Abstract
Hypercholesterolaemia leads to cholesterol accumulation in macrophages and other immune cells, which promotes inflammatory responses, including augmentation of Toll-like receptor (TLR) signalling, inflammasome activation, and the production of monocytes and neutrophils in the bone marrow and spleen. On a cellular level, activation of TLR signalling leads to decreased cholesterol efflux, which results in further cholesterol accumulation and the amplification of inflammatory responses. Although cholesterol accumulation through the promotion of inflammatory responses probably has beneficial effects in the response to infections, it worsens diseases that are associated with chronic metabolic inflammation, including atherosclerosis and obesity. Therapeutic interventions such as increased production or infusion of high-density lipoproteins may sever the links between cholesterol accumulation and inflammation, and have beneficial effects in patients with metabolic diseases.
In industrialized societies, the consumption of high-fat, high-cholesterol diets — known as Western-type diets (WTDs) — can lead to hypercholesterolaemia and atherosclerosis, especially in genetically predisposed individuals. The principal atherogenic lipoprotein in the blood is the low-density lipoprotein (LDL), and increased levels of LDL promote cholesterol accumulation and an inflammatory response in the artery wall, which drives the process of atherosclerosis (BOX 1). By promoting the cellular efflux of cholesterol, high-density lipoprotein (HDL) opposes this process and reduces inflammation. Increased levels of LDL lead to its entry into and retention in the arterial wall, where it may be modified by various processes such as oxidation and aggregation1. This has two key adverse consequences: first, modified LDL functions as a ligand for macrophage pattern recognition receptors, including Toll-like receptors (TLRs), and can thereby directly trigger pro-inflammatory signalling pathways; and second, modified LDL is engulfed by macrophages, causing cellular cholesterol accumulation, which in turn amplifies TLR signalling1–6. Increased TLR activity leads to augmented production of cytokines and chemokines, amplification of the inflammatory process and, when combined with the uptake or intracellular formation of cholesterol crystals, may lead to NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome activation7,8.
Box 1. Atherosclerosis and inflammation.
Atherosclerosis is a chronic disease of large and medium arteries in which cholesterol deposition incites a progressive macrophage-dominated inflammatory response. In atherosclerosis, the recognition and uptake of cholesterol-rich apolipoprotein B (APOB)-containing lipoproteins (mainly low-density lipoprotein (LDL) but also cholesterol-rich, partially lipolysed remnants of the triglyceride-transporting lipoproteins — that is, very low-density lipoprotein (VLDL) and chylomicrons — sometimes referred to as ‘non-HDL cholesterol’) by macrophages, especially when combined with defective high-density lipoprotein (HDL)-mediated cholesterol efflux, leads to a chronic inflammatory response involving both innate and adaptive immune responses1,137–139. After binding to the subendothelial arterial matrix, LDL is modified by oxidation or aggregation, leading to its cellular recognition and uptake by pattern recognition receptors. Macrophages in atherosclerotic plaques may be derived from blood-borne monocytes, which are produced in the bone marrow and the spleen. Hypercholesterolaemia and cholesterol accumulation in haematopoietic stem cells (HSCs) promotes the overproduction of monocytes, which leads to their accumulation in atherosclerotic plaques; this process is opposed by HDL and cholesterol efflux pathways. In the bone marrow, cholesterol accumulation in the plasma membrane of HSCs increases the expression levels and signalling of growth factor receptors, causing the expansion of these populations and the increased production of monocytes, neutrophils and platelets97,139,140. In mouse models of hypercholesterolaemia, HSC mobilization from the bone marrow leads to extramedullary haematopoiesis in the spleen103, which is an important reservoir for the production of monocytes141 that may help to heal the heart after myocardial infarction142 but that may also contribute to atherogenesis143.
Counter-regulatory mechanisms oppose cholesterol accumulation and inflammation in macrophages. In particular, accumulating levels of cellular cholesterol lead to the formation of specific sterols that activate the liver X receptor (LXR)–retinoid X receptor (RXR) heterodimeric transcription factors. The LXR–RXR heterodimers have a range of anti-inflammatory activities — including upregulating the expression of ATP-binding cassette transporters (ABC transporters) ABC subfamily A member 1 (ABCA1) and ABCG1, and promoting the efflux of cholesterol from macrophages — and thus may counter the amplification of TLR signalling by cellular cholesterol accumulation. ABCA1 and ABCG1 promote efflux of cholesterol onto HDL particles or onto the lipid-poor form of the main HDL protein, apolipoprotein A1 (APOA1), and initiate the process of reverse cholesterol transport (RCT), in which cholesterol is transported from peripheral tissues back to the liver via the lymphatics and bloodstream, followed by its excretion into bile and faeces9,10. Of note, TLR activation suppresses LXR activity on its target genes, causing decreased macrophage cholesterol efflux11, which probably results in an amplification of TLR signalling.
Thus, there is a feedforward mechanism in which the acute phase response effects changes in cellular cholesterol homeostasis, which amplifies the inflammatory response. More generally, the acute phase response downregulates the RCT pathway12, which suggests that the innate immune system modifies cholesterol homeostasis as a way to amplify the inflammatory response. This may be beneficial as part of the overall immune response to infections or wound healing; however, excessive or prolonged cholesterol-facilitated immune responses can become associated with disease, notably atherosclerosis. Accordingly, chronic infections, such as with HIV-1 (REFS 13,14), and autoimmune disorders, such as systemic lupus erythematosus, rheumatoid arthritis and psoriasis15, are often associated with reduced levels of HDL, increased levels of atherogenic lipoproteins and accelerated atherosclerosis.
Although the links between cholesterol and inflammation are best exemplified by atherosclerosis, similar mechanisms may also contribute to other metabolic disorders such as obesity or to autoimmune diseases. For example, in obesity, activation of TLRs and NOD-like receptors (NLRs) on adipose macrophages in response to lipids, such as ceramides or saturated fatty acids, may lead to chronic inflammation, insulin resistance and fatty liver disease16,17. These processes seem to be enhanced by cholesterol accumulation in adipose tissue and are reversed by the activation of RCT18,19. In autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, defects in cholesterol efflux pathways seem to worsen the underlying condition20,21. The emphasis of this Review is on alterations in immune signalling in response to cholesterol accumulation in cells that are relevant to atherosclerosis. In particular, we emphasize the relationships between inflammation and RCT, as well as the role of LXRs in counter-regulating cellular cholesterol accumulation and TLR-induced inflammatory responses.
RCT and the inflammatory response
The acute phase response inhibits RCT
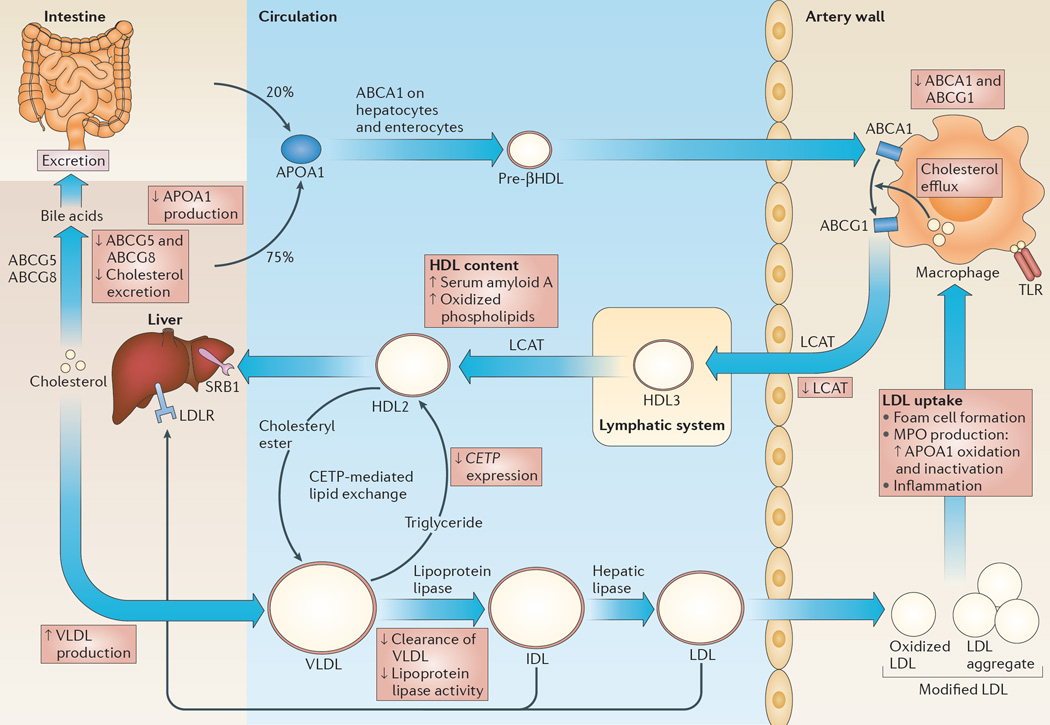
The efflux of cholesterol from cholesterol-loaded macrophages in the arterial wall is primarily mediated by ABCA1 and ABCG1 (FIG. 1). These transporters promote unidirectional cholesterol efflux from macrophages onto lipid-poor APOA1 (in the case of ABCA1) or HDL (in the case of ABCG1), which initiates the process of RCT9,10,22–25 (FIG. 1). HDL is the major lipoprotein in lymph and recent studies have shown that the lymphatic system is crucial for RCT from multiple tissues, including from the atherosclerotic aortic wall26,27. HDL-free cholesterol is esterified by the lecithin–cholesterol acyltransferase (LCAT) enzyme. Free cholesterol or cholesteryl ester in HDL may be directly cleared in the liver via scavenger receptor B1 (SRB1; also known as SCARB1), which mediates a process of selective cholesterol or cholesteryl ester uptake without degradation of HDL28. In humans, cholesteryl ester transfer protein (CETP) in the plasma mediates the transfer of cholesteryl ester from HDL to triglyceride-rich lipoproteins, such as very low-density lipoprotein (VLDL) and LDL, in exchange for triglyceride29 and thus facilitates RCT. The triglycerides in triglyceride-rich lipoproteins are hydrolysed by lipoprotein lipase and hepatic lipase, and the cholesteryl ester-rich remnant particles are either cleared in the liver or further metabolized to LDL. Cholesterol deposited in the liver by RCT can be recycled in the form of secreted lipoproteins or can undergo net excretion into the bile by ABCG5 and ABCG8 (REF. 30) (FIG. 1).
