Abstract
In the present work we report on the click synthesis of a new camptothecin (CPT) prodrug based on anionic polyamidoamine (PAMAM) dendrimer intended for cancer therapy. We applied ‘click’ chemistry to improve polymer-drug coupling reaction efficiency. Specifically, CPT was functionalized with a spacer, 1-azido-3,6,9,12,15-pentaoxaoctadecan-18-oic acid (APO), via EDC/DMAP coupling reaction. In parallel, propargylamine (PPA) and methoxypoly(ethylene glycol) amine were conjugated to PAMAM dendrimer G4.5 in sequence using an effective coupling agent 4-(4,6-dimethoxy-(1,3,5)triazin-2-yl)-4-methyl-morpholinium chloride (DMTMM). CPT-APO was then coupled to PEGylated PAMAM dendrimer G4.5-PPA via a click reaction using copper bromide/2,2’-bipyridine/ dimethyl sulfoxide (catalyst/ligand/solvent). Human glioma cells were exposed to the CPT-conjugate to determine toxicity and cell cycle effects using WST-1 assay and flow cytometry. The CPT-conjugate displayed a dose-dependent toxicity with an IC50 of 5 μM, a 185-fold increase relative to free CPT, presumably as a result of slow release. As expected, conjugated CPT resulted in G2/M arrest and cell death while the dendrimer itself had little to no toxicity. Altogether, highly efficient click chemistry allows for the synthesis of multifunctional dendrimers for sustained drug delivery.
INTRODUCTION
Dendrimers have attracted increasing attention as drug carriers in that they possess a high degree of molecular uniformity, high drug loading capacity, and the ability to accommodate various functional entities.1-3Thus, various dendrimer-based drug delivery platforms have been developed4-7, and their utility for delivering anticancer drugs _ENREF_18_ENREF_19has been actively explored and yielded promising results.8-14_ENREF_20 _ENREF_18Regardless, a commonly encountered problem is the heterogeneity of ligand and drug on the dendrimer surface during chemical synthesis.15 It is essential to obtain a uniform distribution of ligand and drug for standardizing therapeutic effects and for the successful translation of dendrimer-based nanomedicines to clinical application. Robust and efficient coupling methods must be applied to overcome such issues.
Camptothecin is a plant alkaloid isolated from Camptotheca acuminata of the Nyssaceae family with remarkable anticancer activity by inhibiting both DNA and RNA synthesis.16 Camptothecin has been used for the treatment of many different types of cancer despite its low water solubility and poor stability of the lactone form, a required form for therapeutic activity. 17 In particular, it is of advantage to use CPT to treat cancer as it selectively kills proliferating (S-phase) cells, thus exerting little to no toxicity to non-dividing normal cells resident of the brain.18, 19 Polymer-CPT conjugates have been made by various methods.20-22 Although several dendrimer-CPT derivatives have been made in the past, the efficiency using high capacity of dendrimer to deliver CPT was low. _ENREF_9For instance, Thiagarajan et al. applied EDC/NHS chemistry to conjugate CPT to the dendrimer via a glycine spacer.23_ENREF_9 Less than 20% of CPT used in the reaction was successfully attached to the dendrimer. Copper-catalyzed azide-alkyne cycloaddition (CuAAC), the best known example of a “click” reaction, is a highly efficient and selective synthetic method. It has proven to be a powerful strategy for precisely loading drugs to various polymeric carriers including dendrimers.22, 24, 25_ENREF_30 The current study reports on the use of click chemistry for the synthesis of water soluble polyamidoamine (PAMAM) dendrimer-camptothecin (CPT) prodrug conjugates towards development of an enabling modular dendrimer-based delivery system for cancer therapy. In particular, we applied CuAAC for improving coupling reaction efficiency and to synthesize CPT-dendrimer conjugates. Anionic PAMAM dendrimer G4.5 was used as the carrier because of low toxicity and low non-specific cellular uptake. It was modified with polyethylene glycol (PEG) for improved water solubility and cytocompatibility. CPT was click coupled to the dendrimer carrier via a short heterobifunctional spacer. The click synthesis and characterization of the resulting dendrimer-based CPT prodrug and its therapeutic activity are reported herein.
EXPERIMENTAL SECTION
Materials
EDA core PAMAM dendrimer G4.5 caboxylate sodium salt was purchased from Dendritech (Midland, MI). 2,2’-Bipyridine, 4-dimethylaminopyridine (DMAP), copper(II) sulfate (CuSO4), (+)-sodium L-ascorbate, 4-(4,6-dimethoxy-(1,3,5)triazin-2-yl)-4-methyl-morpholinium chloride (DMTMM), deuterated solvents, dichloromethane (DCM), dimethyl sulfoxide (DMSO), and other organic solvents were purchased from Acros (Morris Plains, NJ). (S)-(+)-Camptothecin (CPT), propargylamine (PPA), copper bromide (CuBr), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), trifluoroacetic acid (TFA) and silica gel 60 (40-63 μm, 230-400 mesh) were purchased from Sigma-Aldrich (St. Louis, MO). 1-Azido-3,6,9,12,15-pentaoxaoctadecan-18-oic acid (APO) and methoxypoly(ethylene glycol) amine (mPEG-NH2, 2000 g/mol) were purchased from Biomatrik (Jiaxing, Zhejiang, China) and JenKem Technology USA (Plano, TX), respectively. SnakeSkin dialysis tubing 3.5 kDa and 7 kDa MWCO, acetonitrile (ACN), HPLC grade water, phosphate-buffered saline (PBS), and magnesium sulfate (MgSO4) were purchased from Thermo Fisher Scientific (Pittsburg, PA).
