Abstract
Background
Depression is a major barrier to HIV treatment outcomes.
Objective
Test whether antidepressant management decision support integrated into HIV care improves antiretroviral adherence and depression morbidity.
Design
Pseudo-cluster randomized trial.
Setting
Four US infectious diseases clinics.
Participants
HIV-infected adults with major depressive disorder.
Intervention
Measurement-Based Care: depression care managers used systematic metrics to give HIV primary-care clinicians standardized antidepressant treatment recommendations.
Measurements
Primary: Antiretroviral medication adherence (monthly unannounced telephone-based pill counts for 12m). Primary timepoint: 6m. Secondary: Depressive severity, depression remission, depression-free days, measured quarterly for 12m.
Results
From 2010-2013, 149 participants were randomized to intervention and 155 to usual care. Participants were mostly male, Black non-Hispanic, unemployed, and virally suppressed with high baseline self-reported antiretroviral adherence and depressive severity. Over follow-up, no differences between arms in antiretroviral adherence or other HIV outcomes were apparent. At 6 months, depressive severity was lower among intervention participants than usual care (mean difference −3.7 [95% CI: −5.6, −1.7]), probability of depression remission was higher (risk difference 13% [1%, 25%]), and suicidal ideation was lower (RD −18% [−30%, −6%]). By 12 months the arms had comparable mental health outcomes. Intervention arm participants experienced an average of 29 (95% CI: 1-57) more depression-free days over 12 months.
Conclusions
In the largest trial of its kind among HIV-infected adults, MBC did not improve HIV outcomes, possibly because of high baseline adherence, but achieved clinically significant depression improvements and increased depression-free days. MBC may be an effective, resource-efficient approach to reducing depression morbidity among HIV patients.
Keywords: HIV, depression, Measurement-Based Care depression treatment, randomized trial, antiretroviral adherence, pseudo-cluster randomization
Introduction
Depression is a major barrier to HIV care. Depressive disorders affect an estimated 20-30% of people living with HIV[1-3] and are strongly associated with reduced antiretroviral (ARV) medication adherence, virologic failure, and higher mortality rates.[4-12]
Depressive disorders are cyclical, with approximately half of depressive episodes resolving within 12 months without treatment.[13] However, evidence-based treatments such as antidepressants and psychotherapy are critical in speeding the time to recovery and reducing depression morbidity.[14] Although such treatments have demonstrated efficacy for HIV-infected patients,[15, 16] this population still faces a large mental health treatment gap. Estimates suggest that among HIV-infected patients with depression, only one in five are receiving depression treatment and even fewer are receiving effective (rather than sub-therapeutic) treatment.[17-19] Evidence is needed on the impact of pragmatic, efficient, evidence-based mental health service delivery strategies integrated within HIV primary care.
Despite the associations of depression with adverse HIV outcomes, the impact of effective depression treatment on these outcomes is unclear. Several observational studies as well as trials of counseling interventions have reported a positive association between receipt of depression treatment and ARV adherence,[20-23] while two recent randomized trials of medication-based depression treatment found no effect on ARV adherence or other HIV outcomes.[24, 25] We report the results of a randomized trial to test the effect of Measurement-Based Care, a decision support model for antidepressant management integrated into HIV care, on HIV and mental health outcomes among HIV-infected adults with depression.
Methods
Study design and objective
The Strategies to Link Antidepressant and Antiretroviral Management at Duke University, University of Alabama at Birmingham, Northern Outreach Clinic (Henderson, NC), and University of North Carolina (SLAM DUNC) Study was a single-blind randomized controlled trial with the objective of testing whether evidence-based decision support for antidepressant management, integrated into HIV care, would improve ARV adherence and clinical outcomes (ClinicalTrials.gov NCT01372605).[26] English-speaking adults ages 18-65 receiving HIV care were eligible if they were taking or about to start ARVs, scored ≥10 on the Patient Health Questionnaire-9 (PHQ-9) depression screening instrument,[27, 28] and had a major depressive disorder diagnosis confirmed using the Mini International Neuropsychiatric Interview (MINI).[29] Patients were excluded who met MINI criteria for bipolar or psychotic disorder history, had failed ≥2 antidepressant trials of ≥6 weeks at a moderate/high dose during the current depressive episode, were mentally incompetent, or required immediate psychiatric hospitalization. Patients already taking antidepressants were eligible if they met other criteria, since despite being on treatment they were currently depressed and therefore could plausibly benefit from the active regimen adjustments conferred by the intervention.
Conditions
Participants were randomized to Measurement-Based Care (MBC, “intervention”) or enhanced treatment as usual (“usual care”). In MBC, depression care managers (DCMs) collaborate with medical providers to optimize antidepressant treatment following an evidence-based algorithm.[30, 31] MBC emphasizes vigorous antidepressant dosing combined with careful monitoring of depressive symptoms and medication tolerability using standardized measures. Assessments occur at standardized time points to ensure timely assessment of treatment response and subsequent treatment adjustment if indicated.
DCMs in this study were six licensed clinical social workers, one clinical psychologist, and one PhD public health researcher. In prior work the DCM role has been filled effectively by medical assistants and generalist nurses. DCMs completed a two-day initial training focused on diagnosis of depression and excluding diagnoses, response to suicidality, antidepressant dosing and side effects, use of standardized MBC tools to assess treatment response and tolerability, and use of the MBC algorithm to generate treatment recommendations for HIV providers. Ongoing training, quality assurance, and continuous quality improvement occurred through weekly group supervision with psychiatric supervisors. Supervisors reviewed all DCM patient contacts using a web-based participant registry, focusing on review of safety (suicidality) assessments, congruence of treatment recommendations with the algorithm and reasons for divergence, pros and cons of specific antidepressants in specific situations, and troubleshooting issues such as provider ambivalence about treatment adjustments.