Figure 1. RCT and its regulation by innate immune responses.
The process of reverse cholesterol transport (RCT) is depicted and how inflammation impairs this process is described in the red boxes. Under physiological conditions, apolipoprotein A1 (APOA1), which is the major protein component of high-density lipoprotein (HDL), is secreted by the liver and the intestines, and is assembled into a pre-βHDL particle as a result of its interaction with the ATP-binding cassette transporter ABC subfamily A member 1 (ABCA1) on hepatocytes and enterocytes. ABCA1 on macrophages promotes cholesterol and phospholipid efflux onto these relatively lipid-poor pre-βHDL particles, initiating the process of RCT. ABCG1 promotes further cholesterol efflux onto HDL particles. Free cholesterol in HDL is esterified by the enzyme lecithin–cholesterol acyltransferase (LCAT), which gives rise to cholesteryl esters. Free cholesterol or cholesteryl esters in HDL may be directly cleared in the liver via scavenger receptor B1 (SRB1), which mediates a process of selective free cholesterol or cholesteryl ester uptake in which the lipid moiety of HDL is mostly removed and the protein portion is recycled into the circulation (not shown). Cholesterol deposited in the liver by RCT can either be recycled in the form of secreted triglyceride-rich, very low-density lipoproteins (VLDLs; the main protein component of which is APOB) or can undergo net excretion into the bile via ABCG5 and ABCG8. In humans, plasma cholesteryl ester transfer protein (CETP) mediates the exchange of cholesteryl esters in HDL with triglyceride in VLDL. A lipolytic cascade mediated by lipoprotein lipase and hepatic lipase causes hydrolysis of triglycerides and results in the formation of cholesterol-rich and cholesteryl ester-rich LDL. Although most LDL is cleared in the liver, LDL may supply cholesterol to peripheral tissues and a small proportion is taken up into the arterial wall, where it is modified by oxidation or aggregation, leading to its uptake by macrophages. Modified LDL in the artery wall promotes Toll-like receptor (TLR) signalling in macrophages and it is taken up by these cells, leading to the formation of macrophage foam cells, the production of myeloperoxidase (MPO) and inflammation. IDL, intermediate-density lipoprotein; LDLR, LDL receptor.
The acute phase response induced following lipopolysaccharide (LPS) injection of mice blocks RCT at multiple points12 (FIG. 1). LPS induces the expression of the microRNA miR-33, which causes a reduction in the levels of ABCA1 and ABCG1, and reduces cholesterol efflux from macrophages31. LPS treatment also causes decreased production of APOA1 in the liver12, suppresses hepatic CETP gene expression32 and reduces excretion of cholesterol from the liver via the downregulation of ABCG5, ABCG8 and cholesterol 7α-hydroxylase (CYP7A1) expression12,33. Taken together, these studies show that LPS injection results in impaired RCT, with the block occurring primarily in cholesterol mobilization from the liver to the intestines33. Furthermore, in humans, HDL binds to LPS and thus blocks its ability to stimulate TLR4 signalling in monocytes and macrophages34; thus, the suppression of APOA1 production and HDL levels by LPS12 is pro-inflammatory.
In addition to blocking RCT, LPS causes accumulation of triglycerides within VLDL, which has been attributed to increased hepatic VLDL production and a reduction in the clearance of VLDL from the bloodstream by lipoprotein lipase, a phenomenon that has been designated the ‘lipaemia of sepsis’ (REF. 35). VLDL might sequester LPS, viruses and other toxic compounds to improve host survival, and it is possible that the increase in VLDL might help to maintain lipid levels in peripheral tissues to help suppress infection and to allow for tissue repair.
HDL becomes dysfunctional and pro-inflammatory
During acute infections, there are pro-inflammatory changes in HDL that parallel decreases in the ability of HDL to mediate cholesterol efflux from macrophages. HDL is converted from an anti-inflammatory lipoprotein that suppresses monocyte adhesion to the endothelium into a pro-inflammatory form that does not suppress monocyte adhesion during the acute phase response, for example, during that induced by influenza virus infection in humans36,37. Furthermore, in patients with acute sepsis, cholesterol efflux from cultured macrophages to plasma or to HDL is greatly decreased38. Studies using HDL isolated from healthy individuals following low-dose LPS challenge have shown a decreased cholesterol efflux capacity associated with accumulation of serum amyloid A in HDL, decreased LCAT expression and a decrease in HDL phospholipid content, even without major changes in HDL cholesterol or APOA1 levels33,39. The anti-inflammatory and anti-oxidative activities of HDL are also impaired as a result of the loss of antioxidant proteins and the accumulation of oxidized phospholipids in HDL37.
Reductions in HDL function through oxidation can also be mediated by macrophage myeloperoxidase (MPO), which is induced by inflammatory stimuli in atherosclerotic lesions40,41 (FIG. 1). Although mice have much lower expression levels of macrophage MPO than humans, overexpression of human MPO in macrophages in LDL receptor (LDLR)-deficient mice promotes atherosclerosis42. Human APOA1 that has been oxidized by MPO has an impaired ability to promote cholesterol efflux via ABCA1 on macrophages and has a decreased ability to promote RCT and atherosclerosis regression when injected into mice43. Individuals with coronary artery disease had higher levels of MPO-modified APOA1 than control individuals44; their HDL had an impaired capacity to induce ABCA1-mediated cholesterol efflux from macrophages ex vivo. The levels of modified APOA1 in individuals with coronary artery disease were inversely related to ABCA1 efflux capacity and positively correlated with atherosclerotic disease. Although the plasma levels of MPO-oxidized APOA1 represent a small proportion of the total APOA1, in inflammatory microenvironments within atherosclerotic plaques, the amounts of damaged APOA1 might be sufficient to considerably impair macrophage cholesterol efflux. These observations suggest that a macrophage inflammatory response that is mediated by MPO leads to local inactivation of APOA1 in the arterial wall, reduced macrophage cholesterol efflux and increased atherosclerosis.
Huang et al.45 recently developed a high-affinity monoclonal antibody that specifically recognizes both APOA1 and HDL that have been modified by MPO. An oxindolyl alanine (2-OH-Trp) moiety at tryptophan 72 of APOA1 (oxTrp72-APOA1) was found to be the functionally relevant, immunogenic epitope. APOA1 containing this modification was found at very low levels in the circulation of individuals with coronary heart disease but accounted for 20% of the APOA1 in atherosclerosis-laden arteries. OxTrp72-APOA1 that was recovered from human atheromas was almost entirely devoid of cholesterol acceptor activity and also had pro-inflammatory activity on endothelial cells45. Elevated oxTrp72-APOA1 levels in patients presenting to a cardiology clinic were associated with increased cardiovascular disease risk, which suggests that this could be a biomarker for inflamed atherosclerotic plaques.
In summary, the acute phase response leads to blockage of the RCT process at multiple points. We speculate that this in turn leads to cholesterol accumulation in macrophages and to an enhancement of inflammatory responses. In addition, HDL levels are decreased during the acute phase response and compositional changes in HDL, including MPO-mediated modifications of APOA1, may convert HDL into a dysfunctional form that cannot efficiently mediate cholesterol efflux (or could even perhaps deliver cholesterol to cells) and that becomes pro-inflammatory. Although these changes in HDL and APOA1 are likely to be pro-atherogenic, we believe they may also have a physiological function in the setting of infection by enhancing the inflammatory response. This contention is supported by the observation that mice with macrophage-specific deletion of Abca1 are resistant to Listeria monocytogenes infection46. Moreover, in cells involved in wound healing and tissue repair, maintaining cholesterol in the cell may facilitate enhanced cell proliferation47,48.
In terms of the mechanisms connecting inflammation with decreases in RCT, many of the genes involved in RCT are induced by cellular cholesterol accumulation and the activation of LXR transcription factors. Activation of TLR3 or TLR4 signalling suppresses expression of LXR target genes in macrophages, including Abca1 and Abcg1, via induction of the transcription factor interferon-regulatory factor 3 (IRF3) (REF. 11). In addition, LPS suppresses expression of Lxr and Rxr in hepatocytes49. Thus, suppression of LXR–RXR may be a general mechanism connecting TLR-mediated inflammatory responses to decreased RCT.
Opposing effects of cholesterol in macrophages
There is abundant evidence that the interaction of LDL with macrophages in atherosclerotic plaques leads to an increase in inflammatory gene expression. In hypercholesterolaemic mouse models of atherosclerosis, inflammatory signalling in macrophages and endothelial cells — via TLR2 and TLR4, and myeloid differentiation primary response protein 88 (MYD88)-dependent pathways — promotes cytokine and chemokine gene expression, and atherogenesis50–52. Macrophage foam cells in progressive atherosclerotic plaques have increased expression of inflammatory genes compared with plaques undergoing regression53. Human atherosclerotic plaques express increased levels of cytokines and chemokines that are dependent on MYD88-mediated signalling via various TLRs, especially TLR2 (REF. 54). The most important ligands for TLRs are probably modified forms of LDL. In mouse macrophages, TLR4 and TLR6 function in combination with CD36 to mediate macrophage inflammatory responses2. In contrast to these pro-inflammatory effects of the interaction of modified LDL with macrophages, cholesterol efflux pathways suppress TLR signalling and inflammatory cytokine expression in atherosclerotic plaques4,55,56.
