Abstract
Breast cancer (BC) is the most common cause of cancer-related death among women under the age of 50 years. Established biomarkers, such as hormone receptors (estrogen receptor [ER]/progesterone receptor) and human epidermal growth factor receptor 2 (HER2), play significant roles in the selection of patients for endocrine and trastuzumab therapies. However, the initial treatment response is often followed by tumor relapse with intrinsic resistance to the first-line therapy, so it has been expected to identify novel molecular markers to improve the survival and quality of life of patients. Alternative splicing of pre-messenger RNAs is a ubiquitous and flexible mechanism for the control of gene expression in mammalian cells. It provides cells with the opportunity to create protein isoforms with different, even opposing, functions from a single genomic locus. Aberrant alternative splicing is very common in cancer where emerging tumor cells take advantage of this flexibility to produce proteins that promote cell growth and survival. While a number of splicing alterations have been reported in human cancers, we focus on aberrant splicing of ER, HER2, and CD44 genes from the viewpoint of BC development. ERα36, a splice variant from the ER1 locus, governs nongenomic membrane signaling pathways triggered by estrogen and confers 4-hydroxytamoxifen resistance in BC therapy. The alternative spliced isoform of HER2 lacking exon 20 (Δ16HER2) has been reported in human BC; this isoform is associated with transforming ability than the wild-type HER2 and recapitulates the phenotypes of endocrine therapy-resistant BC. Although both CD44 splice isoforms (CD44s, CD44v) play essential roles in BC development, CD44v is more associated with those with favorable prognosis, such as luminal A subtype, while CD44s is linked to those with poor prognosis, such as HER2 or basal cell subtypes that are often metastatic. Hence, the detection of splice variants from these loci will provide keys to understand the pathogenesis, predict the prognosis, and choose specific therapies for BC.
Keywords: alternative splicing, breast cancer, tumorigenesis, metastasis, estrogen receptor, HER2, CD44, signal transduction, stem cell, prognosis
Introduction
Alternative splicing (AS) is a mechanism through which cells generate multiple messenger RNAs (mRNAs) with different functions from a single genomic locus. This is conducted by the inclusion or exclusion of specific exons in pre-mRNA processing. It occurs in nearly all the mammalian genes that consist of multiple exons and is catalyzed by the spliceosome, a protein complex that consists of five small nuclear ribonucleoproteins.1,2 It is assisted by numerous transacting factors that recognize cis-regulatory sequences within the pre-mRNAs and direct splice variants generated from different mechanisms, including alternative promoters, preferential usage of exons or splice sites, and/or alternative sites for polyadenylation.3
AS gives a significant evolutionary advantage by providing proteomic diversity.4 It is often regulated in a tissue-specific manner and contributes to the remodeling of protein–protein interaction networks.5 The functional classes of genes that are regulated by AS include both those with wide-spread homeostatic activities and those with cell type-specific functions. AS can drive determinative physiological change or can have a permissive role by providing mRNA variability that is used by other regulatory mechanisms.6 AS is pervasive in stem cells and has a fundamental impact on stem cell differentiation by regulating different isoforms of the core pluripotency transcription factors. Additionally, splicing factors can regulate pluripotency by affecting stem cell-specific AS. Thus, the crosstalk between AS and other gene regulatory networks has a fundamental effect on the maintenance and differentiation of stem cell pluripotency.7,8
A common signature of cancer cells is a general loss of splicing fidelity with the concomitant reorganization of splicing profiles and even switching to specific splicing isoforms usually expressed in other cell types to bestow incipient cancer cells a growth advantage; thus, specific splicing errors are detectable in fully developed cancer cells than pathologically normal-looking tissues. Indeed, genome-wide studies have revealed the existence of cancer-specific splicing alterations.9–13 The ability to regulate AS could be beneficial to emerging cancer cells at their early stage of development if splice isoforms encode proteins that stimulate cell proliferation and inhibit apoptosis, driving their uncontrolled cell growth. This switch in splicing preference can be critical as numerous genes possess splice variants that have dominant-negative or even antagonistic activities. Typical examples for these are aberrant splicing for p63 and p73, where oncogenic splice variants are generated from tumor-suppressive loci by aberrant splicing,14 contributing to solid tumors. Splicing abnormalities are also common in hematopoietic malignancies. Yoshida et al15 performed whole-exome sequencing of myelodysplasia specimens and found novel pathway mutations involving multiple components of the RNA splicing machinery. These splicing pathway mutations were frequent (45%–85%) in myeloid neoplasms showing features of myelodysplasia.16
Many onco- and tumor suppressor genes are aberrantly spliced in cancer.9 They include genes that control cell cycle progression (eg, cyclin D1b17,18), proliferation (fibroblast growth factor [FGF] receptor, telomerase19), differentiation (C/EBP20), signal transduction (Ha-Ras, Rac1, Ron9), cell death (Bcl-x, Fas1, caspase 29), angiogenesis (VEGF-A9), tumor suppression (p53, p63, p73, DMP114,21), and invasion and metastasis (ASF/SF2, SRp20, hTra2β1, YB-1, MDM29–13).
Breast cancer (BC) is the most common malignance in women in the US22 and industrialized countries. Although there has been significant progress in the diagnosis and treatment in the past decades, significant number of patients die of relapsed disease, thus improved diagnosis including gene expression and microRNA profiling and stem cell evaluation to decide therapeutic strategy therapy is expected.23–25 BC is categorized into five groups (luminal A, luminal B, human epidermal growth factor receptor 2 [HER2] type, triple negative, and normal like) dependent on the cell surface and other molecular markers, which are critical in predicting the prognosis and deciding therapies (see Refs. 26–30 for review). Aberrant splicing of genomic loci for estrogen receptors (ERs), HER2/neu, Cyclin D1, BRCA1, BARD1, Tenscin-C, and CD44 has been shown to contribute to breast carcinogenesis. This review focuses on the splicing mechanisms for ER, HER2, and CD44 genomic loci, which play essential roles in breast carcinogenesis and development.
Estrogen Receptor
Estrogens play an important role in the development and progression of BC. ERα and ERβ are encoded by two distinct genes, ESR1 (Fig. 1A) and ESR2, that are on human chromosomes 6q24–27 and 14q21–22, respectively. Hormone-activated ERs form homo- or heterodimers, namely, ERα (αα), ERβ (ββ), and ERαβ (αβ). ERα and ERβ show significant overall sequence homology, and both are composed of six domains A–F (Fig. 1B and C). In the absence of estrogen, ERs are largely located in the cytosol. Hormone binding to the receptor triggers a number of events starting with the migration of the receptor from the cytosol into the nucleus, dimerization of the receptor, and subsequent binding of the receptor dimer to specific sequences of DNA known as estrogen-response elements (EREs). The DNA/receptor complex then recruits other proteins that are responsible for gene transcription, resulting in a change in the cellular function. When the ERα and ERβ are coexpressed, each of them mediates specific effects with estrogens in the BC cells (reviewed in Refs. 31–33).
Figure 1.
Human estrogen receptor domain structures and their variants. (A) The genomic structure for the human Estrogen Receptor 1 (ESR1) locus. The human ESRα locus consists of 10 exons with two different promoters. The ATG for ERα66 exists in exon 1, while that for ERα36 and 46 exists in exon 2. The stop codon is in exon 8. The second promoter for ERα36/46 is repressed by ERα66. (B) The domain structure for human ERα66 and its splice variants ERα36 and ERα46 (38, Sotoca 2012). ERα contains two transactivation functions, a weak constitutive activation function (AF-1; A/B domains) and a hormone-dependent activation function (AF-2). AF-2 (E domain) works by recruiting a large coactivator complex, composed of one or more p160s, CBP/p300, and P/CAF via direct contacts with the p160s. The DNA-binding domain (C domain) binds specific EREs on genomic DNA. The D domain is a bridging region that connects the C and E domains. The F domain of ERα is critical for attenuation of E2β-induced receptor dimerization and transcriptional activity. Both ERα36 and ERα46 lack domains A and B responsible for transactivation. ERα36 lacks the C-terminal domain E but has 27 unique amino acid residues (gray). ERα46 has the same domains E and F as those in ERα66. (C) The domain structure for human ERβ1 and its splice variant ERβ2.71 Although ERβ1 binds to E2β, ERβ2 does not bind to it, but blocks the activity of ERα through heterodimerization.
Both ERα (consisting of 595 amino acids [aa]) and ERβ (530 aa) are hormone-responsive nuclear receptors.33 ERα contains two transactivation domains, such as a weak, constitutive activation function (AF-1) and a hormone-dependent activation function (AF-2; Fig. 1B). AF-2 (E domain) works by recruiting a large coactivator complex, composed of one or more p160s, CREB-binding protein (CBP)/p300, and p300 and CBP-associated factor (P/CAF) via direct contact with the p160s.34 The DNA-binding domains (DBDs, C domains) have 96% homology between ERα and ERβ and bind most EREs on genomic DNA. The D domain is a bridging region that connects the C and E domains. The F domain of ERα is critical for the attenuation of 17β-estradiol (E2β)-induced receptor dimerization and transcriptional activity.35
Genomic versus nongenomic (membrane signaling) pathways governed by estrogen
Estrogen-stimulated cell proliferation by E2β is largely mediated by the activation of ERα66 localized in the nucleus. However, earlier studies also reported that estrogen binds to a cell surface receptor and stimulates a rapid generation of cyclic adenosine monophosphate (cAMP).36,37 Subsequently, other reports of a plasma membrane-localized ER that transduces membrane-initiated estrogen signaling appeared; this membrane signaling (ie, non-genomic pathway) was found to activate different cytoplasmic proteins, including adenylate cyclase, G proteins, protein kinase C δ, phospholipase C, and mitogen-activated protein kinase, and phosphatidylinositide 3-kinase/protein kinase B (PI3K/Akt) pathways.38–41 These nongenomic pathways control more genes that are involved in the regulation of cell growth, survival, motility, invasion, and apoptosis than the genomic pathway.
