Abstract
To determine if age-associated vascular dysfunction in older adults with heart failure (HF) is due to insufficient synthesis of nitric oxide (NO), we performed two separate studies: 1) a kinetic study with a stable isotope tracer method to determine in vivo kinetics of NO metabolism, and 2) a vascular function study using a plethysmography method to determine reactive hyperemic forearm blood flow (RH-FBF) in older and young adults in the fasted state and in response to citrulline ingestion. In the fasted state, NO synthesis (per kg body wt) was ∼50% lower in older vs. young adults and was related to a decreased rate of appearance of the NO precursor arginine. Citrulline ingestion (3 g) stimulated de novo arginine synthesis in both older [6.88 ± 0.83 to 35.40 ± 4.90 μmol·kg body wt−1·h−1] and to a greater extent in young adults (12.02 ± 1.01 to 66.26 ± 4.79 μmol·kg body wt−1·h−1). NO synthesis rate increased correspondingly in older (0.17 ± 0.01 to 2.12 ± 0.36 μmol·kg body wt−1·h−1) and to a greater extent in young adults (0.36 ± 0.04 to 3.57 ± 0.47 μmol·kg body wt−1·h−1). Consistent with the kinetic data, RH-FBF in the fasted state was ∼40% reduced in older vs. young adults. However, citrulline ingestion (10 g) failed to increase RH-FBF in either older or young adults. In conclusion, citrulline ingestion improved impaired NO synthesis in older HF adults but not RH-FBF, suggesting that factors other than NO synthesis play a role in the impaired RH-FBF in older HF adults, and/or it may require a longer duration of supplementation to be effective in improving RH-FBF.
Keywords: aging, arginine, endothelial dysfunction, stable isotope tracers
regulation of peripheral blood flow is impaired in older individuals. This condition is commonly referred to as endothelial dysfunction (12). Endothelial dysfunction can occur in older adults (20) and is more pronounced in older adults with heart failure (HF) (35). It is thought that reductions in NO synthesis via endothelial nitric oxide synthase (eNOS) or NO bioavailability play a key role in endothelial dysfunction (21, 36). The importance of NO in endothelial dysfunction is supported by the results of many previous studies using various techniques, including strain gauge plethysmography (35) and Doppler ultrasound (20), in combination with pharmacological treatments. Several potential mechanisms have been suggested to explain endothelial dysfunction. These include impaired NO synthesis, decreased bioavailability resulting from increased production of reactive oxygen species (ROS) (12, 45), increased eNOS inhibitors such as asymmetric dimethylargine (AMDA) (42), increased arginase activity (7, 39), and decreased NOS cofactors including tetrahydrobiopterin (BH4) (19, 46). It is also possible that NO synthesis is limited by reduced bioavailability of arginine (7), the sole precursor of NO synthesis, with advancing age.
Arginine is a “conditionally” essential amino acid. Under normal circumstances, the rate of endogenous arginine synthesis from citrulline, i.e., de novo arginine synthesis, is sufficient to match the rate of degradation of arginine as well as the body's demand for various metabolic pathways. These pathways include the synthesis of agmatine, creatinine, protein, and NO synthesis (27). However, in a number of clinical circumstances such as sepsis (29) and mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome (16), de novo arginine synthesis is insufficient, with a resulting impairment in de novo NO synthesis. Arginine insufficiency can be restored by arginine infusion or dietary supplementation with arginine or citrulline, the precursor of endogenous arginine production, in those clinical conditions. Consistent with this notion, it was shown in short-term studies (∼2 mo) that oral arginine (24, 37) or citrulline supplementation (37) improved heart or vascular function. Furthermore, a recent meta-analysis suggests that arginine supplementation (typically, for 2–4 wk) improved vascular function, assessed by flow-mediated dilation (FMD), in adults with poor baseline FMD (6). Despite these abundant data implicating reduced NO synthesis (or bioavailability) in various clinical settings, NO kinetics in older HF adults has not been previously quantified.
Therefore, we hypothesized 1) that NO synthesis is limited in both young healthy subjects and, to a greater extent, in older individuals with HF by availability of precursor, and we therefore proposed that increasing precursor (i.e., arginine) availability by means of citrulline ingestion would stimulate NO synthesis in the young and to a greater extent in older HF adults; and 2) that stimulation of NO synthesis would increase peripheral vascular function in young, healthy individuals and, to a greater extent, in older individuals with HF. We used citrulline to test the effect of increasing arginine availability, because citrulline is the precursor of de novo arginine synthesis and is normally more effective than ingestion of arginine in increasing plasma arginine concentration (5). The greater effectiveness of citrulline results from the extensive clearance by the gut and liver of orally ingested arginine (5). There is also a practical advantage to citrulline, since a large dose of arginine can cause gastric distress (23).
SUBJECTS AND METHODS
Subjects
We performed two separate studies: 1) the kinetic study and 2) the vascular function study. In the kinetic study, eight healthy young and eight older HF adults participated, and in the vascular function study, seven healthy young and seven older HF adults participated (Table 1). In both studies, a battery of medical tests was performed on their first visit to the laboratory to determine study eligibility. Tests and procedures included medical history, blood count, plasma electrolytes, blood glucose concentration, and liver and renal function tests. Written informed consent was obtained from all subjects, and the study was approved by the Institutional Review Board at the University of Arkansas for Medical Sciences. Inclusion criteria for both studies were male or female aged 21–40 yr for the young or >60 yr for the older adults, diagnosed with stage 2 or 3 HF according to NYHA classification. Older HF adults were taking several medications for heart failure therapy, listed in Table 1. Exclusion criteria included current use of insulin for treatment of diabetes mellitus, history of tobacco use in the past 3 mo, or participation in a regular exercise program.
Table 1.
Subject characteristics and medications for heart failure therapy
| Kinetic Study |
Peripheral Vascular Function Study |
|||
|---|---|---|---|---|
| Older | Young | Older | Young | |
| Subject characteristics | ||||
| Subjects (M/F) | 8(2/6) | 8(5/3) | 7(2/5) | 7(5/2) |
| Age, yr | 76.6 ± 3.1 | 27.0 ± 1.1* | 81 ± 4 | 26 ± 2* |
| Weight, kg | 92.3 ± 3.6 | 69.6 ± 4.6* | 78.8 ± 5.5 | 78.9 ± 9.8 |
| BMI, kg/m2 | 35.2 ± 1.9 | 21.9 ± 0.8* | 29.0 ± 1.9 | 25.6 ± 2.4 |
| FFM, kg | 51.0 ± 2.8 | 51.4 ± 3.4 | N/M | N/M |
| Fat mass, % | 44.8 ± 2.0 | 25.9 ± 2.2* | N/M | N/M |
| eGRF, ml/min/1.73m2 | 75.6 ± 9.8 | N/M | 53.4 ± 12.0 | N/M |
| Medications for heart failure therapy (% of subjects) | ||||
| ACE inhibitor | 25 | 29 | ||
| Beta blocker | 50 | 43 | ||
| Diuretic | 75 | 57 | ||
| Statin | 38 | 43 | ||
| Aspirin | 50 | 43 | ||
| CCB | 25 | 29 | ||
| Isosorbide nitrate | 38 | 29 | ||
| Clopidogrel | 0 | 14 | ||
Values for characteristics are means ± SE. M/F, no. of male and female subjects; BMI, body mass index; FFM, fat-free mass; CCB, calcium channel blocker; nitrate, isosorbide mono- or dinitrate; eGFR, estimated glomerular filtration rate; N/M, not measured.
