Abstract
Purpose
Azathioprine (AZA) is widely used as an immunosuppressive drug in autoimmune diseases, but its use is limited by significant adverse drug reactions (ADRs). Thiopurine S-methyltransferase (TPMT) is an important enzyme involved in AZA metabolism. Several clinical guidelines recommend determining TPMT genotype or phenotype before initiating AZA therapy. Although several studies have investigated the association between TPMT polymorphisms and AZA-induced ADRs, the results are inconsistent. The purpose of this study is to evaluate whether there is an association between TPMT polymorphisms and AZA-induced ADRs using meta-analysis.
Methods
We explored PubMed, Web of Science and Embase for articles on TPMT polymorphisms and AZA-induced ADRs. Studies that compared TPMT polymorphisms with-ADRs and without-ADRs in patients with autoimmune diseases were included. Relevant outcome data from all the included articles were extracted and the pooled odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were calculated using Revman 5.3 software.
Results
Eleven published studies, with a total of 651 patients with autoimmune diseases, investigated associations between TPMT polymorphisms and AZA-induced ADRs, were included in this meta-analysis. Our meta-analysis demonstrated that TPMT polymorphisms were significantly associated with AZA-induced overall ADRs, bone marrow toxicity and gastric intolerance; pooled ORs were 3.12 (1.48–6.56), 3.76 (1.97–7.17) and 6.43 (2.04–20.25), respectively. TPMT polymorphisms were not associated with the development of hepatotoxicity; the corresponding pooled OR was 2.86 (95%CI: 0.32–25.86). However, the association in GI subset could be driven by one single study. After this study was excluded, the OR was 2.11 (95%CI: 0.36–12.42); namely, the association became negative.
Conclusions
Our meta-analysis demonstrated an association of TPMT polymorphisms with overall AZA-induced ADRs, bone marrow toxicity and gastric intolerance, but not with hepatotoxicity. The presence of the normal TPMT genotypes cannot preclude the development of ADRs during AZA treatment, TPMT genotyping prior to commencing AZA therapy cannot replace, may augment, the current practice of regular monitoring of the white blood cell. Because of small sample sizes, large and extensive exploration was required to validate our findings.
Introduction
Autoimmune diseases are a group of heterogeneous maladies in which the patient’s immune homeostasis becomes so deregulated that it mounts a destructive attack against the host’s tissues[1].Such diseases are characterized by the activation of T cells or B cells, or both, in the absence of an ongoing infection or other discernible cause[2, 3].The treatment strategies of such diseases include immunosuppressant—medication that alters thresholds of immune activation[3]. Azathioprine (AZA), a synthetic purine analogue, is widely used as immunosuppressive drug in autoimmune diseases, including rheumatoid arthritis (RA), autoimmune hepatitis (AIH), systemic lupus erythematosus (SLE) and autoimmune bullous diseases. Despite of its efficacy, AZA was documented for adverse drug reactions (ADRs), such as bone marrow toxicity (BMT), gastric intolerance (GI), pancreatitis, hepatotoxicity, etc.
The variable response to, and efficacy of, AZA are related to its pharmacogenetics. AZA is an inactive compound that must be metabolized to 6-thioguanine nucleotides (6-TGNs) to exert both the cytotoxic and therapeutic effects[4]. AZA is a pro-drug that is absorbed into the plasma and rapidly converted into 6-mercaptopurine (6-MP) via a glutathione-dependent process. Thiopurine S-methyltransferase (TPMT) is an important cytoplasmic enzyme catalyzing the methylation of 6-MP, competing with xanthine oxidase (XO) and hypoxanthine guanine phosphoribosyl transferase (HPRT) to determine the amount of 6-MP metabolized to 6-TGNs[5].The gene encoding for TPMT is subject to genetic polymorphisms that have been studied extensively. To date, a total of 37 mutations have been identified [6]. Approximately 4%-11% of individuals are heterozygous for a mutant TPMT allele and have intermediate TPMT activity; whereas approximately 1 in 300 individuals are homozygous or compound heterozygous and have very low or absent TPMT activity[7–9]. Individuals with intermediate TPMT activity accumulate 50% more 6-TGNs when compared with normal or high TPMT activity and thus at increased risk of AZA-induced ADRs [10]. Patients with deficient TPMT activity rapidly accumulate high doses of 6-TGNs, resulting in fatal bone marrow toxicity. Several clinical guidelines recommend determining TPMT genotypes or phenotypes before commencing AZA therapy [11–13].Drug label modifications for AZA approved by the U.S. Food and Drug Administration (FDA) also recommend pretesting, but does not mandate it[14]. The evidence base for these recommendations is unclear, particularly the crucial, direct evidence that pre-therapy TPMT measuring decreases BMT-specific mortality [15]. In addition, it is still controversial whether there is an association between TPMT polymorphisms and AZA-induced ADRs.
AZA, the pro-drug of 6-mercaptopurine, is also widely prescribed to patients with inflammatory bowel disease (IBD). Previous meta-analyses on association between TPMT polymorphisms and thiopurine-induced ADRs in patients with IBD were available [16–18]. However, to the best of our knowledge, there were no similar meta-analyses in patients with auto-immune disease. In the present study, we performed a meta-analysis with the purpose of gaining more insight into a possible association between TPMT polymorphisms and the common AZA-induced ADRs by evaluation of the literature on this subject. The finding of a significant association may become indirect evidence for pretesting TPMT genotype before commencing AZA therapy in patients with autoimmune diseases.