ERα splicing—ERα46 and ERα36
There are at least two physiologically relevant splice variants of full-length ERα (ERα66), ERα46, and ERα36 (Fig. 1). Transcription of ERα36 initiates from a previously unidentified promoter in the first intron of ERα66 (Fig. 1A, ATG2), which continues from exon 2 to exon 6 and skips the final exons 7 and 8 of ERα66. Transcription for ERα46 also starts from ATG2 but uses the same exons 2–8 as those for ERα66. Both ERα46 and ERα36 are truncated in the amino-terminus (173 aa) and lack the first transcriptional activation domain (AF-1). ERα36 lacks the second transcriptional activation domain in AF-2 and has a unique 27-aa sequence at the C-terminus. This extra sequence may change the ligand-binding spectrum of an ERα in a way that it can interact with other factors than estrogens. Conversely, ERα46 is identical to ERα66 in the amino acid sequences for the C–F domains (Fig. 1B). Both ERα46 and ERα36 make homodimers or heterodimers with ERα66. ERα46 has a twofold higher binding affinity to the EREs than ERα66.42 While 74% of ERα66 is in the nucleus, ERα46 is localized in the plasma membrane (26%), cytoplasm (30%), and nucleus (26%), possibly by palmitoylation.43 Overexpression of ERα46 in Michigan Cancer Foundation-7 (MCF-7) cells reduces E2-stimulated endogenous pS2, cyclin D1, nuclear respiratory factor-1, and progesterone receptor (PR) transcription.44 Transfection with ERα46 changed the pharmacology of E2 regulation of oncomiR miR-21 expression from inhibition to stimulation, suggesting that ERα46 inhibits the ERα66 activity.44
Wang et al39 showed that ERα36 is primarily localized on plasma membrane (50%) and cytoplasm (40%) rather than on the nucleus in HEK293 cells. Since ERα36 has three potential myristoylation sites near the N-terminus,38–41,45 instead of the nuclear location signals of ERα66, it may be modified by palmitoylation and located in the plasma membrane/cytoplasm. ERα36 inhibits the traditional nuclear estrogen signaling mediated by ERα66 in a dominant-negative fashion since it has the DBD but lacks the two transactivation domains (Fig. 1B).
The molecular mechanisms for the differential expression of ERα66 and α36 have been studied. ERα36 is highly expressed in the majority of ERα66(−) BCs; overexpression of ERα36 occurs with a decrease of ERα66, indicating that the expression of ERα66 and ERα36 is mutually exclusive.41 This is because, at least in part, ERα66 negatively regulates the promoter activity of ERα36 (Fig. 1A).46 The Wilms’ tumor gene WT1 encodes a zinc-finger protein WT1 that functions as a dual transcription regulator to activate or suppress gene transcription (reviewed in Refs. 47–49). Although the gene has been cloned as a tumor suppressor for the gene in Wilms’ tumor, high levels of wild-type (the phenotype of the nonmutated form of a species as it occurs in nature) WT1 are found in acute leukemias and solid tumors with poor prognosis,50–52 involved in cell proliferation53 and block cellular differentiation when the wild-type is overexpressed.54 High passage ER-positive BC MCF-7 cells were found to express ERα66 and WT1 at higher levels and ERα36 at a very low level.55 MCF-7 cells depleted for WT1 expressed a reduced level of ERα66 with an increased level of ERα36, suggesting that WT1 increases ERα66/ERα36 ratio. WT1 directly activated the promoter of the ERα66 gene while repressing the ERα36 promoter. Thus, WT1 stimulates genomic estrogen signaling (mediated by ERα66) while inhibiting nongenomic (by ERα36) estrogen signaling.55 A similar finding was reported by Nasomyon et al,56 who reported that the WT1 expression correlated with the high expression of ERα and HER2, leading to cell proliferation and might be involved in cancer development and progression.
Another factor that affects the ERα66/ERα36 ratio is synuclein γ (SNCG), which binds to ERα66 or ERα36 depending on the estradiol concentration.57 SNCG is highly expressed in cancer cells but not in normal epithelium. Shi et al showed that SNCG expression enhanced estrogen-induced activation of ERK1/2 and mechanistic target of rapamycin (mTOR). Heat-shock protein 90 (Hsp90) acts as molecular chaperone, a group of proteins that assist the covalent folding/unfolding and the assembly/disassembly of other macromolecular structures, together with several cochaperone molecules.58 Hsp90 binds to its client proteins such as steroid receptors, Cdks, and Akt that regulate cell cycle, survival, and death and promotes their proper protein folding, assembly, and transportation across different cellular compartments.59 Disruption of Hsp90 with 17-N-allylamino-17-demethoxygeldanamycin (17-AAG) significantly reduced ERα36 expression and membrane-initiated estrogen signaling, which was recovered by the expression of SNCG.57 Expression of SNCG also rendered tamoxifen (TAM) resistance, consistent with the clinical observation that ERα36 expression was associated with TAM resistance explained later. In summary, their study indicates that ERα36 mediates membrane-initiated estrogen signaling and that SNCG can replace the function of Hsp90, a molecular chaper-one ERα36, to stimulate ligand-dependent cell growth.57
Expression of ER36 in primary BC samples and their roles in TAM resistance
Under normal conditions, ERs bind to estrogen and then translocate to the nucleus, subsequently binding to specific EREs and regulating transcription of downstream gene expression. With the dynamic regulation of ERα66 and ERα36, genomic and nongenomic estrogen signaling pathways should be coordinated to maintain a balance. An imbalance between ERα66 and ERα36 may result in abnormal proliferation and differentiation, leading to BC and other neoplastic disorders.
The role for ERα36 in nongenomic, membrane signaling of estrogens has been studied in BC cell lines. To understand the role of ERα36 in breast carcinogenesis and drug resistance, it is essential to study the expression of the protein in a primary BC specimen. Lee et al60 studied 31 tissue samples of patients with BC for ERα36 and ERα66 protein expression status by immunohistochemistry and six additional patient tissue samples by Western blotting using an antibody specific to each ERα isoform. They found a cytoplasmic and plasma membrane-associated expression pattern of ERα36 in both ERα66-positive and -negative BC samples. Furthermore, ERα36 expression was associated with decreasing nuclear/cytoplasmic ERα66 expression, suggesting its potential use as a diagnostic and prognostic marker. In conclusion, ERα36 is frequently expressed in ERα66-negative BCs, which may provide additional information for better diagnosis and prognosis of BC.60
Antiestrogens such as TAM have provided a successful treatment for ER-positive BC for the past four decades. However, BCs eventually acquire resistance to TAM therapy.61 The molecular mechanisms for the TAM resistance have been extensively studied to overcome the problem.62–68 It was reported that BCs expressing high concentrations of ERα36 benefited less from TAM therapy than those with low levels, indicating that increased ERα36 levels are one of the underlying mechanisms of TAM resistance.45 Zhang and Wang40 reported that TAM increased ERα36 concentrations, and TAM-resistant BC cells expressed high levels of ERα36. Depletion of ERα36 in TAM-resistant BC cells with short hairpin RNA restored TAM sensitivity. They also found that cells with high concentrations of ERα36 protein were hypersensitive to estrogen, activating ERK phosphorylation at a picomolar range. Thus, elevated ERα36 is one of the mechanisms by which ER-positive BC cells escape TAM therapy and provided a rationale to develop novel therapeutic approaches for TAM-resistant patients by targeting ERα36.
The structure of ERb
ERβ (Fig. 1C) has been identified as the major form of ER in the normal breast that is localized in the luminal epithelium, myoepithelium, and also in the stroma.69–71 Similar to the ERα, ERβ1 binds to E2β with high affinity through its ligand-binding domain (LBD), but they share only moderate homology at the protein level (58% in humans) at the LBD.69 ERβ2 lacks the C-terminal LBD (Fig. 1C) and thus does not bind to E2. Since the homology for the DBD for ERα and ERβ is very high (96% in humans69), they interact with specific EREs and transactivate common ER target genes.72
The biology of ERb: a tumor suppressor in BC?
Approximately 58% of BCs express both ERα and ERβ, 14% express ERα only, and 18% express ERβ only.73 Ectopic expression of ERβ inhibits E2β-stimulated proliferation of the BC cells,74 reduces cell motility and invasion,75 and thus inhibits tumor development in mice.76 Thus, ERβ antago-nizes the tumor-promoting activities of ERα. Honma et al77 showed that the ERβ was associated with better survival in patients with HER2-positive and triple-negative breast cancer (TNBC) with a good response to TAM. Generally, ERβ1 expression is associated with small tumor, lower histological grade, lymph-node negativity, and longer disease-free/overall survival of BC,77–81 suggesting that ERβ1 expression has positive impact on BC survival. Consistently, epidemiologic studies demonstrated a loss of ERβ expression in higher grade BC tissues.82,83 ERβ expression was inversely correlated with Ki67, particularly in high-grade ductal carcinoma in situ.84 Although there is no correlation between loss of heterozygosity (LOH) at 14q22–24 (genomic locus at the ERβ/ESR2 locus) and ERβ1 expression in primary BCs, a negative correlation between ERβ1 mRNA expression and methylation status was observed for the ERβ promoter in BC cells, which was reversed by 5-aza-deoxycytidine and trichostatin A.85 Together, these studies indicate that ERβ functions as a tumor suppressor to inhibit BC initiation and progression.
Interestingly, ERβ1 is often expressed in TNBC, the most aggressive type of BC with limited treatment options because of the lack of expression of a biological target (ERα, PR, HER2). Clinical data have demonstrated a clear correlation between ERβ1 positivity and improved disease-free and overall survival in those patients treated with TAM.86 It was also shown that ERβ1 inversely correlates with PTEN/PI3K/AKT pathway and predicts a favorable prognosis in TNBC.87 Thus, ERβ1 may be worth considering as a potential therapeutic target, particularly in TNBC.
Notwithstanding with these findings that suggest tumor-suppressive roles for ERβ, other studies with BCs lacking ERα demonstrated a positive correlation between high ERβ expression and poor prognosis associated with increased proliferation88,89 since ERβ is widely expressed in basal myo-and luminal epithelium in normal breast. Hou et al90 reported that ERβ increased the proliferation and invasion of MDA-MB-435 cells (ERα-negative) significantly in estradiol-independent fashion in culture. In vivo studies showed that ERβ(+) MDA-MB-435 cells grew much faster and had more pulmonary metastasis than control cells. Thus, ERβ shows differential effects on BC growth and metastasis dependent on ERα levels, which needs further investigation at the molecular level.
ERb splice isoforms and BC prognosis
The human ERβ locus on chromosome 14q21–22 generates two splice variants, namely, ERβ1 and ERβ2 (Fig. 1C). The mode of dimerization for ERβ1 and ERβ2 is similar in heterodimerization but different in homodimerization. ERβ1 forms homo- and heterodimers with other ERβ isoforms as well as with ERα and quenches ERα signaling.91,92 Conversely, ERβ2 molecules do not form homodimers but inhibits ERα signaling through heterodimerization and proteasome-dependent degradation of ERα since ERβ2 has undetectable affinity for E2 and other tested ligands.72,92,93 ERβ isoforms are differentially expressed in BC cells and in normal epithelial and nonepithelial components of breast tissues,94,95 indicating that they have different biological effects on both normal and transformed cells. ERβ1 antagonizes the tumor-promoting activities of ERα and thus is a favorable prognostic marker at least in ERα(+) BC as described earlier Conversely, recent studies show that ERβ2 expression in BCs, especially when it is expressed in the cytoplasm, is associated with worse (disease-free) survival of patients regardless of the ERα status.96–100 Thus, ERβ1 and β2 isoforms have distinct impacts on BC survival.
