Abstract
Objective
To investigate the clinical and MRI features of MOG seropositive pediatric demyelinating syndromes.
Methods
Serum samples collected from 74 children with suspected demyelinating disorders followed at Massachusetts General Hospital, were incubated with MOG-GFP and GFP control transfected Jurkat cell clones. Binding ratios were calculated using flow cytometry. Statistical analysis compared demographic, clinical and radiological features in seropositive and seronegative patients.
Results
13/74 (17.5%) patients were seropositive for MOG. MOG seropositive patients were younger than seronegatives (p=0.049). No single disease category predominated amongst seropositives, nor was one group more likely to have a polyphasic course. 2/4 NMO patients had MOG antibodies; both were serognegative for AQP4. One had monophasic disease, and the other had frequent relapses. There was a bimodal distribution of MOG seropositive patients by age at onset, with a distinct younger group (4–8 years) having high prevalence of encephalopathy and an older group who presented almost exclusively with optic neuritis (13–18 years). MRI analysis demonstrated absence of corpus callosum lesions in seropositive patients (p=0.012). Annualized relapse-rate and EDSS at 2 years did not differ between seropositive and seronegative patients.
Conclusion
MOG antibodies are found across a variety of pediatric demyelinating syndromes with some distinct clinical and MRI features.
Keywords: myelin, oligodendrocyte, glycoprotein, ADEM, NMO, pediatric multiple sclerosis
INTRODUCTION
There is increasing evidence of B-cell autoimmune mechanisms in the pathogenesis of inflammatory demyelinating diseases (DD)1, 2. We and others, have previously reported anti-myelin oligodendrocyte glycoprotein (MOG) Abs in pediatric DD cases, predominantly in children with an ADEM-like first episode3, 4 and in children with MS with onset<10 years of age.5 More recently, high-titers of MOG-IgG were observed in several pediatric cases with recurrent ON,6 seronegative NMO7, 8 and in a group of young patients with ADEM like onset followed by monophasic or recurrent ON8 (ADEM-ON). MOG seropositivity has also been reported in a subset of adults with seronegative NMO and high risk- NMO (recurrent ON/ recurrent LETM).9–12 Given the broadening array of clinical phenotypes described to be associated with MOG antibodies, particularly in the pediatric population, we asked whether there is a common clinical and radiological phenotype and clinical outcome associated with seropositivity for MOG antibodies in children with demyelinating diseases. In addition, we assessed the longitudinal outcomes of seropositive children.
To address these questions, we measured anti-MOG antibodies using our previously described cell-based assay,5 in serum samples from 74 children with acquired CNS inflammation followed at our clinic. We compared the clinico-radiological phenotype and disease course in MOG antibody seropositive versus seronegative patients.
2. METHODS
2.1- Patients
74 patients with acquired demyelination of the CNS and onset prior to age 18 diagnosed between 2004 and 2012, were enrolled in a biomarkers study at the Partners Pediatric MS Center at Massachusetts General Hospital. All eligible patients diagnosed with an acquired demyelinating disease at the clinic in this time frame were enrolled in the biomarkers study and contributed to this analysis. First available blood samples from each of all patients were utilized for this study. The patients had the following diagnoses at last visit: 7 with ADEM, 12 with CIS (ON, TM), 45 with MS, 4 with NMO, 2 with radiologically isolated syndrome (RIS) and, 4 with non-specific demyelinating disease (DD), which did not meet aforementioned criteria. Pediatric MS and ADEM were diagnosed using the International Pediatric MS Study Group (IPMSSG) criteria (polysymptomatic clinical presentation with evidence of encephalopathy)13, 14. Patients with only one first clinical inflammatory event, such as ON or TM, and no evidence of encephalopathy, were diagnosed as CIS according to IPMSSG criteria. Patients were diagnosed as NMO when presenting with ON, TM and at least two of these three criteria: MRI evidence of a contiguous spinal cord lesion (3 or more segments in length), brain MRI non-diagnostic for MS and NMO IgG sero-positivity, consistent with current diagnostic criteria.14, 15 Children with non-specific DD had demyelinating syndromes which did not meet aforementioned criteria. Serum samples from 23 pediatric healthy controls were collected under the same protocol.
Standard Protocol Approvals, Registrations, and Patient Consents: Institutional Review Board approval was granted by the Partners Human Research Committee.
2.2- Sample collection and Storage
Blood samples were collected in plastic heparin-coated plasma/serum tubes and serum was extracted and stored at −80°C within 4 hours of blood draw.
2.3- MOG Ab detection Assay
MOG-GFP and GFP control stably transfected Jurkat clones were generated as described in previous publications3, 5. Cells were stored in liquid nitrogen tanks, thawed and cultured in DMEM medium (Invitrogen) enriched with 10% Fetal Bovine Serum (Fisher/Lonza), penicillin-streptomycin mixture 10 mg/ml (Fisher/Lonza), 35 mM HEPES (Fisher/Lonza), 2 mM Glutamax (Invitrogen), and 1mg Geneticin powder (Invitrogen) to maintain selection. Presence of GFP and MOG antigen in the cells were confirmed by Western-blot (Millipore).