Results
Literature search outcome
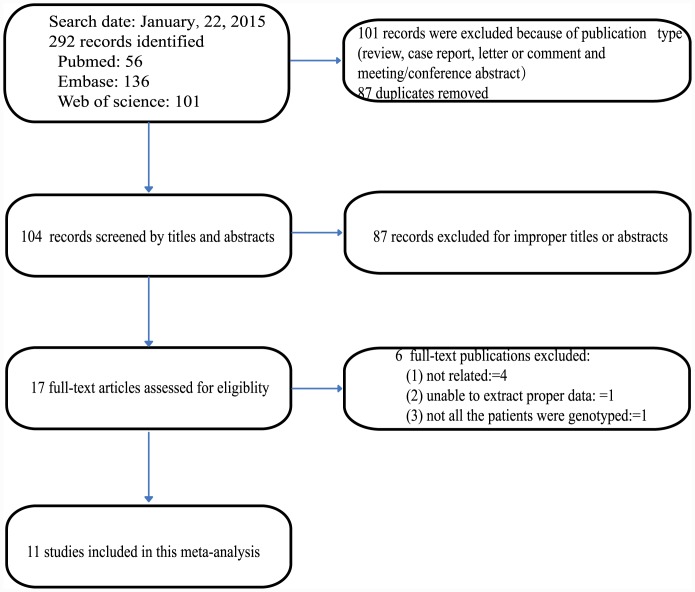
With the aforementioned search strategy, a total of 292 potentially relevant records were retrieved. 101 records were excluded because of publication type (review, case report, letter or comment and meeting/conference abstract). 87 records were excluded because they were duplicates and another 87 records were excluded after reviewing the titles and the abstracts for improper titles or abstracts; 17 full-text papers were deemed to be relevant and were examined in detail. 6 full-text papers were excluded for the reasons described in Fig 1. Finally, 11 studies [19–29] met the inclusion criteria, and were included in this meta-analysis.
Fig 1. Flowchart describing the systematic literature search and study selection process.
Characteristics of included studies
A total of 11 studies with 651 patients with autoimmune diseases were included in our meta-analysis and the average number of patients per study was 59, ranging from 9[26] to 126[29]. A summary of the included studies is listed in Table 1.The earliest studies were reported in 1999 [19, 20], while the latest was in 2014 [29].4 studies[20, 23, 24, 29] were about the association between TPMT polymorphism and AZA-induced ADRs in SLE patients, 3 studies [21, 25, 26] focused on this association in AIH patients, another 3 studies [19, 22, 28] were talking about the association in patients with rheumatic diseases or RA while 1 study [27] concentrated this association in patients with autoimmune bullous diseases. Only one of the eleven included studies reported the occurrence of AZA-induced pancreatitis [25], but data were insufficient to calculate OR and corresponding 95%CI. 6[20–22, 25, 27, 28]of the 11 studies were from research in Caucasian populations of European ancestry, while the other 5[19, 23, 24, 26, 29]studies were from Asian populations. We can see that TPMT*3A is the most common mutant allele in Caucasians while TPMT*3C is the most common in Asians. TPMT*2 was a relatively rare variant allele, which was only found in one study[27]. As can be observed in Table 1, 8 studies determined TPMT*2,*3A, *3B and *3C alleles[19, 20, 22, 24, 26–29], 2 studies determined TPMT*3A, *3B and *3C[21, 25], while 1 study determined TPMT*2,*3A, *3B, *3C and *6 [23]. When all the studies were considered, including a total of 651 patients, three homozygous mutant genotypes were detected, with a frequency of approximately 1/217.
Table 1. Characteristics of 11 studies included in this meta-analysis*.
| Author | Year | Country | Study Design | No. of Patients Included | Disease | TPMT Genotypes Determined | Profile of mutant TPMT polymorphisms | Dose of AZA | No. of Overall ADRs | NO. of BMT | NO. of Hepatotoxicity | NO. of GI | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naughton, M A | 1999 | UK | CS | 78 | SLE | TPMT*2,*3A,*3B,*3C | 1 Homo.*3A; 2 Het. *3A;1 Het.*3C | 25–250 mg/day | 17 | 13 | 3 | 1 | [16] |
| Ishioka, S | 1999 | Japan | CS | 36 | Rheumatic Diseases | TPMT*2,*3A,*3B,*3C | 3 Het. *3C | 50 mg/day | NA | 7 | NA | NA | [17] |
| Langley, P G | 2002 | UK | CS | 53 | AIH | TPMT*3A,*3B,*3C | 7 Het.*3A; 3 Het. *3B | 50–100 mg/day | NA | 3 | NA | NA | [18] |
| Corominas, H | 2003 | Spain | CS | 40 | RA | TPMT*2,*3A,*3B,*3C | 4 Het.*3A;1 Het.*3B | 50–100 mg/day | 6 | 2 | NA | 3 | [19] |
| Jun, J B # | 2005 | Korea | CS | 94 | SLE | TPMT*2,*3A,*3B,*3C,*6 | 5 Het.*3C; 2 Het. *6 | 25–100 mg/day | 23 | 17 | 1 | 4 | [20] |
| Okada, Y | 2005 | Japan | CS | 18 | SLE | TPMT*2,*3A,*3B,*3C | 2 Het.*3C | 50 mg/day | NA | 3 | NA | NA | [21] |
| Heneghan, M A | 2006 | UK | CS | 86 | AIH | TPMT*3A,*3B,*3C | NA | 100 mg/day | 22 | 13 | NA | NA | [22] |
| Tamori, A | 2007 | Japan | CS | 9 | AIH | TPMT*2,*3A,*3B,*3C | 1 Homo.*3C | 50 mg/day | NA | 2 | NA | NA | [23] |
| Bezier, M # | 2008 | France | CS | 33 | Autoimmune Bullous Diseases | TPMT*2,*3A,*3B,*3C | 1 Het. *3C; 1 Het.*2 | 2.7mg/kg/day | NA | 12 | NA | NA | [24] |
| Tani, C | 2009 | Italy | CS | 78 | Rheumatic Diseases | TPMT*2,*3A,*3B,*3C | 1 Homo.*3A; 1 Het.*3A | 1.4mg/kg/day | NA | 1 | NA | NA | [25] |
| Chen, D | 2014 | China | CS | 126 | SLE | TPMT*2,*3A,*3B,*3C | 4 Het. *3C. | 1.4–2.0 mg/kg/day | 44 | 34 | 4 | 4 | [26] |
*: The meta-analysis was performed on the studies looking at TPMT*3 family (including TPMT*3A, TPMT *3B and TPMT *3C).
