Abstract
Purpose
To investigate the hypothesis that increased interferon-γ (IFN-γ) expression is associated with conjunctival goblet cell loss in subjects with tear dysfunction.
Methods
Goblet cell density (GCD) was measured in impression cytology from the temporal bulbar conjunctiva, and gene expression was measured in cytology samples from the nasal bulbar conjunctiva obtained from 68 subjects, including normal control, meibomian gland disease (MGD), non-Sjögren syndrome (non-SSATD)-, and Sjögren syndrome (SSATD)-associated aqueous tear deficiency. Gene expression was evaluated by real-time PCR. Tear meniscus height (TMH) was measured by optical coherence tomography. Fluorescein and lissamine green dye staining evaluated corneal and conjunctival disease, respectively. Between-group mean differences and correlation coefficients were calculated.
Results
Compared to control, IFN-γ expression was significantly higher in both ATD groups, and its receptor was higher in SSATD. Expression of IL-13 and its receptor was similar in all groups. Goblet cell density was lower in the SSATD group; expression of MUC5AC mucin was lower and cornified envelope precursor small proline-rich region (SPRR)-2G higher in both ATD groups. Interferon-γ transcript number was inversely correlated with GCD (r = −0.37, P < 0.04) and TMH (r = −0.37, P = 0.02), and directly correlated with lissamine green staining (r = 0.51, P < 0.001) and SPRR-2G expression (r = 0.32, P < 0.05).
Conclusions
Interferon-γ expression in the conjunctiva was higher in aqueous deficiency and correlated with goblet cell loss and severity of conjunctival disease. These results support findings of animal and culture studies showing that IFN-γ reduces conjunctival goblet cell number and mucin production.
Keywords: dry eye, cytokines, interferon-γ, goblet cells, gene expression
The number of mucin-filled conjunctival goblet cells (GC) has been found to decrease in aqueous-deficient dry eye and certain ocular surface inflammatory conditions, such as Stevens-Johnson syndrome and graft-versus-host disease (GVHD).1–4 The cause for GC loss in these dry eye/ocular surface diseases has not been established, but mouse models suggest it may be due to imbalanced expression of T helper (Th) cytokines, with increased levels of the Th1 cytokine interferon-γ (IFN-γ) and increased ratio of IFN-γ to the Th2 cytokine IL-13 (IFN-γ/IL-13).5,6 Altered ratios of these Th cytokines have been associated with hyperplasia or loss of GC in the airway and gut mucosa.7,8 Interleukin-13 has been found to promote GC differentiation in the conjunctival and airway epithelium, while IFN-γ has caused conjunctival GC loss in mice.6,7,9 Expression of these Th cytokines and their receptors in the conjunctiva and the relationship between levels of these cytokines and goblet cell density (GCD) and expression of cornified envelope precursors have not been studied in patients with tear dysfunction.
The purpose of this study was to investigate the hypothesis that increased IFN-γ expression is associated with conjunctival GC loss and mucin deficiency in subjects with tear dysfunction.
Subjects and Methods
Subjects
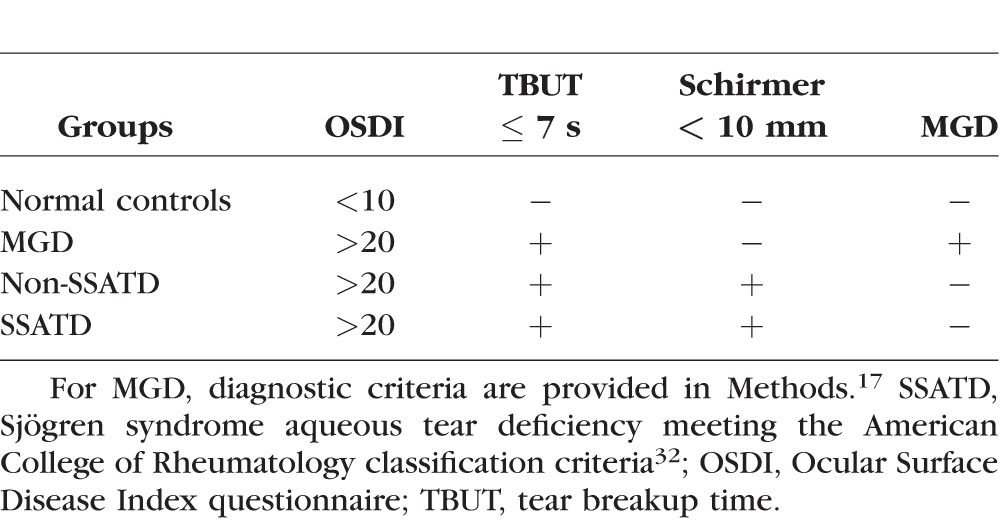
This study protocol to compare cytokine expression, GCD, and markers of cornification in the conjunctiva of subjects with tear dysfunction and normal control subjects was approved by the Baylor College of Medicine Institutional Review Board (IRB). It adhered to the tenets of the Declaration of Helsinki for clinical research and complied with the Health Insurance Portability and Accountability Act. Written informed consent was obtained from all participants after explanation of the purpose and possible consequences of the study. This is a single-institution prospective observational study. One hundred twenty-eight eyes from 68 subjects were used in this study. Tear dysfunction was stratified into meibomian gland disease (MGD), non-Sjögren syndrome aqueous tear deficiency (non-SSATD), and Sjögren syndrome aqueous tear deficiency (SSATD). Subjects with no symptoms or signs of tear dysfunction were recruited as normal controls. Diagnostic criteria used for subject classification are described in Table 1.
