Abstract
We tested the hypothesis that ingestion of sodium bicarbonate (NaHCO3) prior to an acute session of high-intensity interval training (HIIT) would augment signaling cascades and gene expression linked to mitochondrial biogenesis in human skeletal muscle. On two occasions separated by ∼1 wk, nine men (mean ± SD: age 22 ± 2 yr, weight 78 ± 13 kg, V̇o2 peak 48 ± 8 ml·kg−1·min−1) performed 10 × 60-s cycling efforts at an intensity eliciting ∼90% of maximal heart rate (263 ± 40 W), interspersed with 60 s of recovery. In a double-blind, crossover manner, subjects ingested a total of 0.4 g/kg body weight NaHCO3 before exercise (BICARB) or an equimolar amount of a placebo, sodium chloride (PLAC). Venous blood bicarbonate and pH were elevated at all time points after ingestion (P < 0.05) in BICARB vs. PLAC. During exercise, muscle glycogen utilization (126 ± 47 vs. 53 ± 38 mmol/kg dry weight, P < 0.05) and blood lactate accumulation (12.8 ± 2.6 vs. 10.5 ± 2.8 mmol/liter, P < 0.05) were greater in BICARB vs. PLAC. The acute exercise-induced increase in the phosphorylation of acetyl-CoA carboxylase, a downstream marker of AMP-activated protein kinase activity, and p38 mitogen-activated protein kinase were similar between treatments (P > 0.05). However, the increase in PGC-1α mRNA expression after 3 h of recovery was higher in BICARB vs. PLAC (approximately sevenfold vs. fivefold compared with rest, P < 0.05). We conclude that NaHCO3 before HIIT alters the mRNA expression of this key regulatory protein associated with mitochondrial biogenesis. The elevated PGC-1α mRNA response provides a putative mechanism to explain the enhanced mitochondrial adaptation observed after chronic HIIT supplemented with NaHCO3 in rats.
Keywords: high-intensity interval training, mitochondria, glycogen, supplementation
dietary manipulations can influence acute and chronic responses to exercise training, including metabolic adaptation in skeletal muscle (21). Although most studies have focused on traditional aerobic/endurance or strength/resistance exercise, nutrition or supplementation can also alter the response to interval-type exercise. For example, manipulation of carbohydrate (CHO) availability has been shown to alter skeletal muscle metabolic adaptations to interval training (22). The altered muscle adaptive response may be due in part to differences in the activation of signaling proteins linked to skeletal muscle remodeling. The phosphorylation of AMP-activated protein kinase (AMPK) (48), p38 mitogen-activated protein kinase (MAPK) (11), and p53 (3), as well as the expression of peroxisome proliferator-activated receptor-γ coactivator 1-alpha (PGC-1α) (41) are enhanced to a greater extent when interval exercise is performed under conditions of CHO restriction. However, a practical limitation of reduced CHO training protocols is that they can also result in reductions in power output, higher perceptions of effort, and the lack of a clear performance enhancement (23).
Sodium bicarbonate (NaHCO3) ingestion is another nutritional strategy that has been shown to enhance acute high-intensity exercise capacity, with a meta-analysis concluding that performance during a single 60-s sprint is enhanced ∼2% with a further ∼1% modifying effect when sprints are repeated following the consumption of ∼0.3 g/kg NaHCO3 (9). Combining NaHCO3 consumption with high-intensity interval training (HIIT) is potentially beneficial because the capacity to produce work is elevated, which may in turn lead to a greater cumulative training stimulus. Alternatively, there is evidence that NaHCO3 supplementation per se favorably alters the metabolic response to HIIT such that adaptations are enhanced even if total work is held constant. One study showed that team-sport athletes who consumed NaHCO3 before each of three weekly HIIT sessions improved their endurance capacity and lactic threshold relative to the placebo control, even though total training intensity and volume were matched over the 8-wk protocol (15). With respect to a potential underlying mechanism, Bishop et al. (5) reported that mitochondrial respiratory capacity in rats was increased to a greater extent when HIIT was supplemented with NaHCO3 compared with a placebo, which was associated with a 52% greater increase in running time to exhaustion in the NaHCO3 group.
Few data are available regarding the potential mechanistic basis for the superior performance adaptations observed after HIIT is supplemented with NaHCO3 in humans. There is evidence to suggest that acute NaHCO3 ingestion alters fuel metabolism during exercise, with several studies reporting higher rates of skeletal muscle glycogen utilization and elevated accumulation of plasma, muscle lactate, or both (4, 7, 25, 45). These findings suggest an enhanced contribution from nonoxidative energy metabolism, which in turn could alter metabolically sensitive signaling mechanisms during recovery that are linked to cellular remodeling (19). In the present study, we investigated the hypothesis that ingestion of NaHCO3 before an acute session of HIIT would augment signaling cascades and gene expression linked to mitochondrial biogenesis in human skeletal muscle.
