Abstract
Background
There is an abundance of evidence to support the association of damaging neuroinflammation and neurodegeneration across a multitude of diseases. One of the links between these pathological phenomena is the role of chaperone proteins as both neuroprotective and immune-regulatory agents.
Scope of Review
Chaperone proteins are highly expressed at sites of neuroinflammation both in glial cells and in the injured neurons that initiate the immune response. For this reason, the use of chaperones as treatment for various diseases associated with neuroinflammation is a highly active area of investigation. This review explores the various ways that the small heat shock protein chaperones, α-crystallins, can affect glial cell function with a specific focus on their implication in the inflammatory response associated with neurodegenerative disorders, and their potential as therapeutic treatment.
Major Conclusions
Although the mechanisms are still under investigation, a clear link has now been established between alpha-crystallins and neuroinflammation, especially through their roles in microglial and macroglial cells. Interestingly, similar to inflammation in itself, crystallins can have a beneficial or detrimental impact on the CNS based on the context and duration of the condition.
General Significance
Overall this review points out the novel roles that chaperones such as alpha-crystallins can play outside of the classical protein folding pathways, and their potential in the development of new therapies for the treatment of neuroinflammatory/neurodegenerative diseases.
Keywords: crystallins, neuroinflammation, microglia, astrocytes, central nervous system
Introduction
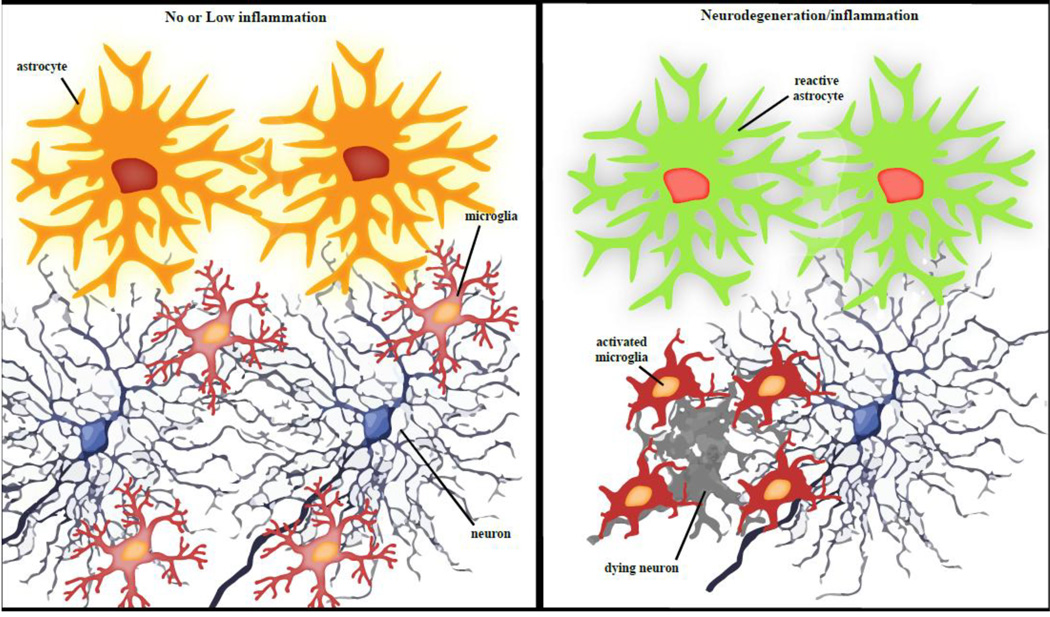
Neuroinflammation is a relatively new field of study focusing on the function and regulation of the immune system in the very unique environment of the long-lived neuronal cells of the central nervous system (CNS). CNS-resident glial cells develop and migrate to their appropriate environments early in embryogenesis, the first demonstration of the critical role these cells play in the central nervous system development and homeostasis. These cells are part of the innate immune response, designed to respond rapidly to any identified threat to the system ranging from foreign pathogens to increased cell death in the course of normal aging, and as such, constitutes a beneficial, pro-survival network aimed at maintaining the health of the highly sensitive neuronal tissue. Unfortunately, the efficacy of neuroinflammation balances precariously as if on the edge of a knife. Chronic insults such as from unresolved infection or injury, changes in metabolism, or increased protein aggregation can tip the balance of neuroinflammation from beneficial to detrimental (overview in Figure 1).
Figure 1. Overview of the neuroglial status in the CNS under normal or pathological conditions.
Schematic of the interaction of microglia and macroglial cells with neurons and the changes induced by neurodegeneration/neuroinflammation conditions which include neuronal cell death as well as astroglia reactivity and microglial activation.
The cellular stress response is key to our understanding of both neuronal cell death and glial cell activation. As the cells’ first line of defense, chaperone proteins and pathways upregulated in response to stress are important for determining not only the fate of individual cells, but of the tissue overall. One class of chaperones, the heat shock proteins, (HSPs), are characterized for their role in promoting proper protein folding and preventing aggregation. HSPs encompass a wide range of sizes and structures, permitting diversity in their clients and partners to better respond to a variety of insults. One family of HSPs is the ATP-independent small HSPs, characterized by their small monomeric size, ability to form elaborate oligomeric structures, and high variability in sequence amongst the family members. These small HSPs were originally identified as co-chaperones to the more recognized ATP-dependent chaperones including Hsp70s and Hsp90s. Due to this designation, independent functions for small HSPs have only relatively recently begun to be investigated. Closer analysis of these “co-chaperones” demonstrated that they have functions entirely of their own and appear to be more prevalent and integral to cellular homeostasis than originally thought. Two of these small HSPs, αA- and αB-crystallin were originally thought to reside only in the ocular lens, but upon discovering a broader tissue expression for these chaperones, an explosion of data has occurred demonstrating their function in the normal stress response as well as key players in several disease states.
Here, we review the role of the molecular chaperones, αA- and αB-crystallin, in regulating inflammation in the CNS. We highlight recent advancements in our understanding of the function of α-crystallins in glial cells, the potential role of α-crystallins in extracellular signaling throughout the glial cell network, and the effect of α-crystallins on glial cell activation. This review not only points out the anti-inflammatory properties of α-crystallin chaperones, but also how these properties could be used to treat inflammatory diseases.
1. Expression of α-crystallins in glial cells
1.1 α-Crystallins in Microglia
Microglial cells are bone marrow-derived cells that resides in the CNS, which are considered to present two distinct states, the resting and the activated state[1]. In the absence of pathogens or extracellular signals, microglia are in a resting state characterized by small cellular bodies and several, thin, and extended processes. The resting microglial cells are surveyor cells, constantly sampling their environment in order to locate any injury or exogenous pathogens and non-self antigens. When the microglial cells detect an insult, they undergo a process termed activation in which they switch from their resting form into an amoeboid form. During activation, the cell bodies expand and the processes shrink and are retracted. Expression of various receptors and enzymes changes, as does the expression of cytokines and other immune response-associated molecules. Activated microglia migrate toward the area of insult and once there, secrete pro-inflammatory cytokines in order to mount an appropriate immune response. If the insult persists, the microglia can undergo another transformation and become phagocytic, removing apoptotic cells and cell debris as well as pathogens. Through phagocytosis, microglia mediate the immune response in the brain.
Both αA- and αB-crystallin have been shown to play a role in microglial cells. αB-crystallin is upregulated in microglial cells in the brains of Alzheimer’s disease patients compared to non-demented controls[2–5]. The increased expression of αB-crystallin was not detected in all activated microglia, but is found in microglia surrounding amyloid plaques suggesting an involvement of αB-crystallin in recognizing and phagocytosing extracellular amyloid deposits. More recently, increased expression of αB-crystallin was shown in the microglia in a model of Parkinson’s disease[6]. Unlike αB-crystallin, expression of αA-crystallin is more limited and as such, no data regarding its expression in microglia in the brain has been reported; however it has been shown to have an important role in microglial activation, an aspect that will be developed later in this review. Additionally, while their level of expression and specific role in microglia remains unclear, αA- and αB-crystallins are highly expressed by other glial cells of the retina under various disease conditions.
