Abstract
Despite significant advances, cardiovascular disease is the leading cause of world-wide mortality, highlighting an important yet unmet clinical need. Understanding the pathophysiological basis underlying cardiovascular tissue injury and repair in therefore of prime importance. Following cardiac tissue injury, the immune system plays an important and complex role throughout the acute inflammatory response and regenerative response. This review will summarize the role of the immune system in cardiovascular disease, and focus on the idea that the immune system evolved to promote tissue homeostasis following tissue injury and/or infection, and that the inherent cost of this evolutionary development is unwanted inflammatory mediated damage. While inflammation induced tissue damage is of little evolutionary consequence in organisms that have limited life spans, as will be discussed below, inflammation plays a major role in the development of cardiovascular disease worldwide in humans.
Introduction
The cardiovascular system evolved ~600 million years ago as a means to transport nutrients and cells within multicellular organisms. Primitive organisms such as Drosophila possess a single chamber that acts as both a pumping tube and a simple vascular system 1. More complex organisms have compartmentalized functions, with venous and arterial vascular systems connected to a multi-chamber muscular myocardium that continually receives and ejects blood components. Despite the more complex nature of the mammalian cardiovascular system, its primary functions remain the same, and its importance to health and disease is underscored by the fact that cardiovascular disease is the leading cause of death world-wide, with an increasing burden over the last decade 2, 3. Therefore, understanding both how cardiac tissue is injured and how cardiac tissue regenerates is of prime importance to global health.
The immune system evolved as both a layered mechanism of host defense against invading pathogens, and as a facilitator of tissue growth during development and repair after sterile tissue injury, including within the myocardium. We will utilize a contemporary immunological framework to review the roles of individual immune subsets and pathways in response to both sterile and infectious cardiac injury. We will also bring to light the idea that immune system evolved to promote tissue homeostasis, although this beneficial evolutionary mechanism also comes at a cost of increased "bystander damage" secondary to over reactivity of immune responses to internal injury signals.
The immune system during tissue growth and regeneration
A careful examination reveals temporal and phylogenetic characteristics that predict the ability of tissue to regenerate in diverse organisms. More primitive organisms such as invertebrates, reptiles and amphibians have a striking regenerative potential when compared with mammals. For example, both the zebrafish and newt heart can fully re-grow after significant injury and the salamander can fully re-grow limbs after amputation, functions not possessed by adult mammals 4–6. During times of rapid growth, such as during development, very young mammals also retain this significant regenerative capacity. For example, the neonatal heart whether through apical resection of the left ventricle (LV), or myocardial infarction or, fully regenerates - which is lost after the first weeks of life 7–9. One important similarity between more primitive organisms and very young mammals is a more limited (primitive) immune system.
Phagocytes are an evolutionary conserved lineage that evolved more than 600 million years ago 10, 11. The macrophage (MΦ) is a specialized mononuclear phagocyte that resides in all tissues from the earliest stages of development 12, 13. Loss of MΦs due to deficiencies in transcription factors or growth signaling leads to increased mortality and stunted growth 14–16. Loss of MΦs also leads to abnormalities focused on remodeling and growth of complex vascular and neuronal networks 17–20. Beyond supporting growth, MΦs also have an important and more generalized role in clearance of senescent cells during embryonic development 21, 22. Importantly, non-selective depletion of all MΦs impairs the ability of primitive organisms and young mammals to regenerate, highlighting the critical role MΦs play in tissue growth and repair 7–9. Together, these data suggest that the MΦ, first identified by Ilya Metchnikoff in primitive organisms, may possess evolutionary conserved functions that aid tissue growth, both during homoeostasis and following injury – a hypothesis that Metchnikoff himself proposed in the late 19th century 11. While MΦs possess important regenerative functions, they can also mediate pathology. Excessive MΦ expansion during ischemic injury impairs tissue healing, indicating that either specific MΦ activation profiles or pathological MΦ subsets can interfere in the regenerative process 23. Understanding when MΦs do or do not promote tissue repair is a critical first step if we are to understand why the adult human heart has only a limited regenerative capacity. Moreover, we need to understand how in more complex organisms, MΦs both regulate, and are regulated by other leukocyte populations – which adds an additional layer of complexity to the interactions between immune cells and the regenerative capacity of the myocardium.
Mechanisms of Cardiac Injury
The myocardium can be injured through a variety of pathophysiological processes, which can be grouped broadly into ischemic and non-ischemic etiologies. In terms of global disease burden, ischemic injury is the primary pathophysiological mechanism of injury 2, 3. Occlusion of a coronary vessel after acute plaque rupture can leads down one of two pathways. The first is permanent anoxic / low nutrient injury from a completed infarction. The second is due in part to advances in timely interventions that re-establish blood flow to ischemic (yet viable) tissue, albeit at the cost of what has been termed “reperfusion injury” (discussed below). Non-ischemic cardiomyopathy is a composite diagnosis that includes myocarditis secondary to viral / bacterial infections or toxin administration. In addition, there are cardiomyopathies that develop secondary to chronic hypertension. All these forms of injury are influenced by genetic predisposition, which can itself lead to early onset cardiac dysfunction 24. Whether through acute ischemic injury or through the gradual impairment of cardiac function secondary to a variety of clinical pathologies, irreversible heart failure often develops. As we are coming to understand, the immune system can contribute both the initial insult and during the chronic phase of cardiac injury – and despite significant investment in understanding the contribution of immune cells to injury and repair, much remains unknown.
Sensing Cardiac Injury
Mammalian hearts use both innate and adaptive immunity to respond to tissue injury resulting from pathogens or environmental injury (e.g. ischemia or hemodynamic overloading). Resident cardiac immune cells are triggered by the detection of pathogen associated molecular patterns (PAMPs) or damage associated molecular patterns (DAMPs) by a fixed number of germ-line encoded pattern recognition receptors (PRRs). Classic examples of pathogen-associated molecular patterns include the lipopolysaccharides (LPS) of Gram-negative organisms, the teichoic acids of Gram positive organisms, the zymosans of yeast, the glycolipids of mycobacterium, or the double-stranded RNAs of viruses (Fig 1). More recently it has become clear that cardiac PRRs also recognize the molecular patterns of endogenous host material released by dying or injured myocardial cells. Cells that die by accidental necrosis, regulated necrosis (necroptosis), and/or secondary apoptosis release their cytosolic contents into the extracellular space, thereby initiating a brisk inflammatory response through engagement of an ensemble of extracellular or intracellular PRRs 25. The time course of the inflammatory response that ensues following tissue injury is remarkably consistent, irrespective of the specific cause of cell injury, and is associated with the rapid influx of neutrophils, and subsequently monocytes into the area of tissue injury. This inflammatory response has been referred to as “sterile inflammation,” insofar as the inflammation following tissue injury occurs in the absence of a known pathogenic infection 26, 27.
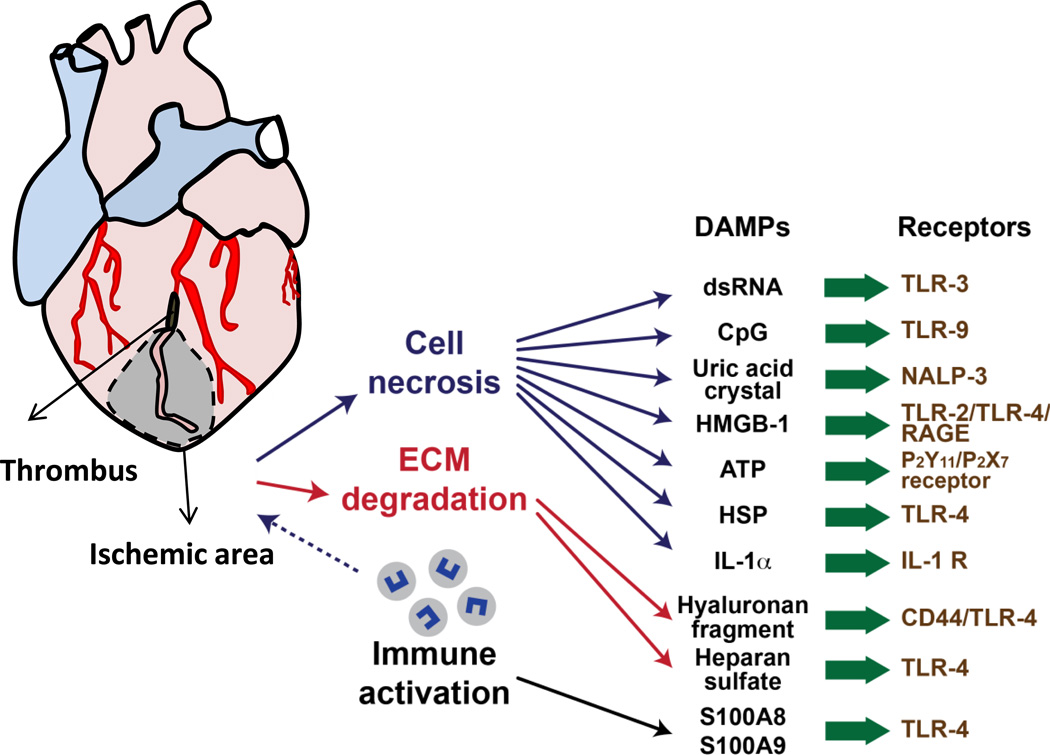
Figure 1. Cardiac injury and sensing damaged tissue.
