Abstract
Background
To study the change of cardiac-specific microRNA-208 (miRNA-208) level in acute myocardial infarction (AMI) patients and to explore the role of miRNA-208 in AMI progression.
Methods
The consecutive subjects including 42 AMI patients, 22 patients with unstable angina (UA), and 40 healthy subjects in our hospital from January 2013 to December 2013 were enrolled in this study. The peripheral miRNA-208 level was measured with real-time reverse-transcription polymerase chain reaction (RT-PCR), and the plasma cardiac troponin I (cTnI) and creatine kinase-MB (CK-MB) levels were determined using electrogenerated chemiluminescence (ECL) method. Patients in the AMI group were further grouped according to the number of stenosed coronary vessels and primary percutaneous coronary intervention (PCI) or not, and the difference in peripheral miRNA-208 level among these subgroups was analyzed.
Results
The miRNA-208 level was significantly higher in AMI group than in UA group and healthy controls immediately after admission (P<0.01). In the AMI patients, the plasma miRNA-208 level had a positive correlation with serum cTnI level (r=0.700, P=0.000). In 24 AMI patients who had undergone coronary angiography, the expression of miRNA-208 was significantly higher in patients with two or three stenosed coronary vessels. In 17 AMI patients who had successfully received emergent PCI treatment, the 24-h plasma miRNA-208 level was significantly lower than that immediately after admission (P<0.01).
Conclusions
The peripheral plasma miRNA-208 level remarkably increases after cardiac infarction and may dramatically change along with the increase of the myocardial damage. Thus, it may be a new biomarker for AMI.
Keywords: MicroRNA-208 (miRNA-208), acute myocardial infarction (AMI), biomarker
Introduction
MicroRNAs are a group of small, highly conserved non-coding RNA molecules. Composed of more than 20 nucleotides, microRNAs regulate gene expressions by suppressing the translation of the messenger RNA of target genes or by promoting mRNA degradation (1). Recent studies have demonstrated the presence of circulating microRNAs in human peripheral blood. These microRNAs are a class of potential biomarkers and may provide key information for the diagnosis of common diseases including tumors (2), tissue damage (3), and autoimmune diseases (4).
The circulating microRNAs are very stable and have shown potentials as the biomarkers of cardiovascular diseases (5). A recent study has recommended to using microRNA-1, microRNA-133, microRNA-208 (miRNA-208), and microRNA-499 as the valuable biomarkers for acute myocardial infarction (AMI) (6). Some earlier studies have shown that miRNA-208 is specifically expressed in myocardial cells and thus may serve as a potential biomarker for myocardial injury. The subsequent clinical studies also found that miRNA-208 may have a certain diagnostic value for AMI (7,8). However, the relevant findings and conclusions remain controversial. Therefore, more research evidences are required. In our current study, by analyzing the change of plasma miRNA-208 levels before and after coronary angiography and percutaneous coronary intervention (PCI) in AMI patients, we tried to further demonstrate the potential of miRNA-208 as a biomarker for AMI treatment and evaluation.
Materials and methods
Materials
The consecutive subjects including 42 AMI patients, 22 patients with unstable angina (UA), and 40 healthy subjects in our hospital from January 2013 to December 2013 were enrolled in this study. The inclusion criteria were as follows: The diagnostic criteria of AMI were based on the universal definition of myocardial infarction jointly established by European Society of Cardiology (ESC), American Heart Association (AHA), American College of Cardiology Foundation (ACCF), and World Heart Federation (WHF) (9). Patients with chronic liver and/or kidney diseases were ruled out in this study. Twenty healthy subjects who received health check-up during the same period were enrolled as the normal control group. This study was approved by the institutional review board of Chuzhou First People’s Hospital. Informed content forms were obtained from all the subjects.
Blood specimens were collected within 12 and 24 h after the symptom onset in the AMI group and were collected immediately after admission in UA group. In the normal control group, fasting blood samples were collected early in the morning. All blood samples were collected using EDTA-containing tubes. Thus, the blood was centrifuged at 4 °C at 3,000 rpm for 10 min to separate plasma. The supernatants were obtained and then put in RNA enzyme-free EP tubes and stored in a −80 °C freezer.
Data collection
The demographic and clinical data including age, sex, height, weight, smoking status, history of diabetes/hypertension, blood lipids, and creatinine were collected. In the AMI group, any record of PCI and/or coronary angiography was also collected.