The microglial expression of αB-crystallin in the Parkinson’s disease model coincided with increased protein aggregates in the microglia suggesting that αB-crystallin may be upregulated in order to resolve protein aggregation and prevent further protein misfolding[6, 7]. Like many other molecular chaperones, α-crystallins are upregulated in response to stresses such as oxidative stress, changes in metabolic state, and viral and bacterial infection in order to regulate protein folding and prevent aggregation[8, 9]. In addition to their role in maintaining protein homeostasis, α-crystallins also regulate apoptosis by binding pro-apoptotic proteins to prevent cell death from occurring until the situation is resolved or the stress response pathways are overwhelmed and the cell undergoes programmed cell death[10, 11]. One proposed hypothesis for the increased expression of αB-crystallin in microglial cells in the brain is that they may be part of the normal stress response to regulate aberrant protein aggregation, and may prevent apoptosis in the face of the increased phagocytosis and protein aggregate load[12]. Alternatively, another hypothesis is that the switch from the resting state to the activated state may induce a stress response as gene expression changes, processes are retracted, and intercellular communication increases and thus, α-crystallins are upregulated to promote a smooth transition between the resting and activated states. Indeed, since it has been shown that αB-crystallin can interact with several cytoskeletal proteins, increased expression in microglial cells may play a role in process retraction and migration of microglia to the site of injury[13–15]. The role of α-crystallins in microglial cells is only in the preliminary stages of investigation, but the functions and mechanisms associated with these chaperones elucidated from other cell types offer many exciting possibilities moving forward.
1.2 α-Crystallins in Macroglial cells
Aside from the microglial cells, the central nervous system contains several other non-neuronal cells playing more supportive roles for the entire nervous tissue.
1.2.1 Astrocytes
Similar to microglia, astrocytes, which are neuroectodermal in origin, are also known to exist in two primary states, resting and reactive[16]. However, astrocytes and microglia primarily serve very distinct functions in normal neural tissue, as astrocytes are more directly involved in neuronal support and homeostasis regulation. The resting state of astrocytes is characterized by their role in synapse homeostasis, maintaining ion and neurotransmitter levels, and bridging the neuronal and vascular networks for metabolic support. Unlike microglia, astrocytes display a variety of morphologies, from the classic star-like appearance with several, long processes to that of the radial glial characterized by two main processes. The morphology of the astrocytes is highly dependent on where in the CNS the astrocytes are located. In response to pathogens or injury, astrocytes can also transition to a reactive state in which the cell body expands, the processes retract, and proinflammatory and reactive specific genes are highly expressed. Reactive astrocytes can in some cases migrate to the area of injury to provide immune support. More generally the astrocytes already residing at the damage site can react and form a glial scar, and aid with neuronal recovery after resolution of the insult.
Increased levels of expression of α-crystallins in astrocytes have been reported in several models of disease-associated neuroinflammation. Indeed it has been shown in reactive astrogliosis associated with Alzheimer’s disease[3, 5], Parkinson’s disease[5, 6], Alexander disease[17], prion disease[18], tauopathies[7], multiple sclerosis (MS)[19, 20], stroke[21], traumatic injury[22], and retinopathy as a complication of diabetes[23, 24]. Analogous to microglia, α-crystallin expression increases during or following activation of astrocytes suggesting a role for the chaperones in regulating the function of reactive astrocytes in response to stress including pro-inflammatory signals. Expression of α-crystallins, as well as other heat shock chaperones, can be induced in astrocytes by cytokines including TNF-α, TGF-β2, and several members of the interleukin family[25–27]. Induced expression by pro-inflammatory cytokines, in conjunction with their upregulation in diabetic retinopathy and MS suggests an important role for α-crystallins in the ongoing activation of astrocytes during chronic, pathological neuroinflammation.
As α-crystallin chaperones can regulate apoptosis by binding pro-apoptotic proteins, one hypothesis for the function of increased α-crystallin expression in astrocytes is to prevent apoptosis. Indeed, overexpression of either αA- or αB-crystallin in cultured astrocytes prevented cell death induced by ceramide and staurosporine[28] or H2O2[29]. This anti-apoptotic function has been linked to the p38 MAPK signaling cascade, the activation of which has been shown to result from inflammation. Interestingly, prevention of apoptosis in astrocytes by increased α-crystallins may be dependent on its phosphorylation state, an observation consistent with previous findings that phosphorylation of α-crystallins take place in response to various types of stress and regulates their chaperone activity[30–33].
One interesting role of αB-crystallin in astrocytes is exemplified by the pathology of Alexander disease. In this disease, mutations in glial fibrillary acidic protein (GFAP), an intermediate filament upregulated in reactive astrocytes, cause the protein to become insoluble and aggregate in the cytoplasm of astrocytes resulting in cell death. αB-crystallin is upregulated in this disease[17] and localizes to the GFAP inclusions[34]. Overexpression of αB-crystallin in cultured astrocytes with GFAP inclusions results in resolubilization of the GFAP and enhanced cell survival[34]. These data support a role for αB-crystallin in cytoskeletal organization within reactive astrocytes to prevent aggregation. Although these GFAP inclusions are specific to this disease, αB-crystallin may still function to organize the cytoskeletal network to promote efficient activation and migration of the glial cells in other disease conditions.
The chaperone functions proposed for α-crystallins in astrocytes have all been described in non-glial cell types, but α-crystallins may also play a role in mechanisms specific to glial cells. For example, in cultured astrocytes lacking αB-crystallin, expression of pro-inflammatory molecules was significantly increased compared to controls[35]. Consistent with this observation, astrocytes from αB-crystallin deficient mice are highly activated even in absence of stress, though it is not associated with neuronal cell loss[35]. These data suggest that αB-crystallin may play a role outside its normal chaperone function that regulates astrogliosis and activation through a novel pathway. In a recent study, Shao et al. used a mouse model of aging that lacks the dopamine receptor, DRD2[35]. Correlative with aging and the progressive impairment of cognitive and motor skills is the downregulation of DRD2 in specific regions of the brain such as the substantia nigra. Astrocytes lacking DRD2 are actually hyper-responsive, becoming more highly activated in response to stimulus. Loss of DRD2 in both cultured astrocytes and animal models significantly decreased expression of αB-crystallin at the same time, while increasing the expression of pro-inflammatory molecules. Furthermore, overexpression of αB-crystallin in the Drd2-null astrocytes reversed the effects of the loss of DRD2. These novel findings suggest that astrocytes may play an integral role in the aging brain and that modulation of their role in neuroinflammation, specifically through pathways involving αB-crystallin, may slow the progression of cognitive and motor function impairment associated with both normal aging and age-onset neurodegenerative disease.
1.2.2 Specialized macroglial cells: Oligodendrocytes and Müller cells
Oligodendrocytes have the specific function, distinct from those of astrocytes and microglia, of producing myelin, which makes up the myelin sheath protecting the axons of neurons in the CNS. Oligodendrocytes have several processes that connect to the myelin segments on axons and are responsible for secreting both myelin and its various binding partners. Recurring loss of the myelin sheath, such as is the case in MS, leads to increased neurodegeneration and accumulation of immune cells such as infiltrating lymphocytes, macrophages, and activated microglia and astrocytes. In fact, αB-crystallin was identified not only as a binding partner of myelin, but also an important antigen leading to the increased inflammation and phagocytosis of myelin sheaths[20]. In active lesions, αB-crystallin expression is significantly increased in both oligodendrocytes and astrocytes and may be involved in the stress response in these cells, though the role of αB-crystallin specifically in oligodendrocytes has not been elucidated[19]. In addition to its impact in MS, αB-crystallin is also involved in oligodendrocyte survival as a result of ischemia of the optic nerve. In experimental anterior ischemic optic neuropathy, oligodendrocytes undergo apoptosis within the first week and there is progressive demyelination within two weeks. During these first weeks, αB-crystallin is upregulated in the optic nerve, correlative with increased microglial activation, suggesting activation of an inflammatory and stress response at the optic nerve[36]. Additionally, intravenous or intravitreal injections of purified αB-crystallin led to a decrease in microglial activation in response to experimental ischemia as well as an increase in oligodendrocyte survival[36]. Whether the effect on oligodendrocyte survival is a direct effect of αB-crystallin interaction with the oligodendrocytes or an indirect result of the chaperone’s regulation of the local inflammatory environment is still unknown. In this model, administration of αB-crystallin did not increase ganglion cell survival, but other reports using optic nerve injury models to investigate α-crystallin function in neuroprotection provide contradicting evidence. Addition of α-crystallin promoted axon survival and regeneration in an optic nerve axotomy model[37] and prevented ganglion cell death in an optic nerve crush model[38]. All of these reports, however, highlight the role of α-crystallin as an anti-inflammatory agent in the immune response to optic nerve injury.
