Abstract
Mast cell proteases are thought to be involved with tumor progression and neo-vascularization. However, their exact role is still unclear. The present study was undertaken to further elucidate the function of specific subtypes of recombinant mouse mast cell proteases (rmMCP-6 and 7) in neo-vascularization. SVEC4-10 cells were cultured on Geltrex® with either rmMCP-6 or 7 and tube formation was analyzed by fluorescence microscopy and scanning electron microscopy. Additionally, the capacity of these proteases to induce the release of angiogenic factors and pro and anti-angiogenic proteins was analyzed. Both rmMCP-6 and 7 were able to stimulate tube formation. Scanning electron microscopy showed that incubation with the proteases induced SVEC4-10 cells to invade the gel matrix. However, the expression and activity of metalloproteases were not altered by incubation with the mast cell proteases. Furthermore, rmMCP-6 and rmMCP-7 were able to induce the differential release of angiogenic factors from the SVEC4-10 cells. rmMCP-7 was more efficient in stimulating tube formation and release of angiogenic factors than rmMCP-6. These results suggest that the subtypes of proteases released by mast cells may influence endothelial cells during in vivo neo-vascularization.
Introduction
Mast cells are connective tissue cells that are involved in allergy, inflammation and host defense [1–5]. The location of the mast cell as well as their ability to produce and release a variety of chemical mediators is essential in the pathophysiology of allergic and inflammatory reactions [6–9]. A number of studies have functionally linked mast cells to tumor angiogenesis [10–14]. Mast cells have been shown to accumulate around several types of tumors and are generally the first inflammatory cells to infiltrate tumors [15, 16]. Preformed mast cell mediators such as heparin, histamine, TNF-α, and bFGF have been shown to stimulate the proliferation of endothelial cells [13, 17–19], thus suggesting that mast cell mediators could be important for blood vessel formation and/or maintenance [20–23]. However, some preformed mast cell mediators are also produced by other cell types such as macrophages, endothelial cells, and fibroblasts, which impedes delineation of the specific role of mast cells in angiogenesis.
The major constituents of mast cell secretory granules are the mast cell specific proteases: chymase, tryptase, and CPA3 (carboxypeptidase A3) [6, 24–29]. The majority of recent investigations on the role of mast cells in tumor angiogenesis have focused on the ability of mast cells to synthesize, store, and release mast cell specific chymases and tryptases. Several these studies have shown that tryptase can act directly or indirectly in the degradation and remodeling of the extracellular matrix during angiogenesis [30, 31]. Zhi and colleagues [32] have shown that tryptase induces cell proliferation, migration, and tube formation in mouse brain endothelial cells, suggesting a role for tryptase in microvessel formation. Furthermore, mMCP-6 (mouse mast cell protease 6) and mMCP-7 (mouse mast cell protease 7), both tryptases, were able to induce spreading and tube formation in SVEC4-10 endothelial cells [33].
The previous results noted that the tryptase subtypes have differing efficiencies in promoting spreading and tube formation, suggesting that they may have different physiological and pathological roles in angiogenesis. The present study was undertaken to further elucidate the mechanisms by which the specific subtypes of mast cell tryptases stimulate endothelial cells during angiogenesis. The current investigation confirms that rmMCP-6 and rmMCP-7 have differing effects on endothelial cells, both in their ability to induce tube formation and in their capacity to release angiogenic factors.
Materials and Methods
Ethics Statement
The research was conducted in accordance with Ethical principles in the use of experimental animals adopted by the Brazilian College of Animal Experimentation. Experimental protocols were approved by the Commission on Ethics on Animal Experimentation of the Ribeirão Preto Medical School (Protocol number 033/2007).
Cell Lines
The murine endothelial cell line SVEC4-10 (CRL-2181) was purchased from the American Type Culture Collection (ATCC; Manassas, VA). The cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM) plus 10% heat inactivated fetal bovine serum (FBS) according to ATCC guidelines. The cells were cultured in a humidified environment containing 5% CO2 in air. All reagents used for cell culture were purchased from Life Technologies (Carlsbad, CA).
Primary Culture of Bone Marrow-derived Murine Mast Cells (BMMC)
Three young (8 to 12 weeks) male BALB/c mice were anesthetized with ketamine 80 mg/kg plus xylazine 12 mg/kg (Sigma-Aldrich, St.Louis, MO). Bone marrow was removed from the femurs and cultured according to Jamur and colleagues [34]. After 21 days in the culture, all the cells were mast cells. These mast cells were used for production of pre-formed mast cell mediators.
Pre-formed Mast Cell Mediators
To obtain pre-formed mast cell mediators [26], BMMC cells were incubated with 0.1 μM calcium ionophore-A23187 (Sigma-Aldrich) for 45 min at 37°C and the supernatant collected and used in tube formation assays. To confirm the release of mediators, the supernatant was analyzed by western blot for mMCP-6.
In vitro angiogenesis—Tube Formation Assay
10μl of Geltrex® (Life Technologies) was added to each well of μ-slides Angiogenesis® (IBIDI, Martinsried, Germany) and allowed to solidify at 37°C for 30 min. After the gel solidified, SVEC4-10 cells (1x104) in 50μl of DMEM supplemented with 10% FBS were added to each well. The cells were incubated at 37°C in a humidified atmosphere (95% air/5% CO2) for 5 h in the presence or absence of rmMCP-6 or 7 (20ng/well; R&D Systems Inc., Minneapolis, MN). In some experiments, the proteases were pre-incubated using antibodies against mMCP-6 and 7 (kindly provided by Dr. Michael F. Gurish, Division of Immunology, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA). The proteases (20ng) were incubated with the respective antibody (1μg) for 30 min before adding them to the cell culture. The antibody concentration was determined using a dose-response curve for blocking tube formation. For fluorescence experiments, the cells were fixed, permeabilized, and then incubated with 2.6 U/ml Phalloidin-Alexa 488 (Life Technologies) for 30 min [35]. Following staining, the samples were washed in PBS and observed with an inverted fluorescence microscope (Nikon Eclipse TE2000-U, Nikon instruments Inc., NY, USA) and images acquired using a Nikon DS-1QM digital camera. The images were acquired using a 10X objective and the measurements are expressed in pixels (800x600) where 1 pixel is equal to 0.069mm2. Tube formation was quantified using WimTube (Wimasis Image Analysis, Munich, Germany).
The parameters [36–39] used for quantification were:
Covered area (%): the area covered by cells that are part of a tubular structure.
Tubes: part of a tubular structure that go from one branching point to another branching point or to a loose end.
Branching points: the points where three or more tubes converge
Loops: enclosed (or almost enclosed) areas inside the tubular structure that fulfill roundness conditions.
In vivo chick embryo chorioallantoic membrane (CAM) assay
Fifteen fertilized eggs were incubated at 37°C with 60% humidity. On day 2 of incubation 3 ml of albumen were removed using a syringe with a 21g needle. A square window was then opened in the eggshell and the window covered with Scotch® Magic™ tape (3M, St. Paul, MN) to prevent dehydration and contamination. At day 8 of incubation, 13 mm Thermanox™ (Thermo Scientific™ Nunc™, Rochester, NY) rings were placed on the CAM under sterile conditions. 100 ng rmMCP-6 or 7 in 20μL of assay buffer (50 nM MES, 1M NaCl, pH 6.5) was placed into the Thermanox™ rings. For controls 20 μL of assay buffer only were placed in the Thermanox™ rings. On day 12 of incubation, the CAM was fixed in situ with 2% paraformaldehyde for 20 min. The Thermanox™ rings with the underlying CAM were cut out and transferred to a petri dish containing PBS. Samples were stained with hematoxylin and eosin. The samples were mounted on glass slides and a minimum of five fields/sample was analyzed with a 4x objective on an Olympus BX-50 microscope (Olympus America Inc., Melville, NY) equipped with a SPOT RT3 digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI).
Scanning Electron Microscopy
Geltrex® was placed on 12-mm-round coverslips coated with Biobond (Electron Microscopy Sciences, Hatfield, PA). After the gel solidified, SVEC4-10 cells (1x104) were added in 50μl of DMEM supplemented with 10% FBS. The cells were incubated at 37°C in a humidified atmosphere (5% CO2 in air) for 5 h in the presence or absence of rmMCP-6 or 7 (20ng; R&D Systems Inc.). The samples were then rinsed in warm PBS (37°C) and fixed in 2% glutaraldehyde (Ladd Research Industries; Burlington, VT) in warm PBS for 2 hours at room temperature. Samples were postfixed in 1% OsO4 (EM Sciences) for 2 hours, rinsed in Milli-Q water, and incubated with a saturated solution of thiocarbohydrazide (EM Sciences), followed by 1% OsO4. This step was repeated once. The samples were dehydrated in a graded ethanol series and critically point-dried with liquid CO2 in a Tousimis Autosandri-810 (Tousimis Research Co., Rockville, MD), mounted on aluminum stubs with silver paint (EM Sciences), and coated with gold in a BAL-TEC SCD 050 Sputter Coater (BAL-TEC AG). Samples were examined with a JEOL JSM-6610LV scanning electron microscope (JEOL, Ltd.; Tokyo, Japan).
