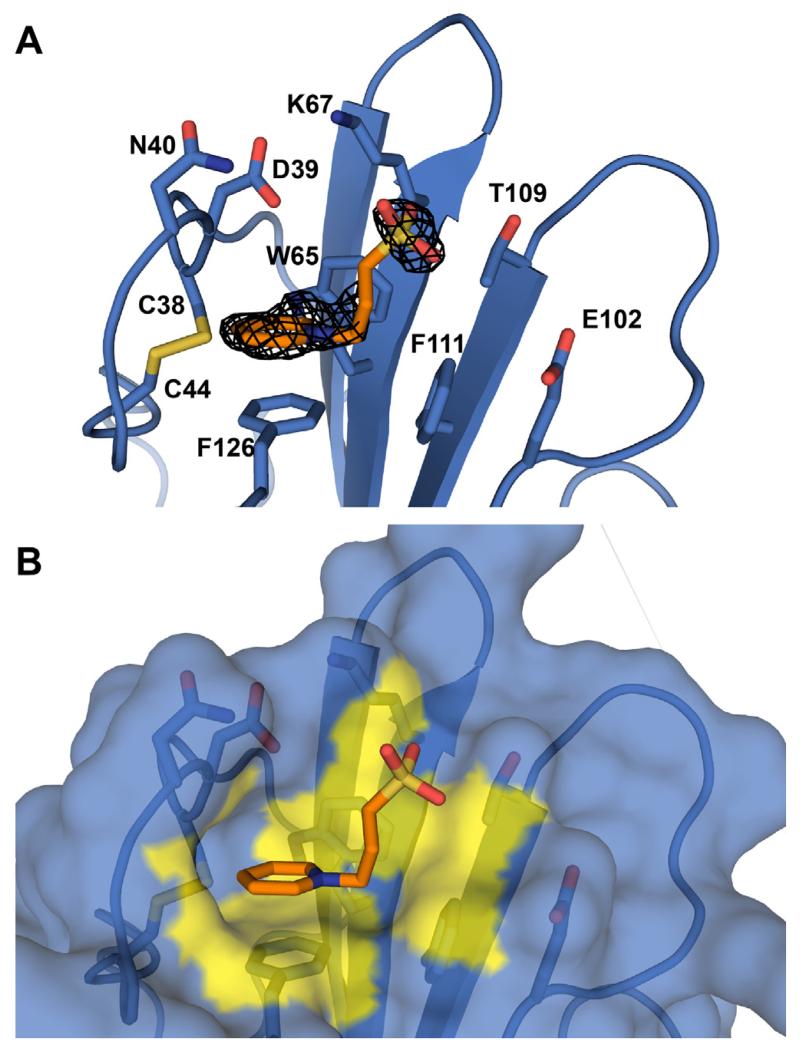
We used X-ray crystallography to examine whether NDSB-201 binds to the TBRII fusion protein. Purified TBRII-ECD-PR could be crystallized using previously reported conditions [21]. When NDSB-201 was added, protein crystals grew and reached full size in 2–3 days as opposed to 1–2 weeks. Analysis by X-ray crystallography revealed a molecule of NDSB-201 bound to TBRII-ECD-PR, as the electron density of the pyridinium ring and the sulfonate group were apparent in the difference density map (Fig. 5). The NDSB-201 interaction site is a hydrophobic pocket formed by W65, F111, F126, and a disulfide bridge involving cysteine residues at positions 38 and 44. The cationic pyridinium ring of NDSB-201 stacks onto the phenyl ring π system of F126 in a staggered conformation. The pyridinium ring also interacts with W65 in an edge-on manner. There are additional contacts to several adjacent hydrophilic residues (D39, N40, K67, E102, and T109); however, functional group atoms of these side chains are more than 4 Å away from the sulfonate group of NDSB-201 and may not contribute substantially to the NDSB-201 binding. The total buried surface area of the complex is 375 Å2 (PISA) [39]. These results indicate that NDSB-201 occupies a binding pocket and engages in favorable interactions with TBRII-ECD.
Fig. 5.