In the intervention arm, DCMs met with participants to measure depressive severity (PHQ-9[27]) and side effect burden (Frequency, Intensity, and Burden of Side Effects Rating scale[32]) at weeks 4, 8, and 12 and, guided by the MBC algorithm, provided treatment recommendations to the HIV medical provider (e.g., maintain dose; increase dose; change antidepressant).[30] Typically, if depression remission had not been achieved and the medication was being tolerated, a dose increase was recommended. If side effects were bothersome, possible strategies included dose timing adjustment, dose decrease, or medication switch. Side effects were also assessed at interim 2, 6, and 10-week contacts. Participants who entered the study already on an antidepressant (but still meeting the eligibility criterion of current major depressive disorder) were handled similarly, with an initial recommendation to either increase dose or switch medication based on duration, dose, and tolerability of current medication. At 12 weeks, participants who had achieved remission entered a maintenance phase (3-monthly assessments to confirm continued response; if a relapse occurred, a new acute phase cycle was initiated). For non-remitting participants, recommendations were made for antidepressant switch/augmentation or an optional 4-week extension of the current regimen. All final treatment decisions were the purview of the treating HIV provider. Intervention participants also received three motivational interviewing sessions focused on barriers to ARV adherence at 7, 8, and 10 months, adapted from the PACT intervention.[33] While completion of the PHQ-9 and discussion of depressive symptoms and side effects during the DCM assessment sometimes led to a broader discussion of the participant's wellbeing, DCMs did not provide any structured cognitive-behavioral or other psychotherapeutic depression treatment during study contacts.
Usual care was enhanced with initial training of all HIV providers in MBC principles, provision of diagnosis information at enrollment, and availability of the MBC algorithm for providers to consult on their own. HIV medical providers could prescribe antidepressants or refer to mental health services but received no DCM decision support.
Randomization
Pseudo-cluster randomization[34, 35] was employed to balance competing concerns of contamination and referral bias.[36] Providers were randomly assigned at baseline to an imbalanced case mix of either 80% intervention/20% usual care or 20% intervention/80% usual care patients. Using a random number generator, providers were randomized 1:1 in blocks of 4 stratified by site and depression treatment experience level. Depression treatment experience was assessed at baseline via a semi-structured interview asking about providers’ approaches to antidepressant prescription, monitoring of response, and treatment adjustment.[37] Responses were compared to evidence-based guidelines and scored in standardized fashion. For randomization, depression treatment experience was classified as high, medium, or low based on tertiles of the resulting total scores.
Each provider was then assigned patient slots in blocks of ten (8 intervention/2 usual care or 2 intervention/8 usual care, randomly ordered). Provider allocation and patient slot order were masked to everyone except the study statistician (BWP). The statistician maintained a document showing each provider's next three open slots, with access granted to five coordinating center staff with no involvement in enrollment. For each new participant, enrollment staff called the coordinating center, identified the participant's provider, and were given the arm assignment for that provider's next slot. Regular checks ensured that arm assignments were given in the correct order.
Blinding
Blinded research assessors collected all research outcomes. Participants, DCMs, medical providers, and investigators were unblinded.
Data collection
The primary outcome was ARV adherence, measured monthly for 12 months by unannounced telephone-based pill count. Unannounced telephone-based pill counts have been shown to provide valid, accurate measures of adherence[38] and are more sensitive than self-report to changes over time.[39] Adherence was calculated as (observed pills taken / expected pills taken). “Observed pills taken” was defined as the pills at the previous count minus the pills at the current count, corrected for pills gained (e.g. new bottles) or lost (e.g. given away) in the interim. “Expected pills taken” was defined as the prescribed number of daily pills times the number of days between counts. Extensive probes about pills gained or lost were used to maximize accuracy.
Secondary outcomes included depressive severity (Hamilton Rating Scale for Depression [HRSD]),[40-42] depression remission (HRSD<8), self-reported ARV adherence (30-day visual analog scale),[43] and HIV-related symptoms (recent headaches, fever, pain in gums, white patches in mouth, rashes, nausea, trouble with eyes, sinus pain, numbness in hands or feet, cough, diarrhea, or weight loss) ,[1] measured at 3, 6, 9, and 12 months; virologic suppression (viral load <50 copies/mL) and physical and mental health-related quality of life (SF-12),[44, 45] measured at 6 and 12 months; and depression-free days[46, 47] and HIV medical appointment adherence (proportion of kept visits), measured cumulatively over the 12-month follow-up period. An annualized measure of depression-free days (DFD) was calculated by first assigning a value of 1 (depression-free) to HRSD values <8 and a value of 0 (severely depressed) to HRSD values >21 at each of the 0, 3, 6, 9, and 12-month assessments.[46, 47] HRSD scores 8-21 were assigned a proportionately weighted value. To annualize DFDs, for each three-month interval the DFD values on each side were averaged and multiplied by the interval duration, and then the values for all intervals were summed.
Data quality assurance
Each pill count was reviewed by the original and a second data collector. Pill counts were ruled invalid if insufficient information was available to link to the previous count (e.g., >1 month had elapsed and the number of bottles dispensed was unknown) or if the data collector noted substantially conflicting or suspicious information (e.g., hostile behavior; open bottles that were not newly dispensed but had not been presented in previous counts). If the two reviewers disagreed on validity, all data collectors and the PI jointly reviewed the pill count (blinded to arm) and came to consensus.