Somewhat unexpectedly, peritoneal macrophages isolated from WTD-fed Ldlr−/− mice had an overall reduction in inflammatory gene expression compared with macrophages from wild-type mice that were fed a control diet57. This reduction in inflammatory gene expression was related to the accumulation of desmosterol, which is the penultimate molecule in the cholesterol biosynthetic pathway and is a known activator of LXRs58. Desmosterol probably accumulates in peritoneal macrophage foam cells because the expression of desmosterol reductase, which is the last enzyme in the cholesterol biosynthetic pathway, is more repressed by cellular cholesterol loading than the enzymes that are involved in the earlier steps in the pathway, leading to disproportionate biosynthesis of desmosterol. These observations of suppressed inflammation in peritoneal macrophage foam cells highlight the importance of the specific environment of the arterial wall in the macrophage inflammatory response in atherosclerosis. The artery provides a unique environment for the interaction of LDL with macrophages, which results both in the activation of TLRs and in cholesterol accumulation in macrophages, leading to progressive atherosclerosis. The net effect of macrophage cholesterol accumulation on inflammation may thus depend on a balance of factors, such as the activation of TLRs, the localization of accumulating cholesterol within the cell, cholesterol crystal formation and inflammasome activation, and the phenotype of the macrophages involved.
Inflammatory effects of cholesterol accumulation
Promotion of TLR signalling
There is evidence that plasma membrane cholesterol enrichment promotes the formation of TLR4–MD2 (REF. 4) and TLR4–CD14 complexes59, which enhances the response to TLR4 ligands such as LPS. Conversely, ABCA1 and ABCG1 promote macrophage cholesterol efflux and suppress macrophage inflammatory responses via TLR2, TLR3 and TLR4 (REF. 4). This may involve decreased formation of cholesterol-enriched lipid rafts in the plasma membrane and in the endosomal system. Mice that are deficient in ABCA1 and ABCG1 accumulate free and esterified cholesterol in peritoneal macrophages, and have enhanced inflammatory responses to TLR ligands and increased apoptosis when exposed to oxidized LDL3. This is in contrast to the observations of decreased inflammatory gene expression in peritoneal macrophage foam cells from Ldlr−/− mice57, which indicates that the mode of accumulating free cholesterol within the cell may be important in determining the inflammatory response. Even without cholesterol efflux, ABCA1 and ABCG1 promote the trans-bilayer movement of cholesterol at the plasma membrane60; when this is defective in Abca1−/−Abcg1−/− macrophages, inflammatory changes ensue. Macrophage-specific deficiency in ABCA1 and ABCG1 resulted in increased atherosclerosis, and laser capture microdissection of macrophage-rich areas of plaques shows increased expression of the inflammatory chemokines CC-chemokine ligand 2 (CCL2) and CCL3 (REF. 56), which confirms that cholesterol efflux pathways in macrophages have anti-inflammatory activity in the context of the atherosclerotic plaque.
Inflammasome activation
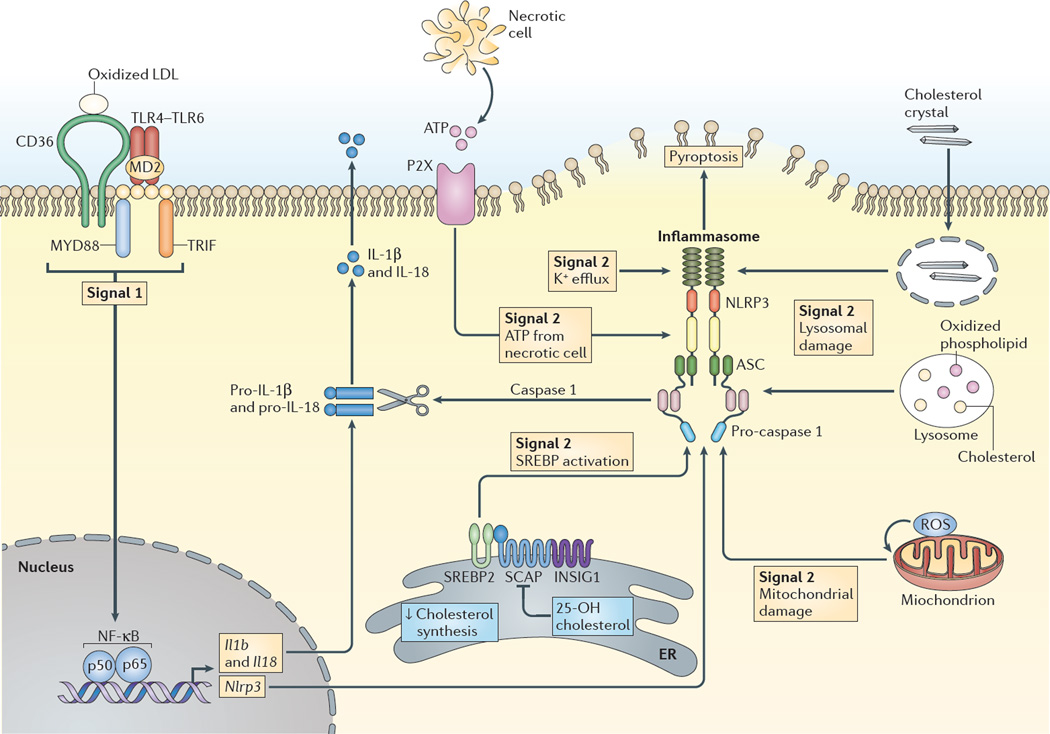
Accumulating cholesterol in atherosclerotic plaques may give rise to cholesterol crystal formation. Cholesterol crystal uptake or formation in macrophages has been shown to promote inflammasome activation and atherogenesis. Inflammasome activation requires two signals — a priming signal that is typically mediated by TLR4 activation and a second signal involving potassium ion efflux, lysosomal damage or reactive oxygen species (ROS) generation61 (FIG. 2). The priming signal may result from pattern recognition receptor activation — for example, the induction of TLR4–TLR6–CD36 signalling by oxidized LDL8 — whereas the second signal can be mediated by cholesterol crystals in lysosomes, either as a result of phagocytosis of extracellular cholesterol crystals or via CD36-mediated uptake of modified LDL and free cholesterol release from the LDL8. Inflammasome activation leads to the secretion of the pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18.
Figure 2. Inflammasome activation by sterols.
The NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome is activated by many signals, including infectious agents and stress- or injury-induced host factors, leading to caspase 1 activation, cleavage of pro-interleukin-1β (pro-IL-1β) and pro-IL-18, and the secretion of the active cytokines, as well as in some cases resulting in a form of cell death termed pyroptosis. A priming stimulus (signal 1), acting through nuclear factor-κB (NF-κB), induces the expression of Il1b, Il18 and Nlrp3, and precedes assembly of the inflammasome complex. In atherosclerosis, priming may result from pattern recognition receptor activation; for example, combinatorial Toll-like receptor 4 (TLR4)–TLR6–CD36 signalling induced by oxidized low-density lipoprotein (LDL). The second stimulus (signal 2) may arise from various stressors. In atherosclerosis, one such signal is lysosomal damage or dysfunction, which may result from phagocytosis of extracellular cholesterol crystals via the CD36-mediated uptake of modified LDL (not shown) and free cholesterol release and crystallization in lysosomes. Other signals may result from mitochondrial damage, oxidative stress-induced production of reactive oxygen species (ROS) or ATP release from dying cells. 25-hydroxycholesterol (25-OH cholesterol) suppresses inflammasome activation by reduced sterol regulatory element-binding protein 2 (SREBP2) cleavage mediated by SREBP cleavage-activating protein (SCAP) in the endoplasmic reticulum (ER). INSIG1, insulin-induced gene 1; MYD88, myeloid differentiation primary response protein 88; TRIF, TIR domain-containing adaptor protein inducing IFNβ.
Duewell et al.7 used confocal reflectance microscopy to show refractile material in early mouse atherosclerotic lesions, interpreted as small free cholesterol crystals. They showed decreased lesion formation in WTD-fed Ldlr−/− mice following irradiation and transplantation of bone marrow that was deficient in key components of the NLRP3 inflammasome or in Il1a and Il1b. These data suggest that cholesterol crystals and minimally oxidized LDL could induce inflammasome activation in macrophages and the authors proposed a prominent role for cholesterol crystals in activating the inflammasome, even in early atherosclerotic lesions. In addition, increased atherosclerosis in Apoe−/− mice in which immune cells were deficient in autophagy protein 5 (ATG5) was proposed to reflect inflammasome activation62. This could be due to the ability of autophagy to suppress the inflammasome63,64 or to promote cholesterol efflux and thus to oppose cholesterol crystal formation65. Some but not all studies have shown that IL-1β signalling is pro-atherogenic in mouse models of atherosclerosis66. In addition, IL-18 levels in the blood predict cardiovascular death in patients with coronary heart disease67. Administration of IL-18 to Apoe−/− mice increased the size of atherosclerotic lesions, and their T cell content and MHC class II expression, in an interferon-γ (IFNγ)-dependent manner68, whereas antagonism of IL-18 reduced atherosclerosis69. Thus, several lines of evidence support an important role of cholesterol crystal-induced inflammasome activation and inflammasome products in mouse models of atherosclerosis and possibly in human coronary heart disease.
In contrast with the above studies, Menu et al.70 found no effect on atherosclerosis in Apoe−/− mice deficient for components of the NLRP3 inflammasome that had been fed a WTD. Nevertheless, more recent reports have confirmed an anti-atherogenic effect of caspase 1 (REFS 71,72) or NLRP3 deficiency73 in Apoe−/− mice. The lack of effect on atherosclerosis reported by Menu et al.70 may have been due to the analysis of very advanced atherosclerotic lesions66. Although numerous reports support the activation of the NLRP3 inflammation by cholesterol crystals taken up by macrophages, some literature seems to be inconsistent with the presence of free cholesterol crystals in early atherosclerotic lesions7. Small et al.74,75 analysed the composition of early human atherosclerotic plaques and, on the basis of the physical state of lipids that have been described in model systems, did not predict that a separate free cholesterol crystalline phase occurred in humans. Furthermore, a cholesterol crystalline phase was not observed using a polarized light microscope with a heating and cooling stage. During these studies, it was noted that plaque cholesteryl esters that were always liquid or liquid crystalline at body temperature readily formed small crystals upon cooling, raising the issue that cholesteryl ester crystal formation could be an artefact of refrigerated samples of atherosclerotic arteries. However, Rothblat and colleagues76,77 produced authentic, highly elongated free cholesterol crystals within lysosomes of peritoneal macrophages and the formation of these crystals was reversed by addition of APOA1 or APOE to the cultures. The authors found that substantial crystal formation required extensive cholesterol loading using acetylated LDL and the presence of an inhibitor of cholesterol esterification.