Crystallographic analysis of TBRII-ECD:NDSB-201 complex. (A) Structure of NDSB-201 (orange) bound to TBRII-ECD-PR (blue). The mFo – DFc difference map (black mesh, contoured 3.0 σ above the mean) was calculated after the ligand was removed and the structure re-refined. (B) Surface representation of the binding site. TBRII-ECD-PR surface atoms that are within 4 Å of the ligand are highlighted in yellow. Functional groups of hydrophilic side chains are not close enough to interact with NDSB-201. Most interactions are cationic π-π stacking and hydrophobic. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Discussion and conclusions
An on-plate assay was developed to rapidly identify conditions that promote folding of the Trx-TBRII-ECD. The most effective conditions from the plate assay were also useful in folding untagged TBRII (TBRII-ECD-PR) in solution, indicating that the results from the higher throughput assay translated well to solution refolding. The assay provided valuable information about chemical sensitivity of TBRII-ECD during the folding process. Specifically, a low concentration of urea is highly effective at folding TBRII-ECD, but the efficiency of that process rapidly dropped with increasing urea concentration (Fig. 4A). These results highlight the importance of controlling urea concentration when applying the previously published protocol [12]. Because our screen covers significant chemical space of known refolding reagents, it could readily be applied to other monomeric receptors or enzymes for which an appropriate detection system for functionally folded protein is available. Small amounts of candidate folding buffers can readily be generated by mixing stock solutions in 96-well blocks and transferring them directly to the folding plate; accordingly, this assay and screening format are readily adaptable for a high-throughput platform using liquid handling robots.
The additives we identified, urea and members of the NDSB series, are aggregation suppressors [40,41], suggesting that a major bottleneck of TBRII-ECD folding is aggregation. Still, NDSB-201 and NDSB-256 outperformed other NDSBs in this screen, which led us to test for a binding site on the receptor. NDSB-201 and NDSB-256 contain pyridinium and benzyl groups, respectively, that can engage in arene–arene interactions with accessible aromatic amino acids [42]. The less effective zwitterionic additives, NDSB-195 and NDSB-221, possess quaternary ammonium groups, but lack an aromatic substituent that can participate in aromatic stacking (Fig. 4B).
Addition of NDSB-201 to crystallization solutions affords a complex of NDSB-201 and TBRII-ECD-PR; electron density maps indicate NDSB-201 interacts with TBRII-ECD-PR in a shallow hydrophobic pocket composed of aromatic residues. The cationic pyridinium is oriented such that it stacks upon the aromatic phenylalanine in the binding site. Through this mode of interaction, NDSB-201 may stabilize the folded product in addition to preventing protein aggregation during folding, highlighting its potential to function as a pharmacological chaperone. NDSB-201 binding should therefore favor the native disulfide bond in the pocket and mask hydrophobic surfaces within TBRII-ECD to stabilize the folded state and prevent protein aggregation.
To date, there are six structures of protein:NDSB-201 complexes in the Protein Data Bank [43-48], and in each of them, NDSB-201 interacts with aromatic residues. These interactions provide a rationale by which this compound promotes crystallization. In these previously reported structures, however, NDSB-201 was used primarily as a crystallization additive or a cryoprotectant after the correctly folded protein has been obtained by other means. Our results illustrate that NDSB-201 can serve the dual roles of folding agent and crystallization additive. We postulate that for proteins that can be folded or refolded with NDSB-201, it will be advantageous to include NDSB-201 in crystallization trials.
The presence of a pocket for NDSB-201 on TBRII, suggests derivatives of this pyridinium-containing compound could be further optimized to generate probes of TGF-β signaling. Alignment of our NDSB-201-bound structure with that of the compounds of TBRII, TBRI and TGF-β3 (PDB ID 2PJY, Fig. 6) reveals that the identified NDSB-201 binding site on TBRII does not overlap with the regions bound by TGF-β3 or TBRI-ECD. Thus, the NDSB-201 binding site is distinct. These data are intriguing in that they suggest NDSB-201 is unlikely to block TGF-β binding. Still, as demonstrated previously [8,9], molecules that bind at a remote site can modulate TGF-β signaling. Our research not only suggests an alternative folding mechanism of TBRII-ECD by NDSB-201, but also reveals a potential small-molecule, binding pocket on TBRII-ECD that could be targeted for inhibitor or activator development.
