Abstract
Current evidence suggests that anxiety disorders have developmental origins. Early insults to the circuits that sub-serve emotional regulation are thought to cause disease later in life. Evidence from studies in mice demonstrate that the serotonergic system in general, and 5-HT1A receptors in particular, are critical during the early postnatal period for the normal development of circuits that subserve anxious behavior. However, little is known about the role of serotonin signaling through 5-HT1A receptors between the emergence of normal anxiety behavior after weaning, and the mature adult phenotype. Here, we use both transgenic and pharmacological approaches in male mice, to identify a sensitive period for 5-HT1A function in the stabilization of circuits mediating anxious behavior during adolescence. Using a transgenic approach we show that suppression of 5-HT1A receptor expression beginning in early adolescence results in an anxiety-like phenotype in the open field test. We further demonstrate that treatment with the 5-HT1A antagonist WAY 100,635 between postnatal day (P)35 and P50 but not at later timepoints, results in altered anxiety in ethologically based conflict tests like the open field test and elevated plus maze. This change in anxiety behavior occurs without impacting behavior in the more depression related sucrose preference test or forced swim test. The treatment with WAY 100,635 does not affect adult 5-HT1A expression levels, but leads to increased expression of the serotonin transporter in the raphe, along with enhanced serotonin levels in both the prefrontal cortex and raphe that correlate with the behavioral changes observed in adult mice. This work demonstrates that signaling through 5-HT1A receptors during adolescence (a time when pathological anxiety emerges), but not early adulthood, is critical in regulating anxiety setpoints. These data suggest the possibility that brief interventions in the serotonergic system during adolescence could lead to profound and enduring changes in physiology and behavior.
Keywords: 5-HT1A, serotonin, anxiety, depression, adolescence, development
1.1
Over the past decade, there has been a growing appreciation that anxiety disorders are neurodevelopmental in origin (Ansorge et al., 2007, Leonardo and Hen, 2008). In this view, aberrant maturation of circuits that sub-serve important emotional functions leads to increased risk for the later expression of illness (Caspi and Moffitt, 2006). Anxiety disorders are amongst the first psychiatric disorders to manifest typically during the adolescence, with a mean age of onset of 11 and 75% manifesting by age 21 (Kessler et al., 2005). Studies to date examining the developmental origins of anxiety have largely focused on early events that might lead to a vulnerability to these disorders. Remarkably, later developmental periods, when anxiety disorders emerge, have been largely overlooked. Thus, a full understanding of the pathophysiology of anxiety will require an understanding of its full developmental trajectory, including its increasing prevalence during adolescence and early adulthood.
Brain circuits mature at different rates, with a general posterior to anterior gradient (Tau and Peterson, 2010). Thus, brainstem and midbrain functions mature relatively early, and frontal cortical areas mature last (Miller and Cohen, 2001). Similarly, neurotransmitter systems mature at different times, with the more caudal serotonergic system maturing relatively early and with the more rostral noradrenergic and dopaminergic systems maturing later (Bylund et al., 2001, Murrin et al., 2007).
While excess serotonin (5-HT) early in murine development (P2-P11), leads to decreased novelty investigation, increased anxiety, anhedonia, and a helpless state in response to stress, no such effect is seen after 5-HT transporter (SERT) blockade in adult mice (Caspi et al., 2003, Ansorge et al., 2007, Ansorge et al., 2008, Oberlander et al., 2009). The 5-HT system matures during early postnatal development. At this time it plays an important role in establishing circuits that mediate anxious behavior (Rood et al., 2014). Once the 5-HT system has matured, it is well positioned to shape development of more rostral systems. Less is known, in adolescence, when the rostral systems mature and anxiety disorders are emerge. Interestingly, a recent study in mice has demonstrated that 5-HT reuptake inhibitors (SSRI’s) administered during adolescence (P35-P49) results in a mix phenotype, with decreased depression-like behavior along with enhanced anxiety-like responses in adulthood (Iniguez et al., 2014). Thus, the consequences of disrupting the 5-HT system are dependent on the timing of the insult.
Within the 5-HT system, signaling through the inhibitory serotonergic 1A receptor (5-HT1A), is required for the normal development of circuits that subserve anxiety-like behavior in mice (Heisler et al., 1998, Parks et al., 1998, Ramboz et al., 1998, Gross et al., 2002). The evidence from transgenic approaches suggests that normal expression of 5-HT1A receptors is required in the 2nd and 3rd week of life for the emergence of normal anxiety (Gross et al., 2002, Leonardo and Hen, 2008). Similar results are observed as result of pharmacological blockade of 5-HT1A receptors from postnatal day P0-P21 or from P13-P34 (Lo Iacono and Gross, 2008, Vinkers et al., 2010). Furthermore, pharmacological and genetic mouse models also suggest that the receptor is dispensable for normal anxiety-like behavior in adult animals. Thus, evidence available to date, indicates that once formed, the circuits are either sufficiently stable to withstand the loss of 5-HT1A receptors, or that 5-HT1A receptors play a different role in adulthood than they do in development. Surprisingly, little is known about the function of 5-HT1A receptors in regulating anxiety between the post weaning period and the fully mature adult phenotype. Indeed, numerous mental processes undergo a shift in the structures that support their function during development. For example, fear extinction during early development depends primarily on the amygdala, whereas joint roles for the amygdala, medial prefrontal cortex and the hippocampus emerge later (Shechner et al., 2014). Thus, it is possible, that 5-HT1A receptors play a distinct role in maturation/stabilization of circuits during the adolescent period, after conflict based anxiety has been established, but before full maturity.
Here, we test the hypothesis that 5-HT signaling through 5-HT1A receptors during adolescence regulates lifelong setpoints in ethologically based conflict tests (Bailey and Crawley, 2009).