The retina contains specialized radial glia called Müller glia, with a characteristic morphology, extending processes among the various retinal layers. Müller glia play an important role in the retina as the architectural support for the neurons and in regulation of neuronal homeostasis, including recycling neurotransmitters, maintaining the ionic environment, and cleaning neuronal debris after injury. Like the other glial cells, Müller glia are activated in response to disease and injury. Interestingly, α-crystallins are increased in the Müller glia in both rodent models of diabetes[23] as well as in diabetic human donor tissues (our unpublished data). These data suggest that similar to other glial cells, α-crystallins may play a role in survival or activation of these specialized glia.
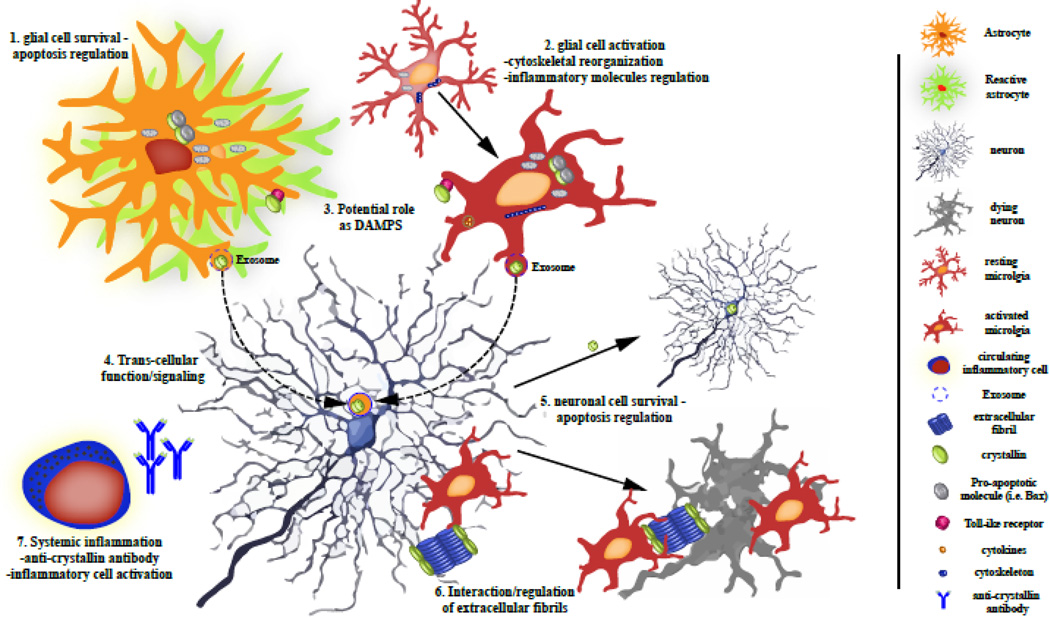
Despite the increased expression of α-crystallins in all types of glial cells, reported data suggest that α-crystallins may have distinct roles in each cell type (overview in Figure 2). As each cell type may respond differently to stress and has an individual role in the inflammatory response, it is understandable that α-crystallins function differently, even among glial cells. Furthermore, various types of neuroinflammatory insults may also trigger distinct cell signaling pathways, which then direct α-crystallin to specific functions. Therefore, the function of α-crystallin in astrocytes in response to amyloid aggregates in the brain may be very different from the function of α-crystallin in astrocytes in reactive MS lesions and much work is needed to elucidate how these chaperones can be targeted therapeutically in each specific disease state.
Figure 2. Overview of the different roles that crystallin proteins can play in the CNS, especially during neuroinflammation.
Schematic representation of the various roles and implications that crystallin proteins can have in neuronal survival, CNS tissue homeostasis, and glial cell function.
2. Regulation of Glial Cells function by α-Crystallins
2.1 The role of α-crystallins in microglial activation
The α-crystallin chaperones affect microglial and astrocytic activation, most often in an anti-inflammatory manner resulting in decreased cytokine production and tempering of the innate immune response. However, as discussed in the first part of this review, α-crystallin expression can be upregulated in activated glial cells in response to various conditions and may therefore also be involved in regulating cytokine production from within the glial cells. In any given condition, only a fraction or sub-population of the glial cell population tends to express α-crystallin, a population often localized to a specific region near or at the injury site. Moreover, a growing number of reports have described the ability of exogenously applied α-crystallin to control glial cell activation and attenuate the immune response. One of the first reports to show that α-crystallins played a role in microglial activation incubated cultured primary microglia cells from newborn rats with purified α-crystallin and examined the levels of cytokine production[39]. Interestingly, this report showed that both αA- and αB-crystallin independently increased production of TNFα and iNOS in non-activated microglia suggesting a role for regulation of the innate immune response by extracellular α-crystallins. Of note, this initial report suggested that α-crystallins are promoting inflammation by inducing expression of pro-inflammatory cytokines. Interestingly, a report ten years later observed a similar effect of α-crystallins, but noticed that increased cytokine production only occurred in non-activated microglia and not in microglia already activated by injury or natural triggers (lipopolysaccharides)[40]. Several more recent reports showed that mice lacking α-crystallins showed increased basal inflammation, while additional reports determined that treatment of mouse models of inflammation and treatment of cultured cells with α-crystallins results in a decrease in overall cytokine production and glial cell activation[36, 38, 40–43]. Thus, the vast majority of studies suggest that α-crystallins are anti-inflammatory signals and play a role in attenuating the immune response during disease, though they may also contribute to the initial microglial activation.
As described previously, glial cell activation primarily occurs through signals originating from the extracellular environment directing glia to respond to pathogens or neuronal injury in the CNS. How, then, could an intracellular molecular chaperone known for its role in protein folding and regulation of apoptosis, not only make it out into the extracellular space, but also then bind to glial cells to cause an anti-inflammatory signaling cascade? Several theories have been investigated as to how α-crystallins get into the extracellular space, and once there, what role they may have, especially in the context of inflammatory diseases. This will be the focus of the next part of this review.
2.2 Exosome-mediated Secretion of α-Crystallins
Intercellular signaling is a critical process in the immune response, guiding activation, cytokine secretion, phagocytosis, and attenuation. One of the key pathways involved in these processes is the exosome-mediated pathway, in which target molecules to be secreted are encapsulated in membrane-bound vesicles called exosomes, often including a sampling of the cytoplasm as well. Exosomes themselves are then collected in larger membrane-bound vesicles, which can then fuse with the plasma membrane, spilling their contents into the extracellular space. Once in the extracellular space, these exosomes can interact with the plasma membrane of neighboring cells, and through an as yet not well-understood process, be taken up into the cell where they can affect signaling pathways by releasing their content. This pathway is clearly important in cellular communication between inflammatory cells, as several types of glial cells have been shown to secrete exosomes, thus perhaps coordinating an immune response among several different cell types[44].
Several members of the HSP family of chaperones have been shown to be present in exosomes and actively secreted into the extracellular space by a wide variety of cell types including macrophages and B lymphocytes[45–47]. Among them, αB-crystallin, specifically, has been shown to be secreted by exosomes. In two independent studies, researchers showed that another important non-neuronal cell type of the retina, retinal pigment epithelial (RPE) cells, can secrete αB-crystallin via exosomes, both under basal conditions as well as in response to oxidative stress, in which secretion is exacerbated[48, 49]. These data suggest that secretion of α-crystallins may be a normal aspect of the stress response. Interestingly, in polarized RPE cells, αB-crystallin was preferentially secreted from the apical side, or the interface of the RPE with the photoreceptor cells. Further analysis showed that under stress conditions, exosomes carrying αB-crystallin were taken up by both photoreceptors and neighboring RPE cells suggesting that secretion of αB-crystallin is stress-dependent and possibly neuroprotective[49].
More recently, a study has shown that secretion of αB-crystallin from astrocytes also occurs via an exosome-mediated pathway[50]. These tumor-derived astrocytes are a model for glioblastoma multiforme, an aggressive and common form of human brain cancer in which inflammation drives progression of the disease. In this study, the authors showed that both expression and secretion of αB-crystallin was intensified when cells were treated with TNF-α or IL-1β, mimicking the pathophysiological state. This study also demonstrated for the first time that glial cells can secrete αB-crystallin in response to pro-inflammatory cytokines and suggests that αB-crystallin may play a role in the normal intercellular signaling pathways between different types of inflammatory cells or between inflammatory cells and surrounding neurons. Given its role as an anti-inflammatory molecule, one could speculate that secretion of αB-crystallin is part of a biofeedback network between different types of cells involved in the inflammatory response to prevent chronic, damaging neuroinflammation. Contrarily, exosomes loaded with αB-crystallin from glial cells may be targeted for uptake by neurons and may thus play more of a neuroprotective role by directly promoting survival of neurons. Though these two hypotheses are not mutually exclusive, further investigation is needed to determine the impact of exosome-mediated secretion of α-crystallins on both glial and neuronal cells.
