Abstract
In utero, fetal lung epithelial cells actively secrete Cl− ions into the lung air spaces while Na+ ions follow passively to maintain electroneutrality. This process, driven by an electrochemical gradient generated by the Na+-K+-ATPase, is responsible for the secretion of fetal fluid that is essential for normal lung development. Shortly before birth, a significant upregulation of amiloride-sensitive epithelial channels (ENaCs) on the apical side of the lung epithelial cells results in upregulation of active Na+ transport. This process is critical for the reabsorption of fetal lung fluid and the establishment of optimum gas exchange. In the adult lung, active Na+ reabsorption across distal lung epithelial cells limits the degree of alveolar edema in patients with acute lung injury and cardiogenic edema. Cl− ions are transported either paracellularly or transcellularly to preserve electroneutrality. An increase in Cl− secretion across the distal lung epithelium has been reported following an acute increase in left atrial pressure and may result in pulmonary edema. In contrast, airway epithelial cells secrete Cl− through apical cystic fibrosis transmembrane conductance regulator and Ca2+-activated Cl− channels and absorb Na+. Thus the coordinated action of Cl− secretion and Na+ absorption is essential for maintenance of the volume of epithelial lining fluid that, in turn, maximizes mucociliary clearance and facilitates clearance of bacteria and debris from the lungs. Any factor that interferes with Na+ or Cl− transport or dramatically upregulates ENaC activity in airway epithelial cells has been associated with lung diseases such as cystic fibrosis or chronic obstructive lung disease. In this review we focus on the role of the ENaC, the mechanisms involved in ENaC regulation, and how ENaC dysregulation can lead to lung pathology.
Keywords: oxidative stress, plasminogen activator, steroid hormones, stem cells, β-adrenergic agonists
the most important ion transport process of normal alveolar epithelial cells is active transport of Na+ from the alveolar lining fluid (ALF) to the interstitium, down an electrochemical gradient created by the basolateral Na+-K+-ATPase (5, 94, 98). Cl− ions move passively across paracellular junctions of alveolar epithelial cells or transcellularly to maintain electroneutrality (98). The vectorial movement of Na+ and Cl− creates an osmotic gradient, which causes fluid to move passively from the air space to the interstitium [alveolar fluid clearance (AFC)]. This process is crucial for the reabsorption of alveolar edema following alveolar flooding, which occurs when alveolar permeability to fluid and plasma proteins is increased, resulting in more efficient gas exchange. For example, patients with acute lung injury (ALI) or adult respiratory distress syndrome (ARDS) with normal AFC have lower morbidity and mortality than those with compromised AFC (98, 99). Furthermore, Na+ transport through epithelial Na+ channels (ENaCs) is also essential for the resolution of high-altitude edema (130). In mouse models, inhibition of α-ENaC (the pore-forming subunit of ENaC) by siRNA in the adult lung decreased basal fluid clearance, as well as terbutaline-stimulated clearance (87). Complete knockout (KO) of α-ENaC is lethal, because these mice are unable to clear lung fluid at birth (61). Conditions that increase Cl− secretion across alveolar epithelial cells (such as an acute increase in left atrial pressure) may result in pulmonary edema (138). Novel methodologies using imaging techniques to assess Na+-driven AFC have been reviewed recently by Gammon et al. (43).
In contrast, airway epithelial cells absorb Na+ and actively secrete Cl−, which enter the basolateral membranes through Na+-K+-2Cl− transporters (94), are transported down an electrochemical gradient generated by the basolateral Na+-K+-ATPase, and exit through apical Cl− channels, including the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) (124) and transmembrane protein 16a (TMEM16A). TMEM16A, a Cl− channel and member of the anoctamin family, is thought to compensate for CFTR in the mouse lung, potentially providing a rationale for the lack of a lung phenotype in CFTR KO mice (126). However, other channels, such as CLCN2 (131), must play a role, since up to 40% of the UTP-stimulated Ca2+-activated Cl− channel activity persists in TMEM16A KO mice. Interestingly, recent experiments have shown CFTR to play a direct role in Ca2+-activated Cl− transport (126).
The airway surface liquid (ASL), which is composed of the periciliary layer (PCL) and the mucus layer, coats the lining of the airways and functions to trap and expel inhaled pathogens and particulate matter (reviewed in Ref. 89). Under normal conditions, the PCL is ∼7 μm thick; this layer is critical for cilia-mediated transport of the mucus to the larynx, and effective mucociliary transport clears the airway surface of pathogens and other noxious substances (89). The ENaCs, the CFTR, and Ca2+-activated Cl− channels work in concert to regulate PCL height, viscosity, and/or pH and, therefore, are critical components of mucociliary clearance (MCC) (Fig. 1) (8, 23, 24, 37, 44, 72, 96, 118, 137, 152). Overexpression of the ENaC β-subunit in mice produces CF-like lung disease because of an excess amount of fluid absorption (93). Compromised MCC leads to chronic bacterial infections and subsequent inflammatory responses in a number of lung diseases, including CF and chronic obstructive pulmonary disease (COPD) (1, 119, 123). The focus of this review is on the role of ENaC in surface hydration (23) and the factors that influence its expression and function, rather than on how changes in pH in the PCL influence mucus viscosity and elasticity due to changes in bicarbonate secretion (16, 25, 30, 118).
Fig. 1.
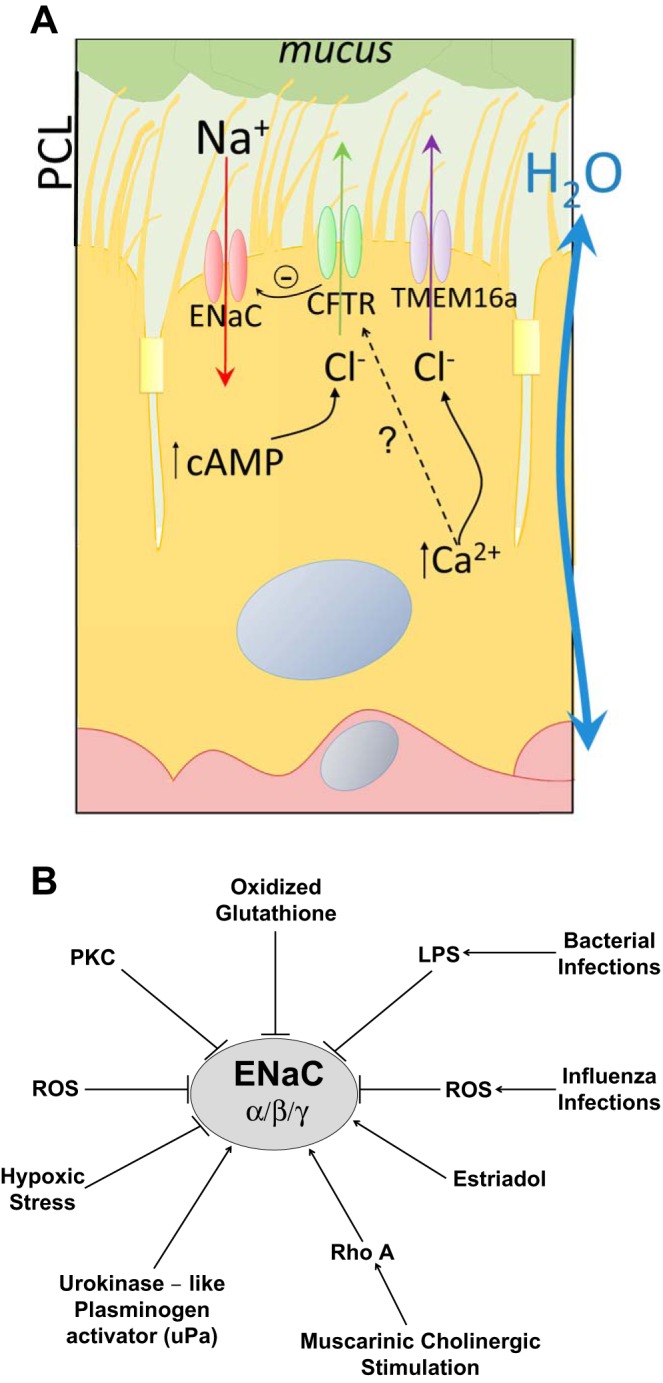
A: apical Na+ and Cl−/bicarbonate channels in alveolar epithelia and their role in regulating airway surface liquid levels in the lung. Three channels in alveolar epithelial cells regulate ion composition in the airway surface liquid. In turn, ion composition regulates the water levels and pH required for efficient gas exchange and mucociliary clearance. Alterations in Na+ channel function often lead to a number of lung diseases, and one prominent model, the volume hypothesis, suggests that decreases in cystic fibrosis transmembrane conductance regulator (CFTR) expression and/or function lead to elevated epithelial Na+ channel (ENaC) function, increased Na+ absorption, and subsequently, lung dehydration and defects in mucociliary transport. This chronic bronchitis phenotype would be illustrated in cystic fibrosis and chronic obstructive pulmonary disease patients and lead to bacterial infections, inflammation, and, eventually, decreases in lung function. PCL, periciliary layer. B: factors that elevate or inhibit ENaC channel expression and/or function in alveolar cells. A number of factors enhance or inhibit ENaC activity, and each of those shown is discussed in this review. LPS, lipopolysaccharide; PKC, protein kinase C; ROS, reactive oxygen species.