Schematic demonstrating coronary artery occlusion (black) that leads to ischemic tissue injury (grey zone). From within the ischemic zone, cell necrosis, extracellular matrix (ECM) degradation and recruitment of immune cells all leads to production specific damage associated molecular patterns (DAMP)s, which are recognized by pattern recognition receptors (PRR)s, leading to the generation of inflammatory responses to internal injury signals.
Many PRRs encountering PAMPs and DAMPs trigger signaling cascades that activate nuclear factor-κB, activator protein 1, and interferon regulatory factors transcription factors, that in turn regulate target genes that encode pro-inflammatory cytokines and interferons in the heart 28. Another subset of PRRs in the heart trigger a distinct pro-inflammatory mechanism that requires assembly of cytosolic protein complexes called inflammasomes 29. Canonical inflammasomes convert procaspase-1 into the catalytically active protease that is responsible the production of interleukin 1β (IL-1β) and IL-18, which are sufficient to trigger inflammatory responses in the heart 29.
PRRs can be subdivided into two major classes based on their subcellular localization. Toll-like receptors (TLRs) and C-type lectin receptors are found on plasma membranes or endosomes, where they can detect the presence of PAMPs or DAMPs. A second class of PRRs resides in intracellular compartments, and includes RIG (retinoic acid inducible gene)-I-like receptors, also called RLRs, nucleotide binding and oligomerization domain (NOD) like receptors (NLRs) and absent-in-melanoma (AIM) 2 receptors 30, 31.
Messenger RNA for TLRs 1 – 10 has been identified in the human heart, with TLR4 and TLR2 being the most abundant 32. Although expression levels of TLRs have not been identified in human myocytes, TLR-2, 3, 4 , 6 mRNA has been identified in cardiac myocytes from neonatal rats 33. Little is known with regard to the regulation of TLR expression in the heart, however TLR4 appears to be upregulated in the failing human heart and on circulating monocytes at the time of myocardial infarction 34–36. In animal studies, loss of TLR4 is hematopoietic cells is protective in the setting of sepsis-induced cardiac dysfunction, and while loss of TLR4 is also protective following ischemic injury, it is not know if this too is in hematopoietic or other cell types 37–40. Alternatively, loss of TLR2 in hematopoietic cells is protective during ischemic injury 41. In the setting hemodynamic stress, mitochondria are typically damaged, however if degradation of mitochondrial DNA (mtDNA) is inhibited, a TLR9-dependent inflammation-induced cardiomyopathy develops 42. C-type lectin receptors are calcium-dependent carbohydrate-binding receptors, while expressed in human and murine heart tissue, very little is known about their role in cardiac tissue injury 43.
NLRs act as cytosolic sensors to intracellular DAMPs and PAMPs. In humans, the NLR family is composed of 22 intracellular pattern recognition molecules that share a central NACHT domain and a carboxy-terminal leucine rich repeat region 44. Analysis of human heart tissues has revealed that NOD2, NOD1 and NLR family members NLRP2, NLRP3 are expressed. Both NOD1 and NLRP3 have been shown to activate cannonical inflammasomes in the heart, and play an important role in adverse cardiac remodeling following ischemia reperfusion injury and myocardial infarction, however the cell types involved are not known 45, 46. Interestingly, potent inflammatory responses (inflammasome activation) require integration from two separate signals. The first (through TLRs) leads to upregulation of mRNA and translation of pro-IL-1β and pro-IL-18, while the second signal (such as NLRP3 sensing ATP) generates the second signal required for inflammasome assembly and cleavage of into mature IL-1β and IL-18 47.
The RLR family is composed of RIG-I, melanoma differentiation-associated gene 5 (MDA5), and LGP2. RLRs are localized in the cytoplasm and recognize the genomic RNA of double stranded (ds)RNA viruses and dsRNA generated as the replication intermediate of ssRNA viruses. The expression of RLRs is greatly enhanced in response to type I IFN stimulation or virus infection. MDA5 is the best characterized receptor in this family, with loss in cardiomyocytes leading to uncontrolled viral replication and rapid death, while over-expression resulting in protection from lethal myocarditis 48–50. One important issue which has only been partially addressed thus far is the delineation of cell-types specific roles for PRRs. Beyond the few example given here, it is not clear what differential roles TLR4 or NOD2 play within individual immune and non-immune subsets during the process of tissue injury and repair, which represents an important avenue for further investigations.
Innate immunity cell activation following tissue injury
Acute ischemic injury is the best characterized model of cardiac injury and repair. Following injury, necrotic cell death leads to activation of most leukocyte populations, which initiates an inflammatory response characterized initially by the activation of ensembles of pro-inflammatory cytokines and chemokines driven by resident immune and none-immune cells that are responsible for initiating the recruitment of leukocytes into the area of tissue injury. The initial inflammatory phase is followed by a proliferative phase characterized by the expansion of neutrophils and MΦs that are responsible for removing dead cells and matrix debris, as well as releasing cytokines and growth factors that lead to the formation of a highly vascularized granulation tissue, comprised of connective tissue and new blood vessels. The final maturation phase is characterized by fibroblast activation and endothelial cell proliferation, culminating in reparative myocardial fibrosis and angiogenesis.
During the very early stages following ischemic injury mast cells and soluble complement proteins become important initiators of inflammation – a process amplified following coronary reperfusion. Immediately after blood flow is restored, resident cardiac mast cells release preformed pro-inflammatory mediators (TNF-α, histamine and various proteases) that initiate an amplification loop involving adjacent cells, such as endothelium, resident macrophages and subsequently, infiltrating neutrophils 51. Timely restoration of blood flow to viable tissue is critical to prevent cardiomyocytes death, however reperfusion comes at the cost of introducing complement proteins to injured / inflamed endothelial cells and myocardium (see review 52). Activated (cleaved) complement proteins trigger further mast cell degranulation, release of histamine and vasogenic edema 52. Cleaved complement proteins such as C5a both attract neutrophils and induce their transendothelial migration into the injured tissue via the CD11b/CD18 complex 53 (Fig 2A). If reperfusion is not established, cardiomyocyte cell death will ensue through a variety of pathways that lead to additional DAMP liberation. The exact role of resident mast cells is in part inferred, since models of mast cell deficiency revolve around using Kit−/− animals, which have other immune cell deficits 54–56. However, mast cells, the complement cascade, oxidative stress and proinflammatory cytokine / chemokine production immediately after injury initiate a complex interplay between innate and adaptive immune cells.
