Abstract
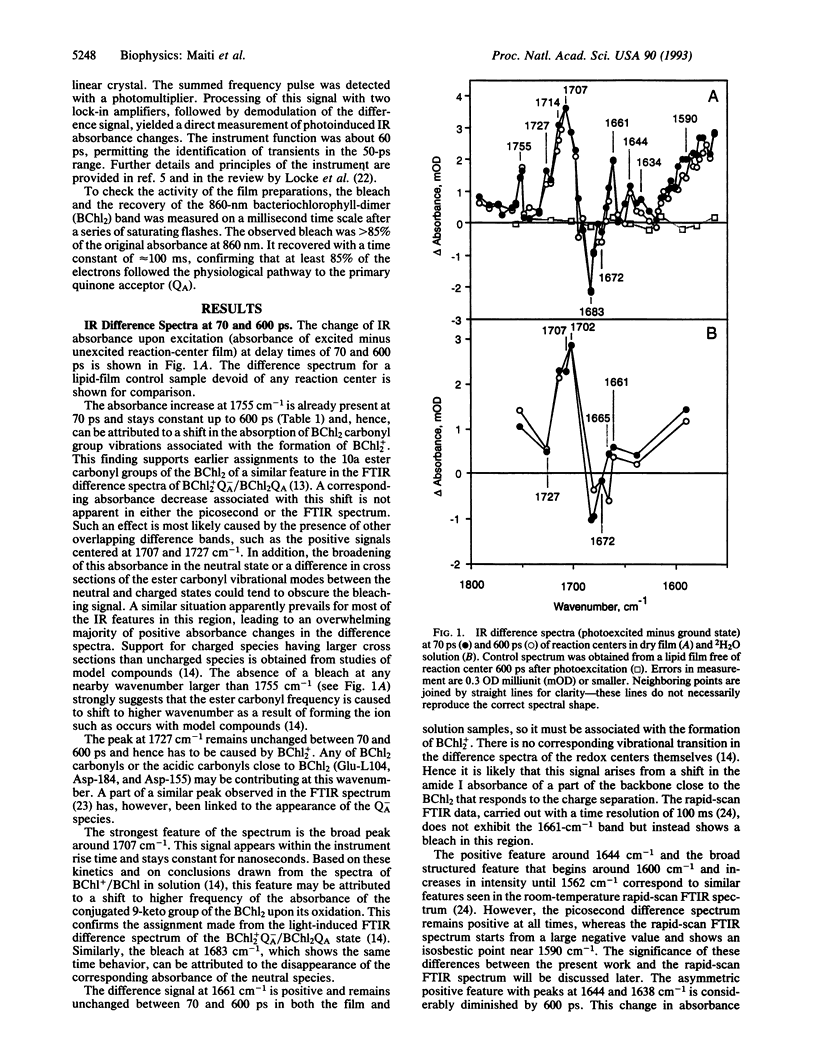
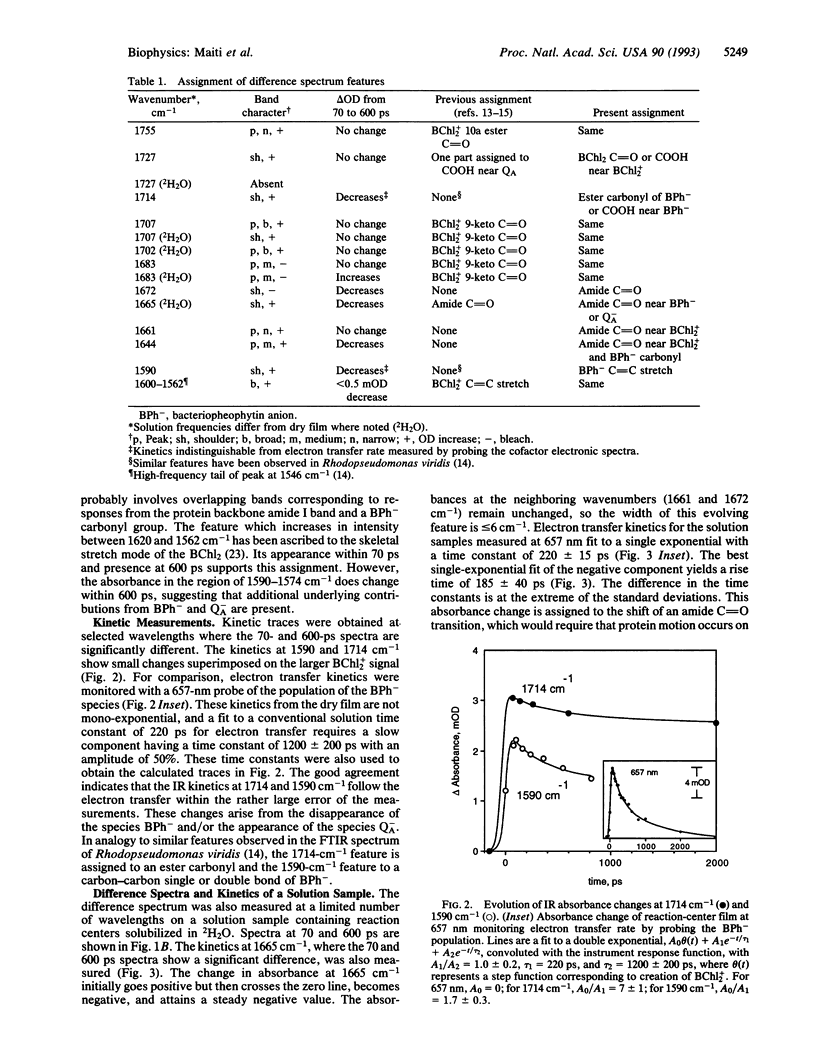
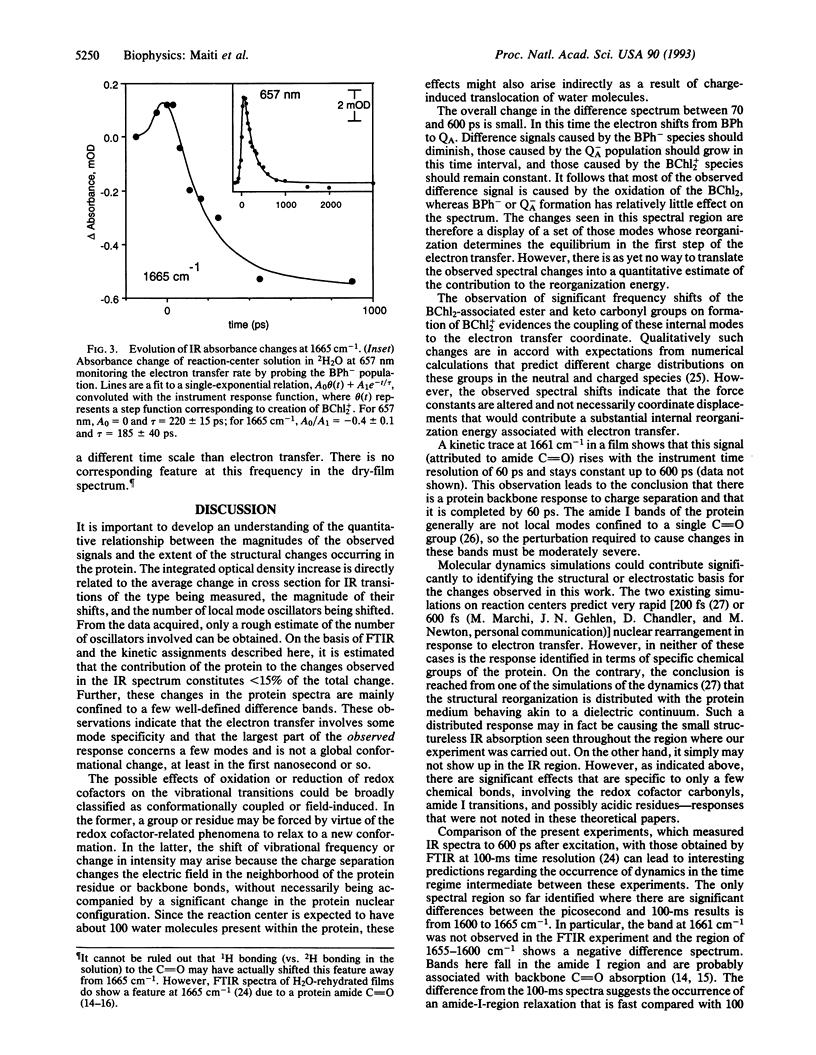
The changes in the vibrational transitions of the protein and redox cofactors of the photosynthetic reaction center were examined by picosecond infrared spectroscopy. The spectra in the vibrational mid-infrared region (1800-1550 cm-1) of hydrated and partially dehydrated reaction centers were investigated from 50 ps to 4 ns after photoinitiation of the electron transfer. Features in the infrared difference spectra were identified with both protein and redox cofactor vibrational modes and correlated with electron transfer events whose kinetics were measured in the infrared and visible regions. The observed protein response is confined to a few amide I transitions (1644 cm-1, 1661 cm-1, 1665 cm-1) and carboxylic residues (1727 cm-1). About 85% of the observed signal corresponded to alterations in the cofactor-associated ester and keto carbonyls. The amide I and carboxylic transitions appeared prior to 50 ps, suggesting that the primary electron transfer event is coupled with a specific piece of the protein backbone and to glutamic or aspartic residues nearby the special pair. Infrared absorption changes accompanying bacteriochlorophyll-dimer cation formation dominated the signal at all times investigated. Infrared spectral changes observed in hydrated and partially dehydrated reaction centers were distinctly different; a band at 1665 cm-1 with a spectral width of 6 cm-1 in the hydrated protein, corresponding to a protein amide I bleach, was not present in the dehydrated film. These differences are discussed in terms of the markedly different electron transfer kinetics observed in the presence of water.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinrud P. A., Han C., Hochstrasser R. M. Direct observations of ligand dynamics in hemoglobin by subpicosecond infrared spectroscopy. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8387–8391. doi: 10.1073/pnas.86.21.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. High-resolution structures of photosynthetic reaction centers. Annu Rev Biophys Biophys Chem. 1991;20:247–266. doi: 10.1146/annurev.bb.20.060191.001335. [DOI] [PubMed] [Google Scholar]
