Abstract
The choroid plexus maintains the homeostasis of critical molecules in the brain by regulating their transport between the blood and cerebrospinal fluid (CSF). The current study was designed to investigate the potential role of the blood-CSF barrier (BCSFB) in α-synuclein (a-Syn) transport in the brain as affected by exposure to manganese (Mn), the toxic metal implicated in Parkinsonian disorders. Immunohistochemistry was used to identify intracellular a-Syn expression at the BCSFB. Quantitative real-time PCR was used to quantify the change in a-Syn mRNA expression following Mn treatments at the BCSFB in vitro. ELISA was used to quantify a-Syn levels following in vivo and in vitro treatments of Mn, copper (Cu), and/or external a-Syn. Thioflavin-T assay was used to investigate a-Syn aggregation after incubating with Mn and/or Cu in vitro. A two-chamber Transwell system was used to study a-Syn transport by BCSFB monolayer. Data revealed the expression of endogenous a-Syn in rat choroid plexus tissue and immortalized choroidal epithelial Z310 cells. The cultured primary choroidal epithelia from rats showed the ability to take up a-Syn from extracellular medium and transport a-Syn across the cellular monolayer from the donor to receiver chamber. Exposure of cells with Mn induced intracellular a-Syn accumulation without causing any significant changes in a-Syn mRNA expression. A significant increase in a-Syn aggregation in a cell-free system was observed with the presence of Mn. Moreover, Mn exposure resulted in a significant uptake of a-Syn by primary cells. These data indicate that the BCSFB expresses a-Syn endogenously and is capable of transporting a-Syn across the BCSFB monolayer; Mn exposure apparently increases a-Syn accumulation in the BCSFB by facilitating its uptake and intracellular aggregation.
Keywords: a-synuclein transport, cerebrospinal fluid, blood-cerebrospinal fluid barrier, manganese exposure
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder behind Alzheimer’s disease and is characterized by dopaminergic neurodegeneration in the substantia nigra with the following hallmark symptoms: bradykinesia, rigidity, postural instability, and resting tremors [1]. Dysfunction and aggregation of the protein, α-synuclein (a-Syn) has been associated with PD pathophysiology [2]. The protein a-Syn is small (14.5 kDa), natively unfolded, and expressed in a wide range of cell types. The exact function of wild-type a-Syn is uncertain. Some researchers have suggested potential functions of a-Syn in synaptic plasticity [3], synaptic pool maintenance [4], regulation of dopamine synthesis [5], and vesicular dynamics, e.g. stabilization and exocytic fusion at the presynapse [2,6,7].
The aggregation of a-Syn within neurons results in the efflux of aggregate species from the cell to the interstitial fluid (ISF) [8,9], which merges with the cerebrospinal fluid (CSF). The CSF is mainly produced by the choroid plexus located in brain ventricles. The choroid plexus forms the blood-CSF barrier (BCSFB), which separates the blood circulation from the CSF. For its continuous cycle throughout brain structure, the CSF functions as a “sink”, where waste products can be collected from the ISF and removed from the brain via the BCSFB or subarachnoid drainage [10,11]. The a-Syn molecules present in the CSF are believed to originate primarily from neurons and neuroglia [12], and the a-Syn concentration in the CSF has been compared against a-Syn in blood as potential biomarkers for PD progression [13–15]. Literature evidence suggests that the transport of a-Syn aggregates between neurons and astrocytes of the blood-brain barrier (BBB) is by endocytosis and exocytosis [8,9,16,17]. Since the a-Syn level in the blood is over 1000-fold higher than that in the CSF in healthy humans [12,14,18], any possible transport of a-Syn from the CSF back to the blood must be energy dependent and against this large concentration gradient. These transport properties, however, have yet to be thoroughly investigated at the BCSFB or BBB. For detailed discussions, please see a recent review by Bates and Zheng [19].
Manganese (Mn) is an essential trace element utilized for a variety of functions in the brain including glutamate and GABA metabolism, astrocyte morphology and migration, and the neutralization of reactive oxidative species [20]. Mn overexposure occurs primarily in the occupational setting for welders, smelters, and others with related professions [21,22], but it also occurs in premature newborns via medically administered paternal nutrition [20]. Excess Mn has been shown to accumulate primarily in the globus pallidus; more recent data have shown that Mn also accumulates in the substantia nigra [23–25]. In humans and rats, excess Mn has been associated with increased GABA levels at the basal ganglia, impaired iron and copper homeostasis, and mitochondrial dysfunction [26–30]. Individuals suffering from Mn intoxication display clinical symptoms including impaired balance and gait, kinetic tremors, dystonia, cognitive problems, memory loss, anxiety, and aggression [31,32].
Previous findings in the literature showed that the choroid plexus accumulates Mn to a great extent after Mn exposure [22,33,34]. The BCSFB is known to favor Mn influx to the brain with a slow rate of Mn efflux from the CSF [22,35,36]. This may contribute to the high Mn storage and relatively long half-life of Mn in the brain [37]. Studies have also indicated that Mn can interact with a-Syn, promoting the aggregation of the protein into oligomers in vitro [38,39] and overexpression of a-Syn in PC12 and SH-SY5Y cells [40,41]. The question as to whether or not Mn may interfere with a-Syn production, aggregation and transport in the BCSFB remains unanswered.
The purposes of the current study were to 1) determine the endogenous expression of a-Syn in the BCSFB as affected by Mn exposure, 2) investigate the uptake of a-Syn by the BCSFB and the effect of Mn exposure, 3) study the direct interaction between Mn and a-Syn in a cell-free system, and 4) explore the directional transport properties of wild-type a-Syn monomer by the BCSFB and effects of Mn exposure. The findings of these works establish a foundation for understanding the role of the BCSFB in regulating a-Syn homeostasis in the brain, the effects of toxic Mn exposure, and the potential implications of these relationships in parkinsonian disorders.
Experimental
Materials
Chemicals and reagents were acquired from the following sources: Mn chloride tetrahydrate (MnCl2•4H2O) from Fisher Scientific (Pittsburgh, PA); sodium chloride (NaCl) from Avantor Performance Materials (Center Valley, PA); sodium phosphate (Na2HPO4), potassium chloride (KCl), and potassium phosphate (KH2PO4) from Mallinckrodt Chemicals (Phillipsburg, NJ); copper chloride (CuCl2), paraformaldehyde (PFA), phenylmethylsulfonyl fluoride (PMSF), epidermal growth factor (EGF), cis-4-hydroxyl-L-proline (Cis-HP), 0.25 % trypsin-EDTA from Sigma (St. Louis, MO); Hank’s balanced salt solution (HBSS), Fluor Alexa-555 conjugated goat anti-mouse secondary antibody, Fluor Alexa-488 conjugated goat anti-rabbit secondary antibody, TRIzol reagent, collagen I (rat tail), fetal bovine serum (FBS), penicillin, streptomycin, amphotericin, gentamycin, and Human a-Syn ELISA kit from Life Technologies (Carlsbad, CA); fetal bovine serum (FBS), and Dulbecco’s modified Eagle’s medium (DMEM) from Cellgro (Manassas, VA); pronase, protease, and protease inhibitor cocktail from Calbiochem (San Diego, CA); sodium dodecyl sulfate (SDS), Triton X-100, cDNA synthesis kit, and iTaq Universal SYBR Green Supermix from Bio-Rad (Hercules, CA); collagen-coated Transwell-COL inserts from Corning (Cambridge, MA); rabbit polyclonal a-Syn primary antibody from Cell Signaling Technologies (2642S) (Danvers, MA). All reagents were of analytical grade, HPLC grade, or the best pharmaceutical grade available.