#: All data being combined were the results from the same association model, thus Het*6 and Het*2 were not included in this meta-analysis.
CS: cross sectional; SLE: systemic lupus erythematosus; AIH: autoimmune hepatitis; RA: rheumatoid arthritis; AZA: azathioprine; TPMT: thiopurine S-methyltransferase; Homo.: homozygous; Het.: heterozygous; ADR: adverse drug reaction; NA: not available; GI: gastric intolerance.
In order to make the study clearer to readers, the definitions of AZA-induced ADRs are summarized as below: AZA-induced BMT markedly varied between studies, but the threshold for the number of leucopenia was generally set at 3–4×109/L, and the number of neutrophils at 1.5×109/L. The definitions of AZA-induced hepatotoxicity also differed between studies, with the level of alanine transaminase (ALT) set at >2 times the upper limit of normal (ULN). Gastric intolerance was defined as occurrence of any or a combination of the following: nausea, vomiting, dyspepsia and abdominal pain with normal amylase and normal abdominal ultrasound. Positive TPMT polymorphisms were defined as: with one or more mutant TPMT alleles (TPMT *3A, TPMT *3B and TPMT *3C).
Meta-analysis outcomes
TPMT polymorphisms and AZA-induced overall ADRs
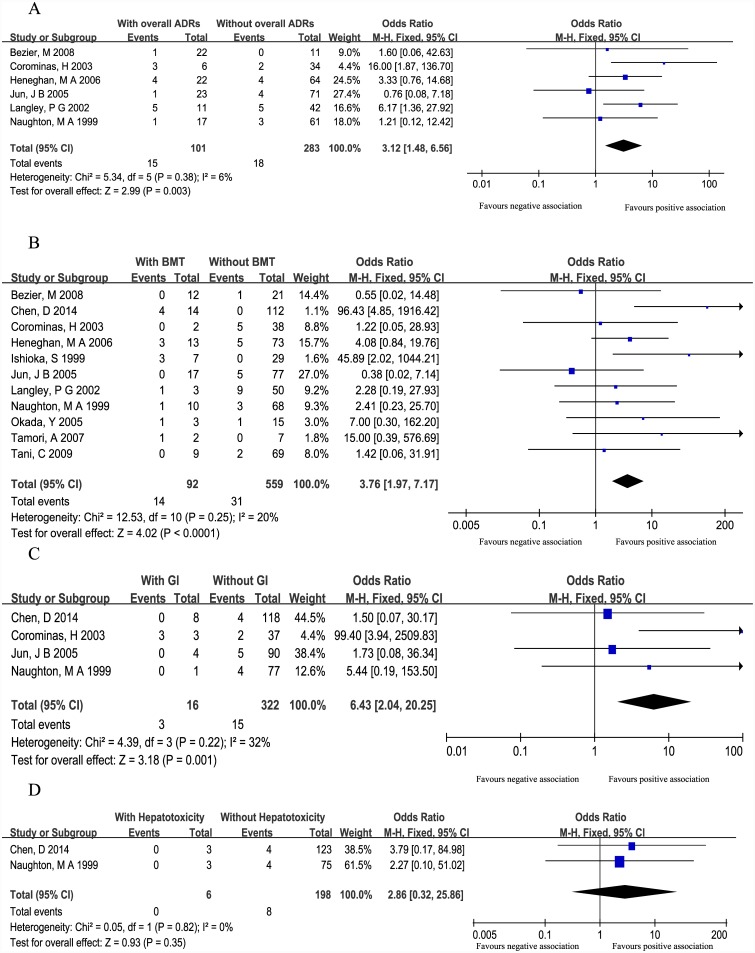
6 studies [19, 21–23,25, 27], including 384 patients, analyzed the association between TPMT polymorphisms and overall ADRs. Of the 101 patients with overall ADRs, 15 (14.9%) patients were TPMT polymorphism positive and18 (6.4%) out of 283 patients without overall ADRs were TPMT polymorphisms positive. The pooled OR (3.12, 95%CI: 1.48–6.56) indicated a significant association between TPMT polymorphisms and AZA-induced overall ADRs (Fig 2A).
Fig 2. Forest plots of association between TPMT polymorphisms and AZA-induced overall ADRs (A), bone marrow toxicity (B), hepatotoxicity (C) and gastric intolerance (D).
Total: total number of patients with or without ADRs. Events: number of patients with one or more mutant TPMT alleles (TPMT*3A, TPMT*3B and TPMT*3C) within the ADRs or no ADRs group.
TPMT polymorphisms and AZA-induced BMT
All the included studies, with 651 patients, reported the association between TPMT polymorphisms and BMT. Of 92 patients with BMT, 14(15.2%) were TPMT polymorphisms positive, compared with 31(5.6%) of the 559 patients without BMT. There was a significant association between TPMT polymorphisms and BMT (pooled OR = 3.76, 95%CI = 1.97–7.17) (Fig 2B).