Oxidative Stress Effects on Na+ Channel Activity
Oxidative stress is a common component in many lung diseases, including ALI, COPD, asthma, and lung cancer (78, 108, 112, 120, 144, 145). To combat oxidative stress in the lower respiratory tract, a number of antioxidants, including ascorbate, urate, and glutathione (GSH), are present in high levels in the alveolar epithelial lining fluid (9, 13, 59, 74, 86, 140, 149). The redox potential of the GSH-to-oxidized GSH (GSSG) ratio is known to correlate with the ability of the lungs to suppress an oxidative insult (11, 150), and GSSG is increased in the alveolar fluid of patients with ARDS (12). Recently, Downs and colleagues (32, 33) provided a mechanism for why this is a problem. They found that GSSG inhibits ENaC activity in primary alveolar epithelial cells (32, 33). They examined the effect of decreasing the ratio of GSH to GSSG on ENaC activity by examining single-channel recordings from primary alveolar type 1 (AT1) and type 2 (AT2) cells from lung slices or isolated from rat lungs (33). The oxidized redox potentials decreased ENaC activity, and in vivo studies demonstrated compromised fluid clearance in GSSG-instilled mouse lungs. These studies demonstrated that the redox potential of the lung directly affected lung fluid balance (32) through modulation of ENaC activity and suggest that the regulation of the redox potential by antioxidants needs to be tested as a potential therapy, given the demonstrated inhibitory effects of GSSG on ENaC activity. Indeed, antioxidants prevented and reversed Cl−-induced decreases in amiloride-sensitive short-circuit currents across confluent monolayers of rat alveolar epithelial cells mounted in Ussing chambers (79).
Cholinergic Regulation of Na+ channels
Cholinergic receptors in bronchial smooth muscle are well-established targets in COPD and asthma (49, 53, 127). Takemura and colleagues (143) examined the role of cholinergic regulation of ENaC in alveolar epithelial cells. Using single-channel analysis and biochemical approaches, they found that carbachol and oxotremorine activated muscarinic receptors in AT2 cells and activated ENaC in a dose-dependent manner, whereas nicotine did not. This activity could be blocked with atropine, supporting the view that ENaC activity was induced by activation of muscarinic cholinergic receptors in the AT2 cells. Takemura and colleagues also showed that the AT2 cells had muscarinic M2 and M3 cholinergic receptors and that the cholinergic agonists activated RhoA in these cells. Finally, an inhibitor of Rho-associated protein kinase, Y-27632, was shown to block carbachol-induced activation of ENaC, as well as even basal ENaC activity, suggesting that RhoA activity is essential for this process in rat AT2 cells (143). The connection between RhoA activity and ENaC activity may be in part due to the known effects of RhoA on the cytoskeleton and in promoting ENaC trafficking to the plasma membrane (115). The importance of these studies is highlighted by the fact that, in COPD, where cholinergic regulation is dominant, hypersecretion occurs in the airway, whereas the present results suggest that Na+ reabsorption and water in the alveolar space could partially compensate for this effect (143). However, activation of RhoA is known to disrupt lung barrier function (67, 102, 114, 132). These studies emphasize the need to understand the overall regulation of secretion and absorption in the various locations in the lung in different lung pathologies (92).
Plasminogen Activator Effects on Na+ Channels
Urokinase-like plasminogen activator (uPA) is a serine protease that converts plasminogen to active plasmin, triggering a proteolysis cascade that promotes fibrinolysis. uPA is expressed in the airways, and its activity is readily detectable in lung lavage fluids of normal animals (62, 97, 136). Perhaps more importantly, the majority of plasminogen activator activity in the lung lavage fluids is from uPA, rather than tissue plasminogen activator (76). Given that fibrinolytic proteases such as uPA have been implicated in a number of lung diseases (129), Chen et al. (21) measured ENaC activity in primary murine tracheal epithelial cells from uPA−/− mice. In Ussing chamber experiments, they demonstrated that both basal and cAMP-activated ENaC were reduced in monolayers of tracheal epithelial cells from uPA-deficient mice. The fluid height of the mouse tracheal epithelial cell cultures was more than double that of the uPA−/− cells compared with wild-type controls. Causality was demonstrated by the addition of uPA and plasmin to the uPA−/− cells, which was able to partially reverse this effect. uPA is known to exert its effects through multiple processes, including the direct proteolysis of the γ-chain of ENaC, regulation of Na+-K+-ATPase activity, and modulation of ERK1/2 signaling (7, 60, 83, 113, 135). This study represents the first evidence that uPA upregulates ENaC activity in vitro and in vivo and indicates that alteration of uPA activity could lead to increases in ASL through decreases in ENaC activity. Furthermore, it suggests that changes in uPA activity in injured lungs could directly influence ENaC activity and, therefore, alter the composition or levels of airway fluid, thus further exacerbating lung injury. On the other hand, increased ENaC activity will limit the degree of alveolar edema in ALI (98, 100).
Steroid Hormone Effects on Alveolar Fluid Clearance
Sex differences show up in many aspects of physiology, including lung pathology (14, 55, 70, 77). A good example is the greater incidence of bacterial lung infections and higher risk of hospitalizations for female than male CF patients (109, 141, 153). Furthermore, this increased susceptibility to severe and often fatal complications from lung influenza infections also appears to be the case in young adult female patients with influenza-associated pneumonia compared with male patients (71, 133). A common feature of influenza infections and CF is the presence of ENaC dysregulation (20, 23). Given that female patients have poorer outcomes in respiratory disorders, Greenlee and coworkers (52) examined the effect of estradiol on ENaC in rat alveolar cells. Cell-attached patch-clamp analysis was used to monitor single-channel ENaC activity in a rat alveolar cell line [L2, which has an AT2 phenotype (31)] after overnight treatment with estradiol or progesterone. They established that estradiol, but not progesterone, increased ENaC activity by increasing the open probability (Po) and number of channels. Comparison of lung homogenates from female rats in proestrus vs. diestrus revealed that when serum estradiol levels are highest (in proestrus), the plasma membrane levels of ENaC are at their highest. Estradiol primarily affected the activity of the nonselective ENaC channels, and these effects were mediated through the G protein-coupled estrogen receptor (52). Greenlee and coworkers point out an interesting correlation between estradiol and complications in females from influenza-associated pneumonia. In female rats, influenza is known to cause a constant state of diestrus, when the estradiol levels are at their lowest, and treatment of these animals with estradiol attenuates the mortality (125). Greenlee and coworkers propose that low levels of estradiol in female influenza patients lead to reduced AFC and that the mechanisms involved here should be investigated further given the potential therapeutic implications.
Bacterial Infections and ENaC Activity
A number of factors influence ENaC activity and expression in alveolar epithelial cells. ENaC expression is increased by glucocorticoids (26, 28, 64, 84) and decreased by interleukin-1β (128), interleukin-4 (41), and transforming growth factor-β (38). The effects of tumor necrosis factor (TNF)-α are more controversial, with some studies showing that TNFα increases ENaC activity (40) and others showing that TNFα inhibits it. This controversy was reconciled by Braun et al. (10), who reported results indicating that the receptor-binding sites of TNF inhibit ENaC, whereas its lectin-like domain activates ENaC.