Significantly different from older adults with the same study (P < 0.05).
Experimental Protocol
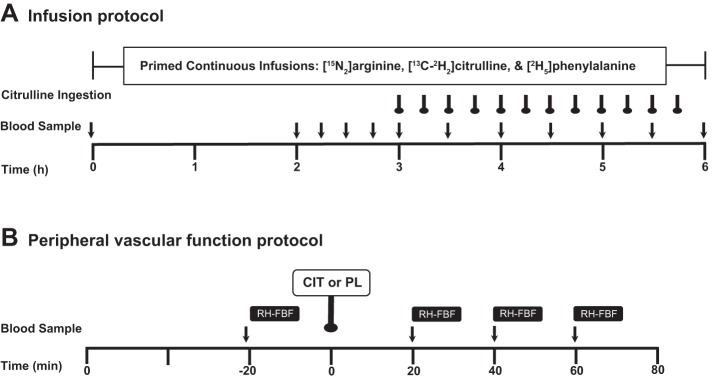
Subjects were instructed to maintain their normal diet and to abstain from vigorous physical activity for >72 h before the start of their study. Subjects were studied in the Reynolds Institute on Aging (RIOA) at the University of Arkansas for Medical Sciences. During the screening process for the kinetic study, dual-energy X-ray absorptiometry (QDR-4500A; Hologic, Waltham, MA) was performed for determination of fat-free mass. In the kinetic study, after an overnight fast an isotope tracer infusion study was performed in both the basal state and during citrulline ingestions over a 6-h experimental time period. Subjects remained in the fasted state for the first 3 h and then consumed a small amount (250 mg/100 ml) of citrulline dissolved in distilled water every 15 min for the next 3 h (3 g total) while isotope tracers were primed and continuously infused throughout the 6-h time period (see Tracer Infusion Protocol). Blood samples were obtained before and during the tracer infusion period (Fig. 1). Blood samples were analyzed for determination of plasma isotopic enrichment using liquid chromatography-mass spectrometry as described previously (15, 18, 28). In the vascular function study, each subject was studied (see Vascular function measurement protocol) on two occasions in a randomized placebo controlled crossover experimental design with >2-day interval between the treatments (Fig. 1). Upon their arrival to the IOA after an overnight fast, subjects rested supine for ≥40 min on a bed. Then, forearm blood flow (FBF) was determined at baseline and following 3-min arterial occlusion 20 min before (basal fasted state) and 20, 40, and 60 min after ingestion of either 10 g of citrulline or placebo (equal parts l-alanine-l-glycine-l-serine).
Fig. 1.
Experimental protocols. A: in the kinetic study, primed continuous infusions of tracers of arginine, citrulline, and phenylalanine were performed to determine NO and its precursor metabolism and protein breakdown in the fasted state and in response to citrulline sip ingestions. B: in the peripheral vascular function study, reactive hyperemic forearm blood flow responses (RH-FBF) were determined in the fasted state and in response to a bolus ingestion (10 g) of citrulline (CIT) or placebo (equal parts serine, glycine, and alanine).
Tracer Infusion Protocol
The 6-h isotope tracer infusion protocol is presented in Fig. 1A. On the infusion study day, subjects reported to the IOA after an overnight (after 2200) fast. Two 18-gauge catheters were inserted, one into a forearm vein for infusion of three stable-isotope tracers and the other into a forearm vein for arterialized venous blood sampling using a heating box (1). Before the initiation of the tracer infusion, a baseline blood sample was collected to determine background isotopic enrichments and blood amino acid concentrations. For determination of kinetics of NO, arginine and citrulline at the whole body level, primed continuous infusions of l-[guanidine-15N2]arginine (prime, 3.65 μmol/kg; infusion rate, 3.75 μmol·kg−1·h−1) and l-[ureido-13C,5,5-2H2]citrulline (prime, 0.88 μmol/kg; infusion rate, 0.45 μmol·kg−1·h−1) were given. Infusion rates of the citrulline tracer during the citrulline ingestions were increased to 0.675 μmol·kg−1·h−1 (1.5-fold) to minimize the diluting effect of unlabeled citrulline ingestion on citrulline enrichment in the blood. Since protein breakdown is the major source of the systemic appearance of arginine (∼80%) (27), we additionally infused l-[ring-2H5]phenylalanine tracer with a priming dose (prime, 2.19 μmol/kg; infusion rate, 3.60 μmol·kg−1·h−1) to quantify the contribution of protein breakdown to the systemic appearance of arginine. All isotope tracers were purchased from Cambridge Isotopes (Andover, MA). Blood samples were taken at specific times: 0, 120, 135, 150, 165, 180, 210, 240, 270, 300, 330, and 360 min to ascertain changes in tracer enrichment and to measure plasma concentrations of amino acids. A total of 12 blood samples were taken during the stable isotope infusion study (∼100 ml).
Vascular Function Measurement Protocol
To determine peripheral endothelial function, forearm blood flow (FBF) following arterial occlusion was measured using the strain gauge plethysmography technique (AI6 Arterial Inflow system; Hokanson, Bellevue, WA) according to a previous study, with a slight modification (38) (Fig. 1B). Briefly, each FBF measurement consisted of 7-s inflation and then 8-s deflation at the upper arm; FBF was measured during the 7 s of inflation. The cuff on the upper arm was inflated to 50 mmHg pressure, which allows arterial blood inflow but prevents venous blood outflow. First, baseline FBF measurements were obtained for 2 min. Following a 4-min rest, the pressure cuff at the upper arm was inflated to a pressure 30 mmHg higher than the baseline systolic pressure of subjects (suprasystolic pressure) to completely occlude arterial flow for 3 min. FBF measurement (the same 7-s inflation/8-s deflation cycle) was repeated after the end of the 3-min arterial occlusion (reactive hyperemic forearm blood flow, RH-FBF). After the fasted FBF measurements, subjects ingested 10 g of citrulline or placebo dissolved in 100 ml of plain water over 1 min. The placebo was also a total of 10 g of amino acids (equal parts serine, glycine, and alanine). The FBF protocol was repeated every 20 min following the ingestion of the citrulline or placebo. Blood samples were collected before each FBF measurement: 20 min before and 20, 40, and 60 min after the ingestion of the citrulline or placebo.