Sample size
The target sample of 390 (195/arm) was calculated to have 80% power to detect a 10 percentage point improvement in ARV adherence at 6 months. Power calculations assumed pooled standard deviation=25-29%,[22], two-tailed α=0.05, design effect=1.04, 5-10% contamination, and 80% 6-month retention. The design effect assumed a mean of 9 patients per provider, intraclass correlation coefficient ρ=0.02,[48-51] and an 80%/20% within-provider intervention allocation ratio in the pseudo-cluster design.[35]
Statistical analysis
The primary time point was defined as 6 months; data collection continued through 12 months to assess longer-term outcomes. The primary intention-to-treat analysis addressed the pseudo-cluster randomization design with clustering by provider and fixed effects for site and provider depression treatment experience level. To address missing data, the Statistical Analysis Plan, approved by the Data Safety and Monitoring Board, adopted a direct modeling approach.[52] Correlates of missingness and earlier (months 1-5) pill count measures were modeled as additional outcome variables along with the primary outcome using a multivariate normal distribution. If the included correlates of missingness capture the important differences between those with and without outcome data, this approach yields an effect estimate corrected for selection bias from the missing data. This approach was implemented using linear mixed models with PROC MIXED (SAS 9.3, Cary NC), specifying random intercepts for providers and for patients within providers. For secondary binary outcomes, risk differences were estimated using generalized linear models (identity link, binomial error distribution). Since the linear mixed model approach was inappropriate for binary outcomes, correction for missing data was implemented using inverse probability of observation weighting, with correlates of missingness serving as explanatory variables in the weight estimation model (see e-Appendix).
Ethical review
Each site's Institutional Review Board approved all study activities. Equipoise was reasonably present with respect to the study's primary endpoint, and providers were free to prescribe antidepressants to usual care participants or refer them to other mental health services.
Results
Enrollment occurred from April 2010-October 2013 and follow-up continued through April 2014. As recruitment was slower than expected, the trial ended due to funding constraints before achieving full enrollment. A total of 304 participants were randomized to intervention (n=149) or usual care (n=155) (Figure 1). Seventy-seven percent of participants had at least one valid ARV adherence measure (78% intervention/76% usual care); 60% had a valid primary (6-month) outcome measure (58% intervention/61% usual care).
Figure 1.
Total PHQ-9 screens and positive screens include multiple screens per patient. Participants were defined as receiving the allocated intervention if their provider received at least one antidepressant treatment recommendation from the depression care manager (intervention arm) or received no antidepressant treatment recommendations from the depression care manager (usual care arm).
Participants
The majority of participants were 30-55 years, male, black non-Hispanic, and unemployed (Table 1). Participants had high depressive severity and prevalence of psychiatric comorbidities. Participants had strong HIV clinical indicators and high self-reported ARV adherence. There were small differences between arms in certain demographic characteristics, but the arms were well balanced on baseline physical and mental health measures.
Table 1.
Comparison of baseline participant characteristics by study arm assignment.
| Intervention (n = 149) |
Usual care (n = 155) |
|||
|---|---|---|---|---|
| % (n) | Mean (SD) | % (n) | Mean (SD) | |
| Sociodemographics | ||||
| Age (years) | 42.8 (10.3) | 44.9 (9.9) | ||
| Present sex | ||||
| Male | 75% (112) | 64% (100) | ||
| Female | 24% (35) | 34% (52) | ||
| Transgender and other | 1% (2) | 2% (3) | ||
| Sexual orientation | ||||
| Heterosexual | 40% (58) | 53% (81) | ||
| Gay/Lesbian | 46% (67) | 34% (51) | ||
| Bisexual | 11% (17) | 10% (16) | ||
| Other | 3% (4) | 3% (4) | ||
| Race/ethnicity | ||||
| White non-Hispanic | 36% (54) | 25% (39) | ||
| Black non-Hispanic | 56% (83) | 68% (105) | ||
| Hispanic | 6% (9) | 3% (4) | ||
| Other | 2% (3) | 4% (7) | ||
| Employment status | ||||
| Employed full time | 15% (22) | 14% (22) | ||
| Employed part time | 12% (17) | 13% (20) | ||
| Unemployed | 73% (108) | 73% (111) | ||
| Mental health indicators | ||||
| Depressive severity (HAM-D) | 20.3 (6.9) | 19.9 (6.9) | ||
| Suicidality | 23% (32) | 20% (26) | ||
| Comorbid anxiety disorder | 59% (88) | 64% (99) | ||
| Comorbid substance use disorder | 32% (47) | 25% (38) | ||
| SF-12 mental functioning score | 30.5 (9.4) | 30.3 (10.4) | ||
| HIV-related indicators | ||||
| CD4 count, cells/mm3 | 607 (371) | 569 (354) | ||
| HIV-RNA viral load <50 copies/mL | 69% (91) | 68% (98) | ||
| Self-reported ARV adherence, % | 85.8 (23.3) | 87.2 (22.2) | ||
| Number of HIV symptoms | 5.2 (2.9) | 5.1 (3.1) | ||
| SF-12 physical functioning score | 44.1 (11.8) | 43.8 (12.1) | ||
Treatment
Intervention participants had a mean of 8.9 DCM contacts (Table 2). At study enrollment, 44% of intervention and 40% of usual care participants were already on an antidepressant; 17% of intervention and 21% of usual care participants were on a moderate/high antidepressant dose (as defined previously based on standard dosing guidelines;[30, 53]). Antidepressant prescription and moderate/high dosing increased in both arms during the study, but more rapidly and substantially in the intervention arm. External mental health referrals and non-study treatment sessions were similar between arms.
Table 2.
