Abstract
Acute lung injury/acute respiratory distress syndrome (ALI/ARDS), an illness characterized by life-threatening vascular leak, is a significant cause of morbidity and mortality in critically ill patients. Recent preclinical studies and clinical observations have suggested a potential role for the chemotherapeutic agent imatinib in restoring vascular integrity. Our prior work demonstrates differential effects of imatinib in mouse models of ALI, namely attenuation of LPS-induced lung injury but exacerbation of ventilator-induced lung injury (VILI). Because of the critical role of mechanical ventilation in the care of patients with ARDS, in the present study we pursued an assessment of the effectiveness of imatinib in a “two-hit” model of ALI caused by combined LPS and VILI. Imatinib significantly decreased bronchoalveolar lavage protein, total cells, neutrophils, and TNF-α levels in mice exposed to LPS plus VILI, indicating that it attenuates ALI in this clinically relevant model. In subsequent experiments focusing on its protective role in LPS-induced lung injury, imatinib attenuated ALI when given 4 h after LPS, suggesting potential therapeutic effectiveness when given after the onset of injury. Mechanistic studies in mouse lung tissue and human lung endothelial cells revealed that imatinib inhibits LPS-induced NF-κB expression and activation. Overall, these results further characterize the therapeutic potential of imatinib against inflammatory vascular leak.
Keywords: acute lung injury, acute respiratory distress syndrome, imatinib, endothelium, lipopolysaccharide, mechanical ventilation, NF-κB
acute lung injury/acute respiratory distress syndrome (ALI/ARDS) is a devastating disease process characterized by severe pulmonary inflammation and vascular leak that causes hypoxemic respiratory failure in critically ill patients (51). Despite multiple clinical trials over the past few decades, targeted pharmacologic therapies are still lacking, and the mortality rate remains at 30–35% (20). The most common causes of ARDS are sepsis and severe pneumonia; however, other inflammatory conditions can initiate this disease process, including trauma, pancreatitis, and aspiration of gastric contents (51). Multiple endogenous inflammatory mediators, as well as exogenous agents such as LPS, alter the pulmonary endothelial cell (EC) structure, leading to paracellular gap formation and vascular leak (19). Care for these patients is largely supportive, and low-tidal-volume mechanical ventilation (MV) is the only intervention proven to decrease mortality (1). However, the biophysical forces present during MV can contribute to both the inflammatory and the permeability aspects of ARDS, a process known as ventilator-induced lung injury (VILI) (44).
Recent work by multiple groups indicates that the FDA-approved tyrosine kinase inhibitor, imatinib, attenuates vascular permeability (5, 10, 27, 29, 45). Although imatinib was originally designed to inhibit the BCR-Abl fusion protein that causes chronic myelogenous leukemia, it also inhibits several other kinases including c-Abl, Abl-related gene (Arg), c-kit, and platelet-derived growth factor receptor (PDGFR), suggesting that it may have pleiotropic effects on vascular function (39). Accordingly, recent reports indicate that imatinib attenuates the vascular leak induced by diverse stimuli including thrombin, histamine, VEGF, LPS, and reactive oxygen species (ROS) (5, 10, 27, 29, 45). These studies have indicated that multiple targets of imatinib, including c-Abl, Arg, and PDGFR, are involved in regulating the cytoskeletal rearrangements that mediate vascular permeability. Further support for a role of imatinib in the treatment of vascular leak is provided by a series of case reports in which imatinib therapy was associated with rapid resolution of pulmonary and systemic vascular leak (4, 8, 36). Although these observations suggest that imatinib may be a promising treatment for inflammatory vascular leak, the mechanisms underlying these effects remain incompletely understood. Additionally, contrary to its barrier-protective effects, imatinib has well-established side effects of periorbital and subcutaneous edema, as well as pleural and pericardial effusions in patients, suggesting that it can worsen vascular leak in certain clinical contexts (17, 23, 41).
Consistent with this potential for imatinib to exert both barrier-protective and edemagenic effects, we recently reported that imatinib attenuates LPS-induced lung injury but exacerbates VILI both in vitro and in vivo (29). Because of the necessity of MV in the treatment of patients with ARDS, these observations are clinically relevant and suggest that imatinib has the potential to worsen ARDS in some situations. To address this concern, in the present study, we investigated the efficacy of imatinib using a “two-hit” preclinical murine model of ALI that combines intratracheal LPS administration followed by high-tidal-volume MV (HTVMV). Additionally, we assessed the therapeutic potential of imatinib when given after the onset of inflammatory lung injury, a critical step in the preclinical testing of interventions aimed at treating existing injury. We also characterized the effects of imatinib on LPS-induced NF-κB signaling in vitro and in vivo. Our data indicate that imatinib has potent anti-inflammatory effects, in addition to its previously described effects on EC permeability, and further support its potential use as a therapeutic option for inflammatory vascular leak syndromes.
MATERIALS AND METHODS
Animal care and preclinical models of ALI.
Male C57BL/6J mice aged 8–12 wk (Jackson Laboratories, Bar Harbor, ME) were used for all experiments. Mice were anesthetized with intraperitoneal ketamine (100 mg/kg) and xylazine (5 mg/kg) before LPS and VILI protocols, with additional doses given as needed during the VILI protocol to ensure adequate anesthetic depth. For experiments utilizing the two-hit lung ALI model, animals were given intratracheal (it) LPS (0.5 mg/kg), allowed to recover for 20 h, and were then reintubated and placed on MV (Harvard Apparatus, Boston, MA) with room air for 4 h using the following parameters: tidal volume (VT) 30 ml/kg, respiratory rate 75 breaths/min, and positive-end expiratory pressure 0 cm H2O. Spontaneously breathing (SB) control animals were given intratracheally PBS instead of LPS and were intubated but allowed to breathe spontaneously for the 4 h. Imatinib (75 mg/kg, ip) (LC Laboratories, Woburn, MA) or vehicle (water) was given 30 min before LPS administration and again 30 min before the initiation of MV. For experiments in which imatinib was administered post injury, imatinib (75 mg/kg, ip) or vehicle was given 4 h after LPS (1 mg/kg, it) or PBS. The dosage of imatinib used was chosen to be 75 mg/kg to be consistent with our previous work and within the range of what other groups have used in mice (5, 27, 29). The dosage of imatinib used in cancer therapy ranges from 11–15 mg/kg depending on the particular malignancy (16, 37). Although this dose is lower than what we used in the mouse models in this study (75 mg/kg), there are substantial differences in the metabolism of imatinib between mice and humans that strongly suggest that the dosage used in this study is reasonable and clinically relevant. The most striking difference between the pharmacokinetics of imatinib in humans and mice is that the half-life of imatinib in mice is 2.4 h, compared with 18 h in humans (37, 39, 47). Thus, to attain an effective plasma concentration of imatinib in the time frame necessary for our studies, a higher dose of imatinib was necessary than that used clinically in humans.