Table 1.
Criteria Used to Define Tear Dysfunction and Control Groups
Conjunctival Gene Expression
Cells were obtained by impression cytology of the nasal bulbar conjunctiva of each eye using the EyePrim device (Opia Tech, Paris, France) that applies a porous membrane with uniform pressure to the conjunctival surface. Both membranes were placed in 0.5 mL RNA lysis buffer (Qiagen, Valencia, CA, USA) containing 1% 2-mercaptoethanol and stored at −80°C. Total RNA was isolated from the membranes using an RNeasy Mini Kit (Qiagen) following the manufacturer's protocol. Briefly, samples were heated at 37°C for 10 minutes, vortexed, and passed through a QIAshredder column (Qiagen). Samples were applied to an RNeasy mini spin column and washed with the two buffers provided in the kit. The RNA was eluted by pipetting 50 μL RNase-free water directly onto the center of the silica-gel membrane. The RNA concentration was measured by its absorption at 260 nm using a spectrophotometer (NanoDrop 2000; Thermo Scientific, Wilmington, DE, USA), and first strand cDNA was synthesized with random hexamer using M-MuLV reverse transcriptase.
Absolute real-time PCR was performed to measure the number of IFN-γ, IL-13, IL-17A, MUC5AC, small proline-rich region 1A (SPRR-1A), SPRR-2F, and SPRR-2G and cytokeratin 7 (K7) RNA transcripts. The PCR primer identification numbers are provided in Table 2. Templates to create standard curves to measure transcript copy number were initially prepared for each gene by conventional PCR, and these products were electrophoresed on a 1.5% agarose gel and stained with ethidium bromide to confirm amplification of a single gene product. The size and identity of these PCR products were verified by cloning each into a sequencing vector using a TOPO TA Cloning Kit for Sequencing (Invitrogen, Grand Island, NY, USA), following the manufacturer's protocol. The DNA was sequenced, and sequences were verified using the BLAST program (National Library of Congress, Bethesda, MD, USA). Polymerase chain reaction products were cleaned and their concentrations were measured and converted from nanograms per microliter to copy number per microliter. Serial dilutions were performed starting from 109 to 100 for each DNA template. Absolute real-time PCR was performed using two replicates of each subject's cDNA sample and each dilution point in the standard curve. The results are presented as the mean ± SEM number of copies.
Table 2.