Fig. 6.
Alignment of the NDSB-201-TBRII-ECD-PR complex structure to a structure of the TGF-β signaling complex. The location of NDSB-201 is indicated by the boxes.
Acknowledgments
This research was supported by NIGMS (GM055984). We thank Dr. Andrew P. Hinck for pET32a containing TBRII (15-136), advice, and various protocols. X-ray diffraction data collection was assisted by Dr. Kenneth A. Satyshur. K.W. was supported by the Development and Promotion of Science and Technology Talents Project of Thailand. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817). We thank Sayaka Masuko, Darryl A. Wesener, and Anna W. Baker for critical comments on the manuscript.
Footnotes
Abbreviations used: TBRII-ECD, extracellular domain of type II TGF-β receptor; TBRII-ECD-PR, protease resistant variant of TBRII-ECD; NDSB, non-detergent sulfobetaine; TGF-β, transforming growth factor β; TBRII, type II TGF-β receptor; TBRI, type I TGF-β receptor; Trx, thioredoxin; GSH, reduced glutathione; GSSG, oxidized glutathione.
References
- [1].Massague J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- [2].Groppe J, Hinck CS, Samavarchi-Tehrani P, Zubieta C, Schuermann JP, Taylor AB, Schwarz PM, Wrana JL, Hinck AP. Cooperative assembly of TGF-β superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol. Cell. 2008;29:157–168. doi: 10.1016/j.molcel.2007.11.039. [DOI] [PubMed] [Google Scholar]
- [3].Massague J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Massague J. How cells read TGF-β signals. Nat. Rev. Mol. Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- [5].Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N. Engl. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- [6].Halder SK, Beauchamp RD, Datta PK. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7:509–521. doi: 10.1593/neo.04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- [8].Li L, Orner BP, Huang T, Hinck AP, Kiessling LL. Peptide ligands that use a novel binding site to target both TGF-β receptors. Mol. BioSyst. 2010;6:2392–2402. doi: 10.1039/c0mb00115e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li L, Klim JR, Derda R, Courtney AH, Kiessling LL. Spatial control of cell fate using synthetic surfaces to potentiate TGF-β signaling. Proc. Natl. Acad. Sci. U.S.A. 2011;108:11745–11750. doi: 10.1073/pnas.1101454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pedersen RO, Loboa EG, LaBean TH. Sensitization of transforming growth factor-beta signaling by multiple peptides patterned on DNA nanostructures. Biomacromolecules. 2013;14:4157–4160. doi: 10.1021/bm4011722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Boesen CC, Motyka SA, Patamawenu A, Sun PD. Development of a recombinant bacterial expression system for the active form of a human transforming growth factor β type II receptor ligand binding domain. Protein Expr. Purif. 2000;20:98–104. doi: 10.1006/prep.2000.1306. [DOI] [PubMed] [Google Scholar]
- [12].Hinck AP, Kerfoot I, Walker P, Martina NR, Deepa S, Hinck CS, Freedberg DI. Sequential resonance assignments of the extracellular ligand binding domain of the human TGF-β type II receptor. J. Biomol. NMR. 2000;18:369–370. doi: 10.1023/a:1026775321886. [DOI] [PubMed] [Google Scholar]
- [13].Gasparian ME, Elistratov PA, Yakimov SA, Dolgikh DA, Kirpichnikov MP. An efficient method for expression in Escherichia coli and purification of the extracellular ligand binding domain of the human TGFβ type II receptor. J. Biotechnol. 2010;148:113–118. doi: 10.1016/j.jbiotec.2010.04.013. [DOI] [PubMed] [Google Scholar]