The cells were harvested, washed in PBS (Fisher/Lonza) with 1% BSA (Sigma-Aldrich) and resuspended to 1 million cells per mL. 1 uL of serum sample and 50,000 MOG-GFP or GFP-control cells per well were incubated in 96 well U-bottom plates at 4°C for one hour. After washing twice with staining buffer, the cells were incubated in R-Phycoerythrin-conjugated F (ab’) 2 fragment donkey anti-human IgG (Jackson) diluted to a 1:1000 ratio in PBS/BSA (Fisher Lonza) and 10% BSA. Cells were washed again twice and then incubated in a live/dead cell solution reconstructed with 1 ul of violet fluorescent reactive dye (Invitrogen) in 1ml of diH2O and 9 ml of PBS for 20 minutes at 4°C. We used mouse monoclonal anti-MOG IgG, clone 8-18C5, (Millipore) as a primary ab for our positive controls. 1% paraformaldehyde was used for fixation during 10 minutes. The cells were resuspended in PBS/BSA until acquisition in BD LSR-II flow cytometer. The results were analyzed with Tree Star Flowjo 8.8.6 software.
To classify patients as MOG positive/negative, samples from pediatric healthy controls were run along with the patient samples on each plate. MOG binding ratios were corrected for background by subtracting the plate specific mean binding ratios for healthy controls to avoid differences between plates. Patients with corrected binding ratios greater than 5 (more than 3 SD from controls) were considered positive for all analyses as per our prior study.5
2.4- Clinical Data
We have prospectively collected the number, symptoms and location of each demyelinating attack, and this data is stored in our longitudinal pediatric demyelinating diseases electronic Oracle-based database,16 with posterior validation by the authors. Symptoms were classified as visual, motor, sensory, cerebellar, bowel/bladder dysfunction and encephalopathy. Attack locations were classified as optic nerve, brainstem-cerebellar, cerebrum and spinal cord. Disability scores and attack severity were measured using the EDSS scale.
2.5- MRI Analysis
Analysis of brain and spine MRIs was performed by a pediatric neurologist (C.F) blinded to clinical diagnosis, who underwent MRI lesion assessment training by senior authors (CRG, TC) and assessed lesion distribution by a qualitative protocol presented in previous work.17, 18 We reviewed T2 and FLAIR sequences from MRIs available in our computerized system, performed within 6 months of serum sampling. Brain lesion location was classified as cortical (1), subcortical (2), periventricular (3), corpus callosum (4), optic chiasm (5), thalamus (6), brainstem (7), area postrema (8) and cerebellum (9). Spine lesions were categorized according to level involved and extension, both longitudinal and cross-sectional: whole spine (1), partial (2) and central grey matter (3). If a lesion was bigger enough to occupy two locations, it was counted twice (example, a periventricular lesion involving corpus callosum was counted as ‘periventricular’ and ‘callosal’).
2.6- Statistical analysis
All analyses compared MOG seropositive subjects to MOG seronegative subjects. Continuous and ordinal variables were compared using a Wilcoxon rank sum test, and dichotomous/categorical variables were compared using Fisher’s exact test. To compare the relapse rate after the blood draw in the two groups, a Poisson regression model with an overdispersion parameter was used. To compare the EDSS scores in the two groups, a Wilcoxon rank sum test was used. Finally, Silverman’s test was used to assess if there was statistical evidence that age distribution in the MOG positive subjects had more than one mode, and this test was fit using the silvermantest library in the statistical package R (www.r-project.org).19, 20
3. RESULTS
Demographic features
The baseline demographic characteristics of the MOG positive and negative subjects are presented in Table 1. Thirteen of the 74 subjects were seropositive at the time of the blood draw. MOG seropositive patients were significantly younger at the time of blood draw (Table 1) and younger at disease onset compared to seronegative patients (Table 2). The groups did not differ significantly in terms of gender, race or ethnicity. The two groups were also not significantly different in terms of family history of MS or other autoimmune diseases.
Table 1.
Demographic and baseline clinical characteristics of MOG antibody positive and negative subjects
| MOG Positive |
MOG Negative | P-value | |
|---|---|---|---|
| N | 13 | 61 | |
| Female (N (%)) | 8 (61.5) | 44 (72.1) | 0.510 |
| Ethnicity (N (%)) | 0.719 | ||
| Hispanic | 2 (15.4) | 15 (24.6) | |
| Non-Hispanic | 11 (84.6) | 46 (75.4) | |
| Race (N (%)) | 1 | ||
| White | 9 (69.2) | 45 (73.8) | |
| Black/African American | 2 (15.4) | 8 (13.1) | |
| Other+ | 2 (15.4) | 8 (13.1) | |
| Age at blood sample (Mean (SD)) | 12.1 (5.1) | 15.7 (2.4) | 0.020 |
| Disease duration at blood sample | 2.15 (3.02) | 1.89 (2.43) | 0.81 |
| Duration of follow-up from disease onset | 4.53 (3.88) | 4.86 (3.06) | 0.500 |
| Disease Category N(%) | |||
| MS | 4 (30.77) | 41 (67.21) | 0.026 |
| CIS | 2 (15.38) | 10 (16.39) | 1 |
| ADEM | 3 (23.1) | 4 (6.56) | 0.099 |
| NMO | 2 (15.38) | 2 (3.28) | 0.14 |
| RIS | 0 | 2 (3.28) | 1 |
| Non-specific DD | 2 (15.38) | 2 (3.28) | 0.14 |
| Family history of MS (N (%))* | 0 (0.0) | 3 (5.08) | 1 |
| Family history of other autoimmune diseases (N (%))** | 6 (46.15) | 43 (71.67) | 0.105 |
Missing for 2 Patients
Missing for 1 Patient;
Abbreviations: DD=demyelinating diseases
Table 2.