Analytic Methods
Plasma samples were analyzed as previously described (15, 18, 28). Briefly, plasma samples were precipitated with 10% sulfosalicylic acid (SSA) containing internal standards of stable isotopomers with a high mass (>M+5) to avoid issues of overlapping spectra contribution to the isotope tracers infused, followed by centrifugation. Plasma free amino acids were first extracted by cation exchange chromatrography (Strata-X-C; Phenomenext, Torrance, CA) and dried under Speed Vac (Savant Instruments, Farmingdale, NY). Enrichments of arginine, citrulline, and phenylalanine and amino acid concentrations were determined on the 9-fluorenylmethoxycarbonyl (FMOC) derivatives using liquid chromatography-electron spray ionization-mass spectrometry (QTrap 5500 MS; AB Sciex) with liquid chromatography device ExpressHT Ultra LC (Eksigent Div; AB Sciex). Plasma insulin concentrations in the kinetic study were determined before (at 180 min) and during citrulline ingestion (at 210, 240, and 300 min) by means of a commercially available human insulin ELISA kit (Alpco Diagnostics).
Calculations
Whole body kinetics.
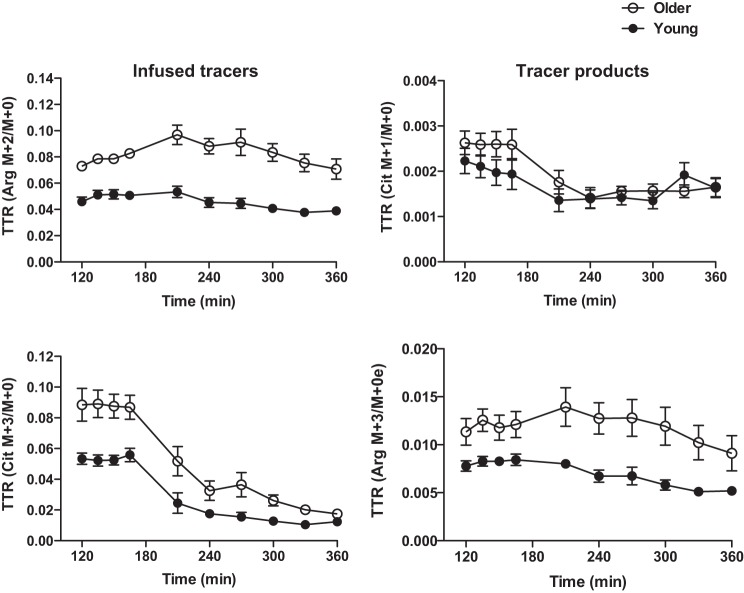
Calculations of whole body (WB) kinetics were performed based on the determinations of the rate of appearance (Ra) into the plasma of arginine (or phenylalanine for determination of protein breakdown rate) and citrulline and the fraction of Ra of endogenous citrulline or arginine derived from arginine or citrulline, respectively (13, 44). All the kinetic measurements were normalized to kilograms of body weight or of fat-free mass (FFM). Plasma enrichments of tracers of arginine and citrulline either infused or derived (Fig. 2) were averaged for the calculation for the fasted values and were curve-fitted with a polynomial method using Graphad Prism 5 for Mac (Graphpad Software, La Jolla, CA) for calculation of values during citrulline ingestion. In the fasted state, Ra of arginine, citrulline, and phenylalanine were calculated as infusion rates (F, μmol·kg−1·h−1) of l-[guanidine-15N2]arginine, l-[ureido-13C-5,5-2H2]citrulline, and l-[ring-2H5]phenylalanine divided by arterial plateau enrichments (TTR) of the respective tracers:
Fig. 2.
Plasma enrichments of infused tracers and compounds derived from infused tracers before and during sip ingestions of citrulline in the kinetic study. Tracer infusion rates (μmol·kg body wt−1·h−1) were the same for both age groups. Data are presented as means ± SE.
During citrulline ingestion, the Steele equation (41) modified for stable-isotope tracer (44) was used to determine Ra of arginine (Ra ARG) and citrulline (Ra Cit) (μmol·kg−1·h−1) in the non-steady-state kinetics calculations:
where F represents the infusion rate of tracer; pV is the effective volume of distribution for substrate of interest, for which 40 ml/kg was used (44); C1 and C2 are plasma concentrations of substrate of interest at specific times t1 and t2, respectively, and E1 and E2 are plasma enrichment of arginine or citrulline tracers at specific times t1 and t2, respectively.
De novo arginine synthesis.
Whole body de novo synthesis rate of arginine from citrulline (de novo arginine, μmol·kg−1·h−1) was calculated as the product of WB Ra Arg and the fraction of WB Ra Arg derived from citrulline, as described by Castillo et al. (13):
where ArgM+3 is [13C-2H2]arginine derived from the infused [13C-2H2]citrulline.
NO synthesis.
Whole body NO synthesis rate (μmol·kg−1·h−1) was determined from the calculation of whole body citrulline synthesis from arginine, since NO and citrulline are produced from arginine at a 1:1 stoichiometry via NOS. Thus, the rate of whole body citrulline synthesis from arginine (= rate of NO synthesis) is calculated as the product of WB Ra Cit and the fraction of Ra Cit converted from arginine as described (13):
where CitM+1 is [15N1]citrulline derived from [15N2]arginine tracer infused.
Protein breakdown.
Calculations of whole body protein breakdown rate (mg·kg−1·h−1) in the fasted state and during citrulline ingestion were performed as in the case of NO kinetics for the steady state, since enrichment of phenylalanine (Phe) was not affected during the citrulline ingestions using following equations:
where EPhe is plasma plateau enrichment of l-[ring-2H5]phenylalanine, and 25 is the correction factor for the average contribution of phenylalanine to protein (8). FPhe is the infusion rate of the phenylalanine tracer.
Reactive Hyperemic Responses
Reactive hyperemic forearm blood flow responses (RH-FBF, ml/100 ml tissue volume), including baseline and 3-min responses to a 3-min arterial occlusion, were first curve-fitted with a polynomial method and then calculated as area under the curve (AUC) using Graphpad Prism 5 for Mac.
Statistical Analysis
In the kinetic study, a repeated-measures analysis of variance (RM-ANOVA) model was used to evaluate the fixed effects of age (Young vs. Older HF adults) and time (fasted vs. citrulline-fed states) on measures of whole body kinetics. Specifically, for the measures of NO synthesis rate and %Ra arginine to NO, log transformation was performed before the statistical analysis to normalize the data. For the statistical comparisons for plasma amino acids (the kinetic study), a RM-ANOVA model was used to evaluate the effect of age (Young vs. Older HF adults) and the effect of time (fasted and 6 postfeeding time points) on each of the amino acids.
In the vascular function study, three-factor RM-ANOVA models were used to evaluate the effects of age (Young vs. Older HF adults), time (1 pretreatment & 3 posttreatment time points), and treatment (Citrulline vs. Placebo) on RH-FBF and plasma amino acid responses. Three-way and two-way interactions between the factors were assessed and were removed from the model if their P values were greater than 0.10. Estimates of means and standard errors along with the pairwise comparisons of the age, treatment, and time effects were tabulated for each response.