Instrumentation
1H NMR and 13C NMR spectra were recorded on a Bruker AVANCEIII 600 MHz spectrometer. Mass spectra were obtained on AB Sciex 5800 MALDI TOF-TOF using α-Cyano-4-hydroxycinnamic acid (4-HCCA) as matrix. UV/Vis spectra were acquired on an Agilent 8453 spectrophotometer. The dendrimer conjugates in solutions were characterized with Malvern Zetasizer Nano ZS90 (Malvern Instruments, Worcestershire, U.K.).
Synthesis of CPT-APO
To a suspension of CPT (200 mg, 0.57 mmol) in 60 mL of DCM were added EDC (330 mg, 1.73 mmol) and DMAP (140 mg, 1.14 mmol) followed by APO (290 mg, 0.86 mmol) pre-dissolved in 5 mL of DCM. After having been stirred for 24 h at room temperature, the reaction mixture was poured into 50 mL of water. The organic phase was collected. The aqueous phase was extracted with DCM two times. The DCM fractions were combined and dried over MgSO4. Upon the removal of DCM by rotary evaporation, the obtained CPT-APO was further purified by column chromatography on silica gel using a DCM/methanol mixture (97.3%/2.7%, v/v). Yield 67%. 1H NMR (d6-DMSO, 600 MHz): δ (ppm) 8.68 (s, 1H), 8.15 (d, J=8.5 Hz, 1H), 8.12 (d, J=8.2 Hz, 1H), 7.86 (t, J= 7.9Hz, 1H), 7.71 (t, J=7.5 Hz, 1H), 7.16 (s, 1H,), 5.50 (s, 2H), 5.28 (q, 2H), 3.83-3.33 (m, 22H), 2.81 (m, 1H), 2.66 (m, 1H), 2.14 (m, 2H), 0.94 (t, J =7.4Hz, 3H). 13C NMR (CDCl3, 600 MHz): δ (ppm) 170. 81, 167.63, 157.57, 152.61, 149.04, 146.36, 146.26, 131.33, 130.83, 129.80, 128.66, 128.39, 128.34, 128.20, 120.25, 96.54, 76.25, 70.86, 70.79, 70.70, 70.19, 67.19, 66.54, 50.86, 50.10, 35.12, 31.92, 7.79. MALDI-TOF MS (m/z): [M+H]+ Calculated for C33H40N5O10: 666.27, Found: 666.22.
Synthesis of G4.5-PPA
To a solution of PAMAM dendrimer G4.5 in carboxyl form (50 mg, 2.1 μmol) in 3 mL of 0.1M NaHCO3 was added DMTMM (58 mg, 0.21 mmol) followed by addition of 0.5 mL of DMF containing 0.13 mmol PPA. The reaction mixture was stirred overnight. Upon removal of the solvent under reduced pressure, the remaining residue was dialyzed against water using dialysis tube with MWCO 3.5 kDa and freeze-dried to yield 53 mg of G4.5-PPA. 1H NMR (D2O, 600 MHz): δ (ppm) 3.98 (m, 2H, CH2CCH), 2.43-3.69 (m, methylene protons of G4.5).
Synthesis of PPA-G4.5-PEG Conjugates
To a solution of G4.5-PPA (40 mg, 1.7 μmol) and DMTMM (17 mg, 0.061 mmol) in 4 mL of 0.1M NaHCO3 was added mPEG-NH2 (102 mg, 51 μmol). The obtained mixture was stirred overnight at room temperature, dialyzed against water using dialysis tubing with 7.0 kDa MWCO for 48 h, and then freeze-dried to obtain 86 mg of PPA-G4.5-PEG. 1H NMR (D2O, 600 MHz): δ (ppm) 3.99 (m, 2H), 3.73 (br.s, methylene protons in PEG repeat units), 3.41 (s, 3H), 3.40-2.42 (m, methylene protons of G4.5).