Determination of miRNA-208
Plasma total RNA was extracted using Qiagen’s miRNeasy RNA isolation kit, followed by the reverse transcription using Taq-Man miRNA Reverse Transcription kit (Applied BioSystems). Finally, the plasma miRNA-208 and U6 levels were determined using the miRNA-208 and U6 detection kit (Applied BioSystems). The tests repeated three times for each sample. Relative miRNA-208 expression was calculated using the 2−∆∆CT method and then applied for log2−∆∆CT conversion (10,11). ∆∆CT = intervention group (CTMicroRNA-208-CTu6) − control group (CT MicroRNA-208-CTu6). Here the −∆∆CT was applied to represent the relative expression level of miRNA-208.
Determination of plasma cardiac troponin I (cTnI) and creatine kinase-MB (CK-MB) levels
OLYMPUS AU5421 automated biochemical analyzer was used to determine the CK-MB and cTnI levels.
Statistical analysis
Categorical variables were compared using Chi square test or Fisher exact test. Continuous variables are expressed as means ± standard deviations. Univariate analysis of variance or Kruskal-Wallis H test was applied to compare the differences among multiple groups. Differences in paired sample means was compared using paired sample t test, whereas comparisons between two groups were based on LSD-t method. A P value of <0.05 was considered statistically significant.
Results
General data of the subjects
This study enrolled 42 AMI patients, 22 UA patients, and 20 healthy subjects. Their data are summarized in Table 1. The baseline clinical features (e.g., gender and age) showed no significant difference among these three groups (P>0.05).
Table 1. Baseline characteristics of the patients and healthy control.
| Groups | AMI group | UA group | Normal control group | P value |
|---|---|---|---|---|
| Sample size (n) | 42 | 22 | 20 | – |
| Age (years) | 69.2±7.4 | 67.4±5.4 | 68.1±6.3 | 0.560# |
| Gender (male/female) | 34/8 | 19/3 | 14/6 | 0.418# |
| History of hypertension (%) | 26 (61.9) | 10 (45.5) | 2 (10.0) | 0.001# |
| History of diabetes (%) | 9 (21.4) | 6 (27.2) | 0 (0) | 0.079# |
| Smoking history (%) | 26 (61.9) | 12 (54.5) | 7 (35) | 0.138# |
| TC (mmol/L) | 4.12±0.99 | 4.06±1.11 | 3.72±0.89 | 0.344# |
| TG (mmol/L) | 1.69±0.82 | 1.95±1.19 | 1.44±0.78 | 0.211# |
| HDL (mmol/L) | 1.11±0.27 | 1.12±0.36 | 1.23±0.41 | 0.393# |
| LDL (mmol/L) | 2.69±0.96 | 2.57±1 | 2.20±0.81 | 0.159# |
| Creatinine (μmol/L) | 71.6±13.47 | 69.2±14.52 | 67.4±11.5 | 0.483# |
#, the overall P value of three groups (normal control group, UA group, and AMI group). AMI, acute myocardial infarction; UA, unstable angina; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Abundance of plasma miRNA-208 in acute myocardial infarction (AMI) patients
As shown in Figure 1, the plasma miRNA-208 level was significantly higher in AMI group than in UA group and normal control group (both P<0.01).
Figure 1.
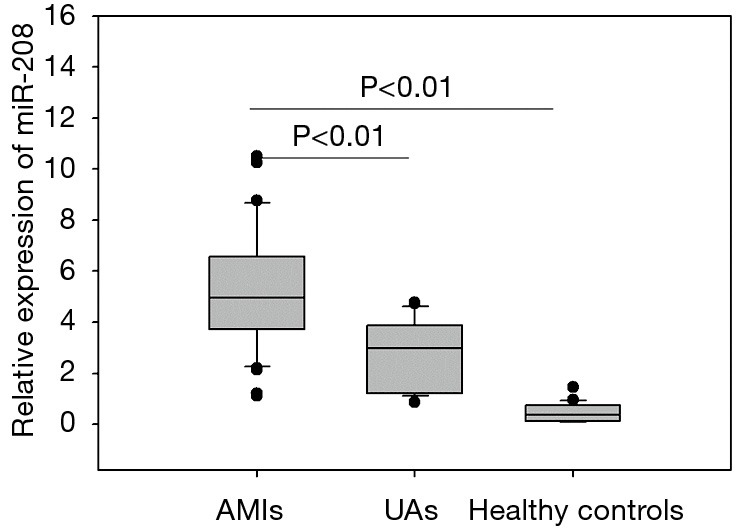
Plasma microRNA-208 levels in the patients.