For both studies, P values were adjusted for multiple comparisons using Hommel's method (25) to maintain the overall Type I error rate. Data were expressed as means ± SE. Statistical significance was declared when P value was less than the 5% level. The statistical analyses were performed using SAS (v. 9.4; SAS Institute, Cary, NC).
RESULTS
Whole Body Kinetics
NO and its precursor metabolism.
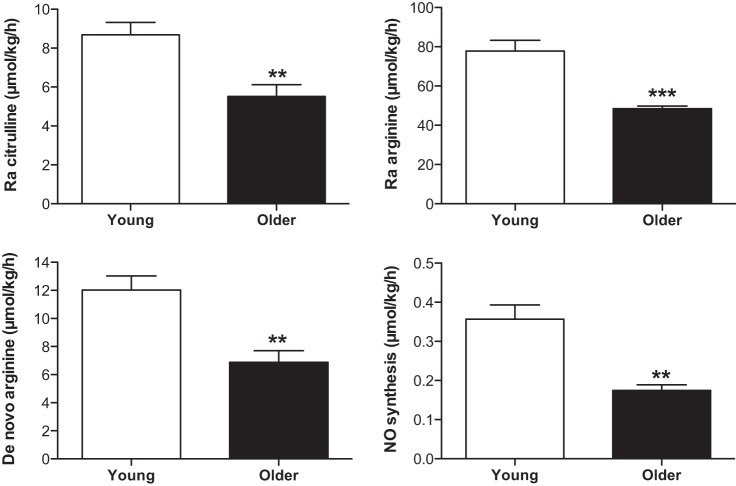
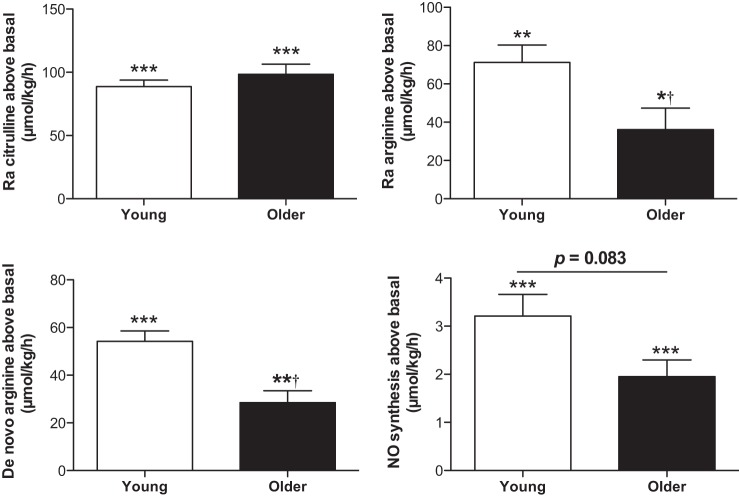
NO and its precursor kinetics (μmol/kg bw/h) are present in Table 2 and Figs. 3 and 4. In the fasted state, Ra Arg and Ra Cit, de novo arginine synthesis rate (de novo arginine), and NO synthesis rate were significantly lower in older HF adults than in young adults (for all, P < 0.01) (Fig. 3). Percentage of Ra Arg that contributed to NO synthesis (%Ra Arg to NO) tended to be lower in older HF adults than in young adults (0.37 ± 0.03 and 0.46 ± 0.05%, respectively, P = 0.0974). During the sip ingestions of citrulline (3 g total), Ra Arg, Ra Cit, de novo arginine, and NO synthesis rate were all significantly increased from the fasted state (for all, P < 0.01 for the young; for all, P < 0.05 for older HF adults) (Fig. 4). Differences between young and older HF adults in Ra Cit observed in the fasted state disappeared during the citrulline ingestion (P = 0.7080). However, Ra Arg and de novo arginine remained significantly lower in older HF adults (for both, P < 0.005). Ra Arg, directly reflecting systemic arginine availability, was consistent with arginine availability index (AAI) (43), expressed as the ratio of plasma concentrations of arginine to the sum of ornithine and lysine. AAI was not different between groups in the fasted state but significantly increased in both groups (for both, P < 0.0001) and to a greater extent in young adults (for both, P < 0.01) during the citrulline ingestion. Similarly, NO synthesis during citrulline ingestion was 41% lower in older HF adults than in young adults, although this reduction was not statistically significant (P = 0.0830). %Ra Arg to NO tended to be higher in the young than in the older HF adults at the fasted sate (P = 0.0974), and the citrulline ingestion significantly increased %Ra Arg to NO in the older (0.36 ± 0.03 to 2.49 ± 0.23%, P < 0.0001) and the young (0.46 ± 0.05 to 2.42 ± 0.32%, P < 0.0001) in a similar magnitude (P = 0.7080). Metabolic clearance rate of arginine (MCR Arg), expressed as Ra Arg divided by plasma arginine concentration, was lower in the older HF (0.82 ± 0.04 to 0.80 ± 0.06 l·kg−1·h−1) than in the young adults (0.58 ± 0.04 to 0.46 ± 0.04 l·kg−1·h−1) in the fasted state and during the citrulline ingestion, respectively, (main effect of group: P < 0.001) with no significant changes during the citrulline ingestion above the basal, fasted state (main effect of time: P = 0.1306).
Table 2.
NO and its precursor kinetics, expressed per kg body wt or FFM
| Older |
Young |
|||||||
|---|---|---|---|---|---|---|---|---|
| Fasted |
Citrulline ingestion |
Fasted |
Citrulline ingestion |
|||||
| Body Wt | FFM | Body Wt | FFM | Body Wt | FFM | Body Wt | FFM | |
| Ra arginine, | 48.44 ± 1.362 | 88.28 ± 3.09 | 84.55 ± 11.571,2 | 155.79 ± 23.251 | 77.78 ± 5.52 | 105.26 ± 7.66 | 149.01 ± 10.721 | 201.61 ± 15.191 |
| Ra citrulline | 5.52 ± 0.602 | 10.08 ± 1.19 | 103.95 ± 8.121 | 188.50 ± 14.361,2 | 8.69 ± 0.63 | 11.70 ± 0.72 | 97.38 ± 5.271 | 131.61 ± 6.641 |
| De novo arginine | 6.88 ± 0.832 | 12.54 ± 1.59 | 35.40 ± 4.901,2 | 65.51 ± 10.401 | 12.02 ± 1.01 | 16.26 ± 1.43 | 66.26 ± 4.791 | 89.85 ± 7.101 |
| NO synthesis | 0.17 ± 0.012 | 0.32 ± 0.023 | 2.12 ± 0.361,4 | 3.90 ± 0.731 | 0.36 ± 0.04 | 0.49 ± 0.05 | 3.57 ± 0.471 | 4.81 ± 0.641 |
Values are means ± SE in μmol·kg−1·h−1. 1Significantly different from the fasted state within same age group for the same unit (P < 0.05). 2Significantly different from the young within same time frame for the same unit (P < 0.05). 3,4Tended to be different from the young within same time frame for the same unit (P = 0.112 and P = 0.083, respectively).