2.1 Experimental Procedures
Animal husbandry
Male mice were housed in groups of 3–5 per cage and had ad libitum access to food and water. Animals were maintained on a 12:12 light/dark schedule. Animal protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance to the NIH Guide for the Care and Use of Laboratory Animals. Non-Transgenic mice were all 129SvEv. Transgenic mice were maintained in a 129SvEv/C57 mixed background
Generation of the conditional 5-HT1A receptor knock-out (KO) mice
To generate an inducible whole-brain 5-HT1A KO mouse, tetO-1A male mice were bred to a transgenic mouse line with tTS expression driven from human β-actin promoter fragments as previously described (Richardson-Jones et al., 2010). Htr1atetO/tetO βact-tTS+ mice maintained in the presence of doxycycline (DOX) displayed no receptor suppression and are indistinguishable from WT, while the same mice maintained in the absence of DOX display full receptor suppression and are indistinguishable from 5-HT1A KO mice (β-actin system).
Osmotic pump implantation
For pharmacological blockade experiments, mice (129SvEv/Tac) were bred at the New York State Psychiatric Institute. Briefly, osmotic minipumps (0.25 µl/h; model 1002; Alzet) were implanted subcutaneously to deliver WAY100635 (Sigma-Aldrich, St. Louis, MO) or saline (0.9% NaCl) continuously for 14 d. Pumps were filled with WAY100635 to deliver a dose of 0.15 mg/h per kg of body weight (Lo Iacono and Gross, 2008). Mice were anesthetized with isoflurane, pumps were subcutaneously implanted in the dorsal thoracic area, and wounds were closed with a 9 mm stainless-steel clip. After 14 d of treatment, mice were anesthetized, pumps removed, and the wounds were closed with a clip. WAY 100,635 is the protypical high affinity 5-HT1A silent antagonist although it also has agonist effects at D4 receptors at higher concentrations (Chemel et al., 2006).
Behavioral and physiological studies
All animals used for behavioral and physiological testing were age matched within two weeks. Animals were initially tested at 14–16 weeks of age. Baseline anxiety tests were completed before other behavioral tests, followed by forced swim stress. Mice recovered for one week before being tested in the sucrose preference test (see Table 1).
Table 1.
Experimental cohort of mice
| Figure | Strain | n(per group) |
Intervention | Testing time | Procedures |
|---|---|---|---|---|---|
| 1 | B-Actin | 17–21 | DOX remove at P28 |
14–16 weeks | OF |
| 2 | 129SVE | 5–7 | Osmotic pump | P35-P49, P50-P64 |
DPAT hypothermia |
| 3 | 129SVE | 19–28 | Osmotic pump | P35-P49, P50-P64 |
OF, EPM, NSF, FST, SUCPREF |
| 4 | 129SVE | 8–13 | Osmotic pump | P35-P49, P50-P64 |
CORT AM-PM, FST induced CORT |
| 5 | 129SVE | 7 | Osmotic pump | P35-P49 | HPLC |
| 5 | 129SVE | 4–5 | Osmotic pump | P35-P49 | QPCR |
OF: Open Field, EPM: Elevated Plus Maze, NSF, Novelty suppressed feeding test, FST, Forced Swim Test, SUCPREF, Sucrose Preference. Procedures are listed in the order in which they were administered. In cohort 3 tests were as follows: OF Day 1, EPM Day 3, NSF Day 5. FST Day 9–10, Sucrose Preference Day 17–25.
8-OH-DPAT-induced hypothermia
Mice were singly housed in clean cages for 10 min and three baseline body temperature measurements were taken. Ten minutes after the third baseline measurement, animals received 1mg/kg 8-OH-DPAT (Sigma-Aldrich, St. Louis, MO) intraperitoneally. Change in core temperature was assessed using a rectal probe every 10 min for 60 min as previously described. (Richardson-Jones et al., 2010, Richardson-Jones et al., 2011). 8-OH-DPAT is the prototypical 5-HT1A full agonist, although it does have some activity at 5-HT7 receptors (Landry et al., 2006). 8-OH-DPAT induced hypothermia in mice is dependent on functional 5-HT1A autoreceptors (Richardson-Jones et al., 2011).
Open-field test
Exploration in response to a novel open field was measured as described (Weisstaub et al., 2006, Richardson-Jones et al., 2010, Richardson-Jones et al., 2011) with the following minor modifications: (1) animals were habituate to the testing room for at least 30 min prior to testing, (2) light levels in the open field chambers were maintained at 30–40 lux to encourage exploration of the full environment, (3) animals were placed in a corner of the maze and allowed to explore the center at will, and (4) the test was conducted for a total of 30 min. Center of the arena was defined as a square area occupying the center 50% of the total arena. Dependent measures were total path length (cm), time in the center and percent center distance (distance travelled in the center divided by the total distance travelled).
Elevated-plus maze
After animals were habituated to the testing room for at least 30 min, animals were placed into the central area facing one open arm and allowed to explore the maze for 5 min. Testing took place at 90–100 lux. The videos were analyzed with TopScan software. Dependent measures were total number of entries into the open arms, time in the open arms and percent time in the open arms (time in the open arms divided by the total time).
Novelty suppressed feeding test
Testing was performed as previously described (David et al., 2009, Richardson-Jones et al., 2010). Briefly, animals were food restricted for 24 hours and the latency (dependent measure) to begin chewing a food pellet placed on a white piece of filter paper (12.5 cm diameter) in the center of brightly lit arena was recorded (40 × 60 cm arena with 2 cm of new corn cob bedding; 800–900 lux). The trial was terminated either when an animal began chewing or 300 seconds transpired. Immediately after terminating the trial, animals were then placed in their home cage and the amount of food consumed in 5 minutes was measured (home cage consumption), followed by an assessment of post-restriction weight. Percentage body weight lost during food deprivation prior to the testing was assessed to ensure both groups lost similar amounts of weight, and home cage consumption immediately after testing was assessed as a relative measure of hunger.