In rat alveolar epithelial cells, an inducer of cellular stress, cycloheximide, and inflammatory responses to lipopolysaccharide (LPS), a glycolipid from the outermost membrane of gram-negative bacteria, were recently shown to downregulate α-ENaC mRNA levels (103). To understand the mechanisms involved, Migneault and colleagues (103) followed a time course of treatments and found that both methods decreased α-ENaC by ∼50% in as little as 4 h. Interestingly, the effect of cycloheximide was not dependent on its ability to inhibit translation. Both treatments downregulated α-ENaC message via the ERK and p38 MAPK pathways, although LPS appeared to inhibit α-ENaC promoter activity, whereas cycloheximide inhibited ENaC through posttranscriptional effects. The authors posit that the LPS effects could be due to a proinflammatory response, whereas the cycloheximide response appeared to be due to a nonspecific stress response that did not involve reactive oxygen species (ROS) but apparently did affect proinflammatory cytokines (103). A difference between the two effectors was clear, since inhibition of either pathway was sufficient to block the cycloheximide effects, whereas simultaneous inhibition of both pathways was required to block the LPS effect. Given that Pseudomonas aeruginosa is the most common bacterial infection in CF and that P. aeruginosa is known to downregulate ENaC in infected mouse lungs (27), this could suggest that any inflammatory or stress condition in the lungs would have a detrimental effect on ENaC activity, even without invoking reactive species, which are known to inhibit ENaC (32, 155).
Protein Kinase C and ENaC Activity
An important signaling molecule for regulating ENaC activity is protein kinase C (PKC). PKC activation inhibits ENaC activity (39, 88, 142), and, conversely, PKC inhibition enhances ENaC activity (88, 151). Although there is significant information about the role of PKC phosphorylation in the kidney (4, 39), there is only one isoform in the principal cells (69). There are, however, multiple isoforms of PKC in the lung (147), suggesting that there could be differences in PKC regulation in the lung. To test the role of PKC phosphorylation in the lung, Eaton et al. (35) used a mouse PKCα KO model. In cell-attached patch-clamp studies in AT2 cells, they demonstrated that the ENaC Po and the number of channels were significantly decreased in the PKCα KO mice compared with controls. Using biochemical approaches, they demonstrated that all three ENaC subunits (α, β, and γ) were expressed at lower levels in the KO mouse lung and that this correlated with decreased Na+ transport in AT2 cells (35). Because alveolar tissues are exposed to high levels of oxygen, they used a fluorescent superoxide reporter and lung slices to show that the PKCα KO animals produce nearly twofold more superoxide than the wild-type animals. The superoxide dismutase activities were also decreased in the cytosol and mitochondria in the KO animals. ERK1/2 activity was elevated in the KO lung (35), and ERK1/2 phosphorylation of ENaC promotes Nedd4-2 interactions with ENaC (7), which leads to ENaC ubiquitination and subsequent degradation. The ROS activates PKCδ, which reduces ERK1/2 dephosphorylation and leads to decreased amounts of ENaC. Eaton et al. also showed that the decreased ENaC Po was attributed to PKCδ phosphorylation of myristoylated alanine-rich PKC substrate. Myristoylated alanine-rich PKC substrate (36), which acts as a scaffolding protein at the plasma membrane that promotes phosphatidylinositol phosphate interactions with ENaC and enhances activity, dissociates from the membrane after phosphorylation, which lowers the phosphatidylinositol phosphate concentration and the ENaC activity. To establish the role of ROS and PKCδ in this process, a ROS scavenger or PKCδ inhibitor was used in patch-clamp experiments, and these manipulations blocked these effects (35). From these data, Eaton et al. speculate that a PKCα-PKCδ double KO would cause ENaC activity to increase. There is evidence to indicate that reactive intermediates damage ENaC and inhibit Na+-driven AFC in vivo (18, 34, 56, 139). On the other hand, it has been shown that small amounts of superoxide are capable of upregulating ENaC and stimulating Na+-driven AFC in vivo (32, 47).
There is also evidence that another isoform of PKC (PKCζ) is involved in the downregulation of ENaC. Davis et al. (29) reported that ENaC was not activated by β2-agonists in respiratory syncytial virus-infected mice, because KC (IL-8) promotes hydrolysis of GTP by the inhibitory α-subunit of the receptor-associated heterotrimeric G protein (Giα), which then activates PKCζ. In turn, PKCζ phosphorylates and activates G protein-coupled receptor kinase 2, which serine phosphorylates β2-adrenergic receptor, uncoupling it from Gsα. Upon binding of β-agonist, these phosphorylated receptors are unable to activate adenylyl cyclase, and no AFC response is detected (29). Ji et al. (65) reported that severe acute respiratory syndrome coronavirus proteins decrease levels and activity of human ENaC via activation of PKCα/β and PKCζ. Lazrak et al. (81) showed that activation of a number of PKC isoforms (α, β1, and ζ) plays an essential role in the downregulation of ENaC by the M2 protein of influenza virus. Infection of lung epithelial cells with inactivated influenza virus A/PR/8/34 (PR8;H1N1) inhibited ENaC function and decreased Na+ transport across airway murine distal epithelial cells, and these events were prevented by pretreatment with PKC inhibitors (20).
CF and a Mutation in the ENaC β-Subunit
ASL levels and composition are regulated by a critical balance between Na+ absorption and Cl−/bicarbonate secretion (23). In CF, the Cl− and bicarbonate channel CFTR has severely compromised surface expression and/or function, which leads to mucociliary transport defects, bacterial infections, inflammatory responses, and, eventually, lung failure. Furthermore, loss of CFTR function leads to dysregulated ENaC function (82). In an interesting study, Rauh et al. (122) examined a mutation in the β-subunit of ENaC (βV348M) that had been identified in a patient with severe CF-like symptoms (105). To fully characterize this mutation, they expressed the αβγ-ENaC channels with wild-type ENaC or with the mutant β-subunit in Xenopus laevis oocytes and monitored whole cell and single-channel conductances. The analysis revealed that the Po was increased nearly twofold with the βV348M mutation (122). This gain-of-function mutation was confirmed in transfected HEK 293 cells. Computational channel modeling, along with functional analysis, suggested that the gain-of-function open channel was caused by a destabilized closed-channel state. These studies are significant, since they clearly point to how elevation of ENaC activity in the presence of one mutant CF allele can promote a severe CF phenotype and support the idea that enhanced ENaC activity contributes to the pathophysiology of CF.
In that regard, it is interesting that an 18-amino acid peptide from residue G22–A39 of a natural inhibitor of ENaC in airway epithelial, short palate lung and nasal epithelial clone 1 (SPLUNC1) has been shown to be useful in treating Na+ hyperabsorption in CF airway epithelial cultures (57). Treatment of CF has often involved correction of Cl− channel activity or suppression of ENaC activity, and this was further supported by studies of Lazrak and colleagues (83), who examined the role of inter-α-inhibitor (IαI), a protease inhibitor, on ENaC activity in CF. There are two pools of ENaC channels at the cell surface, an active proteolytically cleaved pool and an inactive noncleaved pool. The results show that IαI is present in the bronchoalveolar lavage fluid of children with CF (83), suggesting that it could potentially inhibit ENaC activity in CF patients and, thus, block Na+ hyperabsorption. The results of Lazrak and colleagues, in lung slices from ΔF508 CF mice, showed that IαI was an effective inhibitor of ENaC proteolysis and was able to decrease ENaC activity in the lung epithelial cells from ΔF508 CF mice. Since reactive species activate PKC (32), these two signaling molecules may act in synergy to downregulate ENaC in a number of pathological conditions.
Other ENaC Subunits: δ-ENaC
The fourth subunit of ENaC, the δ-subunit, was recently cloned in human and monkey (reviewed in Ref. 66). The δ-subunit is expressed in epithelial and nonepithelial tissues and has an expression profile similar to the γ-subunit (66). High levels of expression of the δ-subunit are found in a number of human tissues, including liver, heart, skeletal muscle, prostate, pituitary, smooth muscle, and lung. A prevailing view is that a functional ENaC is composed of at least one α-like subunit (α or δ) and that the β- and γ-subunits are required to amplify channel activity (66). In the lung, the δ-ENaC subunit has been found in human primary alveolar cells and shown to contribute ∼50% of the amiloride-sensitive salt transport across human nasal epithelial cells (3). Interestingly, but unfortunately, the δ-subunit is not expressed in the mouse (46), making functional analysis of this subunit more difficult. Highly selective Na+ channels have been reported to contain α-, β-, and γ-ENaCs, whereas channels consisting of other combinations of subunits have decreased selectivity for Na+ over K+ and higher amiloride IC50 (18, 154). Furthermore, ENaC channels containing δ-ENaC are activated by PKGII and cGMP, in addition to cAMP (106). Elevated levels of the δ-subunit have been associated with chronic sinusitis and allergic disorders, and reduced expression has been associated with rhinovirus diseases (reviewed in Ref. 66). Children with the genetic deletion of this subunit are predisposed to respiratory infections and nasal congestion. Finally, whether channels containing δ-ENaC are essential in lung fluid homeostasis will require in vivo and ex vivo studies in primates.