TPMT polymorphisms and AZA-induced GI
4 studies [20, 22, 23, 29] which included 338 patients, reported the correlation between TPMT polymorphisms and gastric intolerance. Of the 16 patients with GI, 3 (18.8%) patients were TPMT polymorphisms positive, compared with 15(4.7%) of the 322 patients without GI. The pooled OR (95%CI) was 6.43 (2.04–20.25) indicated a significant association between TPMT polymorphisms and AZA-induced GI (Fig 2C).
TPMT polymorphisms and AZA-induced hepatotoxicity
2 studies [20, 29], which included 204 patients, reported the correlation between TPMT polymorphisms and hepatotoxicity. Of the 6 patients with hepatotoxicity, no patients were TPMT polymorphisms positive, compared with 8 (4.0%) of the 198 patients without hepatotoxicity. The overall OR (2.86, 95%CI: 0.32–25.86) demonstrated that TPMT polymorphisms did not predict AZA-induced hepatotoxicity (Fig 2D).
Subgroup analysis
Subgroup analysis according to ethnicity
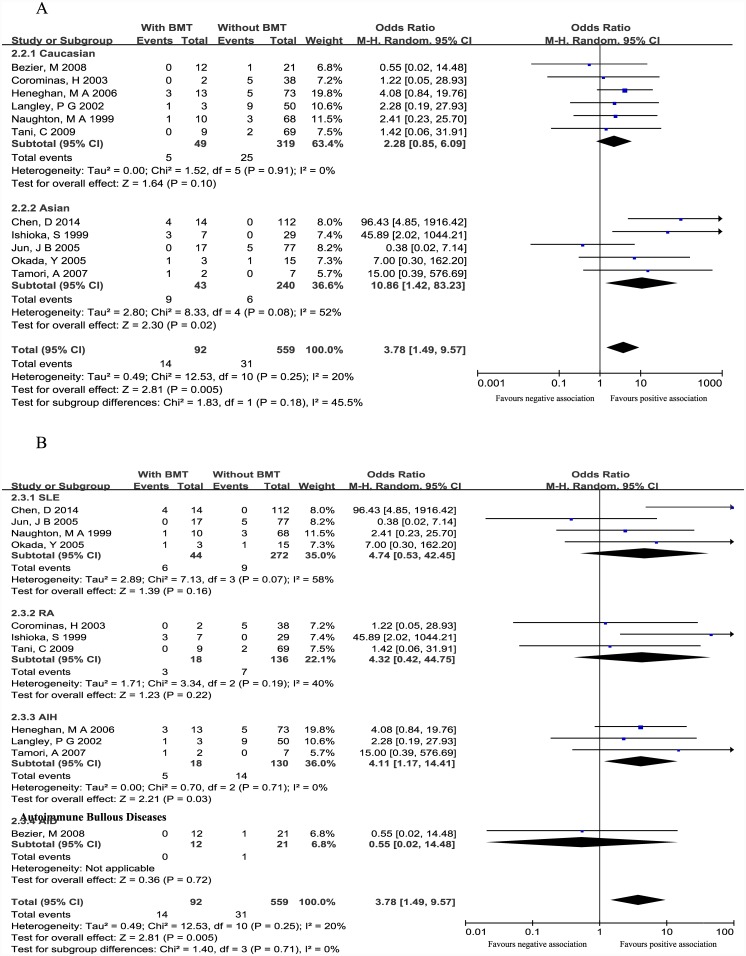
We performed subgroup analysis according to ethnicity in order to investigate whether the association signal differs among different ethnic origin. From the results, we can see that the pooled ORs (95%CI) of Caucasian population subgroup and Asian population subgroup in BMT subset were 2.28(0.85–6.09) and 3.78 (1.49–9.57), respectively. These results still showed a significant association between TPMT polymorphisms and AZA-induce BMT in Asian populations while the association in Caucasian populations was not significant (Fig 3A).
Fig 3. Forest plots of subgroup analysis according to ethnicity (A) and disease (B) in BMT subset.
SLE: systematic lupus erythematosus. AIH: autoimmune hepatitis. RA: rheumatoid arthritis. Total: total number of patients with or without ADRs. Events: number of patients with one or more mutant TPMT alleles (TPMT*3A, TPMT*3B and TPMT*3C) within the ADRs or no ADRs group.
Because of small sample size, heterozygous and homozygous patients were grouped together as TPMT polymorphism positive. In order to better investigate the association between TPMT heterosigosity and AZA-induced BMT, an extra meta-analysis by excluding individuals with homozygosity genotypes of the genotyped TPMT polymorphisms was performed. The pooled OR (95%CI) of BMT subset was 3.47(1.78–6.79). This result was consistent with the original result, which indicated that TPMT heterosigosity was also associated with AZA-induced overall BMT.
Subgroup analysis according to disease
We also performed subgroup analysis according to disease in order to investigate whether the association signal differs among different disease sources. From the results, we can see that the pooled ORs (95%CI) of SLE subgroup, AIH subgroup, RA subgroup and autoimmune bullous diseases subgroup in BMT subset were 4.16 (1.59–6.88), 5.18 (1.36–19.69), 4.21 (1.25–14.15) and 0.31 (0.01–7.05), respectively (Fig 3B).
Sensitivity analysis and publication bias
Sensitivity analysis was performed to examine the influence set by the individual study on the overall ORs by deleting each study once in every subset. Results in overall ADRs, BMT and hepatotoxicity subsets were consistent with the original results. However, the association in GI subset could be driven by one single study [22]. After this study was excluded, the OR (95%CI) was 2.31 (0.36–12.42), namely, the association became negative.