Fig. 3.
In vivo kinetics of NO and its precursor metabolism in the fasted state. Ra arginine is derived from de novo arginine and from protein breakdown. Significantly increased from the young (**P < 0.01 and ***P < 0.001, respectively). Data are presented as means ± SE.
Fig. 4.
In vivo kinetics of NO and its precursor metabolism above the fasted state in response to sip ingestions of citrulline (3 g total). Ra arginine is derived from de novo arginine and from protein breakdown. Significantly increased from the fasted state (*P < 0.05, **P < 0.001, and ***P < 0.0001, respectively) (see Fig. 5); †significantly different from the young, P < 0.005. Data are presented as means ± SE.
The rationale for normalizing results by body weight is discussed below. Alternatively, the results could be normalized by the FFM of each individual. When the kinetics were expressed per kilogram FFM (Table 2), there were no age differences in any kinetic variable, either at rest or during the citrulline ingestion. However, citrulline ingestion significantly increased Ra Arg, Ra Cit, de novo arginine, and NO synthesis in both age groups. There was no difference in %Ra Arg to NO between groups in the fasted state and during the citrulline ingestion (for both, P > 0.288). However, the citrulline ingestion significantly increased %Ra Arg to NO in the older HF adults (0.36 ± 0.03 to 2.49 ± 0.23%, P < 0.0001) and the young adults (0.46 ± 0.05 to 2.42 ± 0.32%, P < 0.0001).
Protein breakdown.
Whole body protein breakdown rate was significantly lower in the older HF adults compared with the young adults in the fasted state (120.4 ± 7.5 vs. 163.9 ± 9.2 mg·kg body wt−1·h−1, P = 0.0014). The sip ingestions of citrulline did not significantly change the whole body protein breakdown rate in the young adults (163.9 ± 9.2 to 155.2 ± 7.1 mg·kg body wt−1·h−1, P = 0.4034) or in the older HF adults (120.4 ± 7.5 to 106.2 ± 3.0 mg·kg body wt−1·h−1 P = 0.2385). The rate of protein breakdown in the fed state remained lower in the older HF adults compared with the young during the citrulline ingestion (P = 0.0008). When the kinetics were expressed per kg FFM, whole body protein breakdown rate was not different in the fasted state and during the citrulline fed state between the older HF adults and the young adults. Citrulline ingestion slightly but significantly reduced rate of protein breakdown in the young (220.6 ± 8.5 to 209.3 ± 6.2 mg·kg FFM−1·h−1, P = 0.0259), but not in the older HF adults (220.5 ± 17.8 to 193.5 ± 6.8 mg·kg FFM−1·h−1, P = 0.241).
Reactive Hyperemic Responses
RH-FBF in the fasted state and following a bolus ingestion of citrulline (10 g) or placebo are shown in Table 3. We found a significant age effect (P = 0.009) and an age × time interaction for RH-FBF (P = 0.047). RH-FBF was significantly lower in older HF adults than in young adults in the fasted state and all posttreatment time points following the bolus ingestion of citrulline (for all, P < 0.05). However, there was no significant treatment (citrulline vs. placebo) effect in either age group (P = 0.5564).
Table 3.
Reactive hyperemic forearm blood flow responses in the fasted state and following a bolus ingestion of citrulline in the young and older adults
| Pre |
Post |
||||
|---|---|---|---|---|---|
| Treatment | −20 min | 20 min | 40 min | 60 min | |
| Young | CIT | 11.92 ± 2.18 | 12.24 ± 2.18 | 12.24 ± 2.35 | 12.20 ± 1.99 |
| PL | 11.12 ± 1.42 | 12.02 ± 1.80 | 12.23 ± 1.71 | 12.14 ± 1.34 | |
| Older | CIT | 7.33 ± 1.28 | 6.91 ± 1.68 | 5.99 ± 0.89 | 6.91 ± 1.63 |
| PL | 6.55 ± 0.60 | 6.42 ± 1.20 | 5.77 ± 1.00 | 6.01 ± 1.12 | |
Values are means ± SE. Forearm blood flow response (ml/100 ml tissue volume) is calculated area under curve of baseline and in 3-min response to 3-min arterial occlusion at upper arm before and 20, 40, and 60 min following either 10 g of citrulline (CIT) or placebo (PL, equal parts l-alanine, l-glycine, and l-serine).
Significantly different from older adults with the same study (P < 0.05). There was a significant age by time interaction (P = 0.0479). There were no treatment (P = 0.5564) and time effects (P = 0.8293); however, there was a significant age effect (P = 0.0090).
Plasma Amino Acid Concentrations
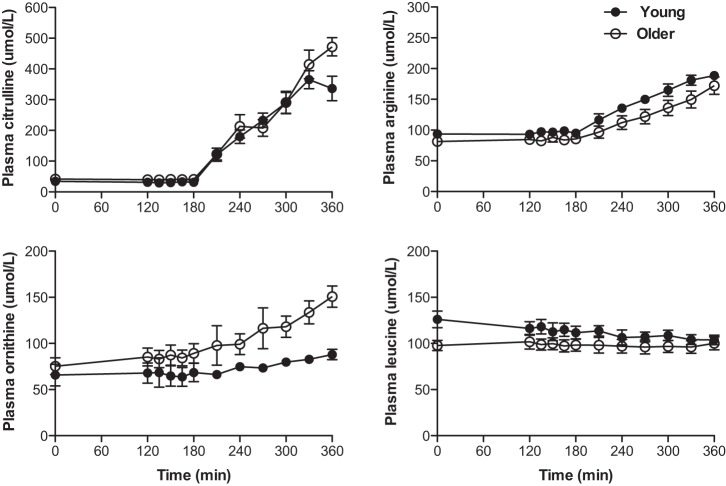
Plasma responses of citrulline, arginine, ornithine, and leucine in response to sip ingestions of citrulline (3 g total) are presented in Fig. 5. There were no differences in arginine and citrulline in the fasted state between the age groups, and the concentrations of both increased similarly during the citrulline sip ingestions. Plasma concentrations of ornithine, which is derived from arginine via the arginase enzyme reaction in the urea cycle, was not different in the fasted state but gradually increased with ingestions of citrulline in older HF (P = 0.0620) and young adults (P = 0.0208), with the older HF adults being significantly higher than the young (for the kinetic study, P < 0.01). There were no differences in leucine concentration in the fasted state between the age groups, and leucine did not significantly increase in response to the citrulline ingestion. The plasma responses of the respective amino acids in the vascular function study were similar to those in the kinetic study (Table 4). Plasma insulin than in young adults in the fasted state (11.4 ± 2.0 vs. 5.2 ± 0.9 μIU/ml) and during ingestion of citrulline (mean insulin concentrations: 11.4 ± 2.0 vs. 5.2 ± 0.9 μIU/ml) (for both, P > 0.05). However, citrulline ingestion did not significantly change plasma insulin concentrations from the fasted state to the citrulline ingestion in both groups (for both, P > 0.10).