Modified forced-swim test
Behavioral response to forced swimming (FST) was assayed as described previously (David et al., 2007, Richardson-Jones et al., 2010). After 30 min habituation to the testing room, mice were placed into clear plastic buckets 20 cm in diameter and 23 cm deep filled 2/3 of the way with 26°C water and videotaped from the side for 6 min. Only the last 4 minutes were scored. All animals were exposed to the swim test on two consecutive days, as this increases sensitivity for serotonergic manipulations (Ramboz et al., 1998, Wellman et al., 2007). Scoring was done using an automated Viewpoint Videotrack software package (Montreal, Canada), which was validated before by manual scoring. Dependent variables were immobility, swimming and climbing. Behavior was scored as total time in seconds.
Sucrose Preference
An 8-day sucrose preference protocol was performed during the light phase as previously described (Kirshenbaum et al., 2014) with minor modifications. On days 1 and 2, mice were presented with two identical bottles filled with water (water/water) for 2 h and 1 h respectively. On days 3 and 4, both bottles contained 1% sucrose solution dissolved in the drinking water (sucrose/sucrose) for 1 h and 30 min respectively. On days 5–8, one bottle was filled with water and the other was filled with 1% sucrose solution for 30 min each day (water/sucrose). Preference on each day was calculated as: (weight bottle sucrose/ (weight bottle sucrose+ weight bottle water) ×100).
Corticosterone AM-PM levels and stress evoked increase of corticosterone levels
Circadian fluctuations in baseline corticosterone was obtained as previously described (Snyder et al., 2011). For stress evoked corticosterone, experiments were performed starting at 12.00 where mice were exposed to FST for 6 min and blood was drawn from the submandibular vein 12 min later as previously described (David et al., 2009). Corticosterone levels were assessed as previously described (Kirshenbaum et al., 2014).
Quantitative PCR
Total RNA from the tissues was extracted using TRIzol (Invitrogen). The SuperScript® III First-Strand Synthesis System (Invitrogen) was used to synthesize cDNA, and PCR was performed and quantified using SYBR Green real-time PCR Master Mix (Applied Biosystems). Data was normalized by mRNA levels of GAPDH. For 5-HT1A, the sense primer was 5’- GACCCTTCCTGTTCACTCA-3’ and the antisense primer was 5’-AAAAGCACTGTCCCCTTAA-3’. For SERT, the sense primer was 5’-CATAGGAACATCGTCTGTCATC-3’ and the antisense primer was 5’-ATTTCCGTTGGTGTTTCAG-3’
Neurotransmitter brain levels
The concentrations of 5-HT and its metabolite 5-hydroxyindoleacetic (5-HIAA) were determined by HPLC. Specifically, all assays were carried out on a Waters Xevo TQ MS ACQUITY UPLC system (Waters, Milford, MA, USA). Concentrations of compounds in the samples were quantified by comparing integrated peak areas for those of each compound against those of known amounts of purified standards. Loss during extraction was accounted for by adjusting for the recovery of the internal standard added before extraction. The results were normalized by protein contents in the samples, which were measured by using Bio-Rad Protein Assay Kit II (Bio-Rad, #500-0002).
Statistical Analysis
Results from data analyses were expressed as mean ± SEM. p<0.05 was used as the threshold for significance. Transgenic groups differences were analyzed using a one-way ANOVA unless otherwise stated. Time dependent pharmacological blockade experiments were analyzed as planned comparisons for treatment (SAL vs WAY) within each age group (P35 or P50). No comparisons across age groups were done. Repeated measures ANOVA was used for 8-OH-DPAT induced hypothermia and for sucrose preference test.
3.1 Results
3.1.1 Increased anxiety-like behavior in mice lacking 5-HT1A receptors after postnatal day 28
Here, we ask whether ongoing expression of 5-HT1A receptors is required after the emergence of normal anxious behavior but prior to full maturity, by taking advantage of a tTS based conditional whole-brain 5-HT1A receptor KO mouse that we have previously described (Mallo et al., 2003, Richardson-Jones et al., 2010). In this system, Htr1atetO/tetO βact-tTS+ mice maintained on doxycycline (DOX) express WT levels of 5-HT1A receptors, while in the absence of DOX, 5-HT1A expression is suppressed (Figure 1A). The experiment comprised three groups of male mice: 1) whole-life WT for 5-HT1A (ON DOX whole life), 2) whole-life KO for 5-HT1A (OFF DOX whole life), and 3) P28-KO mice (on DOX until p28) (Figure 1B). Removal of DOX at p28 results in receptor disappearance with an 8 day half life; thus significant loss of receptor is seen by P35 and less than 10% of wild-type 5-HT1A levels remain after 4 weeks of suppression (Richardson-Jones et al., 2010).
Figure 1.
Suppression of 5-HT1A receptor expression beginning in early adolescence results in increased anxiety similar to that seen in whole life 5-HT1A KO mice. Htr1atetO/tetO βact-tTS+ mice maintained in the presence of doxycycline (DOX) express 5-HT1A receptors at levels that are indistinguishable from WT as assessed by 125I-labeled MPPI autoradiography, while the same mice maintained in the absence of DOX are display no detectable 5-HT1A receptors (A). Experimental timeline for β-actin tTS interventions (B). At 14–16 weeks of age, Htr1atetO/tetO βact-tTS+ mice were tested in the open field, where both, whole-life 5-HT1A KO and P28 KO mice, showed decreased total path (C) and percentage center distance (D) when compared to WT control (n=17–21 mice per group) ( **p<0.01).