Cell Cultures and Treatments
The choroidal epithelial Z310 cell line was derived from rodent choroid plexus by this laboratory; the culturing and maintenance procedures of this cell line have been previously described [42–44]. Z310 cells were grown in DMEM medium supplemented with 10 % FBS, 100 U/mL penicillin-streptomycin, 100 μg/mL gentamicin sulfate, and 10 ng/mL EGF, in a humidified incubator with 95% air-5 % CO2 at 37 °C. 0.25 % Trypsin-EDTA was used to dissociate cells during maintenance. Z310 cells were passaged twice a week.
For culturing primary choroidal epithelial cells from rats, male Sprague-Dawley rats (3-week old, 35–49 g) were used. Rats were purchased from Harlan Laboratories (Indianapolis, IN) and euthanized with CO2. Choroid plexuses from the lateral and third ventricles were dissected, minced down to cubes 1 mm in length, and digested in 1 mL HBSS containing 0.2 % pronase at 37 °C for 5–10 min. Digestion was stopped by adding 4 mL of HBSS. Cells were pelleted via centrifugation (800 × g for 5 min), washed with fresh HBSS, and centrifuged again (800 × g for 5 min). Cells were passed 13–14 times through a 20-gauge needle attached to a 10 mL syringe and counted, seeded on wells pre-coated with collagen (Gibco/Life Technologies Cat. No. A1048301), and incubated at 37 °C with 95 % air-5 % CO2 for 2–3 days without disturbing. Medium was replaced every other day with a fresh growth medium. On the 4th day, cells were cultured with the growth medium supplemented with 25 μg/mL Cis-HP to inhibit the growth of fibroblastic cells. This protocol has been used extensively in previous research performed by this lab [27,29,45–47].
Autoclaved 25 mM MnCl2 and 25 mM CuCl2 stock solutions were prepared by dissolving MnCl2•4H2O and CuCl2 in sterile, double-deionized water, respectively. Z310 cells were treated with 50 or 100 μM MnCl2 or CuCl2 for 24 h, or 100 μM MnCl2 for 24 and 48 h for the immunohistochemical studies. Z310 cells were treated with 25, 50, or 100 μM MnCl2 for 24 h for the quantitative RT-PCR study. The dose regimen, used in the following studies, was chosen according to previous research performed in this lab or by other groups [27,29,42,48].
Immunohistochemical (IHC) Staining
Freshly harvested choroid plexus tissues were immediately fixed with 4 % paraformaldehyde (pH 7.4) for 10 min, permeabilized with 0.1 % Triton X-100, and blocked in 1 % bovine serum albumin (BSA) at room temperature for 1 h. Tissues were washed three times with PBS (10 min/wash) after the fixing and permeabilizing steps. Tissues were incubated with polyclonal rabbit anti-a-Syn antibody (1:100) in 1 % BSA at 4 °C for 48 h, followed by repeated washes with PBS (3 × 10 min/wash), and then incubated with Alexa Fluor -488 conjugated goat anti-rabbit secondary antibody (1:1000) in 1 % BSA at room temperature for 1 h. Tissues were rinsed with PBS (3 × 10 min/wash) and then mounted to the objective slides using ProLong Gold Anti-Fade reagent (Life Technologies, Carlsbad, CA) to prevent fluorescent bleaching for confocal microscopy examination.
Glass coverslips (0.17 mm in thickness) were sterilized with 75 % ethanol. Z310 cells were seeded on glass coverslips at a density of 2 × 105/well in a 6-well-plate. Rat plexus primary cells were seeded on glass coverslips at a density of 5 × 104/well in a 24-well plate and allowed to grow for 24 h to achieve 50 % confluence, and then treated with 100 μM MnCl2 for 12, 24, or 48 h. At the end of treatment, cells were fixed in 4 % paraformaldehyde and permeabilized in 0.1 % Triton X-100 at room temperature and washed with PBS (3 × 5 min/wash) after the fixing and permeating steps. Cells were then blocked with 1% BSA for 1 h at room temperature followed by incubation with rabbit polyclonal a-Syn antibody (1:1000) at 4 °C for 48 h. Cells were washed with PBS (3 × 5 min/wash) before being incubated in Alexa Fluor-488 conjugated goat anti-rabbit secondary antibody (1:1000) for 24 h. Glass coverslips were mounted to objective slides for confocal microscopy observation. Slides were prepared using Gold Anti-Fade to avoid fluorescent bleaching for confocal microscopic examination. All slides were dried in a hood, with no exposure to light, at room temperature overnight. The negative control was established by using only the secondary antibody to reflect non-specific staining on the background.
Confocal Microscopy and Quantification
To acquire images, pre-dried slides containing experimental specimens were mounted on the stage of a Nikon inverted confocal laser-scanning microscope and viewed through a 60x oil-immersion objective (Plan Apo, 60x/1.40 oil, ∞ 0.17, DIC), with a 488-nm laser and a 562-nm laser source for excitation. Lower laser intensity was used to avoid photo bleaching. Each slide was examined under reduced transmitted-light illumination; the area containing undamaged epithelium with underlying vasculature was chosen for analyses. Approximately 10–20 areas of epithelium were selected for image collection and analysis. Image data reported were the results of a single experiment representative of three to four experiments. Images were scanned with the Nikon EZ-C1 (Version 3.90) confocal imaging program.
Signal intensities were quantified using the NIS-Elements BR (Version 3.10) program. Each cell or group of cells was isolated as a region of interest (ROI). Cells that were partially imaged within the image frame were excluded to improve accuracy and reliability of quantification. Mean intensity values were calculated as the sum of intensities within the corresponding ROI divided by the area of the ROI. A minimum of three images per treatment group was used for confocal quantification.
a-Synuclein mRNA Quantitation by Quantitative Real-time RT-PCR (qPCR)
The transcription levels of mRNA encoding rat a-Syn (Snca) were quantified using qPCR. Z310 cells were seeded and cultured for 24 h prior to incubation with 25, 50, or 100 μM MnCl2 for 24 h. Total RNA was isolated from the cells using TRIzol, following the manufacturer’s directions. RNA samples were reverse-transcribed using the BioRad iScript cDNA synthesis kit (Hercules, CA). The iTaq Universal SYBR Green Supermix (Hercules, CA) was used for qPCR analysis. Snca cDNA was amplified using the following nucleotide sequences:
Forward primer: 5′-AGA TCT GCC CAG GTG TTC TTCC-3′
Reverse primer: 5′-AGG ACT CCG ATC ACT GCT GATG-3′
The primers were designed using the Primer Express 3.0 software. All primers were obtained from Integrated DNA Technologies (Coralville, IA). The amplification was performed in the CFX ConnectTM Real-Time PCR Detection system (BioRad, Hercules, CA).