- Diller R., Iannone M., Bogomolni R., Hochstrasser R. M. Ultrafast infrared spectroscopy of bacteriorhodopsin. Biophys J. 1991 Jul;60(1):286–289. doi: 10.1016/S0006-3495(91)82050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller R., Iannone M., Cowen B. R., Maiti S., Bogomolni R. A., Hochstrasser R. M. Picosecond dynamics of bacteriorhodopsin, probed by time-resolved infrared spectroscopy. Biochemistry. 1992 Jun 23;31(24):5567–5572. doi: 10.1021/bi00139a020. [DOI] [PubMed] [Google Scholar]
- Du M., Rosenthal S. J., Xie X., DiMagno T. J., Schmidt M., Hanson D. K., Schiffer M., Norris J. R., Fleming G. R. Femtosecond spontaneous-emission studies of reaction centers from photosynthetic bacteria. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8517–8521. doi: 10.1073/pnas.89.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hienerwadel R., Thibodeau D., Lenz F., Nabedryk E., Breton J., Kreutz W., Mäntele W. Time-resolved infrared spectroscopy of electron transfer in bacterial photosynthetic reaction centers: dynamics of binding and interaction upon QA and QB reduction. Biochemistry. 1992 Jun 30;31(25):5799–5808. doi: 10.1021/bi00140a016. [DOI] [PubMed] [Google Scholar]
- Holzapfel W., Finkele U., Kaiser W., Oesterhelt D., Scheer H., Stilz H. U., Zinth W. Initial electron-transfer in the reaction center from Rhodobacter sphaeroides. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5168–5172. doi: 10.1073/pnas.87.13.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmaier C., Holten D. Evidence that a distribution of bacterial reaction centers underlies the temperature and detection-wavelength dependence of the rates of the primary electron-transfer reactions. Proc Natl Acad Sci U S A. 1990 May;87(9):3552–3556. doi: 10.1073/pnas.87.9.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimm S., Bandekar J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv Protein Chem. 1986;38:181–364. doi: 10.1016/s0065-3233(08)60528-8. [DOI] [PubMed] [Google Scholar]