Real-Time PCR Primers
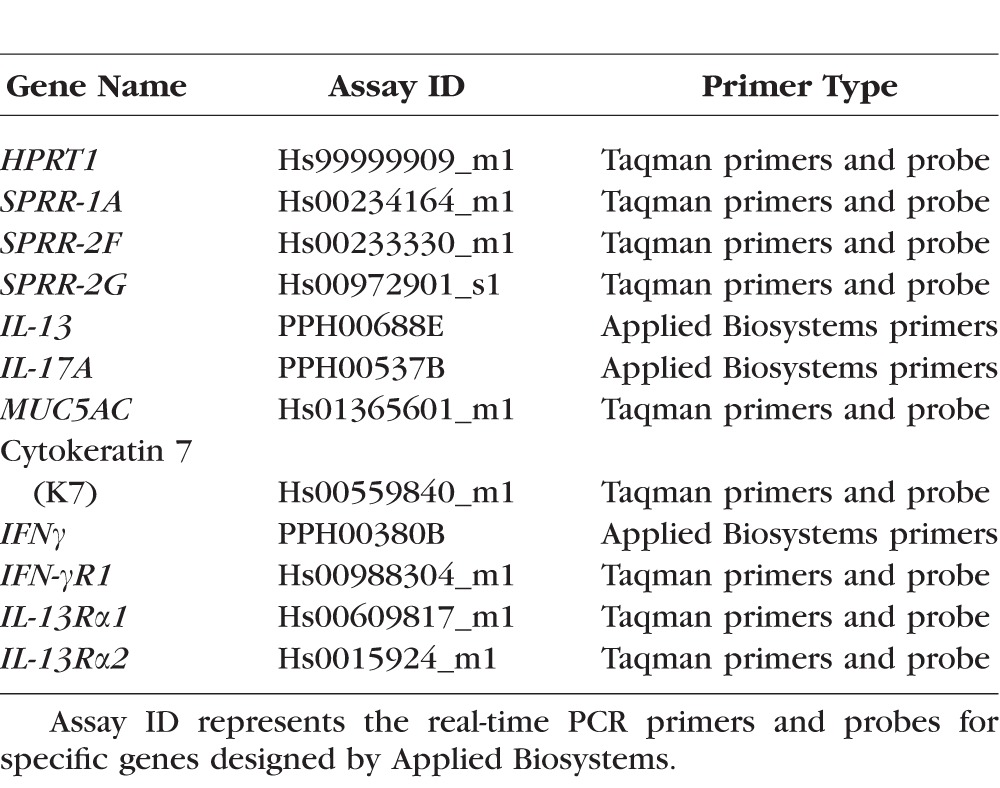
Quantitative real-time PCR was performed to evaluate expression of IFN-gamma receptor 1 (IFN-γR1), IL-13 receptor alpha 1 (IL-13Rα1), and Il-13Rα2 using Taqman Fast Universal PCR Master Mix and specific Taqman probes (Table 2; Applied Biosystems, Grand Island, NY, USA). The housekeeping gene hypoxanthine phosphoribosyltransferase 1 (HPRT-1) was amplified to normalize levels of expression. No-template or RNA controls were performed for evidence of contamination. Results were analyzed using the comparative threshold cycle method using values from the normal healthy subjects as calibrator.
Conjunctival Goblet Cell Density
Goblet cell density was measured in impression cytology specimens taken from the temporal bulbar conjunctiva of the eye with the worse lissamine green dye staining. Membranes were fixed and stained by periodic acid Schiff (PAS) reagent as previously described.10 Goblet cells were counted in five representative images taken using image analysis software (Nikon Elements, Garden City, NY, USA), normalized per area, and expressed as GC/mm2.
Statistical Analysis
Sample size calculations performed with StatMate (GraphPad, La Jolla, CA, USA) using the results of a prior study predicted that a sample size of seven subjects per group would have a 90% power of detecting a statistical difference (P = 0.05) in level of IFN-γ expression between groups.11 Between-group differences in levels of gene expression, GCD, and clinical parameters were compared by ANOVA with Tukey post hoc testing using Prism 6.0 (GraphPad). Pearson's correlation coefficients (R) were calculated to assess the relationships between levels of gene expression, GCD, and clinical parameters within the entire cohort.