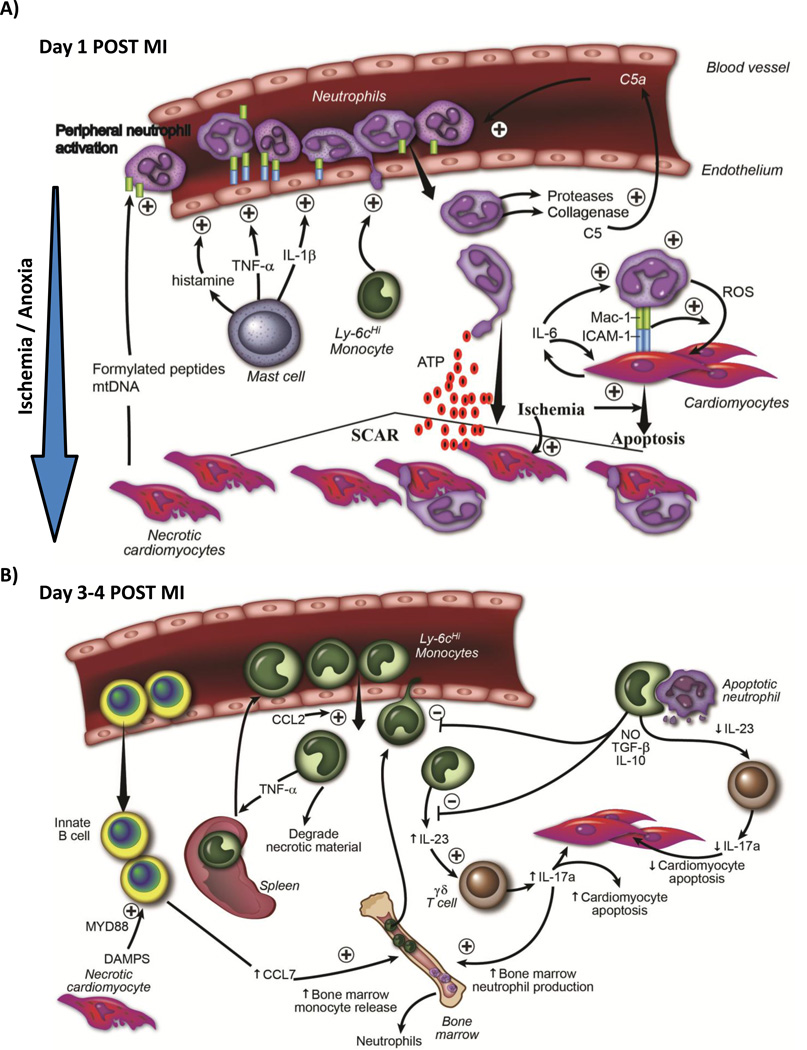
Figure 2. Immune Response To Ischemic Injury in Adult Animals.
A) Temporal schematic demonstrating that early after ischemic injury (first 24 hrs), internal DAMP signals released from necrotic cardiomyocytes activate resident mast cells, causing degranulation and release of preformed pro-inflammatory cytokines and vasogenic compounds such as histamine, which activate endothelial cells. Necrotic cardiomyocytes also release mitochondrial DAMPS (formylated peptides and mtDNA) into circulation, which causes systemic neutrophil activation. Activated neutrophils adhere to activated endothelium, transmigrate into tissue following a chemokine gradient. Neutrophils secrete proteases that digest tissue (and also activate chemokines such as C5a), which further potentiates leukocyte recruitment. Early recruitment of monocytes aids neutrophil recruitment. Neutrophils are directed to ischemic areas by following DAMP gradient (such as ATP). Neutrophils then both phagocytose dying cells, but can also induce apoptosis in live cardiomyocytes themselves through a MAC-1 : ICAM-1 interaction and release of reactive oxygen species. B) Schematic demonstrating that later after ischemic injury (24-96 hrs), there is recruitment of Ly6cHi monocytes from the blood into ischemic cardiac tissue. Some of the monocytes originated from the spleen. IgM/IgD+ innate B cells are also recruited into the myocardium, and through a CCL7-dependent fashion, promote further monocyte recruitment 86. Innate B cell activation is Myd88 dependent (suggesting TLR / DAMP involvement). Recruited monocytes secrete pro-inflammatory cytokines and chemokines, and drive inflammatory processes. A proportion of recruited monocytes ingested apoptotic material including neutrophils, which serves to increase secretion of anti-inflammatory cytokines such as TGF-B and IL-10, and thereby decrease leukocyte recruitment. Monocytes produce IL-23, which drives innate γδ T cells to produce IL-17a. IL-17a has two roles; it drives neutrophil production in the bone marrow and causes cardiomyocytes death. As inflammatory responses diminish, less IL-23 is produced.
The Neutrophil - recruitment, oxidative damage and cell death
Following either cardiac ischemic injury or pressure overload, neutrophils are the first innate immune cell recruited to the myocardium in large numbers 57. Patients deficient in neutrophils or neutrophil function suffer from devastating disseminated bacterial infections, indicating a clear requirement for this cell type to prevent expansion of otherwise harmless pathogens 58. Yet, their role in the response to injury is almost entirely pathologic, and as such, neutrophils serve as the best example of adaptations that promote overall longevity, yet in the setting of sterile injury, have no known protective role.
Neutrophil recruitment is mediated in two phases. The first phase is peripheral activation prior to infiltration. Mitochondria in all cell types, including cardiomyocytes, contain formylated peptides and mitochondrial DNA - both of which are structurally similar to bacterial components. In an analogous system (skeletal muscle necrosis), these mitochondrial DAMPs are released. Formylated peptides are sensed by formyl peptide receptor 1 (FPR1) and mtDNA is sensed by TLR9, which leads not only lead to neutrophil activation, but both act as chemoattractants that lead to neutrophil tissue infiltration homing 42. The ability to sense mitochondrial motifs is not surprising since mitochondria are endosymbionts, and related to microbes in many ways. The second phase of neutrophil activation is dependent on cardiac endothelial cells, Cardiac endothelium is activated by proinflammatory cytokines such as TNF-α, IL-1β and histamine which activate endothelium and induce upregulation of adhesion molecules allowing for neutrophils transmigration between and through endothelial cells to reach the site of tissue injury 51, 59. Detailed imaging studies in other organ systems have helped our understanding of how neutrophils enter damaged tissue. Following necrotic tissue injury in the liver, neutrophils adhere to more remote sites, where viable tissue is present. Initially, migration is dependent on chemokines and subsequently neutrophils follow necrotic signals, such as liberated intracellular ATP - also a DAMP - in order to precisely home to the site of tissue injury 60. The reason neutrophils (and perhaps other recruited cells) take this more convoluted path is that necrotic tissue is usually non- (or under) perfused, and transendothelial migration at the site of injury is not possible. Unfortunately, such detailed imaging studies have yet to be performed in the injured beating heart, however techniques to image are being developed 61. It would also be highly informative to understand the spatial dynamics of resident MΦs, recruited neutrophils and monocytes during the evolution of ischemic cardiac injury.
An important early mediator of tissue injury appears to be IL-6, which is produced in an autocrine fashion by cardiomyocytes and recruited myeloid cells (both neutrophils and macrophages) 62, 63. IL-6 upregulates ICAM-1 on cardiomyocytes, whose expression induces neutrophil binding and stimulates cytotoxic activity 64–66. The role of neutrophils following cardiac injury may be more pronounced when significant areas of the myocardium are at risk of cell death (but have not yet died), such as following ischemia-reperfusion injury. Neutrophils produce numerous proteases which contribute to injury and blockade of neutrophil recruitment appears to be most effective during more limited episodes of ischemia, rather than during longer episodes where cardiomyocyte death is largely secondary to anoxia and nutrient deprivation 67–69.
Circulating monocytes and tissue macrophages - rediscovered
The dominant view for the last half century has been that bone marrow derived hematopoietic stem cells (HSC)s produce circulating blood monocytes which enter into tissue and become tissue macrophages 70. However, in the last few years a series of more definitive publications have drastically revised our understanding of monocyte and MΦ origin by demonstrating that many resident tissue MΦs are established during embryonic development, and are maintained through self-renewal, rather than through blood monocyte input 13, 71–76. Given the importance of monocytes and MΦs to cardiovascular disease, we will review recent insights into the origin of functions of these subsets, and how they contribute to cardiac tissue injury and repair.
There are two principle subsets of circulating monocytes in mice (Ly6cHi and Lyc6Low). Ly6c+ monocyte progenitors give rise to Ly6cHi monocytes, and through an Nr4a1-dependent transcriptional program, Ly6cHi monocytes differentiate into Ly6cLow monocytes 72, 77, 78. Global transcriptional profiling has revealed these subsets are conserved in humans 79. Not only do cell surface markers differ between monocyte subsets, but these subsets have very different roles. Ly6cLow monocytes adhere to and move along the endothelium, both clearing damaged cells and trigger inflammatory responses without entering tissue 80, 81. In the setting of cardiac stress, Ly6cHi monocytes are the primary subset recruited in the heart, either following ischemic injury or hypertensive stress, while Ly6cLow monocytes do not appear to be directly recruited into the myocardium 73, 74, 76, 82, 83. Monocytes recruited into ischemic myocardium are found in the blood, but the spleen also represents a monocyte reservoir that can be utilized when blood and bone marrow stores are insufficient 84, 85. Monocyte recruitment appears to be dependent on innate B cells, which are also recruited to the ischemic myocardium, and drive monocyte expansion through a CCL7-dependent fashion 86. Many studies have examined the role of monocytes and MΦs and analyzed them a single cell population, but as we will describe below – these subsets represent different ontological lineages, and those differences have important functional implications.
