It is indisputable that natural killer (NK) immune surveillance is of crucial importance for hematological and solid tumors. However, the outcome of NK cell-based immune therapies appears disappointing in many trials. Limitations include poor in vivo survival or lack of specificity and are often attributed to tumor-associated immune escape mechanisms. NK cells are terminally activated by ligation of the activating NK receptors NKG2D, NKp30 or NKp46 through their corresponding ligands, which are up-regulated on the cell surface of dangerous cells (1-5). There is evidence that malignant cells bypass the NK surveillance by releasing these ligands as soluble proteins, as shown for NKG2D and NKp30 ligands (6-10). In most cases, the ligands are released by metalloproteinase-dependent shedding. The relevance of this mechanism was clearly confirmed in a transgenic animal model, showing spontaneous growth of tumors with the shedding-proficient NKG2D ligand MICA but tumor-free survival of animals with a shedding-resistant NKG2D ligand (11).
The NKG2D/NKG2D-L system has been studied extensively and its role in tumor immunity and immune escape is well understood. There are many clinical and experimental reports demonstrating that the surface expression of NKG2D-L may activate an effective anti-tumor immune response in early stages, whereas a sustained NKG2D-L expression and shedding of soluble ligands counteracts NKG2D-dependent NK cell activity in later stages. This depends on a passive blocking of the receptors and also on a down-regulation of the receptors on the cell surface.
Neuroblastoma (NB) is a pediatric extracranial tumor, which is characterized by a down-regulation of the human leukocyte antigen (HLA) class I. Because the loss of HLA class I is an additional trigger for NK cells, this disease is an appropriate target for NK-mediated immunotherapies. First evidence for a NKG2D-targeted immune escape mechanism in NB was described already more than a decade ago. A soluble form of the NKG2D-ligand MICA (sMICA) was identified in most patient sera and in some cell line supernatants, which masked the receptor and moreover down-regulated NKG2D surface expression (12).
In contrast to the knowledge on the relevance and regulation of NKG2D/NKG2D-L in tumor immunology, our understanding of the natural cytotoxicity receptors NKp46 and NKp30 and their ligands remains enigmatic. A recent study revealed that NB tumor cell-derived factors affect the chemokine receptor repertoire of human resting NK cells and caused a down regulation of NKp30 in a TGF-β1-dependent manner (13). A critical role of NKp30 in immune surveillance was recently demonstrated in gastrointestinal tumors (14). Inhibitory NKp30 splice variants were identified that affect the prognosis of gastrointestinal sarcoma upon treatment with NK cell-stimulatory KIT tyrosine kinase inhibitors (14). Complementary, a significant relevance for the NKp30-ligand BAG6 (15) in anti-tumor immunity was reported for patients with chronic lymphocytic leukemia (16). Now, Semeraro and colleges come up with a study that presents evidence for a clinical impact of NKp30 and its ligand B7-H6 (17) in patients with high risk neuroblastoma (HR-NB) (18).
Here they show that the expression level of the immunosuppressive NKp30C isoform and the activating isoforms NKp30A/B affected NK cell functions and correlated with the event free survival. However, low NKp30B/C ratio in GIST was explained by a higher transcription of isoform C, while low NKp30A/C ratio in HR-NB was attributed to a diminished isoform B expression suggesting differential regulation in these diseases.
A proportion of the patients revealed high serum level of the soluble NKp30-ligand B7-H6, which was associated with NKp30 down-regulation and NK cell dysfunction and this correlated to NB dissemination and chemoresistance (18). Other factors that might be involved, e.g., in cases with low sB7-H6 levels were not investigated in this study. Moreover, the question whether soluble or surface B7-H6 has a direct impact on the transcription of NKp30 in general or on specific isoforms remains open.
One strong candidate, which might be involved in this scenario is the NKp30-ligand BAG6. Consistent with a fundamental role of NKp30 as a target for tumor immune escape, soluble inhibitory NKp30-ligand BAG6 levels were significantly elevated in patients with hematological diseases (16,19). In contrast to the soluble ligand, BAG6 has another functionality when released in a membrane-associated context on the surface of exosomes (20). Interestingly, an exosome phase II trial in NSCLC stage IV patients revealed that BAG6 was the relevant ligand for NKp30-dependent NK cell-activity (21). Exosomes from dendritic cells, which harbour the activating form of BAG6 (Dex), triggered NKp30-dependent NK cell functions in NSCLC patients presenting with defective NKp30 expression. Importantly, the BAG6 expression level on exosomes correlated with NKp30-dependent NK functions, the latter being associated with longer progression-free survival (21). Patients presenting with high sBAG6 serum level were those prone to respond to Dex therapy with Dex harbouring BAG6 molecules (21).