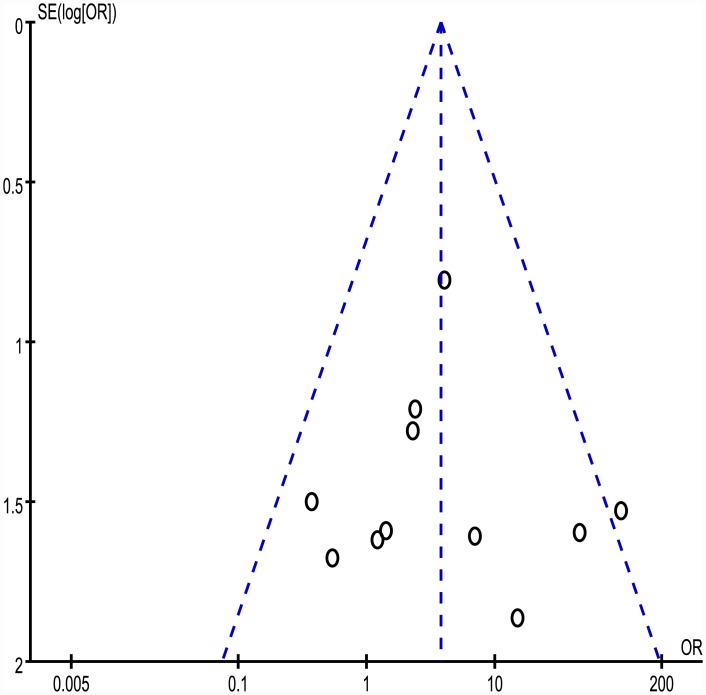
As a recommendation, tests for funnel plot asymmetry should not be used when there are fewer than 10 studies in the meta-analysis [30], thus only funnel plot of BMT subset is shown in Fig 4. Egger’s test was used to provide statistical evidence of potential publication bias. The results did not suggest any evidence of publication bias.
Fig 4. Funnel plot of BMT subset.
The dotted vertical line indicates the overall OR. S.E. = standard error, OR = odds ratio. Each circle represents an eligible study.
Discussion
AZA is widely used as immunosuppressive drug in autoimmune diseases. For instance, it has most often been prescribed as an alternative to cyclophosphamide and methotrexate (MTX) in SLE and RA [23], respectively. However, safety concerns do exist, because moderate to serious adverse events may occur. Gastric intolerance, bone marrow toxicity, hepatotoxicity and pancreatitis were among the most frequently reported clinically relevant adverse events. These events may be divided into dose-independent idiosyncratic reactions and dose-related, pharmacologically explainable toxicity [31].The results of our meta-analysis demonstrated that patients who were TPMT polymorphism positive were more likely to experience overall ADRs, BMT and GI, but not hepatotoxicity. However, several aspects have to be taken into consideration when interpreting the results of our study.
First, we identified that there have been a number of variants tested in the included studies for our meta-analysis. All the included studies determined the TPMT*3 family, while some studies tested additional variants, such as, a study of Korean population determined TPMT*6[23].TPMT*3A is the most common variant allele in Caucasian populations, while TPMT*3C is the most common mutant allele in Asian and African populations [32, 33]. TPMT*6 may be a potentially unique mutant allele within the Korean population, which was previously found in Korean children [34].Genotyping for the TPMT*3 family of variant alleles (TPMT*3A, TPMT*3B and TPMT*3C) will detect over 92% of low activity alleles and inclusion of TPMT*2 pushes this to over 95%[14]. However, all the studies tested the most common polymorphisms and these would miss rare variants, which may lead to the underestimation of the effect of TPMT polymorphisms on AZA-induced ADRs.
Second, TPMT polymorphism can explain a variable proportion of AZA-related ADRs, but in no way explain all episodes of ADRs [15]. In other words, the majority cases of ADRs were not TPMT-related, thus TPMT genotyping prior to commencing AZA therapy cannot replace, may augment, the current practice of regular monitoring of the white blood cell. Our study showed that TPMT polymorphism positive had specificity of 94.10% (526/559), but at the expense of a sensitivity of 16.30% (15/92) for predicting AZA-induced BMT. The specificity and sensitivity of TPMT polymorphism positive for predicting overall ADRs were 92.93% (263/283) and 14.85% (15/101).Several reasons may account for the gap between TPMT polymorphism and ADRs. First, up to 37 mutations in TPMT have been reported[6]. In included studies, 3 to 5 inactive TPMT alleles were investigated. We assume that patients with ADRs had one of the rare inactive TPMT alleles that were not examined. Another factor is the influence of variations in other genes, such as hypoxanthine guanine phosphoribosyltransferase(HPRT)[26]and inosine triphosphate pyrophosphatase(ITPA)[35].The development of AZA-induced ADRs is a multi-factorial event, caused by a co-influence of factors, other than variants in TPMT[36, 37], and a combined evaluation of the potential factors may enhance the correlation with ADRs. If possible, a genome-wide association study (GWAS) was required to investigate the associations between potential genes and ADRs. For instance, a study by Zalbala [37] reported variants associated with thiopurine-related BMT that was identified by GWAS. They indentified that rs372996 in interleukia 6 singnal transducer (IL6ST) gene and re3749598 in follistatin-like 5 (FSTL5) gene as new bone marrow toxicity susceptibility candidate genes after thiopurine treatment in IBD patients. The ORs (95%CI) were 3.41 (1.71–6.78) and 3.67 (1.68–8.01), respectively. Another GWAS association study published in Nature Genetics revealed variants associated with thiopurine-induced pancreatitis in patients with IBD [38]. Strong evidence of association within the class II HLA region were reported, with the most significant association identified at rs2647087 [OR (95%CI) 2.59 (2.07–3.26), p value of 2× 10−16].