Using genetic fate mapping, parabiosis and adoptive transplant studies, resident cardiac MΦs have recently been defined in much more detail. Rather than a single homogenous population, resident cardiac MΦs are composed of three discrete subsets, with different origins and functions (Fig 3A) 74. These three MΦ subsets are defined by two cell surface markers [major histocompatibility class II (MHC-II) and C-C chemokine receptor 2 (CCR2)]. MHC-IIHi and MHC-IILow cardiac MΦs are both CCR2−, numerically are the dominant subsets, and are derived primarily from embryonic progenitors, but also contain adult-monocyte-derived MΦs 74. These embryonic-derived subsets originate both from both primitive yolk sac precursors and fetal monocytes, and renew in situ. MHC-IIHi and MHC-IILow cardiac MΦs represent two transcriptionally distinct subsets, yet each are comprise of a complex coexistence of MΦs from multiple lineages 74. Immediately after birth, MHC-IILow MΦs are the primary subset, and MHC-IIHi MΦs develop from MHC-IILow, as well as monocyte recruitment. After birth there is some dilution of embryonic-derived MΦs by recruited monocyte-derived MΦs in the heart, however in adult animals (20 weeks old), the majority of resident cardiac MΦs remain of embryonic origin 74, 87.
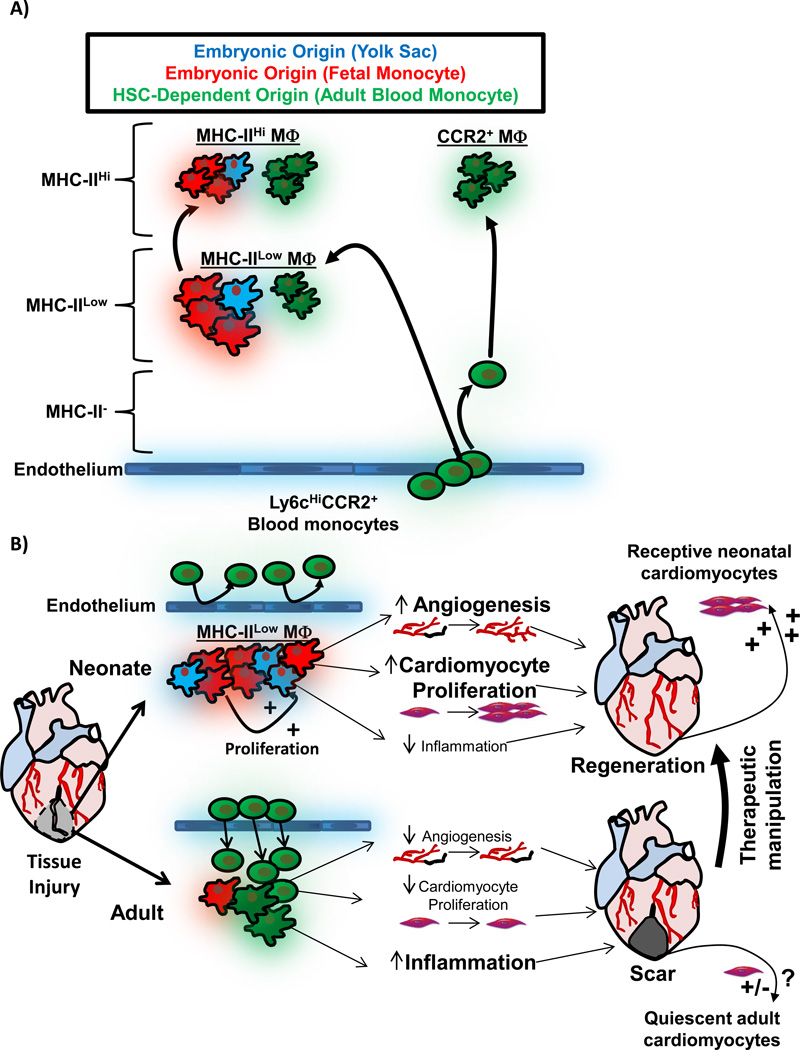
Figure 3. Role of embryonic-derived MΦs during tissue injury and repair.
A) Schematic depicting cardiac MΦ origins. During embryonic development, initially extra-embryonic yolk-sac derived MΦs seed the developing heart (blue), and expressed low levels of MHC-II. Later during development, fetal-monocyte-derived MΦs also infiltrate the heart (red), and both embryonic MΦ populations expand by proliferation during development and after birth. MHC-IIHi MΦs develop from MHC-IILow MΦs after weaning 74, 87. During this time, HSC-derived monocytes infiltrate the heart and also differentiate into MHC-IIHi and MHC-IILow MΦs (green). MHC-IIHi and MHC-IILow MΦ live as ontologically mixed groups, primarily made up of embryonic-derived MΦs 74. Monocytes infiltrate the heart and become shorter lived CCR2+ MΦs, which are entirely derived from blood monocytes 74. B) Comparison of the MΦ response to tissue injury in neonatal animals (which regenerate cardiac tissue) and adult animals (minimal regeneration). In the neonate, resident embryonic-derived populations expand without significant monocyte input. Embryonic-derived MΦs promote angiogenesis, cardiomyocyte proliferation and produce minimal inflammation when stimulated by DAMPs, which together facilitate cardiac regeneration 9. In injury in the adult, large numbers expansion of CCR2+ monocytes and MΦs, which possess a limited ability to promote angiogenesis and cardiomyocyte expansion, but have significant capacity to drive inflammatory responses, which impedes regeneration 9, 74. Importantly, neonatal cardiomyocyte are primed to divide and therefore "receptive" to regenerative signals 8, 103, 104. Adult cardiomyocytes are much less receptive to regenerative signals, and growth signals from embryonic-derived cardiac MΦs are but one important component to enhancing cardiac regeneration in adult tissues. To exploit this understanding therapeutically, the goal would be model the neonatal response to injury (embryonic-derived MΦs and receptive cardiomyocytes) in the adult.
The third cardiac MΦ subset is made up of CCR2+ MΦs, which are derived from, and slowly replenished by circulating blood monocytes. Detailed studies during hypertensive stress indicate that embryonic-derived MΦs expand solely through in situ proliferation, while monocyte-derived MΦs require monocyte input prior to proliferative expansion in tissue 74. CCR2+ MΦs are enriched in NLPR3 inflammasome genes, which are required to process and deliver IL-1β to the heart during cardiac stress 74. Previously it has been demonstrated that inflammasome activation promotes adverse cardiac remodeling following ischemic injury, genetic cardiac hypertrophy and hypertensive cardiac disease, and it may be that CCR2+ MΦs are an important contributor to inflammasome activation irrespective of the mechanism of cardiac injury 29, 88, 89. Recruited CCR2+ MΦs and their robust pro-inflammatory signature likely evolved as a mechanism to control invading pathogen expansion, which is activated inappropriately during sterile inflammatory responses 90, 91. One of the interesting questions in the setting of cardiac tissue injury is trying to understand what role recruited monocytes and monocytes-derived MΦs play, since the ability to separate monocyte-derived MΦs and embryonic-derived MΦs has only recently been developed.
Using non-selective depletion strategies that target all monocyte and MΦ subsets has revealed that in the absence of both monocytes and MΦs, scar formation is impaired after cardiac ischemic injury, with decreased collagen production, decreased angiogenesis and increased mortality due to myocardial rupture 92, 93. Alternatively, increased MΦ expansion in ApoE deficient mice suggests that excessive MΦ expansion also impairs infarct healing, leading to excessive inflammation and impaired cardiac function 23. Models of monocyte ablation (Ccr2−/− mice), which lack circulating monocytes, or impaired monocyte function indicate monocyte recruitment and the resultant inflammatory response leads to pathology 94–96. One simple interpretation of these data is that either too many or too few monocytes / MΦs impair infarct healing. However given our new understanding that cardiac embryonic-derived resident MΦs exist, some clarity emerges about studies that produce seemingly paradoxical findings. For example, studies which target Ly6cHi monocytes (Ccr2−/− mice), are in fact targeting adult monocyte-derived cells, but leaving the embryonic MΦs untouched. Loss of Ly6cHi monocytes prevents hypertension induced cardiac fibrosis and improves LV function after myocardial infarction, suggesting recruited monocytes play a pathological role in the setting sterile injury, as long as resident, embryonic-derived MΦs are not targeted 82, 92, 97.