At 14–16 weeks of age the mice were tested in the open field paradigm, a conflict-anxiety test that is reliably affected in 5-HT1A KO mice (Heisler et al., 1998, Parks et al., 1998, Ramboz et al., 1998). Compared to whole-life WT animals, whole-life KO and p28-KO animals displayed significantly decreased exploration and percent distance travelled in the center of the open field in relation to the rest of the arena (main effect of group. Total path: F1,49=5.044; p=0.0293; post hoc between WT whole life and KO whole life, p=0.0002; WT whole life and P28KO, p =0.0021; Main effect of group % center distance: F1,49=5.866.; p=0.0053; post hoc between WT whole life and KO whole life, p=0.0018; WT whole life and P28KO p=0.0044) (Figure 1C,D). This phenotype is consistent with both the decreased exploratory behavior and increased anxietylike behavior that has been observed in 5-HT1A KO mice on at least 3 separate genetic backgrounds (Heisler et al., 1998, Parks et al., 1998, Ramboz et al., 1998). Importantly, these results were not an effect of DOX, as littermate control animals lacking the tTs transgene but fed the same chow displayed no detectable differences in any of these measures (Data not shown). Thus, loss of 5-HT1A receptors beginning after P28 is sufficient to result in an anxiety phenotype.
3.1.2 Pharmacological blockade of 5-HT1A receptors before but not after P50 lead to sustained anxiety throughout life
Here, we test whether a time limited blockade of 5-HT1A receptors is sufficient to reproduce the effect seen with continuous suppression after P28. We used a pharmacological approach that provides increased time resolution over the transgenic approach. Using osmotic minipumps, we continuously treated mice with WAY 100,635, a 5-HT1A antagonist or saline vehicle during two contiguous two week periods P35-P49 or P50-P64. The P35 initial timepoint coincides with the beginning of adolescence and parallels the timing of receptor suppression seen in the P28 transgenic experiment described above. The P50 timepoint is an immediately contiguous two-week timepoint that marks the transition from adolescence to adulthood.
In order to confirm that the dosing of WAY 100,635 was adequate, we tested the animals by injecting 8-OH-DPAT (a 5-HT1A agonist) after osmotic pump implantation. The characteristic hypothermic response to 1mg/Kg 8-OH-DPAT was completely blocked 1 day after implantation (Anova repeated measures main effect of drug: P35: F1,5=161.253; p<0.001; P50; F1,5=61.526; p<0.001) (Figure 2A,B), remained absent throughout the treatment period (Anova repeated measures main effect of drug: P35: F1,5=67.412 p<0.001;P50; F1,5=87.428; p<0.001) (Figure 2C,D), and was restored 1 day after minipump explantation (Anova repeated measures main effect of drug: P35: F1,5=0.242 p=0.9416; P50; F1,5=1.160; p=0.347) (Figure 2E,F). These results confirm that WAY 100,635 delivered via osmotic pumps treatment rapidly and persistently blocks 5-HT1A function.
Figure 2.
Hypothermic response to an injection of the 5-HT1A agonist 8-OH-DPAT (DPAT). Time course shown is minutes after injection. The hypothermic response is blocked within 24 hours of osmotic pump implantation; by P36–37 in P35 WAY mice (A) and by P51–52 in P50 WAY mice (B). Blockade of 5-HT1A receptors was maintained until P48–49 in P35 WAY mice (C) as well as until P63–64 in P50 WAY mice (D). The hypothermic response was restored by P50–51 in P35 WAY mice (E) and by P65–66 in P50 WAY mice (F). (n=5–7 mice per group). (*p<0.05; **p<0.01).
In order to test whether 5-HT1A receptor blockade during adolescence has an impact on lifelong anxiety, we tested the mice in two conflict-based tests: the OF, and the elevated plus maze test (EPM) when the mice were well into adulthood (14–16 weeks)(Figure 3A). In the OF, P35 WAY mice displayed decreased total path, decreased time in the center of the arena as well as decreased percent distance travelled in the center, compared to P35 Saline (main effect of drug: Total path: F1,50=4.980; p=0.0302; Center Time: F1,50=5.840; p=0.0194; % Center Distance: F1,50=6.034; p=0.0141) (Figure 3B,C,D). No such differences were detected when comparing the P50 WAY to P50 Saline treated mice (main effect of drug: Total path: F1,38=0.400; p=0.5311; Center Time: F1,38=0.187; p=0.6676; % Center Distance: F1,38=0.377; p=0.5430) (Figure 3B,C,D). In the EPM, a decreased percentage time spent in the open arms was detected when comparing P35 WAY mice to P35 Saline (main effect of drug: F1,47=7.344; p=0.0094), while no difference was detected in P50 animals (main effect of drug: F1,34=0.398; p=0.5323) (Figure 3E). We next tested the mice in the novelty-suppressed feeding (NSF) paradigm, a test of hyponeophagia that measures the latency of a mouse to consume food placed in the middle of a brightly lit, aversive arena (Bodnoff et al., 1988, Richardson-Jones et al., 2010). In this paradigm, P35 WAY mice showed an increased latency to feed relative to their saline control group (main effect of drug: F1,52=66.744; p<0.001), while no differences were detected between P50 Saline and WAY mice (main effect of drug: F1,36=0.067; p=0.7978)(Figure 3F).
Figure 3.
Increased anxiety-like behavior in adult mice after adolescent but not adult blockade of 5-HT1A receptors with WAY 100,635 delivered by osmotic pumps. Experimental timeline (A). Anxiety-like behavior was assessed in mice using the open field test (B,C,D), the elevated plus maze (E) and the novelty-suppressed feeding test (F), while depressive-like behavior was assessed by forced swim stress over two days (G) and sucrose preference test (H). Specifically, pharmacological 5-HT1A blockade from P35-P49 reduced the total path (B), time in the center (C) as well as % center distance (D) when compared to control mice treated with saline from P35-P49. 5-HT1A blockade from P35–P49 also decreased the % duration in the open arms of the EPM (E) and latency to feed (F) when compared to control mice treated with saline from P35–P49. No differences were detected in the P50-P64 group in the above measures. No effect of 5-HT1A pharmacological blockade was detected in either the P35-P49 or P50-P64 groups, in mobility time (G) or in sucrose preference (w; water, s; sucrose 1%) (H). (n=19–28 mice per group). (*p<0.05; **p<0.01).