At the termination of each of the animal experiments, bronchoalveolar lavage (BAL) fluid was collected by instilling 1 ml of HBSS (Invitrogen, Grand Island, NY) through the tracheal cannula into the lungs, followed by slow recovery of the fluid. Cells were recovered from the resulting BAL fluid by centrifugation (500 g, 20 min, 4°C) and counted using an automated cell counter (TC20; Bio-Rad, Hercules, CA). Manual differential cell counts (500 cells/sample) were performed by a blinded investigator on cytospin samples (Shandon, Cytospin 4; Thermo Fisher Scientific, Rockford, IL) after staining with Kwik Diff Stain (Thermo Scientific). The BAL fluid supernatant was centrifuged again (17,000 g, 10 min, 4°C) and stored at −80°C for further analysis. Lung tissues were snap-frozen in liquid nitrogen or embedded in paraformaldehyde (PFA) for histology. Select animals in both studies were given Evans blue dye (EBD) (30 mg/kg) 1 h before harvest via an intrajugular injection. BAL was not performed on animals that were given EBD before harvest. All animal care procedures and experiments were approved by the University of Illinois at Chicago Animal Care and Use Committee.
Analysis of mouse samples.
BAL fluid protein was measured using a bicinchoninic acid assay kit (Thermo Scientific), and BAL TNF-α, IL-6, and IL-1β levels were measured using ELISA kits obtained from eBiosciences (San Diego, CA) according to the manufacturer's protocols. Lung tissues were homogenized in radioimmunoprecipitation assay (RIPA) buffer supplemented with protease and phosphatase inhibitors using a hand-held homogenizer (Qiagen, Venlo, The Netherlands), subjected to three freeze-thaw cycles, and sonicated before protein quantification and Western blotting. Lung tissue and BAL albumin concentrations were measured using an ELISA kit (Bethyl Laboratories, Montgomery, TX).
Wet:dry lung weight measurements and EBD measurements were conducted on mice that did not undergo BAL. In these animals, mice were given an intrajugular injection of EBD (30 mg/kg) 1 h before harvest. Following perfusion of the vasculature, the left lungs were immediately excised and weighed for determination of wet lung weight. The lungs were then placed in an oven at 21°C for 48 h and then weighed again for determination of dry lung weight. The right lungs of these animals were frozen in liquid nitrogen and then homogenized in 1 ml of PBS using a hand-held homogenizer (Qiagen). The samples were then incubated in a 60°C water bath overnight, after the addition of 2 ml of formamide to each sample. The following morning, the samples were centrifuged (12,000 g, 20 min, room temperature). The EBD concentration in the resulting supernatant was determined by spectrophotometry as previously described (33).
Lung histopathology and immunohistochemistry.
To assess alterations in the lung tissue morphology, lungs were fixed in formalin, embedded in paraffin, sectioned, mounted onto slides, and stained with hematoxylin-eosin (H and E). Separate slides for immunohistochemistry (IHC) were hydrated through a xylene and alcohol gradient before antigen unmasking with an EDTA-based retrieval solution. Slides were then blocked with hydrogen peroxide blocking reagent (20 min, room temperature), probed with NF-κB antibody (Abcam, Cambridge, MA) (1:100, 60 min, room temperature), and then incubated in anti-rabbit horseradish peroxidase (HRP) secondary antibody (Biocare, Concord, CA) (20 min, room temperature). NF-κB staining was detected by 3,3′-diaminobenzidine (Cell Marque, Rockland, CA) for 10 min. All slides were scanned using an Aperio ScanScope (Leica, Buffalo Grove, IL), and representative images were taken at ×100 by an individual blinded to experimental condition.
Endothelial cell culture and Western blotting.
Human pulmonary artery endothelial cells (HPAEC) were purchased from Lonza (Walkersville, MD) and cultured in endothelial growth medium-2 supplemented with 10% FBS (Sigma, St. Louis, MO). Cells were maintained at 37°C in a 5% CO2 incubator and used at passages 6–8. For all experiments, the media containing 10% FBS was replaced with media containing 2% FBS 3 h before the indicated treatment. HPAEC were treated with imatinib (40 μM) or vehicle (water) for 1 h before challenge with LPS (E0127:B8, Sigma no. L3880) (1 μg/ml, 0–60 min). The in vitro dosage of imatinib was chosen to be consistent with the dosage used in our previous publication (29). Although this dose is slightly higher than that used in other in vitro studies on the effects of imatinib on vascular integrity (10 μM) (5, 10), these studies solubilized the drug in DMSO, which increases permeability of the plasma membrane and thus increases the effective concentration of imatinib in the cells (15). Following the indicated treatments, EC were washed in ice-cold PBS and then harvested in RIPA buffer supplemented with protease and phosphatase inhibitors. Protein samples were then prepared in Laemmli SDS sample buffer (Boston Bioproducts, Ashland, MA), boiled, subjected to SDS-PAGE, and transferred to polyvinylidene difluoride membrane. The membranes were blocked in 5% BSA and incubated in the indicated antibodies (phospho-NF-κB, total NF-κB, and IκB were purchased from Cell Signaling, Danvers, MA) (overnight, 4°C). Next, HRP-conjugated secondary antibodies (Cell signaling) were added to the membranes (60 min, room temperature), and the Pierce enhanced chemiluminescence detection system (Thermo Scientific) was used to visualize the bands. Western blots on homogenized mouse lung samples were conducted identically. Band densities were determined using the Image J software (http://imagej.nih.gov/ij/) (National Institutes of Health).
Immunofluorescence microscopy.
HPAEC grown on gelatin-coated coverslips were treated with imatinib and LPS as described above, fixed in 4% PFA (20 min, room temperature), permeabilized with 0.1% Triton-X in PBS, and incubated with NF-κB p65 antibody (overnight, 4°C). Coverslips were then washed, incubated with fluorescently labeled secondary antibody, and mounted on glass slides using 4′,6-diamidino-2-phenylindole Prolong Gold anti-fade reagent (Invitrogen). Images were acquired by a blinded investigator using Nikon Eclipse TE2000 microscope at ×63 with an oil emersion lens.
Statistical analysis.
Results were expressed as mean ± SE with three to seven animals per group in each experiment. The data for BAL albumin, lung tissue albumin, EBD extravasation, and lung tissue NF-κB expression are presented as values normalized to either the LPS + VILI + Veh (2-hit model), or to LPS + Veh (postinjury studies). Only three animals per group were used in the SB groups (2-hit model studies) and the PBS injection groups (postinjury studies) because of the very small variation between samples. All in vitro experiments were repeated at least three independent times. Student's t-test was used to compare between two groups, and one-way ANOVA with Tukey's post hoc test was used to compare between multiple groups. All statistical analyses were performed using GraphPad Prism 6 software with the significance level set to P < 0.05 for all experiments.
RESULTS
Imatinib attenuates vascular leak and inflammation in a clinically relevant two-hit model of ALI.