2.3 The role of α-crystallins as DAMPs
The local innate immune response is propagated on the recognition of “non-self” and “altered self” patterns, also called “danger signals,” by molecules and receptors expressed by glial and neuronal cells[51]. These molecules and receptors are called pattern recognition receptors (PRRs). PRRs can be either soluble or membrane-bound and the outcome is based on the type of ligand and receptor that interact. There are several classes of receptors expressed on glial cells responsible for recognizing both self and non-self molecules, and each receptor can bind multiple ligands suggesting a large number of outcomes that could result. In order to make better sense of the complexity of possible interactions, combinations of ligands and receptors have been classified into groups termed “molecular patterns” and designated by the initial immune-activating signal. For example, pathogenic-associated molecular patterns (PAMPs), in which a foreign or microbial invader is the cause of the immune response, are the network of cytokines, ligands, and receptors involved in responding to foreign pathogens. A less well-characterized class are the danger-associated molecular patterns (DAMPs) in which dying cells express certain “altered self” signals which promote clearance of dead and dying cells to maintain tissue homeostasis. The best-characterized DAMP signal is phosphatidyl-serine, which is expressed on the plasma membrane of apoptotic cells, but other signals include HMGB-1, nucleic acids, sugars, and oxidized LPLs[52].
Many types of neurodegenerative diseases display associated neuroinflammation suggesting that apoptotic neurons may signal neighboring glial cells and may do this through the expression or release of DAMPs. As HSP chaperones are upregulated in response to stress and often localize to the site of the insult, they are logical candidates as DAMPs. Several HSPs, including Hsp60 and Hsp70 are expressed in injured axons, and can be released by cells undergoing necrosis or apoptosis [53–56]. A growing body of evidence suggests that HSPs can bind to Toll-like receptors (TLRs), one class of PRRs that recognize other known DAMPs and are integral to glial cell signaling and cytokine production[57–59]. Interestingly, both αA- and αB-crystallin are expressed by retinal neurons under stress condition[23, 60, 61], and have also been shown to bind TLR4 and TLR2 suggesting that α-crystallins may also regulate glial cells as a result of release from dying neurons[62–64]. Therefore, another function for the extracelllular localization of α-crystallins is its potential involvement in such signaling from neurons to the glial cells to promote clearance of cell debris.
Interestingly, a very new theory suggests that some HSPs belong to a new class of immune signals termed resolution-associated molecular patterns (RAMPs)[65, 66]. These immunoregulatory chaperones are involved in restoring immunological homeostasis and thus may be important targets for treating chronic neuroinflammation during disease.
2.4 Amyloid Fibrils and α-crystallins
Several neurodegenerative diseases associated with neuroinflammation including Alzheimer’s disease, Parkinson’s disease, and tauopathies are characterized by the deposition of amyloid plaques in the extracellular space. Several types of amyloid fibrils have been shown to trigger the innate immune response suggesting that the accumulation of amyloidogenic protein into aggregates may represent a novel DAMP[67–74]. Although the mechanism driving secretion of amyloid fibrils is still highly debated, one of the hypotheses is that their presence in the extracellular space is a neuroprotective event resulting in these toxic species being secreted by neurons, and inducing glial cell activation and clearance of the fibrils by phagocytosis. One of the major innate immune pathways triggered by amyloid fibrils is the activation of the inflammasome, which can be activated by a variety of signals including ATP, bacterial toxins, and various crystals[75, 76]. Therefore, amyloid fibrils seem to be another endogenous ‘danger’ signal. Further investigation are underway to establish whether the ability of amyloid fibrils to trigger an immune response is beneficial or harmful, what other pathways or receptors are targeted by these aggregates, and whether these plaques will be useful therapeutic targets in preventing chronic neuroinflammation.
As molecular chaperones, one of the primary functions of αA- and αB-crystallin is to prevent protein misfolding by binding to exposed hydrophobic regions and preventing aggregation[77]. Both αA- and αB-crystallin can prevent amyloid fibril formation by various proteins including amyloid-β, β2-microglobulin, and α-synuclein[78–83]. In disease conditions, α-crystallins can be found localized to these extracellular aggregates including amyloid-β plaques and neurofibrillary tangles composed of tau in Alzheimer’s disease[3, 84]. It is possible that this localization of α-crystallins to the extracellular amyloid plaques would have a critical impact on the associated glial cell activation but this remains to be clearly established. While studies have shown that fibrils of purified amyloidogenic protein are sufficient for glial cell activation in vitro, the presence of α-crystallins in amyloid aggregates in vivo may contribute to the glial response, perhaps as a negative regulator[85]. Recent studies have shown a correlation between the chaperone activity of αB-crystallin and its immune-regulatory ability, specifically its ability to bind proinflammatory proteins and reduce aggregation[86]. Additionally, a short region of the chaperone, aa73–92, was shown to be sufficient to reduce paralysis and ameliorate neuroinflammation in an EAE model [70]. Of all the peptides tested, only the peptides that exhibited chaperone activity were able to reduce inflammation, suggesting that the immune-regulatory activity of α-crystallins may be intricately connected to the chaperone’s ability to bind client proteins.
Interestingly, short peptides analogous to those used in the previous study to demonstrate that chaperone activity was critical for therapeutic function were also shown in an independent study to form amyloid fibrils[87]. In fact, Tanaka and colleagues demonstrated that the chaperone activity of this short peptide of αA-crystallin was dependent on its ability to form amyloid fibrils. Following this discovery, several hexapeptides of αB-crystallin were shown to not only form amyloid fibrils, but in doing so were able to bind and precipitate pro-inflammatory proteins[70]. This new data correlated with previous data that amyloid-β fibrils were able to precipitate similar plasma proteins[88]. Therefore, amyloid fibrils are endogenous regulators of the innate immune response and this ability of proteins, including α-crystallins, to form amyloid fibrils can be utilized when designing therapies to reduce inflammation.
2.5 α-Crystallins as auto-antigens
A major finding that brought the role of α-crystallins in immune regulation into light, was the study performed by van Noort and colleagues in 1995 showing that αB-crystallin was enriched in the myelin in the brain of patients with MS and was a potent antigen for T cells from both healthy and MS patients[20]. Since then, the role of αB-crystallin as an autoantigen in inflammatory diseases has been under intense investigation. One of the confounding parts of the data on human T cell activation by αB-crystallin is that T cells from both healthy and MS patients react to αB-crystallin as an antigen suggesting this response is not exclusively disease-associated. Several studies have been reported investigating autoantibodies against αB-crystallin[89–93]. Unfortunately, these reports have failed to conclusively demonstrate if antibodies against αB-crystallin are increased in patients with a wide range of chronic disease conditions including diabetes, cancer, neuropathies, and cardiomyopathies. These cumulative data suggest that αB-crystallin can indeed induce an adaptive immune response, and to this end, immunodominant epitopes within α-crystallins have been identified that are recognized by antibodies from patients[94]. At first glance, these data appears to be somewhat in contradiction with the anti-inflammatory role of crystallins depicted in the previous sections of this review but could reflect the impact of unresolved long-lasting inflammation.
One hypothesis put forth to resolve this question is based on the very definitions of the innate and adaptive immune responses. The innate immune response is the first response to pathogens or insult and as such is critical in the activation of the adaptive response. If the innate immune response is sufficient to efficiently resolve the situation, recruitment and activation of the adaptive immune network is unnecessary. However, in cases where the innate network is overwhelmed by the infection or injury, a secondary and more specific response is needed leading to activation of the adaptive immune response. The sum of the reported studies suggest that αB-crystallin is a critical regulator of the innate immune response, but its accumulation could also play a pivotal role in determining when and if an adaptive immune response should be mounted. As the glial cells accumulate at the site of infection or injury in the CNS, so do α-crystallin proteins[20, 91, 95, 96]. Thus the accumulation of autoantibodies against α-crystallins could result from the increased local concentration and increased risk that α-crystallins are taken up by antigen-presenting cells and used to signal T cell activation. This hypothesis has led to suggest that α-crystallins circulating antibodies could be a specific marker of an overwhelmed immune response, and thus a potentially valuable marker in measuring chronic advert neuroinflammation.