Clinical characteristics at time of first symptom
| MOG Positive |
MOG Negative | P-value | |
|---|---|---|---|
| N | 13 | 59 | |
| Age at first symptom (Mean (SD); Median) | 9.9 (5.7); 7.7 | 13.7 (3.5); 14.9 | 0.046 |
| First symptom | |||
| Visual | 9 (69.2) | 26 (44.1) | 0.13 |
| Motor | 8 (61.5) | 26 (44.1) | 0.36 |
| Sensory | 4 (30.8) | 31 (52.5) | 0.22 |
| Bowel-bladder | 1 (7.7) | 4 (6.8) | 1 |
| Fatigue | 2 (15.4) | 7 (11.9) | 0.66 |
| Coordination | 3 (23.1) | 14 (23.7) | 1 |
| Cognitive | 1 (7.7) | 1 (1.7) | 0.33 |
| Encephalopathy | 5 (38.5) | 5 (8.5) | 0.014 |
| Other | 3 (23.1) | 10 (16.9) | 0.69 |
| More than one symptom | 8 (61.5) | 33 (55.9) | 0.77 |
| First symptom location | |||
| Optic nerve | 8 (61.5) | 20 (33.9) | 0.11 |
| Brainstem/cerebellar | 4 (30.8) | 21 (35.6) | 1 |
| Spinal cord | 5 (38.5) | 25 (42.4) | 1 |
| Cerebrum | 6 (46.2) | 32 (54.2) | 0.76 |
| No defined location | 1 (7.7) | 2 (3.4) | 0.45 |
| Multiple locations | 8 (61.5) | 33 (55.9) | 0.77 |
| Relapse rate after blood draw* | 0.42 | 0.71 | 0.24 |
| EDSS at blood draw (Median (range))# | 1.75 (0, 3) | 1 (0, 9) | 0.42 |
| EDSS two years after blood draw+ (Median (range)) | 2 (1, 3) | 1 (0, 8.5) | 0.081 |
Legend: Two subjects with RIS were not included in this table because first symptom details were not available.
64 subjects had visits after the blood draw date to contribute to the relapse rate analysis.
71 subjects had a visit with an EDSS score within 100 days of the blood draw.
40 subjects had a visit with an EDSS score within 100 days of the two-year post blood draw time point.
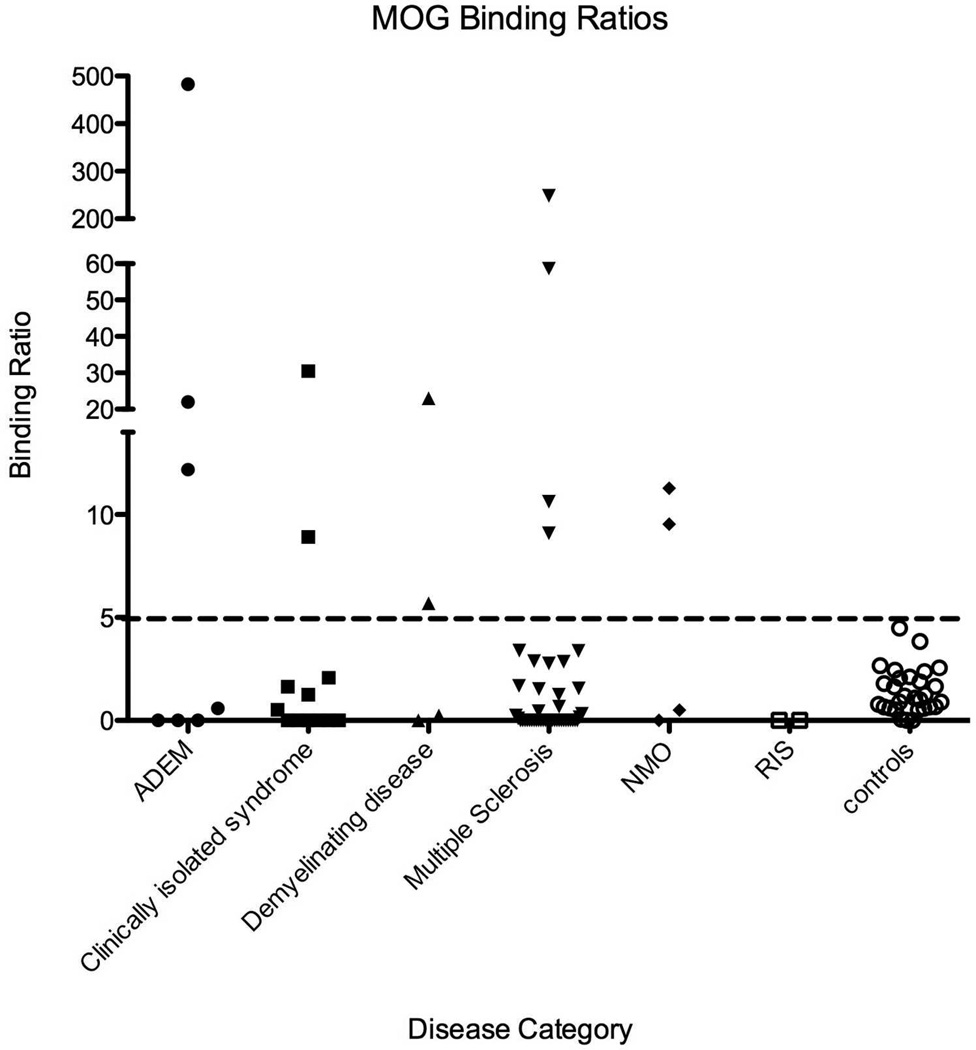
MOG antibody binding ratios by disease category are presented in Figure 1. Overall final disease category differed between seropositive and seronegative patients (p=0.021), and seronegative subjects were significantly more likely to have MS or CIS than seropositive (p=0.0078).