Interestingly, the neonatal heart has a remarkable capacity to regenerate in response to multiple forms of tissue injury - and the regenerative process is lost when resident, embryonic-derived MΦs are eliminated 7–9. In fact, the neonatal heart expands resident embryonic MΦ populations, rather than recruits monocytes, which appears to be a fundamental difference between neonatal and adult hearts. Neonatal cardiac MΦs promote endothelial cell activation and cardiomyocyte growth, and generate minimal inflammation after stimulation through TLR and inflammasome pathways 9. These data suggest that that the neonatal heart avoids excessive inflammatory responses generated in the adult, which may be a critical factor that aids the regenerative process (Fig 3B). The generalized immunosuppressive nature of neonatal animals may be related to their limited ability to recruit monocytes, and while the reliance on embryonic-derived MΦs for growth and regeneration after injury is clearly beneficial, it comes at the cost of being susceptible to infections 98.
Embryonic-enriched MΦ subsets (MHC-IIHi and MHC-IILow) are efficient at internalizing debris and engulfing apoptotic cardiomyocytes, suggesting important homeostatic roles that are reminiscent embryonic MΦ function during development, and could suggest some of these functions are “hard-wired” into the adult 21, 22, 74. The uptake of dead / dying cells is an important function in the setting ischemic injury. The Mer tyrosine kinase (MerTK) – a phagocytic receptor (and highly specific marker of tissue macrophages) is upregulated on cardiac MΦ after myocardial infarction and loss of MerTK leads to accumulation of apoptotic cardiomyocytes, increased neutrophil persistence and decreased levels of the anti-inflammatory cytokine IL-10 in the myocardium indicative of ongoing inflammation, which ultimately results in decreased cardiac function 99, 100. The act of phagocytosing apoptotic cells (efferocytosis) in skeletal muscle results in their transition to a more anti-inflammatory state through the intracellular signaling kinase AMPK, which is associated with downregulation of proinflammatory genes (i.e. TNF-α) and upregulation of anti-inflammatory genes (i.e. IL-10) 101, 102 (Fig 3B). ). Therefore, impaired phagocytic clearance of apoptotic debris can act increase inflammation through a feed-forward mechanism. Macrophages that cannot phagocytose dying cells secrete excessive pro-inflammatory cytokines , while excess apoptotic cells accumulate leading to secondary necrosis, which itself further stimulates MΦ activation through DAMP signaling, thereby perpetuating inflammation.
Together, these data suggest strategies that spare resident MΦs may yield therapeutic benefit. Given the new found heterogeneity of cardiac MΦs, it would be interesting to determine how ontologically distinct macrophage subsets behave. How do embryonically derived cardiac MΦs promote regeneration? Can you instruct an infiltrating monocyte-derived MΦ to behave in a fashion similar to resident, embryonic-derived MΦs? Given that MΦs during development promote tissue growth and differentiation, are these functions "hard-wired" and retained in adult hearts 21, 22. Alternatively, given that adult-derived CCR2+ MΦs are enriched in inflammatory genes and are dependent on blood monocytes - can strategies be developed to exclusively target monocyte expansion only, while leaving resident MΦs untouched prevent injury? While embryonic-derived cardiac MΦs are a critical for triggering cardiomyocyte growth after injury in the neonate, on their own, they may not be sufficient to trigger a robust proliferative program in relatively quiescent adult cardiomyocytes, as neonatal cardiomyocytes loss are much more receptive to growth signals 8, 103, 104.
Neutrophils, monocytes and macrophages - Intertwined inflammatory amplification and de-amplification
Neutrophils and recruited monocytes may act within an amplification loop to both trigger synergistic inflammation early after cardiac tissue injury and promote rapid resolution of inflammation. Imaging studies have revealed following ischemic injury in lung tissue, very early recruited Ly6cHi monocytes interact with and activate neutrophils - a process required for neutrophil transendothelial migration 105 (Fig 3A). Once recruited, neutrophils propagate inflammation through the release of preformed mediators that induce the recruitment of monocytes (proteoglycans, cathepsin G), release of neutrophil proteases that digest monocyte chemokines (which can serve to attenuate or enhance chemokine activity) or in the correct context, the direct release of chemokines themselves (as reviewed in 106).
Monocytes and MΦs also control neutrophil numbers through at least two mechanisms. The first involves production of neutrophils in the bone through the action of IL-17a, which itself is regulated by IL-23 107. Increased levels of apoptotic tissue neutrophils are sensed by tissues MΦs, and by phagocytosis apoptotic neutrophils, tissue MΦs decrease their own secretion of IL-23, which decreases IL-17a levels, and thereby decrease neutrophil production 107–109. Neutrophil apoptosis in tissue is regulated by a number of factors including reactive oxygen species, TNF-α, Bcl-2 and FasL 110–112. IL-23 appears to act on innate γδ T cells, which are an important source of IL-17a production 107. Blockade of IL-17a improves LV function, decreases neutrophil recruitment and cardiomyocyte apoptosis 113, highlighting not only the important role IL-17a plays, but how production of IL-17a acts as a integration node for multiple cell types and pathways (Fig 2B).
In inflammation, apoptotic neutrophils release chemotactic cues that attract monocytes and MΦs 106. In addition to reduced IL-23 production, MΦs that have ingested neutrophils also increase production of anti-inflammatory cytokines and decrease production of pro-inflammatory cytokines such as IL-1β and TNF-α 114, 115. Following ischemic injury, if all cardiac MΦs are absent, or they if they lack the phagocytic receptor MerTK, there is neutrophil persistence within the infarcted tissue and ongoing inflammation, suggesting phagocytosis of neutrophils and likely other necrotic elements by resident and/or recruited MΦs is critical to limit inflammation and promote wound healing 74, 92, 99. The specific contributions from either embryonic-derived cardiac MΦs or recruited monocytes / MΦs to the maintenance of tissue homeostasis in the heart is not currently known.
Myocarditis as a Model of Cardiac Inflammation
Myocarditis is an excellent model of dissecting inflammatory processes in the heart. It typifies the balance of innate and acquired immune mechanisms in response to injury, and in turn determines the outcomes of progression to heart failure versus repair and regeneration. Myocarditis is most often induced by infection with viruses, although other infectious pathogens can also be involved, such as bacteria or the protozoa Trypanosoma cruzii (causing Chagas’ disease, the commonest cause of heart failure in South America) 116, 117. During infection, PAMP production triggers innate immune response through pattern recognition receptors. Myocarditis can also result from non-infectious triggers produced by DAMPs that activate the innate immune response in the susceptible individual leading to sterile inflammation. These can follow cardiac injury stimuli such as myocardial infarction, cardiac surgery, allergic reactions to drugs or chemicals or excessive stress, and can be mimicked in the laboratory by exposure of intracellular proteins such as myosin in the presence of Freund’s adjuvant 118.
Clinically, myocarditis accounts for about 1 in 9 cases of heart failure of non-ischemic etiology, and remains one of the most common reasons for heart transplantation worldwide 119. Currently there are no specific treatments for viral myocarditis, except for generalized supportive therapy. However, the pathophysiological underpinnings of myocarditis profile the interplay amongst the different immune signaling pathways in the heart, and can help to guide personalized treatment strategies 120.
The commonest viruses which are known to cause myocarditis include enteroviruses such as Coxsackievirus B3/4 strains and adenoviruses, with a periodicity to its prevalence in the population, possibly related to herd immunity. European studies also demonstrate a significant contribution of Parvovirus 19, with chronic tropism for endothelial cells and the bone marrow 121. The coxsackieviruses and adenoviruses target the host tissue, including the immune, cardiovascular and neurological systems through the internalizing coxsackie-adeno receptor (CAR), an immune regulated tight junction protein expressed in the target tissues 122, 123. The internalization is assisted by its co-receptor, decay accelerating factor or CD55, that also inhibits complement activation 124. However, while viral entry and subsequent proliferation trigger disease, they are not necessarily the primary determinants of disease outcomes. Both the innate and adaptive immune responses play critical roles in progression viral myocarditis, evidenced by findings that genetic deletion of innate immune toll-receptor intermediates such as MyD88 or IRAK4, or T-cell receptor tyrosine kinase p56lck, can all ameliorate myocardial inflammation and survival despite viral proliferation 50, 125–127.