Results
Clinical Features
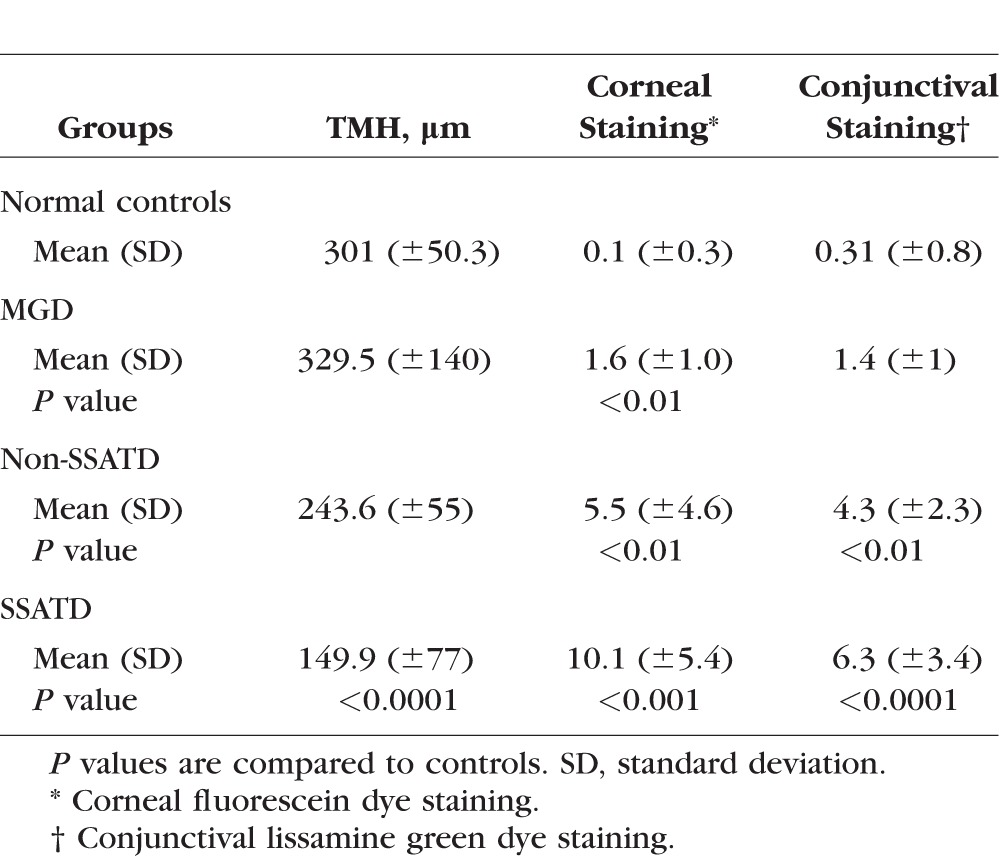
This study evaluated three groups of tear dysfunction (MGD, non-SSATD, and SSATD) and a group of normal subjects without evidence of dry eye. The demographic characteristics of the four study groups are provided in Table 3. The mean age of the control group was significantly lower than that of all of the tear dysfunction groups, but the mean age in all tear dysfunction groups was similar. The majority of subjects in all groups were female. Tear meniscus height (TMH) as a measure of tear volume and severity of cornea and conjunctival dye staining are provided in Table 4. Subjects with SSATD had a significantly lower mean TMH, and both ATD groups had greater cornea fluorescein and conjunctival lissamine green dye staining than the control group.
Table 3.
Demographic Characteristics of Study Groups
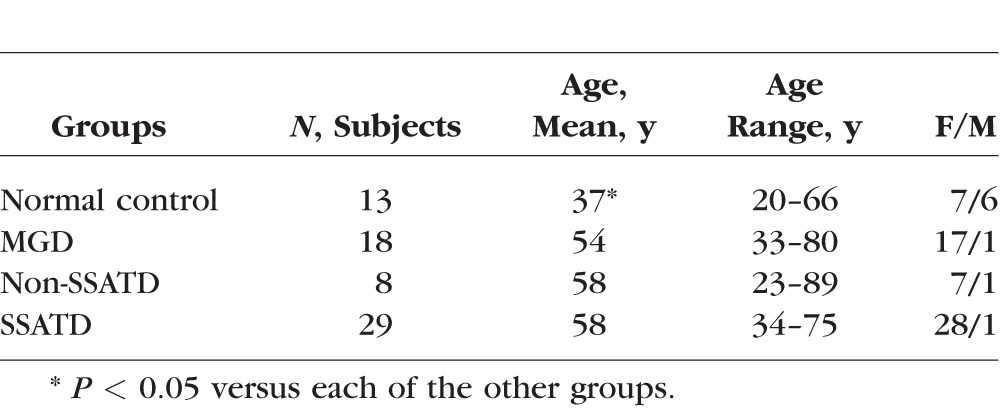
Table 4.
Summary of Clinical Data
Expression of Th Cytokine and Receptor Genes
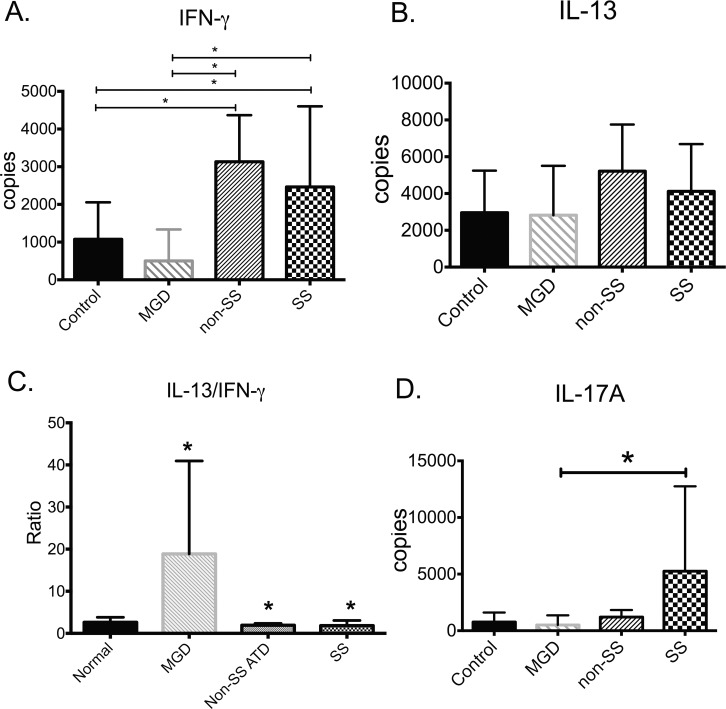
Expression of the signature Th1 and Th2 cytokines, IFN-γ and IL-13, respectively, and their receptors was measured in conjunctival cells obtained by impression cytology by quantitative real-time PCR. Levels of Th cytokines are compared in Figure 1. Compared to control, IFN-γ expression was higher in both ATD groups (P < 0.05), while expression of IL-13 was similar in all groups. The IL-13/IFN-γ ratio was higher in MGD and lower in both aqueous-deficient groups compared to control. For comparative purposes, expression of the Th17 cytokine IL-17A was measured and found to be increased in Sjögren syndrome (SS) compared to MGD.