Synthesis of CPT-G4.5-PEG Conjugates
PPA-G4.5-PEG (20 mg, 0.26 μmol), CPT-APO (2.9 mg, 4.4 μmol), and 2,2’-bipyridine (0.6 mg, 3.8 μmol) were mixed in 1 mL of DMSO. The molar feed ratio of CPT to dendrimer in this reaction was 17:1, which was determined based on availability assessment for the alkyne groups on the dendrimer (see Supporting Information at http://www.rsc.org/suppdata/c5/ra/c5ra07987j/c5ra07987j1.pdf). The obtained mixture underwent 3 times of freeze-pump-thaw cycling for degassing. Afterwards, CuBr (0.3 mg, 2.1 μmol) dissolved in 40 μL of DMSO was added to the mixture solution. The reaction mixture under nitrogen was stirred in dark overnight at room temperature and then poured into 5 mL of water. After 1 h-stirring, the solvents were removed under reduced pressure. Extraction of unreacted CPT-APO was conducted by vortexing the obtained solid residue with ether (1 mL each time) followed by centrifugation for liquid-solid separation. The extraction procedure was repeated until CPT-APO became undetectable in ether by UV-Vis spectrophotometer. The remaining solid was dissolved in 1 mL of water followed by centrifugation. The liquid phase was collected and freeze-dried to yield CPT-G4.5-PEG. UV-Vis spectroscopy analysis confirmed that conjugated CPT accounted for 6.4 wt.% of the product. 1H NMR (d6-DMSO, 600 MHz): δ (ppm) 8.64 (br.s, 1H), 8.12 (br.s, 2H), 7.85 (br.s, 2H), 7.68 (br.s, 1H), 7.14 (br.s, 1H), 5.50 (s, 2H), 5.26 (br.s, 2H), 4.44 (br.s, 2H), 4.26 (br.s, 2H), 3.50 (br.s, methylene protons in the repeat unit of PEG), 3.23 (s, 3H), 2.06-2.95 (m, methylene protons of G4.5), 0.94 (s, 3H).
In Vitro Drug Release
The CPT-containing polymers (0.5 mg/mL) were incubated in pH 7.4 PBS at 37 °C. Aliquots (20 μL) of drug release medium were taken at different time points and analyzed with reverse-phase high-performance liquid chromatography (RP-HPLC) (Waters, Milford, MA). The RP-HPLC system was equipped with Waters 717plus autosampler, XTerra RP18 column (5 μm, 4.6×150 mm), Waters 1515 isocratic HPLC pump, and Waters 2487 dual absorbance detector. The mobile phase was comprised of 74.97 v% H2O, 24.99 v% ACN and 0.04 v% TFA. The eluent with a flow rate at 1.0 mL/min was monitored by the UV detector at a wavelength of 370 nm for CPT.
Cell Culture
Human glioma U1242 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% Cosmic calf serum at 37 °C in 95% air/5% CO2.26
Cytotoxicity Assay
Human glioma U1242 cells were seeded at a density of 1×104 cells/well in a 96-well cell culture plate and cultured for 1 day to allow cell attachment. The cells were then treated with various concentrations (0-50 μM) of CPT in either free or conjugated form for 2 days. For comparison, toxicity of PPA-G4.5-PEG conjugates at the same molar concentrations as CPT-G4.5-PEG conjugates was used as control. Cell viability relative to untreated and control-treated cells was then determined by WST-1 proliferation assay. GraphPad Prism 5 was used to perform the curve fitting and then determine the 50% maximal inhibitory concentrations of free CPT (IC50free) and conjugated CPT (IC50conjugated).
Cell Cycle Analysis
Human glioma U1242 cells (1×106) were seeded in a 100-mm cell culture dish and cultured for 1 day to allow cell attachment. The cells were treated with CPT at the concentration of 2×IC50free, conjugated CPT at the concentration of 2×IC50cojugated and PPA-G4.5-PEG conjugates at the equivalent concentration of CPT-G4.5-PEG conjugates for various lengths of time (6, 12, and 24 h). The cells treated with PBS were used as a control. At the end of each treatment, the cells were washed with PBS and re-suspended in fresh cell culture medium following trypsinization. Following the centrifugal removal of the medium, the cells were fixed with cold 70% ethanol and maintained at 4°C for 1 h. Finally, the cells were washed with PBS three times and incubated with RNase at a final concentration of 1 μg/mL and propidium iodide at a final concentration of 50 μg/mL at 37 °C for 30 min. The cells were then immediately analyzed by flow cytometry using a Guava EasyCyte mini flow cytometry system (Millipore, Billerica, MA).27
RESULTS AND DISCUSSION
Characterization
We synthesized clickable PAMAM dendrimer G4.5 and used it as a carrier to deliver CPT as half generation PAMAM dendrimers are generally less toxic and have lower nonspecific cellular uptake as opposed to full generation dendrimers.28, 29 Given the hydrophobicity of CPT, the dendrimer drug loading degree should be carefully controlled to avoid generating water insoluble entities. The incorporation of hydrophilic molecules such as PEG onto the dendrimer surface has proven effective in improving water solubility and enhancing cytocompatibility. 29, 30 Thus, this step was applied in the synthesis of our dendrimer-CPT conjugates. A short hetero-bifunctional spacer, i.e., APO bearing azide and carboxyl groups, was used to modify CPT via EDC/DMAP chemistry. The reaction proceeded successfully in methylene chloride. The purity of CPT-APO and CPT-G4.5-PEG conjugates was verified based on the RP-HPLC analysis. As shown in Figure S-1 (Supporting Information), unreacted CPT (retention time 1.90 minutes) was successfully removed from CPT-APO (retention time 2.02 minutes) and CPT-G4.5-PEG conjugates (retention time 1.02 minutes). PAMAM dendrimer G4.5 bearing carboxylic acid terminals (0.5 mg/mL) was found to possess a zeta potential of −18.5 mV. The zeta potential of the resulting conjugates (0.5 mg/mL) was −8.3 mV. The less negative zeta potential indicated dendrimer surface property change as a result of PEGylation and CPT coupling.