Fig. 5.
Plasma concentrations of citrulline, arginine, ornithine, and leucine in the fasted state and during sip ingestions of citrulline in the kinetic study. There were no significant age-by-time interactions for citrulline, arginine, and ornithine. However, there were significant differences with respect to time for arginine and citrulline (for all, P < 0.0001; for ornithine P = 0.0203) and with respect to age only for ornithine (P = 0.0280). Data are presented as means ± SE.
Table 4.
Plasma responses of citrulline, arginine, ornithine, and leucine in response to 10 g citrulline
| Treatments |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citrulline |
Placebo |
||||||||
| PRE | POST20 | POST40 | POST60 | PRE | POST20 | POST40 | POST60 | ||
| Citrulline | Young | 29 ± 3 | 501 ± 1641 | 1,156 ± 1621 | 1,528 ± 1641 | 26 ± 2 | 25 ± 3 | 26 ± 3 | 31 ± 4 |
| Older | 36 ± 4 | 541 ± 1031 | 1,181 ± 1931 | 1,533 ± 1501 | 36 ± 3 | 33 ± 4 | 32 ± 4 | 34 ± 4 | |
| Arginine | Young | 97 ± 8 | 130 ± 121,3 | 172 ± 161,3 | 215 ± 141,3 | 92 ± 8 | 95 ± 8 | 97 ± 7 | 102 ± 7 |
| Older | 75 ± 6 | 96 ± 51,2 | 130 ± 81,2,3 | 172 ± 91,2,3 | 74 ± 6 | 76 ± 6 | 77 ± 6 | 79 ± 7 | |
| Ornithine | Young | 46 ± 3 | 56 ± 7 | 75 ± 101 | 98 ± 101,3 | 48 ± 7 | 51 ± 6 | 52 ± 4 | 53 ± 5 |
| Older | 61 ± 4 | 66 ± 4 | 90 ± 51,3 | 125 ± 91,3 | 76 ± 102 | 64 ± 51 | 62 ± 4 | 59 ± 5 | |
| Leucine4 | Young | 121 ± 9 | 121 ± 8 | 116 ± 10 | 106 ± 11 | 116 ± 9 | 122 ± 10 | 120 ± 8 | 120 ± 9 |
| Older | 98 ± 8 | 94 ± 8 | 90 ± 10 | 90 ± 10 | 95 ± 10 | 96 ± 7 | 98 ± 6 | 98 ± 6 | |
Values are means ± SE. PRE, POST20, POST40, and POST60 are pre- and 3 postingestion measurements at time points of −20, 20, 40, and 60 min, respectively. 1Significantly different from Pre within the same treatment of the same age group, P < 0.05. 2Significantly different from the young at the same time point within the same treatment, P < 0.05. 3Significantly different from Placebo at the same time point within age group, P < 0.05. 4The older tended to be lower vs. young at all time points, P = 0.1047.
DISCUSSION
In this study, we observed impaired NO synthesis in older HF adults primarily due to a reduction in NO precursor bioavailability. Consistent with the kinetic data, there was a corresponding reduction in reactive hyperemic blood flow response in the older HF adults. Acute ingestion of 3 g of citrulline stimulated NO synthesis ∼10-fold in both the older HF adults and the young adults. However, the reactive hyperemic blood flow response was not increased by ingestion of 10 g citrulline. These findings suggest that factors other than NO synthesis may be responsible for the reduction in peripheral vascular function in older HF adults, and/or long-term supplementation may be required to induce favorable effects. Our conclusions rely on normalization of the kinetic data to kilograms of body weight (μmol·kg body wt−1·h−1) instead of kilograms of fat free mass. It has been shown that adipose tissue blood flow is quantitatively similar to that of skeletal muscle in resting conditions, particularly in the fasted state, when expressed per tissue mass (21). Furthermore, NO has been shown to be a major regulator of preprandial adipose tissue blood flow (3) via eNOS (2, 17). Consequently, we felt it reasonable to account for fat mass in the expression of the kinetic data.
In accord with previous findings (30, 35), we also observed persistent impairments in vascular dysfunction in the older HF adults compared with the young adults. Many previous studies using various techniques including strain gauge plethysmography (35) and Doppler ultrasound (20) in combination with pharmacological treatments indicate impaired NO synthesis or bioavailability in the vascular endothelial dysfunction in older adults with or without HF. However, this paper provides the first direct evidence of a reduction in NO synthesis in vascular endothelial dysfunction in older HF adults. The reduction in NO synthesis rate in our study corresponded to a reduction in Ra Arg, indicating a deficiency of NO precursor availability in the older HF adults. Ra Arg is derived from synthesis from citrulline (i.e., de novo arginine synthesis) and from protein breakdown (27). Reduced Ra Arg in the older HF individuals may have been due, in part, to a limitation in the production of citrulline, as we found that Ra Cit was also reduced in the older HF adults compared with the young. Despite the relatively small percentage of Ra Cit derived from de novo synthesis (27), impaired citrulline synthesis may limit NO synthesis in certain clinical conditions. For example, El-Hattab et al. (16) have shown in patients with MELAS that reduced Ra Cit was accompanied by reductions in de novo arginine and NO synthesis (13), which were reversed by citrulline supplementation to rates exceeding the baseline values of the normal subjects. A reduced rate of protein breakdown also contributed to the lower Ra Arg in the older HF adults. Since the rate of protein breakdown was not different between groups when the data were expressed per kilogram of FFM, the reduced total lean body mass in the older HF individuals likely contributed the reduction in Ra Arg (at the whole body level) and thus NO synthesis. This notion is supported by the findings of Eskurza et al. (19) that FMD was lower by ∼45% in sedentary older adults, but not in endurance-trained older adults, who had similar relative lean body mass, compared with sedentary healthy young adults. While the results of this study are consistent with our findings, it is hard to distinguish the effect of body composition from the effect of exercise training in the Eskura, et al. (19) study. Endurance training, per se, improves vascular endothelial function in sedentary young adults (4).
In the kinetic study, subjects were given multiple sip ingestions of small amounts of citrulline (3 g) over a 3-h time period (total 3 g). This approach was chosen to increase Ra Arg in a manner that would minimize alterations in plasma enrichments of infused tracers. In a separate study, we determined RH-FBF following a bolus ingestion of 10 g of citrulline. The dose of citrulline in the second study (i.e., 10 g) was selected because a previous study had shown this to maximize the renal capacity to convert citrulline to arginine (34). Furthermore, since 3 g of ingested citrulline was sufficient to increase NO synthesis 10-fold, we were confident that this would also be the case with ingestion of 10 g of citrulline. We observed that plasma concentrations of citrulline, and to a lesser extent arginine, were significantly and gradually increased by ∼2.5-fold for arginine and by more than 10-fold for citrulline in both age groups with the sip ingestions of citrulline. The changes in plasma arginine and citrulline concentrations were accompanied by similar increases in Ra Cit in both age groups, reflecting that the sip ingestions of citrulline were effective in increasing NO precursor availability. Despite the similar increases in precursor availability during the citrulline ingestion in the two age groups, de novo arginine synthesis was increased more in the young than in the older HF adults. This response indicated a reduced renal capacity for conversion of citrulline to arginine with advancing age. Most importantly, we found that citrulline ingestion increased NO synthesis rate ∼10-fold above the fasted state in both groups. These results indicate that precursor availability is limiting for NO synthesis in both groups.