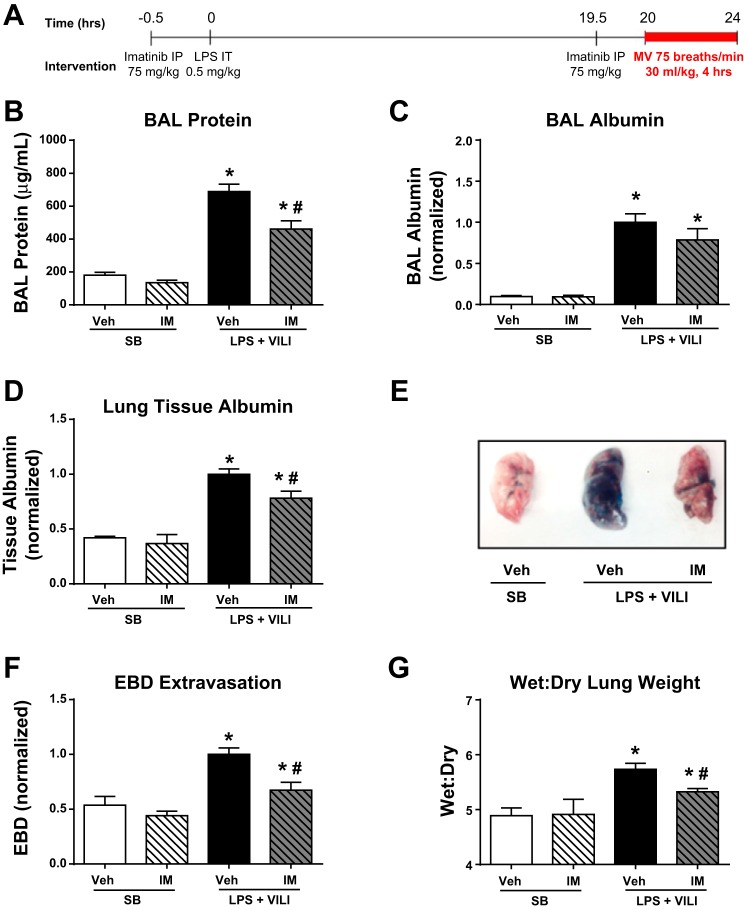
We recently reported that imatinib attenuates LPS-induced ALI but exacerbates VILI in both cell culture and murine models (29). However, because of the necessity of MV in the treatment of ARDS, inflammation- and mechanical force-induced cellular injuries commonly coexist in patients. In this study, we utilized a clinically relevant two-hit murine model of LPS combined with VILI (22, 31) to further assess the efficacy of imatinib in ALI. In these studies, mice were challenged with LPS (0.5 mg/kg, it) (t = 0 h), followed by HTVMV (VT = 30 ml/kg) (t = 20–24 h). Imatinib (or vehicle) was given 30 min before LPS injection (t = −0.5 h) and again 30 min before MV (t = 19.5 h) (Fig. 1A). In this study, we utilized identical imatinib treatments and MV parameters as in our previous work (29) and therefore have not included redundant data here on the effects of pretreatment with imatinib in LPS-induced lung injury or VILI alone. As expected, mice exposed to the two-hit model displayed increased BAL protein (∼4-fold), indicative of increased vascular leak. Imatinib was protective in this model and reduced BAL protein levels by 33% (P < 0.01) (Fig. 1B). To further quantify the vascular leak, albumin levels in BAL fluid and lung tissue were quantified. Imatinib attenuated the increase in lung tissue albumin induced by LPS plus VILI by 22% (P < 0.05) (Fig. 1C). We also observed a trend toward decreased BAL albumin in imatinib-treated animals (22% decline, P = 0.2) (Fig. 1D). Additionally, EBD extravasation into the lungs, another well-characterized measurement of vascular leak (21), was decreased by 33% (P < 0.05) in animals receiving imatinib (Fig. 1, E and F). Furthermore, LPS plus VILI significantly increased lung wet:dry weight ratio, a measure of pulmonary edema, and imatinib attenuated this increase by 62% (P < 0.05) (Fig. 1G). Together, these data indicate that imatinib attenuates vascular leak in this two-hit model of ALI.
Fig. 1.
Imatinib attenuates vascular leak in mice challenged with a 2-hit model of LPS and mechanical ventilation (MV). Mice were challenged with LPS [0.5 mg/kg, intratracheally (it)] (t = 0 h) and MV (respiratory rate 75, tidal volume 30 ml/kg, positive end-expiratory pressure 0 cm H2O) (t = 20–24 h) as outlined in A. Spontaneously breathing (SB) control mice received PBS (vs. LPS) and were intubated for 4 h without MV. Bronchoalveolar lavage (BAL) fluid, plasma, and lungs were harvested from the animals immediately after MV. Imatinib (IM) (75 mg/kg, ip) or vehicle was administered 0.5 h before LPS administration and before the initiation of MV. Lung permeability was assessed by BAL protein content (B), BAL fluid albumin (C), and lung tissue albumin (D). Additionally, in separate animals, Evans Blue dye (EBD) was injected (30 mg/kg, iv) 1 h before harvest, and representative extravasation into harvested lung tissue is shown (E) and quantified in multiple samples (F). The left lung of each of these animals was used for calculation of lung wet:dry ratio (G). SB (n = 3), SB + imatinib (n = 3), LPS + ventilator-induced lung injury (VILI) (n = 3–11) and LPS + VILI + imatinib (n = 3–6). *P < 0.05 compared with SB controls and #P < 0.05 compared with untreated animals.
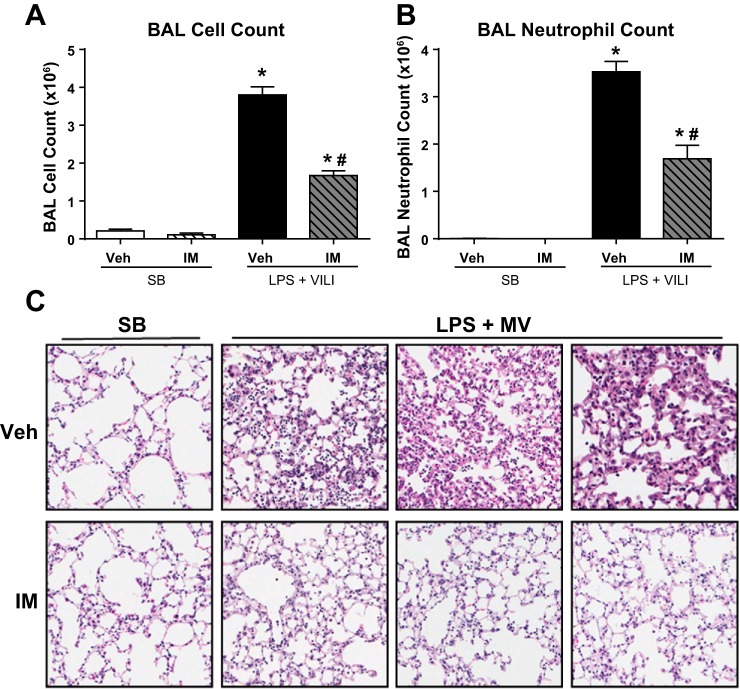
We next assessed pulmonary inflammation in these animals by measuring BAL cell counts and lung histology. Compared with control mice, the two-hit injury model caused a dramatic increase in total BAL cells and BAL neutrophils, which were decreased by 56 and 52%, respectively, in imatinib-treated animals (P < 0.001 for both) (Fig. 2, A and B). Histologic analyses of H and E-stained lung tissue revealed that mice challenged with LPS plus MV displayed architectural distortion and neutrophil invasion, which were substantially decreased in the imatinib-treated animals as evidenced by representative images from three independent mice treated with imatinib, along with LPS plus VILI, and three vehicle-treated control animals that were also subjected to LPS plus VILI (Fig. 2C). Together these data demonstrate that imatinib attenuates inflammation in this clinically relevant two-hit model of ALI.
Fig. 2.
Imatinib decreases lung inflammation in mice challenged with LPS and MV. Mice were subjected to the 2-hit lung injury model (LPS + VILI), and lung inflammation was quantified by BAL total cell counts (A) and BAL neutrophil counts (B). Representative hematoxylin and eosin (H and E)-stained lung sections are shown (C). Each H and E image was obtained from a different animal. SB (n = 3), SB + imatinib (n = 3), LPS + VILI (n = 3–11), and LPS + VILI + imatinib (n = 3–6). *P < 0.05 compared with SB controls and #P < 0.05 compared with untreated animals.