Following viral entry into the target cell, such as an immune cell or the myocyte, the virus can engage intracellular NLR’s including RIG-I and MDA5. The endosomal degradation of the virus can lead to activation of TLR3 and TLR7s, with the participation of their downstream signals in the host inflammatory response 48, 50. The TLR signal cascade activation, when exuberant, can have detrimental consequences for the host.
Genetic deletion of TLR adaptor MyD88 or its downstream tyrosine kinase IRAK4, following exposure to CVB3 viral infection, surprisingly showed a very significant protective benefit for the host. This is accomplished by at least 4 different mechanisms: (1) reduction in MyD88-IRAK4 signaling reduced downstream activation of TRAF6, and nuclear translocation of the NF-kB complexes leading to reduced cytokine production and T-cell activation 128; (2) paradoxical increases of IRF3 or IRF5 homodimerization and stat1/stat5 phosphorylation, leading to increased production of protective type I interferons 50, 127; (3) IRAK4 deletion also facilitated the mobilization of protective CCR5+ macrophages from the bone marrow into the myocardium 50; (4) down regulation of the CAR receptor and decreased viral proliferation. Conversely, the genetic deletion of IRF3, of the TRIF or MyD88 independent TLR pathway, leads to much worse outcome with increased mortality and viral proliferation. Mechanistically, this involves decreased type I interferon production while conversely activation of NF-kB translocation and downstream pro-inflammatory signals are increased 129.
These observations have several important implications. The first is that viral receptor number and the degree of viral proliferation are paradoxically facilitated by the host innate immune response – likely an evolutionarily selected advantage for the pathogenic virus at the expense of the host. The second is that the intracellular pathways of innate immune signaling downstream to the TLR or NLR engage in significant cross talk, such that down regulation of IRAK4 signaling up regulates IRF3/IRF5 mediated type I interferon production; conversely IRF3 signaling counter regulates NF-kB activity. The third is that intracellular immune signaling appears to also regulate trafficking of inflammatory cells from the bone marrow, such as the CCR5+ macrophages. Indeed the latter is also the case in sterile inflammation post myocardial infarction, where IRAK4 appears to regulate the trafficking and maturation of dendritic cells into the myocardium, with subsequent orchestration of host inflammatory responses that dictate remodeling and host survival.
The activation of innate immune signaling pathways also sets the stage for the acquired immune T-cell maturation and participation in the host inflammatory response. Genetic deletion studies of the T-cell receptor tyrosine kinase p56lck, where T-cell maturation is impaired, demonstrated almost complete protection of the host against Coxsackievirus B3 infection 125. This is mimicked partially by CD4/CD8 subset deletion 130. This is also replicated by the general leukocyte tyrosine phosphatase CD45 deletion 126. Interestingly, in many of these models, there was a significant reduction in viral proliferation and up regulation of type I interferons. Meanwhile the T regulatory cell subset is usually produced in limited numbers following infection. However, isolated external T regulatory cell expansion and adoptive transfer to infected hosts demonstrated a very significant protective effect with again a decrease in viral proliferation and up regulation of type I interferons 131. Surprisingly there was a general down regulation of TLR related signaling pathways, including MyD88, IRAK, NF-kB and even TLR4 itself (see summary of protective and detrimental pathways during viral myocarditis – Fig 4). Moreover, beyond their role during infections, regulatory T cell also promote recovery via an IL-10 pathway following ischemic injury 132–134. These data suggest that there is close cross-talk between the T regulatory subsets and innate immune signaling pathways during both infectious and sterile injury.
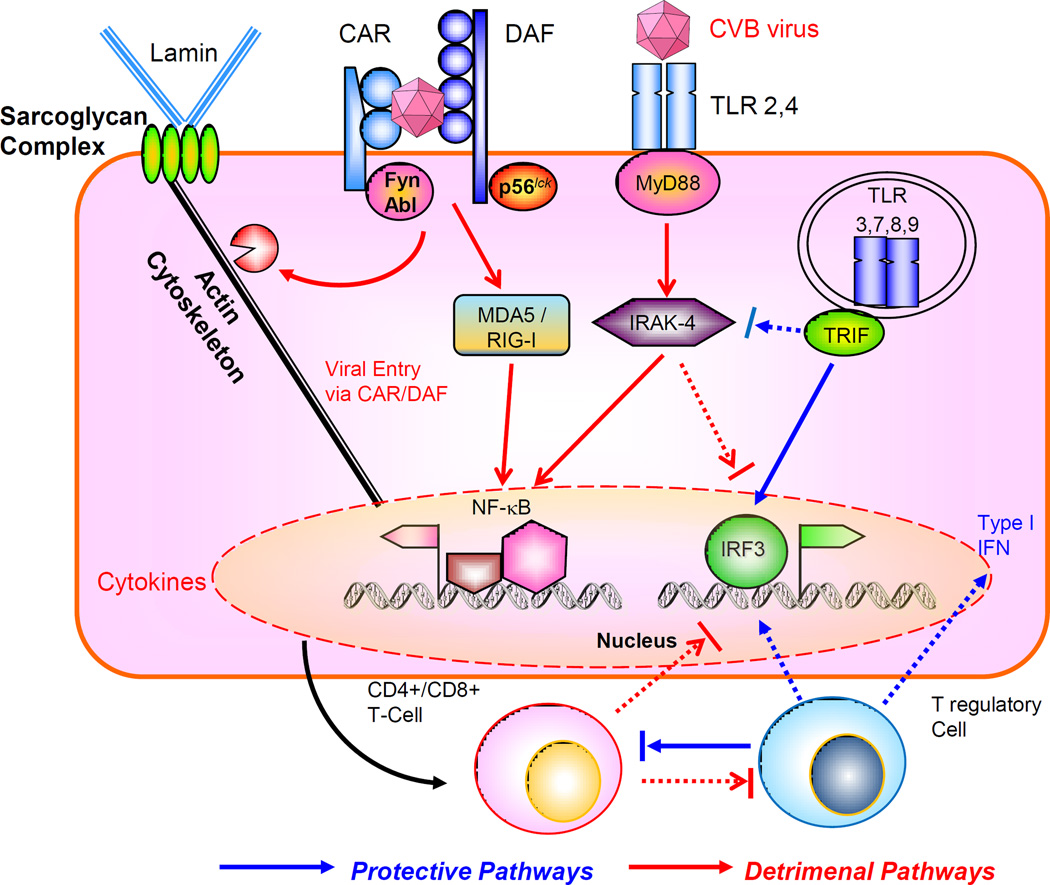
Figure 4. Interaction of Coxsackievirus B (CVB) with the host innate and acquired immune system.
The virus engages the internalizing receptor Coxsackie-Adeno Receptor (CAR) whose tyrosine kinases Fyn and Abl can facilitate viral remodeling of cytoskeleton to gain entry, further aided by co-receptor decay accelerating factor (DAF), associated with tyrosine kinase p56lck. The viral components can interact with MDA5/RIG-I to activate NF-kB, or engage the cell surface toll-like receptors (TLR) through adaptor MyD88 and downstream signal intermediates such as IRAK-4 and TRAF6. These pathways facilitate viral proliferation and host immune tissue damage. On the other hand, activation of TRIF-IRF3/IRF7 pathway leading to type I interferon (IFN) production is protective. There is mutual counter-regulation of the MyD88 –IRAK4 and TRIF-IRF3 pathways. The subsequent maturation of CD4+ / CD8+ T cell subsets is also detrimental for the host, in contrast to the T regulatory cells which are host-protective. Cross talk between acquired and innate immune signaling pathways modulate the host response to viral infection.
The above progress in understanding the myocarditic processes has moved the field forward from the earlier failed effort in treating patients with biopsy proven myocarditis with broad immunosuppressive regimen, demonstrating no net benefit 135. Subsequently, a Phase II trial has demonstrated that in patients with persistent viral proliferation, intravenous interferon has benefits in clearing the virus, and improving symptoms 136. Targeted immunosuppressive therapy may be indicated for patients with persistent immune activation where the viral proliferation phase has already passed. Nevertheless, there are still major gaps in knowledge, including why there are periodic enteroviral outbreaks in the population, what are the susceptibility factors predisposing some patients to develop several viral myocarditis requiring transplantation, vs. those who are able to rebalance the immune response and promptly recover, are there biomarkers to permit one to predict an individual patient’s susceptibility, and for those at risk, is vaccination a viable option?