3. Treatment of inflammatory disease with α-crystallins
As with all disease-associated research, the primary goal is to identify molecules and pathways that are good therapeutic targets in order to prevent or slow disease pathology and the correlated symptoms. In the case of autoimmune and inflammation-associated diseases, the targets are unfortunately more challenging. The human immune response has evolved as a pro-survival mechanism and a way to fight disease and infection; treating an inflammatory disease is thus not as simple as preventing an immune response. Additionally, the immune response is a collection of inter-connected networks of pro- and anti-inflammatory signals with an array of receptors that can be utilized for both purposes. +Treating these diseases will not be as easy as targeting a single pathway or receptor as its role in inflammation must be understood in the context of all the other receptor-mediated pathways that make up the collective response.
The discovery that α-crystallins are negative regulators of neuroinflammation offers a real and novel approach for treating diseases associated with inflammation. Several studies have used the administration of α-crystallins to treat the symptoms of inflammatory disease with promising results. Addition of α-crystallins has been shown to decrease inflammation in models of MS, bacterial infection, stroke, spinal cord injury, optic nerve injury, ischemic optic neuropathy, and COPD[36–38, 41–43, 97–100]. Administration of α-crystallins not only prevents damaging inflammation, but also prevents neurodegeneration in these models, leading to a reduction in symptoms. In addition, since α-crystallins are endogenous regulators, present in many tissues of the body and already function as chaperones, these chaperones are part of the glial and neuronal cells’ normal stress response and, as such, would minimize activation of off-target pathways.
One of the challenges to using proteins therapeutically is the purification and folding of those proteins to ensure their function once administered. Several recent reports have shown that peptides of α-crystallin can be just as functional as the fully folded protein[70, 86, 87, 101–105]. Use of the mini-chaperone peptides engineered by Sharma and colleagues or the amyloidogenic hexapeptides characterized by Steinman and colleagues offer a more controlled and more affordable alternative to fully folded proteins. These peptides can perform similar chaperone activity to the whole protein and can interact with glial cells to attenuate inflammation. These peptides can be easily synthesized in high concentrations with no contaminating factors and are much less susceptible to be targeted by function-altering post-translational modifications. The major phosphorylation sites affecting αB-crystallin chaperone function [106, 107] are indeed outside of the most promising peptide sequence aa73–92 [102]. Furthermore, while the fully folded protein can act as an antigen to activate the adaptive immune response, these peptides can be engineered so as to not contain the epitopes necessary for recognition by antibodies, thus decreasing the risks of activation of the adaptive response. Finally, peptides such as these can be loaded into nanoparticles or micro-vesicles for delivery to specific tissues [99]. Use of a ‘vehicle’ to deliver drugs and small molecules to their target tissues is becoming more common and peptides of α-crystallin are certainly amenable to conjugation with various vehicles to target delivery.
All in all, though much more work needs to be done to determine the mechanisms of α-crystallin function in immune regulation and neuroprotection, the most current data strongly suggest that α-crystallins may be a novel target in treating inflammatory diseases and dampening the effects of chronic neuroinflammation.
4. Concluding Remarks
Though still not well understood, a link between neuroinflammation and the chaperones α-crystallins has clearly been established over the last two decades. The role of α-crystallins in regulation of the immune response has evolved from identification of the chaperone as an antigen in MS models, through its initial characterization as a proinflammatory molecule, to our current understanding of the chaperone as an antiinflammatory agent. Preliminary investigations in mice have even begun to report the use of the chaperone as treatment for inflammatory diseases. While the exact nature of the role of α-crystallins in the immune response is being pursued, this raises the question of the role of other small heat shock proteins in the innate immune response. Though not discussed here, Hsp27 and Hsp22 have also been shown to function in immune regulation. These studies have shed light on new roles for chaperones outside of the classical protein folding pathways and offer intriguing opportunities for the identification of novel immune-regulatory pathways endogenous to the CNS.
Though our initial understanding of the immune response as a beneficial network still holds true, we are beginning to understand how a good system can be corrupted over time and promote disease pathology rather than prevent it. As the number and type of diseases associated with detrimental inflammation grows, our basic understanding of the pro- and anti-inflammatory networks must also expand to include novel pathways and to connect the known pathways propagated by many different cells simultaneously. Although attaining this understanding will be considerably challenging, the benefits will be far-reaching, promoting multi-discipline approaches to therapeutic research across a wide variety of diseases classically thought of as distinct and independent.
Table 1.
microglial and macroglial cells display upregulation of αB-crystallin in a variety of diseases and conditions.
| Upregulation of αB-crystallin | Reference | |
|---|---|---|
| Microglia | Alzheimer's disease | 2 – 5 |
| Parkinson's disease | 6 | |
| Astrocytes | Alzheimer's disease | 3, 5 |
| Parkinson's disease | 5, 6 | |
| Alexander disease | 17 | |
| Diabetic Retinopathy | 23, 24 | |
| Multiple Sclerosis | 19, 20 | |
| tauopathies | 7 | |
| prion disease | 18 | |
| traumatic brain injury | 22 | |
| stroke | 21 | |
| activation by cytokines | 25 – 27 | |
| Oligodendrocytes | Multiple Sclerosis | 19, 20 |
| Müller glia | Diabetic Retinopathy | 23 (rodent) |
| unpublished (human) |
Highlights.
-
→
Alpha-crystallins are expressed during neuroinflammation
-
→
Alpha-crystallins are expressed by microglia and macroglial cells
-
→
Alpha-crystallins are intrinsically involved in neuroinflammation regulation
-
→
The exact role of alpha-crystallins in neuroinflammation is complex and still requires a lot of attention
Acknowledgements
This authors were supported by an NEI T32-EY013934 training grant and an international retina research foundation (IRRF) postdoctoral fellowship (JED) and NIH-NEI EY020895 (P.E.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annual review of immunology. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowe J, Landon M, Pike I, Spendlove I, McDermott H, Mayer RJ. Dementia with beta-amyloid deposition: involvement of alpha B-crystallin supports two main diseases. Lancet. 1990;336:515–516. doi: 10.1016/0140-6736(90)92075-s. [DOI] [PubMed] [Google Scholar]
- 3.Renkawek K, Voorter CE, Bosman GJ, van Workum FP, de Jong WW. Expression of alpha B-crystallin in Alzheimer’s disease. Acta neuropathologica. 1994;87:155–160. doi: 10.1007/BF00296185. [DOI] [PubMed] [Google Scholar]
- 4.Iwaki T, Wisniewski T, Iwaki A, Corbin E, Tomokane N, Tateishi J, Goldman JE. Accumulation of alpha B-crystallin in central nervous system glia and neurons in pathologic conditions. The American journal of pathology. 1992;140:345–356. [PMC free article] [PubMed] [Google Scholar]
- 5.Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson’s disease. Neuroreport. 1999;10:2273–2276. doi: 10.1097/00001756-199908020-00009. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Zhou Q, Tang M, Fu N, Shao W, Zhang S, Yin Y, Zeng R, Wang X, Hu G, Zhou J. Upregulation of alphaB-crystallin expression in the substantia nigra of patients with Parkinson's disease. Neurobiology of aging. 2015 doi: 10.1016/j.neurobiolaging.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Gonzalez I, Carmona M, Arregui L, Kovacs GG, Ferrer I. alphaB-crystallin and HSP27 in glial cells in tauopathies. Neuropathology : official journal of the Japanese Society of Neuropathology. 2014;34:517–526. doi: 10.1111/neup.12134. [DOI] [PubMed] [Google Scholar]