Immunoblotting and Zymography
Following the tube formation assay, the culture supernatants were used for immunoblotting to analyze metalloprotease expression and for zymograms to determine metalloprotease activity. The Geltrex® on the μ-slides after the tube formation assay was used to analyze the presence of laminin and collagen IV. The attached cells were removed from the Geltrex® by incubation for 20 min with TrypLE™ Express (Life Technologies). The Geltrex® was recovered using 50μl of lysis buffer. The protein concentration of the sample (Geltrex® plus lysate buffer) was determined using the BCA Protein Assay Kit (Pierce, Thermo Fisher Scientific, Rockford, IL). 10 μg of sample (Geltrex® plus lysate buffer) were boiled for 5 min in 1x SDS sample buffer (50 mM Tris-HCl pH 6.8, 12.5% glycerol, 1% sodium dodecylsulfate, 0.01% bromophenol blue), and applied to 10% polyacrylamide gels. Immunoblotting was performed as previously described [33] using anti-laminin (SC16589; Abcam Cambridge, MA) and anti-collagen IV (ab6586); Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. The secondary antibodies goat anti-rabbit IgG conjugated to HRP and rabbit anti-goat IgG conjugated to HRP were purchased from Jackson ImmunoResearch (West Grove, PA).
The supernatants from the tube formation assays were used for zymography. The supernatants were centrifuged at 2800 g at 4°C for 30 min. The protein concentration of the supernatants and lysates were determinate using the BCA Protein Assay. Quantitative gelatinolytic zymography was done using the method of Leber and Balkwill [40]. The samples were subjected to electrophoresis under nonreducing conditions on 7.5% SDS-polyacrylamide gels copolymerized with 2 g/L porcine skin gelatin (Sigma Aldrich). After electrophoresis, gels were washed in 2.5% Triton-X 100 (Sigma Aldrich) with agitation and then incubated for at least 24 hours at 37°C in enzyme incubation buffer (50 mM Tris-HCl, pH 7.5, containing 5 mM CaCl2, 100 mM NaCl, 0.01% Triton X-100, 0.1 mM ZnCl2, 0.2% Brij (Sigma Aldrich), and 0.002% NaN3). Gels were stained in 0.2% Coomassie Brilliant Blue R-250 (Sigma Aldrich) and then destained in water. The optical density of the bands was determined using Adobe Photoshop (Adobe Systems, San Jose, CA).
Expression Profile of Angiogenesis Related Proteins
The expression profile of angiogenesis-related proteins was analyzed using the Proteome Profiler™ Mouse Angiogenesis Antibody Array (R&D Systems). After the tube formation assay, the supernatants were collected and processed according to the manufacturer’s instructions. Briefly, supernatants were mixed with a cocktail of biotinylated detection antibodies and then incubated with the membrane containing immobilized angiogenesis-related antibodies. Bound protein was detected with streptavidin conjugated to HRP. Membranes were washed and developed using ECL™ Western Blotting Detection Reagent RPN2106 (GE Healthcare, Piscataway, NJ). Supernatants from tube formation assays performed without proteases served as controls.
Statistical Analysis
Values are expressed as the mean ± SD. Student’s t-test was used to compare data. p-values of ≤0.05 were considered significant. The data is expressed as mean ± SD from three independent experiments.
Results
Pre-formed mast cell mediators induced tube formation
In order to confirm that mast cell mediators stimulate tube formation, the ability of the released pre-formed mast cell mediators to induce tube formation was tested. Bone marrow derived mast cells were stimulated with calcium ionophore A23187 and SVEC4-10 cells cultured with the medium containing the released mast cell mediators. The mast cell mediators were highly efficient in stimulating tube and loop formation (Fig 1A) in SVEC4-10 cells when compared to the SVEC4-10 cells cultured in the absence of mast cell mediators (Fig 1B). Quantitative analysis confirmed the data obtained by fluorescence microscopy (Fig 1C), suggesting that pre-formed mast cell mediators, including tryptases, can stimulate in vitro angiogenesis.
Fig 1. In the presence of pre-formed mast cell mediators, SVEC4-10 cells form tubes and loops.
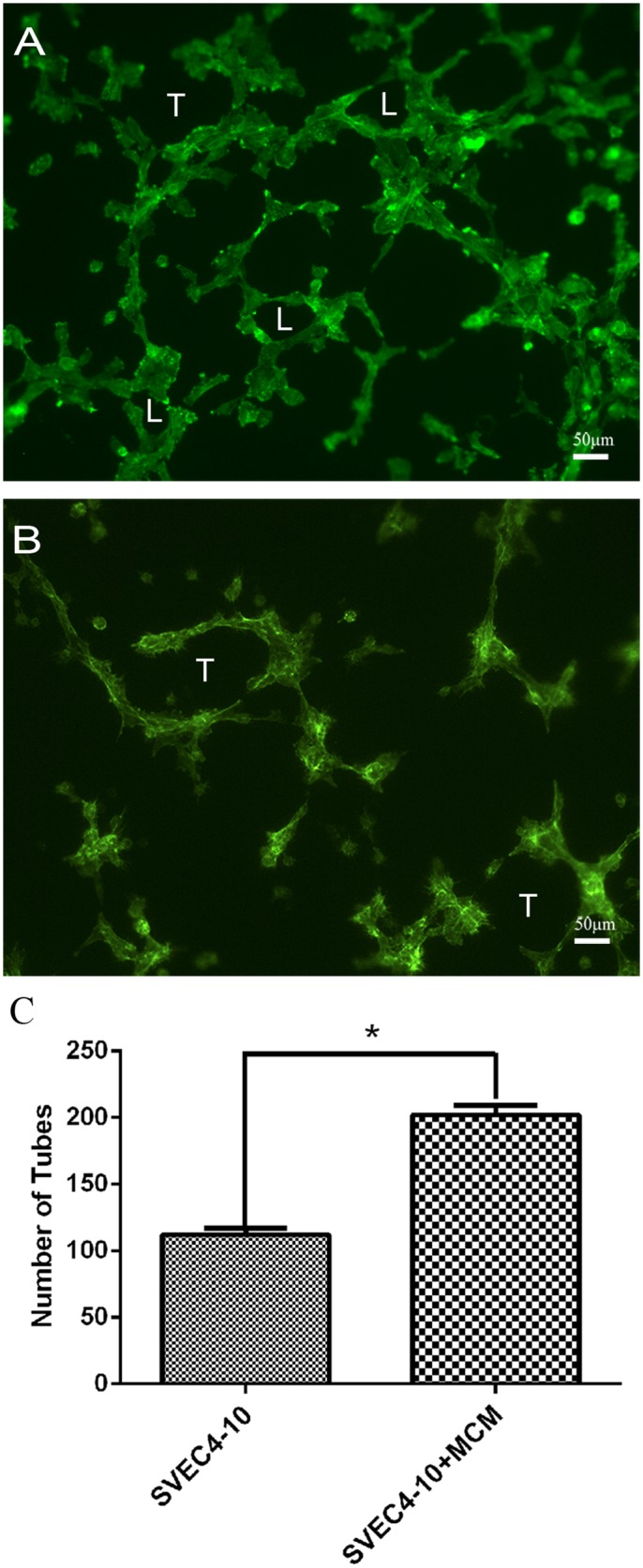
The cells were cultured for 5 hours at 37°C on Geltrex® in the presence of Mast Cell Mediators (MCM) from bone marrow derived mast cell. (A) When the SVEC4-10 cells were cultured in the presence of MCM, most of the cells were spread on the substrate, and formed both tubes (T) and loops (L). (B) When the cells were incubated in absence of mast cell mediators, the cells were spread on the substrate forming tubes (T), but there was no loop formation. (C) Quantitative analysis shown that the tube formation was higher in presence of mast cell mediators. The significance was determined by Student's t-test *p ≤ 0.05.
Mast cell tryptases increased tube formation
To directly evaluate the ability of specific mast cell tryptases, rmMCP 6 and 7, to induce tube formation an in vitro angiogenesis assay was performed using SVEC4-10 cells. After 5 hours of incubation with rmMCP-6 most SVEC4–10 cells were spread on the substrate, and formed both tubes and loops (Fig 2A). When the cells were incubated with rmMCP-7 there was an increase in the number of tubes and loops when compared with incubation with rmMCP-6 (Fig 2B). After 5 hours of culture in the absence of proteases, only a few SVEC4–10 cells were spread on the substrate forming tubes, but there was no loop formation (Fig 2C).