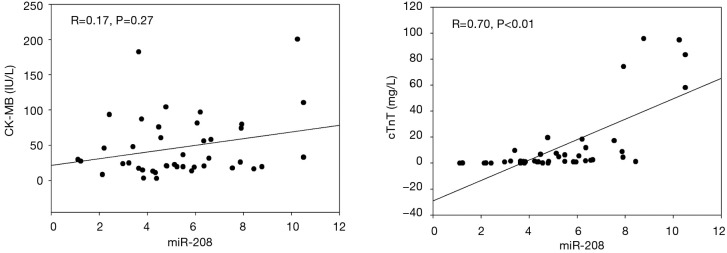
Correlation of miRNA-208 with cardiac troponin (cTn) and creatine kinase-MB (CK-MB)
Correlation analysis showed that miRNA-208 had significant correlation with cTnT (r=0.700, P=0.000) but not with CK-MB (r=0.27, P=0.17) (Figure 2).
Figure 2.
The correlations between microRNA-208 and concentrations of cTn and CK-MB. cTnT, cardiac troponin T; CK-MB, creatine kinase-MB.
Impact of different coronary lesions on miRNA-208 level in acute myocardial infarction (AMI) patients
Of these 42 AMI patients, 24 had undergone coronary angiography. These 24 patients were divided into three subgroups according to the number of stenosed coronary vessels (n=1, 2, or 3), in which the relative expression level of miRNA-208 was 3.49±1.76, 5.74±2.76, and 7.51±2.4, respectively. Obviously, the plasma miRNA-208 level was significantly higher in AMI patients with 2 or 3 stenosed coronary vessels than those in AMI patients with 1 stenosed coronary vessel (both P<0.01) (Figure 3).
Figure 3.
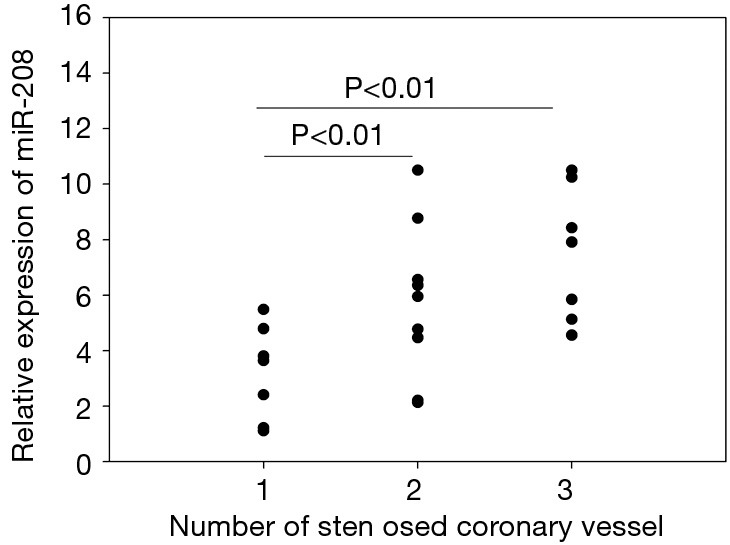
Relationship between microRNA-208 and the severity of coronary atherosclerosis.
Change of miRNA-208 level before and after percutaneous coronary intervention (PCI)
Of these 42 AMI patients, 17 successfully underwent emergency PCI. As shown in Figure 4, the relative expression level of circulating miRNA-208 was (2.81±1.19) 24 h after PCI, which was significantly lower than that (6.5±2.3) immediately after admission (P<0.01).
Figure 4.
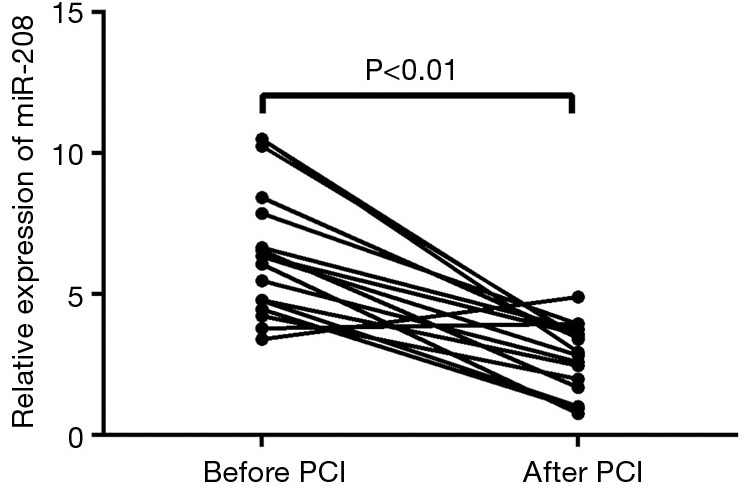
microRNA-208 expression in AMI patients after emergency PCI. AMI, acute myocardial infarction; PCI, percutaneous coronary intervention.