HER2
The transmembrane HER2/ErbB2/neu gene encodes an 185-kDa glycoprotein with protein tyrosine kinase activity.101–103 It belongs to the epidermal growth factor receptor (EGFR) family together with HER1, HER3, and HER4 (for reviews, see Refs. 104, 105). Overexpression and gene amplification of ErbB2 are frequently observed in human malignancies, in particular in ~30% of primary BCs,106 which correlate with enhanced tumor aggressiveness, lymph node metastasis, and poor clinical outcomes of patients. It is widely accepted that wild-type HER2 gene amplification is necessary but not sufficient to induce transformation.107
Bargmann et al101 isolated complementary DNA (cDNA) clones of the normal and transforming neu gene through NIH 3T3 focus forming assay of neuro- and glioblastomas of BXID rats. Then they created constructs in vitro between the normal and transforming cDNAs for Rat neu to determine the mutation responsible for the activation of the neu gene, which was the substitution of Val664 to Glu664.108 The Val664 was in the transmembrane domain of the predicted neu protein p185. Segatto et al109 then mutated the corresponding valine to glutamine in HER2 and demonstrated that this mutant protein had dramatically increased protein tyrosine kinase activity with 15-fold increase in transforming efficiency. Consistently, MMTV-neu mice with point mutation of neu develop aggressive mammary carcinomas at the latency of seven months, while MMTV-ErbB2 mice (wild-type) develop mammary carcinomas at the latency of 15 months,110 indicating increased oncogenicity of HER2/neu by the transmembrane point mutation. The oncogenic potential by mutant HER2/neu is quenched by the Arf–p53 pathway since it transactivates both Dmp1 and Arf promoters111–113 (for Dmp1, see Refs. 114–121). Although mutations at the transmembrane region have not been reported in human BCs, several studies have reported the expression of an HER2 alternatively spliced isoform in normal mammary cells and in human breast carcinomas.122–124 The in-frame deletion of 16 amino acids at exon 20 (aa 619–634; Fig. 2B) in the extracellular domain by aberrant splicing induces the formation of ΔErbB2 that displays a stronger transforming activity than wild-type ErbB2. This has been demonstrated by the development of ER(−), high-grade, and metastatic mammary tumors in ΔHER2 transgenic mice with short latency, driven by the MMTV promoter.125,126
Figure 2.
Activation of the HER2 gene by alternative splicing at exon 20. (A) HER2 splicing and generation of D16HER2.202 Constitutive expression of a human HER2 alternative splice isoform carries an in-frame deletion in the same mutated region of the rat neu proto-oncogene.122 This splice variant produces an aberrant receptor that lacks exon 20 encoding 16 amino acids (Δ16HER2). (B) The 16 amino acids (aa 619–634) in the HER2 extracellular domain deleted in Δ16HER2 include two relevant cysteine residues close to the T-binding epitope. This deletion results in stable, constitutively active homodimer formation, enhanced multisignaling activity, and accelerated transformation.
Although extensive research has been done to identify/isolate the ligand(s) for c-ErbB2, it is now called an orphan receptor due to the lack of any known ligands.127 A structural biological study for HER2 revealed that HER2 by itself had an activated conformation similar to that of the EGFR–ligand complex, which was very different from that seen in the unligand forms of HER1 or HER3.128 The electrostatic repulsions possibly prevent homodimerization of HER2 explaining its inability to bind known ligands, which also explains why HER2 fails to form homodimers.128 Interestingly, HER2 makes heterodimer with HER3 that has an authentic ligand heregulin but is kinase-dead.127,129 Vaught et al130 demonstrated the importance of HER3 in all stages of HER2-mediated mammary epithelial transformation and metastasis through the analyses of gene-engineered mouse models. HER2:HER3 heterodimerization is critically important in the progression of HER2(+) BC since heregulin–HER3 binding initiates HER2:HER3 dimerization, causing epithelial–mesenchymal transition (EMT) via phosphorylation of AKT-heat shock factor 1 (HSF1)-SLUG, eventually leading to cancer metastasis.131
Splicing in HER2 is supposed to trigger the kinase activity by promoting intermolecular disulfide bonding, which, in turn, forms homodimers capable of transforming cells.132 Since the levels of the HER2 splice variant represent only 9% of those observed with the wild-type receptor, HER2 protein overexpression caused by gene amplification (or other mechanisms) in primary human BCs will therefore increases the levels of this oncogenic variant above the critical threshold, allowing it to contribute to BC progression.124 Δ16HER2 is expressed in many HER2-positive BCs, where it has been linked with resistance to the HER2-targeting monoclonal antibody trastuzumab in metastatic BC,133 but the impact of Δ16HER2 on tumor pathobiology and therapeutic response in patients with operable BC remains to be determined. Castagnoli et al134 provided genetic evidence in transgenic mice that expression of Δ16HER2 was sufficient to accelerate mammary tumorigenesis and improve the response to trastuzumab (Herceptin®), a monoclonal antibody that interferes with the HER2 receptor. A comparative analysis of effector signaling pathways activated by Δ16HER2 and wild-type HER2 revealed that Δ16HER2 was optimally functional through a link to SRC activation (pSRC). Clinically, HER2-positive BCs from patients who received trastuzumab as an adjuvant therapy showed a positive correlation in Δ16HER2 and pSRC abundance, consistent with the results from the mouse study. Moreover, patients expressing high pSRC or Δ16HER2 received the greatest benefit from trastuzumab therapy with BC patients in an adjuvant setting.134 Thus, the Δ16HER2 and pSRC abundance should be predetermined in tumors when making therapy decisions. They speculate that although HER2-positive primary BCs expressing high levels of pSRC are initially dependent on HER2 and all its potential driver isoforms and are, thus, responsive to trastuzumab, the progression of such BCs due to a high HER2-dependent growth rate might lead to the accumulation of genetic alterations that result in less HER2 dependency, which, in turn, results in significantly less or even no responsiveness to trastuzumab as reported by Zhang et al.133
Cittelly et al135 studied the mechanisms of resistance with endocrine therapy in BCs with Δ16HER2 in relationship to microRNA and BCL-2. They showed that Δ16HER2 was expressed in >30% of ER-positive BCs, which promoted TAM resistance and estrogen independence of MCF-7 xenografts. MCF-7/Δ16HER2 cells evade TAM through upregulation of BCL-2, which was targeted by miR-15a and miR-16. Reintroduction of miR-15a/16 reduced TAM-induced BCL-2 expression and sensitized MCF-7/Δ16HER2 to TAM. Hence, their preclinical models of BC with Δ16HER2 overexpres-sion recapitulate numerous phenotypes of endocrine-resistant human breast tumors.135
Huynh and Jones136 also studied the contribution of altered microRNA expression in Δ16HER2-mediated tumorigenesis and trastuzumab resistance. Using a gene array strategy comparing microRNA expression profiles of MCF-7 with MCF-7/Δ16HER2 cells, they found that Δ16HER2 caused a fivefold suppression of the miR-7 tumor suppressor. Re-expression of miR-7 in the MCF-7/Δ16HER2 cell line caused a G1 cell cycle arrest and reduced both colony formation and cell migration to levels of parental cells. MiR-7 inhibited MCF-7/Δ16HER2 cell migration through EGFR and the inactivation of the SRC kinase. Together miR-7- and -15a/16-regulated signaling pathways involving BCL-2, EGFR, and/or SRC kinase can be future targets for therapeutic intervention of Δ16HER2-driven BC.
After the discovery of Δ16HER2, another mechanism was proposed to mediate resistance to trastuzumab: a truncated form of the HER2 receptor, p95-HER2.137,138 The amino terminal-truncated p95-HER2 is a constitutively active kinase that can form heterodimers with other HER family proteins and activates the downstream signaling pathways. Since p95-HER2 lacks the trastuzumab-binding site, its expression is associated with trastuzumab resistance and poor prognosis but maintains sensitivity to the HER2 kinase inhibitor lapatinib.137,138 Thus, it is essential to determine the levels of p95-HER2 levels in BC with HER2 overexpression before making decisions in therapy. We do not discuss this issue further since this mutant is not considered to be a splice variant for HER2.
CD44
BC is characterized by a remarkable biological heterogeneity within tumors, which has been demonstrated by GeneChip microarray analyses of gene expression.139,140 Early studies have identified a subpopulation of cells with stem cell activity in CD44+/CD24−/low/lineage(−)25,141 fraction, and more recently, aldehyde dehydrogenase (ALDH) activity was shown to mark normal as well as malignant human mammary stem cells.142,143 These cancer stem cells (CSCs) have enhanced invasiveness,144 resistance to radio-145 or chemotherapy,146 and are associated with poor prognosis.142,147,148 The presence of CD44+/CD24−/low/lineage(−) tumor cells has been associated with the basal-like subtype of BC, especially those with hereditary mutations for BRCA1.149 The role of CD44 as a marker for CSCs will be discussed later in this section.
The CD44 gene and splice variants
The human CD44 gene is located on chromosome 11p13 and consists of 20 coding exons of which 10, located between constant exons 5 and 6 (colored pink), can be alternatively spliced into many different isoforms with tissue- and differentiation-specific expression (Fig. 3A).150,151 The standard isoform of CD44 (CD44s) contains none of the 10 variable exons, whereas the CD44v2–v10 isoform includes all of them (exon v1 is not expressed in humans152). The protein products for CD44 are shown in Figure 3B. The CD44v3–v10 isoform has one less exon, and the CD44v8–v10 isoform includes only the last three of the variable exons. Other isoforms are formed by AS, and various posttranslational modifications further increase the heterogeneity of the CD44 proteins.
Figure 3.
Alternative splicing for the human CD44 locus and its protein products. (A) The genomic structure for the human CD44 locus. It has 10 constant exons (exons 1–5 and 15–19) shown in tan that encode the extracellular, transmembrane, and cytoplasmic tail sequences and 10 variable exons for the extracellular domain (shown in silver). (B) Protein structures for the standard (CD44s) and variant CD44 (CD44v) proteins. CD44v has insertion of amino acid sequences between those encoded by exons 5 and 15 (exons 5a–14). The exon 5a (=exon v1) is not expressed in human tissues.
CD44 protein structure
CD44 is a multifunctional transmembrane glycoprotein that participates in many cellular processes including cell division, survival, migration, and adhesion153 through the binding of its major ligand, hyaluronic acid (HA; Fig. 4). HA is a polymer of disaccharides, themselves composed of d-glucuronic acid and d-N-acetylglucosamine, linked via alternating β-1,4- and β-1,3-glycosidic bonds.154,155 HA is synthesized by a class of integral membrane proteins called hyaluronan synthases, that is, HAS1, HAS2, and HAS3. These enzymes lengthen HA by adding glucuronic acid and N-acetylglucosamine repeatedly to the growing polysaccharide, and the final products are extruded into the extracellular space via ATP-binding cassette (ABC) transporter through the plasma membrane. CD44 can act as a coreceptor to mediate signaling of receptor-type protein tyrosine kinases (RTKs) by making functional complexes, which will be explained later (see “CD44 promotes tumorigenesis” section). CD44 also provides a link between the plasma membrane and the actin cytoskeleton, modulating cellular shape and motility (Fig. 4).155,156
Figure 4.