A clinical relevance of the recently described NKp30-ligand galectin-3 (Gal-3) (22) for NKp30-dependent NK cell-activity remains to be demonstrated. Gal-3 is a β-galactoside-binding protein expressed by tumor cells, mainly in a soluble form. It was shown to bind specifically to NK cells and NKp30 and to inhibit NKp30-mediated activation and cytolysis (22). However, expression of Gal-3 seemed to inhibit proliferation and differentiation of NB cells and was associated with a better prognosis for NB (23).
Thus future work to dissect the impact of these factors either in their soluble or in their membrane form on NKp30-dependent NK cell function and possibly on NKp30 expression level will shed more light on the underlying mechanistic pathways. Not much is known on the mechanisms that direct the presentation of NKp30-ligands either on the surface of target cells, on bystander cells or upon secretion in the body fluids on the surface of exosomes or as a soluble protein. B7-H6 was described as a target of shedding (8), whereas BAG6 seems to be released via unconventional protein secretion pathways independently from cell surface shedding (personal observation). Clarification of these mechanisms and a better basic understanding of NKp30 interaction with its ligands BAG6, B7-H6 and Gal-3 are urgently required considering their clinical relevance, as nicely confirmed for NKp30/B7-H6 in the study by Semeraro and colleagues (18).
A model (Figure 1) based on these data and other reports implicates that a therapeutic intervention with NK cells aiming at a NK cell-dependent anti-tumor immune response should tackle distinct pathways to circumvent immune evasion. This includes—but is not restricted—(I) to a shift of NKp30C expression towards NKp30A/B isoforms; (II) to an increase of B7-H6 surface expression on target cells; (III) to an enhancement of the secretion of BAG6-expressiong immune stimulatory exosomes; (IV) a depletion of immune compromising soluble ligands (sB7-H6, sBAG6) and chemokines; and (V) an enhancement of inflammatory cytokines, e.g., via T cell activation using check point inhibitors.
Figure 1.
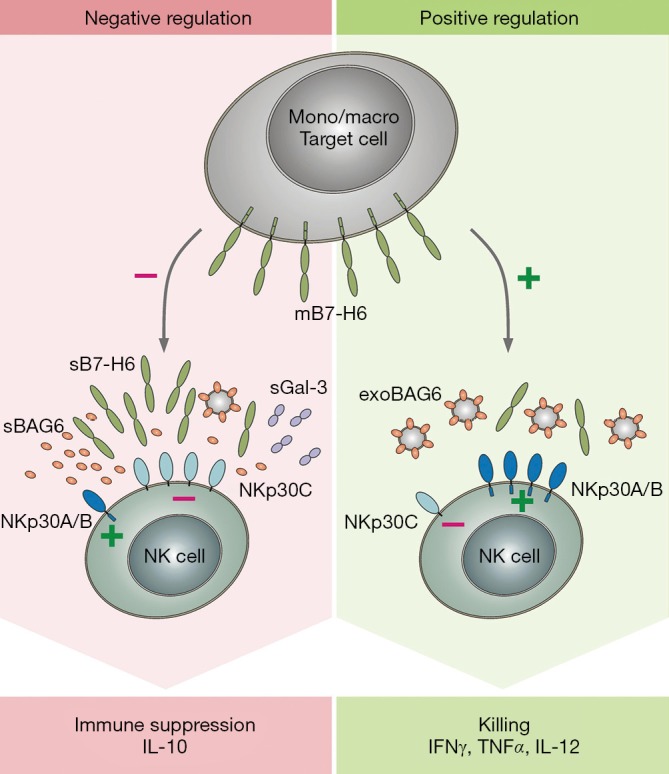
Model depicting the role of NKp30 isoforms and ligands for the detection and elimination of target cells, see text for explanation. Mono/macro, monocytes/macrophages.
So far, the relation of soluble ligands and the NKp30 isoform transcription level, e.g., in cooperation with chemokines such as TGFβ have not been investigated. The expected findings will give a more detailed insight on tumor immune escape mechanisms involving soluble NKp30-ligands. These soluble ligands and their association with defects in NK cell-activity might arise as a prognostic factor and as a start point for combined immunotherapies (neutralisation of the soluble ligands, e.g., using recombinant NKp30-Fc proteins). Ideally, the results of further investigations on NKp30/NKp30-L will allow the development of new therapeutic approaches to circumvent this evasion strategy of tumor cells. Preliminary results suggest a beneficial clinical effect dependent on a NK cell-dependent graft versus tumor effect in haplo-stem cell transplantation in pediatric solid tumors (24). A better molecular knowledge of immune escape strategies in these settings will probably allow further improvement of promising approaches using combination therapies.