To test whether the effects of suppressing 5-HT1A receptors in adolescence was limited to anxiety-like behaviors or extended to other mood related behaviors, we tested these mice in the forced swim test (FST), a model of behavioral despair. No differences were detected when comparing P35 WAY or P50 WAY with their respective saline treated mice on both test days (main effect of drug: P35 Day1: F1,50=2.842;p=0.0981; P35 Day2: F1,50=0.375;p=0.5432; P50 Day1: F1,50=0.005; p=0.9425; P50 Day2: F1,50=0.016;p=0.8997) (Figure 3G). We next tested the mice in the sucrose preference test, a measure of anhedonia, a core and common symptom of depression (Treadway and Zald, 2011). Again, no differences were detected in P35 WAY (Anova repeated measures main effect of drug: F1,3=0.090; p=0.9646) and P50 WAY mice (Anova repeated measures main effect of drug: F1,6=0.362; p=0.8959) when compared to their respective saline treated mice during the testing days (Days 5–8) (Figure 3H).
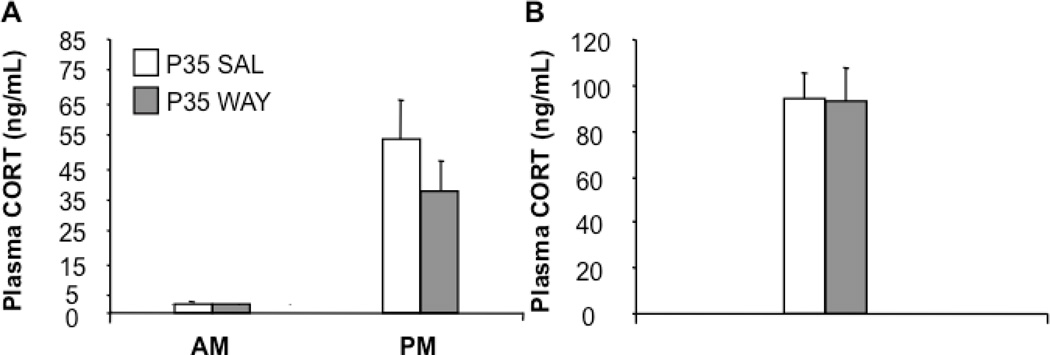
3.1.3 P35 WAY mice showed normal corticosterone levels at baseline and after stress
5-HT1A receptors are known to play key roles in reciprocal interactions between the HPA axis and the 5-HT system (Laaris et al., 1995, Laaris et al., 1999, Chaouloff, 2000). We therefore examined baseline and stress induced corticosterone levels, in adult P35 Saline and WAY mice. All groups showed equivalent levels of corticosterone at the onset of both the light and the dark phase (main effect of group: AM: F1,12=0.003; p=0.9567; PM: F1,12=01042; p=0.3225) (Figure 4A). Furthermore, while corticosterone elicited by FST was nearly double the normal PM peak, no differences were detected between groups (main effect of group: F1,21=0.002; p=0.9644) (Figure 4B).
Figure 4.
P35 SAL and P35 WAY mice had equivalent levels of corticosterone at the onset of both the light and the dark phase (A). Post forced swim stress induce CORT release was not different between P35 SAL and WAY mice (B). (n=24–25 mice per group). (*p<0.05; **p<0.01).
3.1.4 Transient 5-HT1A adolescent blockade leads to long-term changes in the 5-HT system
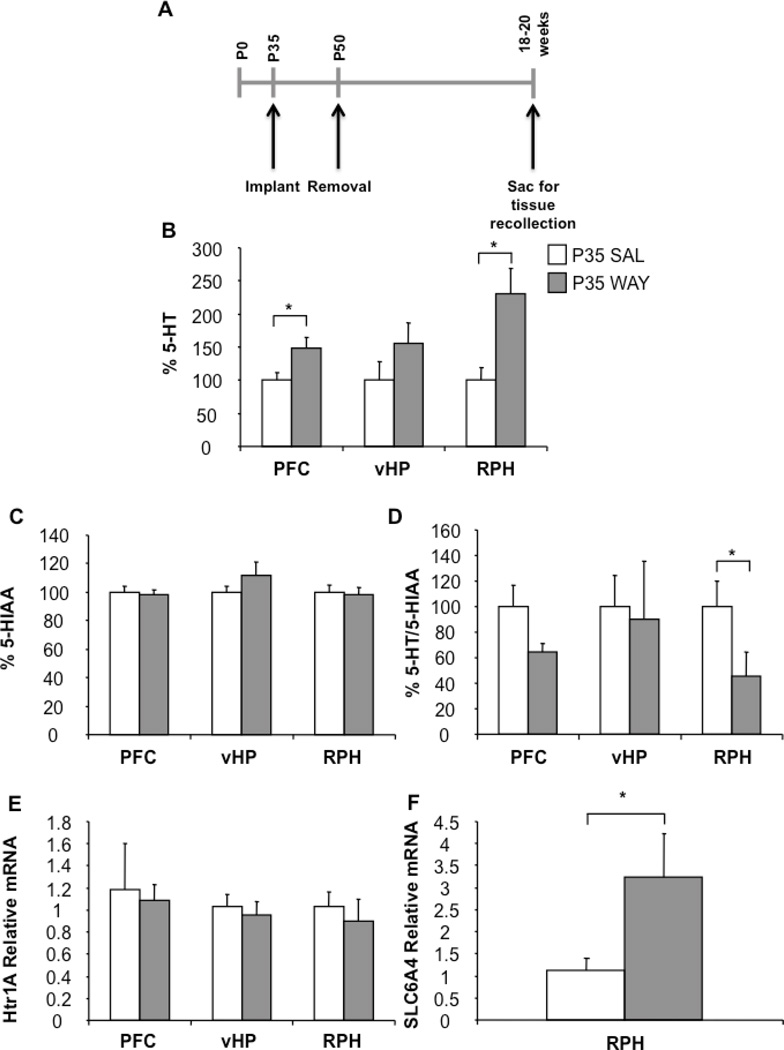
As 5-HT1A receptors have a critical role in setting overall 5-HT tone in the brain through negative feedback, we hypothesized that adolescent blockade of 5-HT1A receptors could lead to long-term changes in adult 5-HT levels and its metabolites. Thus, we surveyed the content of 5-HT and 5-HIAA in two areas rich in serotonergic inputs and 5-HT1A receptors, the prefrontal cortex and ventral hippocampus, as well and brainstem containing the serotonergic raphe nucleus (Garcia-Garcia et al., 2014). Specifically, we treated wild-type mice chronically with either saline or WAY 100,635 from P35 to P49 using subcutaneous osmotic minipumps and sacrificed them at 18–20 weeks for tissue processing (Figure 5A). Adolescent WAY treatment resulted in an increase in 5-HT content in the prefrontal cortex, no change in the ventral hippocampus and an increase in the raphe (main effect of group; prefrontal cortex: F1,12=5.683; p=0.0345; ventral hippocampus: F1,12=1.780; p=0.2070; raphe: F1,11=8.257; p=0.0151) when compared with saline treated controls (Figure 5B). Furthermore, although no differences were detected between groups in 5-HIAA levels in the studied brain regions (main effect of group; prefrontal cortex: F1,12=0.133; p=0.7220; ventral hippocampus: F1,12=1.318; p=0.2733; raphe: F1,11=0.052; p=0.8235) (Figure 5C), there was a trend for a lower 5-HIAA/5-HT ratio, in the prefrontal cortex and ventral hippocampus and a significant decrease in the raphe of P35 WAY mice when compared to their respective Saline controls (main effect of group; prefrontal cortex: F1,12=3.932; p=0.0707; ventral hippocampus: F1,12=4.021; p=0.0702; raphe: F1,11=6.513; p=0.0269) (Figure 5D).