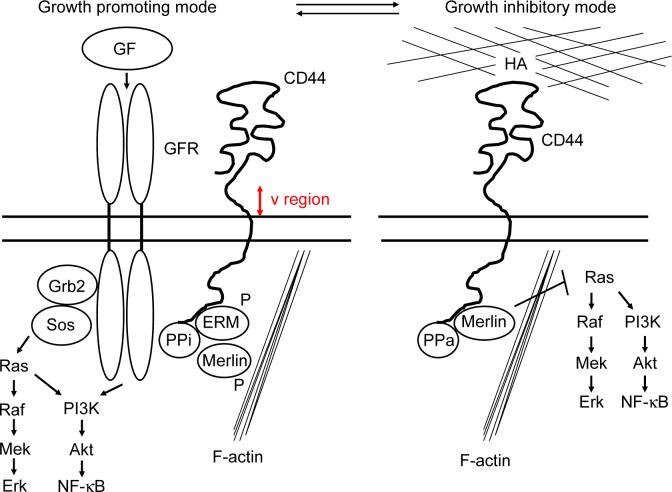
The model for CD44 action in logarithmic (growth-promoting mode) and confluent (growth-inhibitory mode) growth conditions.157 Specific ligands determine two functional states of CD44 that influence the cytoplasmic complexes. The ligands of the growth mode have not been defined for CD44. It is, however, known that CD44s, and particularly the larger splice variants CD44v, serve as a platform for the activation of growth factors (GF). GFR, growth factor receptor; PPi, inactive protein phosphatase; PPa, active protein phosphatase; ERM, ezrin, radixin, and moesin. Merlin (NF2), which is an inhibitor for the Ras–Raf–Mek–Erk pathway, is inactivated by phosphorylation in the growth-promoting mode for CD44.
The CD44 molecule consists of an amino-terminal extracellular and LBD, a membrane-proximal stem loop including the variable region (shown in red) and a transmembrane region, and a cytoplasmic tail that attaches to actin, ankyrin, ezrin, radixin, and moesin (ERM) in the cytoskeleton (Fig. 4).150,151 The epitope recognized by the CD44 monoclonal antibodies commonly used for the isolation of CSCs is located in the amino-terminal region of CD44 consisting of the nonvariable exons 1–5, indicating that all CD44 isoforms should be detected by these antibodies.156
CD44 contributes to both cell proliferation (Fig. 4, growth-promoting mode, left) and growth inhibition (growth-inhibitory mode, right), dependent on the biological conditions of cells. The neurofibromatosis-2 (NF2) gene encodes merlin, an ERM-related protein that functions as a tumor suppressor that inhibits the Ras–Raf–Mek–Erk cell growth pathway.157 At low cell density, merlin is phosphorylated and growth permissive and exists in a complex with ERM and CD44 (Fig. 4, left). CD44s- or CD44v-mediated activation of mitogenic and antiapoptotic proteins is initiated through their association with RTKs. At high cell density, merlin becomes hypophosphorylated and inhibits cell growth in response to HA in the extracellular matrix (Fig. 4, right). Merlin’s growth-inhibitory activity depends on specific interaction with the cytoplasmic tail of CD44 that interacts with an active protein phosphatase. The hypo-phosphorylated merlin will directly associate with CD44 and inhibit the Ras–Raf–Mek–Erk and PI3K–Akt mitogenic pathways (Fig. 4, right).
CD44 promotes tumorigenesis
CD44 promotes tumorigenesis through a variety of major signaling pathways, including the Ras–Raf–Mek–Erk–cyclin D1 and PI3K–Akt pathways for stimulating cell growth, survival, and invasion, and Rho GTPases for cytoskeletal remodeling and invasion.151 CD44 makes complexes with growth factor receptors such as EGFR (HER1), HER2, HER3, and HER4. The association of CD44 with HER2 and HER3 mediates heterodimerization and activates the receptor in response to neuregulin, which strongly endows apoptosis resistance in cancer-initiating cells. ERBB protein activation stimulates growth factor receptor-bound protein 2 (GRB2) and son of sevenless (SOS) proteins, which subsequently activate the Ras–Raf–Mek–Erk (proliferative) and PI3K–Akt–NF-κB pathways (antiapoptotic; Fig. 4, left).
CD44 regulates other RTKs through physical association. CD44 promotes MET phosphorylation via CD44v3-bound hepatocyte growth factor (HGF), which is important in colorectal cancer tumorigenesis.151 CD44v6 also initiates MET activation through HGF binding.158 Likewise, CD44 interacts with insulin-like growth factor 1 receptor, platelet-derived growth factor receptor, and transforming growth factor beta (TGFβ) receptor,151 demonstrating its broad activity in receptor-mediated signaling.
In addition to these RTK-related activities, CD44 serves as a docking molecule for matrix metalloproteases (MMPs), which are matrix-modifying enzymes that degrade basement membrane and promote cell migration.159 MMP2 and 9, in turn, cleave TGFβ for activation, which promotes angiogene-sis and invasion.160 Interestingly, Kuo et al161 later showed that TGFβ induced membrane type 1 MMP expression in MDA-MB-435s BC cells, which caused CD44 cleavage. Cleaved CD44 then promoted the migration of tumor cells, indicating the significant role of the CD44–MMP–TGFβ axis in cancer invasion and metastasis. CD44 also interacts with multidrug resistance 1 to confer drug resistance.151 Although crosstalk between the p53 tumor suppressor pathway and CD44 has not been extensively studied, Godar et al162 showed that p53 inhibited expression of the CD44 cell-surface molecule via binding to a noncanonical p53-binding sequence in the CD44 promoter. In the absence of p53 function, increased CD44 expression accelerated the growth and tumor-initiating ability of highly tumorigenic human mammary epithelial cells. Thus, CD44 is a key tumor-promoting agent in transformed tumor cells lacking p53 function.
Several groups have assessed the role of CD44 in BC progression in vivo using mouse models. Ouhtit et al163 developed a tetracycline-regulated CD44s expression system in the weakly metastatic BC cell MCF-7. Induction of CD44s alone increased their abilities to proliferate, migrate, and invade in vitro. They then developed a doxycycline (DOX)-repressed CD44s BC xenograft model.164 Although induction of CD44s did not affect the growth rate or local invasion of the primary tumor, 8 of 11 mice from the DOX(−) group expressing CD44s developed secondary tumors to the liver. They showed that TGFβ2 was a novel target for CD44 that promoted BC invasion.164 Consistently, treatment with a CD44-blocking monoclonal antibody P245 dramatically inhibited tumor growth and prevented recurrence in human BC xenografts after treatment with doxorubicin and cyclophosphamide, demonstrating growth-promoting activities of CD44 in vivo.165
CD44 as a marker for CSCs—role of EMT in cancer metastasis
Normal stem cells renew themselves through asymmetrical cell division while simultaneously generating committed progenitor cells whose descendants will eventually differentiate and execute tissue-specific functions.166,167 More recently, studies of cancer cells have provided evidence of self-renewing, stem-like cells within tumors, which have been called CSCs (reviewed in Refs. 25, 168–170). CSCs were first identified in hematopoietic malignancies;171,172 later, they have also been discovered in solid tumors, such as those found in the breast, colon, and brain.141,173–176
The process of tumor metastasis is often enabled by EMT,177,178 where cancer cells require self-renewal capability. This raises the possibility that the EMT process, which enables cancer cell dissemination, may also bestow a self-renewal capacity to the cancer cells. EMT is transcriptionally regulated by a family of transcription repressors that suppress E-cadherin expression and by microRNAs (reviewed in Refs. 179–184). Successful colonization of cancer cells in secondary sites requires the ability of the cells to avoid the mechanisms that interfere with metastasis where CD44 plays a critical role. Yae et al185 showed that orthotropic transplantation of a CD44v(+) subpopulation of 4T1 BC cells, but not that of a CD44v(−) subpopulation, in mice results in efficient lung metastasis accompanied by expansion of stem-like cancer cells proving the role of the variant isoform in cancer metastasis.185 Other studies support the finding of the Yae study concluding that some CD44v isoforms mediate cancer metastasis.186,187
In good contrast to these studies, other groups demonstrated the importance of CD44s rather than CD44v in cancer progression. Brown et al188 demonstrated the role of CD44s in BC progression. Most importantly, they showed that CD44v and CD44s were differentially regulated during EMT, resulting in a switch from CD44v isoform to CD44s.188 The expression of CD44s accelerated both EMT and BC progression through activation of Akt. Although both CD44s and CD44v were upregulated in BCs in comparison to normal tissues, the expression of CD44s was significantly higher in advanced tumors and correlated with N-cadherin expression. Thus, the regulation of AS for CD44 constitutes a critical mechanism in controlling EMT and cancer progression.188 Xu et al189 showed that the RNA-binding protein heterogeneous nuclear ribonucleoprotein M (hnRNPM) promoted BC metastasis by activating the switch of AS from the CD44v to CD44s isoform during EMT. Genome-wide deep sequencing analysis showed that hnRNPM potentiated TGFβ signaling and identified CD44 as a key downstream target of hnRNPM.189 They also showed that the hnRNPM expression was associated with aggressive BC in primary samples. Thus, tumor metastasis is accelerated by the hnRNPM-mediated splicing program, which increased relative expression of CD44s over CD44v.189
EMT, which causes invasion and metastasis of carcinoma, is driven by the transcription factor ZEB1 that promotes tumor-initiating capacity. Remarkably, EMT-induced repression of epithelial splicing regulatory protein 1 (ESRP1) controls AS of CD44, causing a shift in the expression from the variant CD44v to CD44s isoform.190 Intriguingly, CD44s itself activates the expression of ZEB1, resulting in a self-sustaining ZEB1 and CD44s expression. Activation of this CD44s–ZEB1 regulatory loop has functional impact on tumor cells, as evident by increased tumor sphere initiation, drug resistance, and tumor recurrence. Their study again emphasizes the importance of CD44s in tumor cell stemness independent of external stimuli as ZEB1 downregulates ESRP1, further promoting CD44s isoform synthesis.190
Olsson et al191 studied the correlation between CD44 isoforms and BC subtypes. They found that BCs with a strong expression of the CSC marker ALDH1 had elevated expression of CD44s. A high expression of the CD44v2–v10 and CD44v3–v10 isoforms (Fig. 3B) correlated with positive hormone receptor (ER, PR) status, low proliferation, and luminal A subtype. High expression of CD44v8–v10 correlated with positive EGFR, negative/low HER2 status, and basal-like subtype. High expression of CD44s was associated with strong HER2 expression and also a basal-like phenotype. Thus, individual CD44 splice isoforms can be associated with particular BC subtypes and clinical markers.191 Taken together, although both CD44 splice isoforms have been reported to play essential roles in BC development and progression, CD44v (especially CD44v2–v10, v3–v10) is more likely to be associated with BCs with good prognosis, such as luminal A, while CD44s is linked to BCs with poor prognosis, such as HER2 or basal cell subtypes that are often metastatic.