Fig 2. rmMCP-6 and 7 induce tube formation by endothelial cells.
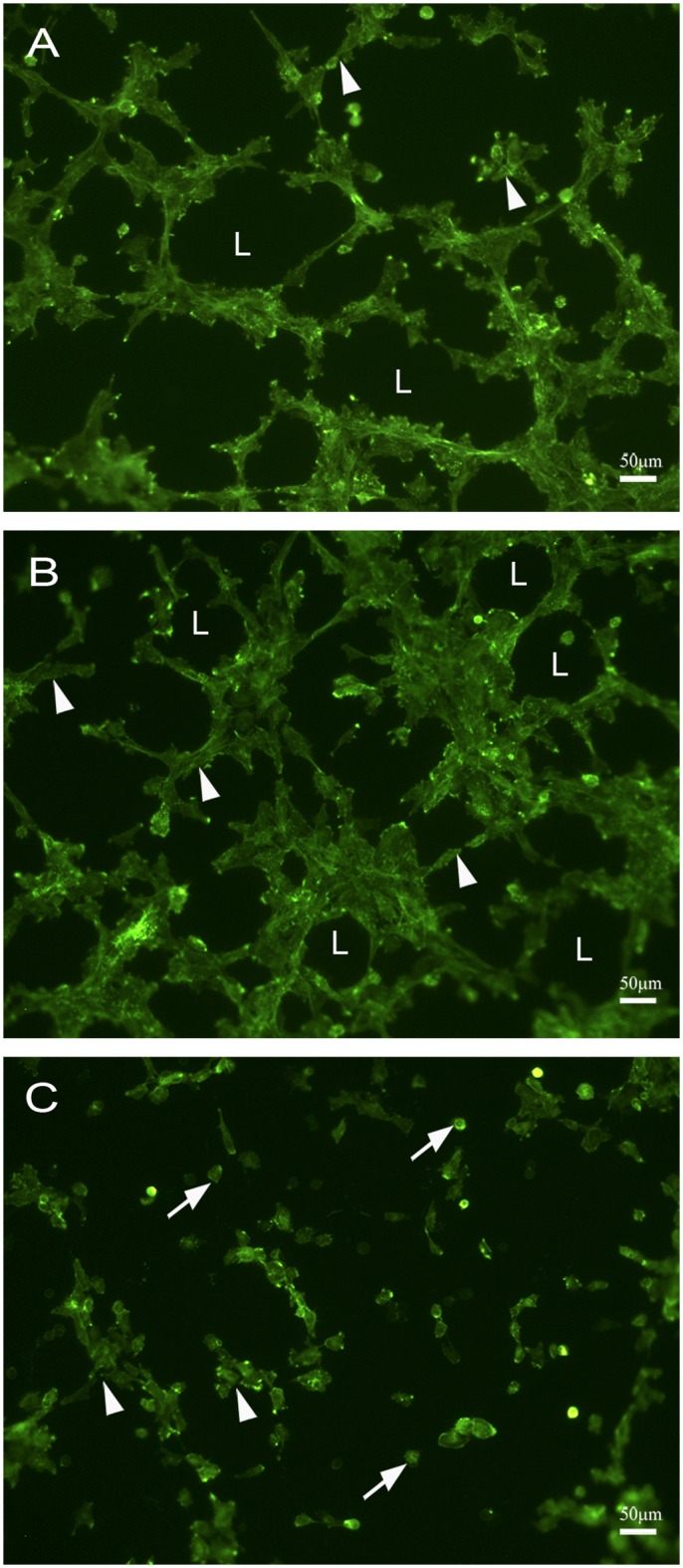
SVEC4-10 cells were cultured for 5 hours at 37°C on Geltrex® in the presence of rmMCP-6 (A), rmMCP-7 (B) or in the absence of tryptases (C). (A) In the presence of rmMCP-6 most of the SVEC4-10 cells were spread on the substrate, tube formation was observed (arrowhead) and loops were also present (L). (B) When the SVEC4-10 cells were cultured with rmMCP-7, tubes (arrowheads) and loops (L) were more prevalent. (C) When SVEC4-10 cells are cultured in the absence of tryptases (control) only a few cells were seen in the initial phase of tube formation (arrowhead) and most of the cells remained unspread (arrow). Cells were stained with phalloidin conjugated to Alexa 488. Five independent experiments were performed.
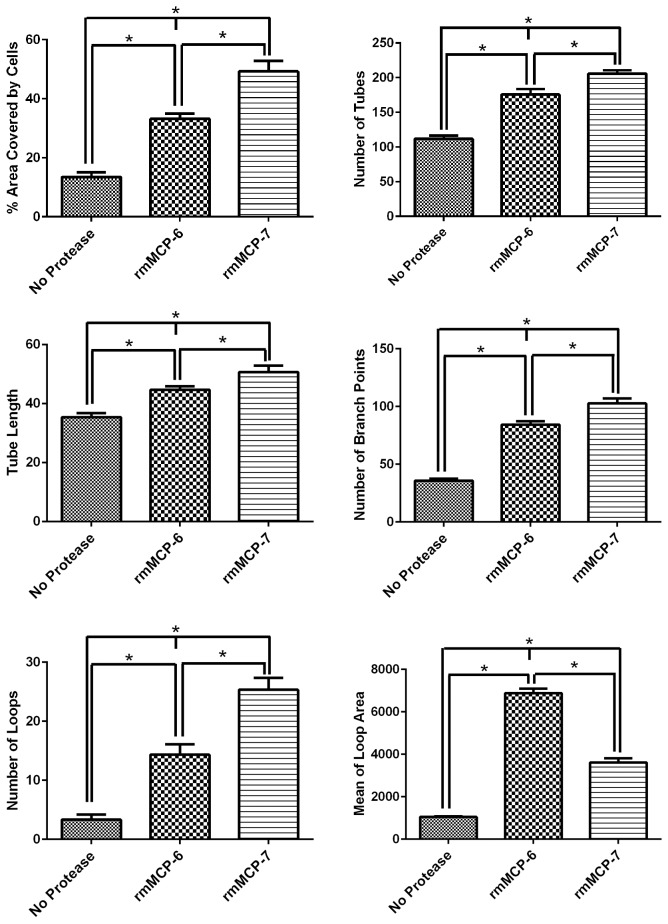
A quantitative analysis confirmed the data obtained by fluorescence microscopy (Fig 3). The area covered by tubes, the number of tubes, the tube length, branches and loops were significantly higher when SVEC4-10 cells were cultured with rmMCP-7. On the other hand, the average area occupied by loops was higher when endothelial cells were cultured with rmMCP-6 in comparison to culture with rmMCP-7. To further confirm the direct action of these proteases on tube formation the proteases were pre-incubated with antibodies against either mMCP-6 or mMCP-7.
Fig 3. rmMCP-7 is more effective in inducing tube formation by endothelial cells in vitro.
SVEC4-10 cells were cultured for 5 hours at 37°C on Geltrex® in the presence of rmMCP-6, rmMCP-7 or in the absence of tryptases. The area covered by tubes, the tube length, number of tubes, loops and branching points, the average area occupied by the loops were quantified using Wimasis WimTube. Tube length and mean of loop area are expressed in number of pixels. Data are presented as mean ± SD from five independent experiments. *p ≤ 0.05.
When the SVEC4-10 cells were cultured with proteases that had been preincubated with specific antibodies (Fig 4), the number of tubes formed by the SVEC4-10 cells was reduced to control levels, confirming the direct action of the proteases on in vitro angiogenesis.
Fig 4. Blocking of tryptases with anti-mMCP-6 and anti-mMCP-7 reduced the number of tubes formed.