Figure 5.
Serotonin system adaptations in adults after adolescent 5-HT1A blockade. Experimental timeline (A). WAY treated mice during adolescence display increased 5-HT levels in the prefrontal cortex and raphe (B), no changes in 5-HIAA levels (C) and decreased 5-HT turnover in the raphe (D) in adulthood when compared with Saline controls. Values were normalized to P35 Saline treated mice. Chronic 5-HT1A blockade during adolescence does not affect Htr1A mRNA levels in the adulthood (E), but increases SLC6A4 expression in the raphe (F). (n=4–7 mice per group). (*p<0.05; **p<0.01). (Htr1A: 5-HT1A gene and SLC6A4: SERT gene) (PFC: Prefrontal cortex; vHP: Ventral hippocampus; RPH: Raphe).
Further, we hypothesized that the observed changes in adult 5-HT levels in P35 WAY mice could be linked to persistent changes in expression of Htr1A (5-HT1A gene) and SLC6A4 (SERT gene). Adolescent blockade of 5-HT1A receptor has no effects on 5-HT1A mRNA expression in the studied brain regions (main effect of group; prefrontal cortex: F1,5=0.073; p=0.7979; ventral hippocampus: F1,8=0.206; p=0.6621; raphe: F1,7=0.272; p=0.6181) (Figure 5E), suggesting that at the time of testing in adulthood P35 WAY mice 5-HT1A receptor expression is normal. Indeed, no differences were detected in hypothermic responses to 8-OH-DPAT between P35 WAY and Saline treated mice when they were tested in the adulthood, an effect that is thought to be mediated by 5-HT1A autoreceptors in mice (Richardson-Jones et al., 2011)(Figure 2E). As we saw no changes in 5-HT1A receptor mRNA levels, we hypothesized that adolescent pharmacological blockade of 5-HT1A receptors may affect other regulators of 5-HT tone. We thus examined SERT mRNA expression, which was increased in the raphe of P35 WAY mice (main effect of group; raphe: F1,7=5.532; p=0.05) (Figure 5F). In sum, these results indicate that 5-HT1A adolescent blockade raises 5-HT content and decreases 5-HT turnover in a region dependent manner.
5-HT levels in select brain regions correlate with distinct behavioral measures. As P35 WAY mice were more anxious than their respective P35 Saline controls, and since the P35 WAY mice had increased levels of 5-HT when they reached adulthood, we wondered whether the increased 5-HT levels could account for the behavioral changes we observed. We thus examined the relationship between behavioral measures of individuals in the P35 treated cohorts and their respective serotonin levels. Regression analysis demonstrates that total path in the open field correlates specifically with ventral hippocampal serotonin (R2=0.44,p=0.008) but not raphe (R2=0.09, p=0.3) or prefrontal cortex (R2=0.09, p=0.28) (Fig 6A-C). In contrast, we observe that latency to feed in the novelty suppressed feeding paradigm correlates with 5-HT levels in the prefrontal cortex (R2=0.4, p=0.01), and in the raphe (R2=0.54, p=0.003), but not with levels seen in the ventral hippocampus (R2=0.16, p=0.15). Other behavioral measures such as % center distance in the open field and time in the open arms of the elevated plus maze showed no significant correlation with 5-HT in any of the regions (data not shown).
Figure 6.
Correlation between behavioral outcomes and 5-HT levels. Total path in the open field compared to baseline serotonin content in the PFC (A) vHP (B) and RPH (C). Linear regression is significant for vHP but not PFC or RPH. Latency to feed in the novelty suppressed feeding compared to baseline serotonin content in PFC (D), vHP (E) and raphe (F). Linear regression is significant for PFC and RPH but not vHP. 5-HT measured as pmol/gram of total protein in each sample (PFC: Prefrontal cortex; vHP: Ventral hippocampus; RPH: Raphe).