METHODS
Participants and Ethics
Ten healthy active men were recruited for the study. The purpose and potential risks of participation were explained to all subjects, and written informed consent was obtained prior to their participation. Subjects also completed a Physical Activity Readiness Questionnaire, which was designed to identify any issues that might preclude their participation (46). The study protocol was approved by the Hamilton Integrated Research Ethics Board. One subject was subsequently removed as a precaution, owing to an adverse reaction to the initial muscle biopsy procedure. Descriptive characteristics for the nine men who completed the study are presented in Table 1.
Table 1.
Physical characteristics and performance test data
| Characteristic | Means ± SD |
|---|---|
| Age, yr | 22 ± 2 |
| Height, cm | 180 ± 6 |
| Body mass, kg | 78 ± 13 |
| Body fat, % | 15 ± 5 |
| V̇o2peak, ml·min−1·kg−1 | 48.1 ± 8.0 |
| Wmax, W | 345 ± 53 |
| HR max, beats/min | 193 ± 7 |
Wmax, maximum workload; HRmax, maximum heart rate.
Pre-experimental Procedures
Subjects completed baseline testing and familiarization procedures a minimum of 1 wk before the main experimental trials. Fat and fat-free mass were determined through air-displacement plethysmography (BodPod; COSMED, Concord, CA). A ramp test to volitional fatigue was performed on a cycle ergometer (Lode Excalibur Sport; Groningen, the Netherlands) to determine peak oxygen uptake (V̇o2 peak) using continuous online gas collection (Moxus modular oxygen uptake system; AEI Technologies, Pittsburgh, PA), as well as maximum power output (Wmax) and maximal heart rate (HRmax). The protocol involved a 2-min warm-up at 50 W and thereafter resistance was increased by 1 W/2 s until volitional exhaustion, as previously described (11). Heart rate (HR) was assessed continually using telemetry (Polar A3; Lake Success, NY). Subjects were also familiarized for ∼30 min with the use of an Actiheart device (CamNtech; Boerne, TX), which permits the noninvasive estimation of habitual energy expenditure on the basis of activity counts, determined by an accelerometer and heart rate (13). On a separate day, subjects completed a familiarization trial that consisted of four 60-s cycling efforts, which mimicked the HIIT intervention (see Experimental Protocol for further details).
Experimental Protocol
Subjects completed two trials in a random, counterbalanced order, separated by ∼1 wk. Each trial involved an acute session of HIIT, with repeated blood and muscle biopsy sampling to assess the metabolic response to exercise. The only difference between trials was the nature of the supplement that was ingested prior to exercise. On one occasion, subjects ingested 0.2 g/kg body weight NaHCO3 at 90 and 60 min before exercise for a total dose of 0.4 g/kg body weight (BICARB). On the other occasion, subjects ingested an equimolar amount of NaCl, which served as the placebo treatment (PLAC). The BICARB and PLAC capsules were indistinguishable. This dosing protocol was modeled after a previous study that revealed treatment differences while minimizing the potential for gastrointestinal discomfort (15).
Subjects reported to the laboratory 24 h before each trial to be fitted with the Actiheart device as we have previously described (44). They were also provided with an activity log and asked to abstain from physical activity other than tasks of daily living before the trial. The Actiheart data and self-reported activity logs reflected no differences in the 24-h activity before BICARB and PLAC trials. In addition, diet logs were provided along with instructions to record all food and drink consumption. Before the second trial, subjects were encouraged to replicate the same nutritional pattern. Subjects were asked to refrain from alcohol and caffeine 24 h before each trial. All diet logs were subsequently analyzed using an on-line diet analysis tool (myfitnesspal; San Francisco, CA) to estimate total energy intake and macronutrient composition. There were no differences between trials in total energy intake (BICARB vs. PLAC: 2,220 ± 501 vs. 2,249 ± 520 kcal, P = 0.71) or macronutrient composition (BICARB vs. PLAC: 52 ± 10 vs. 51 ± 8% CHO, P = 0.58; 26 ± 8 vs. 31 ± 11% fat, P = 0.20; 22 ± 9 vs. 18 ± 6% protein, P = 0.17).
For each trial, subjects reported to the laboratory in the morning after a 10-h overnight fast. A catheter was inserted in an antecubital vein, and a fasting blood sample was obtained. Subjects then ingested a standardized breakfast, which consisted of ∼600 kcal derived from ∼59% CHO, ∼25% fat, and ∼16% protein. Immediately following the breakfast, subjects ingested the first dose of either BICARB or PLAC, followed 30 min later by the second dose. Ninety minutes after the initial dose, a second blood sample was collected and a resting muscle biopsy was obtained from the vastus lateralis muscle under local anesthesia (1% lidocaine) using a Bergström needle adapted for manual suction as previously described (18). Muscle tissue was quickly removed from the needle, cleaned of excess blood (when rarely needed), sectioned, frozen in liquid nitrogen, and stored at −80°C for subsequent analyses.