The weight of evidence supports an important role for cholesterol- or oxidized LDL-induced inflammasome activation in atherosclerosis; however, the effect of inflammasome activation at different stages of the disease and the underlying mechanisms deserve further investigation. Although the major focus of these studies has been on the links between inflammasome activation in cholesterol-loaded macrophages and atherosclerosis, we speculate that cholesterol accumulation in macrophages as a result of innate immune responses may contribute to inflammasome activation in the spleen during microbial infection, which could have beneficial effects on the outcome.
25-hydroxycholesterol and the inflammasome
In macrophages and dendritic cells, the enzyme that synthesizes 25-hydroxycholesterol (25-OH cholesterol) — cholesterol 25-hydroxylase — is induced by type I IFNs downstream of TLR3 or TLR4 activation78–80. 25-OH cholesterol has broad activity against enveloped viruses — including HIV, herpes simplex virus and Ebola virus — by preventing fusion of the viral membrane with cells81, but it can also lead to detrimental tissue damage in some settings, such as during infection with influenza virus82. A recent report shows that 25-OH cholesterol decreases inflammasome activation in macrophages83. Accordingly, cholesterol 25-hydroxylase-deficient mice showed increased sensitivity to septic shock and exaggerated autoimmune encephalomyelitis but showed a stronger ability to repress bacterial growth after bacterial infection83. Decreased cholesterol and/or sterol accumulation following 25-cholesterol hydroxylase induction is associated with decreased expression of Il1b mRNA and decreased caspase 1 activation. The underlying mechanism probably involves the suppression of cholesterol biosynthetic genes by 25-OH cholesterol. The expression of these genes is controlled by the nuclear active form of the transcription factor sterol regulatory element-binding protein 2 (SREBP2). 25-OH cholesterol binds to insulin-induced gene 1 (INSIG1) protein in the endoplasmic reticulum (ER), which prevents the SREBP cleavage-activating protein (SCAP)-mediated transport of SREBP2 from the ER to the Golgi and its subsequent cleavage into the nuclear active form83. This study implies that following TLR activation and the induction of cholesterol 25-hydroxylase, decreased active SREBP2 and decreased accumulation of cholesterol or other sterols leads to inflammasome suppression. This is in contrast to the idea presented above that the acute phase response promotes macrophage cholesterol accumulation, leading to an enhanced inflammasome response. However, these ideas can be reconciled by proposing that the effects occur sequentially and that the immune system uses these various changes in macrophage cholesterol homeostasis to activate the inflammasome and then to turn it off.
Although these studies imply that increased SREBP2 activity may promote caspase 1 activation83, SREBPs may in turn be cleaved and activated by caspase 1 — for example, when pore-forming bacterial toxins activate the inflammasome84 — which suggests that there may be a positive feedback loop that promotes inflammasome activation and lipid synthesis for membrane repair. In endothelial cells, oscillatory blood flow induces inflammasome activation through the SREBP2-induced expression of Nlrp3 and Casp1 (the gene encoding caspase 1) via direct promoter activation; this induction seems to be independent of the effects of SREBP2 on cellular sterols85.
Oscillatory or turbulent blood flow occurs in regions of the aorta such as the lesser curvature of the aortic arch and at vessel branch points. These sites are susceptible to atherosclerosis and, because of their altered blood flow, may also be adapted for the capture of pathogens by antigen-presenting cells86. We speculate that inflammasome activation at sites of disturbed arterial blood flow, perhaps involving both endothelial cells and resident arterial macrophages87, may have evolved to promote the clearance of pathogens; however, under conditions of hypercholesterolaemia, these same responses may promote atherogenesis.
Counteracting cholesterol-mediated inflammation
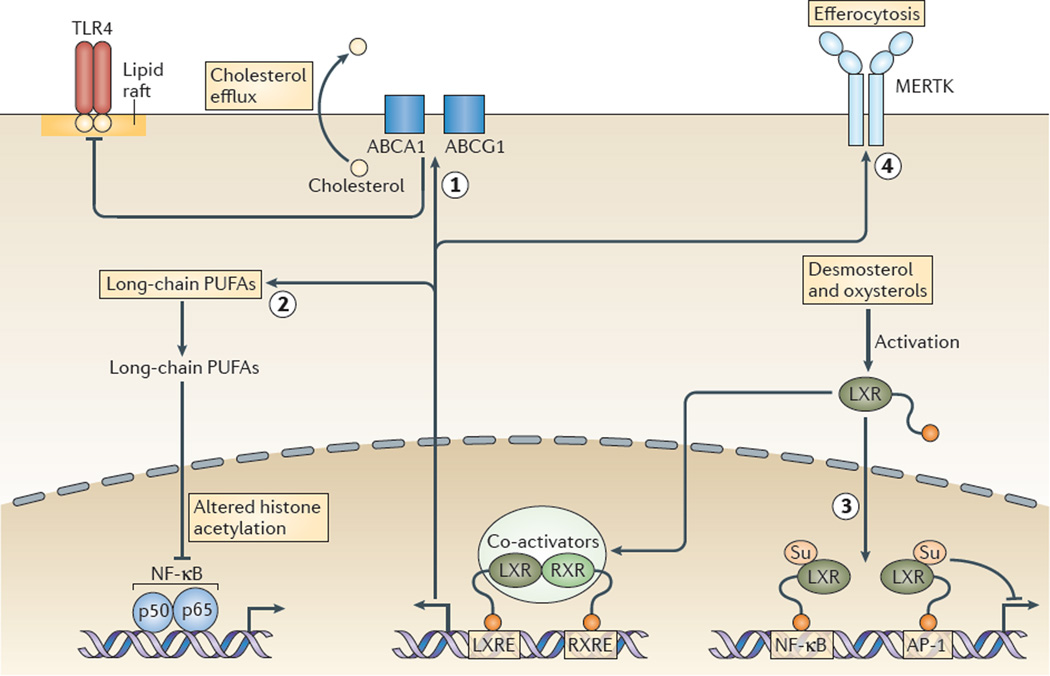
As mentioned above, LXR transcription factors exert potent anti-inflammatory effects and thus provide a mechanism that counter-regulates modified LDL-induced TLR activation and macrophage cholesterol accumulation (FIG. 3). Multiple mechanisms are likely to be involved.
Figure 3. Molecular mechanisms underlying anti-inflammatory effects of LXR activation.
Liver X receptors (LXRs) promote macrophage cholesterol efflux via the induction of ABC subfamily A member 1 (ABCA1) and ABCG1 expression, which suppresses Toll-like receptor (TLR)-mediated inflammatory responses, possibly by disrupting membrane lipid rafts (labelled 1 in the figure). LXRs induce the expression of genes mediating elongation and unsaturation of fatty acids, leading to the synthesis of long-chain polyunsaturated fatty acids (PUFAs) including omega 3 fatty acids, as well as specialized pro-resolving lipid mediators. Long-chain PUFAs mediate decreased transcriptional responses of nuclear factor-κB (NF-κB) target genes as a result of altered histone acetylation in their enhancer and/or promoter regions, without changes in nuclear p65 levels (labelled 2 in the figure). Activation of LXRs by desmosterol and oxysterols causes sumoylation (Su) of specific residues in the ligand-binding pocket of LXR, leading to binding of LXR (without retinoid X receptor (RXR)) to NF-κB and AP-1 response elements, blunting the inflammatory responses that are mediated by these transcription factors (labelled 3 in the figure). LXRs increase expression of the tyrosine protein kinase MER (MERTK), which enhances the uptake of apoptotic cells by macrophages (in a process known as efferocytosis; labelled 4 in the figure) and this leads to a suppression of TLR4-mediated inflammatory responses (not shown). Efferocytosis also causes marked LXR-dependent upregulation of ABCA1 and ABCG1, which is probably an important contributor to the anti-inflammatory effect. LXRE, LXR response element; RXRE, RXR response element.
First, LXRs promote cholesterol efflux from macrophages via the induction of ABCA1 and ABCG1, and given that cholesterol efflux via these transporters suppresses TLR-mediated inflammatory responses3,4, this seems to be a key anti-inflammatory effect of LXR activation.
Second, LXRs induce the expression of several genes that mediate the elongation and the unsaturation of fatty acids, which leads to synthesis of long-chain polyunsaturated fatty acids (PUFAs) including omega 3 fatty acids88. The increase in long-chain PUFAs was shown to result in decreased transcriptional responses of nuclear factor-κB (NF-κB) target genes as a result of altered histone acetylation in their enhancer and/or promoter regions, without changes in nuclear p65 levels88. Long-chain PUFAs may also function as substrates for the enzymes that synthesize eicosanoids and specialized pro-resolving lipid mediators, such as resolvins and protectins, that promote the resolution of inflammation89. Moreover, LXRα in the liver induces lysophospholipid acyltransferase 5 (LPCAT3), which is an enzyme that mediates the synthesis of phospholipids containing long-chain PUFAs, leading to decreased ER stress and inflammatory responses90.
Third, activation of LXRs by desmosterol and other oxysterols causes the sumoylation of specific residues in their ligand-binding pocket, leading to the binding of LXR (without RXR) to NF-κB and AP-1 response elements, which reduces the inflammatory responses that are mediated by these transcription factors91.
Fourth, LXRs increase expression of the tyrosine protein kinase MER (MERTK), which enhances the uptake of apoptotic cells by macrophages (a process termed efferocytosis) and this leads to a suppression of TLR4-mediated inflammatory responses92. Efferocytosis also causes marked LXR-dependent upregulation of ABCA1 and ABCG1 (REF. 93), which is probably an important contributor to the anti-inflammatory effect.
Finally, LXRs are highly expressed by haematopoietic stem cells (HSCs) and myeloid progenitor cells, in which they promote cholesterol efflux via upregulating the expression of ABCA1, ABCG1 and APOE, and decrease the proliferative responses of these cells to IL-3 and granulocyte–macrophage colony-stimulating factor (GM-CSF), thus reducing the production of inflammatory cells94.