Indicators of mental health treatment by study arm assignment.
| Intervention (n = 149) |
Usual care (n = 155) |
|||
|---|---|---|---|---|
| Range | Mean (SD) or % (n) | Range | Mean (SD) or % (n) | |
| DCM contacts, 12 months | 1-21 | 8.9 (4.4) | n/a | n/a |
| Duration, weeks 4, 8, 12, minutes | 5-165 | 36 (20) | n/a | n/a |
| Duration, weeks 2, 6, 10, minutes | 3-90 | 23 (17) | n/a | n/a |
| On antidepressant | ||||
| Baseline | 44% (66) | 39% (60) | ||
| 6 months | 79% (112) | 53% (78) | ||
| 12 months | 79% (98) | 53% (71) | ||
| On moderate to high* antidepressant dose | ||||
| Baseline | 17% (25) | 20% (31) | ||
| 6 months | 39% (55) | 28% (42) | ||
| 12 months | 37% (46) | 33% (44) | ||
| Non-study mental health counseling sessions, 12 months** | 0-294 | 6.9 (29.8) | 0-66 | 4.6 (11.1) |
| Referred for mental health treatment*** | 20% (30) | 18% (28) | ||
| Baseline antidepressant | ||||
| Citalopram | 12% (18) | 12% (19) | ||
| Sertraline | 5% (7) | 4% (6) | ||
| Mirtazapine | 5% (7) | 3% (5) | ||
| Bupropion | 5% (7) | 3% (4) | ||
| Fluoxetine | 3% (4) | 3% (4) | ||
| Venlafaxine | 3% (4) | 2% (3) | ||
| Other**** | 11% (16) | 4% (6) | ||
| Multiple | 2% (3) | 9% (14) | ||
As defined previously based on standard dosing guidelines;[30, 53]e.g. ≥40mg citalopram or ≥100mg sertraline daily.
One intervention arm participant started daily outpatient mental health and substance use treatment after study enrollment. Excluding this outlying participant, mean (SD) number of mental health counseling sessions in the intervention arm was 4.5 (13.9).
Any referral for non-study related mental health treatment within or outside the ID clinic
In both arms combined, 6 or fewer participants taking amitriptyline, duloxetine, escitalopram, or paroxetine.
HIV-related outcomes
When comparing crude (not accounting for design effects or missingness) outcome measures by original arm assignment, ARV adherence measured by unannounced telephone-based pill count was high overall and showed little change in either arm over time (Figure 2A). Self-reported ARV adherence, viral load, appointment attendance, HIV-related symptoms, physical health-related functioning, emergency department use, and hospitalizations were also similar between the arms over time (supplemental figures).
Figure 2.
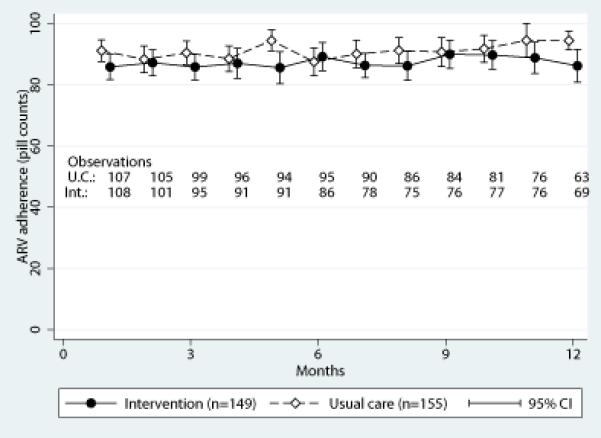


HIV medication adherence (A), depressive severity (B), and suicidality (C) over time by study arm (uncorrected for design or missing data).
Mental health outcomes
When comparing crude outcome measures by original arm assignment, the arms had comparable baseline depressive severity (Figure 2B). Intervention arm scores improved 7 points at 6 and 12 months. Usual care scores improved 3 points at 6 months and 5 points at 12 months. Other mental health indicators also showed early gains in the intervention group, with the usual care group mostly closing the gap by 12 months (Figure 2C; supplemental figures). The intervention group experienced an average of 160 depression-free days during the 12 months of follow-up compared to 136 days among usual care participants.
Effect estimates
In intent-to-treat analyses adjusted for design elements and corrected for missingness, no effect was evident on ARV adherence (the primary outcome) or other HIV-related outcomes at the primary time point, 6 months (Table 3). At the primary 6-month time point, the intervention demonstrated an effect on depressive severity, achievement of depression remission, and suicidality as well as a trend toward an effect on mental health-related functioning. By 12 months the usual care group had achieved comparable mental health outcomes to the intervention group on most indicators, but participants in the intervention group maintained their advantage in depression remission (relative advantage of 16.0 [2.6, 29.4] percentage points) (data not shown). Over 12 months, the intervention arm experienced 29 (1, 57) more depression-free days. Correction for missing data had little impact on most effect estimates.
Table 3.
Crude and corrected differences in HIV-related and mental health outcomes and healthcare utilization between arms at 6 months (primary time-point).