Following the first biopsy, subjects commenced the HIIT intervention, which involved a 5-min warm-up at 50 W, followed by 10 × 60-s cycling intervals at an individualized absolute workload selected to elicit ∼90% HRmax (263 ± 40 W, 76 ± 2%Wmax), interspersed with 60 s of active recovery at 50 W. The intervals were completed on a cycle ergometer set in constant-watt mode (LifeCycle R1; Life Fitness, Schiller Park, IL). HR was continuously measured throughout each trial by telemetry (Polar A3). Subjects were asked for a rating of perceived exertion (RPE) on a scale of 6 to 20 after the completion of each interval, as previously described (6).
Immediately upon completion of exercise, a blood sample was collected, and a second muscle biopsy was obtained ∼5 cm distal to the first site. Additional blood samples were collected after 2 and 3 h of recovery, followed by a third and final muscle biopsy from the contralateral leg 3 h after exercise. Subjects also completed a 15-item questionnaire that was designed to assess potential gastrointestinal or other discomforts on a 1-to-10 Likert scale, with total discomfort scores ranging between 15 and 150, as previously described (10). Subjects were permitted to consume water ad libitum throughout the first trial day, with the approximate timing and volume recorded to match the pattern of intake during the second trial.
Blood Analyses
Venous blood was collected in a lithium heparin syringe, stored on ice, and analyzed within 30 min of collection by the Hamilton Regional Medicine Program laboratory for acid-base variables using a blood-gas analyzer (Radiometer ABL 800; Radiometer Medical, Bronshoj, Denmark). Plasma, serum and whole blood samples were collected with appropriate collection tubes, processed as per the manufacturer's recommendations (BD Vacutainer; Becton Dickinson, Franklin Lakes, NJ), aliquoted, and stored at −20°C for analysis. Sodium fluoride- and potassium oxalate-treated samples were analyzed for glucose and lactate concentrations using a YSI 2300 STAT Plus Glucose and Lactate Analyzer (Xylem; White Plains, NY).
Muscle Analyses
Glycogen.
One piece of each muscle sample was freeze-dried, powdered, and dissected free of blood and connective tissue for determination of glycogen as previously described (11). Briefly, ∼2 mg of powdered muscle was incubated for 2 h at 98°C in 2 M HCl to hydrolyze glycogen to glucose. Samples were cooled to room temperature, and an equal volume of 2 M sodium hydroxide was subsequently added to neutralize samples. Glucose concentration was determined using a commercially available hexokinase enzymatic assay kit (Pointe Scientific, Canton, MI).
Signaling proteins.
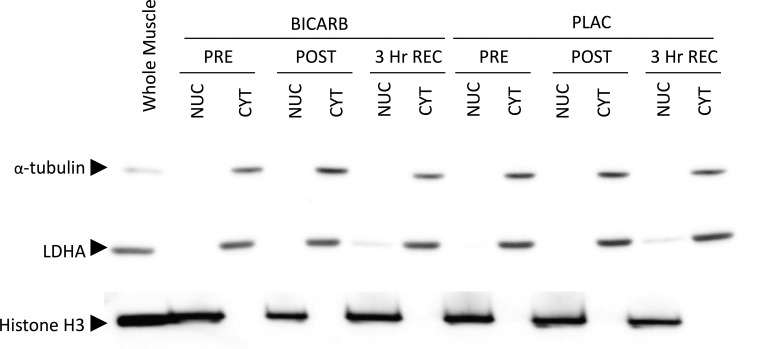
Cytosolic and enriched nuclear fractions were prepared from ∼50 mg of wet muscle using a commercially available kit (NE-PER; Pierce, Rockford, IL) as previously described with additional modifications (31). All incubations and centrifugations were carried out on ice or at 4°C, respectively. Homogenization was performed in CER-I buffer supplemented with protease (cOmplete mini; Roche Applied Science, Laval, PQ, Canada) and phosphatase (PhosStop; Roche Applied Science) inhibitors using a FastPrep-24 Tissue and Cell Homogenizer (MP Biomedicals, Solon, OH). Homogenates were vortexed for 15 s at maximum speed to ensure full suspension and then incubated for 15 min. CER-II was then added to the homogenate. Samples were vortexed 5 s, incubated for 5 min, and vortexed an additional 5 s. A crude cytosolic fraction was obtained by removing the supernatant after samples were centrifuged at 20,000 g for 10 min. The pellets containing nuclei were cleaned of contamination by resuspending them in PBS supplemented with the previously mentioned inhibitors and centrifuging at 20,000 g for 10 min. Additional purifications were performed by gently washing the tube and pellet with excess PBS three times. Washed pellets were then resuspended in NER buffer by vigorous pipetting followed by repeated 15-s vortexes over a 40-min incubation. Samples were centrifuged at 20,000 g for 10 min and then disrupted by gentle agitation with a micropestle and repeated 1-s pulses of sonication. A final 10-min centrifugation at 20,000 g removed insoluble membranes and the supernatant was taken as the enriched nuclear fraction. Crude cytosolic fractions were purified by repeatedly centrifuging at 20,000 g for 5 min and transferring the supernatant to clean tubes. Aliquots were taken before storage at −80°C to quantify protein concentration with a commercially available bicinchoninic acid assay kit (Pierce). The absence of LDH and α-tubulin, and the abundance of histone H3 determined by Western blotting were used as confirmation for the enrichment of nuclear fractions (Fig. 1).