Imatinib is protective after the onset of inflammation in LPS induced-lung injury.
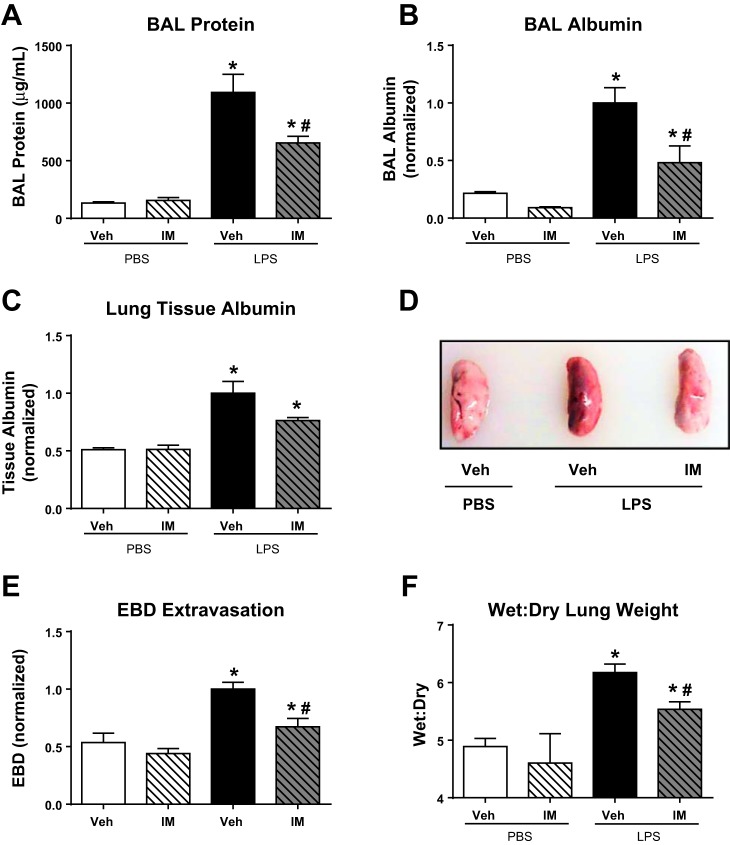
Given that the efficacy of imatinib in the two-hit model is similar to that observed in LPS-induced ALI alone, we next employed this more easily interpreted LPS-only model to evaluate the potential for imatinib to attenuate ALI after the onset of injury. For these studies, mice were challenged with LPS (1.0 mg/kg, it) or PBS and then received imatinib (75 mg/kg, ip) or vehicle 4 h later. The dosage of LPS was increased in these experiments compared with the two-hit injury model to cause a more robust injury in the setting of a single-hit model. Animals were harvested 18 h after LPS administration, and BAL and lung tissue were collected and analyzed identically to the two-hit model studies. As expected, BAL protein, BAL albumin, and lung tissue albumin levels were all increased in the LPS-challenged mice compared with PBS-treated control animals. Additionally, despite being given 4 h after the onset of injury, imatinib retained its protective effects and attenuated vascular leak as measured by BAL total protein content (40% decline, P < 0.05) and BAL albumin (51% decline, P < 0.05) (Fig. 3, A and B). We also observed a trend toward decreased lung tissue albumin (24% decline, P = 0.15) compared with LPS alone (Fig. 3C). These data were supported by measurements of EBD extravasation and wet:dry lung weight. The leakage of EBD into the lung parenchyma was decreased by 25% (P < 0.05) in the animals receiving imatinib after LPS (Fig. 3, D and E), and the wet:dry lung weight ratio was decreased by 50% (P < 0.05) in the imatinib-treated animals (Fig. 3F).
Fig. 3.
Imatinib decreases LPS-induced vascular leak when given after injury onset. Mice were challenged with LPS (1.0 mg/kg, it) (vs. PBS) and then received imatinib (75 mg/kg, ip) (vs. vehicle) 4 h later. Samples were harvested 18 h after LPS administration. Pulmonary vascular permeability was quantified in these animals by measuring BAL protein (A), BAL albumin (B), and lung tissue albumin (C). In separate animals, EBD was injected (30 mg/kg, iv) 1 h before harvest, and representative extravasation into harvested lung tissue is shown (D) and quantified in multiple samples (E). The left lung of each of these animals was used for calculation of lung wet:dry ratio (F). SB (n = 3), SB + imatinib (n = 3), LPS (n = 3–6), and LPS + imatinib (n = 3–6). *P < 0.05 compared with SB controls and #P < 0.05 compared with untreated animals.
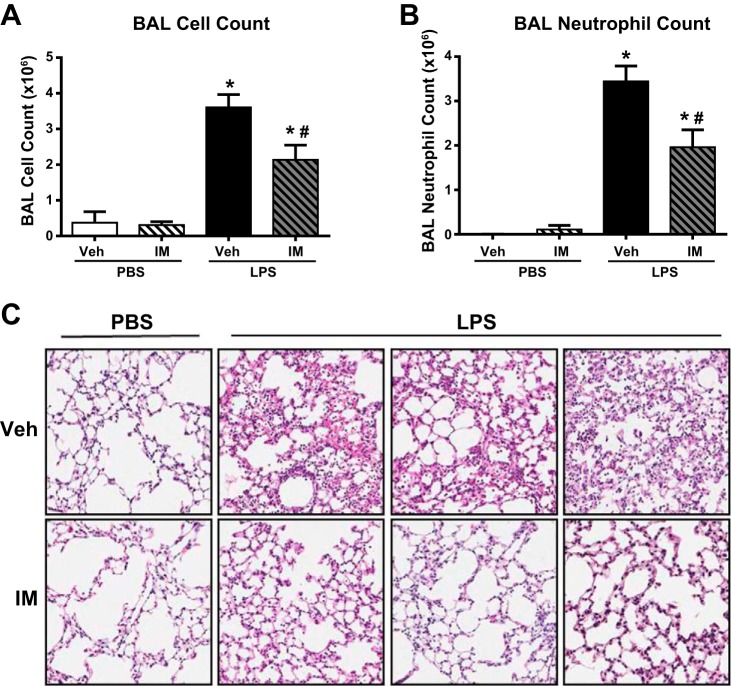
We then measured the BAL cell counts and carried out histological analyses of the lung tissue to measure the inflammatory damage present in these animals. As expected, the LPS-challenged mice displayed drastically higher BAL cell counts (Fig. 4A) and neutrophil numbers (Fig. 4B) than the PBS-treated control animals. Similar to previous experiments in which the imatinib was administered 30 min before the time of injury, imatinib given 4 h after LPS still attenuated LPS-induced BAL cell counts by 41% (P < 0.05) and neutrophil counts by 43% (P < 0.05). In agreement with these data, histological assessment of H and E-stained lung sections from multiple independent animals demonstrated decreased edema formation, neutrophil invasion, and disruption of lung tissue morphology in the mice receiving imatinib (Fig. 4C). Together, these studies suggest that imatinib retains barrier-protective and anti-inflammatory effects in LPS-induced ALI when given after the onset of injury.
Fig. 4.