- [14].Janovick JA, Stewart MD, Jacob D, Martin LD, Deng JM, Stewart CA, Wang Y, Cornea A, Chavali L, Lopez S, Mitalipov S, Kang E, Lee HS, Manna PR, Stocco DM, Behringer RR, Conn PM. Restoration of testis function in hypogonadotropic hypogonadal mice harboring a misfolded GnRHR mutant by pharmacoperone drug therapy. Proc. Natl. Acad. Sci. U.S.A. 2013;110:21030–21035. doi: 10.1073/pnas.1315194110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mecozzi VJ, Berman DE, Simoes S, Vetanovetz C, Awal MR, Patel VM, Schneider RT, Petsko GA, Ringe D, Small SA. Pharmacological chaperones stabilize retromer to limit APP processing. Nat. Chem. Biol. 2014;10:443–449. doi: 10.1038/nchembio.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Oh M, Lee JH, Wang W, Lee HS, Lee WS, Burlak C, Im W, Hoang QQ, Lim HS. Potential pharmacological chaperones targeting cancer-associated MCL-1 and Parkinson disease-associated alpha-synuclein. Proc. Natl. Acad. Sci. U.S.A. 2014;111:11007–11012. doi: 10.1073/pnas.1320556111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mu TW, Ong DST, Wang YJ, Balch WE, Yates JR, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134:769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Makley LN, Gestwicki JE. Expanding the number of ‘druggable’ targets: non-enzymes and protein–protein interactions. Chem. Biol. Drug Des. 2013;81:22–32. doi: 10.1111/cbdd.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huang T, David L, Mendoza V, Yang Y, Villarreal M, De K, Sun L, Fang X, Lopez-Casillas F, Wrana JL, Hinck AP. TGF-β signalling is mediated by two autonomously functioning TβRI:TβRII pairs. EMBO J. 2011;30:1263–1276. doi: 10.1038/emboj.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- [21].Boesen CC, Motyka SA, Patamawenua A, Sun PD. Crystallization and preliminary crystallographic studies of human TGF-β type II receptor ligand-binding domain. Acta Crystallogr. D. 2002;58:1214–1216. doi: 10.1107/s0907444902007357. [DOI] [PubMed] [Google Scholar]
- [22].Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. In: Carter JCW, Sweet RM, editors. Methods in Enzymology: Macromolecular Crystallography, Part A. Academic Press; New York: 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- [23].Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwar PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Boesen CC, Radaev S, Motyka SA, Patamawenu A, Sun PD. The 1.1 Å crystal structure of human TGF-β type II receptor ligand binding domain. Structure. 2002;10:913–919. doi: 10.1016/s0969-2126(02)00780-3. [DOI] [PubMed] [Google Scholar]
- [26].Moriarty N, Grosse-Kunstleve R, Adams P. Electronic ligand builder and optimization workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D. 2009;65:1074–1080. doi: 10.1107/S0907444909029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of coot. Acta Crystallogr. D. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Afonine PV, Grosse-Kunstleve RW, Adams PD. The Phenix refinement framework. CCP4 Newsl. 2005;42:8. [Google Scholar]
- [29].Chen VB, Arendall WB, III, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].The PyMOL Molecular Graphics System, Version 1.3. Schrödinger LLC; 2010. [Google Scholar]
- [31].Glansbeek HL, Beuningen H.M.v., Vitters EL, Kraan P.M.v.d., Berg W.B.v.d. Expression of recombinant human soluble type II transforming growth factor-β receptor in Pichia pastoris and Escherichia coli: two powerful systems to express a potent inhibitor of transforming growth factor-b1. Protein Expr. Purif. 1998;12:201–207. doi: 10.1006/prep.1997.0819. [DOI] [PubMed] [Google Scholar]
- [32].Marlow MS, Brown CB, Barnett JV, Krezel AM. Solution structure of the chick TGFβ type II receptor ligand-binding domain. J. Mol. Biol. 2003;326:989–997. doi: 10.1016/s0022-2836(03)00023-8. [DOI] [PubMed] [Google Scholar]