β-Adrenergic Effects on Anion Secretion
ASL is regulated by Na+ channel absorption and Cl− channel secretion, and Shamsuddin and Quinton (134) questioned the hypothesis that absorption and secretion have to occur in the same cells. In studies in Calu-3 cells, which are a model for serous cells of airway submucosal glands, Banga at al. (2) examined the role of epinephrine in cells that do not normally express ENaC. Interestingly, CFTR in Calu-3 cells is stimulated by nitric oxide and compounds that increase cGMP (17, 19). The results confirmed the lack of ENaC in these cells and showed that this β-adrenergic receptor agonist stimulated two Cl− channels, CFTR and TMEM16A. TMEM16A was shown recently to be present in serous cells and Calu-3 cells (72), as well as human and mouse lung airway smooth muscle cells (42). Furthermore, isolated trachea from TMEM16A KO mice revealed reduced MCC (86), suggesting that both CFTR and TMEM16A are required for normal hydration in mice. Using inhibitors of CFTR and TMEM16A, Ousingsawat et al. (111) also found that the combination of inhibitors had a more profound effect than the additive effect of the separate inhibitors. This result supports the view that CFTR and TMEM16A are functionally linked, as proposed by Kunzelmann et al. (75), and that this linkage may involve Ca2+ elevations that may also stimulate CFTR (6). In addition, in human airway smooth muscle cells, activation of TMEM16A by intracellular Ca2+ secondary to oxidant stress results in membrane depolarization, which may contribute to increased airway reactivity (80).
Therapies: Stem Cells and Ion Transport
The epithelial lining of the lung must maintain a thin ASL for efficient gas exchange. Furthermore, fluid absorption out of the alveolar lumen requires active transport of Na+ from the apical surface of the pulmonary epithelium, across the apical and basolateral membranes, and into the interstitial space and/or bloodstream. Any factor that disrupts Na+ transport results in fluid accumulation and inefficient gas exchange. The importance of this transport process in the lung is clearly demonstrated in a number of human disease processes, where decreased Na+ absorption across the alveolar epithelium contributes to the pathophysiology of pulmonary diseases (reviewed in Refs. 94 and 98), including ALI and ARDS (54, 63, 98).
One potential therapy that holds a lot of promise is the use of bone marrow-derived mesenchymal stem cells (MSCs) because of their ability to differentiate into a number of cell types (51, 63, 117). For example, intravenous and intra-alveolar administrations of MSCs have been shown to decrease the severity of lung damage in a variety of models of ALI (51, 63, 73, 85). Interestingly, although earlier studies (110) reported increased engraftment of systemically administered MSCs in the alveolar epithelium after bleomycin lung injury, a number of other studies found protective effects of MSC therapy, despite low engraftment rates. Furthermore, the lung recovery rates were rapid (1–2 days), suggesting that the protective effects were not due to the MSCs themselves but, rather, their secreted products (51, 63, 73, 85).
With that idea in mind, Ionescu and colleagues (63) demonstrated that treatment with conditioned medium could improve ALI in mice. More recently, Goolaerts and colleagues (48) tested paracrine factors secreted by MSCs in an in vitro model of acute alveolar injury to determine the effectiveness of the treatments but, more importantly, to determine which factors were important for the therapeutic benefit. In this model, primary rat alveolar cells were exposed to hypoxia (3% O2) plus a mixture of cytokines present in ALI pulmonary edema (IL-1β, TNF-α, and IFNγ). Two types of conditioned media were used in these studies, MSC-conditioned medium from human MSCs exposed for 12 h to normoxia (MSC-M) and conditioned medium from MSCs exposed to hypoxia plus the cytokine mixture (HCYT-MSC-M). The HCYT-MSC-M used in this model was tested to mimic the effects of MSCs in the injured lung. The results from this study demonstrated that the inflammatory and hypoxic stress to the alveolar epithelial cells increased transepithelial permeability to albumin and decreased ENaC activity at the apical surface and that keratinocyte growth factor (KGF) secretion by MSCs, which was reduced in the HCYT-MSC-M samples, was required for recovery of AFC due to active Na+ reabsorption (48). These results support the idea that appropriate paracrine factors produced by MSCs in the injured lung hold therapeutic promise by restoring normal Na+ channel function and preventing alveolar flooding.
The role of KGF was supported by another study by McAuley and colleagues (101) in a human ex vivo lung model using lungs that were unsuitable for transplantation and had undergone a prolonged period of ischemia. They tested whether treatment of the lungs with human MSCs could restore normal AFC. Treatment with MSCs restored normal AFC but, more interestingly, KGF was required, since intrabronchial administration of a neutralizing antibody to KGF blocked AFC recovery. The authors suggest that these studies are significant, because they demonstrate that MSCs can enhance AFC in donor lungs with impaired AFC, and this is significant given that only 15–25% of donor lungs are transplanted (101, 116).
Influenza infection is known to damage ENaC and CFTR and decrease their activity in vitro and in vivo (20, 81, 91, 146, 148). Given the positive effects reported in other models of lung injury, Gotts and colleagues (50) investigated whether murine and human bone marrow-derived MSCs administered intravenously during the rapid phase of lung injury in influenza-infected mice would be beneficial in reducing epithelial injury. Although they did not directly measure AFC, their results indicate that administration of stem cells failed to improve lung water levels, bronchoalveolar lavage inflammation, or histology. One possible reason for this negative result comes from studies that show that swine bone marrow-derived MSCs express sialic acid receptors for hemagglutinin binding and, when infected by influenza, secrete proinflammatory cytokines, including TNF-α and IL-6 (68). The influenza-induced alveolar damage in the mouse lung peaked at 1 wk but appeared to last longer than 3 wk (50). These changes appeared to mimic those of ARDS, which highlights the profound effect of influenza infection on lung function.
Inhibition of ENaC to Enhance ASL Hydration
As mentioned above, hydration of the airways is regulated by active transport of Na+ and Cl−, as well as bicarbonate transport, which maintains a ∼7-mm PCL to facilitate cilia function and acts as a lubricant to keep the mucus layer away from the alveolar epithelial cell surface (1). This is a critical function, since hydration of the ASL influences the effectiveness of MCC and dehydration is believed to be a common feature of CF and COPD (1, 22, 23). Given that cigarette smoke decreases CFTR function (22, 95, 104, 121) and loss of CFTR elevates ENaC activity (15, 45, 58, 82, 90), Astrand and colleagues (1) tested the hypothesis that an ENaC inhibitor could be used to properly rehydrate airway cultures that were exposed to cigarette smoke and, thus, restore normal MCC. They tested the ability of a compound that was a potent ENaC inhibitor but, just as importantly, had a long half-life (>20 h) in rats (compound A) to reverse cigarette smoke-induced injury in lung epithelial cells. Their results demonstrate that pretreatment with compound A blocked cigarette smoke-induced ASL dehydration in the bronchial epithelial cultures and that the increased airway height correlated with increased MCC in vivo, supporting the view that ENaC inhibition is a viable approach for enhancing MCC and, therefore, a viable strategy for treatment of patients with chronic bronchitis. Although use of an ENaC inhibitor has been suggested for chronic bronchitis, the concern has always been that inhibitors such as amiloride were too short-acting to be effective in vivo, and importantly, any drug would have to be carefully tested to ensure that it had minimal effects on the kidney (107). In this case, compound A was used to demonstrate that inhibition of ENaC is a viable strategy in chronic lung diseases, but, unfortunately, it would be unsuitable as a therapeutic because of its effects on renal handling of potassium (1).
Summary and Conclusions
Therapeutic strategies for curing or ameliorating lung pathologies such as acute lung disease, CF, and COPD require an understanding of the molecular mechanisms that control normal airway hydration and MCC and the factors that regulate these processes. One clear target is the ENaC in alveolar epithelial cells. Although significant progress is being made in developing long-acting ENaC inhibitors, finding one that does not have untoward effects in other tissues has proven to be difficult.