Thioflavin T Assay
Recombinant human a-Syn was produced using the methods by Zhang et al. [49]. The protein was dialyzed in 1x PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4) overnight prior to use in order to remove excess salt. The recombinant human a-Syn protein (70 μM) was incubated with 100 μM MnCl2, CuCl2, or a mixture of MnCl2 and CuCl2 (each at 100 μM) in a 200 μL solution of 20 mM 2-(N-morpholino)ethanosulfonate (MES) buffer containing 200 μM Thioflavin T for 72 h at 37 °C under constant stirring with a Teflon ball. The combination of MnCl2 and CuCl2 at 100 μM without a-Syn are used as a negative control. Fluorescence readings were measured with a Tecan GENios plate reader (San Jose, CA) every 15 min (Excitation: 440 nm; Emission: 480 nm). Tecan Magellan software (San Jose, CA) was used for recording the data. Concentrations of metal ions and a-Syn were chosen based on previous literature [38,39].
Cellular Uptake of a-Syn and ELISA Quantification
Primary rat plexus cells were seeded and cultured as described above. Once the cells reached 70–80 % confluence, they were exposed to 25, 50, or 100 μM MnCl2 for 24 h. Cells were then washed with PBS and incubated with 0.5 mg/mL recombinant human a-Syn for 2 h in serum-free DMEM medium. Following treatments, cells were washed and collected. Cell lysates were analyzed for a-Syn content via ELISA quantification.
Protein lysates were extracted from cell samples in a homogenization buffer containing 20 mM Tris, pH 7.5, 5 mM EGTA, 1 % Triton X-100, 0.1 % SDS, and protease inhibitor cocktail (Calbiochem, San Diego, CA). Samples were sonicated, centrifuged, and quantified for protein concentration using the Bradford protein assay. Medium and cell samples were diluted 25-fold in Diluent Buffer included in the Human a-Synuclein ELISA kit (Product KHB0061, Invitrogen, Frederick, MD) in accordance with the product’s protocol. Samples were measured for fluorescence at 450 nm with an M2e Plate Reader(Molecular Devices, Sunnyvale, CA) and the SoftMax Pro analysis software (Version 5.4, Molecular Devices, Sunnyvale, CA) within 2 h of the addition of the stop solution.
Transport of a-Syn across BCSFB Monolayer in Two-Chamber Transwell System
Please refer to Fu et al. [29] for a more detailed description of this procedure. Rat choroid plexus primary cells, obtained as previously described were seeded on collagen-coated laminin membranes in the inner chamber of a two-chamber Transwell system. Figure S1 illustrates the concept of this transport system. A 1.0 mL aliquot of growth DMEM medium was added to the inner chamber. The inner chamber was then immersed in 1.0 mL of medium in the outer chamber. Cells were cultured in the medium for 7–10 days to the confluence. Wells were then divided into 2 main sets, i.e., one set for the influx studies and the other for the efflux studies. Each set was further divided into 3 time points: 6, 12, and 24 h. Each time point was comprised of a control (no treatment) and a Mn-Treated group.
Transepithelial electrical resistance (TEER), an indicator of barrier tightness, was used to monitor the development and the tightness of the barrier monolayer on the laminin membrane. TEER readings were taken every 2 days until the resistance reached 50–60 Ω cm2, which confirms the presence of tight junctions throughout the barrier [27,29,46]. Once the cell monolayer was confluent and completely developed, cells designated for Mn treatment were treated with 100 μM MnCl2 for 24 h. Cells were incubated with 1.0 mg/mL a-Syn dissolved in serum-free DMEM medium. a-Syn was added to the outer chamber for the “Influx” study; for the “Efflux” study, a-Syn was added to the inner chamber only. A 20-μL aliquot of both chambers and cells was collected at each time point and immediately frozen at −80 °C until being thawed for ELISA quantification.
Statistical Analysis
Confocal quantification and the Transwell Chamber studies were analyzed using One-Way or Two-ANOVA with Tukey’s post-hoc test as necessary. qPCR data were analyzed with One-Way ANOVA and the Dunnett’s post-hoc test. The a-Syn Uptake studies quantified with ELISA were analyzed with One-Way ANOVA and the Tukey’s post-hoc test. Any p values equal to or less than 0.05 were considered statistically significant.
Results
Expression of a-Syn in the Choroidal Epithelia and Effects of Mn Exposure
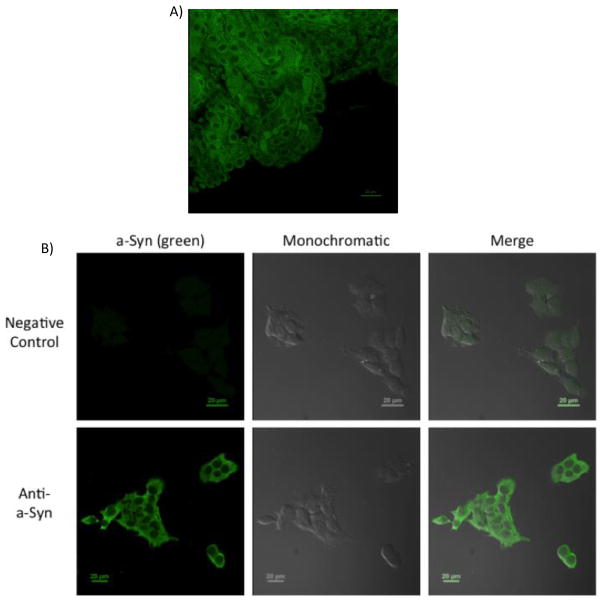
Data from freshly isolated choroid plexus tissue (Fig. 1A) and choroidal epithelial Z310 cells (Fig. 1B) showed that the choroid plexus cells expressed strong fluorescent signals representing a-Syn; furthermore, the fluorescent signal was present primarily in the cytoplasm of these cells. This cytoplasmic expression of a-Syn is consistent with the localization of a-Syn in other cell types described previously in the literature [2,50]. Our data clearly show that choroidal epithelial cells possess the ability to express endogenous a-Syn.
Figure 1.
Expression of endogenous a-Syn in choroid plexus. (A) Presence of a-Syn in rat choroid plexus tissue. Choroid plexuses were dissected from rats and stained with primary anti-a-Syn. A typical image from n = 4 is presented. (B) Presence of a-Syn in choroidal Z310 cells. Z310 cells were fixed and stained with primary anti-a-Syn. A typical image from n = 4 is presented. In both tissues and cells, a-Syn immunoreactivity is evident in the cytosol. Scale bar: 20 μm.