Third, sample sizes of included studies were relatively small, ranging from 9 to 126 patients, which will increase uncertainty. The results of sensitivity analysis in our study showed that the association could be driven by one single study in GI subset. After this study was excluded, the association in GI subset became negative. In this study, three patients with TPMT polymorphism positive were observed to have GI reactions. Extensive exploration was required to support whether this was a chance phenomenon or not. Besides, a point of weakness in our study is the wide heterogeneity of patient cohort in terms of diagnosis. Although the pooled OR (95%CI) in BMT subset was 3.76 (1.97–7.17), subgroup analysis in BMT subset showed the pooled ORs (95%CI) of SLE subgroup, AIH subgroup, RA subgroup and autoimmune bullous diseases subgroup in BMT subset were 4.74 (0.53–42.25), 4.32 (0.42–44.75), 4.11 (1.17–14.41) and 3.78 (1.49–9.57), respectively.
Fourth, disease-related BMT episodes are often difficult to distinguish from those caused by drugs, such as BMT is a feature of SLE disease activity. The study by Naughton et al. [20]detected that 13 patients with BMT, however subsequent investigations found that, in three of these cases, the BMT was disease related, while AZA was strongly implicated in the remaining 10 cases.
Fifth, there is no doubt in the literature that patients who are homozygous or compound heterozygous for a variant allele confer a very high-risk of early severe BMT. This was confirmed by our study; as 2 out of the three homozygotes detected in all the 651 patients experienced early, severe leucopenia requiring hospital management. Such susceptible patients can be identified by TPMT genotyping prior to commencing AZA therapy, thus avoiding potentially fatal consequences. Prospective studies are required, however, to explore the cost-effectiveness of this approach due to low frequency of homozygous individuals.
Sixth,because of the life threatening nature of AZA-induced BMT, pretesting for TPMT genotype before the initiation of AZA therapy has increasingly been accepted clinically. Several guidelines recommend determining TPMT status before AZA therapy. However, these recommendations are considered to be premature from an evidence-based perspective, due to the absence of direct and crucial evidence that TPMT pretreatment testing decreases BMT-specific mortality [15].
In conclusion, our meta-analysis demonstrated an association of TPMT polymorphisms with overall AZA-induced ADRs, bone marrow toxicity and gastric intolerance, but not with hepatotoxicity. Because of small sample sizes and wide heterogeneity of patient cohort in terms of diagnosis, large and extensive exploration was required to support whether these findings were chance phenomena or not. TPMT polymorphism can explain a variable proportion of AZA-related ADRs, but in no way explain all episodes of ADRs. In other words, the presence of the normal TPMT genotype cannot preclude the development of ADRs during AZA treatment, TPMT genotyping prior to commencing AZA therapy cannot replace, may augment, the current practice of regular monitoring of the white blood cell.
Materials and Methods
Literature search strategy
Medline (using PubMed as the search engine), The Excerpta Medica Database (Embase) and Web of science were searched to identify relevant publications published in English with an end date of January 22, 2015. Only human-related literature was searched. We employed both MeSH terms and free text words (in Title/Abstract fields) for ‘TPMT’ or ‘thiopurine S-methyltransferase’ or ‘thiopurine methytransferase’ AND ‘azathioprine ‘ or ‘imuran’ or ‘6-mercaptopurine’ AND (‘autoimmune diseases’ OR ‘SLE’ or ‘systemic lupus erythematosus’ or ‘lupus erythematosus disseminatus’ or ‘libman scks disease’ OR ‘RA’ or ‘Rheumatoid Arthritis’ OR ‘AIH’ or ‘Autoimmune Hepatitis’). We also performed a manual search of the references listed in the articles identified in the search for additional eligible studies. The search was conducted independently by two reviewers (YPL and HQX).
Inclusion and exclusion criteria
The abstracts and full texts were read independently by the two reviewers (YPL and HQX). The following inclusion criteria were used: 1) studies that compared TPMT polymorphisms between with-ADRs and without-ADRs in patients with autoimmune diseases; 2) articles published in English and being human-related were included; 3) expert opinions supported by a preliminary literature review indicated that there was likely to be very few randomized, controlled trials (RCTs) on this topic; therefore, any study design (cross-sectional cohort, prospective cohort and case control studies) were included in this meta-analysis [39]; 4) all patients included in this meta-analysis were genotyped for TPMT polymorphisms; 5) studies that tested at least TPMT*3A, TPMT *3B, TPMT *3C, regardless of whether they tested additional mutant alleles. Studies on non-autoimmune diseases patients were excluded. Reviews, letters, comments, and conference abstracts were also excluded because of limited data. Further, publications identified as duplicates were excluded.
Data extraction strategy
Two reviewers (YPL and HQX) independently extracted relevant data from each eligible study. The following data were collected: author’s name, publication year, country, study type, number of enrolled patients, disease, profile of mutant TPMT polymorphisms, AZA dose, number of patients that were mutant-type TPMT with and without an ADR, TPMT polymorphism type, number of homozygous mutant-type TPMT, and definitions of ADRs. Disagreements between reviewers were resolved by discussion or by consensus including a third author (QH).
Statistical analysis
In order to make results of the present meta-analysis more robust and reasonable, all data being combined were the results from the same association model. In addition, TPMT*3B is usually in tight linkage disequilibrium with the *3C SNP, resulting in the common allele, *3A[40]. Thus, the meta-analysis was performed on the studies looking at TPMT*3 family (including TPMT*3A, TPMT *3B and TPMT *3C). OR and 95% CIs were calculated to mainly evaluate the strength of associations between TPMT*3A/TPMT*3B/ TPMT*3C and AZA-induced ADRs. Not all studies reported all ADRs analyzed in this meta-analysis, and so only studies that reported the adverse events of interest were analyzed for the association between TPMT polymorphisms and that adverse event. The included studies displayed heterogeneity concerning diagnosis, the time to onset of AZA-induced ADRs, definitions of the ADRs, and study designs. The degrees of included studies’ heterogeneity were explored using the chi-squared test of heterogeneity, and inconsistency index (I2). Considering the low statistical power of these tests, a p-value of <0.10 or an I2>50% was defined as significant heterogeneity. ORs from different groups were combined using fixed or random effects models, which depends on the absence or presence of significant heterogeneity.