Inhibition of cancer progression by CD44
Although the majority of in vitro researches described earlier suggest the role of CD44 in cancer progression, other reports have shown that CD44 can respond to signals from the microenvironment, often in response to high molecular weight (HMW, >500 kDa) HA, to inhibit growth and invasion in cancer cells (Fig. 4, right). Consistent with the tumor-suppressive role of CD44, loss of CD44 has been reported in Burkitt’s lymphoma, neuroblastoma, prostate cancer, and BC.192 CD44 binding to merlin acts as a growth/arrest sensor in response to signals from the microenvironment and plays a role in contact inhibition, which is lost in cancer cells.192 Similarly, Louderbough et al193,194 showed that collagen-embedded HMW HA interfered with the activation of EGFR and prevented filopodia formation on collagen in a BC cell line, inhibiting the invasion of tumor cells. CD44 has also been implicated in the inhibition of angiogenesis, particularly by HMW HA,195 suggesting its role in the inhibition of metastasis.
Tumor-suppressive activities for CD44 have been demonstrated in vivo using CD44-deficient mice. SV40-transformed CD44-null fibroblasts injected subcutaneously into nude BALB/C mice were highly tumorigenic, whereas the introduction of CD44s into these cells resulted in a dramatic inhibition of tumor growth.196 In a mouse model of spontaneously metastasizing BC (MMTV-polyoma middle T), Lopez et al197 found that loss of CD44 promoted tumor metastasis to the lung, but not the onset, suggesting its suppressive role for tumor progression. CD44 was expressed in the myoepithelium of the developing mammary gland in mice. The loss of CD44 resulted in defective luminal–myoepithelial cell–cell adhesion and promoted the mixing of luminal and myoepithelial layers, disrupting epithelial bilayer organization.198 The ductal outgrowth and terminal end bud formation were delayed/impaired in CD44-null mice. In BCs, CD44 was expressed in the basal cells of early-stage tumor cells but exhibited altered localization with the development of the disease. Collectively, global depletion of all the CD44 isoforms leads to acceleration of tumorigenesis and metastasis, suggesting its tumor-suppressive role in vivo.
Possible mechanisms for the dual roles of CD44 in cancer
Research findings described earlier indicate that CD44 has dual roles, that is, it either promotes or inhibits cancer progression dependent on the experimental conditions used.194 In the case of HA–CD44 signaling, the molecular weight of HA will decide the biological consequences, that is, HMW HA inhibits metastasis-promoting activity of CD44, whereas LMW HA does the opposite. Thus, environmental factors significantly influence the biological activity of CD44 in cell growth.
Contradictory roles of CD44 in cancer progression can also be attributed to the expression of the standard and alternatively spliced isoforms with different activities. Indeed, a high expression of the CD44v2–v10 and v3–v10 isoform correlated with positive ER/PR status, low proliferation, and luminal A subtype, while a high expression of CD44v8–v10 correlated with positive EGFR, negative/low HER2 status, and basal-like subtype in BCs.191 In advanced ovarian cancer, CD44v6 was associated with peritoneal dissemination and poor prognosis.187 Thus, each splice variant has different biological activity. It is hypothesized that CD44s and CD44v have to be expressed at certain ratio/level in a relatively narrow range in each cell type/tissue to maintain the normal homeostasis, and any events that affect the CD44 transcription, pre-mRNA splicing, translation, or posttrans-lational modifications result in increased predisposition to cancer.
The third possibility to explain the dual roles of CD44 in cancer is its crosstalk with the TGFβ pathway since TGFβ has both tumor-suppressive (early stage) and tumor-promoting (advanced stage) activities dependent on the level of tumor development.199,200 Indeed, published studies have shown the importance of the CD44–MMP–TGFβ axis in tumor cell invasion and metastasis.159–161 It will thus be essential to elucidate the molecular interactions between CD44 and TGFβ signaling cascades since both of these molecules are cleaved and activated by MMPs.
Conclusive Remarks and Future Directions
Estrogen has both genomic and nongenomic pathways for signaling. Published studies have shown that ERα36 is a potential regulator for membrane-initiated mitogenic sig-naling and is a promising diagnostic/prognostic biomarker for therapy-resistant cancer. Conversely, ERα66 expression is generally associated with good prognosis of cancer. Thus, molecular characterization of signaling cascades that regulate ERα36/66 ratio will have significant impacts on cancer therapy. It will also be needed to characterize the signaling pathways governed by ERα46 that has different C-terminal structure from ERα36.
Although the HER2 mutation that corresponds to neu has not been reported in human cancers, the Δ16HER2 variant has revealed its oncogenic activity. Since the levels of Δ16HER2 significantly affect the therapeutic response of BC patients to the monoclonal antibody therapy, Δ16HER2 level should be predetermined using tissues obtained by biopsy or surgery before making therapeutic decisions. Investigation for microRNA-regulated signaling pathways will give novel therapeutic modalities to treat BC refractory to the antibody therapy.
The molecular mechanisms for the role of CD44 in cancer development look very complicated since CD44 has multiple isoforms that may have conflicting activities in tumor initiation or progression. Although Schmits et al196 demonstrated the tumorigenicity of CD44-deficient fibroblasts in vivo, the interpretation of their results is difficult since both Rb and p53 tumor suppressors had already been inactivated by the SV40 T antigen, which does not frequently happen in naturally occurring human cancers. Interestingly, the same laboratory later reported that the absence of CD44 prevented sarcoma metastasis using the more physiological min mutation model for colon cancer, demonstrating the pro-metastatic potential for CD44.201 There has been no report on increased spontaneous or carcinogen/irradiation-induced tumor incidence in CD44-deficient mice. Since CD44 has multiple splice variants with possible conflicting functions, it will be necessary to establish Dox-inducible transgenic mouse models for each variant or create isoform-specific knockout mice to elucidate the role of each CD44 isoform in cancer development or prevention.
Acknowledgments
We thank all members of Dr Inoue’s laboratory for sharing research findings on BC.
Footnotes
ACADEMIC EDITOR: Christian Bronner, editor in chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 919 words, excluding any confidential comments to the academic editor.
FUNDING: K. Inoue was supported by NIH/NCI 2R01CA106314, ACS RSG-07-207-01-MGO, and KG080179. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Contributed to the conceptual designing, writing of the text, creating figures, and collecting literatures: KI and EAF. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Wahl M, Will C, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Matera AG, Wang Z. A day in the life of the spliceosome. Nat Rev Mol Cell Biol. 2014;15:108–121. doi: 10.1038/nrm3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornblihtt AR, Schor IE, Alló M, Dujardin G, Petrillo E, Muñoz MJ. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nat Rev Mol Cell Biol. 2013;14:153–165. doi: 10.1038/nrm3525. [DOI] [PubMed] [Google Scholar]
- 4.Keren H, Lev-Maor G, Ast G. Alternative splicing and evolution: diversification, exon definition and function. Nat Rev Genet. 2010;11:345–355. doi: 10.1038/nrg2776. [DOI] [PubMed] [Google Scholar]
- 5.Ellis JD, Barrios-Rodiles M, Colak R, et al. Tissue-specific alternative splicing remodels protein-protein interaction networks. Mol Cell. 2012;46:884–892. doi: 10.1016/j.molcel.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 6.Kalsotra A, Cooper TA. Functional consequences of developmentally regulated alternative splicing. Nat Rev Genet. 2011;12:715–729. doi: 10.1038/nrg3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen K, Dai X, Wu J. Alternative splicing: an important mechanism in stem cell biology. World J Stem Cells. 2015;7:1–10. doi: 10.4252/wjsc.v7.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He L, Bai Q, Tang L. Alternative splicing regulates pluripotent state in pluripotent stem cells. Curr Stem Cell Res Ther. 2015;10:159–165. doi: 10.2174/1574888x09666141112115525. [DOI] [PubMed] [Google Scholar]
- 9.David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev. 2010;24:2343–2364. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonomi S, Gallo S, Catillo M, Pignataro D, Biamonti G, Ghigna C. Oncogenic alternative splicing switches: role in cancer progression and prospects for therapy. Int J Cell Biol. 2013;2013:962038. doi: 10.1155/2013/962038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Weiss WA. Alternative splicing in cancer: implications for biology and therapy. Oncogene. 2015;34:1–14. doi: 10.1038/onc.2013.570. [DOI] [PubMed] [Google Scholar]
- 12.Silipo M, Gautrey H, Tyson-Capper A. Deregulation of splicing factors and breast cancer development. J Mol Cell Biol. 2015;7:388–401. doi: 10.1093/jmcb/mjv027. [DOI] [PubMed] [Google Scholar]
- 13.Sveen A, Kilpinen S, Ruusulehto A, Lothe RA, Skotheim RI. Aberrant RNA splicing in cancer; expression changes and driver mutations of splicing factor genes. Oncogene. 2015 Aug 24; doi: 10.1038/onc.2015.318. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Inoue K, Fry EA. Alterations of p63 and p73 in human cancers. Subcell Biochem. 2014;85:17–40. doi: 10.1007/978-94-017-9211-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida K, Sanada M, Shiraishi Y, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa S. Splicing factor mutations in myelodysplasia. Int J Hematol. 2012;96:438–442. doi: 10.1007/s12185-012-1182-y. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen KE. The cyclin D1b splice variant: an old oncogene learns new tricks. Cell Div. 2006;1:15. doi: 10.1186/1747-1028-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue K, Fry EA. Aberrant expression of cyclin D1 in human cancer. Sign Transduct Insights. 2015;4:1–13. doi: 10.4137/STI.S30306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong W, Qian Y, Yang L. Telomerase, hTERT and splice variants in patients with myelodysplastic syndromes. Leuk Res. 2014;38:830–835. doi: 10.1016/j.leukres.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Anand S, Ebner J, Warren CB, et al. C/EBP transcription factors in human squamous cell carcinoma: selective changes in expression of isoforms correlate with the neoplastic state. PLoS One. 2014;9:e112073. doi: 10.1371/journal.pone.0112073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maglic D, Stovall DB, Cline JM, et al. DMP1β, a splice isoform of the tumor suppressor DMP1 locus, induces proliferation and progression of breast cancer. J Pathol. 2015;236:90–102. doi: 10.1002/path.