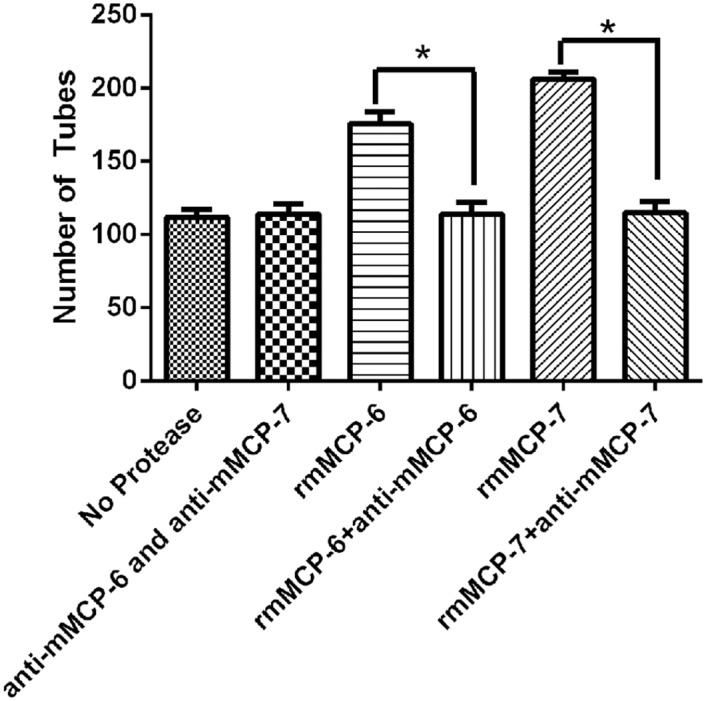
The proteases were incubated with the respective antibody for 30 min. After pre-incubation the complex (proteases and antibodies) were added and the cells were cultured for 5 hours. Controls cells were cultured for 5 hours in the absence of proteases but in the presence of antibodies. After incubation, tube formation was quantified. Data are presented as mean ± SD from three independent experiments. *p ≤ 0.05.
rmMCP-6 and rmMCP-7 induced angiogenesis in vivo
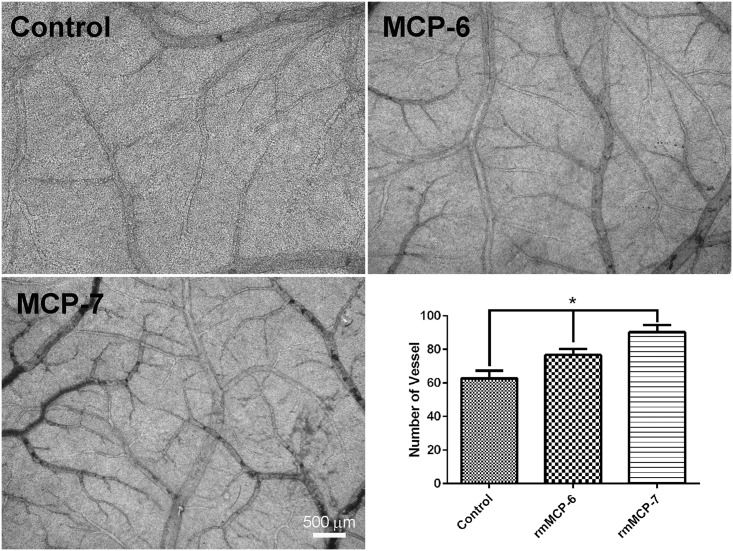
A chick embryo chorioallantoic membrane (CAM) assay was performed to assess the in vivo angiogenic ability of rmMCP-6 and 7. The CAM in the presence or absence of rmMCP-6 and 7 was examined on day 12 of incubation in order to analyze angiogenesis. In the presence of rmMCP-6 and 7 an increase in the number and caliber of blood vessels in the CAM was observed (Fig 5) when compared to controls. These results confirm the previous study by Ribatti et al [41] using human mast cell tryptases 6 and 7.
Fig 5. Mast cell tryptases induce an increase of angiogenesis in chicken chorioallantoic membrane.
When compared to the control CAM (no tryptases), CAM incubated in presence of rmMCP-6 and 7 showed an increase in the number of blood vessels. Moreover, an increase in blood vessel caliber was also seen in the presence of the tryptases. The graph shows the number of vessel per field (6.5mm2) from each sample.
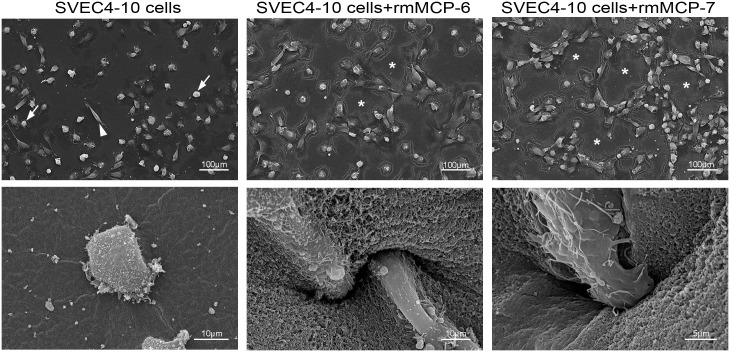
Mast cell tryptases stimulated invasion of the Geltrex® by SVEC4-10 cells
The effect of rmMCP-6, rmMCP-7 on tube formation was then analyzed by scanning electron microscopy. When cultured for 5 hours on Geltrex®, most SVEC4-10 endothelial cells were round, and only a few cells were spread on the substrate (Fig 6). In contrast, when endothelial cells were cultured for 5 hours in the presence of rmMCP-6 or -7, nearly all the cells were fusiform, spread on the substrate and arranged to form loops and tubes. Additionally, the SVEC4–10 cells invaded the Geltrex® in the presence of the tryptases (Fig 6).
Fig 6. In the presence of rmMCP-6 and rmMCP-7 the endothelial cells invade the Geltrex®.
SVEC4-10 cells were cultured for 5 hours at 37°C on Geltrex® in the presence of rmMCP-6, rmMCP-7 or in the absence of tryptases. In the absence of rmMCP-6 or -7, most of the SVEC4-10 cells remained rounded (arrows) and few cells spread on the substrate (arrowheads). In the presence of rmMCP-6 or -7 almost all endothelial cells were spread on the substrate and were organized into tubes (asterisks). Additionally, the SVEC4–10 cells invaded the Geltrex® only in the presence of the tryptases.
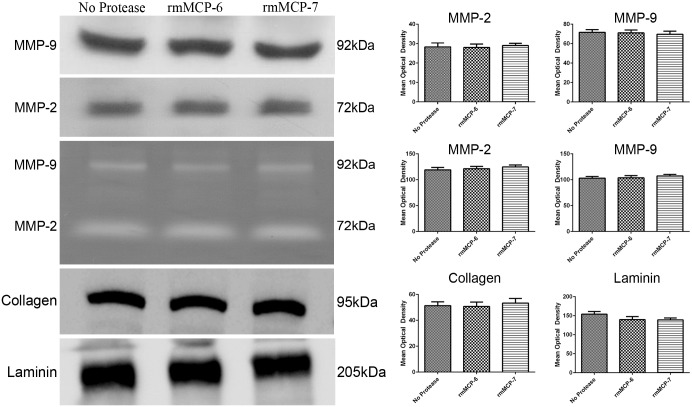
rmMCP-6 and rmMCP-7 did not degrade the Geltrex®
The results of the scanning electron microscopy suggested that the Geltrex® may have been partially degraded. To better understand this process, the expression and activity of metalloproteinases (MMP-2 and -9) from the culture supernatant were analyzed. After 5 hours of culture with or without proteases, no changes in expression or activity of metalloproteases were observed (Fig 7). To investigate gel degradation independent of metalloprotease activity, the main components of Geltrex® (laminin and collagen IV) were examined after 5 hours of incubation with or without rmMCP-6 or rmMCP-7. No changes were seen in the amount of laminin or collagen IV in the gel following incubation (Fig 7).
Fig 7. The expression and activity of metalloprotease or gel compound of are not altered in the presence of mast cell tryptases.
The cells were cultured for 5 hours at 37°C on Geltrex® in the presence of rmMCP-6, rmMCP-7 or in the absence of tryptases. After incubation, the culture supernatants were used for immunoblotting to analyze metalloprotease expression and for zymograms to determine metalloprotease activity. For gel compounds, after incubation, the Geltrex® was recovered using a lyses buffer and used for immunoblotting to analyze their components. Data are presented as mean ± SD from three independent experiments. *p ≤ 0.05.
rmMCP-6 and rmMCP-7 induce the release of angiogenic factors
To investigate whether rmMCP-6 and -7 were able to induce SVEC4–10 cells to release angiogenic factors, the supernatants from the tube formation cultures were analyzed using The Proteome Profiler™ Mouse Angiogenesis Array Kit as described in Materials and Methods. The presence of rmMCP-6 induced the release of a total of 40 angiogenic factors and rmMCP-7 stimulated the release of 47 factors. Thirty-six factors were released by both tryptases. Only 4 cytokines, angiogenin, endostatin, IGFBP-3 (Insulin-like Growth Factor-Binding Protein 3) and MCP-1 (Monocyte Chemoattractant Protein-1) were detected in the absence of rmMCP-6 or -7 (Table 1, and S1 Array). Therefore, rmMCP-6 and rmMCP-7 have the ability to stimulate release of angiogenic factors from endothelial cells.