The important role of NKp30 and its ligands in tumor immunology is emerging and we just begin to understand how the NKp30/NKp30-L system works and how it impacts on tumor development.
Acknowledgements
Funding: This work was supported by the Deutsche Forschungsgemeinschaft (grant KFO286, RP4 to E.P.v.S) and by the Deutsche Jose Carreras Leukämie-Stiftung e.V. (grant to E.P.v.S.).
Footnotes
Provenance: This is a Guest Editorial commissioned by Guest Editor Zhao-Hui Huang, MD, PhD (Oncology Institute, The Affiliated Hospital of Jiangnan University, Wuxi, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Horton NC, Mathew PA. NKp44 and Natural Cytotoxicity Receptors as Damage-Associated Molecular Pattern Recognition Receptors. Front Immunol 2015;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iannello A, Raulet DH. Immune surveillance of unhealthy cells by natural killer cells. Cold Spring Harb Symp Quant Biol 2013;78:249-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam AR, Le Bert N, Ho SS, et al. RAE1 ligands for the NKG2D receptor are regulated by STING-dependent DNA sensor pathways in lymphoma. Cancer Res 2014;74:2193-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanier LL. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol Res 2015;3:575-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mistry AR, O'Callaghan CA. Regulation of ligands for the activating receptor NKG2D. Immunology 2007;121:439-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloess S, Huenecke S, Piechulek D, et al. IL-2-activated haploidentical NK cells restore NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by scavenging of plasma MICA. Eur J Immunol 2010;40:3255-67. [DOI] [PubMed] [Google Scholar]
- 7.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol 2002;169:4098-102. [DOI] [PubMed] [Google Scholar]
- 8.Schlecker E, Fiegler N, Arnold A, et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res 2014;74:3429-40. [DOI] [PubMed] [Google Scholar]
- 9.Waldhauer I, Goehlsdorf D, Gieseke F, et al. Tumor-associated MICA is shed by ADAM proteases. Cancer Res 2008;68:6368-76. [DOI] [PubMed] [Google Scholar]
- 10.Waldhauer I, Steinle A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res 2006;66:2520-6. [DOI] [PubMed] [Google Scholar]
- 11.Liu G, Lu S, Wang X, et al. Perturbation of NK cell peripheral homeostasis accelerates prostate carcinoma metastasis. J Clin Invest 2013;123:4410-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raffaghello L, Prigione I, Airoldi I, et al. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia 2004;6:558-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castriconi R, Dondero A, Bellora F, et al. Neuroblastoma-derived TGF-β1 modulates the chemokine receptor repertoire of human resting NK cells. J Immunol 2013;190:5321-8. [DOI] [PubMed] [Google Scholar]
- 14.Delahaye NF, Rusakiewicz S, Martins I, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 2011;17:700-7. [DOI] [PubMed] [Google Scholar]
- 15.Pogge von Strandmann E, Simhadri VR, von Tresckow B, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 2007;27:965-74. [DOI] [PubMed] [Google Scholar]
- 16.Reiners KS, Topolar D, Henke A, et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 2013;121:3658-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandt CS, Baratin M, Yi EC, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med 2009;206:1495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semeraro M, Rusakiewicz S, Minard-Colin V, et al. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci Transl Med 2015;7:283ra55. [DOI] [PubMed]
- 19.Reiners KS, Kessler J, Sauer M, et al. Rescue of impaired NK cell activity in hodgkin lymphoma with bispecific antibodies in vitro and in patients. Mol Ther 2013;21:895-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simhadri VR, Reiners KS, Hansen HP, et al. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One 2008;3:e3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besse B, Charrier M, Lapierre V, et al. Dendritic Cell-derived Exosomes as Maintenance Immunotherapy after First Line Chemotherapy in NSCLC. Oncoimmunology 2015. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Guo H, Geng J, et al. Tumor-released Galectin-3, a soluble inhibitory ligand of human NKp30, plays an important role in tumor escape from NK cell attack. J Biol Chem 2014;289:33311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veschi V, Petroni M, Bartolazzi A, et al. Galectin-3 is a marker of favorable prognosis and a biologically relevant molecule in neuroblastic tumors. Cell Death Dis 2014;5:e1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Martínez A, de Prada Vicente I, Fernández L, et al. Natural killer cells can exert a graft-vs-tumor effect in haploidentical stem cell transplantation for pediatric solid tumors. Exp Hematol 2012;40:882-91.e1. [DOI] [PubMed]