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 23.Kittaneh M, Montero AJ, Glück S. Molecular profiling for breast cancer: a comprehensive review. Biomark Cancer. 2013;5:61–70. doi: 10.4137/BIC.S9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire A, Brown JA, Kerin MJ. Metastatic breast cancer: the potential of miRNA for diagnosis and treatment monitoring. Cancer Metastasis Rev. 2015;34:145–155. doi: 10.1007/s10555-015-9551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajani JA, Song S, Hochster HS, Steinberg IB. Cancer stem cells: the promise and the potential. Semin Oncol. 2015;42(suppl 1):S3–S17. doi: 10.1053/j.seminoncol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 27.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 29.Maglic D, Zhu S, Fry EA, et al. Prognostic value of the hDMP1-ARF-Hdm2-p53 pathway in breast cancer. Oncogene. 2013;32:4120–4129. doi: 10.1038/onc.2012.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taneja P, Maglic D, Kai F, et al. Classical and novel molecular prognostic markers for human breast cancer and their clinical significance. Clin Med Insights Oncol. 2010;4:15–34. doi: 10.4137/cmo.s4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang B, Warner M, Gustafsson JA. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol Cell Endocrinol. 2014 Nov 26; doi: 10.1016/j.mce.2014.11.015. pii: S0303-7207(14)00371-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids. 2014;90:13–29. doi: 10.1016/j.steroids.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas C, Gustafsson JÅ. Estrogen receptor mutations and functional consequences for breast cancer. Trends Endocrinol Metab. 2015;26:467–476. doi: 10.1016/j.tem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Webb P, Nguyen P, Shinsako J, et al. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol. 1998;12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Singleton DW, Shaughnessy EA, Khan SA. The F-domain of estrogen receptor-alpha inhibits ligand induced receptor dimerization. Mol Cell Endocrinol. 2008;295(1–2):94–100. doi: 10.1016/j.mce.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci U S A. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, anovel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336:1023–1027. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-α, hER-α36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Wang ZY. Estrogen receptor-α variant, ER-α36, is involved in tamoxifen resistance and estrogen hypersensitivity. Endocrinology. 2013;154:1990–1998. doi: 10.1210/en.2013-1116. [DOI] [PubMed] [Google Scholar]
- 41.Su X, Xu X, Li G, Lin B, Cao J, Teng L. ER-α36: a novel biomarker and potential therapeutic target in breast cancer. Onco Targets Ther. 2014;7:1525–1533. doi: 10.2147/OTT.S65345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penot G, Le PC, Merot Y, et al. The human estrogen receptor-α isoform hERα46 antagonizes the proliferative influence of hERα66 in MCF7 breast cancer cells. Endocrinology. 2005;146:5474–5484. doi: 10.1210/en.2005-0866. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klinge CM, Riggs KA, Wickramasinghe NS, et al. Estrogen receptor alpha 46 is reduced in tamoxifen resistant breast cancer cells and re-expression inhibits cell proliferation and estrogen receptor alpha 66-regulated target gene transcription. Mol Cell Endocrinol. 2010;323:268–276. doi: 10.1016/j.mce.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang ZY, Yin L. Estrogen receptor alpha-36 (ER-α36): a new player in human breast cancer. Mol Cell Endocrinol. 2015 Apr 24; doi: 10.1016/j.mce.2015.04.017. pii: S0303-7207(15)00208-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Zou Y, Ding L, Coleman M, Wang Z. Estrogen receptor-alpha (ER-alpha) suppresses expression of its variant ER-alpha 36. FEBS Lett. 2009;583:1368–1374. doi: 10.1016/j.febslet.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huff V. Wilms’ tumours: about tumour suppressor genes, an oncogene and a chameleon gene. Nat Rev Cancer. 2011;11:111–121. doi: 10.1038/nrc3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindstedt I, Lindgren MA, Andersson E, Engström W. The WT1 gene—its role in tumourigenesis and prospects for immunotherapeutic advances. In Vivo. 2014;28:675–681. [PubMed] [Google Scholar]
- 49.Toska E, Roberts SG. Mechanisms of transcriptional regulation by WT1 (Wilms’ tumour 1) Biochem J. 2014;461:15–32. doi: 10.1042/BJ20131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inoue K, Sugiyama H, Ogawa H, et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994;84:3071–3079. [PubMed] [Google Scholar]
- 51.Inoue K, Ogawa H, Sonoda Y, et al. Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia. Blood. 1997;89:1405–1412. [PubMed] [Google Scholar]
- 52.Oji Y, Ogawa H, Tamaki H, et al. Expression of the Wilms’ tumor gene WT1 in solid tumors and its involvement in tumor cell growth. Jpn J Cancer Res. 1999;90:194–204. doi: 10.1111/j.1349-7006.1999.tb00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamagami T, Sugiyama H, Inoue K, et al. Growth inhibition of human leukemic cells by WT1 (Wilms tumor gene) antisense oligodeoxynucleotides: implications for the involvement of WT1 in leukemogenesis. Blood. 1996;87:2878–2884. [PubMed] [Google Scholar]
- 54.Inoue K, Tamaki H, Ogawa H, et al. Wilms’ tumor gene (WT1) competes with differentiation-inducing signal in hematopoietic progenitor cells. Blood. 1998;91:2969–2976. [PubMed] [Google Scholar]
- 55.Kang L, Wang L, Wang ZY. Opposite regulation of estrogen receptor-α and its variant ER-α36 by the Wilms’ tumor suppressor WT1. Oncol Lett. 2011;2:337–341. doi: 10.3892/ol.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nasomyon T, Samphao S, Sangkhathat S, Mahattanobon S, Graidist P. Correlation of Wilms’ tumor 1 isoforms with HER2 and ER-α and its oncogenic role in breast cancer. Anticancer Res. 2014;34:1333–1342. [PubMed] [Google Scholar]
- 57.Shi YE, Chen Y, Dackour R, et al. Synuclein gamma stimulates membrane-initiated estrogen signaling by chaperoning estrogen receptor (ER)-alpha36, a variant of ER-alpha. Am J Pathol. 2010;177:964–973. doi: 10.2353/ajpath.2010.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14:630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Georgakis GV, Younes A. Heat-shock protein 90 inhibitors in cancer therapy: 17AAG and beyond. Future Oncol. 2005;1:273–281. doi: 10.1517/14796694.1.2.273. [DOI] [PubMed] [Google Scholar]
- 60.Lee LM, Cao J, Deng H, Chen P, Gatalica Z, Wang ZY. ER-alpha36, a novel variant of ER-alpha, is expressed in ER-positive and -negative human breast carcinomas. Anticancer Res. 2008;28:479–483. [PMC free article] [PubMed] [Google Scholar]
- 61.Johnston SR. Enhancing endocrine therapy for hormone receptor-positive advanced breast cancer: cotargeting signaling pathways. J Natl Cancer Inst. 2015;107(10):djv212. doi: 10.1093/jnci/djv212. [DOI] [PubMed] [Google Scholar]
- 62.Jin K, Kong X, Shah T, et al. The HOXB7 protein renders breast cancer cells resistant to tamoxifen through activation of the EGFR pathway. Proc Natl Acad Sci U S A. 2012;109:2736–2741. doi: 10.1073/pnas.1018859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin K, Park S, Teo WW, et al. HOXB7 is an ERα cofactor in the activation of HER2 and multiple ER target genes leading to endocrine resistance. Cancer Discov. 2015;5(9):944–959. doi: 10.1158/2159-8290.CD-15-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmad A, Ginnebaugh KR, Yin S, Bollig-Fischer A, Reddy KB, Sarkar FH. Functional role of miR-10b in tamoxifen resistance of ER-positive breast cancer cells through down-regulation of HDAC4. BMC Cancer. 2015;15:540. doi: 10.1186/s12885-015-1561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui J, Yang Y, Li H, et al. MiR-873 regulates ERα transcriptional activity and tamoxifen resistance via targeting CDK3 in breast cancer cells. Oncogene. 2015;34:3895–3907. doi: 10.1038/onc.2014.430. [DOI] [PubMed] [Google Scholar]
- 66.Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015;12(10):573–583. doi: 10.1038/nrclinonc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karlsson E, Veenstra C, Emin S, et al. Loss of protein tyrosine phosphatase, non-receptor type 2 is associated with activation of AKT and tamoxifen resistance in breast cancer. Breast Cancer Res Treat. 2015;153:31–40. doi: 10.1007/s10549-015-3516-y. [DOI] [PubMed] [Google Scholar]
- 68.Egeland NG, Lunde S, Jonsdottir K, et al. The role of microRNAs as predictors of response to tamoxifen treatment in breast cancer patients. Int J Mol Sci. 2015;16:24243–24275. doi: 10.3390/ijms161024243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mosselman S, Polman J, Dijkema R. ERbeta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 70.Speirs V, Skliris GP, Burdall SE, Carder PJ. Distinct expression patterns of ER alpha and ER beta in normal human mammary gland. J Clin Pathol. 2002;55:371–374. doi: 10.1136/jcp.55.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fox EM, Davis RJ, Shupnik MA. ERbeta in breast cancer—onlooker, passive player, or active protector? Steroids. 2008;73:1039–1051. doi: 10.1016/j.steroids.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramsey TL, Risinger KE, Jernigan SC, Mattingly KA, Klinge CM. Estrogen receptor beta isoforms exhibit differences in ligand-activated transcriptional activity in an estrogen response element sequence-dependent manner. Endocrinology. 2004;145:149–160. doi: 10.1210/en.2003-1043. [DOI] [PubMed] [Google Scholar]
- 73.Skliris GP, Leygue E, Watson PH, Murphy LC. Estrogen receptor alpha negative breast cancer patients: estrogen receptor beta as a therapeutic target. J Steroid Biochem Mol Biol. 2008;109:1–10. doi: 10.1016/j.jsbmb.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 74.Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci U S A. 2004;101:1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lazennec G, Bresson D, Lucas A, Chauveau C, Vignon F. ER beta inhibits proliferation and invasion of breast cancer cells. Endocrinology. 2001;142:4120–4130. doi: 10.1210/endo.142.9.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–428. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- 77.Honma N, Horii R, Iwase T, et al. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727–3734. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 78.Nakopoulou L, Lazaris AC, Panayotopoulou EG, et al. The favourable prognostic value of oestrogen receptor beta immunohistochemical expression in breast cancer. J Clin Pathol. 2004;57:523–528. doi: 10.1136/jcp.2003.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim TJ, Lee A, Choi YJ, Song BJ, Yim HW, Kang CS. Prognostic significance of high expression of ER-beta in surgically treated ER-positive breast cancer following endocrine therapy. J Breast Cancer. 2012;15:79–86. doi: 10.4048/jbc.2012.15.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H, Zhang Z, Xuan L, et al. Evaluation of ER-α, ER-B1 and ER-B2 expression and correlation with clinicopathologic factors in invasive luminal subtype breast cancers. Clin Transl Oncol. 2012;14:225–231. doi: 10.1007/s12094-012-0788-0. [DOI] [PubMed] [Google Scholar]