Table 1. Angiogenic factors released in the presence of rmMCP-6 or rmMCP-7.
| Factor | SVEC4-10 | SVEC4-10 + rmMCP-6 | SVEC4-10 + rmMCP-7 |
|---|---|---|---|
| ADAMTS1 | − | − | + |
| Amphiregulin | − | − | + |
| Angiogenin | + | + | + |
| Angiopoietin-1 | − | + | + |
| Angiopoietin-3 | − | + | + |
| Coag. Factor III | − | + | + |
| CXCL16 | − | + | + |
| Cyr61 | − | − | + |
| DLL4 | − | − | + |
| DPPIV | − | + | + |
| Endoglin | − | + | + |
| Endostatin | + | + | + |
| Endothelin-1 | − | + | + |
| FGF acidic | − | + | + |
| FGF basic | − | − | + |
| KGF | − | − | + |
| Fractalkine | − | − | + |
| GM-CSF | − | + | − |
| HB-EGF | − | + | + |
| HGF | − | + | + |
| IGFBP-1 | − | + | + |
| IGFBP-2 | − | + | + |
| IGFBP-3 | + | + | + |
| IL-1α | − | + | + |
| IL-10 | − | − | + |
| IP-10 | − | − | + |
| KC | − | + | + |
| Leptin | − | + | + |
| MCP-1 | + | + | + |
| MIP-1α | − | + | + |
| MMP-3 | − | + | + |
| MMP-8 | − | + | + |
| MMP-9 | − | + | + |
| NOV/ IGFBP-9 | − | − | + |
| Osteopontin | − | + | + |
| PD-ECGF | − | + | + |
| PDGF-AA | − | + | + |
| PDGF-AB | − | + | + |
| Pentraxin-3 | − | + | + |
| Platelet Factor 4 | − | + | + |
| PlGF-2 | − | + | + |
| Prolactin | − | + | + |
| Proliferin | − | − | + |
| SDF-1 | − | + | − |
| Serpin E1 | − | + | + |
| Serpin F1 | − | + | − |
| Thrombospondin-2 | − | + | − |
| TIMP-1 | − | + | + |
| TIMP-4 | − | + | + |
| VEGF | − | + | + |
| VEGF-B | − | + | + |
The Proteome Profiler™ Mouse Angiogenesis Array Kit was used to simultaneously assess the relative levels of mouse angiogenesis-related proteins of the supernatant from the tube formation assay in the presence or absence of rmMCP-6 and rmMCP -7.
rmMCP-6 and rmMCP-7 induced the differential release of angiogenic factors
The profile of the factors released after incubation with rmMCP-6 was different from that seen with rmMCP-7. Four factors released by control SVEC4-10 cells were significantly increased in the presence of the tryptases (Fig 8 and S1 Array). This difference may be a reflection of their physiological functions (Table 2).
Fig 8. In the presence of rmMCP-6 and rmMCP-7 SVEC4-10 cells show a differential release of angiogenesis related cytokines/proteins.
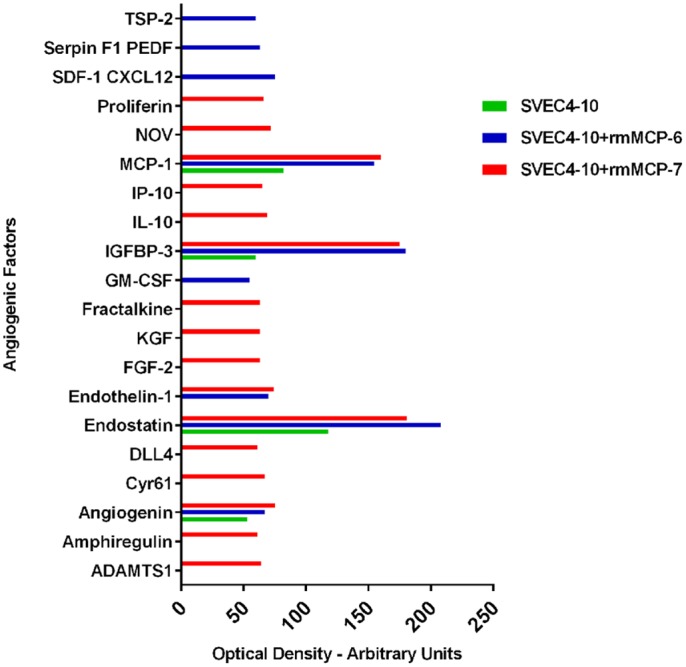
The graph shows the cytokines/proteins that were released exclusively by rmMCP-6 (blue) or rmMCP-7 (red). Four cytokines/proteins that were released by control cells were increased in the presence of the tryptases (green). The cells were cultured for 5 hours at 37°C on Geltrex® in the presence of rmMCP-6, rmMCP-7 or in the absence of tryptases. After incubation, a mouse cytokine array kit was used to analyze the protein expression of different pro- and anti-angiogenic factors in the culture supernatants. The data shown is the mean spot pixel density that was quantified from the arrays using the image analysis software Adobe Photoshop CS6 V 13.0.
Table 2. Action of differentially released angiogenic factors.
| Factors released by SVEC4-10 cells | ||
|---|---|---|
| Angiogenic Fator | Role in Angiogenesis | Reference |
| Angiogenin | Induces the proliferation and migration of endothelial cells | [42, 43] |
| Endostatin | Inhibits endothelial cells proliferation, migration and angiogenesis | [44, 45] |
| IGFBP-3 | Promotes angiogenesis and cell motility | [42] |
| MCP-1 | Induces migration, sprouting of endothelial cells, and induces vascular-like tunnel formation in vivo | [46, 47] |
| Factors Released Exclusively in the Presence of rmMCP-6 | ||
| GM-CSF | Induces proliferation and migration of endothelial cells | [42] |
| SDF-/CXCL12 | Induces the activation of matrix metalloproteinase-9 (MMP-9) | [48] |
| Serpin F1 | Stimulates VEGF secretion, activates integrins, interferes with VEGF signaling thereby inhibiting angiogenesis | [49, 50] |
| Thrombospondin -2 | Inhibits endothelial cell function | [51] |
| Factors Released Exclusively in the Presence of rmMCP-7 | ||
| ADAMTS1 | Induces degradation of venular basement membrane versican in VEGF-induced pathological angiogenesis | [52, 53] |
| Amphiregulin | Induces the proliferation of rat vascular smooth muscle cells, possibly implicated in arterial remodeling | [54, 55] |
| Cyr61 | Induces angiogenesis in vivo, supports cell adhesion, promotes cell migration, and enhances growth factor-stimulated mitogenesis in endothelial cells. | [56, 57] |
| Dll4 | Regulates the specification of endothelial cells into tip and stalk cells during angiogenic sprouting | [58, 59] |
| FGFb | Regulates proliferation, migration and differentiation of endothelial cells | [60] |
| KGF | Directly stimulates capillary endothelial cells’ proliferation and migration | [61, 62] |
| Fractalkine | Stimulates ex vivo and in vivo angiogenesis, may act as a direct angiogenic modulator in endothelial cells without inducing VEGF expression | [63] |
| NOV/IGFBP-9 | Acts directly upon endothelial cells to stimulate pro-angiogenic activities, and induces angiogenesis in vivo | [64, 65] |
| IL-10 | Promotes pathological angiogenesis and interferes with endothelial differentiation | [66, 67] |
| IP-10 | Potent inhibitor of angiogenesis in vivo | [68] |
| Proliferin | Stimulates endothelial cell migration, invasion, and tube formation in vitro, and angiogenesis in vivo | [69] |
Biological function of angiogenic factors shown in Fig 8.
Discussion
The results of this study show that the tryptases, rmMCP-6 and -7, play an important role in angiogenesis, and can act directly on endothelial cells independently of other mediators released by mast cells. Furthermore, rmMCP-7 is more effective in accelerating this process.
During tube formation, the endothelial cells aggregated faster in the presence of rmMCP-7, thus suggesting that rmMCP-7 may induce or accelerate the anastomosis of endothelial cells during angiogenesis. In contrast, the mean area occupied by the loops was increased when the endothelial cells were cultured with rmMCP-6. The area occupied by the loops is directly related to vessel diameter, thus suggesting that mMCP-6 can influence the diameter of the vessels during angiogenesis. The diameter of new capillary sprouts can be influenced by the local concentration and the action of angiogenic factors on endothelial cells [70]. Angiogenic factors may also play a role in determining lumen diameter, as well as vessel length during angiogenesis [71, 72]. The fact that rmMCP-6 and 7 also induced endothelial cells to invade the Geltrex®, suggests that these proteases stimulate endothelial cells to migrate contributing to the formation of new blood vessels. This phenomenon was not observed when the cells are cultured without proteases.
No changes were seen in the amount of laminin or collagen IV in the gel following incubation, however, the results of the scanning electron microscopy showed that the endothelial cells invaded the Geltrex®. These findings suggest that the invasion occurs independently of matrix degradation. However, a small amount of degradation may be occurring that is not detected by Western blot. Invasion independent of degradation was also observed by other investigators. Wolf and colleagues [73] demonstrated that amoeboid migration appears to use morphodynamic supramolecular mechanisms to bypass tissue barriers independent of ECM degradation. Furthermore, cortactin and cofilin appear to regulate invadopodial elongation in MDA-MB-231 cells independent of degradation [74].