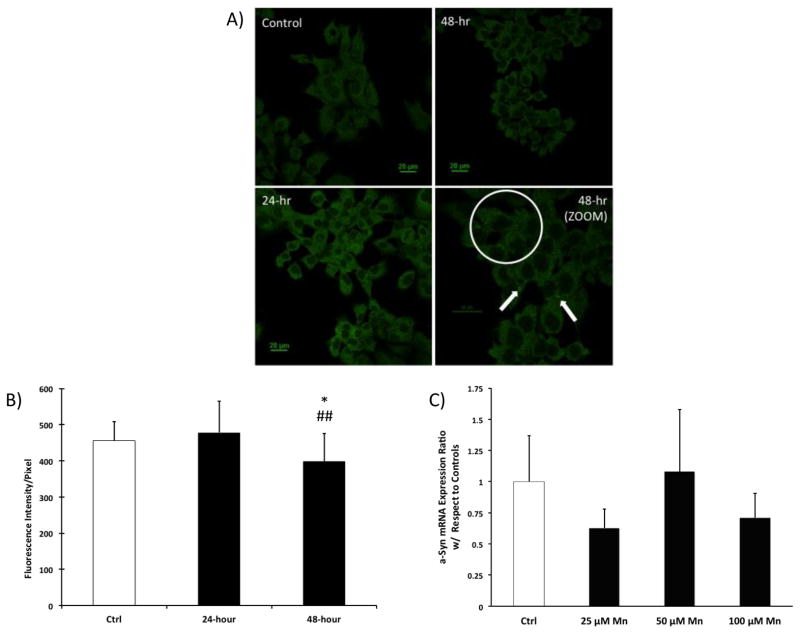
Following incubation of Z310 cells with 100 μM MnCl2 for 48 h, some small, yet bright green dots appeared to form in the cytoplasm (Fig. 2A). Control Z310 cells did not display these dots. However, quantitation of the intensity of a-Syn signals in these cells did not reveal a significant difference between control and Mn-treated groups at 24 h but did show a significant decrease at 48 h (Fig. 2B). This decrease could be a result of the clearing of a-Syn aggregates, which has been shown to occur in the presence of Mn in neurons [50]. The decrease in a-Syn signals, however, is contradictory to some literature reports, where a-Syn overexpression following Mn exposure was observed in other cell types [40, 41].
Figure 2.
Endogenous a-Syn expression in Z310 cells following Mn exposure. (A) IHC staining of a-Syn in Z310 cells. Z310 cells were treated with 100 μM MnCl2 for 24 and 48 h. The white circle indicates that at 48 h after Mn treatment, bright green puncta were formed in the cytoplasm. Data represent a typical study of n = 4. Scale bar: 20 μm. (B) Confocal quantification of endogenous a-Syn expression in Z310 cells following Mn exposure. Data represent mean ±SD. n = 15–27. *: p<0.05 as compared to Ctrl. ##: p<0.01 as compared to data at 24 h. (C) Expression of endogenous a-Syn mRNA in Z310 cells following Mn exposure. Z310 cells were treated with 25, 50, or 100 μM MnCl2 for 24 h. The mRNA levels were quantified by qPCR. Data represent mean ± SD; n = 4.
We further used qPCR to quantify a-Syn mRNA expression in Z310 cells with various concentrations of Mn. After a 24 h treatment, the qPCR analysis did not reveal any significant change in a-Syn mRNA expression (Fig. 2C). These data were consistent with the confocal quantitation results, supporting the view that Mn exposure did not induce a-Syn overexpression at either the transcriptional or translational level. The changes in a-Syn signals in Z310 cells after Mn exposure may reflect a direct interaction of Mn with a-Syn.
Effects of Cu Treatment on Endogenous Expression of a-Syn at the BCSFB
Recent findings in our lab have shown that Mn accumulation in the choroid plexus can cause Cu dyshomeostasis in the BCSFB [29]. Cu itself has been shown to induce a-Syn aggregation and dysfunction [38,39,51]. To compare with the Mn effects outlined in the previous section, we sought to investigate the effect of Cu on endogenous a-Syn expression in the plexus cells.
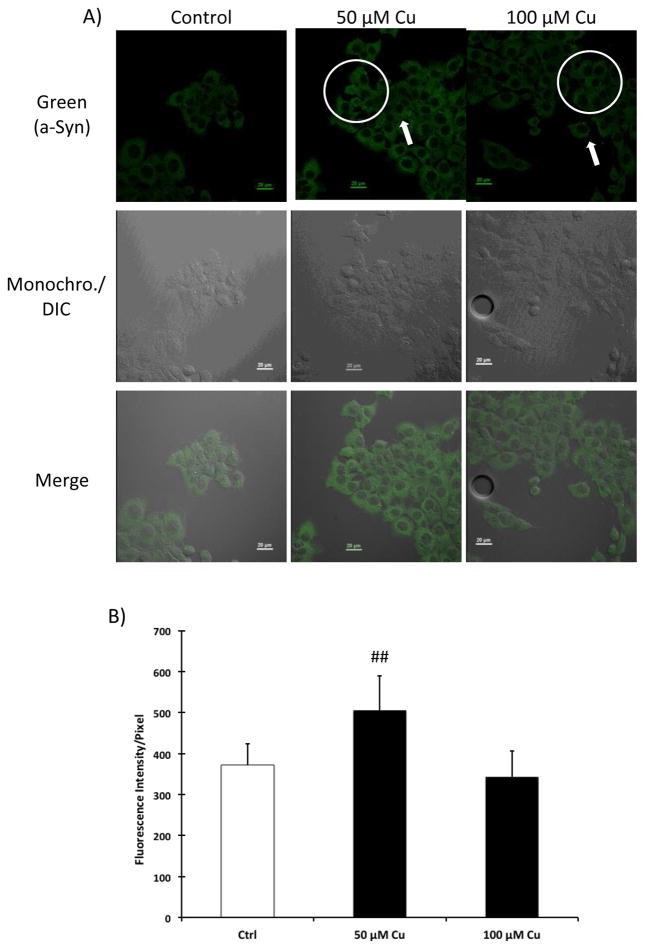
After Z310 cells were incubated with 50 and 100 μM CuCl2 for 24 h, bright dots were also observed in the cytoplasm of Z310 cells, similar to those in the Mn exposed group (Fig. 3A). Confocal quantitation of a-Syn signals revealed that cells exposed to 50 μM CuCl2 displayed a significant increase in a-Syn intensity (Fig. 3B).
Figure 3.
Expression of a-Syn in Z310 cells following Cu treatment. (A) IHC staining of a-Syn in Z310 cells. Z310 cells were treated with 50 or 100 μM CuCl2 for 24 h. The white circles indicate that after Cu treatment, bright green puncta were formed in the cytoplasm. Data represent a typical study of n = 4. (B) Confocal quantification of endogenous a-Syn expression in Z310 cells following Cu treatment. Data represent mean ± SD. n = 12–20. **: p<0.01 as compared to control.
Aggregation of a-Syn Following Mn and Cu Treatment
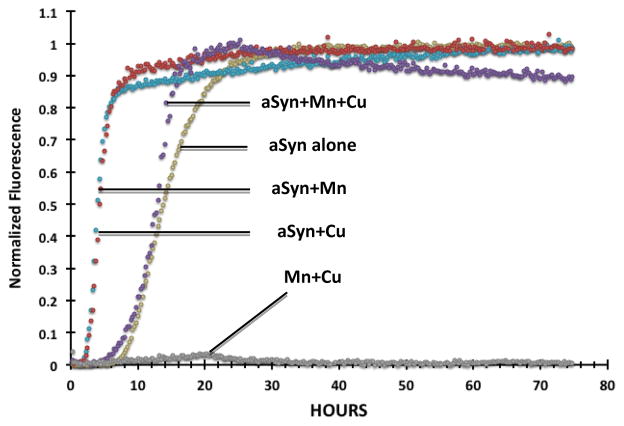
Results so far appeared to suggest a direct, physicochemical interaction between a-Syn, Mn, and/or Cu. Thioflavin T (ThioT) has been used extensively in previous studies to assess metal-induced aggregation of a-Syn [38,39]. In the current study, we incubated recombinant human a-Syn with Mn, Cu, or a combination of Mn and Cu at a concentration of 100 μM [38,39] and used ThioT to determine the metal effects on a-Syn aggregation in vitro. Results presented in Fig. 4 showed a drastic decrease in the maximal (final) fluorescence of a-Syn when incubated with Mn, Cu, or the combination of the two metals (data not shown). This quenching of fluorescence was an indication of a structural change of a-Syn and its aggregated forms in the presence of these metals [38]. When the groups were normalized to their respective maximum fluorescence intensities, the results revealed that the emergence of a-Syn fluorescence occurred much earlier, within 5 h when a-Syn was incubated with Mn or Cu alone, compared to a-Syn alone at about 10 h (Fig. 4). Unexpectedly, however, co-incubation of a-Syn with both Mn and Cu did not show an additive effect, but rather it counteracted each metal’s individual effect by slowing the increase of a-Syn fluorescence (Fig. 4). This finding suggests that both metals likely bind to a-Syn, inducing altered conformations of a-Syn aggregates. To substantiate this finding, experiments beyond ThioT, e.g., NMR, should be conducted.