In summary, although TLR activation reduces cholesterol efflux and promotes cholesterol accumulation in macrophages, enhancing the inflammatory response, cholesterol accumulation also leads to LXR activation and the eventual suppression of TLR-mediated inflammatory responses. This may be analogous to the role of the TLR-mediated induction of cholesterol 25-hydroxylase in eventually turning off the inflammasome response, as suggested above. Thus, the innate immune system uses changes in cholesterol metabolism to amplify the inflammatory response and then to restore homeostasis.
Increased production of inflammatory cells
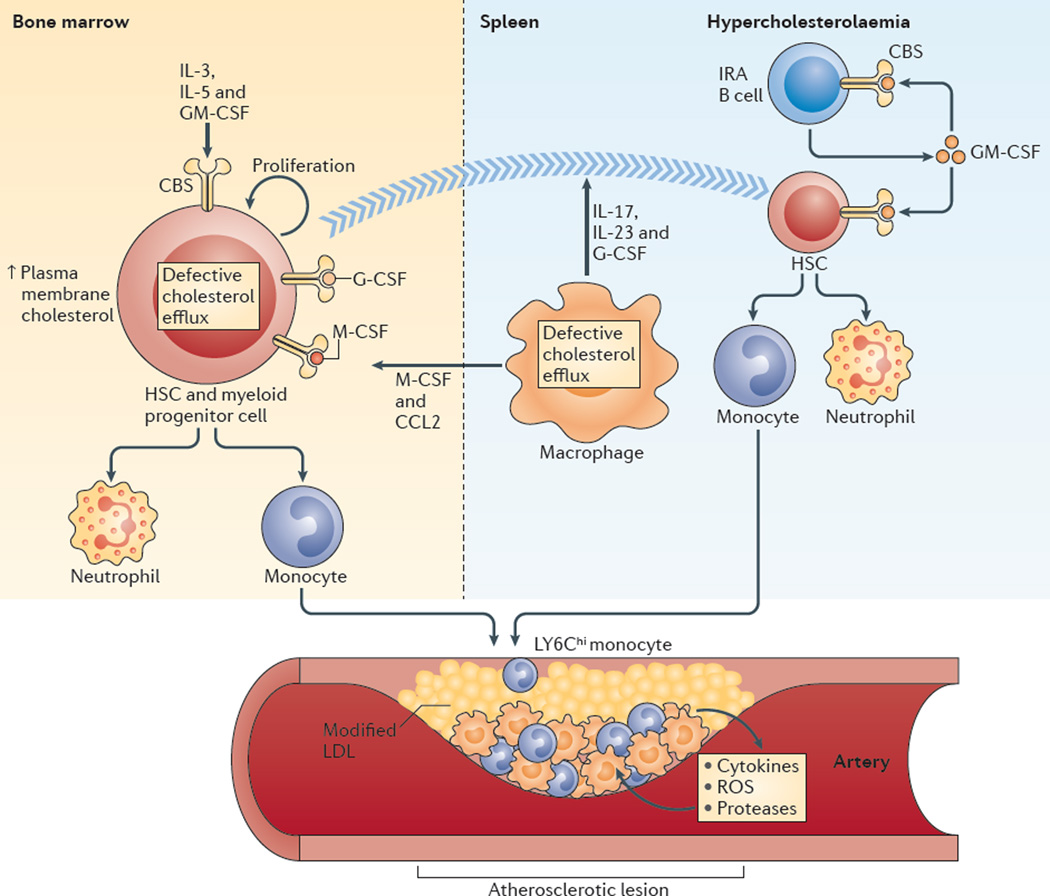
The discussion so far has mainly focused on the links between cholesterol accumulation in macrophages and inflammatory responses in atherosclerotic plaques; however, another important connection occurs at the level of the bone marrow. In both cross-sectional and prospective human population studies, blood monocyte and neutrophil numbers are strongly associated with atherosclerotic cardiovascular disease95, and studies in animal models indicate a causal relationship87,96, which suggests that the excessive production of inflammatory cells in the bone marrow and spleen under hypercholesterolaemic conditions is important in the atherogenic process (FIG. 4).
Figure 4. Hypercholesterolaemia and defective cholesterol efflux promote myelopoiesis and atherosclerosis.
In the bone marrow, increased plasma membrane cholesterol content in haematopoietic stem cells (HSCs) and myeloid progenitor cells as a result of defective cholesterol efflux promotes increased cell surface levels of the common β-subunit (CBS) of the interleukin-3 (IL-3), IL-5 and granulocyte–macrophage colony-stimulating factor (GM-CSF) receptors and increased proliferation in response to these growth factors. Extramedullary haematopoiesis can also occur after HSCs progressively relocate from the bone marrow to the splenic red pulp. Efferocytosis in the setting of defective cholesterol efflux in macrophages fails to suppress the production of IL-17, IL-23 and granulocyte colony-stimulating factor (G-CSF), and these cytokines can promote HSC relocation to the spleen. In the spleen, the number of GM-CSF-producing innate response activator B cells (IRA B cells) increases in mice with hypercholesterolaemia, which causes increased production of monocytes and neutrophils from HSCs. In addition, defective cholesterol efflux in splenic macrophages can promote the development of monocytes from HSCs and myeloid progenitor cells in the bone marrow via macrophage colony-stimulating factor (M-CSF) and CC-chemokine ligand 2 (CCL2). Monocytes, especially LY6Chi monocytes produced in the bone marrow and the spleen, enter the bloodstream and accumulate in atherosclerotic lesions. LDL, low-density lipoprotein; ROS, reactive oxygen species.
Increased bone marrow myelopoiesis promotes monocytosis
In mice, cholesterol efflux pathways mediated by APOE, ABCA1 and ABCG1 suppress the production of inflammatory cells in the bone marrow and the spleen; this is observed in chow-fed mice and becomes more prominent in the setting of hypercholesterolaemia94,97. Transplantation of Abca1−/−Abcg1−/− bone marrow cells into Ldlr−/− mice led to dramatic monocytosis and neutrophilia, infiltration of myeloid cells into multiple organs and accelerated atherosclerosis97. This leukocytosis reflected markedly increased proliferation and expansion of the HSC population in the bone marrow. Increased HSC proliferation, myelopoiesis and atherogenesis were reversed by overexpression of transgenic human APOA1, which probably reflects increased cholesterol efflux from HSCs, possibly by SRB1-facilitated passive efflux98. Subsequent studies showed similar expansion and proliferation of the HSC population in Apoe−/− mice and to a lesser extent in Ldlr−/− mice94. In Ldlr−/− mice, reductions in APOA1 and HDL levels because of Apoa1 haploinsufficiency promoted HSC population expansion and monocytosis99. LDL or oxidized LDL seem to promote HSC proliferation and this proliferation is reversed by HDL99,100. Similarly, children with familial hypercholesterolaemia have an inverse relationship between HDL cholesterol levels and blood monocyte numbers, which suggests that these mouse studies are relevant to humans99.
On a mechanistic level, HSCs from Apoe−/− or Abca1−/−Abcg1−/− mice showed evidence of increased plasma membrane cholesterol, increased cell surface levels of the common β-subunit of the IL-3, IL-5 and GM-CSF receptors (known as cytokine receptor common β-subunit (CBS); encoded by Csf2rb) and increased proliferation in response to these cytokines94,97. The transfer of bone marrow cells from Apoe−/− mice deficient in CBS into Ldlr−/− mice led to a reduction in the number of HSCs and myeloid progenitor cells in the bone marrow and the spleen, a reduction in monocyte and neutrophil numbers in the blood, and reduced macrophage numbers in atherosclerotic lesions101. Thus, in the setting of dietary or genetic hypercholesterolaemia, cholesterol efflux pathways that are mediated by APOE, ABCA1 and ABCG1 in HSCs prevent increases in membrane cholesterol content and in cell-surface expression levels of CBS, and thus prevent the hyperproliferation of HSCs and excessive myelopoiesis.
Recent evidence suggests that WTDs may also induce epigenetic changes in HSCs or myeloid progenitor cells that lead to increased myelopoiesis102. In bone marrow transplantation experiments using Ldlr−/− mice on a chow diet as recipient animals, bone marrow from mice fed a WTD caused increased myeloid cell proliferation compared with bone marrow from chow-fed mice. Bone marrow cells from mice fed a WTD showed hypomethylation of CpG regions in the genes encoding PU.1 and IRF8, which are key regulators of monocyte proliferation and macrophage differentiation102. In the blood of recipients of bone marrow from WTD-fed mice, there was increased monocytosis, their spleens were enlarged and the percentage of F4/80+CD86+ macrophages was increased102. Interestingly, mice reconstituted with bone marrow from WTD-fed mice showed an increase in aortic root plaque size in the absence of changes in serum cholesterol. Although the underlying mechanisms have not yet been established, these findings suggest that a WTD induces transplantable epigenetic changes in cells in the bone marrow that promote the production of inflammatory cells and increase susceptibility to atherosclerosis.
Cholesterol loading in the spleen promotes myelopoiesis in the bone marrow
In addition to the cell autonomous effects of cholesterol accumulation in HSCs, cholesterol build-up in splenic macrophages and dendritic cells promotes bone marrow myelopoiesis and HSC mobilization. In Apoe−/− and Abca1−/− Abcg1−/− mice, macrophages and dendritic cells in the spleen accumulate cholesterol and secrete inflammatory cytokines, which stimulates the production of inflammatory cells in the bone marrow, as well as HSC mobilization and extramedullary haematopoiesis103. Studies in LXR-deficient mice show that the activation of LXRs in splenic macrophages by the uptake of senescent neutrophils represses the production of the pro-inflammatory cytokines IL-17, IL-23 and granulocyte colony-stimulating factor (G-CSF), which controls neutrophil production in the bone marrow104. Similar findings in mice that have a knockout of Abca1 and Abcg1 in dendritic cells or macrophages indicate that these effects of LXRs are probably mediated, at least partly, by upregulation of these transporters and by the promotion of cholesterol efflux103.