| Intervention | Usual care | Adjusted for design** | Adjusted for design** and missing data*** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | n | Mean/% | n | Mean/% | Difference | 95% CI | P value | Difference | 95% CI | P value |
| HIV-related outcomes | ||||||||||
| Percentage adherence, pill count | 86 | 89.2 | 95 | 87.6 | 1.2 | (−4.0, 6.5) | 0.64 | 1.4 | (−3.9, 6.7) | 0.61 |
| Percentage adherence, self-report | 92 | 92.7 | 98 | 93.9 | −0.8 | (−4.6, 3.1) | 0.69 | −1.3 | (−5.8, 3.3) | 0.59 |
| Number of HIV symptoms (range: 0-12) | 92 | 4.5 | 98 | 4.7 | −0.3 | (−1.0, 0.5) | 0.51 | −0.2 | (−0.9, 0.4) | 0.48 |
| Viral load, log-10 | 79 | 1.6 | 98 | 1.9 | −.2 | (−0.5, 0.0) | 0.06 | −0.2 | (−0.6, 0.2) | 0.28 |
| Viral load < 50 copies/ml (%) | 79 | 86.1 | 98 | 76.5 | 9.8 | (−0.9, 20.5) | 0.07 | 9.8 | (−0.9, 20.5) | 0.07 |
| Percentage HIV appointment adherence* | 146 | 81.3 | 151 | 80.1 | 0.8 | (−4.6, 6.2) | 0.77 | 1.2 | (−2.8, 5.3) | 0.56 |
| Physical health-related quality of life (range: 0-100) | 92 | 42.8 | 98 | 43.7 | −1.1 | (−4.4, 2.3) | 0.52 | −1.0 | (−5.0, 3.0) | 0.64 |
| Mental health-related outcomes | ||||||||||
| Depressive severity (range: 0-50) | 92 | 13.5 | 98 | 17.2 | −3.6 | (−5.9, −1.2) | 0.00 | −3.7 | (−5.6, −1.7) | 0.00 |
| Depression remission (%) | 92 | 20.7 | 98 | 17.3 | 0.3 | (−12.1, 12.7) | 0.97 | 12.8 | (1.2, 24.3) | 0.03 |
| Any active suicidality (%) | 92 | 8.7 | 98 | 21.4 | −12.7 | (−23.3, −2.1) | 0.02 | −18.1 | (−29.9, −6.4) | 0.00 |
| Mental health-related quality of life (range: 0-100) | 92 | 41.6 | 98 | 37.6 | 3.7 | (0.3, 7.1) | 0.04 | 3.8 | (−0.1, 7.8) | 0.06 |
| Depression-free days* | 143 | 160 | 137 | 136 | 25 | (1, 49) | 0.04 | 29 | (1, 57) | 0.04 |
| Healthcare utilization | ||||||||||
| Any emergency department visit (%) | 92 | 18.5 | 96 | 20.8 | −5.3 | (−25.2, 14.7) | 0.60 | 0.6 | (−16.0, 17.3) | 0.94 |
| Any hospitalization (%) | 92 | 7.6 | 96 | 4.2 | 3.4 | (−2.4, 9.3) | 0.25 | 5.8 | (−0.9, 12.4) | 0.09 |
Over 12 months of study enrollment
Via ordinary least squares regression (continuous outcomes) or generalized linear model with identity link and binomial error distribution (binary outcomes), with fixed effects for site and depression treatment experience level and clustered by provider
Via linear mixed model (continuous outcomes) or generalized linear model with identity link, binomial error distribution, and inverse probability of observation weighting (binary outcomes), with fixed effects for site and depression treatment experience level and clustered by provider.
Secondary comparisons
In pre-specified secondary comparisons, there was no evidence of stronger intervention effectiveness in an “as treated” analysis (ignoring arm and comparing those who had vs. had not been on antidepressants for ≥90 days) or a “completers” analysis (ignoring arm and comparing those who did vs. did not complete ≥3 treatment adjustment contacts with the DCM) (data not shown). There was no evidence of stronger intervention effectiveness in pre-specified subgroups (those with baseline self-reported adherence <80%, unsuppressed HIV RNA viral load, PHQ-9≥20, or comorbid anxiety/substance use disorders) (data not shown).
Discussion
This study represents the largest trial to date of a collaborative care antidepressant management intervention integrated into HIV primary care, and the first such trial to our knowledge outside of the Veterans Administration (VA) system. The Measurement-Based Care depression management approach was effectively integrated in 4 HIV clinics, with high uptake of antidepressants and timely dose escalation in the intervention arm. No differences between arms were observed in the primary outcome – antiretroviral adherence – or other HIV outcomes, including HIV symptoms, viral load, or appointment adherence. At 6 months, relative to usual care, the intervention had reduced depressive severity by a clinically meaningful margin, increased depression remission, reduced suicidality, and improved mental health-related functioning. By 12 months, the usual care arm had caught up on most mental health outcome measures. However, by shortening the course of depressive episodes, the intervention conferred nearly an additional month of depression-free days over the 12 months of study participation.
Similar to this study, two other randomized trials of antidepressant-focused depression treatment strategies reported substantial improvements in mental health measures but no effect on HIV-related measures. A trial of directly observed weekly fluoxetine compared to referral to standard mental health services among 137 homeless or marginally housed men in San Francisco reported a strong effect of the intervention on depression outcomes but no statistically significant differences in HIV outcomes.[24] A trial of collaborative care for depression relative to usual care among 249 patients at 3 VA HIV clinics found a mental health benefit of the intervention at 6 months, but usual care participants had caught up by 12 months. The intervention lowered HIV symptoms but had no effect on ARV adherence or other HIV outcomes.[25] In contrast, three trials of cognitive behavioral therapy for depression with integrated adherence counseling (CBT-AD) have shown improvements in both depression and adherence among 45 adults with HIV and depression;[22] 89 adults with HIV, depression, and injection drug use histories;[54] and 40 Latino adults with HIV and depression.[55]
Two differences in the above studies are apparent. First, the three trials that identified an effect of depression treatment on ARV adherence[22, 54, 55] were conducted among participants with relatively poor adherence or viral suppression at baseline. In contrast, participants in the present study and the two trials that did not identify such effects[24, 25] had high baseline levels of adherence and rates of viral suppression, potentially introducing a ceiling effect on HIV outcomes. Second, the trials that found effects on adherence deployed an intervention that explicitly targeted both depression and adherence through counseling and/or reminder components, while the present study and the trials that did not find such effects primarily targeted depression through medication management.
In contrast to the mixed trial results, most observational studies have reported a positive association between depression treatment and ARV adherence. A meta-analysis of 29 studies encompassing >12,000 individuals estimated that depression treatment improved the odds of satisfactory ARV adherence by 83%, with a stronger association among observational than experimental studies.[20] Estimates from observational studies may be confounded by characteristics that are difficult to measure. For example, among depressed patients, those willing to initiate depression treatment may be more compliant with medical treatment in general. It is also possible that trials tend to enroll generally compliant patients whose adherence has little room to improve, while observational studies are able to include patients with a wider distribution of adherence.