Despite dramatic increases in NO synthesis during citrulline ingestion in both age groups, there was not a corresponding improvement in the RH-FBF response. It is not surprising that citrulline ingestion had no effect in the healthy young subjects, because of the absence of a preexisting deficiency in responsiveness. However, the lack of improvement in RH-FBF in the older HF adults following the ingestion of 10 g of citrulline was unexpected. On the other hand, our finding is in agreement with that of Churchward-Venne et al. (14), demonstrating no improvement in femoral artery blood flow in older adults following acute ingestion of 10 g of citrulline with whey protein (15 g). Moreover, it has also been shown that an intravenous infusion of a 30-g arginine (1 g/min) dose, which is far greater than that known to stimulate NO synthesis (28), did not improve FMD despite a more than 20-fold increase in plasma arginine concentrations in older adults (22). This suggests the possibility that the acute nature of our study was too short to induce a significant change in response. For example, arginine supplementations for several days (14–30 days) improved FMD in older adults (9, 10, 32). In line with this possibility, Bonner et al. (11) have recently shown that a 3-wk relaxin infusion (rate: 1 mg·kg−1·day−1), but not an acute 6.5-h infusion of relaxin, improved vascular reactivity in mice fed a high-fat diet for 10 wk, due in part to remodeling of the extracellular matrix. It is therefore possible that longer-term supplementation with arginine or citrulline may improve vascular dysfunction by changing vascular environment, including inhibition of leukocyte adhesion and thrombocyte aggregation via NO (12).
Several potential mechanisms may explain the reduced NO synthesis in older HF adults and the lack of an acute effect of citrulline ingestion on RH-FBF despite an ∼10-fold increase in NO synthesis rate. First, it is possible that NO synthesis could increase without corresponding increases in NO bioavailability if the synthesized NO was immediately degraded or converted to other compounds such as peroxynitrite as a result of a reaction between NO and superoxide radicals (12). Second, arginase activity increases with advancing age (7, 39), resulting in reduced arginine bioavailability by converting arginine to ornithine. Consistent with this, we found that the plasma ornithine response to the citrulline ingestion was higher in older adults than in young adults. Third, it is possible that differential menopausal status may explain to some extent the differences in NO synthesis rate between young and older HF female subjects (43). However, we did not observe a difference in response between men and women, and differences in menopausal status would not be expected to explain the lack of improvement in RH-FBF in response to the citrulline ingestion. Fourth, it has been shown that insulin resistance plays a role in vascular dysfunction, and older individuals tend to be more insulin resistant (26). In this regard, we found that plasma insulin concentrations in the fasted state and during citrulline ingestion were higher in the older HF adults than in the young adults without significant changes during citrulline ingestion above the fasted state. This may explain to some extent the differences in vascular function between the groups, but again, not the null effect of citrulline on RH-FBF. Fifth, there might have been higher levels of inhibitors of NO, such as AMDA, in older HF adults (42), that were not measured in the present study. Last, the most likely explanation is decreases in compliance of the arterial system due to loss of elastin in the aging vessel wall (33). Hence, it is possible that, despite the ∼10-fold increase in NO synthesis, the young adults were nonresponsive to NO because of maximal compliance in response to reactive hyperemia without an increase in NO availability, and the older adults were nonresponsive because of restricted compliance of the vessel wall. This suggests that long-term supplementation of citrulline may be required to induce a favorable effect on vascular function (24, 37), probably via remodeling of vascular structure (11).
There are several limitations in the present study. First, NO is produced in the body via several NOS isoforms (31). In this regard, the stable-isotope method used cannot distinguish between sources of NO synthesis but only determine the total NO synthesis rate. Therefore, the extent to which the increase in NO synthesis during the citrulline ingestion was derived via eNOS or other NOS isoforms is unknown. Second, older HF adults in this study took prescribed medications that could have favorably affected vascular function, but those medications could not be stopped. However, it has been shown that impairments in vascular function still persisted in older HF individuals who took those medications compared with “healthy” older adults (30, 35) who also have vascular dysfunctions to some degree as a result of the natural aging process (40).
In conclusion, acute ingestion of citrulline effectively increased arginine availability and NO synthesis in both young and older HF adults. Nonetheless, acute citrulline supplementation was ineffective in improving vascular endothelial dysfunction in the older HF adults. These results imply that factors other than NO synthesis per se may play a role in vascular dysfunction and/or that longer-term supplementation of either arginine or citrulline is required to reverse the impairment in peripheral vascular function in the older HF adults.
GRANTS
This project was supported by Pepper Center Grant PG30-AG-028718 and Award Number UL1-TR-000039 and KL2-TR-000063 from the National Center for Advancing Translational Science (NCATS).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.-Y.K., N.E.D., and R.R.W. conception and design of research; I.-Y.K., S.E.S., and G.A. performed experiments; I.-Y.K. performed calculations of kinetics and blood flow. A.S., and H.S. performed statistical analysis. I.-Y.K., N.E.D., and R.R.W. interpreted results of experiments; I.-Y.K. prepared figures; I.-Y.K. drafted manuscript; I.-Y.K., S.E.S., A.S., H.S., G.A., N.E.D., and R.R.W. edited and revised manuscript; I.-Y.K., S.E.S., A.S., H.S., G.A., N.E.D., and R.R.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the research subjects for their participation in the study. We also thank the research staffs/associates for their support in conducting isotope tracer infusion protocols, Cosby J. Lasely for coordinating study subjects and conducting the isotope infusion study, and Rick Williams for insulin assay, Josh Spore for sample process and John J. Thaden PhD (Texas A&M University) for the LC/MS/MS analysis.