Angiogenesis array analysis showed that there was a differential release of cytokines/angiogenic proteins during the in vitro angiogenesis. In the absence of tryptases only 4 factors were released from the SVEC10-4 cells: angiogenin, endostatin, IGFBP-3, MCP-1. Angiogenin induces the proliferation and migration of endothelial cells [42]. Endostatin down-regulates signaling pathways associated with proangiogenic activity and upregulates many antiangiogenic genes [75]. IGFBP-3 promotes angiogenesis and cell motility [42, 76, 77] while MCP- 1 induces migration and sprouting of endothelial cells, besides induced vascular-like tunnel formation in vivo [46, 47].
The concentration of most cytokines released into the supernatant of SVEC4-10 endothelial cells cultured with either rmMCP-6 or -7 were similar. However, some differences were observed when endothelial cells were cultured with rmMCP-6 or rmMCP-7. In the presence of rmMCP-6, the endothelial cells released GM-CSF, SDF-1, Serpin F1, and Trombopondin-2. These cytokines are directly related to cell proliferation and migration [42]. rmMCP-7 induced the release of cytokines that are considered to be extremely potent angiogenesis inducers such as, ADAMTS1, Amphiregulin, Cyr61, Dll4, FGFb, KGF, Fractalkine, NOV/IGFBP-9, IL-10, IP-10 (Interferon gamma-induced protein 10), and proliferin [78, 79]. Interestingly, rmMCP-7 induced the release of Dll4, which is responsible for the differentiation of endothelial cells into tip or stalk cells via the Notch signaling pathway [80–83]. The Notch pathway controls both normal and pathological angiogenesis by modulating the development of tip cells and stalk cells during the formation of new blood vessels [84]. The release of this cytokine may explain the increased spreading of endothelial cells when rmMCP-7 was present during the tube formation assays.
Several previous studies have shown that tryptase can induce migration and proliferation of endothelial cells [32, 85, 86]. Endothelial cell proliferation induced by tryptase can be in directly attributed to its proteolytic activity on PAR-2 [87, 88]. Tryptase can also indirectly affect angiogenesis by stimulating the α1(I) procollagen synthesis by fibroblast and activating dermal fibroblasts [89]. Ribatti et al. [41] showed that human tryptases stimulate angiogenesis in CAM, similar to what was observed in the present study. The results of both studies confirm the angiogenic activity of these proteases and indicate that tryptase interacts with endothelial cells via unidentified mechanisms to induce angiogenesis.
Taken together, the results of the present study show the extensive angiogenic activity of tryptases and demonstrate that the tryptase subtypes (rmMCP-6 and-7) have different roles during in vitro angiogenesis. Both proteases induced cell migration and adhesion, however, rmMCP-7 also stimulated and accelerated the anastomosis of endothelial cells during the angiogenesis process. Other studies have shown distinct functions for tryptase subtypes in inflammation [30, 90–92].
Understanding the specific role of each tryptase subtype in angiogenesis can be of great importance in developing new therapeutic interventions, or in the improvement of existing ones, that aim to inhibit the formation of blood vessels in pathological processes.
Supporting Information
The cells were cultured for 5 hours at 37°C on Geltrex® in the presence of rmMCP-6, rmMCP-7 or in the absence of tryptases. After incubation, The Proteome Profiler™ Mouse Angiogenesis Array Kit was used to analyze the protein expression of different pro- and anti-angiogenic factors in culture supernatants. Array membrane images are shown. The table gives the mouse angiogenesis array coordinates with a description, location and the mean spot pixel density of each angiogenic factor in the membrane array. The mean spot pixel density was quantified from the arrays using image analysis software Adobe Photoshop CS6 V 13.0.
(TIF)
Red outlines represent the immunoblot sections presented in Fig 6.
(TIF)
Acknowledgments
We would like to thank Anderson Roberto de Souza and Vani Maria Correa for technical assistance, Maria Dolores S. Ferreira and José Augusto Moulin for assistance with the electron microscopy, and Roberta Ribeiro Costa Rosales for assistance with fluorescence microscopy. All from the Department of Cell and Molecular Biology and Pathogenic Bioagents, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, SP.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding was provided by FAPESP (Fundacão de Amparo e Pesquisa do Estado de São Paulo, http://www.fapesp.br/); Post Doctoral Fellowship 11/10363-8 to DASJ and Equipment Grant 09/54013-0 to MCJ; CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, http://www.cnpq.br) Fellowship to MCJ and ACB Post Doctoral Fellowship 150022/2012-3, FAEPA (Fundacão de Apoio ao Ensino, Pesquisa e Assistência, http://www.faepa.br) Grant to MCJ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Reber LL, Sibilano R, Mukai K, Galli SJ. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. 2015. 10.1038/mi.2014.131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wedemeyer J, Galli SJ. Mast cells and basophils in acquired immunity. Br Med Bull. 2000;56(4):936–55. . [DOI] [PubMed] [Google Scholar]
- 3. Krishnaswamy G, Ajitawi O, Chi DS. The human mast cell: an overview. Methods Mol Biol. 2006;315:13–34. . [DOI] [PubMed] [Google Scholar]
- 4. Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37(1):25–33. 10.1016/j.immuni.2012.07.003 . [DOI] [PubMed] [Google Scholar]
- 5. Olivera A, Rivera J. Paradigm shifts in mast cell and basophil biology and function: an emerging view of immune regulation in health and disease. Methods Mol Biol. 2014;1192:3–31. 10.1007/978-1-4939-1173-8_1 . [DOI] [PubMed] [Google Scholar]
- 6. da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014;62(10):698–738. 10.1369/0022155414545334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Souza Junior DA, Santana AC, da Silva EZ, Oliver C, Jamur MC. The Role of Mast Cell Specific Chymases and Tryptases in Tumor Angiogenesis. Biomed Res Int. 2015;2015:142359 10.1155/2015/142359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maciel TT, Moura IC, Hermine O. The role of mast cells in cancers. F1000Prime Rep. 2015;7:09 10.12703/P7-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hallgren J, Gurish MF. Granule maturation in mast cells: histamine in control. Eur J Immunol. 2014;44(1):33–6. 10.1002/eji.201344262 . [DOI] [PubMed] [Google Scholar]
- 10. Marichal T, Tsai M, Galli SJ. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res. 2013;1(5):269–79. 10.1158/2326-6066.CIR-13-0119 . [DOI] [PubMed] [Google Scholar]
- 11. Ribatti D, Ranieri G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp Cell Res. 2015;332(2):157–62. 10.1016/j.yexcr.2014.11.014 . [DOI] [PubMed] [Google Scholar]
- 12. Ammendola M, Leporini C, Marech I, Gadaleta CD, Scognamillo G, Sacco R, et al. Targeting mast cells tryptase in tumor microenvironment: a potential antiangiogenetic strategy. Biomed Res Int. 2014;2014:154702 10.1155/2014/154702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marinaccio C, Ingravallo G, Gaudio F, Perrone T, Nico B, Maoirano E, et al. Microvascular density, CD68 and tryptase expression in human diffuse large B-cell lymphoma. Leuk Res. 2014;38(11):1374–7. 10.1016/j.leukres.2014.09.007 . [DOI] [PubMed] [Google Scholar]
- 14. Ullah E, Nagi AH, Ashraf M. Angiogenesis and mast cell density as predictors of patient survival in squamous cell carcinoma of lung. J Cancer Res Ther. 2013;9(4):701–5. 10.4103/0973-1482.126487 . [DOI] [PubMed] [Google Scholar]
- 15. Ch'ng S, Wallis RA, Yuan L, Davis PF, Tan ST. Mast cells and cutaneous malignancies. Mod Pathol. 2006;19(1):149–59. 10.1038/modpathol.3800474 . [DOI] [PubMed] [Google Scholar]
- 16. Mangia A, Malfettone A, Rossi R, Paradiso A, Ranieri G, Simone G, et al. Tissue remodelling in breast cancer: human mast cell tryptase as an initiator of myofibroblast differentiation. Histopathology. 2011;58(7):1096–106. 10.1111/j.1365-2559.2011.03842.x . [DOI] [PubMed] [Google Scholar]
- 17. Dyduch G, Kaczmarczyk K, Okoń K. Mast cells and cancer: enemies or allies? Pol J Pathol. 2012;63(1):1–7. . [PubMed] [Google Scholar]
- 18. Tahir A, Nagi AH, Ullah E, Janjua OS. The role of mast cells and angiogenesis in well-differentiated oral squamous cell carcinoma. J Cancer Res Ther. 2013;9(3):387–91. 10.4103/0973-1482.119311 . [DOI] [PubMed] [Google Scholar]