GRANTS
This work was supported by National Institutes of Health Grants 2R01 HL-0311971 and R01 DK-060065, as well as the CounterACT Program, National Institutes of Health Office of the Director, and National Institutes of Health Grants 5R21 ES-024027-02, R21 ES-025423-01, and 1U01 ES-026458-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M., R.B., and J.F.C. drafted the manuscript; S.M., R.B., and J.F.C. edited and revised the manuscript; S.M., R.B., and J.F.C. approved the final version of the manuscript.
REFERENCES
- 1.Astrand AB, Hemmerling M, Root J, Wingren C, Pesic J, Johansson E, Garland AL, Ghosh A, Tarran R. Linking increased airway hydration, ciliary beating, and mucociliary clearance through ENaC inhibition. Am J Physiol Lung Cell Mol Physiol 308: L22–L32, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banga A, Flaig S, Lewis S, Winfree S, Blazer-Yost BL. Epinephrine stimulation of anion secretion in the Calu-3 serous cell model. Am J Physiol Lung Cell Mol Physiol 306: L937–L946, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangel-Ruland N, Sobczak K, Christmann T, Kentrup D, Langhorst H, Kusche-Vihrog K, Weber WM. Characterization of the epithelial sodium channel δ-subunit in human nasal epithelium. Am J Respir Cell Mol Biol 42: 498–505, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Bao HF, Thai TL, Yue Q, Ma HP, Eaton AF, Cai H, Klein JD, Sands JM, Eaton DC. ENaC activity is increased in isolated, split-open cortical collecting ducts from protein kinase Cα knockout mice. Am J Physiol Renal Physiol 306: F309–F320, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthiaume Y, Matthay MA. Alveolar edema fluid clearance and acute lung injury. Respir Physiol Neurobiol 159: 350–359, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billet A, Hanrahan JW. The secret life of CFTR as a calcium-activated chloride channel. J Physiol 591: 5273–5278, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth RE, Stockand JD. Targeted degradation of ENaC in response to PKC activation of the ERK1/2 cascade. Am J Physiol Renal Physiol 284: F938–F947, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Boucher RC, Cotton CU, Gatzy JT, Knowles MR, Yankaskas JR. Evidence for reduced Cl− and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol 405: 77–103, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bracher A, Doran SF, Squadrito GL, Postlethwait EM, Bowen L, Matalon S. Targeted aerosolized delivery of ascorbate in the lungs of chlorine-exposed rats. J Aerosol Med 25: 333–341, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun C, Hamacher J, Morel DR, Wendel A, Lucas R. Dichotomal role of TNF in experimental pulmonary edema reabsorption. J Immunol 175: 3402–3408, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Brown LA, Ping XD, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol 292: L824–L832, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Bunnell E, Pacht ER. Oxidized glutathione is increased in the alveolar fluid of patients with the adult respiratory distress syndrome. Am Rev Respir Dis 148: 1174–1178, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Cantin AM, Begin R. Glutathione and inflammatory disorders of the lung. Lung 169: 123–138, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey MA, Card JW, Voltz JW, Arbes SJ Jr, Germolec DR, Korach KS, Zeldin DC. It's all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 18: 308–313, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen EY, Sun A, Chen CS, Mintz AJ, Chin WC. Nicotine alters mucin rheological properties. Am J Physiol Lung Cell Mol Physiol 307: L149–L157, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen EY, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol 299: L542–L549, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Bosworth CA, Pico T, Collawn JF, Varga K, Gao Z, Clancy JP, Fortenberry JA, Lancaster JR Jr, Matalon S. DETANO and nitrated lipids increase chloride secretion across lung airway cells. Am J Respir Cell Mol Biol 39: 150–162, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Fuller CM, Kleyman TR, Matalon S. Mutations in the extracellular loop of α-rENaC alter sensitivity to amiloride and reactive species. Am J Physiol Renal Physiol 286: F1202–F1208, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Patel RP, Teng X, Bosworth CA, Lancaster JR Jr, Matalon S. Mechanisms of cystic fibrosis transmembrane conductance regulator activation by S-nitrosoglutathione. J Biol Chem 281: 9190–9199, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Chen XJ, Seth S, Yue G, Kamat P, Compans RW, Guidot D, Brown LA, Eaton DC, Jain L. Influenza virus inhibits ENaC and lung fluid clearance. Am J Physiol Lung Cell Mol Physiol 287: L366–L373, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Zhao R, Zhao M, Liang X, Bhattarai D, Dhiman R, Shetty S, Idell S, Ji HL. Regulation of epithelial sodium channels in urokinase plasminogen activator deficiency. Am J Physiol Lung Cell Mol Physiol 307: L609–L617, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, Bennett WD, Riordan JR, Boucher RC, Tarran R. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J 26: 533–545, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collawn JF, Lazrak A, Bebok Z, Matalon S. The CFTR and ENaC debate: how important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol 302: L1141–L1146, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collawn JF, Matalon S. CFTR and lung homeostasis. Am J Physiol Lung Cell Mol Physiol 307: L917–L923, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper JL, Quinton PM, Ballard ST. Mucociliary transport in porcine trachea: differential effects of inhibiting chloride and bicarbonate secretion. Am J Physiol Lung Cell Mol Physiol 304: L184–L190, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dagenais A, Frechette R, Clermont ME, Masse C, Prive A, Brochiero E, Berthiaume Y. Dexamethasone inhibits the action of TNF on ENaC expression and activity. Am J Physiol Lung Cell Mol Physiol 291: L1220–L1231, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Dagenais A, Frechette R, Yamagata Y, Yamagata T, Carmel JF, Clermont ME, Brochiero E, Masse C, Berthiaume Y. Downregulation of ENaC activity and expression by TNF-α in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L301–L311, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Dagenais A, Gosselin D, Guilbault C, Radzioch D, Berthiaume Y. Modulation of epithelial sodium channel (ENaC) expression in mouse lung infected with Pseudomonas aeruginosa. Respir Res 6: 2, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis IC, Xu A, Gao Z, Hickman-Davis JM, Factor P, Sullender WM, Matalon S. Respiratory syncytial virus induces insensitivity to β-adrenergic agonists in mouse lung epithelium in vivo. Am J Physiol Lung Cell Mol Physiol 293: L281–L289, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derichs N, Jin BJ, Song Y, Finkbeiner WE, Verkman AS. Hyperviscous airway periciliary and mucous liquid layers in cystic fibrosis measured by confocal fluorescence photobleaching. FASEB J 25: 2325–2332, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douglas WH, Chapple PJ. Characterization of monolayer cultures of type II alveolar pneumonocytes that produce pulmonary surfactant in vitro. Dev Biol Stand 37: 71–76, 1976. [PubMed] [Google Scholar]