Imatinib decreases LPS-induced inflammation when given after injury onset. Mice were challenged with LPS (1.0 mg/kg, it) (vs. PBS) and then received imatinib (75 mg/kg, ip) (vs. vehicle) 4 h later. Lung inflammation was then quantified by BAL total cell counts (A) and BAL neutrophil counts (B). Representative H and E-stained lung sections are shown (C). Each H and E image was obtained from a different animal. SB (n = 3), SB + imatinib (n = 3), LPS (n = 3–6), and LPS + imatinib (n = 3–6). *P < 0.05 compared with SB controls and #P < 0.05 compared with untreated animals.
Imatinib attenuates LPS-induced TNF-α production in vivo.
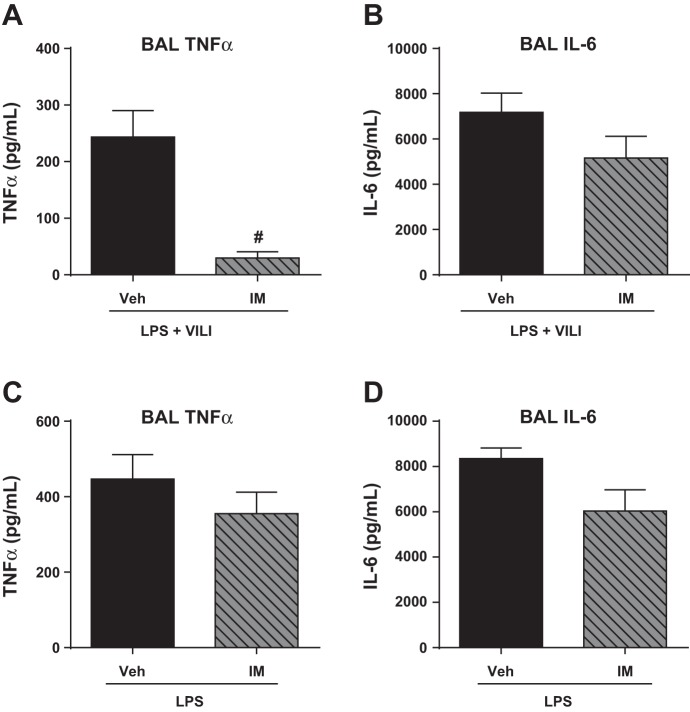
To further characterize the anti-inflammatory effects of imatinib, we next utilized ELISAs to measure its effects on production of the inflammatory cytokines TNF-α, IL-6, and IL-1β. Imatinib reduced BAL levels of TNF-α in the two-hit injury model (88%, P = 0.001) (Fig. 5A). We also observed a trend toward decreased TNF-α in the LPS-only postinjury model (21%, P = 0.33) (Fig. 5C). In both injury models there also was a trend that did not reach statistical significance toward decreased BAL IL-6 in imatinib-treated mice (Fig. 5, B and D), consistent with our previous observations (29). Neither IL-6 nor TNF-α was detected in the BAL of the control animals in either model (data not shown). IL-1β was increased in both ALI models, but imatinib treatment did not alter its levels (data not shown).
Fig. 5.
Imatinib decreases BAL TNF-α levels in a clinically relevant mouse model of acute lung injury (ALI). Inflammatory cytokines implicated in ALI (TNF-α, IL-6) were measured in the BAL fluid of mice challenged with the 2-hit model (LPS + VILI) (A and B) or the LPS-only postinjury model (C and D). 2-hit injury data represent SB (n = 3), SB + imatinib (n = 3), LPS + MV (n = 6), and LPS + MV + imatinib (n = 7). Post-LPS data represent SB (n = 3), SB + imatinib (n = 3), LPS (n = 3–6), and LPS + imatinib (n = 3–6). #P < 0.05.
Imatinib decreases LPS-induced NF-κB activation in HPAEC and expression in mouse lung tissue.
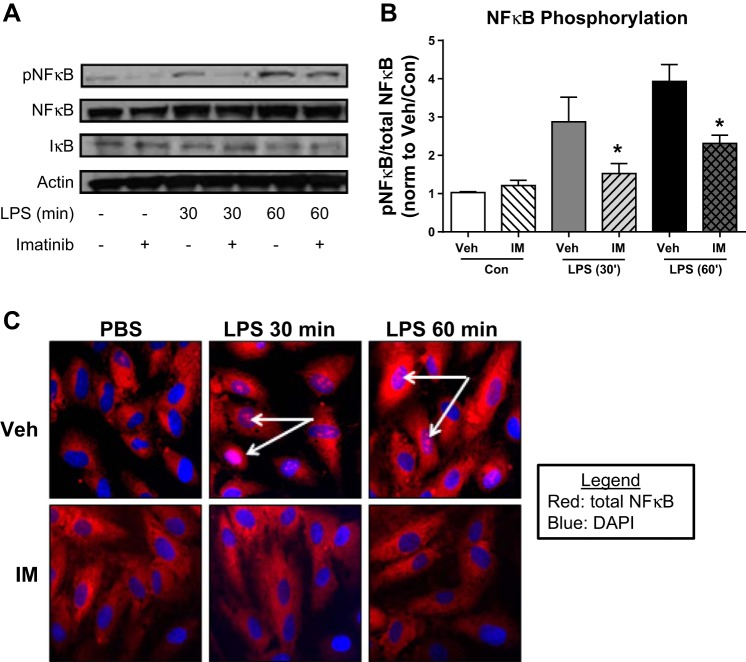
Imatinib inhibits LPS-induced VCAM-1 expression and cytokine production in cultured human lung EC (29). To determine whether these effects are mediated by decreased NF-κB activation, HPAEC were pretreated with imatinib (40 μM, 60 min) and then challenged with LPS (1 μg/ml, 0–60 min). Cell lysates were then assessed by Western blotting for NF-κB phosphorylated at Ser536, indicative of activated NF-κB (40). LPS increased NF-κB phosphorylation threefold at 30 min and fourfold at 60 min, whereas imatinib attenuated this LPS-induced NF-κB activation at both time points (P < 0.05) (Figs. 6, A and B). These changes were found to be independent of levels of IκB (Fig. 6A). In confirmatory immunofluorescence experiments, imatinib inhibited LPS-induced translocation of NF-κB p65 to the nuclei in HPAEC (Fig. 6C), which is indicative of decreased transcriptional activation (49).
Fig. 6.
Imatinib inhibits LPS-induced NF-κB phosphorylation and nuclear translocation in vitro. Human pulmonary artery endothelial cells (HPAEC) were treated with imatinib (40 μM, 60 min) and then challenged with LPS (1 μg/ml, 0–60 min). Lysates were harvested for Western blots for phosphorylated NF-κB p65 (S536), total NF-κB p65 protein, IκB, and actin and quantified by densitometry (A and B). HPAEC plated on glass coverslips were subjected to identical conditions, and immunofluorescence microscopy was conducted to determine the localization of total NF-κB p65 protein (red). 4′,6-diamidino-2-phenylindole (DAPI) (blue) was used to stain the nuclei (white arrows) (C). Data are representative of 3 independent experiments. *P < 0.05 compared with nonchallenged samples.