- 81.Rosin G, de Boniface J, Karthik GM, Frisell J, Bergh J, Hartman J. Oestrogen receptors β1 and βcx have divergent roles in breast cancer survival and lymph node metastasis. Br J Cancer. 2014;111:918–926. doi: 10.1038/bjc.2014.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaw JA, Udokang K, Mosquera JM, Chauhan H, Jones JL, Walker RA. Oestrogen receptors alpha and beta differ in normal human breast and breast carcinomas. J Pathol. 2002;198:450–457. doi: 10.1002/path.1230. [DOI] [PubMed] [Google Scholar]
- 83.Skliris GP, Munot K, Bell SM, et al. Reduced expression of oestrogen receptor beta in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol. 2003;201:213–220. doi: 10.1002/path.1436. [DOI] [PubMed] [Google Scholar]
- 84.Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61:2537–2541. [PubMed] [Google Scholar]
- 85.Al-Nakhle H, Smith L, Bell SM, et al. Regulation of estrogen receptor β1 expression in breast cancer by epigenetic modification of the 5′ regulatory region. Int J Oncol. 2013;43:2039–2045. doi: 10.3892/ijo.2013.2112. [DOI] [PubMed] [Google Scholar]
- 86.Smart E, Hughes T, Smith L, Speirs V. Estrogen receptor β: putting a positive into triple negative breast cancer? Horm Mol Biol Clin Investig. 2013;16:117–123. doi: 10.1515/hmbci-2013-0042. [DOI] [PubMed] [Google Scholar]
- 87.Wang J, Zhang C, Chen K, et al. ERβ1 inversely correlates with PTEN/PI3K/AKT pathway and predicts a favorable prognosis in triple-negative breast cancer. Breast Cancer Res Treat. 2015;152:255–269. doi: 10.1007/s10549-015-3467-3. [DOI] [PubMed] [Google Scholar]
- 88.Jensen EV, Cheng G, Palmieri C, et al. Estrogen receptors and proliferation markers in primary and recurrent breast cancer. Proc Natl Acad Sci U S A. 2001;98:15197–15202. doi: 10.1073/pnas.211556298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Neill PA, Davies MP, Shaaban AM, et al. Wild-type oestrogen receptor beta (ERbeta1) mRNA and protein expression in tamoxifen-treated post-menopausal breast cancers. Br J Cancer. 2004;91:1694–1702. doi: 10.1038/sj.bjc.6602183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hou YF, Yuan ST, Li HC, et al. ERbeta exerts multiple stimulative effects on human breast carcinoma cells. Oncogene. 2004;23:5799–5806. doi: 10.1038/sj.onc.1207765. [DOI] [PubMed] [Google Scholar]
- 91.Peng B, Lu B, Leygue E, Murphy LC. Putative functional characteristics of human estrogen receptor-beta isoforms. J Mol Endocrinol. 2003;30:13–29. doi: 10.1677/jme.0.0300013. [DOI] [PubMed] [Google Scholar]
- 92.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci U S A. 2006;103:13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao C, Matthews J, Tujague M, et al. Estrogen receptor beta2 negatively regulates the transactivation of estrogen receptor alpha in human breast cancer cells. Cancer Res. 2007;67:3955–3962. doi: 10.1158/0008-5472.CAN-06-3505. [DOI] [PubMed] [Google Scholar]
- 94.Chi A, Chen X, Chirala M, Younes M. Differential expression of estrogen receptor beta isoforms in human breast cancer tissue. Anticancer Res. 2003;23:211–216. [PubMed] [Google Scholar]
- 95.Huang B, Omoto Y, Iwase H, et al. Differential expression of estrogen receptor α, β1, and β2 in lobular and ductal breast cancer. Proc Natl Acad Sci U S A. 2014;111:1933–1938. doi: 10.1073/pnas.1323719111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shaaban AM, Green AR, Karthik S, et al. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008;14:5228–5235. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- 97.Yan M, Rayoo M, Takano EA, kConFab Investigators, Fox SB Nuclear and cytoplasmic expressions of ERβ1 and ERβ2 are predictive of response to therapy and alters prognosis in familial breast cancers. Breast Cancer Res Treat. 2011;126:395–405. doi: 10.1007/s10549-010-0941-9. [DOI] [PubMed] [Google Scholar]
- 98.Chantzi NI, Tiniakos DG, Palaiologou M, et al. Estrogen receptor beta 2 is associated with poor prognosis in estrogen receptor alpha-negative breast carcinoma. J Cancer Res Clin Oncol. 2013;139:1489–1498. doi: 10.1007/s00432-013-1467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dhimolea E, Tiniakos DG, Chantzi NI, et al. Estrogen receptors β1 and β2 are associated with distinct responses of estrogen receptor α-positive breast carcinoma to adjuvant endocrine therapy. Cancer Lett. 2015;358:37–42. doi: 10.1016/j.canlet.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 100.Baek JM, Chae BJ, Song BJ, Jung SS. The potential role of estrogen receptor β2 in breast cancer. Int J Surg. 2015;14:17–22. doi: 10.1016/j.ijsu.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 101.Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319:226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 102.Yamamoto T, Ikawa S, Akiyama T, et al. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986;319:230–234. doi: 10.1038/319230a0. [DOI] [PubMed] [Google Scholar]
- 103.Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- 104.Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. doi: 10.1155/2014/852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krishnamurti U, Silverman JF. HER2 in breast cancer: a review and update. Adv Anat Pathol. 2014;21:100–107. doi: 10.1097/PAP.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 106.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 107.Ghedini GC, Ciravolo V, Castagnoli L, Tagliabue E, Pupa SM. delta16HER2 splice variant and its role in HER2-overexpressing breast cancer. Atlas Genet Cytogenet Oncol Haematol. 2014;18:526–531. [Google Scholar]
- 108.Bargmann CI, Hung MC, Weinberg RA. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986;45:649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- 109.Segatto O, King CR, Pierce JH, Di Fiore PP, Aaronson SA. Different structural alterations upregulate in vitro tyrosine kinase activity and transforming potency of the erbB-2 gene. Mol Cell Biol. 1988;8:5570–5574. doi: 10.1128/mcb.8.12.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taneja P, Frazier DP, Kendig RD, et al. MMTV mouse models and the diagnostic values of MMTV-like sequences in human breast cancer. Expert Rev Mol Diagn. 2009;9:423–440. doi: 10.1586/ERM.09.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Inoue K, Roussel MF, Sherr CJ. Induction of ARF tumor suppressor gene expression and cell cycle arrest by transcription factor DMP1. Proc Natl Acad Sci U S A. 1999;96:3993–3998. doi: 10.1073/pnas.96.7.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taneja P, Maglic D, Kai F, et al. Critical role of Dmp1 in HER2/neu-p53 signaling and breast carcinogenesis. Cancer Res. 2010;70:9084–9094. doi: 10.1158/0008-5472.CAN-10-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fry EA, Taneja P, Maglic D, Zhu S, Sui G, Inoue K. Dmp1α inhibits HER2/neu-induced mammary tumorigenesis. PLoS One. 2013;8:e77870. doi: 10.1371/journal.pone.0077870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Inoue K, Sherr CJ. Gene expression and cell cycle arrest mediated by transcription factor DMP1 is antagonized by D-type cyclins through a cyclin-dependent-kinase-independent mechanism. Mol Cell Biol. 1998;18:1590–1600. doi: 10.1128/mcb.18.3.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Inoue K, Wen R, Rehg JE, et al. Disruption of the ARF transcriptional activator DMP1 facilitates cell immortalization, Ras transformation, and tumorigenesis. Genes Dev. 2000;14:1797–1809. [PMC free article] [PubMed] [Google Scholar]
- 116.Inoue K, Zindy F, Randle DH, Rehg JE, Sherr CJ. Dmp1 is haplo-insufficient for tumor suppression and modifies the frequencies of Arf and p53 mutations in Myc-induced lymphomas. Genes Dev. 2001;15:2934–1939. doi: 10.1101/gad.929901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sreeramaneni R, Chaudhry A, McMahon M, Sherr CJ, Inoue K. Ras-Raf-Arf signaling critically depends on the Dmp1 transcription factor. Mol Cell Biol. 2005;25:220–232. doi: 10.1128/MCB.25.1.220-232.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mallakin A, Sugiyama T, Taneja P, et al. Mutually exclusive inactivation of DMP1 and ARF/p53 in lung cancer. Cancer Cell. 2007;12:381–394. doi: 10.1016/j.ccr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frazier DP, Kendig RD, Kai F, et al. Dmp1 physically interacts with p53 and positively regulates p53’s stabilization, nuclear localization, and function. Cancer Res. 2012;72:1740–1750. doi: 10.1158/0008-5472.CAN-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Inoue K, Mallakin A, Frazier DP. Dmp1 and tumor suppression. Oncogene. 2007;26:4329–4335. doi: 10.1038/sj.onc.1210226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Inoue K, Fry EA, Frazier DP. Transcription factors that interact with p53 and Mdm2. Int J Cancer. 2015 Jul 1; doi: 10.1002/ijc.29663. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwong KY, Hung MC. A novel splice variant of HER2 with increased transformation activity. Mol Carcinog. 1998;23:62–68. doi: 10.1002/(sici)1098-2744(199810)23:2<62::aid-mc2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 123.Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Castiglioni F, Tagliabue E, Campiglio M, Pupa SM, Balsari A, Ménard S. Role of exon-16-deleted HER2 in breast carcinomas. Endocr Relat Cancer. 2006;13:221–232. doi: 10.1677/erc.1.01047. [DOI] [PubMed] [Google Scholar]
- 125.Marchini C, Gabrielli F, Iezzi M, et al. The human splice variant Delta16HER2 induces rapid tumor onset in a reporter transgenic mouse. PLoS One. 2011;6:e18727. doi: 10.1371/journal.pone.0018727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alajati A, Sausgruber N, Aceto N, et al. Mammary tumor formation and metastasis evoked by a HER2 splice variant. Cancer Res. 2013;73:5320–5327. doi: 10.1158/0008-5472.CAN-12-3186. [DOI] [PubMed] [Google Scholar]
- 127.Fuller SJ, Sivarajah K, Sugden PH. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium. J Mol Cell Cardiol. 2008;44:831–854. doi: 10.1016/j.yjmcc.2008.02.278. [DOI] [PubMed] [Google Scholar]
- 128.Garrett TP, McKern NM, Lou M, et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11:495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 129.Dey N, Williams C, Leyland-Jones B, De P. A critical role for HER3 in HER2-amplified and non-amplified breast cancers: function of a kinase-dead RTK. Am J Transl Res. 2015;7:733–750. [PMC free article] [PubMed] [Google Scholar]
- 130.Vaught DB, Stanford JC, Young C, et al. HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer Res. 2012;72:2672–2682. doi: 10.1158/0008-5472.CAN-11-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carpenter RL, Paw I, Dewhirst MW, Lo HW. Akt phosphorylates and activates HSF-1 independent of heat shock, leading to Slug overexpression and epithelial-mesenchymal transition (EMT) of HER2-overexpressing breast cancer cells. Oncogene. 2015;34:546–557. doi: 10.1038/onc.2013.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Druillennec S, Dorard C, Eychène A. Alternative splicing in oncogenic kinases: from physiological functions to cancer. J Nucleic Acids. 2012;2012:639062. doi: 10.1155/2012/639062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang S, Huang WC, Li P, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17:461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Castagnoli L, Iezzi M, Ghedini GC, et al. Activated d16HER2 homodimers and SRC kinase mediate optimal efficacy for trastuzumab. Cancer Res. 2014;74:6248–6259. doi: 10.1158/0008-5472.CAN-14-0983. [DOI] [PubMed] [Google Scholar]