In addition to effects on neutrophils, ABCA1- and ABCG1-mediated cholesterol efflux in splenic dendritic cells and macrophages leads to the suppression of monocyte numbers apparently via reduced production of macrophage colony-stimulating factor (M-CSF) and CCL2, and diminished bone marrow myelopoiesis56. The expression of Abca1 and Abcg1 is strongly upregulated in macrophages during efferocytosis93, which probably leads to suppression of TLR-dependent inflammatory responses, including the production of IL-23. In Abca1−/−Abcg1−/− and Apoe−/− mice, activation of the IL-17, IL-23 and G-CSF signalling axis in the spleen also leads to the release of HSCs from the bone marrow and to extramedullary haematopoiesis103. Thus, hypercholesterolaemia and defective cholesterol efflux promote monocyte and neutrophil production through both cell-autonomous and cell-extrinsic mechanisms in the bone marrow and the spleen, involving HSC and myeloid progenitor cell proliferation, HSC mobilization and extramedullary haematopoiesis. Although these pathways probably enhance the response to infections, genetic alterations and dietary challenge lead to aberrant responses that promote atherogenesis.
The spleen as a site of extramedullary haematopoiesis
Robbins et al.105 first showed that in Apoe−/− mice fed a WTD, HSCs progressively relocate from the bone marrow to the splenic red pulp, where they encounter GM-CSF and IL-3, which promote myelopoiesis and the production of LY6Chi monocytes. Monocytes produced in the spleen enter the bloodstream, accumulate in atherosclerotic lesions and secrete pro-inflammatory cytokines, ROS and proteases105. A minor population of B cells in the mouse spleen, termed innate response activator B cells (IRA B cells), which are derived from peritoneal B1a cells, is mainly responsible for splenic GM-CSF production106. IRA B cells have an essential role in supporting the increased production of monocytes and neutrophils, and in the survival of mice with polymicrobial sepsis106. The splenic IRA B cell population is also expanded in WTD-fed Apoe−/− mice101,107 and genetic depletion of this population showed a pro-atherogenic role for these cells107. This was attributed to a decrease in the production of conventional dendritic cells — a process that is known to be dependent on GM-CSF — and to the reduced differentiation of IFNγ-secreting T helper 1 (TH1) cells107.
In Apoe−/− mice, the IRA B cell population is dependent on MYD88 signalling107 and has increased levels of CBS on their cell surface, which is probably secondary to defective cholesterol efflux101; CBS deficiency reduced the size of the B1a and IRA B cell populations101. Thus, in Apoe−/− mice, increased signalling of IL-3 and GM-CSF, mediated through increased levels of CBS, has a key role both in HSCs and in IRA B cells, promoting expansion of these populations, increased production of monocytes and neutrophils in the bone marrow and spleen, and probably also influencing dendritic cell maturation and TH cell differentiation. Apoe gene expression is increased by LXR activation108 and is suppressed by LPS109, and thus may be decreased in IRA B cells during the acute phase response, leading to decreased cholesterol efflux, increased CBS expression and enhanced GM-CSF production. As IRA B cells have an essential role in the clearance of microorganisms106, as a result of their production of GM-CSF, these data represent another example of how cellular cholesterol accumulation may lead to an enhanced immune response with beneficial effects in the setting of acute infection.
Targeting cholesterol and inflammation
LXR activators reduce atherosclerosis in mouse models110, which is probably a result of both cholesterol efflux and anti-inflammatory effects111,112. Although molecules that activate LXRs have been developed as anti-atherogenic drugs, their progress in the clinic has been hampered by unwanted side effects such as fatty liver disease and LDL elevation113. Other potential therapeutic applications of LXR activators involving their anti-inflammatory effects are being evaluated, for example, in autoimmune diseases92 and skin diseases114. Recent studies have uncovered specific roles of cholesterol derivatives such as 25-OH cholesterol and 7α,25-OH cholesterol in the immune response to infections, providing new insights into the intimate links between sterol metabolism and immunity, and opening the possibility of sterol-targeted interventions as a means of immunosuppression83,115,116.
LDL cholesterol can be lowered by statins and these drugs remain the mainstay of treatment in atherosclerosis. However, there is a large burden of residual disease in individuals treated with statins, which indicates the need for new therapies. Increasing the production or infusion of APOA1-containing HDL consistently reduces atherosclerosis in animal models117–119 and infusion of APOA1–phosphatidylcholine complexes seems to reduce coronary atherosclerosis in humans120,121. Moreover, the cholesterol efflux capacity of human plasma HDL is inversely correlated with atherosclerotic burden in the coronary and carotid arteries122. These anti-atherogenic effects are probably at least partly related to the anti-inflammatory effects of HDL. In addition to suppressing the production of inflammatory cells, HDL suppresses the expression of tumour necrosis factor (TNF)-induced cell adhesion molecules on endothelial cells that promote the entry of inflammatory cells into plaques123, and HDL pretreatment reduces LPS or oxidized LDL-induced inflammatory responses in macrophages124–126. Although part of the effect of HDL on inflammatory responses may be mediated through cholesterol efflux and the disruption of TLR-mediated signalling, there may be additional mechanisms such as the induction of the transcription factor ATF3, which function to suppress NF-κB signalling126 and atherogenesis127.
On a practical level, it has so far proved difficult to either increase endogenous APOA1 production or to infuse HDL in sufficient amounts to achieve a therapeutic benefit in humans. Infusions of fairly small amounts of sphingomyelin–APOA1 complexes did not reduce the volume of coronary atherosclerotic plaques128. Although larger amounts of phosphatidylcholine–APOA1 complexes reduced plaque volume compared with baseline levels, there were side effects that may have been related to the presence of bile salts in the preparations or to excessive removal of cholesterol from tissues121.
Improved preparations that are efficacious in mediating cholesterol efflux from macrophages are undergoing clinical evaluation129. For example, pegylation of the HDL particle (as opposed to free APOA1) results in preservation of the ability of APOA1 to promote cholesterol efflux via ABCA1, reduced catabolism of APOA1 in vivo and improved anti-atherogenic efficacy, with the potential to allow lower doses of HDL to be infused but retaining efficacy130. RVX-208 is a small molecule that functions as a bromodomain and extraterminal (BET) domain inhibitor, which displaces BET domains from chromatin and increases transcription of the human APOA1 gene, and thus increases APOA1 and HDL-cholesterol levels in humans131–133. Although the magnitude of these responses is too small to be clinically relevant131–133, there is the potential to identify more potent compounds that function through a similar epigenetic mechanism. Moreover, recent studies have identified a long non-coding RNA at the APOA1–APOC3–APOA4 locus that suppresses gene expression through an epigenetic mechanism134. Targeting this locus resulted in increased APOA1 production in monkey and human hepatocytes. Future studies involving transcriptional upregulation of APOA1, improved versions of reconstituted HDL particles or perhaps APOA1-mimetic peptides135,136 will better define their potential therapeutic benefits in patients with atherosclerosis, insulin resistance and autoimmunity.
Summary and perspective
The disruptions of cellular or organismal cholesterol homeostasis that occur as part of innate immune responses may lead to an augmentation of inflammatory responses via enhanced TLR signalling or inflammasome activation. This physiological adaptation, as exemplified by the process of HDL-mediated cholesterol efflux and RCT, becomes dysfunctional in chronic metabolic diseases such as obesity or atherosclerosis. Increasing the production of APOA1-containing HDL or activation of LXRs represent therapeutic intervention strategies that could disrupt this cycle with a potential benefit for patients with atherosclerosis, obesity, insulin resistance and autoimmune diseases.
Acknowledgements
This work was supported by the US National Institutes of Health (HL107653) and by the Leducq Foundation (to A.R.T.), and ATIP-AVENIR and ANR (to L.Y.-C).
Glossary
- Low-density lipoprotein (LDL)
A 20–25 nm low-density (1.016–1.063 g ml−1) lipoprotein with ~45% cholesterol, 20% phospholipids, 10% triglycerides and 25% protein (with apolipoprotein B (APOB) as the major apolipoprotein).
- High-density lipoprotein (HDL)
An 8–11 nm high-density (1.063–1.210 g ml−1) lipoprotein with 40–55% protein (with apolipoprotein A1 (APOA1) as the major apolipoprotein), 25% phospholipids, 15% cholesterol and 5% triglycerides. HDL particles carry cholesterol from peripheral tissues to the liver.
- Liver X receptor (LXR)
LXRα and LXRβ are transcription factors that function as heterodimeric partners with retinoid X receptors (RXRs) on the promoters of many genes that are involved in cholesterol metabolism and lipogenesis. LXRs are activated by cholesterol biosynthetic intermediates, such as desmosterol, and by oxysterols derived from cholesterol. LXRs are key regulators of cellular cholesterol efflux and reverse cholesterol transport and also block the cellular uptake of low-density lipoprotein (LDL) cholesterol through the LDL receptor.
- ABC transporters
A family of membrane transport proteins that use the energy of ATP hydrolysis to transport various molecules, including cholesterol and other lipids, across the membrane.
- Apolipoprotein A1 (APOA1)
The liver and the intestine secrete lipid-poor APOA1, the major protein component of high-density lipoprotein (HDL) particles. APOA1 functions as an acceptor for phospholipids and cholesterol on hepatocytes, enterocytes and macrophages. Thus, it may be involved in HDL formation as well as in the efflux of cholesterol from cells.
- Reverse cholesterol transport (RCT)
A multistep process that results in the net movement of cholesterol from peripheral tissues back to the liver via the blood. Cholesterol from peripheral tissues is transferred to apolipoprotein A1 (APOA1) and high-density lipoprotein (HDL) by the ATP-binding cassette transporters ABCA1 and ABCG1, respectively. The cholesteryl esters present within HDL can then be transferred, with the help of cholesteryl ester transfer protein in exchange for triglycerides, to APOB-rich lipoproteins (such as low-density lipoprotein and very low-density lipoprotein) or can be taken up in the liver by scavenger receptor B1 (SRB1). In the liver, cholesterol can be converted into bile acids for elimination.
- Acute phase response
The early immune response to infection, which results in the production of cytokines and other mediators, and in an increase in the number of peripheral leukocytes.