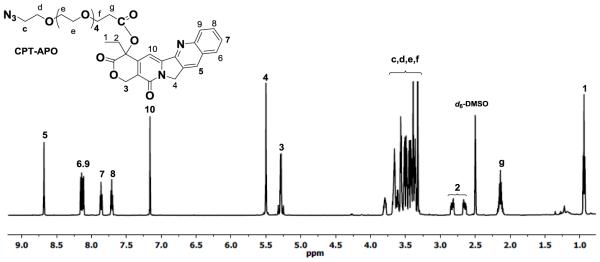
1H NMR spectrum clearly shows proton signals from both CPT and APO (Figure 1). In particular, multiple proton signals between 3.40 and 3.83 ppm are from methylene protons in APO. The signal at 5.28 ppm is assigned to the two methylene protons in the CPT lactone ring, indicating that therapeutically active lactone form remained. A double doublet for the methylene protons in the lactone ring was also clearly seen in the 1H NMR spectrum based on CDCl3 (Figures S-2 and S-3, Supporting Information). 13C NMR spectroscopy (Figures S-4 and S-5) provided complementary information for CPT-APO conjugates.
Figure 1.
1H NMR spectrum of CPT-APO in d6-DMSO.
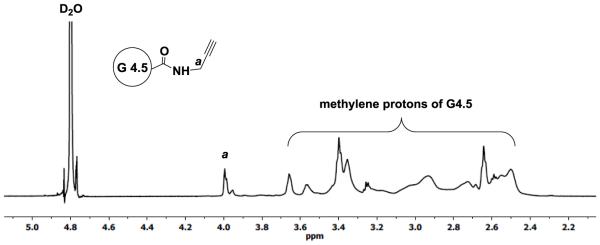
To make clickable dendrimers, alkyne groups were introduced onto the PAMAM dendrimer surface via reaction with PPA using coupling reagent DMTMM. 1H NMR spectrum of G4.5-PPA (Figure 2) shows multiple peaks of dendrimer methylene protons in the range 2.43-3.69 ppm along with multiplet at 3.98 ppm assigned to methylene protons (a) adjacent to the alkyne group of PPA, confirming the successful amide bond formation. Based on the 1H NMR integration analysis, the synthesized G4.5-PPA conjugates carried 30 alkyne groups per dendrimer.
Figure 2.
1H NMR spectrum of G4.5-PPA in D2O.
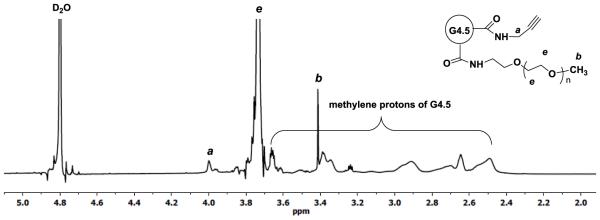
G4.5-PPA was further PEGylated by coupling mPEG-NH2 (2000 Da) to the dendrimer surface in the presence of DMTMM. The 1H NMR spectrum shown in Figure 3 confirms successful covalent attachment of mPEG to G4.5-PPA. In addition to the identification of the proton signals from PPA and dendrimer moieties, a broad singlet centered at 3.73 ppm is assigned to the methylene protons of PEG repeat units while a singlet at 3.41 ppm is due to the methyl protons. Based on the 1H NMR spectrum, the degree of PEGylation was calculated to be 27.
Figure 3.
1H NMR spectrum of PPA-G4.5-PEG in D2O.