Fig. 1.
Representative Western blot images following muscle fractionation to demonstrate nuclear and cytosolic enrichment. Lactose dehydrogenase (LDHA) and α-tubulin were abundant in the cytosolic fractions (CYT) but were lacking in the enriched-nuclear fractions (NUC). In contrast, histone H3 was highly expressed in NUC but absent in CYT. A whole muscle homogenate was used as a positive control. PRE, before ingestion; POST, after ingestion; 3 Hr REC, 3 h after recovery.
Western blotting analysis was performed as previously described (31). Blots containing crude cytosolic fractions were sectioned and incubated with primary antibodies against PGC-1α, phospho-p38 MAPK (Thr180/Tyr182), total-p38 MAPK, phospho-AMPK (Thr172), phospho-acetyl-CoA carboxylase (ACC) (Ser79), phospho-Ca2+/calmodulin-dependent protein kinase (CaMKII) (Thr286), lactate dehydrogenase (LDH), histone H3, α-tubulin (all from Cell Signaling Technology, Beverly, MA) and total-AMPKα2 (Upstate, Millipore, Billerica, MA). Nuclear-enriched fractions were probed for PGC-1α and histone H3. Blots were probed with primary antibodies at a dilution of 1:1,000 overnight at 4°C in 3% fat-free milk or BSA in Tris-buffered saline and Tween-20. After incubation in the appropriate species-specific secondary antibody conjugated to horseradish peroxidase, blots were detected by chemiluminescence (Supersignal, West Dura, Pierce). Band intensity was quantified by spot densitometry using ImageJ software (National Institutes of Health, Bethesda, MD).
Gene expression.
RNA was isolated from ∼30 mg of frozen muscle using a combination of TRIzol reagent (Life Technologies, Carlsbad, CA) and RNeasy kit (Qiagen, Valencia, CA) following the manufacturer's protocol. Briefly, the FastPrep-24 Tissue and Cell Homogenizer (MP Biomedicals) in conjunction with Lysing Matrix D tubes (MP Biomedicals) were used to homogenize samples by rapidly agitating at a speed of 6 m/s for 40 s. Samples were visibly inspected to ensure thorough homogenization and then incubated at room temperature for 5 min followed by the addition of chloroform. After vigorous mixing for 15 s by hand, an additional 5-min incubation at room temperature followed by centrifugation at 12,000 g for 10 min at 4°C forced the partitioning of the aqueous and organic phases. RNA was purified from the aqueous phase using a commercially available kit (RNeasy Mini Kit; Qiagen). To minimize potential genomic DNA contamination, an on-column DNase digestion was performed by coincubating samples with DNase I according to the manufacturer's recommendations (RNase-Free DNase Set; Qiagen). RNA purity and quantity were determined using the Nano-Drop 1000 Spectrophotometer (Thermo Fisher Scientific, Rockville, MD). Furthermore, RNA integrity was quantified using the Agilent 2010 Bioanalyzer (Agilent Technologies, Toronto, ON, Canada). The RNA integrity number (RIN) was 7.4 ± 0.6. Complementary DNA was synthesized using a commercially available high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) in an Eppendorf Mastercycler ep gradient thermal cycler (Eppendorf, Mississauga, ON, Canada) and stored at −20°C. Gene expression was quantified as previously described with slight modification (2, 11). Briefly, quantitative real-time PCR was performed on the prepared cDNA samples using SYBR Green Master Mix (Quanta Biosciences, Gaithersburg, MD) and the corresponding oligonucleotide primers for the genes of interest. Primers used for real-time PCR analysis are listed in Table 2. Reaction mixtures were prepared using the epMotion 5075 Eppendorf automated pipetting system (Eppendorf) and run on an Eppendorf realplex2 Master Cycler ep gradient S. Changes in gene expression were analyzed using the 2−ΔΔCT method as previously described (42). GAPDH was used as a housekeeping gene because it was unchanged across time and treatments and the intraindividual variation was low (4.0% coefficient of variation). Within-subject samples were normalized to their respective pre-exercise placebo values.