- Chylomicrons
50–200 nm diameter lowest density (<1.006 g ml−1) lipoproteins that are composed of 85% triglycerides, 9% phospholipids, 4% cholesterol, and 2% protein (with apolipoprotein B48 (APOB48) as the major apolipoprotein).
- Very low-density lipoprotein (VLDL)
A 30–70 nm very low-density (0.95–1.006 g ml−1) lipoprotein, with ~50% triglycerides, 20% cholesterol, 20% phospholipids and 10% protein (with apolipoprotein B100 (APOB100) as the major apolipoprotein).
- MicroRNA
Small RNA molecules that regulate the expression of genes by binding to the 3′-untranslated regions of specific mRNAs.
- NLRP3 inflammasome
The NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome consists of the NOD-like receptor NLRP3, caspase 1 and the adaptor protein ASC. It is activated by many signals, including microbial products, and stress- and injury-induced host factors, leading to caspase 1 activation, cleavage of pro-interleukin-1β (pro-IL-1β) and pro-IL-18, secretion of IL-1β and IL-18 and, in some cases, pyroptosis, which is a pro-inflammatory and lytic form of cell death.
- 25-hydroxycholesterol (25-OH cholesterol)
An oxysterol formed from cholesterol by the enzyme cholesterol 25-hydroxylase, which is present in the endoplasmic reticulum.
- Sterol regulatory element-binding protein 2 (SREBP2)
A transcription factor that begins as a multi-transmembrane endoplasmic reticulum protein and is cleaved in the Golgi to release the basic helix–loop–helix leucine zipper transcription factor domain that binds to sterol regulatory elements in DNA.
- Sumoylation
The post-translational modification of proteins that involves the covalent attachment of a small ubiquitin-related modifier (SUMO) and that regulates the interactions of those proteins with other macromolecules.
- Innate response activator B cells (IRA B cells)
An effector B cell population and a transitional B1a-derived inflammatory subset that control IgM production and protect against microbial sepsis.
- Statins
A family of inhibitors of hydroxymethylglutaryl-coenzyme A reductase (HMG-CoA reductase), which is an enzyme that catalyses the conversion of HMG-CoA to l-mevalonate. These molecules are mainly used as cholesterol-lowering drugs but they also have immunoregulatory and anti-inflammatory properties. l-Mevalonate and its metabolites are implicated in cholesterol synthesis and other intracellular pathways.
Footnotes
Competing interests statement
The authors declare competing interests: see Web version for details.
References
- 1.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart CR, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nature Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yvan-Charvet L, et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yvan-Charvet L, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J. Immunol. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu X, et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. This study provides the first evidence for cholesterol crystal-induced inflammasome activation in atherosclerotic lesions and shows that genetic deletion of essential inflammasome components results in reduced atherosclerosis.
- 8.Sheedy FJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang NHDL. ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell. Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Rader DJ, Tall AR. The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nature Med. 2012;18:1344–1346. doi: 10.1038/nm.2937. [DOI] [PubMed] [Google Scholar]
- 11. Castrillo A, et al. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell. 2003;12:805–816. doi: 10.1016/s1097-2765(03)00384-8. In this paper, activation of TLRs was shown to suppress LXR-responsive genes, notably those involved in cholesterol efflux from macrophages, providing a molecular mechanism to link the response to infectious organisms to suppression of the RCT pathway.
- 12. Feingold KR, Grunfeld C. The acute phase response inhibits reverse cholesterol transport. J. Lipid Res. 2010;51:682–684. doi: 10.1194/jlr.E005454. This paper is an overview of RCT and the acute phase response showing that the acute phase response suppresses RCT at multiple steps.
- 13.Post WS, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann. Intern. Med. 2014;160:458–467. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Strategies for Management of Antiretroviral Therapy Study Group. CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 15.Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nature Clin. Pract. Rheumatol. 2006;2:99–106. doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- 16.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stienstra R, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc. Natl Acad. Sci. USA. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umemoto T, et al. Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1. Circ. Res. 2013;112:1345–1354. doi: 10.1161/CIRCRESAHA.111.300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung S, et al. Dietary cholesterol promotes adipocyte hypertrophy and adipose tissue inflammation in visceral, but not in subcutaneous, fat in monkeys. Arterioscler. Thromb. Vasc. Biol. 2014;34:1880–1887. doi: 10.1161/ATVBAHA.114.303896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norata GD, Pirillo A, Ammirati E, Catapano AL. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis. 2012;220:11–21. doi: 10.1016/j.atherosclerosis.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 21.Ronda N, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann. Rheum. Dis. 2014;73:609–615. doi: 10.1136/annrheumdis-2012-202914. [DOI] [PubMed] [Google Scholar]
- 22.Rosenson RS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X, et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. This paper uses mice with deficiency of Abca1 and Abcg1 in macrophages to show that these cholesterol efflux-promoting transporters promote the movement of cholesterol from macrophages to the liver followed by excretion in the faeces — so-called ‘macrophage reverse cholesterol transport’.
- 24.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl Acad. Sci. USA. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 2009;50:S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martel C, et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J. Clin. Invest. 2013;123:1571–1579. doi: 10.1172/JCI63685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim HY, et al. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell. Metab. 2013;17:671–684. doi: 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Acton S, et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 29.Inazu A, et al. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N. Engl. J. Med. 1990;323:1234–1238. doi: 10.1056/NEJM199011013231803. [DOI] [PubMed] [Google Scholar]
- 30.Yu L, et al. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao GJ, et al. Antagonism of betulinic acid on LPS-mediated inhibition of ABCA1 and cholesterol efflux through inhibiting nuclear factor-κB signaling pathway and miR-33 expression. PLoS ONE. 2013;8:e74782. doi: 10.1371/journal.pone.0074782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masucci-Magoulas L, et al. Decreased cholesteryl ester transfer protein (CETP) mRNA and protein and increased high density lipoprotein following lipopolysaccharide administration in human CETP transgenic mice. J. Clin. Invest. 1995;95:1587–1594. doi: 10.1172/JCI117832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McGillicuddy FC, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. This paper shows that in humans, the infusion of reconstituted HDL particles suppresses the inflammatory cytokine response to LPS, providing direct evidence for an anti-inflammatory effect of HDL in vivo.
- 34.Pajkrt D, et al. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J. Exp. Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris HW, Gosnell JE, Kumwenda ZL. The lipemia of sepsis: triglyceride-rich lipoproteins as agents of innate immunity. J. Endotoxin Res. 2000;6:421–430. [PubMed] [Google Scholar]
- 36. Van Lenten BJ, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Invest. 1995;96:2758–2767. doi: 10.1172/JCI118345. This paper is an early study to show that HDL becomes pro-inflammatory during the acute phase response.
- 37.Watson AD, et al. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J. Clin. Invest. 1995;96:2882–2891. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Annema W, et al. Myeloperoxidase and serum amyloid A contribute to impaired in vivo reverse cholesterol transport during the acute phase response but not group IIA secretory phospholipase A2. J. Lipid Res. 2010;51:743–754. doi: 10.1194/jlr.M000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de la Llera Moya M, et al. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 2012;222:390–394. doi: 10.1016/j.atherosclerosis.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergt C, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl Acad. Sci. USA. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng L, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMillen TS, Heinecke JW, LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 2005;111:2798–2804. doi: 10.1161/CIRCULATIONAHA.104.516278. [DOI] [PubMed] [Google Scholar]
- 43.Hewing B, et al. Effects of native and myeloperoxidase-modified apolipoprotein a-I on reverse cholesterol transport and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2014;34:779–789. doi: 10.1161/ATVBAHA.113.303044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao B, et al. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ. Res. 2014;114:1733–1742. doi: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y, et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nature Med. 2014;20:193–203. doi: 10.1038/nm.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X, et al. Myeloid cell-specific ABCA1 deletion protects mice from bacterial infection. Circ. Res. 2012;111:1398–1409. doi: 10.1161/CIRCRESAHA.112.269043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown MS, Goldstein JL. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J. Biol. Chem. 1974;249:7306–7314. [PubMed] [Google Scholar]
- 48.Chen HW, Kandutsch AA, Waymouth C. Inhibition of cell growth by oxygenated derivatives of cholesterol. Nature. 1974;251:419–421. doi: 10.1038/251419a0. [DOI] [PubMed] [Google Scholar]
- 49.Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. The acute phase response is associated with retinoid X receptor repression in rodent liver. J. Biol. Chem. 2000;275:16390–16399. doi: 10.1074/jbc.M000953200. [DOI] [PubMed] [Google Scholar]
- 50. Bjorkbacka H, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nature Med. 2004;10:416–421. doi: 10.1038/nm1008. This paper links the TLR signalling pathway to inflammatory cytokine and chemokine production in macrophages in atherosclerotic lesions and to accelerated atherosclerosis in the Apoe−/− mouse model.
- 51.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullick AE, et al. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J. Exp. Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feig JE, et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc. Natl Acad. Sci. USA. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monaco C, et al. Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation. 2009;120:2462–2469. doi: 10.1161/CIRCULATIONAHA.109.851881. [DOI] [PubMed] [Google Scholar]
- 55. Zhu X, et al. Increased cellular free cholesterol in macrophage-specific Abca1-knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. This is an early paper showing that ABCA1 deficiency in macrophages results in increased responses to TLR4 ligands.