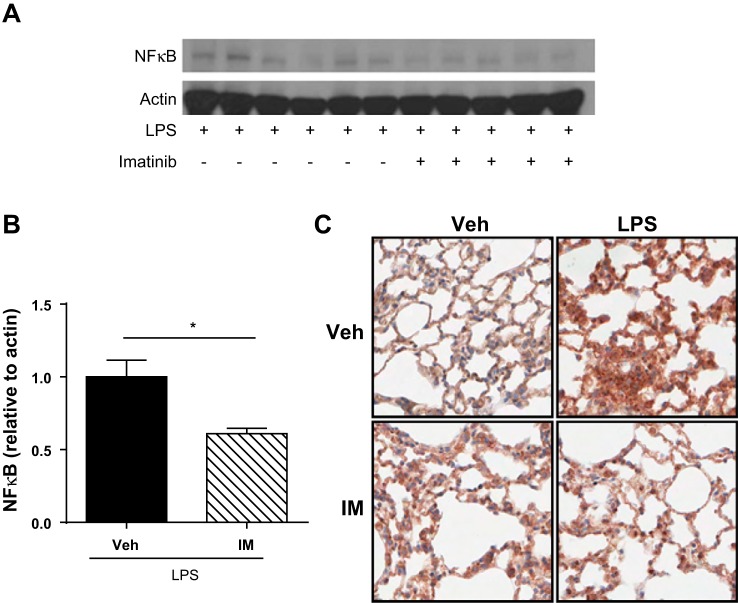
To determine the effects of imatinib on LPS-induced NF-κB expression in vivo, we performed Western blot analysis of whole lung homogenates from LPS-challenged mice that received imatinib (or vehicle control) 4 h after the onset of injury. NF-κB expression that was 39% lower was observed in the whole lung homogenates of mice receiving imatinib compared with the vehicle-treated controls (P < 0.05) (Fig. 7, A and B). In confirmatory experiments, we further evaluated NF-κB expression in vivo using IHC of formalin-fixed, paraffin-embedded sections from LPS-challenged mice receiving imatinib or vehicle. LPS substantially increased NF-κB expression in lung tissue, whereas imatinib attenuated this increase (Fig. 7C). Together, these studies demonstrate that imatinib inhibits LPS-induced NF-κB activation in human pulmonary EC as well as total NF-κB expression in mouse lungs.
Fig. 7.
Imatinib decreases NF-κB expression in mouse lungs after LPS. Mice were challenged with LPS (1.0 mg/kg, it) (vs. PBS) and then received imatinib (75 mg/kg, ip) (vs. vehicle) 4 h later. Lung tissue homogenates were collected, subjected to Western blotting for total NF-κB p65 protein, and quantified by densitometry (A and B). Lanes in A represent lung homogenates from individual animals. Representative immunohistochemistry images are shown for NF-κB p65 taken by an individual blinded to experimental condition (C). *P < 0.05.
DISCUSSION
Inflammatory vascular leak underlies the pathophysiology of several disorders that afflict critically ill patients, including sepsis and ARDS (25, 51). In these disease processes, multiple inflammatory stimuli combine to alter the EC cytoskeleton, which results in intercellular gap formation, increased permeability, and ultimately edema formation (19). Although there are no effective therapies to protect EC barrier function, recent work from several groups has demonstrated that imatinib, an FDA-approved tyrosine kinase inhibitor, attenuates vascular leak induced by multiple inflammatory stimuli (5, 10, 27, 45). Imatinib-mediated barrier protection has also been reported in vivo using the murine cecal ligation and puncture sepsis model and LPS-induced ALI in both immunocompetent and immunocompromised mice (5, 27, 29). Additionally, a recent series of case reports suggests that imatinib may reduce vascular permeability in certain conditions in humans (4, 8, 36), suggesting that imatinib may have therapeutic potential in patients with inflammatory vascular leak syndromes.
However, contrary to these barrier-protective effects, imatinib commonly causes side effects indicative of increased vascular leak, including periorbital and subcutaneous edema, as well as pleural and pericardial effusions (17, 23, 41). Additionally, we recently reported that imatinib exacerbates VILI in both animal and cell culture models of this condition (cyclic stretch and HTVMV, respectively) (29). Because of the crucial role of MV in ARDS treatment, this observation raises significant concerns regarding the potential efficacy of imatinib in patients with ARDS. The present study advances our knowledge concerning the therapeutic potential of imatinib in ALI/ARDS by demonstrating its efficacy in a clinically relevant, two-hit mouse model of ALI induced by a combination of LPS and VILI (Figs. 1 and 2). In these experiments, the ventilator parameters and imatinib treatments were identical to those in our previous work (29), and for this reason the data from VILI alone are not repeated in this study. Although the current ARDS Network lung-protective strategy recommends starting with 6 ml/kg VT (1), we elected to utilize a relatively high VT (30 ml/kg) because of the differences in human vs. mouse lung architecture as well as to maximize the deleterious effects of VILI in this model, thus biasing our study toward accepting the null hypothesis that imatinib is not efficacious in murine ALI. Additionally, the dosage of LPS was lowered from 1.0 mg/kg to 0.5 mg/kg to better titrate the level of lung injury to test this null hypothesis. Given that our study was biased toward identifying the potential deleterious effects of imatinib, its protective effects in this two-hit model are particularly encouraging. These data strongly suggest that the protective effects of imatinib on LPS-induced ALI outweigh its deleterious effects in VILI. Given that our data demonstrate a protective effect of imatinib in the two-hit model despite maximizing its potential deleterious effects (by using a VT that is substantially higher than the ARDS Network recommendation), we believe that further preclinical testing of this therapeutic agent for ARDS is warranted.
Additional experiments demonstrate the potential of imatinib to attenuate ALI when given after the onset of injury (Figs. 3 and 4), an essential characteristic for any intervention being considered as therapy for established disease. Interestingly, the BAL TNF-α and IL-6 levels were much higher in the LPS-only model compared with the two-hit model, which we hypothesize is due to the higher dose of LPS used in the LPS-only experiments. Additionally, we observed that the effect of imatinib on the levels of these cytokines was diminished in the LPS-only model, which suggests that the upregulation of these inflammatory cytokines occurs early in LPS-induced ALI. Although this study focused on the effects of imatinib on LPS-induced NF-κB signaling, it is important to note that VILI also induces NF-κB signaling (28, 32). Thus additional research is necessary to determine the effects of imatinib on NF-κB signaling in the context of MV alone.
The effects of imatinib in several different vascular leak models suggest that multiple imatinib-sensitive kinases may contribute to these effects. Notably, the nonreceptor tyrosine kinase Arg is activated downstream of thrombin, histamine, and VEGF and contributes to vascular leak by inhibiting Rac activation and impairing focal adhesion remodeling (5). Subsequent work demonstrated that the closely related kinase c-Abl also contributes to the cytoskeletal rearrangements that occur downstream of VEGF, thrombin, and histamine via effects on the small GTPases Rac1 and Rap1, as well as by inhibition of actin-myosin contractility in EC (10). Additionally, both c-Abl and Arg are activated in response to oxidative stress (6, 7), and inhibition of c-Abl with imatinib in murine microvascular EC has antioxidant effects, including increasing protein expression of glutathione peroxidase 1 and catalase (45). However, despite these beneficial effects of Abl family kinase inhibition on EC permeability, c-Abl is critically involved in the barrier-enhancing responses to both sphingosine 1-phosphate and FTY720 (18, 50). Additionally, loss of EC c-Abl causes increased apoptosis and vascular dysfunction in EC-specific c-Abl knockout mice (11). Together, these observations support a model in which Abl family kinases are critical mediators of vascular function with both protective and disruptive effects in a stimulus-specific manner.