Table 2.
Primer sequences for RT-PCR
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| PGC-1α | CACTTACAAGCCAAACCAACAACT | CAATAGTCTTGTTCTCAAATGGGGA |
| PPARD | TGACCTGGCCCTATTCATTG | GGAAGAGGTACTGGGCATCA |
| HIF1A | TCTGGGTTGAAACTCAAGCA | TCAACCGGTTTAAGGACACA |
| CS | CCTGCCTAATGACCCCATGTT | CATAATACTGGAGCAGCACCCC |
| COX IV | CGAGCAATTTCCACCTCTGT | GGTCACGCCGATCCATATA |
| TFAM | GGAAGGTCTGGAGCAGAGC | TGGACAACTTGCCAAGACAG |
| PHD2 | CGCAACCCTCATGAAGTACA | TTACCGACCGAATCTGAAGG |
| GAPDH | CCTCCTGCACCACCAACTGCTT | GAGGGGCCATCCACAGTCTTCT |
PGC-1α, peroxisome proliferator activated receptor γ co-activator-1α; PPARD, peroxisome proliferator activated receptor delta; HIF1A, hypoxia-inducible factor 1-alpha; CS, citrate synthase; COX IV, cytochrome oxidase subunit IV; TFAM, mitochondrial transcription factor A; PHD2, prolyl hydroxylase 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analyses
All data are reported as means ± SD. Data on HR, RPE, habitual energy expenditure, and dietary analyses were compared across conditions using a Student's paired t-tests. All other data were analyzed using a two-factor (time × condition) repeated-measures ANOVA. Mauchly's sphericity test was used to validate data sets before interpreting ANOVA results, and the Greenhouse-Geisser correction was applied to data sets that violated the assumption of sphericity. Statistical significance was set at P ≤ 0.05. Significant effects based on ANOVA were further analyzed using Tukey's honestly significant difference post hoc test.
RESULTS
Physiological Responses to Treatments and HIIT