Figure 1.
MOG antibody binding ratios by disease category: 74 children with demyelinating diseases were tested for MOG antibody status., and compared to 23 pediatric healthy controls. Binding ratio>5 was consider positive. Some children within different categories of demyelinating disease including ADEM, CIS, MS and NMO were seropositive for MOG.
Age at onset and onset symptom features
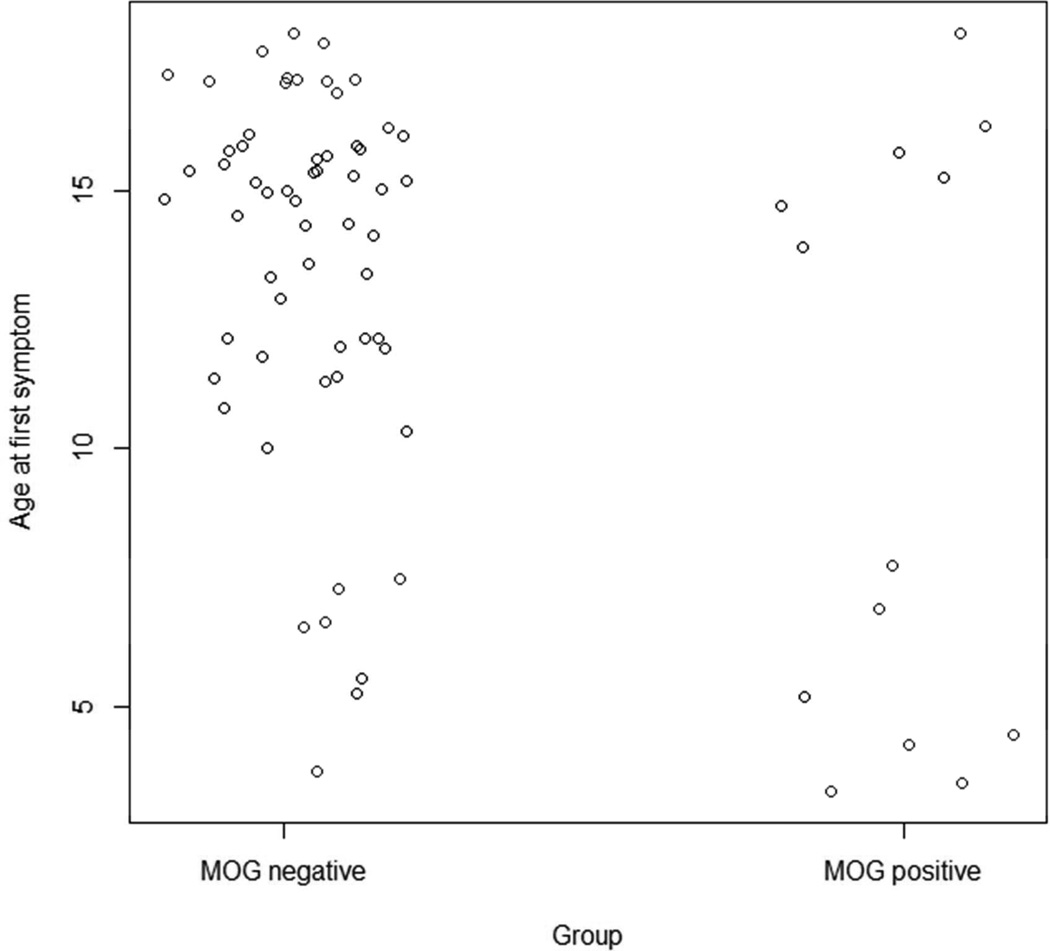
The first symptom type and first symptom location for the seropositive and seronegative patients are presented in Table 2. Seropositive subjects were significantly more likely to have encephalopathy as the first symptom (p=0.012). No other group differences reached statistical significance. Mean age at onset was significantly younger in the seropositive patients (9.9±5.7 versus 13.7±3.5; p=0.046). The distribution of age at onset in the seropositive group was distinctly bimodal (Silverman’s test for one mode: p=0.03), with a subset of seven young patients having onset between ages 3.3–7.7, while six older patients had an onset between 13.8–17.9. This bimodal distribution suggests the potential for two distinct types of seropositive patients (Figure 2). We asked whether first symptoms differ between the younger and older MOG seropositive patients. Interestingly, none of the older seropositive patients presented with encephalopathy as the first symptom, while 71.4% of the younger seropositive patients had encephalopathy as the first symptom was (p=0.02). In contrast 5/6 of the older seropositive children presented with optic neuritis, compared to 3/7 of the younger seropositive children. Of the younger seropositive children, 3/7 had a final diagnosis of MS, 3/7 had a final diagnosis of ADEM, and one had demyelinating disease NOS, meeting the criteria for ADEM-ON. Of the older seropositive patients, 2/6 have a final diagnosis of CIS, 1/6 has MS, 1/6 nonspecific demyelinating disease and 2/6 have a final diagnosis of NMO. Despite a trend towards higher MOG antibody binding ratios in younger children compared to older, this was not statistically significant (p=0.161) (Supplementary Figure 1).
Figure 2.
Age at first symptom for MOG seropositive and seronegative patients: Shows two distinct age groups amongst MOG seropositive patients of children a younger group between the ages of 4–8; and an older group between the ages of 10–13. The distribution of age at onset in the seropositive group was distinctly bimodal (Silverman’s test for one mode: p=0.03), with a subset of seven young patients having onset between ages 3.3–7.7, while six older patients had an onset between 13.8–17.9. None of the older seropositive patients presented with encephalopathy as the first symptom, while 5/7 (71.4%) of the younger seropositive patients had encephalopathy as the first symptom was (p=0.02). In contrast 5/6 of the older seropositive children presented with optic neuritis, compared to 3/7 of the younger seropositive children.