Among this study's strengths are its size, multiple sites, duration of follow-up, objective adherence measure, rigorous pseudo-cluster randomization design, and high level of fidelity to protocol achieved through weekly supervision. An additional strength is the broad inclusion criteria. While many depression treatment trials exclude individuals with anxiety or substance use disorders to achieve a “clean” participant pool, this study did not, since such a set of participants would bear little resemblance to patients with depression in real-world HIV care.[56] Similarly, many adherence trials restrict enrollment to individuals with low adherence or viral failure, but this study did not, since we sought to estimate the impact of a clinic-wide collaborative depression care intervention on HIV outcomes.
A major challenge for this study was missing data. While 77% of participants completed ≥1 follow-up, 60% had a valid 6-month primary outcome measure. Some of those lost may have discontinued ART; this is unknown. Importantly, missingness was balanced between arms and sophisticated missing-data correction methods had little impact on effect estimates. Loss to follow-up could also be related to clinical comorbidities such as immune reconstitution inflammation syndrome (IRIS), although most participants were on stable ART at entry, no instances of IRIS were documented during follow-up, and correction for baseline clinical status did not substantively change effect estimates. An additional limitation is the possibility that contamination may have diluted the true effect size, even with the pseudo-cluster design. While there were large differences in antidepressant prescription and dose escalation between the arms, these measures did improve somewhat among usual care participants over time, suggesting that some of the mental health gains of the usual care group by 12 months could be explained by contamination. Alternatively, this convergence could simply reflect the episodic nature of depressive disorders, for which treatment shortens the course of illness but up to 50% of episodes resolve spontaneously within a year.[13]
Depression is highly prevalent among people living with HIV.[57, 58] Despite the known efficacy of depression treatments in this population,[15, 16] depression remains widely under-diagnosed and untreated or under-treated in HIV primary care.[17, 18] New care models that build on the success of collaborative depression treatment in general primary care[59] are critically needed to address the large mental health treatment gap among people living with HIV. Models such as Measurement-Based Care efficiently leverage clinic staff time to provide antidepressant prescription decision support to HIV medical providers. This trial demonstrates that such a real-world strategy can significantly shorten the course of depressive illness for HIV patients and reduce overall morbidity from depression.
Acknowledgements
Funding/Support: This work was supported by grant R01MH086362 of the National Institute of Mental Health and the National Institute for Nursing Research, National Institutes of Health, Bethesda, MD, USA. Support for the design and conduct of the study was also provided by the NIH-funded Centers for AIDS Research at the University of North Carolina at Chapel Hill, Duke University, and the University of Alabama at Birmingham (P30- AI50410, P30-AI064518, and P30-AI027767).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the NIH.
Footnotes
Prior presentation: This work will be presented at the 10th Annual Conference on HIV Treatment and Prevention Adherence, Miami FL, June 28-30 2015.
Additional contributors: We gratefully acknowledge the contributions of Julie O’Donnell PhD, Angela Bengtson PhD, and Jennifer Lee MPH of the University of North Carolina at Chapel Hill, who were employed as predoctoral trainees and provided key data analysis support for this manuscript, and of the following individuals who were employed by the study and contributed to study management and data collection and analysis: Malaika Edwards MS, Katya Roytburd MPH, Quinn Williams MSW, and Abigail Zeveloff MPH MSW of the University of North Carolina at Chapel Hill; Scotty Elliot MSD, Charita Montgomery BS, Elise Nelson MSc, and Donna Safley MS of Duke University; Riddhi Modi MBBS MPH, Sally Shurbaji MPH, Melonie Walcott BA, and Anne Zinski PhD of the University of Alabama at Birmingham; Kiana Bess MPH of the Virginia State Department of Health; A. Jordan Akerley BA of the Shanti Project (San Francisco CA); Marcus Hawley BA of Durham NC; and R. Scott Pollard MSW RN of the Veritas Collaborative (Durham NC). Gordon Lipscomb MSW, Kathryn Schley MSW, Kathleen Sikkema PhD, and Melissa Watt PhD of Duke University, who did not receive payment, also provided valuable support for study implementation. We are grateful for guidance at the study design stage provided by David Wohl MD and Carol Golin MD of the University of North Carolina at Chapel Hill, who received no payment. Finally, the members of the study's Data Safety and Monitoring Board, who received honoraria for their service, provided invaluable guidance throughout the study on implementation issues and the definition of the statistical analysis plan: David Borasky MPH of Copernicus Group (Chapel Hill NC), Glenn Treisman MD of Johns Hopkins University, Madhukar Trivedi MD of the University of Texas Southwestern, and Stephen Wisniewski PhD of the University of Pittsburgh.
Author contributions:
Study concept and design: Pence, Gaynes, Adams, Thielman, Heine, Mugavero, Quinlivan
Statistical analysis: Pence, Turner
Drafting of the manuscript: Pence
Critical revision of the manuscript for important intellectual content: All authors
Trial Registration: ClinicalTrials.gov #NCT01372605. http://clinicaltrials.gov/ct2/results?term=NCT01372605
Author access to data: The lead author (BWP) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest disclosures: None reported.
Contributor Information
Brian W. PENCE, Epidemiology, UNC-Chapel Hill.
Bradley N. GAYNES, Psychiatry, UNC-Chapel Hill.
Julie L. ADAMS, Pharmaceutical Product Development of Wilmington, NC.
Nathan M. THIELMAN, Infectious Diseases, Duke University.
Amy D. HEINE, Infectious Diseases, UNC-Chapel Hill.
Michael J. MUGAVERO, Infectious Diseases, University of Alabama-Birmingham.