The exercise protocol elicited 89 ± 5 and 88 ± 5% HRmax during the BICARB and PLAC trials, respectively (P = 0.37). RPE was not different between treatments (BICARB vs. PLAC: 15.2 ± 1.6 vs. 14.9 ± 1.8, P = 0.69). Mean ratings of gastrointestinal and other discomforts were low, and the overall scores were not different between trials (BICARB vs. PLAC: 18 ± 2 vs. 18 ± 3, P = 0.53).
Blood Data
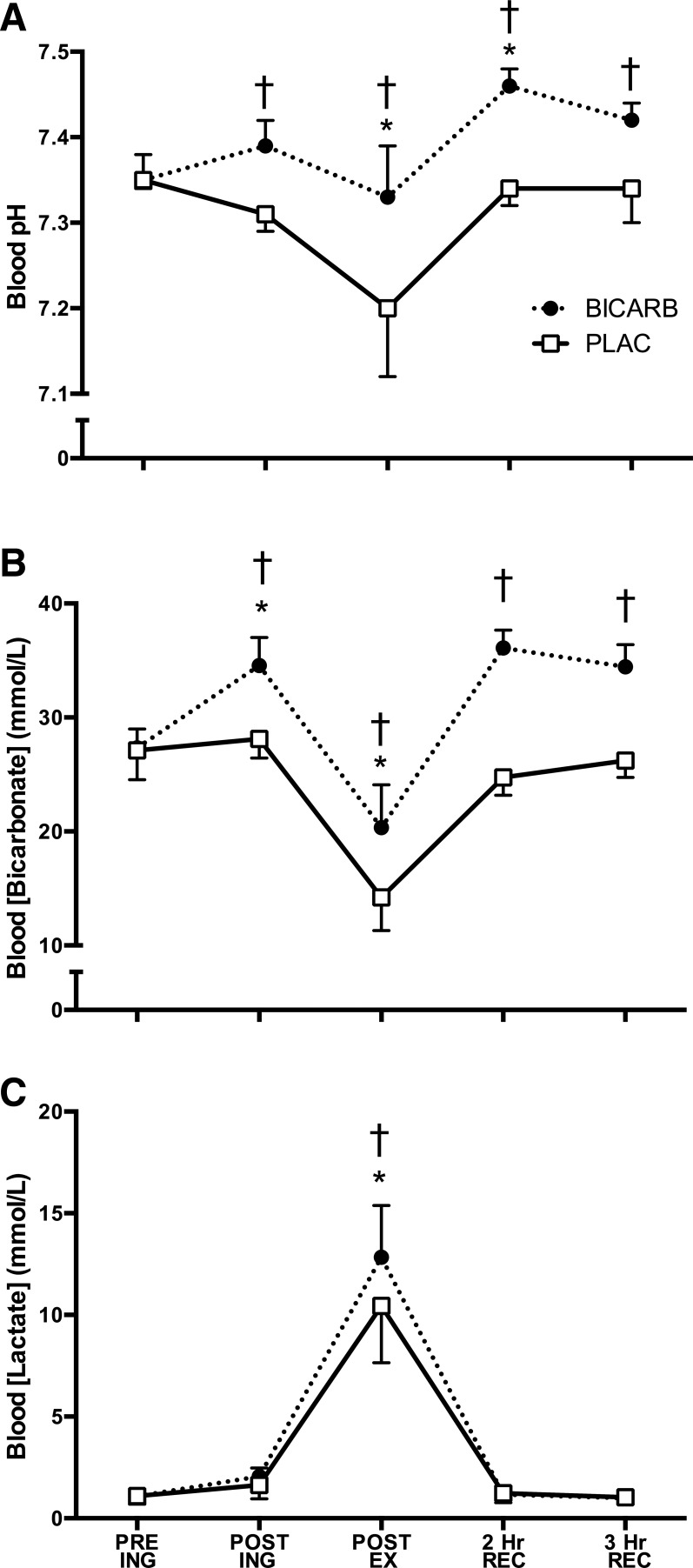
Blood bicarbonate (P < 0.01) and pH (P < 0.01) were higher at all time points in BICARB vs. PLAC after capsule ingestion (Fig. 2, A and B, respectively). The exercise-induced increase in blood lactate was greater in BICARB vs. PLAC (P < 0.05, Fig. 2C).
Fig. 2.
Response of various blood measures to high-intensity interval training (HIIT) with ingestion of sodium bicarbonate (BICARB) or a placebo (PLAC). Blood pH (A) bicarbonate (B) concentrations at five time points: before ingestion (PRE ING), after ingestion, after HIIT, 2 h after recovery, and 3 h after recovery. Blood pH (A), bicarbonate concentration (B), and lactate concentration (C) at five time points. *Main effect for time compared with PRE ING (P < 0.01). †Significant difference between BICARB and PLAC at the time point designated (P < 0.01). Main effect for treatment was observed in blood pH, bicarbonate concentration, and lactate (P < 0.01).
Muscle Data
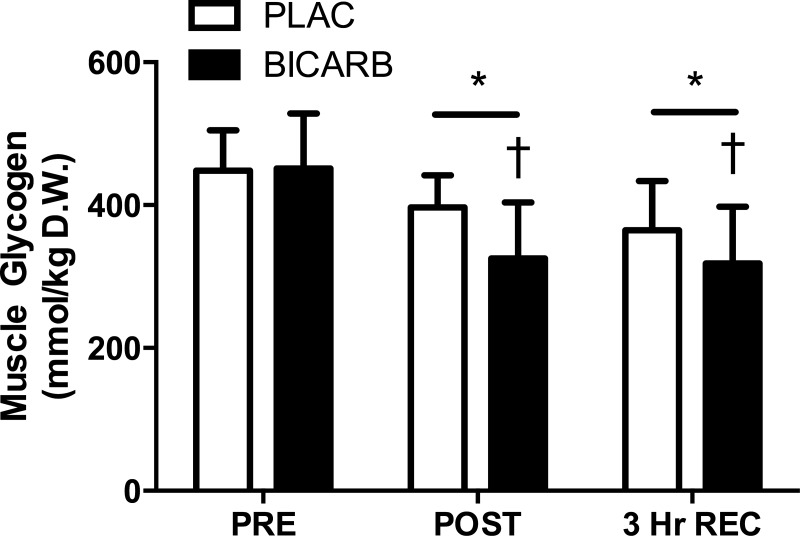
The exercise-induced decrease in muscle glycogen content was greater in BICARB vs. PLAC, and the difference between treatments persisted after 3 h of recovery (P < 0.01, Fig. 3).
Fig. 3.
Muscle glycogen content before (PRE), immediately after (POST), and 3 h into recovery (3 Hr REC) after the ingestion of sodium bicarbonate (BICARB) or a placebo (PLAC) and an acute bout of high-intensity interval training. *Main effect for time compared with PRE (P < 0.01). †Significant difference between BICARB and PLAC at the time point designated (P < 0.01).