Four patients from this cohort met diagnostic criteria for NMO.15 Serum NMO IgG was tested at least twice in all 4 NMO patients. Only 2/4 of these NMO patients, were found to be seropositive for AQP4 IgG. These 2 patients were seronegative for MOG. In contrast, the 2/4 NMO patients who were seronegative for AQP4 IgG, were seropositive for MOG antibodies. One of these NMO patients had a monophasic course, while the other has had an aggressive course with multiple relapses refractory to standard treatment.
Disease course
We asked whether seronegative patients are more likely to have a polyphasic course of disease characterized by more than one attack during the follow-up period (mean 4–5 years since onset-Table 1). We found no difference between the two groups. 79.7% of seronegative patients and 61.5% of seropositive patients had a polyphasic course of disease (p=0. 277).
Relapse rate in the follow-up period after the blood draw was not significantly different between seropositive and seronegative patients (Table 2). EDSS scores at the time of the blood draw and EDSS scores two years after the blood draw did not differ significantly between the two groups (Table 2).
Laboratory features
Laboratory features were measured in subsets of patients, and the available results are summarized in Table 3. The number of white blood cells (WBC) in CSF was significantly higher in MOG seropositive patients (p=0.0004). Intrathecal IgG production and presence of oligoclonal bands did not differ between both groups. Positive EBV serology did not correlate with the presence of MOG antibodies.
Table 3. Laboratory values in MOG antibody seropositive and seronegative patients.
N indicates number of MOG seropositive (sero+) and seronegative (sero−) patients who contributed to each analysis.
| LABS | MOG Seropositive | MOG Seronegative | P-value |
|---|---|---|---|
| CSF WBC Mean(SD) Sero+ N=12 Sero− N=45 |
108.8 (126.5) | 16.2 (32.1) | 0.0004 |
| Positive CSF IGG N(%) Sero+ N=8 Sero− N=32 |
4 (50.00) | 18 (56.25) | 1.000 |
| NMO IgG N(%) Sero+ N=10 Sero− N=25 |
2 (8.0) | 2 (8.00) | 1.000 |
| Oligoclonal bands in CSF N(%) Sero+ N=7 Sero− N=38 |
1 (14.29) | 20 (52.6) | 0.101 |
| ESR Mean(SD) Sero+ N=12 Sero− N=36 |
23.97 (32.47) | 13.59 (14.14) | 0.365 |
| EBV+ N(%) Sero+ N=10 Sero− N=30 |
5 (50.00) | 20 (66.67) | 0.457 |
| ANA N(%) Sero+ N=12 Sero− N=55 |
5 (41.67) | 22 (40.0) | 1 |
MRI features
Brain and spinal MRI characteristics at the scan closest to the blood draw are summarized in Table 4. The only statistically significant difference between the seropositive and seronegative patients was the presence of corpus callosum lesions. Corpus callosum lesions were present in 52% of seronegative patients and 0% of seropositive patients (p=0.012). Interestingly, thoracic lesions were present in 8/46 of the seronegative patients and absent in 0/7 seropositive patients with thoracic MRIs, however the difference between the groups was no statistically significant given the limited sample size. The proportion of LETM was 1/2 (50%) among the seropositive patients compared to 1/22 (6.67%) in the seronegative patients, but this difference was not statistically significant given limited sample size (p=0.16).
Table 4.
Presence of lesion in specific areas of the brain and spinal
| MOG Seropositive | MOG Seronegative | p-value | |
|---|---|---|---|
| Brain | |||
| N | 6 | 45 | |
| Cortical (N (%)) | 0 | 3 (6.7) | 1.00 |
| Subcortical (N (%)) | 5 (83.3) | 34 (75.6) | 1.00 |
| Periventricular (N (%)) | 2 (33.3) | 31 (68.9) | 0.17 |
| Corpus callosum (N (%)) | 0 | 25 (55.5) | 0.023 |
| Optic chiasm (N (%)) | 0 | 1 (2.2) | 1.00 |
| Thalamus (N (%)) | 1 (16.7) | 7 (15.6) | 1.00 |
| Brainstem (N (%)) | 2 (33.3) | 20 (44.4) | 0.69 |
| Area postrema (N (%)) | 0 | 2 (4.4) | 1.00 |
| Cerebellum (N (%)) | 1 (16.7) | 11 (24.4) | 1.00 |
| Spine | |||
| N | 3 | 23 | |
| Cervical spine (N (%)) | 2 (66.7) | 12 (52.2) | 1.00 |
| Thoracic spine (N (%)) | 0 | 8 (34.8) | 0.53 |
DISCUSSION
Anti-MOG antibodies have been described to be associated with several pediatric demyelinating syndromes including ADEM,3, 21, 22 RRMS,5, 23 recurrent optic neuritis,6 NMO-like disease24 and ADEM like onset followed by monophasic or recurrent ON8 (ADEM-ON). The goal of this study was to investigate in an unbiased fashion the clinical and radiologic phenotype of MOG seropositive versus seronegative patients measured at one time-point, in a relatively large cohort of children with demyelinating diseases, in order to assess the clinical significance of this antibody. To address this gap, we compared demographic features, clinical and radiologic characteristics, laboratory findings and outcomes between MOG seropositive and seronegative patients, evaluated using our previously published MOG transfectant flow-cytometry-based assay5. We found that MOG seropositive patients were younger than seronegative patients, however did not differ in other demographic features. There was not one predominant disease category amongst seropositive patients, nor was one group more likely to have a polyphasic course. There was a bimodal distribution of MOG seropositive patients by age at onset, with a distinct younger group having a high prevalence of encephalopathy and an older group who presented almost exclusively with optic neuritis. Analysis of laboratory features showed a higher CSF WBC count in MOG seropositive patients. Analysis of qualitative MRI features showed that corpus callosum lesions were absent in seropositive patients, as well as thoracic cord lesions; however the latter did not reach statistical significance. Relapse rate and EDSS score at 2 years did not differ between groups.