Figure 4.
Thioflavin-T assay of a-Syn aggregation in vitro. Human recombinant a-Syn was incubated in vitro with 100 μM MnCl2, 100 μM CuCl2, or a combination of 100 μM Mn and 100 μM Cu for 72 hours under constant stirring with Teflon ball. Data were normalized to the maximum fluorescent values achieved by each group (n = 3).
Uptake of a-Syn by Plexus Cells and Effect of Mn Exposure
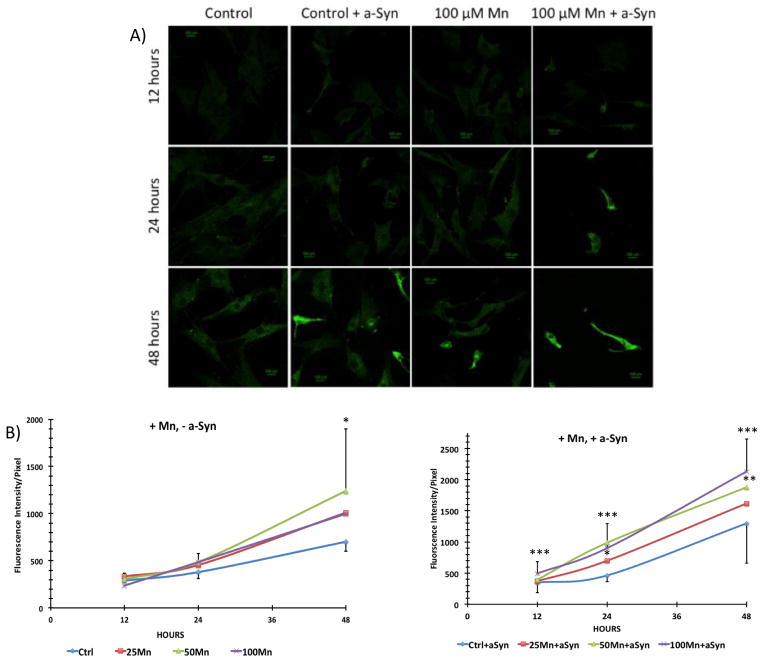
When primary cultures of choroid epithelial cells were exposed to 100 μM MnCl2, without adding a-Syn, the cells displayed a significant increase in a-Syn signals after 48 h (Fig. 5A). With added external a-Syn, the signals became stronger and the presence of intracellular puncta containing a-Syn became apparent (Fig. 5A). Confocal quantifications revealed an increased signal intensity at 48 h in the 50 μM group without added a-Syn (p<0.05) and in both 50 and 100 μM groups with added a-Syn (p<0.01) as compared to controls (Fig. 5B). The a-Syn signals in cells exposed to Mn + a-Syn were significantly greater than those in cells exposed to Mn alone at the same Mn concentration (Supplemental Fig. S2–S3).
Figure 5.
Uptake of a-Syn by primary choroidal epithelial cells in the presence of a-Syn and after Mn exposure. (A) IHC staining of a-Syn in primary plexus cells. Cells were treated with Mn or saline (control) for 12, 24, or 48 h, followed by incubation with 0.5 μM recombinant human a-Syn for 2 h. Cells were stained with primary anti-a-Syn. A typical study of n = 5 is presented. (B) Confocal quantification of a-Syn. “+Mn, −a-Syn”: cells treated with Mn but without added a-Syn; “+Mn, +a-Syn”: cells treated with Mn followed by a-Syn incubation. Data represent mean ± SD. n = 10–24. *: p < 0.05, **: p < 0.01, ***: p < 0.001 as compared to controls at matching time point.
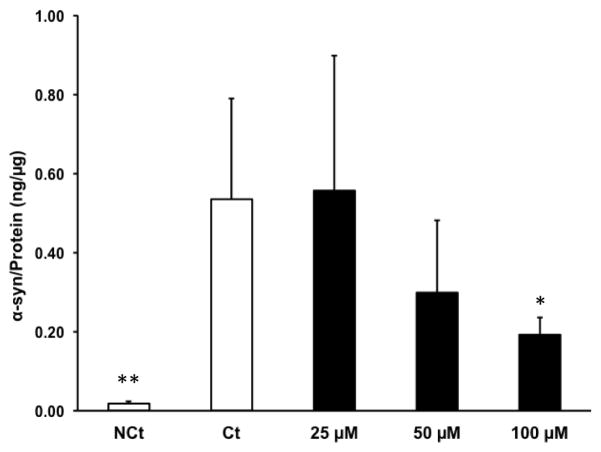
To directly quantify the effect of Mn exposure on a-Syn uptake, we used an ELISA method to determine intracellular a-Syn levels with or without Mn exposure. Primary choroidal epithelial cells treated with various concentrations of Mn for 24 h followed by incubation with a-Syn for an additional 2 h showed a gradual decrease in a-Syn content with an increase in Mn concentration (Fig. 6). A significant decrease was observed at 100 μM Mn (the highest Mn concentration) compared to the control (p<0.05) (Fig. 6). The observed decrease in a-Syn uptake was a direct contrast to the confocal microscopy data. This discrepancy could be explained by the fact that the antibody used in the ELISA method was only capable of detecting human a-Syn monomers but not oligomers. Nonetheless, a significant decrease of a-Syn monomer detected by ELISA, in fact, indirectly suggested an increase in aggregated a-Syn within the cells.
Figure 6.
ELISA quantification of a-Syn uptake by primary choroidal epithelial cells following Mn exposure. Primary cells were pre-treated with 25, 50, or 100 μM MnCl2 or with saline (‘Ct’) for 24 h, followed by incubation with 0.5 mg/mL a-Syn for 2 h. NCt (negative control) was not treated with MnCl2, saline, or a-Syn. Levels of a-Syn were quantified by ELISA. Data represent mean ± SD, n = 5. *: p<0.05, **: p<0.01 as compared with controls.
a-Syn Transport by BCSFB Monolayer in a Two-Chamber Transwell Device
To determine the directional transport of a-Syn across the BCSFB and the effect of Mn exposure, we adapted a two-chamber Transwell transport model (Fig. S1, efflux model is shown). One approach involved adding a-Syn to the outer (donor) chamber and subsequently monitoring the appearance of a-Syn in the inner (receiver) chamber to model the influx of a-Syn from the blood to the CSF. Conversely, the a-Syn was added to the inner chamber and the protein’s appearance in the outer chamber was monitored to model the efflux of a-Syn from the CSF to the blood.