- 135.Cittelly DM, Das PM, Salvo VA, Fonseca JP, Burow ME, Jones FE. Oncogenic HER2{Delta}16 suppresses miR-15a/16 and deregulates BCL-2 to promote endocrine resistance of breast tumors. Carcinogenesis. 2010;31:2049–2057. doi: 10.1093/carcin/bgq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huynh FC, Jones FE. MicroRNA-7 inhibits multiple oncogenic pathways to suppress HER2Δ16 mediated breast tumorigenesis and reverse trastuzumab resistance. PLoS One. 2014;22(9):e114419. doi: 10.1371/journal.pone.0114419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arribas J, Baselga J, Pedersen K, Parra-Palau JL. p95HER2 and breast cancer. Cancer Res. 2011;71:1515–1519. doi: 10.1158/0008-5472.CAN-10-3795. [DOI] [PubMed] [Google Scholar]
- 138.Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11:263–275. doi: 10.1586/era.10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miller LD, Liu ET. Expression genomics in breast cancer research: microarrays at the crossroads of biology and medicine. Breast Cancer Res. 2007;9:206. doi: 10.1186/bcr1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barry WT, Kernagis DN, Dressman HK, et al. Intratumor heterogeneity and precision of microarray-based predictors of breast cancer biology and clinical outcome. J Clin Oncol. 2010;28:2198–2206. doi: 10.1200/JCO.2009.26.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ricardo S, Vieira AF, Gerhard R, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64:937–946. doi: 10.1136/jcp.2011.090456. [DOI] [PubMed] [Google Scholar]
- 144.Ma F, Li H, Wang H, et al. Enriched CD44(+)/CD24(-) population drives the aggressive phenotypes presented in triple-negative breast cancer (TNBC) Cancer Lett. 2014;353:153–159. doi: 10.1016/j.canlet.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 145.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 146.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 147.Liu R, Wang X, Chen GY, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 148.Carrasco E, Alvarez PJ, Prados J, et al. Cancer stem cells and their implication in breast cancer. Eur J Clin Invest. 2014;44:678–687. doi: 10.1111/eci.12276. [DOI] [PubMed] [Google Scholar]
- 149.Honeth G, Bendahl PO, Ringnér M, et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 151.Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 152.Screaton GR, Bell MV, Bell JI, Jackson DG. The identification of a new alternative exon with highly restricted tissue expression in transcripts encoding the mouse Pgp-1 (CD44) homing receptor. Comparison of all 10 variable exons between mouse, human, and rat. J Biol Chem. 1993;268:12235–12238. [PubMed] [Google Scholar]
- 153.Götte M, Yip GW. Heparanase, hyaluronan, and CD44 in cancers: a breast carcinoma perspective. Cancer Res. 2006;66:10233–10237. doi: 10.1158/0008-5472.CAN-06-1464. [DOI] [PubMed] [Google Scholar]
- 154.Jordan AR, Racine RR, Hennig MJ, Lokeshwar VB. The role of CD44 in disease pathophysiology and targeted treatment. Front Immunol. 2015;6:182. doi: 10.3389/fimmu.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Orian-Rousseau V, Ponta H. Perspectives of CD44 targeting therapies. Arch Toxicol. 2015;89:3–14. doi: 10.1007/s00204-014-1424-2. [DOI] [PubMed] [Google Scholar]
- 156.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 2010;46:1271–1277. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 157.Morrison H, Sherman LS, Legg J, et al. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–980. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Orian-Rousseau V, Morrison H, Matzke A, et al. Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol Biol Cell. 2007;18:76–83. doi: 10.1091/mbc.E06-08-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 161.Kuo YC, Su CH, Liu CY, Chen TH, Chen CP, Wang HS. Transforming growth factor-beta induces CD44 cleavage that promotes migration of MDA-MB-435s cells through the up-regulation of membrane type 1-matrix metalloproteinase. Int J Cancer. 2009;124:2568–2576. doi: 10.1002/ijc.24263. [DOI] [PubMed] [Google Scholar]
- 162.Godar S, Ince TA, Bell GW, et al. Growth-inhibitory and tumor-suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134:62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ouhtit A, Abd Elmageed ZY, Abdraboh ME, Lioe TF, Raj MH. In vivo evidence for the role of CD44s in promoting breast cancer metastasis to the liver. Am J Pathol. 2007;171:2033–2039. doi: 10.2353/ajpath.2007.070535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ouhtit A, Madani S, Gupta I, et al. TGF-β2: a novel target of CD44-promoted breast cancer invasion. J Cancer. 2013;4:566–572. doi: 10.7150/jca.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marangoni E, Lecomte N, Durand L, et al. CD44 targeting reduces tumour growth and prevents post-chemotherapy relapse of human breast cancers xenografts. Br J Cancer. 2009;100:918–922. doi: 10.1038/sj.bjc.6604953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 167.Januschke J, Näthke I. Stem cell decisions: a twist of fate or a niche market? Semin Cell Dev Biol. 2014;34:116–123. doi: 10.1016/j.semcdb.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chanmee T, Ontong P, Kimata K, Itano N. Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front Oncol. 2015;5:180. doi: 10.3389/fonc.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rycaj K, Tang DG. Cell-of-origin of cancer versus cancer stem cells: assays and interpretations. Cancer Res. 2015;75(19):4003–4011. doi: 10.1158/0008-5472.CAN-15-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yan Y, Zuo X, Wei D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med. 2015;4:1033–1043. doi: 10.5966/sctm.2015-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 172.Horton SJ, Huntly BJ. Recent advances in acute myeloid leukemia stem cell biology. Haematologica. 2012;97:966–974. doi: 10.3324/haematol.2011.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Filipova A, Seifrtova M, Mokry J, et al. Breast cancer and cancer stem cells: a mini-review. Tumori. 2014;100:363–369. doi: 10.1700/1636.17886. [DOI] [PubMed] [Google Scholar]
- 174.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 175.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 176.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 177.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 178.Bill R, Christofori G. The relevance of EMT in breast cancer metastasis: correlation or causality? FEBS Lett. 2015;589:1577–1587. doi: 10.1016/j.febslet.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 179.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 180.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 181.Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 182.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gwak JM, Kim HJ, Kim EJ, et al. MicroRNA-9 is associated with epithelial-mesenchymal transition, breast cancer stem cell phenotype, and tumor progression in breast cancer. Breast Cancer Res Treat. 2014;147:39–49. doi: 10.1007/s10549-014-3069-5. [DOI] [PubMed] [Google Scholar]
- 184.Yu DD, Lv MM, Chen WX, et al. Role of miR-155 in drug resistance of breast cancer. Tumour Biol. 2015;36:1395–1401. doi: 10.1007/s13277-015-3263-z. [DOI] [PubMed] [Google Scholar]
- 185.Yae T, Tsuchihashi K, Ishimoto T, et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883. doi: 10.1038/ncomms1892. [DOI] [PubMed] [Google Scholar]
- 186.Zhang P, Fu C, Bai H, Song E, Dong C, Song Y. CD44 variant, but not standard CD44 isoforms, mediate disassembly of endothelial VE-cadherin junction on metastatic melanoma cells. FEBS Lett. 2014;588:4573–4582. doi: 10.1016/j.febslet.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 187.Tjhay F, Motohara T, Tayama S, et al. CD44 variant 6 is correlated with peritoneal dissemination and poor prognosis in patients with advanced epithelial ovarian cancer. Cancer Sci. 2015;106(10):1421–1428. doi: 10.1111/cas.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Brown RL, Reinke LM, Damerow MS, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121:1064–1074. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xu Y, Gao XD, Lee JH, et al. Cell type-restricted activity of hnRNPM promotes breast cancer metastasis via regulating alternative splicing. Genes Dev. 2014;28:1191–1203. doi: 10.1101/gad.241968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Preca BT, Bajdak K, Mock K, et al. A self-enforcing CD44s/ZEB1 feedback loop maintains EMT and stemness properties in cancer cells. Int J Cancer. 2015;137(11):2566–2577. doi: 10.1002/ijc.29642. [DOI] [PubMed] [Google Scholar]
- 191.Olsson E, Honeth G, Bendahl PO, et al. CD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markers. BMC Cancer. 2011;11:418. doi: 10.1186/1471-2407-11-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Herrlich P, Morrison H, Sleeman J, et al. CD44 acts both as a growth- and invasiveness-promoting molecule and as a tumor-suppressing cofactor. N Y Acad Sci. 2000;910:106–118. doi: 10.1111/j.1749-6632.2000.tb06704.x. discussion 118–120. [DOI] [PubMed] [Google Scholar]
- 193.Louderbough JM, Lopez JI, Schroeder JA. Matrix hyaluronan alters epidermal growth factor receptor-dependent cell morphology. Cell Adh Migr. 2010;4:26–31. doi: 10.4161/cam.4.1.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Louderbough JM, Schroeder JA. Understanding the dual nature of CD44 in breast cancer progression. Mol Cancer Res. 2011;9:1573–1586. doi: 10.1158/1541-7786.MCR-11-0156. [DOI] [PubMed] [Google Scholar]
- 195.Singleton PA, Mirzapoiazova T, Guo Y, et al. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol. 2010;299:L639–L651. doi: 10.1152/ajplung.00405.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schmits R, Filmus J, Gerwin N, et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–2233. [PubMed] [Google Scholar]
- 197.Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005;65:6755–6763. doi: 10.1158/0008-5472.CAN-05-0863. [DOI] [PubMed] [Google Scholar]
- 198.Louderbough JM, Brown JA, Nagle RB, Schroeder JA. CD44 promotes epithelial mammary gland development and exhibits altered localization during cancer progression. Genes Cancer. 2011;2:771–781. doi: 10.1177/1947601911428223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Leivonen SK, Kähäri VM. Transforming growth factor-beta signaling in cancer invasion and metastasis. Int J Cancer. 2007;121:2119–2124. doi: 10.1002/ijc.23113. [DOI] [PubMed] [Google Scholar]
- 200.Zarzynska JM. Two faces of TGF-beta1 in breast cancer. Mediators Inflamm. 2014;2014:141747. doi: 10.1155/2014/141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Weber GF, Bronson RT, Ilagan J, Cantor H, Schmits R, Mak TW. Absence of the CD44 gene prevents sarcoma metastasis. Cancer Res. 2002;62:2281–2286. [PubMed] [Google Scholar]
- 202.Marchini C, Pietrella L, Kalogris C, et al. HER2–driven carcinogenesis: new mouse models for novel immunotherapies. In: Yahwardiah S, editor. Oncogene and Cancer—From Bench to Clinic. Croatia: InTech; 2013. pp. 151–182. Chap. 2. [Google Scholar]