Subgroup analysis was conducted according to ethnicity or disease in order to investigate whether the association signal differs among different ethnic origin or different diseases.
Sensitivity analysis was performed to assess the stability of the results; namely, a single study in the meta-analysis was deleted each time to reflect the influence of the individual data set to the overall OR. Publication bias was assessed by visual inspection of the funnel plot for symmetry, and formal statistical testing using the Egger test. The meta-analysis was conducted using RevMan 5.3 software.
Supporting Information
(DOC)
(DOCX)
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China(General Program, No.31370853); the Transformation Foundation on Scientific and Technological Achievements of The Third Military Medical University (2012XZH03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from National Natural Science Foundation of China (General Program, No. 31370853), and the Transformation Foundation on Scientific and Technological Achievements of The Third Military Medical University (2012XZH03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Das PK, Elliott G. Conference scene: lessons from animal models of autoimmune diseases: from mechanisms to applications. Immunotherapy. 2011;3(2):147–151. 10.2217/imt.10.102 [DOI] [PubMed] [Google Scholar]
- 2. Sinha AA, Lopez MT, McDevitt HO. Autoimmune diseases: the failure of self tolerance. Science. 1990;248(4961):1380–1388. [DOI] [PubMed] [Google Scholar]
- 3. Goding JW. Autoimmune diseases. N Engl J Med. 2001;345(23):1707–1708. [PubMed] [Google Scholar]
- 4. Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992;43(4):329–339. [DOI] [PubMed] [Google Scholar]
- 5. Lennard L. TPMT in the treatment of Crohn's disease with azathioprine. Gut. 2002;51(2):143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Appell ML, Berg J, Duley J, Evans WE, Kennedy MA, Lennard L, et al. Nomenclature for alleles of the thiopurine methyltransferase gene. Pharmacogenet Genomics. 2013;23(4):242–248. 10.1097/FPC.0b013e32835f1cc0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collie-Duguid ES, Pritchard SC, Powrie RH, Sludden J, Collier DA, Li T, et al. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics. 1999;9(1):37–42. [DOI] [PubMed] [Google Scholar]
- 8. Engen RM, Marsh S, Van Booven DJ, McLeod HL. Ethnic differences in pharmacogenetically relevant genes. Curr Drug Targets. 2006;7(12):1641–1648. [DOI] [PubMed] [Google Scholar]
- 9. Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. American journal of human genetics. 1980;32(5):651–662. [PMC free article] [PubMed] [Google Scholar]
- 10. Egan LJ, Derijks LJJ, Hommes DW. Pharmacogenomics in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4(1):21–28. [DOI] [PubMed] [Google Scholar]
- 11. Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89(3):387–391. 10.1038/clpt.2010.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013;93(4):324–325. 10.1038/clpt.2013.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anstey AV, Wakelin S, Reynolds NJ, British Association of Dermatologists Therapy G, Audit S. Guidelines for prescribing azathioprine in dermatology. Br J Dermatol. 2004;151(6):1123–1132. [DOI] [PubMed] [Google Scholar]
- 14. Lennard L. Implementation of TPMT testing. Br J Clin Pharmacol. 2014;77(4):704–714. 10.1111/bcp.12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Booth RA, Ansari MT, Loit E, Tricco AC, Weeks L, Doucette S, et al. Assessment of thiopurine S-methyltransferase activity in patients prescribed thiopurines: a systematic review. Ann Intern Med. 2011;154(12):814–823, W-295-818. 10.7326/0003-4819-154-12-201106210-00009 [DOI] [PubMed] [Google Scholar]
- 16. Liu YP, Wu HY, Yang X, Xu HQ, Li YC, Shi DC, et al. Association between thiopurine S-methyltransferase polymorphisms and thiopurine-induced adverse drug reactions in patients with inflammatory bowel disease: a meta-analysis. PLoS One. 2015;10(3):e0121745 10.1371/journal.pone.0121745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong XW, Zheng Q, Zhu MM, Tong JL, Ran ZH. Thiopurine S-methyltransferase polymorphisms and thiopurine toxicity in treatment of inflammatory bowel disease. World J Gastroenterol. 2010;16(25):3187–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gisbert JP, Gomollon F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103(7):1783–1800. 10.1111/j.1572-0241.2008.01848.x [DOI] [PubMed] [Google Scholar]
- 19. Ishioka S, Hiyama K, Sato H, Yamanishi Y, McLeod HL, Kumagai K, et al. Thiopurine methyltransferase genotype and the toxicity of azathioprine in Japanese. Intern Med. 1999;38(12):944–947. [DOI] [PubMed] [Google Scholar]
- 20. Naughton MA, Battaglia E, O'Brien S, Walport MJ, Botto M. Identification of thiopurine methyltransferase (TPMT) polymorphisms cannot predict myelosuppression in systemic lupus erythematosus patients taking azathioprine. Rheumatology (Oxford). 1999;38(7):640–644. [DOI] [PubMed] [Google Scholar]
- 21. Langley PG, Underhill J, Tredger JM, Norris S, McFarlane IG. Thiopurine methyltransferase phenotype and genotype in relation to azathioprine therapy in autoimmune hepatitis. J Hepatol. 2002;37(4):441–447. [DOI] [PubMed] [Google Scholar]