- 32.Downs CA, Helms MN. Regulation of ion transport by oxidants. Am J Physiol Lung Cell Mol Physiol 305: L595–L603, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Downs CA, Kreiner L, Zhao XM, Trac P, Johnson NM, Hansen JM, Brown LA, Helms MN. Oxidized glutathione (GSSG) inhibits epithelial sodium channel activity in primary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 308: L943–L952, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duvall MD, Zhu S, Fuller CM, Matalon S. Peroxynitrite inhibits amiloride-sensitive Na+ currents in Xenopus oocytes expressing αβγ-rENaC. Am J Physiol Cell Physiol 274: C1417–C1423, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Eaton AF, Yue Q, Eaton DC, Bao HF. ENaC activity and expression is decreased in the lungs of protein kinase C-α knockout mice. Am J Physiol Lung Cell Mol Physiol 307: L374–L385, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang S, Crews AL, Chen W, Park J, Yin Q, Ren XR, Adler KB. MARCKS and HSP70 interactions regulate mucin secretion by human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 304: L511–L518, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkbeiner WE, Zlock LT, Morikawa M, Lao AY, Dasari V, Widdicombe JH. Cystic fibrosis and the relationship between mucin and chloride secretion by cultures of human airway gland mucous cells. Am J Physiol Lung Cell Mol Physiol 301: L402–L414, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, Howard M, Canessa CM, Fang X, Sheppard D, Matthay MA, Pittet JF. Transforming growth factor-β1 decreases expression of the epithelial sodium channel α-ENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 278: 43939–43950, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Frindt G, Palmer LG, Windhager EE. Feedback regulation of Na channels in rat CCT. IV. Mediation by activation of protein kinase C. Am J Physiol Renal Fluid Electrolyte Physiol 270: F371–F376, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Fukuda N, Jayr C, Lazrak A, Wang Y, Lucas R, Matalon S, Matthay MA. Mechanisms of TNF-α stimulation of amiloride-sensitive sodium transport across alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 280: L1258–L1265, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Galietta LJ, Pagesy P, Folli C, Caci E, Romio L, Costes B, Nicolis E, Cabrini G, Goossens M, Ravazzolo R, Zegarra-Moran O. IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J Immunol 168: 839–845, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Gallos G, Remy KE, Danielsson J, Funayama H, Fu XW, Chang HY, Yim P, Xu D, Emala CW Sr. Functional expression of the TMEM16 family of calcium-activated chloride channels in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 305: L625–L634, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gammon ST, Foje N, Brewer EM, Owers E, Downs CA, Budde MD, Leevy WM, Helms MN. Preclinical anatomical, molecular, and functional imaging of the lung with multiple modalities. Am J Physiol Lung Cell Mol Physiol 306: L897–L914, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Garland AL, Walton WG, Coakley RD, Tan CD, Gilmore RC, Hobbs CA, Tripathy A, Clunes LA, Bencharit S, Stutts MJ, Betts L, Redinbo MR, Tarran R. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc Natl Acad Sci USA 110: 15973–15978, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gentzsch M, Dang H, Dang Y, Garcia-Caballero A, Suchindran H, Boucher RC, Stutts MJ. The cystic fibrosis transmembrane conductance regulator impedes proteolytic stimulation of the epithelial Na+ channel. J Biol Chem 285: 32227–32232, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giraldez T, Rojas P, Jou J, Flores C, Alvarez de la Rosa D. The epithelial sodium channel δ-subunit: new notes for an old song. Am J Physiol Renal Physiol 303: F328–F338, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Goodson P, Kumar A, Jain L, Kundu K, Murthy N, Koval M, Helms MN. NADPH oxidase regulates alveolar epithelial sodium channel activity and lung fluid balance in vivo via O2− signaling. Am J Physiol Lung Cell Mol Physiol 302: L410–L419, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goolaerts A, Pellan-Randrianarison N, Larghero J, Vanneaux V, Uzunhan Y, Gille T, Dard N, Planes C, Matthay MA, Clerici C. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol 306: L975–L985, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 7: 73, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gotts JE, Abbott J, Matthay MA. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol 307: L395–L406, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gotts JE, Matthay MA. Mesenchymal stem cells and the stem cell niche: a new chapter. Am J Physiol Lung Cell Mol Physiol 302: L1147–L1149, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Greenlee MM, Mitzelfelt JD, Yu L, Yue Q, Duke BJ, Harrell CS, Neigh GN, Eaton DC. Estradiol activates epithelial sodium channels in rat alveolar cells through the G protein-coupled estrogen receptor. Am J Physiol Lung Cell Mol Physiol 305: L878–L889, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med 311: 421–425, 1984. [DOI] [PubMed] [Google Scholar]
- 54.Herold S, Gabrielli NM, Vadasz I. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 305: L665–L681, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Herring MJ, Avdalovic MV, Quesenberry CL, Putney LF, Tyler NK, Ventimiglia FF, St George JA, Hyde DM. Accelerated structural decrements in the aging female rhesus macaque lung compared with males. Am J Physiol Lung Cell Mol Physiol 304: L125–L134, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hickman-Davis JM, McNicholas-Bevensee C, Davis IC, Ma HP, Davis GC, Bosworth CA, Matalon S. Reactive species mediate inhibition of alveolar type II sodium transport during Mycoplasma infection. Am J Respir Crit Care Med 173: 334–344, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hobbs CA, Blanchard MG, Alijevic O, Tan CD, Kellenberger S, Bencharit S, Cao R, Kesimer M, Walton WG, Henderson AG, Redinbo MR, Stutts MJ, Tarran R. Identification of the SPLUNC1 ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol 305: L990–L1001, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hobbs CA, Da Tan C, Tarran R. Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease? J Physiol 591: 4377–4387, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Honavar J, Doran S, Oh JY, Steele C, Matalon S, Patel RP. Nitrite therapy improves survival postexposure to chlorine gas. Am J Physiol Lung Cell Mol Physiol 307: L888–L894, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the α- and γ-subunits. J Biol Chem 278: 37073–37082, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in α-ENaC-deficient mice. Nat Genet 12: 325–328, 1996. [DOI] [PubMed] [Google Scholar]
- 62.Idell S, James KK, Levin EG, Schwartz BS, Manchanda N, Maunder RJ, Martin TR, McLarty J, Fair DS. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest 84: 695–705, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thebaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 303: L967–L977, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Itani OA, Auerbach SD, Husted RF, Volk KA, Ageloff S, Knepper MA, Stokes JB, Thomas CP. Glucocorticoid-stimulated lung epithelial Na+ transport is associated with regulated ENaC and SGK1 expression. Am J Physiol Lung Cell Mol Physiol 282: L631–L641, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Ji HL, Song W, Gao Z, Su XF, Nie HG, Jiang Y, Peng JB, He YX, Liao Y, Zhou YJ, Tousson A, Matalon S. SARS-CoV proteins decrease levels and activity of human ENaC via activation of distinct PKC isoforms. Am J Physiol Lung Cell Mol Physiol 296: L372–L383, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ji HL, Zhao RZ, Chen ZX, Shetty S, Idell S, Matalon S. δ-ENaC: a novel divergent amiloride-inhibitable sodium channel. Am J Physiol Lung Cell Mol Physiol 303: L1013–L1026, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joshi AD, Dimitropoulou C, Thangjam G, Snead C, Feldman S, Barabutis N, Fulton D, Hou Y, Kumar S, Patel V, Gorshkov B, Verin AD, Black SM, Catravas JD. Heat shock protein 90 inhibitors prevent LPS-induced endothelial barrier dysfunction by disrupting RhoA signaling. Am J Respir Cell Mol Biol 50: 170–179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khatri M, Saif YM. Influenza virus infects bone marrow mesenchymal stromal cells in vitro: implications for bone marrow transplantation. Cell Transplant 22: 461–468, 2013. [DOI] [PubMed] [Google Scholar]
- 69.Kim WY, Jung JH, Park EY, Yang CW, Kim H, Nielsen S, Madsen KM, Kim J. Expression of protein kinase C isoenzymes α, βI, and δ in subtypes of intercalated cells of mouse kidney. Am J Physiol Renal Physiol 291: F1052–F1060, 2006. [DOI] [PubMed] [Google Scholar]
- 70.King G, Damas JE, Cake MH, Berryman D, Maker GL. Influence of glucocorticoids, neuregulin-1β, and sex on surfactant phospholipid secretion from type II cells. Am J Physiol Lung Cell Mol Physiol 306: L292–L298, 2014. [DOI] [PubMed] [Google Scholar]
- 71.Klein SL, Passaretti C, Anker M, Olukoya P, Pekosz A. The impact of sex, gender and pregnancy on 2009 H1N1 disease. Biol Sex Differences 1: 5, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knowles MR, Stutts MJ, Spock A, Fischer N, Gatzy JT, Boucher RC. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science 221: 1067–1070, 1983. [DOI] [PubMed] [Google Scholar]