Figure 1.
Expression of Th cytokine genes in the conjunctiva in tear dysfunction and control. Among Th cytokines, (A) IFN-γ was increased in non-SS and SS ATD versus control and MGD; (B) IL-13 was similar in all groups; (C) the IL-13/IFN-γ ratio was higher in MGD and lower in both aqueous-deficient groups than control; (D) IL-17 expression was higher in SS versus MGD. MGD, meibomian gland disease; non-SSATD, non-Sjögren syndrome aqueous tear deficiency; SSATD, Sjögren syndrome aqueous tear deficiency; *P ≤ 0.05.
Expression of the IFN-γ receptor 1 was significantly higher in the SSATD group compared to control (2.2 ± 0.25-fold greater than control; P = 0.03). The IL-13 signaling receptor, IL-13Rα1, was similar in all groups, but expression of the IL-13 decoy receptor, IL-13Rα2, was significantly increased in all three tear dysfunction groups compared to control (P ≤ 0.05).
Goblet Cell Density and Expression of Mucin and Cornifying Genes
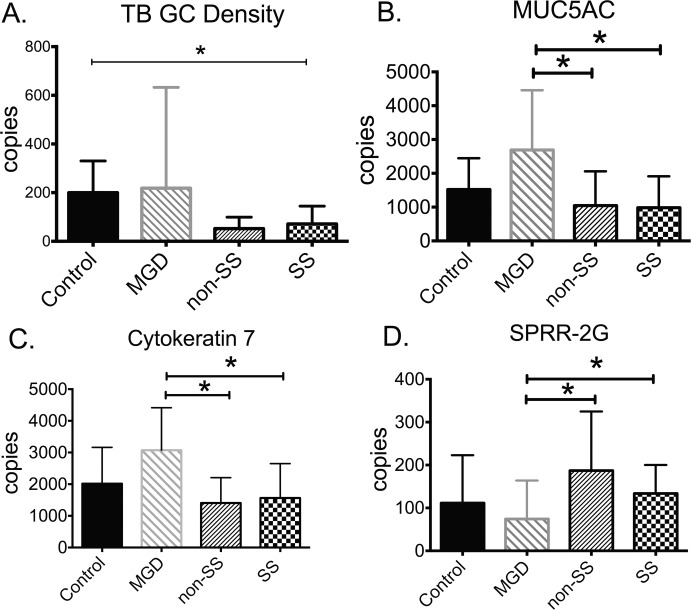
The number of PAS-positive mucin-filled GC in the temporal bulbar conjunctiva and expression of GC-associated (MUC5AC and K7) and cornified envelope precursor (SPRR-1A, -2F, and ‐2G) genes were compared in samples taken from the nasal bulbar conjunctiva of control and tear dysfunction subjects, and the results are presented in Figure 2. Goblet cell density was lowest in SS (P = 0.006). The number of mRNA transcripts encoding GC-associated MUC5AC and K7 was lower in both the ATD groups (non-SSATD and SSATD) compared to MGD. The level of cornified envelope precursors SPRR-1A, ‐2F, and ‐2G found in the epidermis of the skin and in the bulbar conjunctiva of mice with ATD has been measured.12–14 There was no difference in the level of expression of SPRR-1A and ‐2F between groups; however, the number of SPRR-2G transcripts was higher in non-SSATD and SSATD than in MGD (P < 0.05).
Figure 2.
Goblet cell density and expression of goblet cell–associated (MUC5AC and cytokeratin 7) and cornified envelope precursor genes. (A) Goblet cell (GC) density was lower in SSATD than control; (B) number of MUC5AC gene transcripts was lower in both aqueous tear-deficient groups than MGD; (C) number of cytokeratin 7 gene transcripts was lower in both aqueous tear-deficient groups than MGD; (D) number of SPRR-2G transcripts was higher in both aqueous tear-deficient groups than MGD. MGD, meibomian gland disease; non-SSATD, non-Sjögren syndrome aqueous tear deficiency; SSATD, Sjögren syndrome aqueous tear deficiency; *P ≤ 0.05.