- 19. Singer J, Jensen-Jarolim E. IgE-based immunotherapy of cancer: challenges and chances. Allergy. 2014;69(2):137–49. 10.1111/all.12276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ribatti D, Crivellato E, Roccaro AM, Ria R, Vacca A. Mast cell contribution to angiogenesis related to tumour progression. Clin Exp Allergy. 2004;34(11):1660–4. 10.1111/j.1365-2222.2004.02104.x . [DOI] [PubMed] [Google Scholar]
- 21. Crivellato E, Nico B, Ribatti D. Mast cells and tumour angiogenesis: new insight from experimental carcinogenesis. Cancer Lett. 2008;269(1):1–6. 10.1016/j.canlet.2008.03.031 . [DOI] [PubMed] [Google Scholar]
- 22. Ribatti D, Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim Biophys Acta. 2012;1822(1):2–8. 10.1016/j.bbadis.2010.11.010 . [DOI] [PubMed] [Google Scholar]
- 23. Pappa CA, Tsirakis G, Stavroulaki E, Kokonozaki M, Xekalou A, Konsolas I, et al. Mast cells influence the proliferation rate of myeloma plasma cells. Cancer Invest. 2015;33(4):137–41. 10.3109/07357907.2015.1008639 . [DOI] [PubMed] [Google Scholar]
- 24. Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007;95:167–255. 10.1016/S0065-2776(07)95006-3 . [DOI] [PubMed] [Google Scholar]
- 25. Schwartz LB, Bradford TR, Irani AM, Deblois G, Craig SS. The major enzymes of human mast cell secretory granules. Am Rev Respir Dis. 1987;135(5):1186–9. . [DOI] [PubMed] [Google Scholar]
- 26. Lundequist A, Pejler G. Biological implications of preformed mast cell mediators. Cell Mol Life Sci. 2011;68(6):965–75. 10.1007/s00018-010-0587-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cui Y, Dahlin JS, Feinstein R, Bankova LG, Xing W, Shin K, et al. Mouse mast cell protease-6 and MHC are involved in the development of experimental asthma. J Immunol. 2014;193(10):4783–9. 10.4049/jimmunol.1302947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pastorello EA, Morici N, Farioli L, Di Biase M, Losappio LM, Nichelatti M, et al. Serum tryptase: a new biomarker in patients with acute coronary syndrome? Int Arch Allergy Immunol. 2014;164(2):97–105. 10.1159/000360164 . [DOI] [PubMed] [Google Scholar]
- 29. Jamur MC, Oliver C. Origin, maturation and recruitment of mast cell precursors. Front Biosci (Schol Ed). 2011;3:1390–406. . [DOI] [PubMed] [Google Scholar]
- 30. Huang C, Wong GW, Ghildyal N, Gurish MF, Sali A, Matsumoto R, et al. The tryptase, mouse mast cell protease 7, exhibits anticoagulant activity in vivo and in vitro due to its ability to degrade fibrinogen in the presence of the diverse array of protease inhibitors in plasma. J Biol Chem. 1997;272(50):31885–93. . [DOI] [PubMed] [Google Scholar]
- 31. Gruber BL, Marchese MJ, Suzuki K, Schwartz LB, Okada Y, Nagase H, et al. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989;84(5):1657–62. 10.1172/JCI114344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhi X, Xu C, Zhang H, Tian D, Li X, Ning Y, et al. Tryptase promotes atherosclerotic plaque haemorrhage in ApoE-/- mice. PLoS One. 2013;8(4):e60960 10.1371/journal.pone.0060960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Souza DA, Toso VD, Campos MR, Lara VS, Oliver C, Jamur MC. Expression of mast cell proteases correlates with mast cell maturation and angiogenesis during tumor progression. PLoS One. 2012;7(7):e40790 10.1371/journal.pone.0040790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jamur MC, Grodzki AC, Berenstein EH, Hamawy MM, Siraganian RP, Oliver C. Identification and characterization of undifferentiated mast cells in mouse bone marrow. Blood. 2005;105(11):4282–9. 10.1182/blood-2004-02-0756 . [DOI] [PubMed] [Google Scholar]
- 35. Marchini-Alves CM, Nicoletti LM, Mazucato VM, de Souza LB, Hitomi T, Alves CeP, et al. Phospholipase D2: a pivotal player modulating RBL-2H3 mast cell structure. J Histochem Cytochem. 2012;60(5):386–96. 10.1369/0022155412438886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee WS, Park YL, Kim N, Oh HH, Son DJ, Kim MY, et al. Myeloid cell leukemia-1 is associated with tumor progression by inhibiting apoptosis and enhancing angiogenesis in colorectal cancer. Am J Cancer Res. 2015;5(1):101–13. [PMC free article] [PubMed] [Google Scholar]
- 37. Askou AL, Aagaard L, Kostic C, Arsenijevic Y, Hollensen AK, Bek T, et al. Multigenic lentiviral vectors for combined and tissue-specific expression of miRNA- and protein-based antiangiogenic factors. Mol Ther Methods Clin Dev. 2015;2:14064 10.1038/mtm.2014.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khoo CP, Micklem K, Watt SM. A comparison of methods for quantifying angiogenesis in the Matrigel assay in vitro. Tissue Eng Part C Methods. 2011;17(9):895–906. 10.1089/ten.TEC.2011.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Subramanian M, Rao SR, Thacker P, Chatterjee S, Karunagaran D. MiR-29b downregulates canonical Wnt signaling by suppressing coactivators of β-catenin in human colorectal cancer cells. J Cell Biochem. 2014;115(11):1974–84. 10.1002/jcb.24869 . [DOI] [PubMed] [Google Scholar]
- 40. Leber TM, Balkwill FR. Zymography: a single-step staining method for quantitation of proteolytic activity on substrate gels. Anal Biochem. 1997;249(1):24–8. 10.1006/abio.1997.2170 . [DOI] [PubMed] [Google Scholar]
- 41. Ribatti D, Ranieri G, Nico B, Benagiano V, Crivellato E. Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay. Int J Dev Biol. 2011;55(1):99–102. 10.1387/ijdb.103138dr . [DOI] [PubMed] [Google Scholar]
- 42. Distler JH, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47(3):149–61. . [PubMed] [Google Scholar]
- 43. Gao X, Xu Z. Mechanisms of action of angiogenin. Acta Biochim Biophys Sin (Shanghai). 2008;40(7):619–24. . [DOI] [PubMed] [Google Scholar]
- 44. Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, et al. Endostatin induces endothelial cell apoptosis. J Biol Chem. 1999;274(17):11721–6. . [DOI] [PubMed] [Google Scholar]
- 45. Skovseth DK, Veuger MJ, Sorensen DR, De Angelis PM, Haraldsen G. Endostatin dramatically inhibits endothelial cell migration, vascular morphogenesis, and perivascular cell recruitment in vivo. Blood. 2005;105(3):1044–51. 10.1182/blood-2004-03-1164 . [DOI] [PubMed] [Google Scholar]
- 46. Ma J, Wang Q, Fei T, Han JD, Chen YG. MCP-1 mediates TGF-beta-induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood. 2007;109(3):987–94. 10.1182/blood-2006-07-036400 . [DOI] [PubMed] [Google Scholar]
- 47. Niu J, Wang K, Zhelyabovska O, Saad Y, Kolattukudy PE. MCP-1-induced protein promotes endothelial-like and angiogenic properties in human bone marrow monocytic cells. J Pharmacol Exp Ther. 2013;347(2):288–97. 10.1124/jpet.113.207316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28(7):299–307. 10.1016/j.it.2007.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kijowski J, Baj-Krzyworzeka M, Majka M, Reca R, Marquez LA, Christofidou-Solomidou M, et al. The SDF-1-CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells. 2001;19(5):453–66. 10.1634/stemcells.19-5-453 . [DOI] [PubMed] [Google Scholar]
- 50. Bernard A, Gao-Li J, Franco CA, Bouceba T, Huet A, Li Z. Laminin receptor involvement in the anti-angiogenic activity of pigment epithelium-derived factor. J Biol Chem. 2009;284(16):10480–90. 10.1074/jbc.M809259200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6(1):1–12. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fu Y, Nagy JA, Brown LF, Shih SC, Johnson PY, Chan CK, et al. Proteolytic cleavage of versican and involvement of ADAMTS-1 in VEGF-A/VPF-induced pathological angiogenesis. J Histochem Cytochem. 2011;59(5):463–73. 10.1369/0022155411401748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kumar S, Sharghi-Namini S, Rao N, Ge R. ADAMTS5 functions as an anti-angiogenic and anti-tumorigenic protein independent of its proteoglycanase activity. Am J Pathol. 2012;181(3):1056–68. 10.1016/j.ajpath.2012.05.022 . [DOI] [PubMed] [Google Scholar]
- 54. Kato M, Inazu T, Kawai Y, Masamura K, Yoshida M, Tanaka N, et al. Amphiregulin is a potent mitogen for the vascular smooth muscle cell line, A7r5. Biochem Biophys Res Commun. 2003;301(4):1109–15. . [DOI] [PubMed] [Google Scholar]
- 55. Bles N, Di Pietrantonio L, Boeynaems JM, Communi D. ATP confers tumorigenic properties to dendritic cells by inducing amphiregulin secretion. Blood. 2010;116(17):3219–26. 10.1182/blood-2010-01-265611 . [DOI] [PubMed] [Google Scholar]