4.1 Discussion
Our results demonstrate that disruption of normal 5-HT signaling through 5-HT1A receptors during adolescence, results in lifelong increases in conflict-based anxiety. Using the transgenic system to suppress 5-HT1A receptors beginning at P28, we demonstrated that loss of these receptors leads to a phenotype consistent with increased anxiety in the open field test. Notably, this is the most robust effect that is seen in the constitutive 5-HT1A knockout mice (Ramboz et al., 1998). We refined and extended the initial observation from the transgenic model by using a pharmacological blockade approach that affords significantly improved time resolution and rapid reversibility. Using this approach, we show that blockade of 5-HT1A receptors during adolescence but not early adulthood affects a variety of conflict based anxiety measures. These behavioral effects are relatively specific to anxiety, as neither hedonic drive as assessed by the sucrose preference test, nor immobility in the forced swim test were affected. Our results suggest that 5-HT signaling through 5-HT1A receptors during adolescence, but not early adulthood, remains critical in regulating anxiety setpoints. Indeed, it appears that this the role of 5-HT1A receptors in regulating anxiety setpoints ceases somewhat abruptly at P50, suggesting the closure of a sensitive period.
While previous pharmacological and transgenic work has suggested that the effects of 5-HT1A receptors on anxiety are developmentally mediated much of this work has focused on earlier time periods (Gross et al., 2002, Lo Iacono and Gross, 2008, Vinkers et al., 2010, Richardson-Jones et al., 2011). Further evidence suggests that the anxiety phenotype is the result of altered autoreceptor function during these developmental periods (Richardson-Jones et al., 2011, Donaldson et al., 2014). Specifically, disruption of 5-HT1A receptors signaling during the second and third postnatal week prevents proper circuit formation and results in increased anxiety in both juvenile and adult mice (Gross et al., 2002, Richardson-Jones et al., 2011). These interventions occur at a time prior to the emergence of behaviors that are consistent with conflict based anxiety (Murrin et al., 2007), and thus suggest a role for 5-HT signaling through 5-HT1A receptors in the establishment of circuits that mediate anxious behaviors. Our current findings reveal a distinct role for these receptors as adolescent animals exhibit a full complement of conflict based anxiety behaviors. Despite this, our data suggest that 5-HT1A receptors remain critical in maintaining normal levels of anxiety through the adolescent period and in setting life-long baseline reactivity to conflict-anxiety based paradigms. As such results suggest the possibility that, during adolescence, lifelong anxiety setpoints could be fine tuned targeting 5-HT1A receptor activation, but that these setpoints are fixed by P50.
The neurobiological mechanisms underlying the observed adult phenotype after adolescent 5-HT1A receptors blockade are unknown. For example, elevated 5-HT expected by adolescent blockade of raphe 5-HT1A autoreceptors could exert its effects by either affecting the normal developmental trajectory of late maturing targets of 5-HT innervation, or by affecting regulation of the 5-HT system itself. We found increased total 5-HT content in the both prefrontal cortex and in midbrain tissue contain the serotonergic raphe of adult animals that had 5-HT1A blocked in adolescence. These increased levels of 5-HT in both the prefrontal cortex and raphe correlated with latency to feed in the novelty suppressed feeding test, while levels of 5-HT in the ventral hippocampus did not. Interestingly, we see a different pattern in the total path in the open field, where 5-HT levels in the ventral hippocampus correlates behavior while 5-HT levels in the prefrontal cortex and raphe do not. These results suggest that changes in 5-HT1A function during adolescence results in regionally specific enduring changes in 5-HT levels that can account for a significant part of the variance in selected behavioral tasks. Our data also suggest that the effects are not due to changes in adult 5-HT1A mRNA levels, which remain unchanged.
The effects of our intervention in adolescence are easiest to understand in terms of effects on 5-HT tone and thus most likely implicate autoreceptors as the primary mediators of this effect. Interestingly, earlier developmental manipulation of autoreceptors also results in increased anxiety, albeit with significant differences. For example P14 to P30 knockdown of autoreceptors results in increased conflict based anxiety without increased latency in the novelty suppressed feeding (Donaldson et al., 2014). Whether this difference is due to differences in the timing of the intervention, or whether the effect on the novelty suppressed feeding might be mediated through effects of our intervention on 5-HT1A heteroreceptors remains to be investigated.
In addition to differences in 5-HT levels, we found increases in SERT mRNA levels in the P35 WAY treated animals. It is possible that the loss of 5-HT1A mediated feedback in adolescence led to a compensatory increase in SERT that continued in to adulthood. Interestingly, other studies have also reported reciprocal interactions 5-HT1A receptors and SERT, indeed, 5-HT1A autoreceptors are strongly desensitized and their expression down-regulated in the SERT-KO (Fabre et al., 2000, Li et al., 2000, Mannoury la Cour et al., 2001).
5.1 Conclusions
In sum, this study identifies a role for 5-HT1A receptors in maintaining anxiety setpoints in adolescence, suggesting that the circuits mediating anxiety remain plastic through postnatal day 50, and enduring, even permanent changes in these setpoints can be effected until then. In addition to the enduring behavioral consequences, we demonstrate that alteration in 5-HT1A function in adolescence results in long-term changes in key components in the 5-HT system, and that these changes correlate with behavioral outcomes. Whether brief interventions affecting other aspects of the serotonergic system during adolescence could lead to similar profound and enduring changes in behavior remains an open question, although there is evidence demonstrating that treatment with fluoxetine in adolescence may result in adult increase in resilience to some stressors albeit with increased anxiety (Iniguez et al., 2014). Taken together with our results, the data point to the possibility of therapeutically resetting anxiety setpoints in adolescence. This is an exciting prospect as many anxiety disorders emerge in this timeframe. It also reiterates the need to be mindful when treating adolescents with serotonergic drugs given the possibility of long-term unintended consequences.