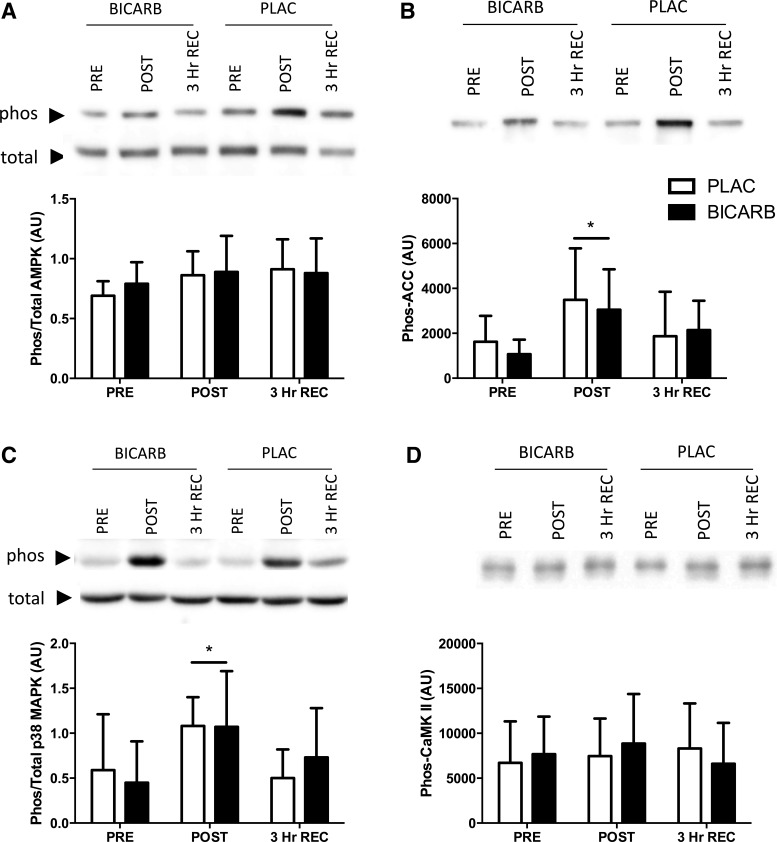
The ratio of phosphorylated (phos-) to total p38 MAPK (P < 0.01, Fig. 4C) was increased immediately following HIIT but returned to resting levels after 3 h of recovery, with no difference between conditions. The ratio of phos- to total AMPK was not significantly elevated after exercise (P = 0.07, Fig. 4A). However, a downstream marker of AMPK activity, phos-ACC, was also elevated immediately following HIIT and returned to baseline after 3 h of recovery (P < 0.01, Fig. 4B). Phos-CaMKII was not increased following exercise and was similar across treatments (Fig. 4D). The nuclear abundance of PGC-1α was not different at any point (Fig. 5).
Fig. 4.
Acute protein phosphorylation in response to high-intensity interval training (HIIT) in conjunction with sodium bicarbonate (BICARB, closed bars) or a placebo (PLAC, open bars) supplementation. Western blots were used to analyze muscle biopsies acquired before commencing HIIT (PRE), immediately upon completion (POST), and 3 h after (3 Hr REC). A: the ratio of phosphorylated AMPK protein kinase (AMPKThr172) to total AMPK (effect for time, P = 0.07). B: phosphorylation of acetyl-CoA carboxylase (phos-ACCSer79). C: ratio of phosphorylated p38 mitogen-activated protein kinase (p38 MAPKThr180/Tyr182) to total p38 MAPK. D: phosphorylation of Ca2+/calmodulin-dependent protein kinase (CaMKIIThr286). *Main effect for time compared with PRE (P < 0.01).
Fig. 5.
The nuclear abundance of peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α) protein in response to high-intensity interval training (HIIT) in conjunction with sodium bicarbonate (BICARB, closed bars) or a placebo (PLAC, open bars) supplementation. Western blots were used to analyze muscle biopsies acquired before commencing HIIT (PRE), immediately upon completion (POST), and 3 h after (3 Hr REC). Protein content of PGC-1α is reported as a ratio to histone H3 protein.