To our knowledge no previous reports have addressed differences in clinical and radiological features between MOG seropositive and seronegative children with demyelinating disease. When only cell-based assays are considered, the prevalence of MOG Abs has been found to be higher in pediatric than in adult patients3–6, 9, 12, 23, 25–27. We previously reported a higher prevalence of anti-MOG Abs among children younger than 10 at disease onset5. Our current work also shows a significant younger mean age among seropositive patients with no significant differences in other demographic features. A recent paper by Baumann et al28 showed that of 19 ADEM children with or without MOG Abs did not differ in their age, sex ratio and range of clinical symptoms. However, they found that MOG antibody positive children with ADEM had large, hazy and bilateral lesions in the absence of well-defined, small lesions. However, our detailed analysis showed two distinct age groups amongst seropositive patients, who differ in clinical presentation, with younger patients more likely to have encephalopathy (as noted in prior studies), and older patients presenting with optic neuritis. Indeed, presence of encephalopathy was a distinguishing feature amongst our MOG seropositive versus seronegative patients, which may also be a function of younger age.29 The dichotomy age group raises questions about the role of development and puberty in clinical presentation.30
Since few experimental studies demonstrated the presence of severe optic neuritis and spinal cord demyelination in animal models over-expressing MOG31–35, several authors have correlated the presence of MOG Abs with optic neuritis (ON) and neuromyelitis optica (NMO). We did not find a statistically significant difference in the presence of optic neuritis as a first symptom location when taking into account all patients, but this was a presenting feature of our older seropositive patients.
In contrast to other studies, we did not find one final demyelinating disease category associated with MOG antibodies in children. We had 4 NMO patients in this study. Interestingly, the 2/4 NMO patients that were MOG Ab positive were negative for AQP4 Abs, while the other 2/4 NMO patients that were seronegative for MOG Abs were AQP4 positive. MOG seropositive adults9–12 and children28 with NMO, have been described to have a more benign course and more frequent optic neuritis. In contrast, although optic neuritis was the predominant presenting symptom in our older MOG seropositive group, one of our two MOG-seropositive NMO patients has an aggressive disease course with frequent relapses. ADEM-ON is a demyelinating disease phenotype that more often presents in children than in adults, and has been previously associated with MOG antibodies.8. One of our seropositive patients met criteria for ADEM-ON, however two patients with this phenotype were MOG seronegative.
MOG seropositive adults have reportedly more frequently had spinal cord lesions distributed in the thoracic and lumbar spinal cord.10 This contrasts to our pediatric MOG seropositive patients who showed a striking absence of thoracic cord lesions, however this finding did not reach statistical significance given the small sample size, and should therefore be interpreted with caution.
Corpus Callosum (CC) involvement is typical in MS and less frequent in other demyelinating diseases. Here we report an absence of CC lesions among seropositive patients compared to seronegative patients, suggesting a difference in CNS targets. In their study of children with ADEM, Baumann et al also suggests the absence of atypical features (such as CC lesions) in MOG seropositive children with ADEM compared to seronegative28. Whether this represents a characteristic radiologic feature of seropositive patients should be addressed in future studies with larger numbers of DD and NMO patients, as this may be helpful to distinguish MOG seropositive TM/NMO from classic NMO. These differential patterns of MRI region involvement may reflect developmental differences in MOG expression that generally proceeds caudal to rostral,36 Given that seropositive patients are more likely to be younger, absence of corpus callosum lesions may reflect a relatively lower expression of MOG in that region, although there is limited data on the temporality of regional MOG expression in humans. Moreover, the observed bimodal distribution of MOG seropositive cases, with younger patients presented with ADEM, while older patients presented with optic neuritis, may reflect the relative levels of regional expression of the MOG antigen in the different age groups. This hypothesis requires further validation through longitudinal studies of MOG expression in humans.
As previously reported28, our MOG seropositive patients had significant higher WBC in CSF, suggesting more inflammatory burden in the acute phase as compared to seronegative patients. This may also be a reflection of the younger age at onset, as higher WBC counts have been described in pre-pubertal children with MS37. We did not find a correlation between EBV and MOG serostatus, in agreement with previous literature.26
In contrast to studies in MOG seropositive NMO pediatric7,28and adult patients12 as well as pediatric ON6 we did not find a difference in relapse frequency and disability scores at 2 years in our seropositive and seronegative cohorts. This maybe because of the diverse phenotypes and disease categories of children studied in our cohort. Further larger studies may explore outcomes in larger clinical subgroups of pediatric patients.
In a longitudinal study, Di Pauli et al4 found that MOG IgG dynamics correlated well to ADEM disease course (IgG declined and disappeared with full ADEM recovery), but not in CIS cases. MOG IgG titers in the MS group showed rather stable low positive levels without significant difference between RRMS and primary progressive MS (PPMS). Probstel et al23 found similar results in a prospective study of 77 pediatric patients with acute demyelinating events. More recently, Baumann et al compared a group of MOG seropositive vs seronegative children with ADEM and found that MOG titers declined with time in the seropositive group28. We did not have sufficient longitudinal samples to assess these findings in this cohort.