- 8.Head MW, Corbin E, Goldman JE. Coordinate and independent regulation of alpha B-crystallin and hsp27 expression in response to physiological stress. Journal of cellular physiology. 1994;159:41–50. doi: 10.1002/jcp.1041590107. [DOI] [PubMed] [Google Scholar]
- 9.Klemenz R, Andres AC, Frohli E, Schafer R, Aoyama A. Expression of the murine small heat shock proteins hsp 25 and alpha B crystallin in the absence of stress. The Journal of cell biology. 1993;120:639–645. doi: 10.1083/jcb.120.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. The Journal of biological chemistry. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 11.Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell death and differentiation. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 12.Kegel KB, Iwaki A, Iwaki T, Goldman JE. AlphaB-crystallin protects glial cells from hypertonic stress. The American journal of physiology. 1996;270:C903–C909. doi: 10.1152/ajpcell.1996.270.3.C903. [DOI] [PubMed] [Google Scholar]
- 13.Bennardini F, Wrzosek A, Chiesi M. Alpha B-crystallin in cardiac tissue. Association with actin and desmin filaments. Circulation research. 1992;71:288–294. doi: 10.1161/01.res.71.2.288. [DOI] [PubMed] [Google Scholar]
- 14.Nicholl ID, Quinlan RA. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. The EMBO journal. 1994;13:945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, Spector A. alpha-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. European journal of biochemistry / FEBS. 1996;242:56–66. doi: 10.1111/j.1432-1033.1996.0056r.x. [DOI] [PubMed] [Google Scholar]
- 16.Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwaki T, Kume-Iwaki A, Liem RK, Goldman JE. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell. 1989;57:71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- 18.Wang K, Zhang J, Xu Y, Ren K, Xie WL, Yan YE, Zhang BY, Shi Q, Liu Y, Dong XP. Abnormally upregulated alphaB-crystallin was highly coincidental with the astrogliosis in the brains of scrapie-infected hamsters and human patients with prion diseases. Journal of molecular neuroscience : MN. 2013;51:734–748. doi: 10.1007/s12031-013-0057-x. [DOI] [PubMed] [Google Scholar]
- 19.Bajramovic JJ, Lassmann H, van Noort JM. Expression of alphaB-crystallin in glia cells during lesional development in multiple sclerosis. Journal of neuroimmunology. 1997;78:143–151. doi: 10.1016/s0165-5728(97)00092-1. [DOI] [PubMed] [Google Scholar]
- 20.van Noort JM, van Sechel AC, Bajramovic JJ, el Ouagmiri M, Polman CH, Lassmann H, Ravid R. The small heat-shock protein alpha B-crystallin as candidate autoantigen in multiple sclerosis. Nature. 1995;375:798–801. doi: 10.1038/375798a0. [DOI] [PubMed] [Google Scholar]
- 21.Piao CS, Kim SW, Kim JB, Lee JK. Co-induction of alphaB-crystallin and MAPKAPK-2 in astrocytes in the penumbra after transient focal cerebral ischemia. Experimental brain research. 2005;163:421–429. doi: 10.1007/s00221-004-2197-2. [DOI] [PubMed] [Google Scholar]
- 22.Ke K, Li L, Rui Y, Zheng H, Tan X, Xu W, Cao J, Xu J, Cui G, Xu G, Cao M. Increased expression of small heat shock protein alphaB-crystallin after intracerebral hemorrhage in adult rats. Journal of molecular neuroscience : MN. 2013;51:159–169. doi: 10.1007/s12031-013-9970-2. [DOI] [PubMed] [Google Scholar]
- 23.Fort PE, Freeman WM, Losiewicz MK, Singh RS, Gardner TW. The retinal proteome in experimental diabetic retinopathy: up-regulation of crystallins and reversal by systemic and periocular insulin. Molecular & cellular proteomics : MCP. 2009;8:767–779. doi: 10.1074/mcp.M800326-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy VS, Raghu G, Reddy SS, Pasupulati AK, Suryanarayana P, Reddy GB. Response of small heat shock proteins in diabetic rat retina. Investigative ophthalmology & visual science. 2013;54:7674–7682. doi: 10.1167/iovs.13-12715. [DOI] [PubMed] [Google Scholar]
- 25.Bajramovic JJ, Bsibsi M, Geutskens SB, Hassankhan R, Verhulst KC, Stege GJ, de Groot CJ, van Noort JM. Differential expression of stress proteins in human adult astrocytes in response to cytokines. Journal of neuroimmunology. 2000;106:14–22. doi: 10.1016/s0165-5728(99)00260-x. [DOI] [PubMed] [Google Scholar]
- 26.Yu AL, Moriniere J, Welge-Lussen U. TGF-beta(2)- and H(2)O(2)-induced biological changes in optic nerve head astrocytes are reduced by the antioxidant alpha-lipoic acid. Ophthalmic research. 2012;48:156–164. doi: 10.1159/000337835. [DOI] [PubMed] [Google Scholar]
- 27.Yu AL, Welge-Lussen U. Antioxidants reduce TGF-beta2-induced gene expressions in human optic nerve head astrocytes. Acta ophthalmologica. 2013;91:e92–e98. doi: 10.1111/aos.12013. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Rohatgi T, Hanck T, Reiser G. Alpha A-crystallin and alpha B-crystallin, newly identified interaction proteins of protease-activated receptor-2, rescue astrocytes from C2-ceramide- and staurosporine-induced cell death. Journal of neurochemistry. 2009;110:1433–1444. doi: 10.1111/j.1471-4159.2009.06226.x. [DOI] [PubMed] [Google Scholar]
- 29.Shin JH, Jeong JY, Jin Y, Kim ID, Lee JK. p38beta MAPK affords cytoprotection against oxidative stress-induced astrocyte apoptosis via induction of alphaB-crystallin and its anti-apoptotic function. Neuroscience letters. 2011;501:132–137. doi: 10.1016/j.neulet.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 30.Bahk SC, Jang JU, Choi CU, Lee SH, Park ZY, Yang JY, Kim JD, Yang YS, Chung HT. Post-translational modification of crystallins in vitreous body from experimental autoimmune uveitis of rats. Journal of proteome research. 2007;6:3891–3898. doi: 10.1021/pr070133k. [DOI] [PubMed] [Google Scholar]
- 31.Li R, Reiser G. Phosphorylation of Ser45 and Ser59 of alphaB-crystallin and p38/extracellular regulated kinase activity determine alphaB-crystallin-mediated protection of rat brain astrocytes from C2-ceramide- and staurosporine-induced cell death. Journal of neurochemistry. 2011;118:354–364. doi: 10.1111/j.1471-4159.2011.07317.x. [DOI] [PubMed] [Google Scholar]
- 32.Li R, Zhu Z, Reiser G. Specific phosphorylation of alphaA-crystallin is required for the alphaA-crystallin-induced protection of astrocytes against staurosporine and C2-ceramide toxicity. Neurochemistry international. 2012;60:652–658. doi: 10.1016/j.neuint.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 33.Kato K, Inaguma Y, Ito H, Iida K, Iwamoto I, Kamei K, Ochi N, Ohta H, Kishikawa M. Ser-59 is the major phosphorylation site in alphaB-crystallin accumulated in the brains of patients with Alexander's disease. Journal of neurochemistry. 2001;76:730–736. doi: 10.1046/j.1471-4159.2001.00038.x. [DOI] [PubMed] [Google Scholar]
- 34.Koyama Y, Goldman JE. Formation of GFAP cytoplasmic inclusions in astrocytes and their disaggregation by alphaB-crystallin. The American journal of pathology. 1999;154:1563–1572. doi: 10.1016/s0002-9440(10)65409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, Chen T, Li SB, Xu M, Zhou JN, Hu G, Zhou JW. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature. 2013;494:90–94. doi: 10.1038/nature11748. [DOI] [PubMed] [Google Scholar]
- 36.Pangratz-Fuehrer S, Kaur K, Ousman SS, Steinman L, Liao YJ. Functional rescue of experimental ischemic optic neuropathy with alphaB-crystallin. Eye. 2011;25:809–817. doi: 10.1038/eye.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying X, Zhang J, Wang Y, Wu N, Wang Y, Yew DT. Alpha-crystallin protected axons from optic nerve degeneration after crushing in rats. Journal of molecular neuroscience : MN. 2008;35:253–258. doi: 10.1007/s12031-007-9010-1. [DOI] [PubMed] [Google Scholar]
- 38.Wu N, Yu J, Chen S, Xu J, Ying X, Ye M, Li Y, Wang Y. alpha-Crystallin protects RGC survival and inhibits microglial activation after optic nerve crush. Life sciences. 2014;94:17–23. doi: 10.1016/j.lfs.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 39.Bhat NR, Sharma KK. Microglial activation by the small heat shock protein, alpha-crystallin. Neuroreport. 1999;10:2869–2873. doi: 10.1097/00001756-199909090-00031. [DOI] [PubMed] [Google Scholar]
- 40.Wu N, Wang YH, Zhao HS, Liu DN, Ying X, Yin ZQ, Wang Y. alpha-Crystallin downregulates the expression of TNF-alpha and iNOS by activated rat retinal microglia in vitro and in vivo. Ophthalmic research. 2009;42:21–28. doi: 10.1159/000219681. [DOI] [PubMed] [Google Scholar]