Future directions
The ultimate goal in terms of understanding how the immune system directs inflammatory and reparative programs following cardiac injury is the development of therapeutic strategies that promote tissue regeneration and repair. Evolutionary pressures drove both the development of primitive phagocytic cells to promote tissue growth during development and wound healing, but also drove the development of innate / adaptive immune subsets and pathways that promote survival of the host in the face of infectious threats. Adaptations to both enhance pathogen clearance, as well as promote tissue regeneration/repair may result in a zero-sum game, whereby the gains in host-defense are counterbalanced by the loss of regenerative potential. Within this context, an important recent advancement has been the discovery of ontologically distinct cardiac MΦ subsets and the pathways that they control. The ability to selectively trigger germ-line encoded reparative programs in resident cardiac immune cells represents a novel approach to modulate tissue damage and repair in patients with a wide range of cardiovascular diseases.
Reference List
- 1.Bier E, Bodmer R. Drosophila, an emerging model for cardiac disease. Gene. 2004;342:1–11. doi: 10.1016/j.gene.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, et al. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 5.Laube F, Heister M, Scholz C, Borchardt T, Braun T. Re-programming of newt cardiomyocytes is induced by tissue regeneration. J. Cell Sci. 2006;119:4719–4729. doi: 10.1242/jcs.03252. [DOI] [PubMed] [Google Scholar]
- 6.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aurora AB, et al. Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavine K, et al. Distinct Macrophage Lineages Contribute to Disparate Patterns of Cardiac Recovery and Remodeling in the Neonatal and Adult Heart. Proc. Natl. Acad. Sci. 2014 doi: 10.1073/pnas.1406508111. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Tauber AI. Metchnikoff and the phagocytosis theory. Nat. Rev. Mol. Cell Biol. 2003;4:897–901. doi: 10.1038/nrm1244. [DOI] [PubMed] [Google Scholar]
- 12.Epelman S, Lavine KJ, Randolph GJ. Origin and Functions of Tissue Macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 14.Dai XM, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 15.McKercher SR, et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- 16.Wiktor-Jedrzejczak W, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nucera S, Biziato D, De PM. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int. J. Dev. Biol. 2011;55:495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- 18.Fantin A, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold T, Betsholtz C. The importance of microglia in the development of the vasculature in the central nervous system. Vasc. Cell. 2013;5:4. doi: 10.1186/2045-824X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lobov IB, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munoz-Espin D, et al. Programmed Cell Senescence during Mammalian Embryonic Development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Storer M, et al. Senescence Is a Developmental Mechanism that Contributes to Embryonic Growth and Patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 23.Panizzi P, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J. Am. Coll. Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacoby D, McKenna WJ. Genetics of inherited cardiomyopathy. Eur. Heart J. 2012;33:296–304. doi: 10.1093/eurheartj/ehr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sangiuliano B, Perez NM, Moreira DF, Belizario JE. Cell death-associated molecular-pattern molecules: inflammatory signaling and control. Mediators. Inflamm. 2014;2014:821043. doi: 10.1155/2014/821043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 27.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Mann DL, Topkara VK, Evans S, Barger PM. Innate immunity in the adult Mammalian heart: for whom the cell tolls. Trans. Am. Clin Climatol. Assoc. 2010;121:34–50. [PMC free article] [PubMed] [Google Scholar]
- 29.Mezzaroma E, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl. Acad. Sci. U. S. A. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol. Pharm. Bull. 2005;28:886–892. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- 33.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor-kappa B by oxidative stress in cardiac myocytes. J. Biol. Chem. 2001;276:5197–5203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- 34.Frantz S, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J. Clin. Invest. 1999;104:271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birks EJ, et al. Increased toll-like receptor 4 in the myocardium of patients requiring left ventricular assist devices. J. Heart Lung Transplant. 2004;23:228–235. doi: 10.1016/S1053-2498(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 36.Kashiwagi M, et al. Differential expression of Toll-like receptor 4 and human monocyte subsets in acute myocardial infarction. Atherosclerosis. 2012;221:249–253. doi: 10.1016/j.atherosclerosis.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 37.Oyama J, et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 38.Tavener SA, et al. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ. Res. 2004;95:700–707. doi: 10.1161/01.RES.0000144175.70140.8c. [DOI] [PubMed] [Google Scholar]
- 39.Fallach R, et al. Cardiomyocyte Toll-like receptor 4 is involved in heart dysfunction following septic shock or myocardial ischemia. J. Mol. Cell Cardiol. 2010;48:1236–1244. doi: 10.1016/j.yjmcc.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Binck BW, et al. Bone marrow-derived cells contribute to contractile dysfunction in endotoxic shock. Am. J. Physiol Heart Circ. Physiol. 2005;288:H577–H583. doi: 10.1152/ajpheart.00745.2004. [DOI] [PubMed] [Google Scholar]
- 41.Arslan F, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 42.Oka T, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012 doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lech M, et al. Quantitative expression of C-type lectin receptors in humans and mice. Int. J. Mol. Sci. 2012;13:10113–10131. doi: 10.3390/ijms130810113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geddes K, Magalhaes JG, Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nat. Rev. Drug Discov. 2009;8:465–479. doi: 10.1038/nrd2783. [DOI] [PubMed] [Google Scholar]
- 45.Mezzaroma E, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl. Acad. Sci. U. S. A. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawaguchi M, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 47.Kono H, Kimura Y, Latz E. Inflammasome activation in response to dead cells and their metabolites. Curr. Opin. Immunol. 2014;30C:91–98. doi: 10.1016/j.coi.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 48.McCartney SA, et al. RNA sensor-induced type I IFN prevents diabetes caused by a beta cell-tropic virus in mice. J. Clin. Invest. 2011;121:1497–1507. doi: 10.1172/JCI44005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philip J, Xu Z, Bowles NE, Vallejo JG. Cardiac-specific overexpression of melanoma differentiation-associated gene-5 protects mice from lethal viral myocarditis. Circ. Heart Fail. 2013;6:326–334. doi: 10.1161/CIRCHEARTFAILURE.112.969402. [DOI] [PubMed] [Google Scholar]
- 50.Valaperti A, et al. Innate immune interleukin-1 receptor-associated kinase 4 exacerbates viral myocarditis by reducing CCR5(+) CD11b(+) monocyte migration and impairing interferon production. Circulation. 2013;128:1542–1554. doi: 10.1161/CIRCULATIONAHA.113.002275. [DOI] [PubMed] [Google Scholar]
- 51.Frangogiannis NG, et al. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 52.Chakraborti T, Mandal A, Mandal M, Das S, Chakraborti S. Complement activation in heart diseases. Role of oxidants. Cell Signal. 2000;12:607–617. doi: 10.1016/s0898-6568(00)00111-x. [DOI] [PubMed] [Google Scholar]
- 53.Foreman KE, Glovsky MM, Warner RL, Horvath SJ, Ward PA. Comparative effect of C3a and C5a on adhesion molecule expression on neutrophils and endothelial cells. Inflammation. 1996;20:1–9. doi: 10.1007/BF01487740. [DOI] [PubMed] [Google Scholar]
- 54.Bhattacharya K, et al. Mast cell deficient W/Wv mice have lower serum IL-6 and less cardiac tissue necrosis than their normal littermates following myocardial ischemia-reperfusion. Int. J. Immunopathol. Pharmacol. 2007;20:69–74. doi: 10.1177/039463200702000108. [DOI] [PubMed] [Google Scholar]
- 55.Ayach BB, et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2304–2309. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waskow C, Paul S, Haller C, Gassmann M, Rodewald HR. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity. 2002;17:277–288. doi: 10.1016/s1074-7613(02)00386-2. [DOI] [PubMed] [Google Scholar]
- 57.Dreyer WJ, et al. Kinetics of C5a release in cardiac lymph of dogs experiencing coronary artery ischemia-reperfusion injury. Circ. Res. 1992;71:1518–1524. doi: 10.1161/01.res.71.6.1518. [DOI] [PubMed] [Google Scholar]
- 58.Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin. Hematol. 2013;50:198–206. doi: 10.1053/j.seminhematol.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh M, Saini HK. Resident cardiac mast cells and ischemia-reperfusion injury. J. Cardiovasc. Pharmacol. Ther. 2003;8:135–148. doi: 10.1177/107424840300800207. [DOI] [PubMed] [Google Scholar]
- 60.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 61.Li W, et al. Intravital 2-photon imaging of leukocyte trafficking in beating heart. J. Clin. Invest. 2012;122:2499–2508. doi: 10.1172/JCI62970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gwechenberger M, et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99:546–551. doi: 10.1161/01.cir.99.4.546. [DOI] [PubMed] [Google Scholar]