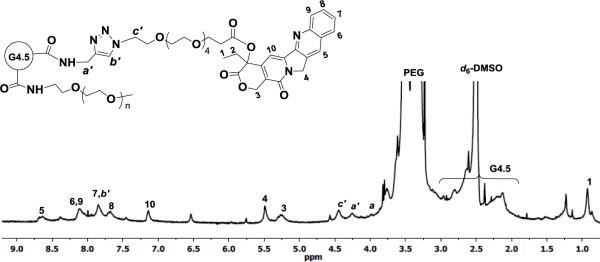
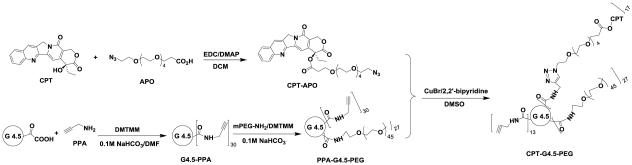
The final step of the synthesis involves a click reaction between alkyne-carrying PEGylated PAMAM dendrimer G4.5 (i.e., PPA-G4.5-PEG) and azide-carrying CPT (i.e., CPT-APO) in the presence of CuBr/2,2’-bipyridine (catalyst/ligand) system in DMSO. Using the alkyne accessibility analysis result as a guide (see Supporting Information Figure S3), the feed molar ratio (17:1) of CPT to dendrimer was used in the click reaction. The resulting CPT-G4.5-PEG conjugates were characterized by 1H NMR spectroscopy. The proton signals of CPT and spacer APO in the conjugates are identified in Figure 4. A further downfield shift of the signal of methylene protons (a’, 4.26 ppm) adjacent to the ring opposed to proton signal (a, 3.99 ppm) resulted from the triazole linkage formation. In addition, methine proton (b’) in the triazole ring appears as a singlet at 7.85 ppm, overlapping the benzene proton signal of CPT. The proton signal at 3.99 ppm (a) indicates the presence of remaining alkyne groups. Because of proton signal interference by the deuterated solvent d6-DMSO in the spectrum, CPT coupled to the conjugates was quantified by using UV-Vis spectroscopy. Based on the UV absorbance at 370 nm (Figure S-6, Supporting Information), the final polymer-drug conjugates carried approximately 17 CPT molecules per dendrimer. The estimated drug loading degree matches the feed molar ratio used in the click reaction, indicating a 100% conversion rate for the utility of CPT in the click reaction. The overall molecular weight of CPT-G4.5-PEG was estimated to be 90,657 g/mol.
Figure 4.
1H NMR spectrum of CPT-G4.5-PEG in d6-DMSO.
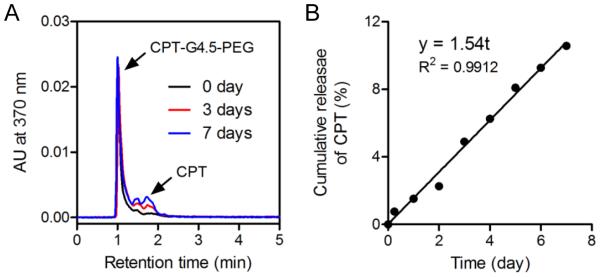
In vitro drug release studies were performed on CPT-G4.5-PEG conjugates to assess CPT release rate and potential utility of the resulting conjugates in delivery applications. RP-HPLC was used to measure CPT release kinetics from the conjugates. CPT was slowly released via hydrolysis of the ester bond between CPT and APO. About 10.6% CPT was released from the conjugates, following zero order kinetics over the course of 7 days (Figure 5). CPT release kinetics may be modulated to achieve a desirable level and duration of drug action in future work. Efforts may include utility of more labile chemical bonds (e.g., pH sensitive bonds, enzymatic sensitive bonds) and more hydrophilic spacer for drug-spacer coupling for faster release, increasing drug loading, and so on.
Figure 5.
(A) Representative RP-HPLC chromatograms of CPT-G4.5-PEG incubated in pH7.4 PBS for various lengths of time (i.e., 0, 3, and 7 days). (B) In vitro release profile of CPT conjugated to PAMAM dendrimer G4.5 based on RP-HPLC measurements.
Drug Activity Evaluation
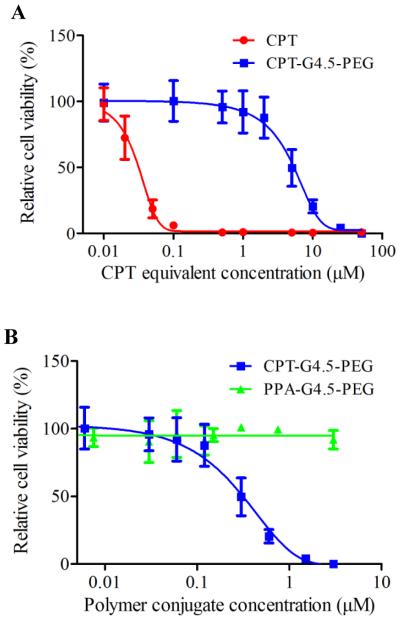
CPT interferes with the breakage-reunion reaction of DNA topoisomerase I (Top1) often referred to as the cleavable complex necessary to relieve DNA stress during DNA replication.16 In doing so, a ternary complex between Top1-CPT-DNA is formed which is converted into single-strand breaks and subsequently into double-strand breaks during DNA replication. The cytotoxicity of the synthesized conjugates to human glioma U1242 cells was then assessed. CPT-G4.5-PEG cytotoxicity was found to be dose-dependent with an IC50 of 5 μM (Figure 5A). In comparison, the IC50 of free CPT was 27 nM. Conjugated CPT is expected to become therapeutically active following hydrolysis of the ester linkage between CPT and the dendrimer. As expected, the conjugated CPT exhibited much lower potency than free CPT at equivalent concentrations because of slow release as presented in Figure 5. Thus, the conjugates are capable of sustaining therapeutic activity for a longer period of time. PEGylated PAMAM dendrimer carrier without CPT is cytocompatible and does not cause toxicity to cells at concentrations up to 50 μM (Figure 5B).