Highlights.
-
-
Temporary loss of 5-HT1A function after P35, but not after P50, results in increased anxiety-like behaviors in adulthood.
-
-
Adolescent blockade of 5-HT1A receptors results in long-term changes serotonin levels.
-
-
Adult anxiety phenotype correlates with increased serotonin levels in select brain regions.
-
-
Brief interventions in the 5-HT system during adolescence lead to profound and enduring changes in behavior.
Acknowledgments
We thank Gila Pilosof and Tamara Briner for technical assistance and Dr. Rene Hen for his support during the early stages of this project.
Funding and Disclosure
This work was supported by a Spanish Ministry of Science postdoctoral fellowship (AGG), NIH R01MH091844 (AD), and NIH RO1 MH91427 (EDL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Current opinion in pharmacology. 2007;7:8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. Boca Raton (FL): 2009. [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Gerety ME, Happe HK, Murrin LC. A robust GTP-induced shift in alpha(2)-adrenoceptor agonist affinity in tissue sections from rat brain. Journal of neuroscience methods. 2001;105:159–166. doi: 10.1016/s0165-0270(00)00358-7. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nature reviews Neuroscience. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Serotonin, stress and corticoids. Journal of psychopharmacology. 2000;14:139–151. doi: 10.1177/026988110001400203. [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology. 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, Craig DA, Zhong H, Swanson CJ, Hegde LG, Ping XI, Dong D, Marzabadi MR, Gerald CP, Hen R. Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphenyl]-2-m ethylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. The Journal of pharmacology and experimental therapeutics. 2007;321:237–248. doi: 10.1124/jpet.106.109678. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Piel DA, Santos TL, Richardson-Jones J, Leonardo ED, Beck SG, Champagne FA, Hen R. Developmental effects of serotonin 1A autoreceptors on anxiety and social behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:291–302. doi: 10.1038/npp.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, Murphy DL, Lanfumey L, Hamon M, Martres MP. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. The European journal of neuroscience. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. 5-HT(1A) [corrected] receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology. 2014;231:623–636. doi: 10.1007/s00213-013-3389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez SD, Alcantara LF, Warren BL, Riggs LM, Parise EM, Vialou V, Wright KN, Dayrit G, Nieto SJ, Wilkinson MB, Lobo MK, Neve RL, Nestler EJ, Bolanos-Guzman CA. Fluoxetine exposure during adolescence alters responses to aversive stimuli in adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:1007–1021. doi: 10.1523/JNEUROSCI.5725-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum GS, Lieberman SR, Briner TJ, Leonardo ED, Dranovsky A. Adolescent but not adult-born neurons are critical for susceptibility to chronic social defeat. Frontiers in behavioral neuroscience. 2014;8:289. doi: 10.3389/fnbeh.2014.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaris N, Haj-Dahmane S, Hamon M, Lanfumey L. Glucocorticoid receptor-mediated inhibition by corticosterone of 5-HT1A autoreceptor functioning in the rat dorsal raphe nucleus. Neuropharmacology. 1995;34:1201–1210. doi: 10.1016/0028-3908(95)00095-n. [DOI] [PubMed] [Google Scholar]
- Laaris N, Le Poul E, Laporte AM, Hamon M, Lanfumey L. Differential effects of stress on presynaptic and postsynaptic 5-hydroxytryptamine-1A receptors in the rat brain: an in vitro electrophysiological study. Neuroscience. 1999;91:947–958. doi: 10.1016/s0306-4522(98)00674-5. [DOI] [PubMed] [Google Scholar]
- Landry ES, Lapointe NP, Rouillard C, Levesque D, Hedlund PB, Guertin PA. Contribution of spinal 5-HT1A and 5-HT7 receptors to locomotor-like movement induced by 8-OH-DPAT in spinal cord-transected mice. The European journal of neuroscience. 2006;24:535–546. doi: 10.1111/j.1460-9568.2006.04917.x. [DOI] [PubMed] [Google Scholar]
- Leonardo ED, Hen R. Anxiety as a developmental disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:134–140. doi: 10.1038/sj.npp.1301569. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Iacono L, Gross C. Alpha-Ca2+/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6250–6257. doi: 10.1523/JNEUROSCI.5219-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo M, Kanzler B, Ohnemus S. Reversible gene inactivation in the mouse. Genomics. 2003;81:356–360. doi: 10.1016/s0888-7543(03)00032-6. [DOI] [PubMed] [Google Scholar]
- Mannoury la Cour C, Boni C, Hanoun N, Lesch KP, Hamon M, Lanfumey L. Functional consequences of 5-HT transporter gene disruption on 5-HT(1a) receptor-mediated regulation of dorsal raphe and hippocampal cell activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:2178–2185. doi: 10.1523/JNEUROSCI.21-06-02178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochemical pharmacology. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clinical pharmacology and therapeutics. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, David DJ, Guiard BP, Beck SG, Hen R, Leonardo ED. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6008–6018. doi: 10.1523/JNEUROSCI.5836-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Calizo LH, Piel D, Spangler ZP, Campbell K, Beck SG. Dorsal raphe serotonin neurons in mice: immature hyperexcitability transitions to adult state during first three postnatal weeks suggesting sensitive period for environmental perturbation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:4809–4821. doi: 10.1523/JNEUROSCI.1498-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Hong M, Britton JC, Pine DS, Fox NA. Fear conditioning and extinction across development: Evidence from human studies and animal models. Biological psychology. 2014 doi: 10.1016/j.biopsycho.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience and biobehavioral reviews. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkers CH, Oosting RS, van Bogaert MJ, Olivier B, Groenink L. Early-life blockade of 5-HT(1A) receptors alters adult anxiety behavior and benzodiazepine sensitivity. Biological psychiatry. 2010;67:309–316. doi: 10.1016/j.biopsych.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]