Our study has several limitations including the relative small number of patients, especially NMO patients, and the limited follow-up period. Approximately 25% of our patients had received steroids or DMT in the 30 days prior to sample, however treatment has not previously been shown to be a determinant of MOG antibody status. In addition, we did not have longitudinal samples in the majority of our patients to assess longitudinal dynamics. Different MOG-Ab detection methods have led to different results even using cell-based assays38. This limitation should be taken in account when comparing assays that use full-length MOG protein versus the extracellular truncated domain used here.
In summary, we found serum MOG antibodies in a broad spectrum of children with demyelinating disease diagnoses. Common underlying features were younger age, presentation with encephalopathy particularly in the younger patients and optic neuritis in older patients, suggesting that the presence of antibodies is associated with constellation of presenting symptoms. NMO patients seropositive for MOG were seronegative for AQP4, and one had an aggressive disease course, which contrasts with MOG seropositive adults with NMO. There were no significant differences in longitudinal outcomes including polyphasic course, ARR and EDSS at 2 years, suggesting that cross-sectional evaluation of MOG antibodies have limited prognostic potential. This study contributes to the growing literature and in future studies we aim to determine assess longitudinal dynamics as well as IgG subtype. Further studies in larger, longitudinal cohorts stratified by specific clinical and radiological presentations are needed to consolidate the long-term prognostic value of MOG antibodies in children with demyelinating diseases.
Supplementary Material
Acknowledgements
We would like to thank Mariann Polgar-Turcsanyi, MS, for her role in managing the Partners MS Center research database.
Brian Healy has received grant support from Merck-Serono and Novartis.
Katherine McLaughlin is currently an employee of Health Advances.
Kai Wucherpfennig is a consultant for Novartis.
Dr. Chitnis has served as an advisor for Biogen-Idec, Novartis, Sanofi-Aventis, Teva Neurosciences, and has received grant support from National MS Society, NIH, Guthy-Jackson Charitable Foundation, Merck-Serono and Novartis.
Sponsorship/Funding: This research was supported by the National Multiple Sclerosis Society Pediatric Regional Centers of Excellence award (TC), the Peabody Foundation (TC) and Caja Madrid Fellowship Grant (CF), and NIH grant to K.W.W. (PO1 AI045757)
Footnotes
Contributions:
Dr. Fernandez-Carbonell contributed to study concept and design, and acquisition of data, analysis and interpretation and prepared the first draft of the manuscript.
Dr. Vargas-Lowy contributed to study concept and design, and acquisition of data, analysis and interpretation.
Mr. Musallam contributed to data analysis and interpretation and critical revision of the manuscript for important intellectual content.
Dr. Healy contributed to study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content.
Dr. McLaughlin acquisition of data and critical revision of the manuscript for important intellectual content.
Dr. Wucherpfennig provided key reagents and contributed to critical revision of the manuscript for important intellectual content.
Dr. Chitnis contributed to study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content and study supervision.
Disclosures:
Cristina Fernandez-Carbonell has no disclosures.
David Vargas-Lowy has no disclosures.
Mr. Musallam has no disclosures.
Charles Guttmann has no disclosures.
References
- 1.Meinl E, Krumbholz M, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Annals of neurology. 2006;59:880–892. doi: 10.1002/ana.20890. [DOI] [PubMed] [Google Scholar]
- 2.Popescu BF, Lucchinetti CF. Pathology of demyelinating diseases. Annual review of pathology. 2012;7:185–217. doi: 10.1146/annurev-pathol-011811-132443. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor KC, McLaughlin KA, De Jager PL, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. 2007;13:211–217. doi: 10.1038/nm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Pauli F, Mader S, Rostasy K, et al. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin Immunol. 2011;138:247–254. doi: 10.1016/j.clim.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin KA, Chitnis T, Newcombe J, et al. Age-dependent B cell autoimmunity to a myelin surface antigen in pediatric multiple sclerosis. J Immunol. 2009;183:4067–4076. doi: 10.4049/jimmunol.0801888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rostasy K, Mader S, Schanda K, et al. Anti-myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch Neurol. 2012;69:752–756. doi: 10.1001/archneurol.2011.2956. [DOI] [PubMed] [Google Scholar]
- 7.Rostasy K, Mader S, Hennes E, et al. Persisting myelin oligodendrocyte glycoprotein antibodies in aquaporin-4 antibody negative pediatric neuromyelitis optica. Mult Scler. 2012 doi: 10.1177/1352458512470310. [DOI] [PubMed] [Google Scholar]
- 8.Huppke P, Rostasy K, Karenfort M, et al. Acute disseminated encephalomyelitis followed by recurrent or monophasic optic neuritis in pediatric patients. Mult Scler. 2013;19:941–946. doi: 10.1177/1352458512466317. [DOI] [PubMed] [Google Scholar]
- 9.Mader S, Gredler V, Schanda K, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation. 2011;8:184. doi: 10.1186/1742-2094-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82:474–481. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitley J, Waters P, Woodhall M, et al. Neuromyelitis Optica Spectrum Disorders With Aquaporin-4 and Myelin-Oligodendrocyte Glycoprotein Antibodies: A Comparative Study. JAMA neurology. 2014 doi: 10.1001/jamaneurol.2013.5857. [DOI] [PubMed] [Google Scholar]