We recently reported that imatinib attenuates LPS-induced VCAM-1 expression and inflammatory cytokine production (IL-6, IL-8) in cultured HPAEC (29). In EC, the process of transcriptional activation of NF-κB mediates the expression of multiple cellular adhesion molecules (CAMs), including ICAM, VCAM-1, and E-selectin, that are involved in neutrophil extravasation (13). Thus, in the current study, we examined the effects of imatinib on NF-κB expression and activation. Imatinib inhibited LPS-induced NF-κB phosphorylation and nuclear translocation in cultured human lung EC (Fig. 6) and NF-κB expression in mouse lung tissue (Fig. 7). Interestingly, imatinib did not alter VCAM-1 expression in the lung tissue of LPS-challenged mice (data not shown). Possible explanations for this observation include the multitude of cell types present in the whole lung tissue as well as the time point at which the animals were harvested relative to the expected increase in VCAM-1 expression.
In support of these data, recent work indicates that imatinib decreases NF-κB activation in macrophages and in human myeloid cells (12, 52), and anti-inflammatory effects of imatinib have been reported in several inflammatory lung diseases including idiopathic pulmonary fibrosis, pulmonary arterial hypertension, and asthma (9, 14, 42), as well as systemic conditions, such as rheumatoid arthritis and multiple sclerosis (2, 3). However, interestingly, the effects of imatinib on inflammation and NF-κB activation appear to vary with cell type and length of exposure. In pancreatic β-cells, imatinib initially increases NF-κB activation, but after a longer exposure it causes a dampened cytokine response (26, 34). Further exploration of the effects of imatinib on inflammatory signaling in pulmonary EC may provide additional insights into its therapeutic potential in ALI/ARDS and other inflammatory lung diseases (Fig. 8). Although in the present study we did not explore the effects of imatinib on NF-κB signaling specifically in the VILI-alone model or the two-hit injury model, NF-κB activity is known to contribute to the pathogenesis of VILI (24, 35, 43), so it will be interesting in future experiments to characterize how imatinib alters VILI-induced NF-κB signaling. However, VILI is an extremely complex process in which multiple pathways are involved, including MAP kinase signaling, actin-cytoskeletal rearrangements, and activation of cyclic adenosine monophosphate regulatory element-binding protein (30, 35, 38, 48), suggesting that the deleterious effects of imatinib reported in the VILI model (29) are likely due to its effects on pathways other than NF-κB signaling.
Fig. 8.
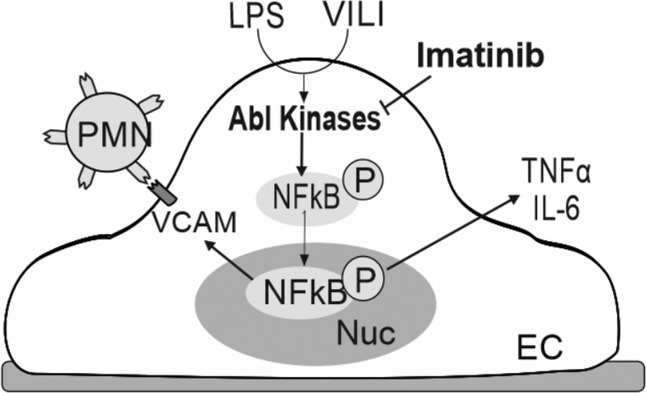
Potential protective effects of imatinib in lung endothelial inflammation induced by LPS plus VILI. In this proposed schema, stimulation of lung endothelial cells (EC) by LPS + VILI results in activation of the Abl kinases and subsequent downstream signaling that includes increased NF-κB activity, upregulation of VCAM-1, release of cytokines (TNF-α, IL-6), and increased pulmonary neutrophil (PMN) recruitment. Imatinib inhibits Abl family kinases to attenuate these effects in vitro and in vivo. Nuc, nucleus.
An important limitation of our work is that imatinib inhibits multiple kinases, and therefore specific conclusions about the mechanism responsible for its protective effects cannot be made. For example, imatinib decreased PDGFR expression in a model of LPS-induced ALI in neutropenic mice (27), and inhibition of PDGFR with imatinib has been implicated in the resolution of vascular leak across the blood-brain barrier in mice (46). In our previous work, we observed that the effect of imatinib on LPS-induced VCAM-1 upregulation was mediated by c-Abl, but not Arg (29). Given that VCAM-1 upregulation occurs downstream of NF-κB activation, we anticipate that the effects of imatinib on NF-κB activation are mediated by c-Abl. However, additional work is necessary to determine the mechanisms underlying these protective effects in ALI, including the pathway through which imatinib inhibits LPS-induced NF-κB activation.
In conclusion, we have demonstrated that imatinib is efficacious in two clinically relevant murine models of ALI. These results suggest that imatinib attenuates the inflammation and vascular leak induced by LPS when combined with VILI and that these protective effects outweigh the deleterious effects of this intervention previously reported in VILI alone. Multiple imatinib targets may be implicated in these effects, and additional studies are required to characterize their roles in the regulation of endothelial barrier integrity and inflammatory signaling pathways. Given that imatinib and several additional Abl family kinase inhibitors are currently in clinical use for multiple medical conditions (39), this work has potential for rapid translation into clinical trials in patients.
GRANTS
This work was supported by NHLBI/NIH HL058064 (J. Garcia), F30HL121982 (A. Rizzo), AHA 14PRE18860021 (A. Rizzo), and University of Illinois institutional funds.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.N.R., J.G.G., and S.M.D. conception and design of research; A.N.R., S.S., A.E.E., and E.L. performed experiments; A.N.R., J.G.G., and E.L. analyzed data; A.N.R., J.R.J., and S.M.D. interpreted results of experiments; A.N.R. prepared figures; A.N.R. drafted manuscript; A.N.R. and S.M.D. approved final version of manuscript; J.R.J., J.G.G., E.L., and S.M.D. edited and revised manuscript.