- Moore J. N., Hansen P. A., Hochstrasser R. M. Iron-carbonyl bond geometries of carboxymyoglobin and carboxyhemoglobin in solution determined by picosecond time-resolved infrared spectroscopy. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5062–5066. doi: 10.1073/pnas.85.14.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntele W. G., Wollenweber A. M., Nabedryk E., Breton J. Infrared spectroelectrochemistry of bacteriochlorophylls and bacteriopheophytins: Implications for the binding of the pigments in the reaction center from photosynthetic bacteria. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8468–8472. doi: 10.1073/pnas.85.22.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabedryk E., Bagley K. A., Thibodeau D. L., Bauscher M., Mäntele W., Breton J. A protein conformational change associated with the photoreduction of the primary and secondary quinones in the bacterial reaction center. FEBS Lett. 1990 Jun 18;266(1-2):59–62. doi: 10.1016/0014-5793(90)81506-j. [DOI] [PubMed] [Google Scholar]
- Nabedryk E., Robles S. J., Goldman E., Youvan D. C., Breton J. Probing the primary donor environment in the histidineM200-->leucine and histidineL173-->leucine heterodimer mutants of Rhodobacter capsulatus by light-induced Fourier transform infrared difference spectroscopy. Biochemistry. 1992 Nov 10;31(44):10852–10858. doi: 10.1021/bi00159a028. [DOI] [PubMed] [Google Scholar]
- Pachence J. M., Dutton P. L., Blasie J. K. Structural studies on reconstituted reaction center-phosphatidylcholine membranes. Biochim Biophys Acta. 1979 Nov 8;548(2):348–373. doi: 10.1016/0005-2728(79)90141-5. [DOI] [PubMed] [Google Scholar]
- Treutlein H., Schulten K., Brünger A. T., Karplus M., Deisenhofer J., Michel H. Chromophore-protein interactions and the function of the photosynthetic reaction center: a molecular dynamics study. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):75–79. doi: 10.1073/pnas.89.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos M. H., Lambry J. C., Robles S. J., Youvan D. C., Breton J., Martin J. L. Femtosecond spectral evolution of the excited state of bacterial reaction centers at 10 K. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):613–617. doi: 10.1073/pnas.89.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]