Teena MCGUINNESS, Psychiatry, University of Alabama-Birmingham.
James L. RAPER, Infectious Diseases, University of Alabama-Birmingham.
James H. WILLIG, Infectious Diseases, University of Alabama-Birmingham.
Kristen G. SHIREY, Psychiatry, Duke University.
Michelle OGLE, Infectious Diseases, Warren-Vance Community Health Center Inc., Henderson NC.
Elizabeth L. TURNER, Biostatistics and Bioinformatics and Duke Global Health Institute, Duke University.
E. Byrd QUINLIVAN, Institute for Global Health and Infectious Diseases, UNC-Chapel Hill.
References
- 1.Bing EG, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry. 2001;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 2.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–30. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 3.Tsai AC. Reliability and validity of depression assessment among persons with HIV in sub-Saharan Africa: systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2014;66(5):503–11. doi: 10.1097/QAI.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez JS, et al. Depression and HIV/AIDS Treatment Nonadherence: A Review and Meta-analysis. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordillo V, et al. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13(13):1763–9. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 6.Horberg MA, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47(3):384–90. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- 7.Mugavero M, et al. Barriers to antiretroviral adherence: the importance of depression, abuse, and other traumatic events. AIDS Patient Care STDS. 2006;20(6):418–28. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- 8.Ironson G, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67(6):1013–21. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ickovics JR, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–74. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 10.Leserman J, et al. Severe stress, depressive symptoms, and changes in lymphocyte subsets in human immunodeficiency virus-infected men. A 2-year follow-up study. Arch Gen Psychiatry. 1997;54(3):279–85. doi: 10.1001/archpsyc.1997.01830150105015. [DOI] [PubMed] [Google Scholar]
- 11.Leserman J, et al. Progression to AIDS: the effects of stress, depressive symptoms, and social support. Psychosom Med. 1999;61(3):397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Mugavero MJ, et al. Predictors of AIDS-related morbidity and mortality in a southern U.S. Cohort. AIDS Patient Care STDS. 2007;21(9):681–90. doi: 10.1089/apc.2006.0167. [DOI] [PubMed] [Google Scholar]
- 13.Whiteford HA, et al. Estimating remission from untreated major depression: a systematic review and meta-analysis. Psychol Med. 2013;43(8):1569–85. doi: 10.1017/S0033291712001717. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association . Practice Guideline for the Treatment of Patients With Major Depressive Disorder. Third Ed. American Psychiatric Association Press; Washington, DC: 2010. [Google Scholar]
- 15.Himelhoch S, Medoff DR. Efficacy of antidepressant medication among HIV-positive individuals with depression: a systematic review and meta-analysis. AIDS Patient Care STDS. 2005;19(12):813–22. doi: 10.1089/apc.2005.19.813. [DOI] [PubMed] [Google Scholar]
- 16.Himelhoch S, Medoff DR, Oyeniyi G. Efficacy of group psychotherapy to reduce depressive symptoms among HIV-infected individuals: a systematic review and meta-analysis. AIDS Patient Care STDS. 2007;21(10):732–9. doi: 10.1089/apc.2007.0012. [DOI] [PubMed] [Google Scholar]
- 17.Pence BW, O'Donnell JK, Gaynes BN. Falling through the cracks: the gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS. 2012;26(5):656–8. doi: 10.1097/QAD.0b013e3283519aae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asch SM, et al. Underdiagnosis of depression in HIV: who are we missing? J Gen Intern Med. 2003;18(6):450–60. doi: 10.1046/j.1525-1497.2003.20938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnam MA, et al. Use of mental health and substance abuse treatment services among adults with HIV in the United States. Arch Gen Psychiatry. 2001;58(8):729–36. doi: 10.1001/archpsyc.58.8.729. [DOI] [PubMed] [Google Scholar]
- 20.Sin NL, Dimatteo MR. Depression Treatment Enhances Adherence to Antiretroviral Therapy: a Meta-Analysis. Ann Behav Med. 2013 doi: 10.1007/s12160-013-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai AC, et al. A marginal structural model to estimate the causal effect of antidepressant medication treatment on viral suppression among homeless and marginally housed persons with HIV. Arch Gen Psychiatry. 2010;67(12):1282–90. doi: 10.1001/archgenpsychiatry.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safren SA, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himelhoch S, et al. Telephone based cognitive behavioral therapy targeting major depression among urban dwelling, low income people living with HIV/AIDS: results of a randomized controlled trial. AIDS Behav. 2013;17(8):2756–64. doi: 10.1007/s10461-013-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai AC, et al. Directly observed antidepressant medication treatment and HIV outcomes among homeless and marginally housed HIV-positive adults: a randomized controlled trial. Am J Public Health. 2013;103(2):308–15. doi: 10.2105/AJPH.2011.300422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyne JM, et al. Effectiveness of collaborative care for depression in human immunodeficiency virus clinics. Arch Intern Med. 2011;171(1):23–31. doi: 10.1001/archinternmed.2010.395. [DOI] [PubMed] [Google Scholar]
- 26.Pence BW, et al. Assessing the effect of Measurement-Based Care depression treatment on HIV medication adherence and health outcomes: rationale and design of the SLAM DUNC Study. Contemp Clin Trials. 2012;33(4):828–38. doi: 10.1016/j.cct.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals. 2002;32(9):509–515. [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- 30.Adams JL, et al. Treating depression within the HIV “medical home”: a guided algorithm for antidepressant management by HIV clinicians. AIDS Patient Care STDS. 2012;26(11):647–54. doi: 10.1089/apc.2012.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trivedi MH, et al. Maximizing the Adequacy of Medication Treatment in Controlled Trials and Clinical Practice: STAR*D Measurement-Based Care. Neuropsychopharmacology. 2007;32:2479–2489. doi: 10.1038/sj.npp.1301390. [DOI] [PubMed] [Google Scholar]