REFERENCES
- 1.Ventilation with lower tidal volumes compared with traditional tidal volumes for acute lung injury, and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342: 1301–1308, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Adzemovic MZ, Zeitelhofer M, Eriksson U, Olsson T, Nilsson I. Imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response. PLoS One 8: e56586, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akashi N, Matsumoto I, Tanaka Y, Inoue A, Yamamoto K, Umeda N, Hayashi T, Goto D, Ito S, Sekiguchi K, Sumida T. Comparative suppressive effects of tyrosine kinase inhibitors imatinib and nilotinib in models of autoimmune arthritis. Mod Rheumatol 21: 267–275, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Aman J, Peters MJ, Weenink C, van Nieuw Amerongen GP, Vonk Noordegraaf A. Reversal of vascular leak with imatinib. Am J Respir Crit Care Med 188: 1171–1173, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Aman J, van Bezu J, Damanafshan A, Huveneers S, Eringa EC, Vogel SM, Groeneveld AB, Vonk Noordegraaf A, van Hinsbergh VW, van Nieuw Amerongen GP. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation 126: 2728–2738, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Cao C, Leng Y, Huang W, Liu X, Kufe D. Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases. J Biol Chem 278: 39609–39614, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Cao C, Leng Y, Kufe D. Catalase activity is regulated by c-Abl and Arg in the oxidative stress response. J Biol Chem 278: 29667–29675, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Carnevale-Schianca F, Gallo S, Rota-Scalabrini D, Sangiolo D, Fizzotti M, Caravelli D, Capaldi A, Anselmetti G, Palesandro E, D'Ambrosio L, Coha V, Obert R, Aglietta M, Grignani G. Complete resolution of life-threatening bleomycin-induced pneumonitis after treatment with imatinib mesylate in a patient with Hodgkin's lymphoma: hope for severe chemotherapy-induced toxicity? J Clin Oncol 29: e691–693, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Chen A, Lee SM, Gao B, Shannon S, Zhu Z, Fang D. c-Abl-mediated tyrosine phosphorylation of the T-bet DNA-binding domain regulates CD4+ T-cell differentiation and allergic lung inflammation. Mol Cell Biol 31: 3445–3456, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chislock EM, Pendergast AM. Abl family kinases regulate endothelial barrier function in vitro and in mice. PLoS One 8: e85231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chislock EM, Ring C, Pendergast AM. Abl kinases are required for vascular function, Tie2 expression, and angiopoietin-1-mediated survival. Proc Natl Acad Sci USA 110: 12432–12437, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciarcia R, Vitiello MT, Galdiero M, Pacilio C, Iovane V, d'Angelo D, Pagnini D, Caparrotti G, Conti D, Tomei V, Florio S, Giordano A. Imatinib treatment inhibit IL-6, IL-8, NF-KB and AP-1 production and modulate intracellular calcium in CML patients. J Cell Physiol 227: 2798–2803, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-κB and cytokine-inducible enhancers. FASEB J 9: 899–909, 1995. [PubMed] [Google Scholar]
- 14.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest 114: 1308–1316, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Ménorval MA, Mir LM, Fernández ML, Reigada R. Effects of dimethyl sulfoxide in cholesterol-containing lipid membranes: a comparative study of experiments in silico and with cells. PLoS One 7: e41733, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA, Investigators I . Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 355: 2408–2417, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344: 1031–1037, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Dudek SM, Chiang ET, Camp SM, Guo Y, Zhao J, Brown ME, Singleton PA, Wang L, Desai A, Arce FT, Lal R, Van Eyk JE, Imam SZ, Garcia JG. Abl tyrosine kinase phosphorylates nonmuscle Myosin light chain kinase to regulate endothelial barrier function. Mol Biol Cell 21: 4042–4056, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD, NIH NHLBI ARDS Network . Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med 37: 1574–1579, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia AN, Vogel SM, Komarova YA, Malik AB. Permeability of endothelial barrier: cell culture and in vivo models. Methods Mol Biol 763: 333–354, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Goldman JL, Sammani S, Kempf C, Saadat L, Letsiou E, Wang T, Moreno-Vinasco L, Rizzo AN, Fortman JD, Garcia JG. Pleiotropic effects of interleukin-6 in a “two-hit” murine model of acute respiratory distress syndrome. Pulm Circ 4: 280–288, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldsby R, Pulsipher M, Adams R, Coffin C, Albritton K, Wagner L. Unexpected pleural effusions in 3 pediatric patients treated with STI-571. J Pediatr Hematol Oncol 24: 694–695, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Held HD, Boettcher S, Hamann L, Uhlig S. Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-kappaB and is blocked by steroids. Am J Respir Crit Care Med 163: 711–716, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Hägerkvist R, Sandler S, Mokhtari D, Welsh N. Amelioration of diabetes by imatinib mesylate (Gleevec): role of β-cell NF-κB activation and anti-apoptotic preconditioning. FASEB J 21: 618–628, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Kim IK, Rhee CK, Yeo CD, Kang HH, Lee DG, Lee SH, Kim JW. Effect of tyrosine kinase inhibitors, imatinib and nilotinib, in murine lipopolysaccharide-induced acute lung injury during neutropenia recovery. Crit Care 17: R114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko YA, Yang MC, Huang HT, Hsu CM, Chen LW. NF-κB activation in myeloid cells mediates ventilator-induced lung injury. Respir Res 14: 69, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Letsiou E, Rizzo AN, Sammani S, Naureckas P, Jacobson JR, Garcia JG, Dudek SM. Differential and opposing effects of imatinib on LPS- and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 308: L259–L269, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li LF, Yu L, Quinn DA. Ventilation-induced neutrophil infiltration depends on c-Jun N-terminal kinase. Am J Respir Crit Care Med 169: 518–524, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Lin SM, Lin HC, Lee KY, Huang CD, Liu CY, Wang CH, Kuo HP. Ventilator-induced injury augments interleukin-1beta production and neutrophil sequestration in lipopolysaccharide-treated lungs. Shock 28: 453–460, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Liu YY, Liao SK, Huang CC, Tsai YH, Quinn DA, Li LF. Role for nuclear factor-kappaB in augmented lung injury because of interaction between hyperoxia and high stretch ventilation. Transl Res 154: 228–240, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Moitra J, Sammani S, Garcia JG. Re-evaluation of Evans Blue dye as a marker of albumin clearance in murine models of acute lung injury. Transl Res 150: 253–265, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Mokhtari D, Li T, Lu T, Welsh N. Effects of Imatinib Mesylate (Gleevec) on human islet NF-kappaB activation and chemokine production in vitro. PLoS One 6: e24831, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ning QM, Wang XR. Activations of mitogen-activated protein kinase and nuclear factor-kappaB by mechanical stretch result in ventilation-induced lung injury. Med Hypotheses 68: 356–360, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Overbeek MJ, van Nieuw Amerongen GP, Boonstra A, Smit EF, Vonk-Noordegraaf A. Possible role of imatinib in clinical pulmonary veno-occlusive disease. Eur Respir J 32: 232–235, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet 44: 879–894, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Pugin J. Molecular mechanisms of lung cell activation induced by cyclic stretch. Crit Care Med 31: S200–S206, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, Köcher T, Superti-Furga G. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 110: 4055–4063, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem 274: 30353–30356, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol 5: 228–231, 2006. [PubMed] [Google Scholar]
- 42.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 115: 2811–2821, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shu YS, Tao W, Miao QB, Zhu YB, Yang YF. Improvement of ventilation-induced lung injury in a rodent model by inhibition of inhibitory κB kinase. J Trauma Acute Care Surg 76: 1417–1424, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 369: 2126–2136, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Stephens RS, Servinsky LE, Rentsendorj O, Kolb TM, Pfeifer A, Pearse DB. Protein kinase G increases antioxidant function in lung microvascular endothelial cells by inhibiting the c-Abl tyrosine kinase. Am J Physiol Cell Physiol 306: C559–C569, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U, Lawrence DA. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med 14: 731–737, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan SY, Kan E, Lim WY, Chay G, Law JH, Soo GW, Bukhari NI, Segarra I. Metronidazole leads to enhanced uptake of imatinib in brain, liver and kidney without affecting its plasma pharmacokinetics in mice. J Pharm Pharmacol 63: 918–925, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Uhlig U, Haitsma JJ, Goldmann T, Poelma DL, Lachmann B, Uhlig S. Ventilation-induced activation of the mitogen-activated protein kinase pathway. Eur Respir J 20: 946–956, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Wadgaonkar R, Linz-McGillem L, Zaiman AL, Garcia JG. Endothelial cell myosin light chain kinase (MLCK) regulates TNFalpha-induced NFkappaB activity. J Cell Biochem 94: 351–364, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Chiang ET, Simmons JT, Garcia JG, Dudek SM. FTY720-induced human pulmonary endothelial barrier enhancement is mediated by c-Abl. Eur Respir J 38: 78–88, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Wolf AM, Wolf D, Rumpold H, Ludwiczek S, Enrich B, Gastl G, Weiss G, Tilg H. The kinase inhibitor imatinib mesylate inhibits TNF-alpha production in vitro and prevents TNF-dependent acute hepatic inflammation. Proc Natl Acad Sci USA 102: 13622–13627, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]