- 41.Masilamoni JG, Jesudason EP, Baben B, Jebaraj CE, Dhandayuthapani S, Jayakumar R. Molecular chaperone alpha-crystallin prevents detrimental effects of neuroinflammation. Biochimica et biophysica acta. 2006;1762:284–293. doi: 10.1016/j.bbadis.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Masilamoni JG, Vignesh S, Kirubagaran R, Jesudason EP, Jayakumar R. The neuroprotective efficacy of alpha-crystallin against acute inflammation in mice. Brain research bulletin. 2005;67:235–241. doi: 10.1016/j.brainresbull.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O'Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 44.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochemical pharmacology. 2011;81:1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. Journal of cell science. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 46.Rayner K, Chen YX, McNulty M, Simard T, Zhao X, Wells DJ, de Belleroche J, O’Brien ER. Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circulation research. 2008;103:133–141. doi: 10.1161/CIRCRESAHA.108.172155. [DOI] [PubMed] [Google Scholar]
- 47.Banerjee S, Lin CF, Skinner KA, Schiffhauer LM, Peacock J, Hicks DG, Redmond EM, Morrow D, Huston A, Shayne M, Langstein HN, Miller-Graziano CL, Strickland J, O’Donoghue L, De AK. Heat shock protein 27 differentiates tolerogenic macrophages that may support human breast cancer progression. Cancer research. 2011;71:318–327. doi: 10.1158/0008-5472.CAN-10-1778. [DOI] [PubMed] [Google Scholar]
- 48.Gangalum RK, Atanasov IC, Zhou ZH, Bhat SP. AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. The Journal of biological chemistry. 2011;286:3261–3269. doi: 10.1074/jbc.M110.160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PloS one. 2010;5:e12578. doi: 10.1371/journal.pone.0012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kore RA, Abraham EC. Inflammatory cytokines, interleukin-1 beta and tumor necrosis factor-alpha, upregulated in glioblastoma multiforme, raise the levels of CRYAB in exosomes secreted by U373 glioma cells. Biochemical and biophysical research communications. 2014;453:326–331. doi: 10.1016/j.bbrc.2014.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffiths M, Neal JW, Gasque P. Innate immunity and protective neuroinflammation: new emphasis on the role of neuroimmune regulatory proteins. International review of neurobiology. 2007;82:29–55. doi: 10.1016/S0074-7742(07)82002-2. [DOI] [PubMed] [Google Scholar]
- 52.Elward K, Gasque P. "Eat me" and "don't eat me" signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Molecular immunology. 2003;40:85–94. doi: 10.1016/s0161-5890(03)00109-3. [DOI] [PubMed] [Google Scholar]
- 53.Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willis D, Li KW, Zheng JQ, Chang JH, Smit AB, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG, Kendall M, Smith DS, Bassell GJ, Twiss JL. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. The Journal of cell biology. 2007;178:965–980. doi: 10.1083/jcb.200703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang D, Sun L, Zhu H, Wang L, Wu W, Xie J, Gu J. Microglial LOX-1 reacts with extracellular HSP60 to bridge neuroinflammation and neurotoxicity. Neurochemistry international. 2012;61:1021–1035. doi: 10.1016/j.neuint.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 57.Takeda K, Akira S. Toll-like receptors in innate immunity. International immunology. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 58.Yu L, Wang L, Chen S. Endogenous toll-like receptor ligands and their biological significance. Journal of cellular and molecular medicine. 2010;14:2592–2603. doi: 10.1111/j.1582-4934.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng Z, Yuan R, Song M, Huo Y, Liu W, Cai X, Zou H, Chen C, Ye J. The toll-like receptor 4-mediated signaling pathway is activated following optic nerve injury in mice. Brain research. 2012;1489:90–97. doi: 10.1016/j.brainres.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 60.Sakaguchi H, Miyagi M, Darrow RM, Crabb JS, Hollyfield JG, Organisciak DT, Crabb JW. Intense light exposure changes the crystallin content in retina. Experimental eye research. 2003;76:131–133. doi: 10.1016/s0014-4835(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 61.Kase S, Meghpara BB, Ishida S, Rao NA. Expression of alpha-crystallin in the retina of human sympathetic ophthalmia. Molecular medicine reports. 2012;5:395–399. doi: 10.3892/mmr.2011.653. [DOI] [PubMed] [Google Scholar]
- 62.Bsibsi M, Holtman IR, Gerritsen WH, Eggen BJ, Boddeke E, van der Valk P, van Noort JM, Amor S. Alpha-B-crystallin induces an immune-regulatory and antiviral microglial response in preactive multiple sclerosis lesions. Journal of neuropathology and experimental neurology. 2013;72:970–979. doi: 10.1097/NEN.0b013e3182a776bf. [DOI] [PubMed] [Google Scholar]
- 63.Oh JY, Choi H, Lee RH, Roddy GW, Ylostalo JH, Wawrousek E, Prockop DJ. Identification of the HSPB4/TLR2/NF-kappaB axis in macrophage as a therapeutic target for sterile inflammation of the cornea. EMBO molecular medicine. 2012;4:435–448. doi: 10.1002/emmm.201200221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz S, Geurts J, Wunderink LU, Schreurs BW, van den Berg WB, Radstake TR. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. Journal of immunology. 2006;176:7021–7027. doi: 10.4049/jimmunol.176.11.7021. [DOI] [PubMed] [Google Scholar]
- 65.Shields AM, Panayi GS, Corrigall VM. Resolution-associated molecular patterns (RAMP): RAMParts defending immunological homeostasis? Clinical and experimental immunology. 2011;165:292–300. doi: 10.1111/j.1365-2249.2011.04433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shields AM, Thompson SJ, Panayi GS, Corrigall VM. Pro-resolution immunological networks: binding immunoglobulin protein and other resolution-associated molecular patterns. Rheumatology. 2012;51:780–788. doi: 10.1093/rheumatology/ker412. [DOI] [PubMed] [Google Scholar]
- 67.Azevedo EP, Guimaraes-Costa AB, Torezani GS, Braga CA, Palhano FL, Kelly JW, Saraiva EM, Foguel D. Amyloid fibrils trigger the release of neutrophil extracellular traps (NETs), causing fibril fragmentation by NET-associated elastase. The Journal of biological chemistry. 2012;287:37206–37218. doi: 10.1074/jbc.M112.369942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grant JL, Ghosn EE, Axtell RC, Herges K, Kuipers HF, Woodling NS, Andreasson K, Herzenberg LA, Herzenberg LA, Steinman L. Reversal of paralysis and reduced inflammation from peripheral administration of beta-amyloid in TH1 and TH17 versions of experimental autoimmune encephalomyelitis. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3004145. 145ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature immunology. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurnellas MP, Adams CM, Sobel RA, Steinman L, Rothbard JB. Amyloid fibrils composed of hexameric peptides attenuate neuroinflammation. Science translational medicine. 2013;5 doi: 10.1126/scitranslmed.3005681. 179ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nature immunology. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinman L, Rothbard JB, Kurnellas MP. Janus faces of amyloid proteins in neuroinflammation. Journal of clinical immunology. 2014;34(Suppl 1):S61–S63. doi: 10.1007/s10875-014-0034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. Journal of neuroimmunology. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 74.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 75.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 76.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 77.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dehle FC, Ecroyd H, Musgrave IF, Carver JA. alphaB-Crystallin inhibits the cell toxicity associated with amyloid fibril formation by kappa-casein and the amyloid-beta peptide. Cell stress & chaperones. 2010;15:1013–1026. doi: 10.1007/s12192-010-0212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Narayan P, Meehan S, Carver JA, Wilson MR, Dobson CM, Klenerman D. Amyloid-beta oligomers are sequestered by both intracellular and extracellular chaperones. Biochemistry. 2012;51:9270–9276. doi: 10.1021/bi301277k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raman B, Ban T, Sakai M, Pasta SY, Ramakrishna T, Naiki H, Goto Y, Rao Ch M. AlphaB-crystallin, a small heat-shock protein, prevents the amyloid fibril growth of an amyloid beta-peptide and beta2-microglobulin. The Biochemical journal. 2005;392:573–581. doi: 10.1042/BJ20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rekas A, Adda CG, Andrew Aquilina J, Barnham KJ, Sunde M, Galatis D, Williamson NA, Masters CL, Anders RF, Robinson CV, Cappai R, Carver JA. Interaction of the molecular chaperone alphaB-crystallin with alpha-synuclein: effects on amyloid fibril formation and chaperone activity. Journal of molecular biology. 2004;340:1167–1183. doi: 10.1016/j.jmb.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 82.Shammas SL, Waudby CA, Wang S, Buell AK, Knowles TP, Ecroyd H, Welland ME, Carver JA, Dobson CM, Meehan S. Binding of the molecular chaperone alphaB-crystallin to Abeta amyloid fibrils inhibits fibril elongation. Biophysical journal. 2011;101:1681–1689. doi: 10.1016/j.bpj.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stege GJ, Renkawek K, Overkamp PS, Verschuure P, van Rijk AF, Reijnen-Aalbers A, Boelens WC, Bosman GJ, de Jong WW. The molecular chaperone alphaB-crystallin enhances amyloid beta neurotoxicity. Biochemical and biophysical research communications. 1999;262:152–156. doi: 10.1006/bbrc.1999.1167. [DOI] [PubMed] [Google Scholar]