- 73.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 302: L1003–L1013, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kunwar A, Haston CK. Basal levels of glutathione peroxidase correlate with onset of radiation induced lung disease in inbred mouse strains. Am J Physiol Lung Cell Mol Physiol 307: L597–L604, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Kunzelmann K, Tian Y, Martins JR, Faria D, Kongsuphol P, Ousingsawat J, Wolf L, Schreiber R. Airway epithelial cells—-functional links between CFTR and anoctamin dependent Cl− secretion. Int J Biochem Cell Biol 44: 1897–1900, 2012. [DOI] [PubMed] [Google Scholar]
- 76.Kuramoto E, Nishiuma T, Kobayashi K, Yamamoto M, Kono Y, Funada Y, Kotani Y, Sisson TH, Simon RH, Nishimura Y. Inhalation of urokinase-type plasminogen activator reduces airway remodeling in a murine asthma model. Am J Physiol Lung Cell Mol Physiol 296: L337–L346, 2009. [DOI] [PubMed] [Google Scholar]
- 77.Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 307: L7–L26, 2014. [DOI] [PubMed] [Google Scholar]
- 78.Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest 122: 314S–320S, 2002. [DOI] [PubMed] [Google Scholar]
- 79.Lazrak A, Chen L, Jurkuvenaite A, Doran SF, Liu G, Li Q, Lancaster JR Jr, Matalon S. Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine-breathing mice. Am J Respir Cell Mol Biol 46: 342–354, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lazrak A, Iles KE, Liu G, Noah DL, Noah JW, Matalon S. Influenza virus M2 protein inhibits epithelial sodium channels by increasing reactive oxygen species. FASEB J 23: 3829–3842, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lazrak A, Jurkuvenaite A, Chen L, Keeling KM, Collawn JF, Bedwell DM, Matalon S. Enhancement of alveolar epithelial sodium channel activity with decreased cystic fibrosis transmembrane conductance regulator expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 301: L557–L567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lazrak A, Jurkuvenaite A, Ness EC, Zhang S, Woodworth BA, Muhlebach MS, Stober VP, Lim YP, Garantziotis S, Matalon S. Inter-α-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in ΔF508 mice. Am J Respir Cell Mol Biol 50: 953–962, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lazrak A, Samanta A, Venetsanou K, Barbry P, Matalon S. Modification of biophysical properties of lung epithelial Na+ channels by dexamethasone. Am J Physiol Cell Physiol 279: C762–C770, 2000. [DOI] [PubMed] [Google Scholar]
- 85.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 106: 16357–16362, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 295: L733–L743, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li T, Folkesson HG. RNA interference for α-ENaC inhibits rat lung fluid absorption in vivo. Am J Physiol Lung Cell Mol Physiol 290: L649–L660, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Ling BN, Eaton DC. Effects of luminal Na+ on single Na+ channels in A6 cells, a regulatory role for protein kinase C. Am J Physiol Renal Fluid Electrolyte Physiol 256: F1094–F1103, 1989. [DOI] [PubMed] [Google Scholar]
- 89.Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol 35: 116–129, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Livraghi-Butrico A, Kelly EJ, Wilkinson KJ, Rogers TD, Gilmore RC, Harkema JR, Randell SH, Boucher RC, O'Neal WK, Grubb BR. Loss of CFTR function exacerbates the phenotype of Na+ hyperabsorption in murine airways. Am J Physiol Lung Cell Mol Physiol 304: L469–L480, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Londino JD, Lazrak A, Jurkuvenaite A, Collawn JF, Noah JW, Matalon S. Influenza matrix protein 2 alters CFTR expression and function through its ion channel activity. Am J Physiol Lung Cell Mol Physiol 304: L582–L592, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Londino JD, Matalon S. Chloride secretion across adult alveolar epithelial cells contributes to cardiogenic edema. Proc Natl Acad Sci USA 110: 10055–10056, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004. [DOI] [PubMed] [Google Scholar]
- 94.Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 61: 627–661, 1999. [DOI] [PubMed] [Google Scholar]
- 95.Mathis C, Poussin C, Weisensee D, Gebel S, Hengstermann A, Sewer A, Belcastro V, Xiang Y, Ansari S, Wagner S, Hoeng J, Peitsch MC. Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air-liquid interface resemble bronchial epithelium from human smokers. Am J Physiol Lung Cell Mol Physiol 304: L489–L503, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95: 1005–1015, 1998. [DOI] [PubMed] [Google Scholar]
- 97.Matsuo O, Sakai T, Bando H, Okada K, Nakajima S, Takagi O, Izaki S. Plasminogen activator in bronchoalveolar fluid. Haemostasis 16: 43–50, 1986. [DOI] [PubMed] [Google Scholar]
- 98.Matthay MA, Folkesson HG, Verkman AS. Salt and water transport across alveolar and distal airway epithelia in the adult lung. Am J Physiol Lung Cell Mol Physiol 270: L487–L503, 1996. [DOI] [PubMed] [Google Scholar]
- 99.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142: 1250–1257, 1990. [DOI] [PubMed] [Google Scholar]
- 100.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 167: 1027–1035, 2003. [DOI] [PubMed] [Google Scholar]
- 101.McAuley DF, Curley GF, Hamid UI, Laffey JG, Abbott J, McKenna DH, Fang X, Matthay MA, Lee JW. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Physiol Lung Cell Mol Physiol 306: L809–L815, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mehta D, Ravindran K, Kuebler WM. Novel regulators of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 307: L924–L935, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Migneault F, Boncoeur E, Morneau F, Pascariu M, Dagenais A, Berthiaume Y. Cycloheximide and lipopolysaccharide downregulate α-ENaC mRNA via different mechanisms in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 305: L747–L755, 2013. [DOI] [PubMed] [Google Scholar]
- 104.Moran AR, Norimatsu Y, Dawson DC, MacDonald KD. Aqueous cigarette smoke extract induces a voltage-dependent inhibition of CFTR expressed in Xenopus oocytes. Am J Physiol Lung Cell Mol Physiol 306: L284–L291, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mutesa L, Azad AK, Verhaeghe C, Segers K, Vanbellinghen JF, Ngendahayo L, Rusingiza EK, Mutwa PR, Rulisa S, Koulischer L, Cassiman JJ, Cuppens H, Bours V. Genetic analysis of Rwandan patients with cystic fibrosis-like symptoms: identification of novel cystic fibrosis transmembrane conductance regulator and epithelial sodium channel gene variants. Chest 135: 1233–1242, 2009. [DOI] [PubMed] [Google Scholar]
- 106.Nie HG, Chen L, Han DY, Li J, Song WF, Wei SP, Fang XH, Gu X, Matalon S, Ji HL. Regulation of epithelial sodium channels by cGMP/PKGII. J Physiol 587: 2663–2676, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O'Riordan TG, Donn KH, Hodsman P, Ansede JH, Newcomb T, Lewis SA, Flitter WD, White VS, Johnson MR, Montgomery AB, Warnock DG, Boucher RC. Acute hyperkalemia associated with inhalation of a potent ENaC antagonist: phase 1 trial of GS-9411. J Aerosol Med Pulm Drug Deliv 27: 200–208, 2014. [DOI] [PubMed] [Google Scholar]
- 108.Odoms K, Shanley TP, Wong HR. Short-term modulation of interleukin-1β signaling by hyperoxia: uncoupling of IκB kinase activation and NF-κB-dependent gene expression. Am J Physiol Lung Cell Mol Physiol 286: L554–L562, 2004. [DOI] [PubMed] [Google Scholar]
- 109.Olesen HV, Pressler T, Hjelte L, Mared L, Lindblad A, Knudsen PK, Laerum BN, Johannesson M, Scandinavian Cystic Fibrosis Study Consortium. Gender differences in the Scandinavian cystic fibrosis population. Pediatr Pulmonol 45: 959–965, 2010. [DOI] [PubMed] [Google Scholar]
- 110.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 100: 8407–8411, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J Biol Chem 284: 28698–28703, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park HS, Kim SR, Lee YC. Impact of oxidative stress on lung diseases. Respirology 14: 27–38, 2009. [DOI] [PubMed] [Google Scholar]
- 113.Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the γ-subunit. J Biol Chem 283: 36586–36591, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pirot N, Delpech H, Deleuze V, Dohet C, Courtade-Saidi M, Basset-Leobon C, Chalhoub E, Mathieu D, Pinet V. Lung endothelial barrier disruption in Lyl1-deficient mice. Am J Physiol Lung Cell Mol Physiol 306: L775–L785, 2014. [DOI] [PubMed] [Google Scholar]
- 115.Pochynyuk O, Medina J, Gamper N, Genth H, Stockand JD, Staruschenko A. Rapid translocation and insertion of the epithelial Na+ channel in response to RhoA signaling. J Biol Chem 281: 26520–26527, 2006. [DOI] [PubMed] [Google Scholar]