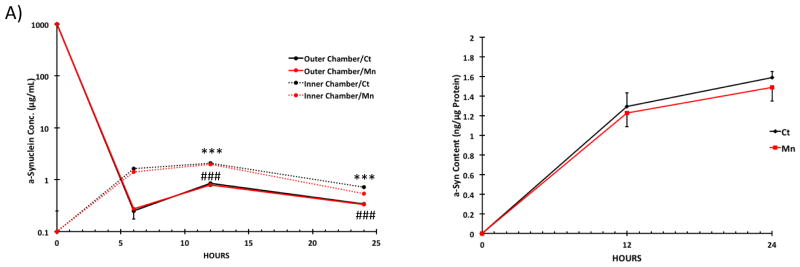
Results from the influx study showed that after a-Syn was added to the outer chamber, there was a significant increase of a-Syn content in the inner chamber between the 0-h and 6-h time points; the increase continued until 12-h in the inner chamber, followed by a gradual decline at 24-h (Fig. 7A). Accordingly, the a-Syn concentration in the outer (donor) chamber decreased significantly between the 0-h and 6-h time points. Interestingly, the a-Syn concentration in the outer chamber began to increase to an apparent steady state between 12 and 24 h (Fig. 7A). Mn exposure had no significant effect on a-Syn concentrations in either the inner or outer chambers at any given time point (Fig. 7A). By quantifying a-Syn concentrations in choroidal epithelial cells, we determined that there was a detectable amount of a-Syn accumulated in the cells in the control group, and this accumulation appeared to continue to increase up to 24 h, although there was no statistical significance between 12 and 24 h (Fig. 7B). Mn treatment had no significant effect on intracellular a-Syn concentrations of the choroidal epithelial monolayer (Fig. 7B).
Figure 7.
Effect of Mn exposure on a-Syn influx transport (from the outer to inner chamber) by choroidal epithelial monolayer. Primary choroidal cells were cultured in the inner chamber. An aliquot of a-Syn was added to the outer chamber after the cells were treated with 100 μM MnCl2 for 24 h. Levels of a-Syn in both chambers and in the cells were quantified by ELISA. (A) Time course. Data represent mean ± SD, n = 6. Means are compared between Inner and Outer Chamber at each time point (6, 12, 24 h). ***: p < 0.001 as compared to 6-hour Inner Chamber/Ct Group. ###: p < 0.001 as compared to 6-hour Outer Chamber/Ct Group. (B) a-Syn concentrations in cell monolayer following Mn exposure. Data represent mean ± S.D., n = 5–6.
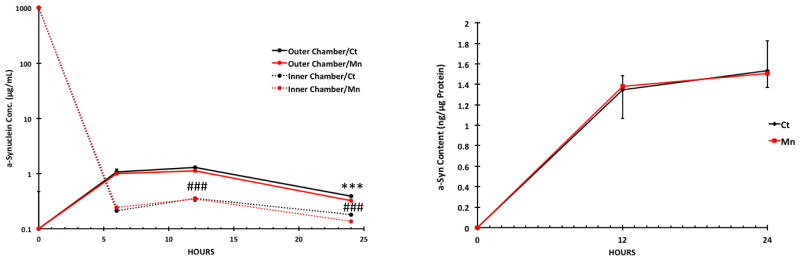
The efflux study showed an increase of a-Syn in the outer (receiver) chamber from the 0-h time point to the 12-h time point, followed by a decrease at 24 h (Fig. 8A). In the inner donor chamber, the a-Syn levels were initially sharply decreased between the 0-h and 6-h time points, followed by a significant, albeit slight, increase in a-Syn content between the 6-h and 12-h time points (Fig. 8A). The a-Syn concentration in the inner chamber appeared to reach a steady state between 12- and 24-h time points (Fig. 8A). Similar to the influx study, Mn exposure had no significant effect on the transport of a-Syn by the BCSFB monolayer (Fig. 8A). Also similar to the influx study, the accumulation of a-Syn in primary choroidal epithelial cells in controls was increased from 0-h to 24 h, although there was no significant change between 12 and 24 h (Fig. 8B). Mn exposure did not significantly alter the intracellular a-Syn concentrations of the BCSFB monolayer (Fig. 8B). The results from these influx/efflux studies suggested that a-Syn molecules were likely transported in either direction across the BCSFB monolayer, and the BCSFB did not appear to have a preferred direction of a-Syn transport. Moreover, Mn exposure had no effect on a-Syn transport by the BCSFB or intracellular a-Syn content.
Figure 8.
Effect of Mn exposure on a-Syn efflux transport (from the inner to outer chamber) by choroidal epithelial monolayer. Primary choroidal cells were cultured in the inner chamber. An aliquot of a-Syn was added to the inner chamber after the cells were treated with 50 μM MnCl2 for 24 h. Levels of a-Syn in both chambers and in the cells were quantified by ELISA. (A) Time course. Data represent mean ± SD. n = 6. Means were compared between Inner and Outer Chamber at each time point (6, 12, 24 h). ***: p < 0.001 as compared to 6-hour Inner Chamber/Ct Group ###: p < 0.001 as compared to 6-hour Outer Chamber/Ct Group. (B) a-Syn concentrations in cell monolayer following Mn exposure. Data represent mean ± S.D., n = 5–6.
Discussion
The data presented in this report suggest that 1) a-Syn is naturally expressed in choroid plexus tissues, particularly within epithelial cells; it is also present in the immortalized choroidal epithelial Z310 cell line; 2) treatment of plexus cells with Mn or Cu induces a-Syn accumulation within the BCB; 3) Mn exposure does not significantly alter a-Syn production at the transcriptional level; 4) in the test tube, incubation of a-Syn with Mn or Cu causes the aggregation of a-Syn; and 5) while Mn exposure does not affect the transport of a-Syn across the BCSFB monolayer, it increases a-Syn uptake by a primary culture of choroidal epithelial cells.
Endogenous expression of a-Syn occurs across a variety of cell types, e.g. neurons, erythrocytes, etc. [52,53]. Here we report the expression of endogenous a-Syn in choroidal epithelial cells in brain ventricles. Multiple functions have been proposed for a-Syn in neurons [54]; but how endogenous a-Syn may function in a cell type constituting a cellular barrier between blood and CSF remains unknown. Interestingly, exposure of these barrier cells to Mn can lead to increased a-Syn fluorescent signals within these cells. Generally, intracellular protein levels increase as a result of (1) increased protein de-novo synthesis, (2) decreased protein degradation, and/or (3) an altered protein transport mechanism. Our qPCR study clearly showed that the levels of mRNAs encoding a-Syn were not affected by Mn exposure in the choroidal Z310 cells. While this observation conflicts with previous reports, which show increased a-Syn expression following Mn exposure in neurons and PC12 cells [40,41], it is possible that different cell types have entirely different responses to Mn treatment. In the current study, we did not study the a-Syn degradation process. Thus, it is unclear whether Mn treatment inhibits or promotes the activity of the enzyme system that metabolizes a-Syn molecules, which could alter intracellular a-Syn levels. Furthermore, the contrasting results between the decrease in a-Syn signal following 48 h of Mn exposure in Z310 cells and the increase in a-Syn signal in primary choroidal epithelia following Mn exposure suggests that the ability to degrade a-Syn aggregates induced by Mn exposure differs between the primary cells and the established cell line. Additionally, our in vitro studies with primary choroidal cells showed a Mn-induced increase of a-Syn uptake in the presence of externally added a-Syn. Thus, it seems likely that Mn exposure may act on some yet unknown mechanisms that transport a-Syn into the cells.