- [33].Zuniga JE, Groppe JC, Cui Y, Hinck CS, Contreras-Shannon V, Pakhomova ON, Yang J, Tang Y, Mendoza V, Lopez-Casillas F, Sun L, Hinck AP. Assembly of TβRI:TβRII:TGFβ ternary complex in vitro with receptor extracellular domains is cooperative and isoform-dependent. J. Mol. Biol. 2005;354:1052–1068. doi: 10.1016/j.jmb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- [34].Abe R, Kudou M, Tanaka Y, Arakawa T, Tsumoto K. Immobilized metal affinity chromatography in the presence of arginine. Biochem. Biophys. Res. Commun. 2009;381:306–310. doi: 10.1016/j.bbrc.2009.01.054. [DOI] [PubMed] [Google Scholar]
- [35].Mishra R, Seckler R, Bhat R. Efficient refolding of aggregation-prone citrate synthase by polyol osmolytes. J. Biol. Chem. 2005;280:15553–15560. doi: 10.1074/jbc.M410947200. [DOI] [PubMed] [Google Scholar]
- [36].Cleland JL, Hedgepeth C, Wang DIC. Polyethylene glycol enhanced refolding of bovine carbonic anhydrase B. J. Biol. Chem. 1992;267:13327–13334. [PubMed] [Google Scholar]
- [37].Karuppiah N, Sharma A. Cyclodextrins as protein folding aids. Biochem. Biophys. Res. Commun. 1995;211:60–66. doi: 10.1006/bbrc.1995.1778. [DOI] [PubMed] [Google Scholar]
- [38].Vuillard L, Rabilloud T, Goldberg ME. Interactions of non-detergent sulfobetaines with early folding intermediates facilitate in vitro protein renaturation. Eur. J. Biochem. 1998;256:128–135. doi: 10.1046/j.1432-1327.1998.2560128.x. [DOI] [PubMed] [Google Scholar]
- [39].Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- [40].Collins T, D’Amico S, Georlette D, Marx JC, Huston AL, Feller G. A nondetergent sulfobetaine prevents protein aggregation in microcalorimetric studies. Anal. Biochem. 2006;352:299–301. doi: 10.1016/j.ab.2006.01.035. [DOI] [PubMed] [Google Scholar]
- [41].D’Amico S, Feller G. A nondetergent sulfobetaine improves protein unfolding reversibility in microcalorimetric studies. Anal. Biochem. 2009;385:389–391. doi: 10.1016/j.ab.2008.11.016. [DOI] [PubMed] [Google Scholar]
- [42].Salonen LM, Ellermann M, Diederich F. Aromatic rings in chemical and biological recognition: energetics and structures. Angew. Chem.-Int. Ed. 2011;50:4808–4842. doi: 10.1002/anie.201007560. [DOI] [PubMed] [Google Scholar]
- [43].Fraser ME, Fujinaga M, Cherney MM, Melton-Celsa AR, Twiddy EM, O’Brien AD, James MNG. Structure of shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J. Biol. Chem. 2004;279:27511–27517. doi: 10.1074/jbc.M401939200. [DOI] [PubMed] [Google Scholar]
- [44].Fraser ME, Cherney MM, Marcato P, Mulvey GL, Armstrong GD, James MNG. Binding of adenine to Stx2, the protein toxin from Escherichia coli O157:H7. Acta Crystallogr. F. 2006;62:627–630. doi: 10.1107/S1744309106021968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang H, Morita M, Yang X, Suzuki T, Yang W, Wang J, Ito K, Wang Q, Zhao C, Bartlam M, Yamamoto T, Rao Z. Crystal structure of the human CNOT6L nuclease domain reveals strict poly(A) substrate specificity. EMBO J. 2010;29:2566–2576. doi: 10.1038/emboj.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jung T-Y, Kim Y-S, Oh B-H, Woo E. Identification of a novel ligand binding site in phosphoserine phosphatase from the hyperthermophilic archaeon Thermococcus onnurineus. Proteins. 2013;81:819–829. doi: 10.1002/prot.24238. [DOI] [PubMed] [Google Scholar]
- [47].Bacik JP, Avvakumov G, Walker JR, Xue S, Dhe-Paganon S. Crystal structure of the N-terminal domains of the ubiquitin specific peptidase 4 (USP4) Structural Genomics Consortium; PDB ID 3JYU. [Google Scholar]
- [48].Chang C, Skarina T, Kagan O, Savchenko A, Edwards AM, Joachimiak A, RHA1, Midwest Center for Structural Genomics . Crystal structure of 3-HSA hydroxylase from Rhodococcus sp. PDB ID 2RFQ. [Google Scholar]