- 12.Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79:1273–1277. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 13.Krupp LB, Banwell B, Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68:S7–S12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 14.Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013 doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 15.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 16.Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol. 2009;66:54–59. doi: 10.1001/archneurol.2008.505. [DOI] [PubMed] [Google Scholar]
- 17.Vishwas MS, Healy BC, Pienaar R, Gorman MP, Grant PE, Chitnis T. Diffusion tensor analysis of pediatric multiple sclerosis and clinically isolated syndromes. AJNR Am J Neuroradiol. 2013;34:417–423. doi: 10.3174/ajnr.A3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorman MP, Tillema JM, Ciliax AM, Guttmann CR, Chitnis T. Daclizumab use in patients with pediatric multiple sclerosis. Arch Neurol. 2012;69:78–81. doi: 10.1001/archneurol.2011.581. [DOI] [PubMed] [Google Scholar]
- 19.Schwaiger F, Holzmann H. Package which implements the silvermantest. Research, R packages [serial online] 2013 Available at: https:// http://www.uni-marburg.de/fb12/stoch/forschung/rpackages/silvermantest_manual.pdf. [Google Scholar]
- 20.Silverman BW. Using Kernel Density Estimates to Investigate Multimodality. Journal of the Royal Statistical Society Series B (Methodological) 1981;43:97–99. [Google Scholar]
- 21.Mayer MC, Breithaupt C, Reindl M, et al. Distinction and temporal stability of conformational epitopes on myelin oligodendrocyte glycoprotein recognized by patients with different inflammatory central nervous system diseases. J Immunol. 2013;191:3594–3604. doi: 10.4049/jimmunol.1301296. [DOI] [PubMed] [Google Scholar]
- 22.Lalive PH, Hausler MG, Maurey H, et al. Highly reactive anti-myelin oligodendrocyte glycoprotein antibodies differentiate demyelinating diseases from viral encephalitis in children. Mult Scler. 2011;17:297–302. doi: 10.1177/1352458510389220. [DOI] [PubMed] [Google Scholar]
- 23.Probstel AK, Dornmair K, Bittner R, et al. Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology. 2011;77:580–588. doi: 10.1212/WNL.0b013e318228c0b1. [DOI] [PubMed] [Google Scholar]
- 24.Rostasy K, Mader S, Hennes EM, et al. Persisting myelin oligodendrocyte glycoprotein antibodies in aquaporin-4 antibody negative pediatric neuromyelitis optica. Mult Scler. 2013;19:1052–1059. doi: 10.1177/1352458512470310. [DOI] [PubMed] [Google Scholar]
- 25.Brilot F, Dale RC, Selter RC, et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Annals of neurology. 2009;66:833–842. doi: 10.1002/ana.21916. [DOI] [PubMed] [Google Scholar]
- 26.Selter RC, Brilot F, Grummel V, et al. Antibody responses to EBV and native MOG in pediatric inflammatory demyelinating CNS diseases. Neurology. 2010;74:1711–1715. doi: 10.1212/WNL.0b013e3181e04096. [DOI] [PubMed] [Google Scholar]
- 27.Lalive PH, Hausler MG, Maurey H, et al. Highly reactive anti-myelin oligodendrocyte glycoprotein antibodies differentiate demyelinating diseases from viral encephalitis in children. Mult Scler. 2011;17:297–302. doi: 10.1177/1352458510389220. [DOI] [PubMed] [Google Scholar]
- 28.Baumann M, Sahin K, Lechner C, et al. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. Journal of neurology, neurosurgery, and psychiatry. 2014 doi: 10.1136/jnnp-2014-308346. [DOI] [PubMed] [Google Scholar]
- 29.Banwell B, Kennedy J, Sadovnick D, et al. Incidence of acquired demyelination of the CNS in Canadian children. Neurology. 2009;72:232–239. doi: 10.1212/01.wnl.0000339482.84392.bd. [DOI] [PubMed] [Google Scholar]
- 30.Chitnis T. Role of puberty in multiple sclerosis risk and course. Clin Immunol. 2013;149:192–200. doi: 10.1016/j.clim.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Storch MK, Stefferl A, Brehm U, et al. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 1998;8:681–694. doi: 10.1111/j.1750-3639.1998.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iglesias A, Bauer J, Litzenburger T, Schubart A, Linington C. T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia. 2001;36:220–234. doi: 10.1002/glia.1111. [DOI] [PubMed] [Google Scholar]
- 33.Sakuma H, Kohyama K, Park IK, Miyakoshi A, Tanuma N, Matsumoto Y. Clinicopathological study of a myelin oligodendrocyte glycoprotein-induced demyelinating disease in LEW.1AV1 rats. Brain : a journal of neurology. 2004;127:2201–2213. doi: 10.1093/brain/awh260. [DOI] [PubMed] [Google Scholar]
- 34.Bettelli E, Baeten D, Jager A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. The Journal of clinical investigation. 2006;116:2385–2392. doi: 10.1172/JCI28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slavin AJ, Johns TG, Orian JM, Bernard CC. Regulation of myelin oligodendrocyte glycoprotein in different species throughout development. Developmental neuroscience. 1997;19:69–78. doi: 10.1159/000111187. [DOI] [PubMed] [Google Scholar]
- 37.Chabas D, Ness J, Belman A, et al. Younger children with MS have a distinct CSF inflammatory profile at disease onset. Neurology. 2010;74:399–405. doi: 10.1212/WNL.0b013e3181ce5db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reindl M, Di Pauli F, Rostasy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nature reviews Neurology. 2013;9:455–461. doi: 10.1038/nrneurol.2013.118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.