- 116.Pomfret EA, Sung RS, Allan J, Kinkhabwala M, Melancon JK, Roberts JP. Solving the organ shortage crisis: The 7th Annual American Society of Transplant Surgeons' State-of-the-Art Winter Symposium. Am J Transplant 8: 745–752, 2008. [DOI] [PubMed] [Google Scholar]
- 117.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276: 71–74, 1997. [DOI] [PubMed] [Google Scholar]
- 118.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372: 415–417, 2008. [DOI] [PubMed] [Google Scholar]
- 119.Rab A, Rowe SM, Raju SV, Bebok Z, Matalon S, Collawn JF. Cigarette smoke and CFTR: implications in the pathogenesis of COPD. Am J Physiol Lung Cell Mol Physiol 305: L530–L541, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 154: 1055–1060, 1996. [DOI] [PubMed] [Google Scholar]
- 121.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, Jones CW, Boydston JA, Clancy JP, Bowen LE, Accurso FJ, Blalock JE, Dransfield MT, Rowe SM. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med 188: 1321–1330, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rauh R, Soell D, Haerteis S, Diakov A, Nesterov V, Krueger B, Sticht H, Korbmacher C. A mutation in the β-subunit of ENaC identified in a patient with cystic fibrosis-like symptoms has a gain-of-function effect. Am J Physiol Lung Cell Mol Physiol 304: L43–L55, 2013. [DOI] [PubMed] [Google Scholar]
- 123.Ribeiro CM, O'Neal WK. Endoplasmic reticulum stress in chronic obstructive lung diseases. Curr Mol Med 12: 872–882, 2012. [DOI] [PubMed] [Google Scholar]
- 124.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073, 1989. [DOI] [PubMed] [Google Scholar]
- 125.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 7: e1002149, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rock JR, O'Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, Grubb BR. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl− secretory channel in mouse airways. J Biol Chem 284: 14875–14880, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roffel AF, Elzinga CR, Van Amsterdam RG, De Zeeuw RA, Zaagsma J. Muscarinic M2 receptors in bovine tracheal smooth muscle: discrepancies between binding and function. Eur J Pharmacol 153: 73–82, 1988. [DOI] [PubMed] [Google Scholar]
- 128.Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, Canessa CM, Pittet JF. Interleukin-1β decreases expression of the epithelial sodium channel α-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem 280: 18579–18589, 2005. [DOI] [PubMed] [Google Scholar]
- 129.Ruppert C, Mahavadi P, Wygrecka M, Weaver TE, Magdolen V, Idell S, Preissner KT, Seeger W, Gunther A, Markart P. Recombinant production of a hybrid plasminogen activator composed of surfactant protein B and low-molecular-weight urokinase. Thromb Haemost 100: 1185–1192, 2008. [PubMed] [Google Scholar]
- 130.Scherrer U, Sartori C, Lepori M, Allemann Y, Duplain H, Trueb L, Nicod P. High-altitude pulmonary edema: from exaggerated pulmonary hypertension to a defect in transepithelial sodium transport. Adv Exp Med Biol 474: 93–107, 1999. [DOI] [PubMed] [Google Scholar]
- 131.Schiffhauer ES, Vij N, Kovbasnjuk O, Kang PW, Walker D, Lee S, Zeitlin PL. Dual activation of CFTR and CLCN2 by lubiprostone in murine nasal epithelia. Am J Physiol Lung Cell Mol Physiol 304: L324–L331, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schmidt TT, Tauseef M, Yue L, Bonini MG, Gothert J, Shen TL, Guan JL, Predescu S, Sadikot R, Mehta D. Conditional deletion of FAK in mice endothelium disrupts lung vascular barrier function due to destabilization of RhoA and Rac1 activities. Am J Physiol Lung Cell Mol Physiol 305: L291–L300, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Serfling RE, Sherman IL, Houseworth WJ. Excess pneumonia-influenza mortality by age and sex in three major influenza A2 epidemics, United States, 1957-58, 1960 and 1963. Am J Epidemiol 86: 433–441, 1967. [DOI] [PubMed] [Google Scholar]
- 134.Shamsuddin AK, Quinton PM. Surface fluid absorption and secretion in small airways. J Physiol 590: 3561–3574, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol 290: F1488–F1496, 2006. [DOI] [PubMed] [Google Scholar]
- 136.Sisson TH, Hanson KE, Subbotina N, Patwardhan A, Hattori N, Simon RH. Inducible lung-specific urokinase expression reduces fibrosis and mortality after lung injury in mice. Am J Physiol Lung Cell Mol Physiol 283: L1023–L1032, 2002. [DOI] [PubMed] [Google Scholar]
- 137.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85: 229–236, 1996. [DOI] [PubMed] [Google Scholar]
- 138.Solymosi EA, Gembrardt SM, Vadasz I, Wang L, Ng CF, Chupin C, Rozowsky S, Ruehl R, Tabuchi A, Schulz H, Kapus A, Morty RE, Kuebler WM. Chloride-transport driven alveolar fluid secretion as a novel mechanism in cardiogenic lung edema. Proc Natl Acad Sci USA 110: E2308–E2316, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Song W, Liu G, Bosworth CA, Walker JR, Megaw GA, Lazrak A, Abraham E, Sullender WM, Matalon S. Respiratory syncytial virus inhibits lung epithelial Na+ channels by up-regulating inducible nitric-oxide synthase. J Biol Chem 284: 7294–7306, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Squadrito GL, Postlethwait EM, Matalon S. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol 299: L289–L300, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stephenson A, Hux J, Tullis E, Austin PC, Corey M, Ray J. Higher risk of hospitalization among females with cystic fibrosis. J Cystic Fibrosis 10: 93–99, 2011. [DOI] [PubMed] [Google Scholar]
- 142.Stockand JD, Bao HF, Schenck J, Malik B, Middleton P, Schlanger LE, Eaton DC. Differential effects of protein kinase C on the levels of epithelial Na+ channel subunit proteins. J Biol Chem 275: 25760–25765, 2000. [DOI] [PubMed] [Google Scholar]
- 143.Takemura Y, Helms MN, Eaton AF, Self J, Ramosevac S, Jain L, Bao HF, Eaton DC. Cholinergic regulation of epithelial sodium channels in rat alveolar type 2 epithelial cells. Am J Physiol Lung Cell Mol Physiol 304: L428–L437, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thannickal VJ, Day RM, Klinz SG, Bastien MC, Larios JM, Fanburg BL. Ras-dependent and -independent regulation of reactive oxygen species by mitogenic growth factors and TGF-β1. FASEB J 14: 1741–1748, 2000. [DOI] [PubMed] [Google Scholar]
- 145.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000. [DOI] [PubMed] [Google Scholar]
- 146.Vohwinkel CU, Vadasz I. Influenza A matrix protein M2 downregulates CFTR: inhibition of chloride transport by a proton channel of the viral envelope. Am J Physiol Lung Cell Mol Physiol 304: L813–L816, 2013. [DOI] [PubMed] [Google Scholar]
- 147.Wetsel WC, Khan WA, Merchenthaler I, Rivera H, Halpern AE, Phung HM, Negro-Vilar A, Hannun YA. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol 117: 121–133, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wolk KE, Lazarowski ER, Traylor ZP, Yu EN, Jewell NA, Durbin RK, Durbin JE, Davis IC. Influenza A virus inhibits alveolar fluid clearance in BALB/c mice. Am J Respir Crit Care Med 178: 969–976, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yadav AK, Doran SF, Samal AA, Sharma R, Vedagiri K, Postlethwait EM, Squadrito GL, Fanucchi MV, Roberts LJ, Patel RP, Matalon S. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. Am J Physiol Lung Cell Mol Physiol 300: L362–L369, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yeligar SM, Harris FL, Hart CM, Brown LA. Glutathione attenuates ethanol-induced alveolar macrophage oxidative stress and dysfunction by downregulating NADPH oxidases. Am J Physiol Lung Cell Mol Physiol 306: L429–L441, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yue G, Edinger RS, Bao HF, Johnson JP, Eaton DC. The effect of rapamycin on single ENaC channel activity and phosphorylation in A6 cells. Am J Physiol Cell Physiol 279: C81–C88, 2000. [DOI] [PubMed] [Google Scholar]
- 152.Zabner J, Smith JJ, Karp PH, Widdicombe JH, Welsh MJ. Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol Cell 2: 397–403, 1998. [DOI] [PubMed] [Google Scholar]
- 153.Zeitlin PL. Cystic fibrosis and estrogens: a perfect storm. J Clin Invest 118: 3841–3844, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao RZ, Nie HG, Su XF, Han DY, Lee A, Huang Y, Chang Y, Matalon S, Ji HL. Characterization of a novel splice variant of δ-ENaC subunit in human lungs. Am J Physiol Lung Cell Mol Physiol 302: L1262–L1272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu S, Ware LB, Geiser T, Matthay MA, Matalon S. Increased levels of nitrate and surfactant protein a nitration in the pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med 163: 166–172, 2001. [DOI] [PubMed] [Google Scholar]