- 56.Westerterp M, et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ. Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spann NJ, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang C, et al. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 2006;281:27816–27826. doi: 10.1074/jbc.M603781200. [DOI] [PubMed] [Google Scholar]
- 59.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 60.Pagler TA, et al. Deletion of ABCA1 and ABCG1 impairs macrophage migration because of increased Rac1 signaling. Circ. Res. 2011;108:194–200. doi: 10.1161/CIRCRESAHA.110.228619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li HB, Jin C, Chen Y, Flavell RA. Inflammasome activation and metabolic disease progression. Cytokine Growth Factor Rev. 2014;25:699–706. doi: 10.1016/j.cytogfr.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 62.Razani B, et al. Autophagy links inflammasomes to atherosclerotic progression. Cell. Metab. 2012;15:534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi CS, et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nature Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ouimet M, et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell. Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheedy FJ, Moore KJ. IL-1 signaling in atherosclerosis: sibling rivalry. Nature Immunol. 2013;14:1030–1032. doi: 10.1038/ni.2711. [DOI] [PubMed] [Google Scholar]
- 67.Blankenberg S, et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24–30. doi: 10.1161/01.cir.0000020546.30940.92. [DOI] [PubMed] [Google Scholar]
- 68.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E−/− mice through release of interferon-γ. Circ. Res. 2002;90:e34–e38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 69.Mallat Z, et al. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ. Res. 2001;89:e41–e45. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
- 70.Menu P, et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Usui F, et al. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 2012;425:162–168. doi: 10.1016/j.bbrc.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 72.Gage J, Hasu M, Thabet M, Whitman SC. Caspase-1 deficiency decreases atherosclerosis in apolipoprotein E-null mice. Can. J. Cardiol. 2012;28:222–229. doi: 10.1016/j.cjca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 73.Zheng F, Xing S, Gong Z, Mu W, Xing Q. Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques in apolipoprotein E-deficient mice. Mediators Inflamm. 2014;2014:507208. doi: 10.1155/2014/507208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Small DM, Shipley GG. Physical-chemical basis of lipid deposition in atherosclerosis. Science. 1974;185:222–229. doi: 10.1126/science.185.4147.222. [DOI] [PubMed] [Google Scholar]
- 75.Small DM. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988;8:103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- 76.Kellner-Weibel G, et al. Crystallization of free cholesterol in model macrophage foam cells. Arterioscler Thromb. Vasc. Biol. 1999;19:1891–1898. doi: 10.1161/01.atv.19.8.1891. [DOI] [PubMed] [Google Scholar]
- 77.Tangirala RK, et al. Formation of cholesterol monohydrate crystals in macrophage-derived foam cells. J. Lipid Res. 1994;35:93–104. [PubMed] [Google Scholar]
- 78.McDonald JG, Russell DW. Editorial: 25-Hydroxycholesterol: a new life in immunology. J. Leukoc. Biol. 2010;88:1071–1072. doi: 10.1189/jlb.0710418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diczfalusy U, et al. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res. 2009;50:2258–2264. doi: 10.1194/jlr.M900107-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park K, Scott AL. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol. 2010;88:1081–1087. doi: 10.1189/jlb.0610318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu SY, et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2013;38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gold ES, et al. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc. Natl Acad. Sci. USA. 2014;111:10666–10671. doi: 10.1073/pnas.1404271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Reboldi A, et al. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345:679–684. doi: 10.1126/science.1254790. This study shows that the synthesis of 25-OH cholesterol that occurs during the acute phase response leads to suppression of inflammasome activity in vivo, probably as a result of the ability of 25-OH cholesterol to inhibit the synthesis of cholesterol or other sterols.
- 84.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 85. Xiao H, et al. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128:632–642. doi: 10.1161/CIRCULATIONAHA.113.002714. This study suggests that the NLRP3 inflammasome is activated in endothelial cells in response to disturbed blood flow.
- 86.Choi JH, et al. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 87.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ. Res. 2014;114:1757–1771. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li P, et al. NCoR repression of LXRs restricts macrophage biosynthesis of insulin-sensitizing omega 3 fatty acids. Cell. 2013;155:200–214. doi: 10.1016/j.cell.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rong X, et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell. Metab. 2013;18:685–697. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghisletti S, et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARγ. Mol. Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.N AG, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gerbod-Giannone MC, et al. TNFα induces ABCA1 through NF-κB in macrophages and in phagocytes ingesting apoptotic cells. Proc. Natl Acad. Sci. USA. 2006;103:3112–3117. doi: 10.1073/pnas.0510345103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Murphy AJ, et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. This study shows that APOE is expressed in HSCs, in which it seems to be bound to cell-surface proteoglycans, promoting cholesterol efflux via ABCA1 and ABCG1 and thus suppressing the proliferation of HSCs and myelopoiesis with anti-atherogenic consequences.
- 95.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler. Thromb. Vasc. Biol. 2005;25:658–670. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 96.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yvan-Charvet L, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. This is the first study to show that cholesterol efflux pathways suppress the proliferation of HSCs, myelopoiesis and atherogenesis.
- 98.Gao M, et al. Regulation of high-density lipoprotein on hematopoietic stem/progenitor cells in atherosclerosis requires scavenger receptor type BI expression. Arterioscler. Thromb. Vasc. Biol. 2014;34:1900–1909. doi: 10.1161/ATVBAHA.114.304006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tolani S, et al. Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: studies in mice and FH children. Atherosclerosis. 2013;229:79–85. doi: 10.1016/j.atherosclerosis.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng Y, et al. Hematopoietic stem/progenitor cell proliferation and differentiation is differentially regulated by high-density and low-density lipoproteins in mice. PLoS ONE. 2012;7:e47286. doi: 10.1371/journal.pone.0047286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang M, et al. Interleukin-3/granulocyte macrophage colony-stimulating factor receptor promotes stem cell expansion, monocytosis, and atheroma macrophage burden in mice with hematopoietic ApoE deficiency. Arterioscler. Thromb. Vasc. Biol. 2014;34:976–984. doi: 10.1161/ATVBAHA.113.303097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Kampen E, Jaminon A, van Berkel TJ, Van Eck M. Diet-induced (epigenetic) changes in bone marrow augment atherosclerosis. J. Leukoc. Biol. 2014;96:833–841. doi: 10.1189/jlb.1A0114-017R. [DOI] [PubMed] [Google Scholar]
- 103. Westerterp M, et al. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. This paper shows that cholesterol efflux pathways suppress the mobilization of HSCs to the spleen and thus extramedullary haematopoiesis, which is relevant to both atherosclerosis and leukaemia.
- 104.Hong C, et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J. Clin. Invest. 2012;122:337–347. doi: 10.1172/JCI58393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robbins CS, et al. Extramedullary hematopoiesis generates Ly-6Chigh monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rauch PJ, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hilgendorf I, et al. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation. 2014;129:1677–1687. doi: 10.1161/CIRCULATIONAHA.113.006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laffitte BA, et al. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl Acad. Sci. USA. 2001;98:507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Werb Z, Chin JR. Endotoxin suppresses expression of apoprotein E by mouse macrophages in vivo and in culture. A biochemical and genetic study. J. Biol. Chem. 1983;258:10642–10648. [PubMed] [Google Scholar]
- 110.Claudel T, et al. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc. Natl Acad. Sci. USA. 2001;98:2610–2615. doi: 10.1073/pnas.041609298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levin N, et al. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler. Thromb. Vasc. Biol. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 112.Kappus MS, et al. Activation of liver X receptor decreases atherosclerosis in Ldlr−/− mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells. Arterioscler. Thromb. Vasc. Biol. 2013;34:279–284. doi: 10.1161/ATVBAHA.113.302781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nature Rev. Drug Discov. 2014;13:433–444. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- 114.Schmuth M, Moosbrugger-Martinz V, Blunder S, Dubrac S. Role of PPAR, LXR, and PXR in epidermal homeostasis and inflammation. Biochim. Biophys. Acta. 2014;1841:463–473. doi: 10.1016/j.bbalip.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 115.Yi T, et al. Oxysterol gradient generation by lymphoid stromal cells guides activated B cell movement during humoral responses. Immunity. 2012;37:535–548. doi: 10.1016/j.immuni.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hannedouche S, et al. Oxysterols direct immune cell migration via EBI2. Nature. 2011;475:524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc. Natl Acad. Sci. USA. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 120.Nissen SE, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 121.Tardif JC, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 122.Khera AV, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler. Thromb. Vasc. Biol. 1995;15:1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 124.Yvan-Charvet L, et al. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler. Thromb. Vasc. Biol. 2010;30:1430–1438. doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suzuki M, et al. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation. 2010;122:1919–1927. doi: 10.1161/CIRCULATIONAHA.110.961193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Nardo D, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nature Immunol. 2014;15:152–160. doi: 10.1038/ni.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gold ES, et al. ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol-induced lipid body formation. J. Exp. Med. 2012;209:807–817. doi: 10.1084/jem.20111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tardif JC, et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur. Heart J. 2014 doi: 10.1093/eurheartj/ehu171. http://dx.doi.org/10.1093/eurheartj/ehu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Diditchenko S, et al. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2013;33:2202–2211. doi: 10.1161/ATVBAHA.113.301981. [DOI] [PubMed] [Google Scholar]
- 130.Murphy AJ, Funt S, Gorman D, Tall AR, Wang N. Pegylation of high-density lipoprotein decreases plasma clearance and enhances antiatherogenic activity. Circ. Res. 2013;113:e1–e9. doi: 10.1161/CIRCRESAHA.113.301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nicholls SJ, et al. ApoA-I induction as a potential cardioprotective strategy: rationale for the SUSTAIN and ASSURE studies. Cardiovasc. Drugs Ther. 2012;26:181–187. doi: 10.1007/s10557-012-6373-5. [DOI] [PubMed] [Google Scholar]
- 132.Picaud S, et al. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc. Natl Acad. Sci. USA. 2013;110:19754–19759. doi: 10.1073/pnas.1310658110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McLure KG, et al. RVX-208, an inducer of ApoA-I in humans, is a BET bromodomain antagonist. PLoS ONE. 2013;8:e83190. doi: 10.1371/journal.pone.0083190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Halley P, et al. Regulation of the apolipoprotein gene cluster by a long noncoding RNA. Cell Rep. 2014;6:222–230. doi: 10.1016/j.celrep.2013.12.015. This paper shows that a long non-coding RNA functions at the APOA1 gene locus to suppress gene expression in rodents and primates, whereas antagonism increases plasma APOA1 and HDL levels.
- 135.Watson CE, et al. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 2011;52:361–373. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bloedon LT, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nature Rev. Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Westerterp M, et al. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ. Res. 2014;114:157–170. doi: 10.1161/CIRCRESAHA.114.300738. [DOI] [PubMed] [Google Scholar]
- 140.Murphy AJ, et al. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nature Med. 2013;19:586–594. doi: 10.1038/nm.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nahrendorf M, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dutta P, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]