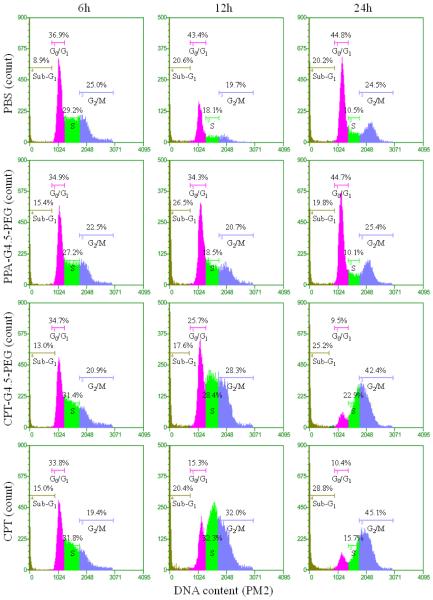
Prolonged exposure to CPT is expected to bring about DNA double-strand breaks during replication hence resulting in an arrest in G2/M of the cell cycle if not repaired.18, 31 We performed cell cycle analyses following treatment with PBS, free CPT, CPT-G4.5-PEG or dendrimer carrier. As shown in Figure 6, cells treated with free CPT or CPT-G4.5-PEG conjugate exhibited a remarkable decrease in the G0/G1 cell population and an increase in G2/M going from a little more than 20% to more than 40% over a period of 24 h. Untreated cells (PBS group) showed a normal cell cycle distribution with a larger fraction in the G0/G1 phase. The cells treated with PPA-G4.5-PEG conjugate showed a similar cell cycle distribution as untreated cells, indicating that the PPA-G4.5-PEG conjugate had little to no effect on cell cycle progression. Consistent with cytotoxicity assessment, cell cycle analyses further demonstrated that CPT-G4.5-PEG induced an arrest in G2/M and lead to apoptosis as indicated by a temporal increase in the sub-G1 population.
Figure 6.
Cytotoxicity of free CPT, CPT-G4.5-PEG and PPA-G4.5-PEG conjugates in U1242 cells. The data points are mean ± SD.
Overall, the current work illustrated the therapeutic activity of dendrimer-based CPT prodrug synthesized via click chemistry and it may exert long-lasting effect through slow release. The presence of a targeting moiety on the surface of a dendrimer molecule whose receptors are overexpressed on cancer cell membrane surface will enable the delivery of PAMAM dendrimer-drug conjugate to the tissue of the interest and improve specific uptake of the drug. The synthesized dendrimer-based CPT prodrug can be further functionalized for targeted therapy of many types of cancer such as glioblastoma multiforme (GBM). GBM is an aggressive form of brain cancer with poor prognosis and a median survival of only 12-15 months. In general, GBMs are _ENREF_5resistant to standard treatment consisting of surgery followed by concurrent chemo- and radiotherapy.32 New therapeutic approaches such as the use of small molecule radiosensitizers and gene therapy are under investigation for the treatment of GBM.33, 34 However, treatment outcomes still depend largely on whether or not sufficient levels of therapeutic agent can be delivered to the brain tumor mass.35 Considering that the blood-brain barrier (BBB), the blood cerebral spinal fluid, and the blood-tumor barrier hamper the administration of the therapeutic to the brain, efficient delivery still remains a challenge, and new technologies and delivery systems need to be developed.36, 37 Despite that a number of carriers have been developed to facilitate drug entry into the brain, 32, 35, 37-39 dendrimers appear to be a suitable platform to deliver drugs along with BBB-specific ligands because of their multivalency. In future studies, coupling tumor- and BBB-specific ligands or other functional moieties to the synthesized dendrimer-CPT conjugates via click chemistry will be explored for directing and enhancing drug uptake by brain tumor cells.
CONCLUSIONS
CPT was coupled to PEGylated PAMAM dendrimer G4.5 via copper-catalyzed click reaction. Nearly 100% of CPT molecules used in the reaction were covalently conjugated to the dendrimer. The resulting conjugates carried an average of 17 CPT molecules per dendrimer and demonstrated dose-dependent toxicity against human glioma U1242 cells by causing an arrest in G2/M and inducing cell death while the carrier itself had no toxicity. Conjugated CPT was found to have an IC50 of 5 μM, a 185-fold increase in comparison to the IC50 of free CPT as a result of slow release. The further utility of click chemistry based coupling strategy would make it possible to make large-scale polymer-drug-ligand conjugates of high quality possessing a uniform loading of ligand and drug on the dendrimer surface, a critical factor for achieving reproducible antitumor efficacy.
Supplementary Material
Figure 7.
Cell cycle analysis. U1242 cells were left untreated (control), CPT at the concentration of 2×IC50free, conjugated CPT at the concentration of 2×IC50cojugated, and PPA-G4.5-PEG conjugates at the equivalent concentration of CPT-G4.5-PEG conjugates for various lengths of time (6, 12, and 24 h).
Scheme 1.
Synthesis of CPT-G4.5-PEG conjugates.
ACKNOWLEDGEMENTS
This work was supported, in part, by the National Science Foundation (CAREER award CBET0954957), National Institutes of Health (R01EY024072, R01NS064593, and R21CA156995), and Virginia Commonwealth University Massey Cancer Center (multi-investigator award 2013-MIP-01).