Our observation of bright green dots in the plexus tissues appears to suggest an aggregation of a-Syn within the plexus cells after Mn exposure. This observation is in a good agreement with previous findings in the literature that a direct incubation of a-Syn with Mn and Cu can result in a-Syn aggregation [38,39,55,56]. The findings from the ThioT assay provide further evidence to support the hypothesis that Mn exposure induces a-Syn aggregation in the BCSFB. Remarkably, however, the aggregation rate of recombinant a-Syn in the ThioT assay did not increase, but rather decreased, upon concomitant incubation with both Mn and Cu, when the data were compared to that obtained for a-Syn incubated with Mn or Cu, alone. Cu has been shown to bind to multiple sites on the a-Syn protein [51,55,57,58] and undergo redox reactions with a-Syn directly, by swapping between Cu(I) and Cu(II) oxidation states [58]. Furthermore, Cu has different preferred binding sites to a-Syn, depending on its oxidation state [53,55]. These facts suggest that Cu can induce a-Syn dysfunction in a variety of ways. Similar research on Mn/a-Syn interactions is practically non-existent. It is possible that Mn may also have multiple binding sites in a-Syn molecules, similar to Cu and other metals [51]. In addition, Mn might undergo direct redox reactions with a-Syn, similar to Cu. These potential interactions between metals themselves and among the metals and the protein may explain our in vitro a-Syn aggregation data. Clearly more research is necessary to depict the underlying mechanisms.
The results from our IHC and ELISA quantifications of a-Syn in rat primary choroidal epithelia following Mn exposure showed conflicting results. While the results from the IHC quantitation demonstrated a Mn-induced a-Syn accumulation and significantly increased uptake of a-Syn from the external environment (compared to controls), the results from the ELISA quantification showed a decrease in a-Syn concentrations following Mn exposure. The ELISA kit, however, is designed to detect a-Syn monomer only. The confocal data from the uptake studies revealed a-Syn accumulation in Mn-treated cells, and the ThioT assay clearly showed a-Syn aggregation occurring in the presence of Mn. Therefore, we propose that the decrease in a-Syn content after Mn treatment determined by ELISA may be the result of a loss of a-Syn monomer caused by Mn-induced aggregation of the protein.
The two-chamber Transwell model cultured with primary choroidal epithelial cells is a useful model to study the directional transport of substances across the BCSFB, because the monolayer of epithelial cells tends to form the polarized cellular architecture with microvilli protruding to the inner chamber culture medium [42]. The results from our Transwell study showed that the BCSFB monolayer was capable of transporting a-Syn in either direction without specific preference toward either chamber and that a-Syn transport by the BCSFB was unaffected by Mn exposure. It is possible that a severe leakage of the monolayer, either due to Mn insults or a-Syn toxicity, may render the barrier inefficient to passing molecules. However, in both influx and efflux studies, the levels of a-Syn in the donor chambers were significantly lower than those in the receiver chambers at nearly all time points; these data appear to suggest an active transport, rather than a passive diffusion, of a-Syn from the donor to receiver chamber. This hypothesis deserves further investigation such as by using ATP production inhibitors.
Quantification of a-Syn levels in chamber cells did not reveal any significant changes after Mn exposure. This observation was in contrast to the results with the same primary plexus cells in the uptake study. In the uptake study, there was an increased accumulation of a-Syn when the cells were exposed to Mn. A plausible reason for this discrepancy is that the primary choroidal cells in the a-Syn uptake experiment did not have a compartment on the basolateral side to expel the excess a-Syn taken up by the cells. This would lead to excessive accumulation of a-Syn in the cells. The primary cells cultured on the membrane of the Transwell inserts, however, possess compartments on both sides of the BCSFB monolayer in the Transwell system. Therefore, the cells could effectively expel the excess a-Syn in either direction to the extracellular culture medium. At present, the mechanism by which a-Syn is expelled out of these BCSFB cells is unknown. Thus, it has become absolutely necessary to investigate how a-Syn is transported by the BCSFB and what are the clinical implications if these naturally present transport machineries are altered due to genetic defects, environmental insults, or both.
In summary, our findings reveal that the BCSFB expresses a-Syn endogenously and is capable of transporting a-Syn between the blood and CSF. Exposure to Mn increases the uptake of a-Syn in choroid epithelial cells, but does not have any apparent effect on a-Syn transport across the BCSFB. The clinical relevance of these observations to Mn-induced Parkinsonian disorder remains unclear. The current study represents the initial steps that have provided the foundation for further investigation of a-Syn transport at the BCSFB in health and disease conditions.
Supplementary Material
Acknowledgments
This research was supported in part by NIH/National Institute of Environmental Health Sciences Grants RO1-ES008146-17. Mr. Christopher Bates has been partially supported by NIH/NIEHS Promotion of Diversity in Health-Related Research Program.
Footnotes
Competing interests
The authors declare that there are no financial or non-financial competing interests.
Authors’ contributions
CB and WZ designed experiments; CB, SF, and DY participated in the acquisition of the data. CB drafted and wrote the manuscript. WZ and JR commented and revised the manuscript. All authors have read and approved the final version of the manuscript.