- 32.Wisniewski SR, et al. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006;12(2):71–9. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Golin CE, et al. A 2-arm, randomized, controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. J Acquir Immune Defic Syndr. 2006;42(1):42–51. doi: 10.1097/01.qai.0000219771.97303.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borm GF, et al. Pseudo cluster randomization: a treatment allocation method to minimize contamination and selection bias. Stat Med. 2005;24(23):3535–47. doi: 10.1002/sim.2200. [DOI] [PubMed] [Google Scholar]
- 35.Teerenstra S, et al. Pseudo cluster randomization dealt with selection bias and contamination in clinical trials. J Clin Epidemiol. 2006;59(4):381–6. doi: 10.1016/j.jclinepi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Pence BW, et al. Balancing contamination and referral bias in a randomized clinical trial: An application of pseudo-cluster randomization. American Journal of Epidemiology. 2015 doi: 10.1093/aje/kwv132. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bess KD, et al. Providers' Attitudes Towards Treating Depression and Self-Reported Depression Treatment Practices in HIV Outpatient Care. AIDS Patient Care STDS. 2013 doi: 10.1089/apc.2012.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalichman SC, et al. Adherence to antiretroviral therapy assessed by unannounced pill counts conducted by telephone. J Gen Intern Med. 2007;22(7):1003–6. doi: 10.1007/s11606-007-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JK, et al. How should we measure medication adherence in clinical trials and practice? Ther Clin Risk Manag. 2007;3(4):685–90. [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton M. Rating depressive patients. J Clin Psychiatry. 1980;41(12 Pt 2):21–4. [PubMed] [Google Scholar]
- 41.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerman M, Chelminski I, Posternak M. A review of studies of the Hamilton depression rating scale in healthy controls: implications for the definition of remission in treatment studies of depression. J Nerv Ment Dis. 2004;192(9):595–601. doi: 10.1097/01.nmd.0000138226.22761.39. [DOI] [PubMed] [Google Scholar]
- 43.Amico KR, et al. Visual analog scale of ART adherence: association with 3-day self-report and adherence barriers. J Acquir Immune Defic Syndr. 2006;42(4):455–9. doi: 10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- 44.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Ware JE, Kosinski M, Keller SD. How to score the SF-12 physical and mental health summaries: a users's manual. The Health Institute, New England Medical Centre; Boston: 1995. [Google Scholar]
- 46.Pyne JM, et al. Depression-free day to utility-weighted score: is it valid? Med Care. 2007;45(4):357–62. doi: 10.1097/01.mlr.0000256971.81184.aa. [DOI] [PubMed] [Google Scholar]
- 47.Lave JR, et al. Cost-effectiveness of treatments for major depression in primary care practice. Arch Gen Psychiatry. 1998;55(7):645–51. doi: 10.1001/archpsyc.55.7.645. [DOI] [PubMed] [Google Scholar]
- 48.Pals SL, et al. Estimates of Intraclass Correlation for Variables Related to Behavioral HIV/STD Prevention in a Predominantly African American and Hispanic Sample of Young Women. Health Education & Behavior. 2009;36(1):182–194. doi: 10.1177/1090198108327731. [DOI] [PubMed] [Google Scholar]
- 49.Parker DR, Evangelou E, Eaton CB. Intraclass correlation coefficients for cluster randomized trials in primary care: The Cholesterol Education and Research Trial (CEART). Controlled Clinical Trials. 2005;26(2):260–7. doi: 10.1016/j.cct.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Smeeth L, Ng ES. Intraclass correlation coefficients for cluster randomized trials in primary care: data from the MRC Trial of the Assessment and Management of Older People in the Community. Control Clin Trials. 2002;23(4):409–21. doi: 10.1016/s0197-2456(02)00208-8. [DOI] [PubMed] [Google Scholar]
- 51.Eldridge SM, et al. Lessons for cluster randomized trials in the twenty-first century: a systematic review of trials in primary care. Clin Trials. 2004;1(1):80–90. doi: 10.1191/1740774504cn006rr. [DOI] [PubMed] [Google Scholar]
- 52.Carpenter JR, Kenward MG. Missing data in randomised controlled trials — a practical guide. London School of Hygiene & Tropical Medicine; London: 2007. [Google Scholar]
- 53.Landis SE, et al. Generalist care managers for the treatment of depressed medicaid patients in North Carolina: a pilot study. BMC Fam Pract. 2007;8:7. doi: 10.1186/1471-2296-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Safren SA, et al. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: a randomized controlled trial. J Consult Clin Psychol. 2012;80(3):404–15. doi: 10.1037/a0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simoni JM, et al. A preliminary RCT of CBT-AD for adherence and depression among HIV-positive Latinos on the U.S.-Mexico border: the Nuevo Dia study. AIDS Behav. 2013;17(8):2816–29. doi: 10.1007/s10461-013-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaynes BN, et al. Prevalence and comorbidity of psychiatric diagnoses based on reference standard in an HIV+ patient population. Psychosom Med. 2008;70(4):505–11. doi: 10.1097/PSY.0b013e31816aa0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pence BW, et al. Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the Southeastern United States. J Acquir Immune Defic Syndr. 2006;42(3):298–306. doi: 10.1097/01.qai.0000219773.82055.aa. [DOI] [PubMed] [Google Scholar]
- 58.Orlando M, et al. Re-estimating the prevalence of psychiatric disorders in a nationally representative sample of persons receiving care for HIV: results from the HIV Cost and Services Utilization Study. Int J Methods Psychiatr Res. 2002;11(2):75–82. doi: 10.1002/mpr.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Archer J, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. doi: 10.1002/14651858.CD006525.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]