Discussion
Timely and rapid diagnosis and treatment of myocardial infarction is crucial. While myocardial infarction biomarkers such as cTnT and CK-MB still play irreplaceable roles in AMI management, they are still not sufficient for the accurate diagnosis and treatment of this fatal condition. Therefore, it is necessary to search for new myocardial infarction biomarkers, so as to further optimize the management of AMI.
In one of our previous studies (12), we have explored the early diagnostic value of microRNA-499 for AMI and found that microRNA-499 was a useful biomarker for the diagnosis of AMI and could provide information beyond cTnI; in fact, the combination of microRNA-499 and cTnT could improve the diagnostic accuracy of AMI. Our current study was designed to investigate whether miRNA-208 could reflect the change of disease conditions and serve as a biomarker for disease monitoring. It was found that, within 12 h after symptom onset, the plasma miRNA-208 level was significantly higher in the AMI group than in UA group and normal control group; meanwhile, it was positively correlated with the expression levels of CK-MB and cTnI. These findings were consistent with Bostjancic et al.’s study, which showed that the miRNA-208 level significantly increased 12 h after Myocardial infarction (13). Therefore, miRNA-208 can be a good biomarker for AMI. In addition, we found the plasma miRNA-208 level was also higher in UA patients than in normal controls, which may be explained by the contraction of ischemic myocardial tissue, which increases the release of miRNA-208, in UA patients.
We also found that the miRNA-208 level was higher in patients with 2 or 3 stenosed coronary vessels than in those with only one stenosed coronary vessel, indicating that miRNA-208 may be predictive for the degree of coronary injury. The possible mechanism may be as follows: the rupture of unstable atherosclerotic plaques formed during AMI can cause the bleeding of coronary artery, and endovascular thrombosis can further block the vessel; finally, the myocardial cells become necrotic due to acuter persistent ischemia and hypoxia.
Finally, we found the miRNA-208 level remarkably decreased 24 h after PCI in AMI patients. Thus, successful clinical interventions can lower miRNA-208 expression in AMI patients, suggesting that miRNA-208 is useful for assessing the effective myocardial reperfusion following myocardial ischemia in AMI patients and thus is valuable for the evaluation of early clinical interventions. Therefore, miRNA-208 can play a role in monitoring clinical conditions.
In summary, circulating miRNA-208 is a useful biomarker for the diagnosis and treatment of myocardial infarction and can provide new evidences in the management of AMI.
Acknowledgements
Funding: This work was funded by Science and Technology Projects of Wuxi City (YGM1119), Clinical Technology Foundation of Jiangsu Province (BL2012042), and National Natural Science Foundation of China (81301503).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ambros V. The functions of animal microRNAs. Nature 2004;431:350-5. [DOI] [PubMed] [Google Scholar]
- 2.Bartels CL, Tsongalis GJ. MicroRNAs: novel biomarkers for human cancer. Clin Chem 2009;55:623-31. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Jia Y, Zheng R, et al. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem 2010;56:1830-8. [DOI] [PubMed] [Google Scholar]
- 4.Carlsen AL, Schetter AJ, Nielsen CT, et al. Circulating microRNA expression profiles associated with systemic lupus erythematosus. Arthritis Rheum 2013;65:1324-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta SK, Bang C, Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet 2010;3:484-8. [DOI] [PubMed] [Google Scholar]
- 6.Rayner K, Dimmeler S, Calin GA, et al. Novel biomarkers for acute myocardial infarction: is microRNA the new kid on the block? Clin Chem 2014;60:812-7. [DOI] [PubMed] [Google Scholar]
- 7.Widera C, Gupta SK, Lorenzen JM, et al. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol 2011;51:872-5. [DOI] [PubMed] [Google Scholar]
- 8.Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol Pharmacol 2011;80:558-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007;116:2634-53. [DOI] [PubMed] [Google Scholar]
- 10.Wang GK, Zhu JQ, Zhang JT, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 2010;31:659-66. [DOI] [PubMed] [Google Scholar]
- 11.Devaux Y, Vausort M, Goretti E, et al. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin Chem 2012;58:559-67. [DOI] [PubMed] [Google Scholar]
- 12.Han ZJ, Shi WQ, Shen HY, et al. Diagnostic performance of plasma miR-499 for acute myocardial infarction. J Chin J Lab Med 2013;36:1096-9. [Google Scholar]
- 13.Bostjancic E, Zidar N, Stajer D, et al. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology 2010;115:163-9. [DOI] [PubMed] [Google Scholar]