- 56. Grzeszkiewicz TM, Lindner V, Chen N, Lam SC, Lau LF. The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin alpha(6)beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology. 2002;143(4):1441–50. 10.1210/endo.143.4.8731 . [DOI] [PubMed] [Google Scholar]
- 57. Maity G, Mehta S, Haque I, Dhar K, Sarkar S, Banerjee SK, et al. Pancreatic tumor cell secreted CCN1/Cyr61 promotes endothelial cell migration and aberrant neovascularization. Sci Rep. 2014;4:4995 10.1038/srep04995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kuhnert F, Kirshner JR, Thurston G. Dll4-Notch signaling as a therapeutic target in tumor angiogenesis. Vasc Cell. 2011;3(1):20 10.1186/2045-824X-3-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu M, Yuan T, Liu H, Chen P. CCAAT/enhancer-binding protein β regulates interleukin-6-induced transmembrane and ubiquitin-like domain containing 1 gene expression in hepatocytes. Mol Med Rep. 2014;10(4):2177–83. 10.3892/mmr.2014.2457 . [DOI] [PubMed] [Google Scholar]
- 60. Lieu C, Heymach J, Overman M, Tran H, Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res. 2011;17(19):6130–9. 10.1158/1078-0432.CCR-11-0659 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gillis P, Savla U, Volpert OV, Jimenez B, Waters CM, Panos RJ, et al. Keratinocyte growth factor induces angiogenesis and protects endothelial barrier function. J Cell Sci. 1999;112 (Pt 12):2049–57. . [DOI] [PubMed] [Google Scholar]
- 62. Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3):235–53. 10.1038/nrd2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Szukiewicz D, Kochanowski J, Pyzlak M, Szewczyk G, Stangret A, Mittal TK. Fractalkine (CX3CL1) and its receptor CX3CR1 may contribute to increased angiogenesis in diabetic placenta. Mediators Inflamm. 2013;2013:437576 10.1155/2013/437576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, et al. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem. 2003;278(26):24200–8. 10.1074/jbc.M302028200 . [DOI] [PubMed] [Google Scholar]
- 65. Gellhaus A, Schmidt M, Dunk C, Lye SJ, Kimmig R, Winterhager E. Decreased expression of the angiogenic regulators CYR61 (CCN1) and NOV (CCN3) in human placenta is associated with pre-eclampsia. Mol Hum Reprod. 2006;12(6):389–99. 10.1093/molehr/gal044 . [DOI] [PubMed] [Google Scholar]
- 66. Dace DS, Khan AA, Kelly J, Apte RS. Interleukin-10 promotes pathological angiogenesis by regulating macrophage response to hypoxia during development. PLoS One. 2008;3(10):e3381 10.1371/journal.pone.0003381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cates AM, Holden VI, Myers EM, Smith CK, Kaplan MJ, Kahlenberg JM. Interleukin 10 hampers endothelial cell differentiation and enhances the effects of interferon α on lupus endothelial cell progenitors. Rheumatology (Oxford). 2014. 10.1093/rheumatology/keu431 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. 2006;98(5):617–25. 10.1161/01.RES.0000209968.66606.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang X, Qiao D, Meyer K, Pier T, Keles S, Friedl A. Angiogenesis induced by signal transducer and activator of transcription 5A (STAT5A) is dependent on autocrine activity of proliferin. J Biol Chem. 2012;287(9):6490–502. 10.1074/jbc.M111.254631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nakatsu MN, Sainson RC, Pérez-del-Pulgar S, Aoto JN, Aitkenhead M, Taylor KL, et al. VEGF(121) and VEGF(165) regulate blood vessel diameter through vascular endothelial growth factor receptor 2 in an in vitro angiogenesis model. Lab Invest. 2003;83(12):1873–85. . [DOI] [PubMed] [Google Scholar]
- 71. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–95. 10.1038/74651 . [DOI] [PubMed] [Google Scholar]
- 72. Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49(3):507–21. . [DOI] [PubMed] [Google Scholar]
- 73. Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160(2):267–77. 10.1083/jcb.200209006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Magalhaes MA, Larson DR, Mader CC, Bravo-Cordero JJ, Gil-Henn H, Oser M, et al. Cortactin phosphorylation regulates cell invasion through a pH-dependent pathway. J Cell Biol. 2011;195(5):903–20. 10.1083/jcb.201103045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Abdollahi A, Lipson KE, Sckell A, Zieher H, Klenke F, Poerschke D, et al. Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res. 2003;63(24):8890–8. . [PubMed] [Google Scholar]
- 76. Granata R, Trovato L, Garbarino G, Taliano M, Ponti R, Sala G, et al. Dual effects of IGFBP-3 on endothelial cell apoptosis and survival: involvement of the sphingolipid signaling pathways. FASEB J. 2004;18(12):1456–8. 10.1096/fj.04-1618fje . [DOI] [PubMed] [Google Scholar]
- 77. Liu B, Lee KW, Anzo M, Zhang B, Zi X, Tao Y, et al. Insulin-like growth factor-binding protein-3 inhibition of prostate cancer growth involves suppression of angiogenesis. Oncogene. 2007;26(12):1811–9. 10.1038/sj.onc.1209977 . [DOI] [PubMed] [Google Scholar]
- 78. Neufeld G, Kessler O, Vadasz Z, Gluzman-Poltorak Z. The contribution of proangiogenic factors to the progression of malignant disease: role of vascular endothelial growth factor and its receptors. Surg Oncol Clin N Am. 2001;10(2):339–56, ix . [PubMed] [Google Scholar]
- 79. Neufeld G, Kessler O. Pro-angiogenic cytokines and their role in tumor angiogenesis. Cancer Metastasis Rev. 2006;25(3):373–85. 10.1007/s10555-006-9011-5 . [DOI] [PubMed] [Google Scholar]
- 80. Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47(8):3595–602. 10.1167/iovs.05-1522 . [DOI] [PubMed] [Google Scholar]
- 81. Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–40. 10.1182/blood-2009-12-257832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A, Betsholtz C. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One. 2011;6(1):e15846 10.1371/journal.pone.0015846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3(1):a006569 10.1101/cshperspect.a006569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kume T. Novel insights into the differential functions of Notch ligands in vascular formation. J Angiogenes Res. 2009;1:8 10.1186/2040-2384-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, et al. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99(11):2691–700. 10.1172/JCI119458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Compton SJ, Cairns JA, Holgate ST, Walls AF. The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: tryptase induces expression of mRNA for IL-1 beta and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J Immunol. 1998;161(4):1939–46. . [PubMed] [Google Scholar]
- 87. Akers IA, Parsons M, Hill MR, Hollenberg MD, Sanjar S, Laurent GJ, et al. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am J Physiol Lung Cell Mol Physiol. 2000;278(1):L193–201. . [DOI] [PubMed] [Google Scholar]
- 88. Lu C, Zhao FD, Li XB, Yin LH. Up regulation of interleukin-8 expressions induced by mast cell tryptase via protease activated receptor-2 in endothelial cell line. Chin Med J (Engl). 2005;118(22):1900–6. . [PubMed] [Google Scholar]
- 89. Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13(11):1382–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Huang C, Friend DS, Qiu WT, Wong GW, Morales G, Hunt J, et al. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J Immunol. 1998;160(4):1910–9. . [PubMed] [Google Scholar]
- 91. McNeil HP, Shin K, Campbell IK, Wicks IP, Adachi R, Lee DM, et al. The mouse mast cell-restricted tetramer-forming tryptases mouse mast cell protease 6 and mouse mast cell protease 7 are critical mediators in inflammatory arthritis. Arthritis Rheum. 2008;58(8):2338–46. 10.1002/art.23639 . [DOI] [PubMed] [Google Scholar]
- 92. Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, et al. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182(1):647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The cells were cultured for 5 hours at 37°C on Geltrex® in the presence of rmMCP-6, rmMCP-7 or in the absence of tryptases. After incubation, The Proteome Profiler™ Mouse Angiogenesis Array Kit was used to analyze the protein expression of different pro- and anti-angiogenic factors in culture supernatants. Array membrane images are shown. The table gives the mouse angiogenesis array coordinates with a description, location and the mean spot pixel density of each angiogenic factor in the membrane array. The mean spot pixel density was quantified from the arrays using image analysis software Adobe Photoshop CS6 V 13.0.
(TIF)
Red outlines represent the immunoblot sections presented in Fig 6.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.