- 22. Corominas H, Domenech M, Laiz A, Gich I, Geli C, Diaz C, et al. Is thiopuriue methyltransferase genetic polymorphism a major factor for withdrawal of azathioprine in rheumatoid arthritis patients? Rheumatology (Oxford). 2003;42(1):40–45. [DOI] [PubMed] [Google Scholar]
- 23. Jun JB, Cho DY, Kang C, Bae SC. Thiopurine S-methyltransferase polymorphisms and the relationship between the mutant alleles and the adverse effects in systemic lupus erythematosus patients taking azathioprine. Clin Exp Rheumatol. 2005;23(6):873–876. [PubMed] [Google Scholar]
- 24. Okada Y, Nakamura K, Kodama T, Ueki K, Tsukada Y, Maezawa A, et al. Thiopurine methyltransferase genotype and phenotype status in Japanese patients with systemic lupus erythematosus. Biol Pharm Bull. 2005;28(11):2117–2119. [DOI] [PubMed] [Google Scholar]
- 25. Heneghan MA, Allan ML, Bornstein JD, Muir AJ, Tendler DA. Utility of thiopurine methyltransferase genotyping and phenotyping, and measurement of azathioprine metabolites in the management of patients with autoimmune hepatitis. J Hepatol. 2006;45(4):584–591. [DOI] [PubMed] [Google Scholar]
- 26. Tamori A, Shinzaki M, Kosaka S, Hayashi T, Iwai S, Enomoto M, et al. Thiopurine S-methyltransferase gene polymorphism in Japanese patients with autoimmune liver diseases. Liver international: official journal of the International Association for the Study of the Liver. 2007;27(1):95–100. [DOI] [PubMed] [Google Scholar]
- 27. Bezier M, Reguiai Z, Vitry F, Broly F, Bernard P. Thiopurine S-methyltransferase genotypic analysis in autoimmune bullous diseases. Eur J Dermatol. 2008;18(5):512–517. 10.1684/ejd.2008.0473 [DOI] [PubMed] [Google Scholar]
- 28. Tani C, Mosca M, Colucci R, Gori G, D'Ascanio A, Ghisu N, et al. Genetic polymorphisms of thiopurine S-methyltransferase in a cohort of patients with systemic autoimmune diseases. Clin Exp Rheumatol. 2009;27(2):321–324. [PubMed] [Google Scholar]
- 29. Chen D, Lian F, Yuan S, Wang Y, Zhan Z, Ye Y, et al. Association of thiopurine methyltransferase status with azathioprine side effects in Chinese patients with systemic lupus erythematosus. Clin Rheumatol. 2014;33(4):499–503. 10.1007/s10067-013-2441-x [DOI] [PubMed] [Google Scholar]
- 30. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 31. de Jong DJ, Derijks LJJ, Naber AHJ, Hooymans PM, Mulder CJJ. Safety of thiopurines in the treatment of inflammatory bowel disease. Scand J Gastroenterol. 2003;38:69–72. [DOI] [PubMed] [Google Scholar]
- 32. Lennard L, Cartwright CS, Wade R, Richards SM, Vora A. Thiopurine methyltransferase genotype-phenotype discordance and thiopurine active metabolite formation in childhood acute lymphoblastic leukaemia. Br J Clin Pharmacol. 2013;76(1):125–136. 10.1111/bcp.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McLeod HL, Pritchard SC, Githang'a J, Indalo A, Ameyaw MM, Powrie RH, et al. Ethnic differences in thiopurine methyltransferase pharmacogenetics: evidence for allele specificity in Caucasian and Kenyan individuals. Pharmacogenetics. 1999;9(6):773–776. [DOI] [PubMed] [Google Scholar]
- 34. Otterness D, Szumlanski C, Lennard L, Klemetsdal B, Aarbakke J, Park-Hah JO, et al. Human thiopurine methyltransferase pharmacogenetics: gene sequence polymorphisms. Clin Pharmacol Ther. 1997;62(1):60–73. [DOI] [PubMed] [Google Scholar]
- 35. Van Dieren JM, Hansen BE, Kuipers EJ, Nieuwenhuis EE, Van der Woude CJ. Meta-analysis: Inosine triphosphate pyrophosphatase polymorphisms and thiopurine toxicity in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2007;26(5):643–652. [DOI] [PubMed] [Google Scholar]
- 36. Zabala-Fernandez W, Barreiro-de Acosta M, Echarri A, Carpio D, Lorenzo A, Castro J, et al. A pharmacogenetics study of TPMT and ITPA genes detects a relationship with side effects and clinical response in patients with inflammatory bowel disease receiving azathioprine. J Gastrointestin Liver Dis. 2011;20(3):247–253. [PubMed] [Google Scholar]
- 37. Zabala W, Cruz R, Barreiro-de Acosta M, Chaparro M, Panes J, Echarri A, et al. New genetic associations in thiopurine-related bone marrow toxicity among inflammatory bowel disease patients. Pharmacogenomics. 2013;14(6):631–640. 10.2217/pgs.13.38 [DOI] [PubMed] [Google Scholar]
- 38. Heap GA, Weedon MN, Bewshea CM, Singh A, Chen M, Satchwell JB, et al. HLA-DQA1-HLA-DRB1 variants confer susceptibility to pancreatitis induced by thiopurine immunosuppressants. Nat Genet. 2014;46(10):1131–1134. 10.1038/ng.3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Higgs JE, Payne K, Roberts C, Newman WG. Are patients with intermediate TPMT activity at increased risk of myelosuppression when taking thiopurine medications? Pharmacogenomics. 2010;11(2):177–188. 10.2217/pgs.09.155 [DOI] [PubMed] [Google Scholar]
- 40. Wang L, Pelleymounter L, Weinshilboum R, Johnson JA, Hebert JM, Altman RB, et al. Very important pharmacogene summary: thiopurine S-methyltransferase. Pharmacogenet Genomics. 2010;20(6):401–405. 10.1097/FPC.0b013e3283352860 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.