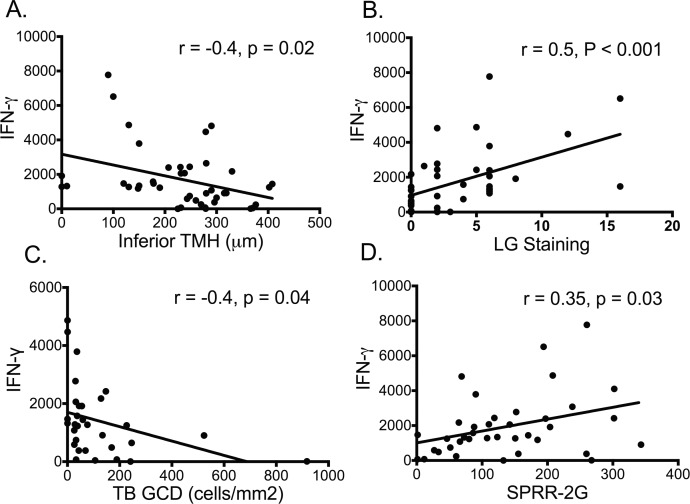
Correlation of IFN-γ With Disease Parameters
Because expression of IFN-γ in the conjunctiva was highest in aqueous-deficient eyes with the lowest GCD and most severe conjunctival disease, the correlation between IFN-γ and clinical parameters (TMH and conjunctival dye staining), GCD, and expression of cornified envelope precursor gene SPRR-2G was evaluated. These correlations are presented in Figure 3. The number of IFN-γ transcripts showed significant correlation with the inferior TMH, a measure of tear volume (Fig. 3A), the severity of conjunctival epithelial disease measured by lissamine green (LG) staining (Fig. 3B), GCD in the temporal bulbar conjunctiva (Fig. 3C), and levels of SPRR-2G transcripts (Fig. 3D). Significant correlation was also observed between number of IFN-γ and SPRR-1A transcripts (R = 0.32, P = 0.05).
Figure 3.
Correlation of IFN-γ with clinical parameters, goblet cell density, and expression of cornified envelope gene. Number of IFN-γ transcripts significantly correlates with (A) inferior tear meniscus height (TMH); (B) conjunctival lissamine green (LG) staining score; (C) temporal bulbar (TB) goblet cell (GC) density; (D) number of small prolene-rich region-2G (SPRR-2G) gene transcripts.
Discussion
This study compared expression of IFN-γ in the conjunctiva of subjects with different types of tear dysfunction and found higher levels of IFN-γ in those with ATD relative to subjects with MGD or normal tear function. Higher IFN-γ expression was associated with greater loss of mucin-filled GC, reduced expression of the GC mucin MUC5AC, and more severe clinically graded conjunctival epithelial disease. These findings are consistent with mouse model studies showing that increased expression of IFN-γ induced by ocular surface dryness promotes conjunctival GC loss.5
Interferon-γ is the only type II interferon; Th1 lymphocytes, CD8 cytotoxic lymphocytes, natural killer (NK) cells, and natural killer T (NKT) cells produce it.15,16 It is critical for innate and adaptive immune responses, including defense against certain bacterial infections (mycobacteria, listeria) and dimorphic yeast and some viral infections.17–19 It is also involved in granuloma formation.20 Elevated IFN-γ expression has been identified in a number of autoimmune diseases, including systemic lupus erythematosis, Sjögren syndrome, inflammatory bowel disease, and multiple sclerosis.15,21,22 We have reported that exposure of the mouse ocular surface to desiccating stress increased IFN-γ production by NK and Th1 cells in the conjunctiva.5,16
We have previously found that the Th2 cytokine IL-13 and Th1 cytokine IFN-γ produced opposite effects on GC differentiation and mucin production in cultured mouse conjunctival GC.9 Interleukin-13 stimulates GC proliferation and production of GC markers (K7, MUC5AC, and MUC2), while IFN-γ decreases GC viability and mucin production in these cells (Coursey TG, et al. IOVS 2014;55:ARVO E-Abstract 2774). In the current study, level of IFN-γ gene transcripts showed significant inverse correlation with conjunctival GCD and direct correlation with severity of conjunctival disease and a marker of squamous metaplasia. Based on the mouse model and culture data, the reduced GCD and expression of GC markers in the conjunctiva of ATD patients could be directly attributed to increased IFN-γ expression. However, it is possible that increased IFN-γ expression in the ATD groups is a consequence of the reduced GCD that develops from ATD. Goblet cells have been found to produce immunomodulatory factors, including TGF-β2, thrombospondin-1, and MUC2 capable of modulating dendritic cells toward a tolergenic phenotype or suppressing production of the Th1-inducing cytokine IL-12 by dendritic cells.9,10,23,24 Goblet cell–associated antigen passages have been identified in the intestinal mucosa that allows passage of molecules from the lumen into the stroma.25 We have also found that under homeostatic conditions, conjunctival GC can serve as conduits for passage of molecules from the tears into the conjunctival stroma (Barbosa FL, et al. IOVS 2015;56:ARVO E-Abstract 3528). It is possible that passage of immunomodulatory factors in tears to the stromal DCs would decrease with the conjunctival GC loss in ATD. Future investigations may determine if loss of lacrimal gland- or GC-derived factors may stimulate development of IFN-γ–producing Th1 cells.