- 63.Youker K, et al. Neutrophil adherence to isolated adult cardiac myocytes. Induction by cardiac lymph collected during ischemia and reperfusion. J. Clin. Invest. 1992;89:602–609. doi: 10.1172/JCI115626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Entman ML, et al. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J. Clin. Invest. 1992;90:1335–1345. doi: 10.1172/JCI115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Entman ML, et al. Neutrophil adherence to isolated adult canine myocytes. Evidence for a CD18-dependent mechanism. J. Clin. Invest. 1990;85:1497–1506. doi: 10.1172/JCI114596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tyagi S, Klickstein LB, Nicholson-Weller A. C5a-stimulated human neutrophils use a subset of beta2 integrins to support the adhesion-dependent phase of superoxide production. J. Leukoc. Biol. 2000;68:679–686. [PubMed] [Google Scholar]
- 67.Kawakami R, et al. Overexpression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase-9 expression after acute myocardial infarction. Circulation. 2004;110:3306–3312. doi: 10.1161/01.CIR.0000147829.78357.C5. [DOI] [PubMed] [Google Scholar]
- 68.Romson JL, et al. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 69.Jolly SR, et al. Reduction of myocardial infarct size by neutrophil depletion: effect of duration of occlusion. Am. Heart J. 1986;112:682–690. doi: 10.1016/0002-8703(86)90461-8. [DOI] [PubMed] [Google Scholar]
- 70.Van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yona S, et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hashimoto D, et al. Tissue-Resident Macrophages Self-Maintain Locally throughout Adult Life with Minimal Contribution from Circulating Monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Epelman S, et al. Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guilliams M, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jakubzick C, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanna RN, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat. Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hettinger J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat. Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 79.Ingersoll MA, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Auffray C, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 81.Carlin LM, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frangogiannis NG, et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 83.Hilgendorf I, et al. Ly-6Chigh Monocytes Depend on Nr4a1 to Balance both Inflammatory and Reparative Phases in the Infarcted Myocardium. Circ. Res. 2014 doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leuschner F, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ. Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zouggari Y, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Molawi K, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 2014 doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bracey NA, et al. Mitochondrial NLRP3 Protein Induces Reactive Oxygen Species to Promote Smad Protein Signaling and Fibrosis Independent from the Inflammasome. J. Biol. Chem. 2014;289:19571–19584. doi: 10.1074/jbc.M114.550624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bracey NA, et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1beta. Exp. Physiol. 2013;98:462–472. doi: 10.1113/expphysiol.2012.068338. [DOI] [PubMed] [Google Scholar]
- 90.Dunay IR, et al. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim YG, et al. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity. 2011;34:769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nahrendorf M, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am. J. Pathol. 2007;170:818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dewald O, et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 95.Leuschner F, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 97.Zhou L, et al. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ. Res. 2006;98:1177–1185. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marodi L. Neonatal innate immunity to infectious agents. Infect. Immun. 2006;74:1999–2006. doi: 10.1128/IAI.74.4.1999-2006.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wan E, et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ. Res. 2013;113:1004–1012. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gautier EL, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mounier R, et al. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18:251–264. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 102.Arnold L, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naqvi N, et al. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157:795–807. doi: 10.1016/j.cell.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kreisel D, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 107.Stark MA, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 108.Tan W, et al. IL-17F/IL-17R interaction stimulates granulopoiesis in mice. Exp. Hematol. 2008;36:1417–1427. doi: 10.1016/j.exphem.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 109.Schwarzenberger P, et al. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J. Immunol. 2000;164:4783–4789. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- 110.Kobayashi SD, Voyich JM, Braughton KR, DeLeo FR. Down-regulation of proinflammatory capacity during apoptosis in human polymorphonuclear leukocytes. J. Immunol. 2003;170:3357–3368. doi: 10.4049/jimmunol.170.6.3357. [DOI] [PubMed] [Google Scholar]
- 111.Kobayashi SD, et al. An apoptosis-differentiation program in human polymorphonuclear leukocytes facilitates resolution of inflammation. J. Leukoc. Biol. 2003;73:315–322. doi: 10.1189/jlb.1002481. [DOI] [PubMed] [Google Scholar]
- 112.Jonsson H, Allen P, Peng SL. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat. Med. 2005;11:666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- 113.Liao YH, et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J. Am. Coll. Cardiol. 2012;59:420–429. doi: 10.1016/j.jacc.2011.10.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Voll RE, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 116.Coura JR, Borges-Pereira J. Chagas disease: 100 years after its discovery. A systemic review. Acta Trop. 2010;115:5–13. doi: 10.1016/j.actatropica.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 117.Kindermann I, et al. Update on myocarditis. J. Am. Coll. Cardiol. 2012;59:779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 118.Neu N, et al. Cardiac myosin induces myocarditis in genetically predisposed mice. J. Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 119.Sagar S, Liu PP, Cooper LT., Jr Myocarditis. Lancet. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schultheiss HP, Kuhl U, Cooper LT. The management of myocarditis. Eur. Heart J. 2011;32:2616–2625. doi: 10.1093/eurheartj/ehr165. [DOI] [PubMed] [Google Scholar]
- 121.Kuhl U, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with"idiopathic" left ventricular dysfunction. Circulation. 2005;111:887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 122.Martino TA, et al. The coxsackie-adenovirus receptor (CAR) is used by reference strains and clinical isolates representing all six serotypes of coxsackievirus group B and by swine vesicular disease virus. Virology. 2000;271:99–108. doi: 10.1006/viro.2000.0324. [DOI] [PubMed] [Google Scholar]
- 123.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 124.Liu PP, Opavsky MA. Viral myocarditis: receptors that bridge the cardiovascular with the immune system? Circ. Res. 2000;86:253–254. doi: 10.1161/01.res.86.3.253. [DOI] [PubMed] [Google Scholar]
- 125.Liu P, et al. The tyrosine kinase p56lck is essential in coxsackievirus B3-mediated heart disease. Nat. Med. 2000;6:429–434. doi: 10.1038/74689. [DOI] [PubMed] [Google Scholar]
- 126.Irie-Sasaki J, et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–354. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- 127.Riad A, et al. Myeloid differentiation factor-88 contributes to TLR9-mediated modulation of acute coxsackievirus B3-induced myocarditis in vivo. Am. J. Physiol Heart Circ. Physiol. 2010;298:H2024–H2031. doi: 10.1152/ajpheart.01188.2009. [DOI] [PubMed] [Google Scholar]
- 128.Fuse K, et al. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation. 2005;112:2276–2285. doi: 10.1161/CIRCULATIONAHA.105.536433. [DOI] [PubMed] [Google Scholar]
- 129.Holm GH, et al. Interferon regulatory factor 3 attenuates reovirus myocarditis and contributes to viral clearance. J. Virol. 2010;84:6900–6908. doi: 10.1128/JVI.01742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Opavsky MA, et al. Susceptibility to myocarditis is dependent on the response of alphabeta T lymphocytes to coxsackieviral infection. Circ. Res. 1999;85:551–558. doi: 10.1161/01.res.85.6.551. [DOI] [PubMed] [Google Scholar]
- 131.Shi Y, et al. Regulatory T cells protect mice against coxsackievirus-induced myocarditis through the transforming growth factor beta-coxsackie-adenovirus receptor pathway. Circulation. 2010;121:2624–2634. doi: 10.1161/CIRCULATIONAHA.109.893248. [DOI] [PubMed] [Google Scholar]
- 132.Matsumoto K, et al. Regulatory T lymphocytes attenuate myocardial infarction-induced ventricular remodeling in mice. Int. Heart J. 2011;52:382–387. doi: 10.1536/ihj.52.382. [DOI] [PubMed] [Google Scholar]
- 133.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am. J. Pathol. 2010;176:2177–2187. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang Z, et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 135.Mason JW, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N. Engl. J. Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 136.Kuhl U, et al. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]