- 84.Wilhelmus MM, Otte-Holler I, Wesseling P, de Waal RM, Boelens WC, Verbeek MM. Specific association of small heat shock proteins with the pathological hallmarks of Alzheimer's disease brains. Neuropathology and applied neurobiology. 2006;32:119–130. doi: 10.1111/j.1365-2990.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- 85.Muth IE, Barthel K, Bahr M, Dalakas MC, Schmidt J. Proinflammatory cell stress in sporadic inclusion body myositis muscle: overexpression of alphaB-crystallin is associated with amyloid precursor protein and accumulation of beta-amyloid. Journal of neurology, neurosurgery, and psychiatry. 2009;80:1344–1349. doi: 10.1136/jnnp.2009.174276. [DOI] [PubMed] [Google Scholar]
- 86.Kurnellas MP, Brownell SE, Su L, Malkovskiy AV, Rajadas J, Dolganov G, Chopra S, Schoolnik GK, Sobel RA, Webster J, Ousman SS, Becker RA, Steinman L, Rothbard JB. Chaperone activity of small heat shock proteins underlies therapeutic efficacy in experimental autoimmune encephalomyelitis. The Journal of biological chemistry. 2012;287:36423–36434. doi: 10.1074/jbc.M112.371229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanaka N, Tanaka R, Tokuhara M, Kunugi S, Lee YF, Hamada D. Amyloid fibril formation and chaperone-like activity of peptides from alphaA-crystallin. Biochemistry. 2008;47:2961–2967. doi: 10.1021/bi701823g. [DOI] [PubMed] [Google Scholar]
- 88.Rothbard JB, Kurnellas MP, Brownell S, Adams CM, Su L, Axtell RC, Chen R, Fathman CG, Robinson WH, Steinman L. Therapeutic effects of systemic administration of chaperone alphaB-crystallin associated with binding proinflammatory plasma proteins. The Journal of biological chemistry. 2012;287:9708–9721. doi: 10.1074/jbc.M111.337691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chauhan VS, Nelson DA, Marriott I, Bost KL. Alpha beta-crystallin expression and presentation following infection with murine gammaherpesvirus 68. Autoimmunity. 2013;46:399–408. doi: 10.3109/08916934.2013.785535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stoevring B, Vang O, Christiansen M. (alpha)B-crystallin in cerebrospinal fluid of patients with multiple sclerosis. Clinica chimica acta; international journal of clinical chemistry. 2005;356:95–101. doi: 10.1016/j.cccn.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 91.van Noort JM, Bsibsi M, Gerritsen WH, van der Valk P, Bajramovic JJ, Steinman L, Amor S. Alphab-crystallin is a target for adaptive immune responses and a trigger of innate responses in preactive multiple sclerosis lesions. Journal of neuropathology and experimental neurology. 2010;69:694–703. doi: 10.1097/NEN.0b013e3181e4939c. [DOI] [PubMed] [Google Scholar]
- 92.van Noort JM, Verbeek R, Meilof JF, Polman CH, Amor S. Autoantibodies against alpha B-crystallin, a candidate autoantigen in multiple sclerosis, are part of a normal human immune repertoire. Multiple sclerosis. 2006;12:287–293. doi: 10.1191/135248506ms1271oa. [DOI] [PubMed] [Google Scholar]
- 93.Wanschitz J, Ehling R, Loscher WN, Kunz B, Deisenhammer F, Kuhle J, Budka H, Reindl M, Berger T. Intrathecal anti-alphaB-crystallin IgG antibody responses: potential inflammatory markers in Guillain-Barre syndrome. Journal of neurology. 2008;255:917–924. doi: 10.1007/s00415-008-0815-9. [DOI] [PubMed] [Google Scholar]
- 94.Doycheva D, Preuss B, Deuter C, Zierhut M, Klein R. Identification of immunodominant epitopes of alpha-crystallins recognized by antibodies in sera of patients with uveitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2012;250:297–305. doi: 10.1007/s00417-011-1758-x. [DOI] [PubMed] [Google Scholar]
- 95.Sinclair C, Mirakhur M, Kirk J, Farrell M, McQuaid S. Up-regulation of osteopontin and alphaBeta-crystallin in the normal-appearing white matter of multiple sclerosis: an immunohistochemical study utilizing tissue microarrays. Neuropathology and applied neurobiology. 2005;31:292–303. doi: 10.1111/j.1365-2990.2004.00638.x. [DOI] [PubMed] [Google Scholar]
- 96.Tajouri L, Mellick AS, Ashton KJ, Tannenberg AE, Nagra RM, Tourtellotte WW, Griffiths LR. Quantitative and qualitative changes in gene expression patterns characterize the activity of plaques in multiple sclerosis. Brain research. Molecular brain research. 2003;119:170–183. doi: 10.1016/j.molbrainres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13287–13292. doi: 10.1073/pnas.1107368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klopstein A, Santos-Nogueira E, Francos-Quijorna I, Redensek A, David S, Navarro X, Lopez-Vales R. Beneficial effects of alphaB-crystallin in spinal cord contusion injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:14478–14488. doi: 10.1523/JNEUROSCI.0923-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Noort JM, Bsibsi M, Nacken PJ, Gerritsen WH, Amor S, Holtman IR, Boddeke E, van Ark I, Leusink-Muis T, Folkerts G, Hennink WE, Amidi M. Activation of an immune-regulatory macrophage response and inhibition of lung inflammation in a mouse model of COPD using heat-shock protein alpha B-crystallin-loaded PLGA microparticles. Biomaterials. 2013;34:831–840. doi: 10.1016/j.biomaterials.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 100.Whiston EA, Sugi N, Kamradt MC, Sack C, Heimer SR, Engelbert M, Wawrousek EF, Gilmore MS, Ksander BR, Gregory MS. alphaB-crystallin protects retinal tissue during Staphylococcus aureus-induced endophthalmitis. Infection and immunity. 2008;76:1781–1790. doi: 10.1128/IAI.01285-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nahomi RB, Wang B, Raghavan CT, Voss O, Doseff AI, Santhoshkumar P, Nagaraj RH. Chaperone peptides of alpha-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. The Journal of biological chemistry. 2013;288:13022–13035. doi: 10.1074/jbc.M112.440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bhattacharyya J, Padmanabha Udupa EG, Wang J, Sharma KK. Mini-alphaB-crystallin: a functional element of alphaB-crystallin with chaperone-like activity. Biochemistry. 2006;45:3069–3076. doi: 10.1021/bi0518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hamann S, Metrailler S, Schorderet DF, Cottet S. Analysis of the cytoprotective role of alpha-crystallins in cell survival and implication of the alphaA-crystallin C-terminal extension domain in preventing Bax-induced apoptosis. PloS one. 2013;8:e55372. doi: 10.1371/journal.pone.0055372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Santhoshkumar P, Sharma KK. Inhibition of amyloid fibrillogenesis and toxicity by a peptide chaperone. Molecular and cellular biochemistry. 2004;267:147–155. doi: 10.1023/b:mcbi.0000049373.15558.b8. [DOI] [PubMed] [Google Scholar]
- 105.Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. The Journal of biological chemistry. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- 106.Ahmad MF, Raman B, Ramakrishna T, Rao Ch M. Effect of phosphorylation on alpha B-crystallin: differences in stability, subunit exchange and chaperone activity of homo and mixed oligomers of alpha B-crystallin and its phosphorylation-mimicking mutant. Journal of molecular biology. 2008;375:1040–1051. doi: 10.1016/j.jmb.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 107.Aquilina JA, Benesch JL, Ding LL, Yaron O, Horwitz J, Robinson CV. Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J Biol Chem. 2004;279:28675–28680. doi: 10.1074/jbc.M403348200. [DOI] [PubMed] [Google Scholar]