These findings suggest that strategies to minimize IFN-γ signaling may increase goblet number and mucin production in eyes with ATD. We have previously reported that systemic or topical administration of IFN-γ neutralizing antibody suppressed conjunctival GC loss and epithelial apoptosis in a mouse dry eye model.26 The increase in GCD that has been observed following topical cyclosporine treatment of human or experimental murine dry eye may be due to inhibition of IFN-γ production by activated Th cells in the conjunctiva.10,27,28
In contrast to our study, a previously reported study by Kawasaki and colleagues29 found reduced expression of IFN-γ receptor (IFN-γR) in the conjunctival epithelium of subjects with SS. However, these authors also found that CXCL9, a chemokine induced by IFN-γ, was one of the most highly expressed genes in the SS conjunctival epithelium as evidence in support of increased IFN-γ signaling. We previously found CXCL9 along with another IFN-γ–inducible chemokine (CXCL10) to be markedly increased in subjects with SS.30 These findings together with those of our current study suggest that ATD promotes an IFN-γ signature pattern of gene expression in the conjunctiva.
One of the strengths of our study was the ability to compare GCD and cytokine gene expression in the exposed bulbar conjunctiva of the same eye using impression cytology. Since initiating this study, there have been refinements in quantitative methods to measure gene expression, such as digital PCR, that might further enhance the ability to evaluate gene expression and cytopathology by impression cytology performed in adjacent areas of the conjunctiva.31 The small number of subjects with non-SSATD is a potential weakness of this study, but the number of subjects in this group still exceeded the calculated sample size.
In summary, eyes with ATD show a unique profile of increased IFN-γ expression that is associated with reduced GCD and mucin production. These findings suggest that inhibition of IFN-γ expression and/or activity may prevent or reverse the conjunctival GC loss in aqueous tear–deficient conditions.
Acknowledgments
Supported by National Institutes of Health (NIH) Grant EY11915 (SCP); NIH Core Grants EY002520 and EY020799; an unrestricted grant from Research to Prevent Blindness, New York, New York, United States (SCP); the Oshman Foundation, Houston, Texas, United States (SCP), the William Stamps Farish Fund, Houston, Texas, United States (SCP); and Hamill Foundation, Houston, Texas, United States (SCP).
Disclosure: S.C. Pflugfelder, None; C.S. De Paiva, None; Q.L. Moore, None; E.A. Volpe, None; D.-Q. Li, None; K. Gumus, None; M.L. Zaheer, None; R.M. Corrales, None
References
- 1. Pflugfelder SC,, Huang AJ,, Feuer W,, Chuchovski PT,, Pereira IC,, Tseng SC. Conjunctival cytologic features of primary Sjogren's syndrome. Ophthalmology. 1990; 97: 985–991. [DOI] [PubMed] [Google Scholar]
- 2. Pflugfelder SC,, Tseng SC,, Yoshino K,, Monroy D,, Felix C,, Reis BL. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997; 104: 223–235. [DOI] [PubMed] [Google Scholar]
- 3. Nelson JD,, Wright JC. Conjunctival goblet cell densities in ocular surface disease. Arch Ophthalmol. 1984; 102: 1049–1051. [DOI] [PubMed] [Google Scholar]
- 4. Tatematsu Y,, Ogawa Y,, Shimmura S,, et al. Mucosal microvilli in dry eye patients with chronic GVHD. Bone Marrow Transplant. 2012; 47: 416–425. [DOI] [PubMed] [Google Scholar]
- 5. De Paiva CS,, Villarreal AL,, Corrales RM,, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007; 48: 2553–2560. [DOI] [PubMed] [Google Scholar]
- 6. De Paiva CS,, Raince JK,, McClellan AJ,, et al. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011; 4: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wills-Karp M,, Luyimbazi J,, Xu X,, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998; 282: 2258–2261. [DOI] [PubMed] [Google Scholar]
- 8. Wilson MS,, Ramalingam TR,, Rivollier A,, et al. Colitis and intestinal inflammation in IL10-/- mice results from IL-13Ralpha2-mediated attenuation of IL-13 activity. Gastroenterology. 2011; 140: 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tukler Henriksson J,, Coursey TG,, Corry DB,, De Paiva CS,, Pflugfelder SC. IL-13 stimulates proliferation and expression of mucins and immunomodulatory gene in cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2015; 56: 9186–9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pflugfelder SC,, De Paiva CS,, Villarreal AL,, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008; 27: 64–69. [DOI] [PubMed] [Google Scholar]