REFERENCES
- 1.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. A new class of polymers: starburst-dendritic macromolecules. Polym. J. (Tokyo) 1985;17(1):117–32. [Google Scholar]
- 2.Tomalia DA. Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog Polym Sci. 2005;30(3-4):294–324. [Google Scholar]
- 3.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 2010;15(5-6):171–85. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Yang H, Kao JW. Synthesis and characterization of nanoscale dendritic RGD clusters for potential applications in tissue engineering and drug delivery. Int J Nanomedicine. 2007;2(1):89–99. doi: 10.2147/nano.2007.2.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H, Lopina ST. Penicillin V-conjugated PEG-PAMAM star polymers. J Biomater Sci Polym Ed. 2003;14(10):1043–56. doi: 10.1163/156856203769231556. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Lopina ST. Extended release of a novel antidepressant, venlafaxine, based on anionic polyamidoamine dendrimers and poly(ethylene glycol)-containing semi-interpenetrating networks. J Biomed Mater Res. 2005;72A(1):107–14. doi: 10.1002/jbm.a.30220. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Morris JJ, Lopina ST. Polyethylene glycol-polyamidoamine dendritic micelle as solubility enhancer and the effect of the length of polyethylene glycol arms on the solubility of pyrene in water. J. Colloid Interface Sci. 2004;273(1):148–154. doi: 10.1016/j.jcis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Gurdag S, Khandare J, Stapels S, Matherly LH, Kannan RM. Activity of dendrimer-methotrexate conjugates on methotrexate-sensitive and -resistant cell lines. Bioconjugate Chem. 2006;17(2):275–283. doi: 10.1021/bc0501855. [DOI] [PubMed] [Google Scholar]
- 9.Huang B, Kukowska-Latallo JF, Tang S, Zong H, Johnson KB, Desai A, Gordon CL, Leroueil PR, Baker JR., Jr. The facile synthesis of multifunctional PAMAM dendrimer conjugates through copper-free click chemistry. Bioorg Med Chem Lett. 2012;22(9):3152–6. doi: 10.1016/j.bmcl.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majoros IJ, Myc A, Thomas T, Mehta CB, Baker JR., Jr. PAMAM dendrimer-based multifunctional conjugate for cancer therapy: synthesis, characterization, and functionality. Biomacromolecules. 2006;7(2):572–9. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]
- 11.Zhu S, Hong M, Zhang L, Tang G, Jiang Y, Pei Y. PEGylated PAMAM dendrimer-doxorubicin conjugates: in vitro evaluation and in vivo tumor accumulation. Pharm Res. 2010;27(1):161–74. doi: 10.1007/s11095-009-9992-1. [DOI] [PubMed] [Google Scholar]
- 12.Huang R, Ke W, Han L, Liu Y, Shao K, Ye L, Lou J, Jiang C, Pei Y. Brain-targeting mechanisms of lactoferrin-modified DNA-loaded nanoparticles. J Cereb Blood Flow Metab. 2009;29(12):1914–23. doi: 10.1038/jcbfm.2009.104. [DOI] [PubMed] [Google Scholar]
- 13.Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4(130):130ra46. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Q, Fu Y, Kao WJ, Janigro D, Yang H. Transbuccal Delivery of CNS Therapeutic Nanoparticles: Synthesis, Characterization, and In Vitro Permeation Studies. ACS Chem Neurosci. 2011;2(11):676–683. doi: 10.1021/cn200078m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi XY, Majoros IJ, Patri AK, Bi XD, Islam MT, Desai A, Ganser TR, Baker JR. Molecular heterogeneity analysis of poly(amidoamine) dendrimer-based mono- and multifunctional nanodevices by capillary electrophoresis. Analyst. 2006;131(3):374–381. doi: 10.1039/b515624f. [DOI] [PubMed] [Google Scholar]
- 16.Liu LF. DNA Topoisomerase Poisons as Antitumor Drugs. Annual Review of Biochemistry. 1989;58(1):351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- 17.Zolotarskaya OY, Wagner AF, Beckta JM, Valerie K, Wynne KJ, Yang H. Synthesis of Water-Soluble Camptothecin-Polyoxetane Conjugates via Click Chemistry. Molecular Pharmaceutics. 2012;9(11):3403–3408. doi: 10.1021/mp3005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng F, Liu J, Teh C, Chong SW, Korzh V, Jiang YJ, Deng LW. Camptothecin-induced downregulation of MLL5 contributes to the activation of tumor suppressor p53. Oncogene. 2011;30(33):3599–3611. doi: 10.1038/onc.2011.71. [DOI] [PubMed] [Google Scholar]
- 19.Liu LF, Desai SD, Li T-K, Mao Y, Sun MEI, Sim S-P. Mechanism of Action of Camptothecin. Annals of the New York Academy of Sciences. 2000;922(1):1–10. doi: 10.1111/j.1749-6632.2000.tb07020.x. [DOI] [PubMed] [Google Scholar]