References
- 1.Kamel F. Science. 2013;341:722–723. doi: 10.1126/science.1243619. [DOI] [PubMed] [Google Scholar]
- 2.Auluck P, Caraveo G, Lindquist S. Ann Rev Cell Dev Biol. 2010;26:211–233. doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
- 3.Clayton DF, George JM. J Neurosci Res. 1999;58:120–129. [PubMed] [Google Scholar]
- 4.Murphy D, Rueter SM, Trojanowski JQ, Lee VMY. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton M, Südhof TC. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon SL, Cousin MA. Traffic. 2014;15:245–254. doi: 10.1111/tra.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Patel S, Lee S. J Neurosci 2005. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Cho E, Lee KW, Kim J, Cho S, Lee S. Exp & Mol Med. 2013;45:1–9. doi: 10.1038/emm.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng W, Aschner M, Ghersi-Egea JF. Toxicol Appl Pharmacol. 2003;192:1–11. doi: 10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansen CE, Duncan JA, Stopa EG, Baird A. Pharma Res. 2005;22:1011–1037. doi: 10.1007/s11095-005-6039-0. [DOI] [PubMed] [Google Scholar]
- 12.Mollenhauer B, Trautmann E, Otte B, Ng J, Spreer A, Lange P, Sixel-Döring F, Hakimi M, VonSattel J, Nussbaum R, Trenkwalder C, Schlossmacher MG. J Neural Transm. 2012;119:739–746. doi: 10.1007/s00702-012-0784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Janković J, Zabetian CP, Leverenz JB, Baird G, Montine TJ, Hancock AM, Hwang H, Pan C, Bradner J, Kang UJ, Jensen PH, Zhang J. Brain. 2010;133:713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foulds PG, Diggle P, Mitchell JD, Parker A, Hasegawa M, Masuda-Suzukake M, Mann DMA, Allsop D. Sci Rep. 2013;3:2540. doi: 10.1038/srep02540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mollenhauer B, Trautmann E, Taylor P, Manniger P, Sixel-Döring F, Ebentheuer J, Trenkwalder C, Schlossmacher MG. J Neurosci Lett. 2013;532:44–48. doi: 10.1016/j.neulet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Suk J, Bae E, Lee J, Paik SR, Lee S. Int J Biochem & Cell Bio. 2008;40:1835–849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Desplats P, Lee H, Bae E, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee S. PNAS. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wennstrom M, Surova Y, Hall S, Nilsson C, Minthon L, Boström F, Hansson O, Nielsen HM. PLoS One. 2013;8:e53250. doi: 10.1371/journal.pone.0053250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates CA, Zheng W. Fluids Barriers. CNS; 2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeWitt MR, Chen P, Aschner M. Biochem Biophys Res Commun. 2013;432:1–4. doi: 10.1016/j.bbrc.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowan DM, Zheng W, Zou Y, Shi X, Chen J, Rosenthal F, Fan Q. Neurotoxicology. 2009;30:1214–1222. doi: 10.1016/j.neuro.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bornhorst J, Wehe CA, Hüwel S, Karst U, Galla HJ, Schwerdtle T. J Biol Chem. 2012;287:17140–17151. doi: 10.1074/jbc.M112.344093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perl DP, Olanow CW. J Neuropathol Exp Neuro. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- 24.Covy JP, Giasson BI. Neurotox. 2011;32:622–629. doi: 10.1016/j.neuro.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robison G, Zakharova T, Fu S, Jiang W, Fulper R, Barrea R, Marcus MA, Zheng W, Pushkar Y. PLoS One. 2012;7:e48899. doi: 10.1371/journal.pone.0048899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guilarte TR, Chen MK. NeuroTox. 2007;28:1147–1152. doi: 10.1016/j.neuro.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Li GJ, Zheng W. Exp Biol Med. 2008;233:1561–1571. doi: 10.3181/0803-RM-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dydak U, Jiang Y, Long L, Zhu H, Chen J, Li W, Edden RAE, Hu S, Fu X, Long Z, Mo X, Meier D, Hareziak J, Aschner M, Murdoch JB, Zheng W. Env Health Perspect. 2011;119:219–224. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu X, Zhang Y, Jiang W, Monnot AD, Bates CA, Zheng W. Toxicol Sci. 2014;139:423–451. doi: 10.1093/toxsci/kfu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long Z, Jiang Y, Li X, Fadel W, Xu J, Yeh C, Long L, Luo H, Harezlak J, Murdoch JB, Zheng W, Dydak U. NeuroTox. 2014;1669:1–8. doi: 10.1016/j.neuro.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crossgrove J, Zheng W. NMR Biomed. 2004;17:544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Racette BA. Neurotox. 2013. In Press. [Google Scholar]
- 33.Takeda A, Akiyama T, Sawashita J, Okada S. Brain Res. 1994;640:341–344. doi: 10.1016/0006-8993(94)91891-0. [DOI] [PubMed] [Google Scholar]
- 34.Zheng W, Jiang Y, Zhang Y, Jiang W, Wang X, Cowan DM. Neurotox. 2009;30:240–248. doi: 10.1016/j.neuro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokel RA, Crossgrove JS, Bukaveckas BL. NeuroTox. 2003;24:15–22. doi: 10.1016/s0161-813x(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt C, Strazielle N, Richaud P, Boubon A, Ghersi-Egea JF. J Neurochem. 2011;117:747–756. doi: 10.1111/j.1471-4159.2011.07246.x. [DOI] [PubMed] [Google Scholar]
- 37.Takeda A, Sawashita J, Okada S. Brain Res. 1995;695:53–58. doi: 10.1016/0006-8993(95)00916-e. [DOI] [PubMed] [Google Scholar]
- 38.Uversky VN, Li J, Fink AL. FEBS Letters. 2001;500:105–108. doi: 10.1016/s0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- 39.Binolfi A, Rasia RM, Bertoncini CW, Ceolin M, Zweckstetter Griesinger C, Jovin TM, Fernândez CO. J Am Chem Soc. 2006;128:9893–9901. doi: 10.1021/ja0618649. [DOI] [PubMed] [Google Scholar]
- 40.Cai T, Yao T, Zheng G, Chen Y, Du K, Cao Y, Shen X, Chen J, Luo W. Brain Res. 2010;1359:201–207. doi: 10.1016/j.brainres.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Sun L, Cai T, Zhang Y, Lv S, Wang Y, Ye L. Brain Res Bull. 2010;81:428–433. doi: 10.1016/j.brainresbull.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Zheng W, Zhao Q. Methods Mol Biol. 2002;188:99–114. doi: 10.1385/1-59259-185-X:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li GJ, Zhao Q, Zheng W. Toxicol Appl Pharmacol. 2005;205:188–200. doi: 10.1016/j.taap.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Li GJ, Zheng W. Brain Res. 2006;1097:1–10. doi: 10.1016/j.brainres.2006.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng W, Zhao Q, Graziano JH. In Vitro Cell Dev Biol. 1998;34:40–45. doi: 10.1007/s11626-998-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi LZ, Zheng W. Brain Res. 2005;1057:37–48. doi: 10.1016/j.brainres.2005.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monnot AD, Behl M, Ho S, Zheng W. Toxicol Appl Pharmacol. 2011;256:249–257. doi: 10.1016/j.taap.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng G, Chen J, Zheng W. Toxicol Appl Pharamcol. 2012;260:285–293. doi: 10.1016/j.taap.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Griggs A, Rochet JC, Stanciu LA. Biophys J. 2013;104:2706–2713. doi: 10.1016/j.bpj.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee H, Khoshaghideh F, Patel S, Lee S. J Neurosci. 2004;24:1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Y, Prudent M, Fauvet B, Lashuel HA, Girault HH. ACS Chem Neurosci. 2011;2:667–675. doi: 10.1021/cn200074d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartels T, Choi JG, Selkoe DJ. Nature. 2011;477:107–111. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fauvet B, Kamdem MM, Fares MB, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, Eliezer D, Moore DJ, Schneider B, Aebischer P, El-Agnaf OM, Masliah E, Lashuel HA. J Biol Chem. 2012:1–35. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuzdas-Wood D, Stefanova N, Jellinger KA, Seppi K, Schlossmacher MG, Poewe W, Wenning GK. Prog Neurobiol. 2014;118:19–35. doi: 10.1016/j.pneurobio.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Binolfi A, Valiente-Gabioud AA, Duran R, Zweckstetter M, Griesinger C, Fernandez CO. J Am Chem Soc. 2011;133:194–196. doi: 10.1021/ja107842f. [DOI] [PubMed] [Google Scholar]
- 56.Guilarte TR. Frontiers in Aging Neuroscience. 2013;5:1–10. doi: 10.3389/fnagi.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmad A, Burns CS, Fink AL, Uversky VN. J Biomol Struc Dyn. 2013;29:825–842. doi: 10.1080/073911012010525023. [DOI] [PubMed] [Google Scholar]
- 58.Camponeschi F, Valensin D, Tessari I, Bubacco L, Dell’Acqua S, Casella L, Monzani E, Gaggelli E, Valensin G. Inorg Chem. 2013;52:1358–1367. doi: 10.1021/ic302050m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.