- 11. Afonso AA,, Sobrin L,, Monroy DC,, Selzer M,, Lokeshwar B,, Pflugfelder SC. Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999; 40: 2506–2512. [PubMed] [Google Scholar]
- 12. Candi E,, Schmidt R,, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005; 6: 328–340. [DOI] [PubMed] [Google Scholar]
- 13. Corrales RM,, de Paiva CS,, Li DQ,, et al. Entrapment of conjunctival goblet cells by desiccation-induced cornification. Invest Ophthalmol Vis Sci. 2011; 52: 3492–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pelegrino FS,, Pflugfelder SC,, De Paiva CS. Low humidity environmental challenge causes barrier disruption and cornification of the mouse corneal epithelium via a c-jun N-terminal kinase 2 (JNK2) pathway. Exp Eye Res. 2012; 94: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pollard KM,, Cauvi DM,, Toomey CB,, Morris KV,, Kono DH. Interferon-gamma and systemic autoimmunity. Discov Med. 2013; 16: 123–131. [PMC free article] [PubMed] [Google Scholar]
- 16. Coursey TG,, Bohat R,, Barbosa FL,, Pflugfelder SC,, de Paiva CS. Desiccating stress-induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE-1 and release of IFN-gamma in experimental dry eye. J Immunol. 2014; 193: 5264–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Newport MJ,, Huxley CM,, Huston S,, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996; 335: 1941–1949. [DOI] [PubMed] [Google Scholar]
- 18. Zerbe CS,, Holland SM. Disseminated histoplasmosis in persons with interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2005; 41: e38–e41. [DOI] [PubMed] [Google Scholar]
- 19. Dorman SE,, Uzel G,, Roesler J,, et al. Viral infections in interferon-gamma receptor deficiency. J Pediatr. 1999; 135: 640–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christophi GP,, Caza T,, Curtiss C,, Gumber D,, Massa PT,, Landas SK. Gene expression profiles in granuloma tissue reveal novel diagnostic markers in sarcoidosis. Exp Mol Pathol. 2014; 96: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blumberg R,, Powrie F. Microbiota, disease, and back to health: a metastable journey. Sci Transl Med. 2012; 4:137rv137. [DOI] [PMC free article] [PubMed]
- 22. Dungan LS,, McGuinness NC,, Boon L,, Lynch MA,, Mills KH. Innate IFN-gamma promotes development of experimental autoimmune encephalomyelitis: a role for NK cells and M1 macrophages. Eur J Immunol. 2014; 44: 2903–2917. [DOI] [PubMed] [Google Scholar]
- 23. Contreras-Ruiz L,, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One. 2015; 10: e0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shan M,, Gentile M,, Yeiser JR,, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013; 342: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McDole JR,, Wheeler LW,, McDonald KG,, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012; 483: 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X,, De Paiva CS,, Su Z,, Volpe EA,, Li DQ,, Pflugfelder SC. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp Eye Res. 2014; 118: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kunert KS,, Tisdale AS,, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002; 120: 330–337. [DOI] [PubMed] [Google Scholar]
- 28. Strong B,, Farley W,, Stern ME,, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005; 24: 80–85. [DOI] [PubMed] [Google Scholar]
- 29. Kawasaki S,, Kawamoto S,, Yokoi N,, et al. Up-regulated gene expression in the conjunctival epithelium of patients with Sjogren's syndrome. Exp Eye Res. 2003; 77: 17–26. [DOI] [PubMed] [Google Scholar]
- 30. Yoon KC,, Park CS,, You IC,, et al. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010; 51: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore QL,, De Paiva CS,, Pflugfelder SC. Effects of dry eye therapies on environmentally induced ocular surface disease. Am J Ophthalmol. 2015; 160: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shiboski SC,, Shiboski CH,, Criswell L,, et al. American College of Rheumatology classification criteria for Sjogren's syndrome: a data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 2012; 64: 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]