REFERENCES
- 1.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metab Clin Exp 30: 936–940, 1981. [DOI] [PubMed] [Google Scholar]
- 2.Andersson K, Gaudiot N, Ribiere C, Elizalde M, Giudicelli Y, Arner P. A nitric oxide-mediated mechanism regulates lipolysis in human adipose tissue in vivo. Br J Pharmacol 126: 1639–1645, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardilouze JL, Fielding BA, Currie JM, Frayn KN, Karpe F. Nitric oxide and beta-adrenergic stimulation are major regulators of preprandial and postprandial subcutaneous adipose tissue blood flow in humans. Circulation 109: 47–52, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, Mathers JC. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med 45: 279–296, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Bahri S, Zerrouk N, Aussel C, Moinard C, Crenn P, Curis E, Chaumeil JC, Cynober L, Sfar S. Citrulline: from metabolism to therapeutic use. Nutrition 29: 479–484, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Bai Y, Sun L, Yang T, Sun K, Chen J, Hui R. Increase in fasting vascular endothelial function after short-term oral l-arginine is effective when baseline flow-mediated dilation is low: a meta-analysis of randomized controlled trials. Am J Clin Nutr 89: 77–84, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108: 2000–2006, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab 268: E75–E84, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Blum A, Hathaway L, Mincemoyer R, Schenke WH, Kirby M, Csako G, Waclawiw MA, Panza JA, Cannon RO. Oral l-arginine in patients with coronary artery disease on medical management. Circulation 101: 2160–2164, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Bode-Böger SM, Muke J, Surdacki A, Brabant G, Böger RH, Frölich JC. Oral l-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med 8: 77–81, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Bonner JS, Lantier L, Hocking KM, Kang L, Owolabi M, James FD, Bracy DP, Brophy CM, Wasserman DH. Relaxin treatment reverses insulin resistance in mice fed a high-fat diet. Diabetes 62: 3251–3260, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res 66: 286–294, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N] arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci USA 93: 11460–11465, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Churchward-Venne TA, Cotie LM, MacDonald MJ, Mitchell CJ, Prior T, Baker SK, Phillips SM. Citrulline does not enhance blood flow, microvascular circulation, or myofibrillar protein synthesis in elderly men at rest or following exercise. Am J Physiol Endocrinol Metab 307: E71–E83, 2014. [DOI] [PubMed] [Google Scholar]
- 15.de Betue CTI, Joosten KFM, Deutz NEP, Vreugdenhil ACE, van Waardenburg DA. Arginine appearance and nitric oxide synthesis in critically ill infants can be increased with a protein-energy-enriched enteral formula. Am J Clin Nutr 98: 907–916, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Hattab AW, Hsu JW, Emrick LT, Wong LJC, Craigen WJ, Jahoor F, Scaglia F. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Mol Genet Metab 105: 607–614, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elizalde M, Rydén M, van Harmelen V, Eneroth P, Gyllenhammar H, Holm C, Ramel S, Olund A, Arner P, Andersson K. Expression of nitric oxide synthases in subcutaneous adipose tissue of nonobese and obese humans. J Lipid Res 41: 1244–1251, 2000. [PubMed] [Google Scholar]
- 18.Engelen MPKJ, Com G, Luiking YC, Deutz NEP. Stimulated nitric oxide production and arginine deficiency in children with cystic fibrosis with nutritional failure. J Pediatr 163: 369–375, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol (Lond) 556: 315–324, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol (Lond) 568: 1057–1065, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming I, Busse R. Signal transduction of eNOS activation. Cardiovasc Res 43: 532–541, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol 102: 63–71, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Grimble GK. Adverse gastrointestinal effects of arginine and related amino acids. J Nutr 137: 1693S–1701S, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Hambrecht R, Hilbrich L, Erbs S, Gielen S, Fiehn E, Schoene N, Schuler G. Correction of endothelial dysfunction in chronic heart failure: additional effects of exercise training and oral l-arginine supplementation. J Am Coll Cardiol 35: 706–713, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 75: 383–386, 1988. [Google Scholar]
- 26.Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin North Am 42: 333–347, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luiking YC, Have Ten GAM, Wolfe RR, Deutz NEP. Arginine de novo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab 303: E1177–E1189, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luiking YC, Poeze M, Deutz NE. Arginine infusion in patients with septic shock increases nitric oxide production without haemodynamic instability. Clin Sci 128: 57–67, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Luiking YC, Poeze M, Ramsay G, Deutz NEP. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr 89: 142–152, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda Y, Akita H, Terashima M, Shiga N, Kanazawa K, Yokoyama M. Carvedilol improves endothelium-dependent dilatation in patients with coronary artery disease. Am Heart J 140: 753–759, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Mattila JT, Thomas AC. Nitric oxide synthase: non-canonical expression patterns. Front Immunol 5: 478, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxwell AJ, Zapien MP, Pearce GL, MacCallum G, Stone PH. Randomized trial of a medical food for the dietary management of chronic, stable angina. J Am Coll Cardiol 39: 37–45, 2002. [DOI] [PubMed] [Google Scholar]
- 33.McEniery CM, Wilkinson IB, Avolio AP. Age, hypertension and arterial function. Clin Exp Pharmacol Physiol 34: 665–671, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Moinard C, Nicolis I, Neveux N, Darquy S, Bénazeth S, Cynober L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: the Citrudose pharmacokinetic study. Br J Nutr 99: 855–862, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Morgan DR, Dixon LJ, Hanratty CG, Hughes SMT, Leahey WJ, Rooney KP, Johnston GD, McVeigh GE. Impaired endothelium-dependent and -independent vasodilation in elderly patients with chronic heart failure. Eur J Heart Fail 6: 901–908, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Münzel T, Gori T, Keaney JF, Maack C, Daiber A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J (July 4, 2015). doi: 10.1093/eurheartj/ehv305 [Epub ahead of print] Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orozco-Gutiérrez JJ, Castillo-Martínez L, Orea-Tejeda A, Vázquez-Díaz O, Valdespino-Trejo A, Narváez-David R, Keirns-Davis C, Carrasco-Ortiz O, Navarro-Navarro A, Sánchez-Santillán R. Effect of l-arginine or l-citrulline oral supplementation on blood pressure and right ventricular function in heart failure patients with preserved ejection fraction. Cardiol J 17: 612–618, 2010. [PubMed] [Google Scholar]
- 38.Raff U, Ott C, John S, Schmidt BMW, Fleischmann EH, Schmieder RE. Nitric oxide and reactive hyperemia: role of location and duration of ischemia. Am J Hypertens 23: 865–869, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Santhanam L, Christianson DW, Nyhan D, Berkowitz DE. Arginase and vascular aging. J Appl Physiol 105: 1632–1642, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci 120: 357–375, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steele R, Altszuler N, Wall JS, Dunn A, De Bodo RC. Influence of adrenalectomy on glucose turnover and conversion to CO2: studies with C14 glucose in the dog. Am J Physiol 196: 221–230, 1959. [DOI] [PubMed] [Google Scholar]
- 42.Sydow K, Schwedhelm E, Arakawa N, Bode-Böger SM, Tsikas D, Hornig B, Frölich JC, Böger RH. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of l-arginine and B vitamins. Cardiovasc Res 57: 244–252, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Wijnands KAP, Meesters DM, van Barneveld KWY, Visschers RGJ, Briedé JJ, Vandendriessche B, van Eijk HMH, Bessems BAFM, Hoven NVD, Wintersdorff von CJH, Brouckaert P, Bouvy ND, Lamers WH, Cauwels A, Poeze M. Citrulline supplementation improves organ perfusion and arginine availability under conditions with enhanced arginase activity. Nutrients 7: 5217–5238, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research (2nd Ed). Hoboken, NJ: Wiley & Sons, 2005. [Google Scholar]
- 45.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MAH, Ives SJ, Barrett-O'Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297: H1829–H1836, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]