- 20.Schultz KM, Campo-Deano L, Baldwin AD, Kiick KL, Clasen C, Furst EM. Electrospinning covalently cross-linking biocompatible hydrogelators. Polymer. 2013;54(1):363–371. doi: 10.1016/j.polymer.2012.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dal Pozzo A, Ni MH, Esposito E, Dallavalle S, Musso L, Bargiotti A, Pisano C, Vesci L, Bucci F, Castorina M, Fodera R, Giannini G, Aulicino C, Penco S. Novel tumor-targeted RGD peptide-camptothecin conjugates: synthesis and biological evaluation. Bioorg Med Chem. 2010;18(1):64–72. doi: 10.1016/j.bmc.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Zolotarskaya OY, Wagner AF, Beckta JM, Valerie K, Wynne KJ, Yang H. Synthesis of Water-Soluble Camptothecin-Polyoxetane Conjugates via Click Chemistry. Mol Pharm. 2012;9(11):3403–3408. doi: 10.1021/mp3005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiagarajan G, Ray A, Malugin A, Ghandehari H. PAMAM-Camptothecin Conjugate Inhibits Proliferation and Induces Nuclear Fragmentation in Colorectal Carcinoma Cells. Pharmaceut Res. 2010;27(11):2307–2316. doi: 10.1007/s11095-010-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lallana E, Sousa-Herves A, Fernandez-Trillo F, Riguera R, Fernandez-Megia E. Click Chemistry for Drug Delivery Nanosystems. Pharmaceut Res. 2012;29(1):1–34. doi: 10.1007/s11095-011-0568-5. [DOI] [PubMed] [Google Scholar]
- 25.Huang B, Desai A, Zong H, Tang S, Leroueil P, Baker JR., Jr. Copper-free click conjugation of methotrexate to a PAMAM dendrimer platform. Tetrahedron Lett. 2011;52(13):1411–1414. doi: 10.1016/j.tetlet.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golding SE, Rosenberg E, Adams BR, Wignarajah S, Beckta JM, O'Connor MJ, Valerie K. Dynamic inhibition of ATM kinase provides a strategy for glioblastoma multiforme radiosensitization and growth control. Cell Cycle. 2012;11(6):1167–1173. doi: 10.4161/cc.11.6.19576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan Q, Yeudall WA, Yang H. PEGylated Polyamidoamine Dendrimers with Bis-Aryl Hydrazone Linkages for Enhanced Gene Delivery. Biomacromolecules. 2010;11(8):1940–1947. doi: 10.1021/bm100589g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D'Emanuele A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm. 2003;252(1-2):263–6. doi: 10.1016/s0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- 29.Yang H, Lopina ST, DiPersio LP, Schmidt SP. Stealth dendrimers for drug delivery: correlation between PEGylation, cytocompatibility, and drug payload. J. Mater. Sci.: Mater. Med. 2008;19(5):1991–7. doi: 10.1007/s10856-007-3278-0. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Lopina ST. In vitro enzymatic stability of dendritic peptides. J Biomed Mater Res, Part A. 2006;76A(2):398–407. doi: 10.1002/jbm.a.30529. [DOI] [PubMed] [Google Scholar]
- 31.Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc. Natl. Acad. Sci. U. S. A. 2002;99(24):15387–15392. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biddlestone-Thorpe L, Marchi N, Guo K, Ghosh C, Janigro D, Valerie K, Yang H. Nanomaterial-mediated CNS delivery of diagnostic and therapeutic agents. Adv Drug Deliv Rev. 2012;64(7):605–13. doi: 10.1016/j.addr.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biddlestone-Thorpe L, Sajjad M, Rosenberg E, Beckta JM, Valerie NCK, Tokarz M, Adams BR, Wagner AF, Khalil A, Gilfor D, Golding SE, Deb S, Temesi DG, Lau A, O'Connor MJ, Choe KS, Parada LF, Lim SK, Mukhopadhyay ND, Valerie K. ATM Kinase Inhibition Preferentially Sensitizes p53-Mutant Glioma to Ionizing Radiation. Clinical Cancer Research. 2013;19(12):3189–3200. doi: 10.1158/1078-0432.CCR-12-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golding SE, Rosenberg E, Valerie N, Hussaini I, Frigerio M, Cockcroft XF, Chong WY, Hummersone M, Rigoreau L, Menear KA, O'Connor MJ, Povirk LF, van Meter T, Valerie K. Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Molecular Cancer Therapeutics. 2009;8(10):2894–2902. doi: 10.1158/1535-7163.MCT-09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H. Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm Res. 2010;27(9):1759–71. doi: 10.1007/s11095-010-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat Rev Drug Discov. 2004;3(6):499–508. doi: 10.1038/nrd1414. [DOI] [PubMed] [Google Scholar]
- 37.Huynh GH, Deen DF, Szoka FC. Barriers to carrier mediated drug and gene delivery to brain tumors. Journal of Controlled Release. 2006;110(2):236–259. doi: 10.1016/j.jconrel.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 38.Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv Drug Deliver Rev. 2014;71:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Pardridge WM. shRNA and siRNA delivery to the brain. Adv Drug Deliver Rev. 2007;59(2-3):141–152. doi: 10.1016/j.addr.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.