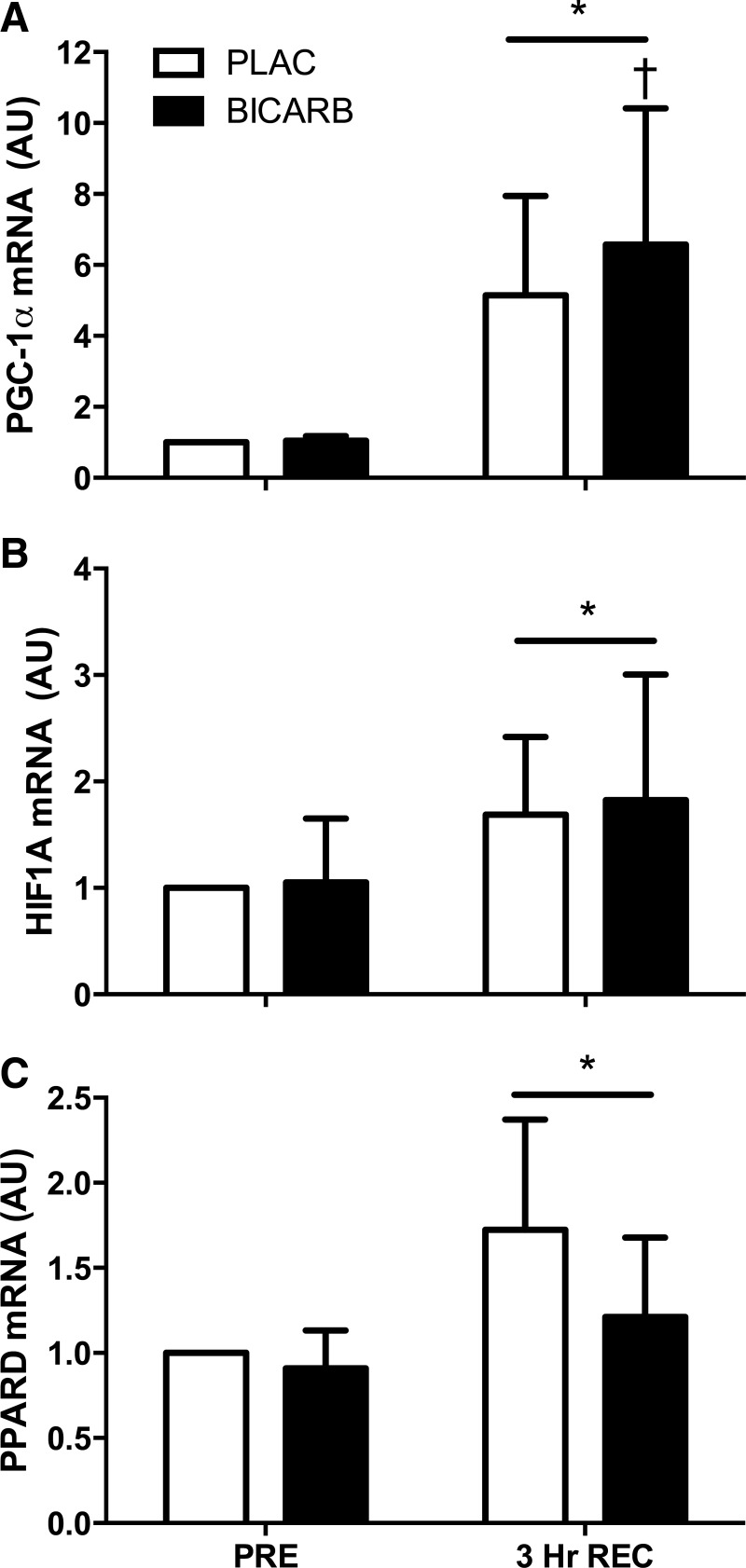
PGC-1α mRNA expression was higher after 3 h of recovery compared with rest (P = 0.02), and the increase was greater in BICARB compared with PLAC (P = 0.035, Fig. 6A). Exercise also increased HIF1A expression (P < 0.01, Fig. 6B) and PPARD (P < 0.01, Fig. 6C), but there were no differences between treatments. There was no effect of exercise or supplementation on the expression of CS, COX IV, PHD2, or TFAM (Table 3).
Fig. 6.
Messenger RNA expression after an acute bout of high-intensity interval training (HIIT) supplemented with sodium bicarbonate (BICARB, closed bars) or a placebo (PLAC, open bars). A: peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α); B: hypoxia-inducible factor 1α (HIF1A); and C: peroxisome proliferator activated receptor delta (PPARD) mRNA expression before (PRE) and 3 h after (3 Hr REC) an acute bout of HIIT. *Main effect for time compared with PRE (P < 0.05). †Significant difference between BICARB and PLAC at time point designated (P < 0.05).
Table 3.
mRNA expression relative to PRE PLAC
| Gene | PRE | 3 Hr REC | |
|---|---|---|---|
| CS | PLAC | 1.0 | 1.0 ± 0.2 |
| BICARB | 1.0 ± 0.2 | 1.0 ± 0.2 | |
| COX IV | PLAC | 1.0 | 1.3 ± 0.7 |
| BICARB | 1.2 ± 0.3 | 1.0 ± 0.2 | |
| PHD2 | PLAC | 1.0 | 1.1 ± 0.3 |
| BICARB | 1.1 ± 0.5 | 1.1 ± 0.4 | |
| TFAM | PLAC | 1.0 | 1.0 ± 0.3 |
| BICARB | 1.0 ± 0.3 | 0.9 ± 0.3 |
CS, citrate synthase; COX IV, cytochrome oxidase sub-unit IV; TFAM, mitochondrial transcription factor A; PHD2, prolyl hydroxylase 2.
DISCUSSION
The major novel finding from this study was that NaHCO3 supplementation before an acute bout of interval exercise increased PGC-1α mRNA expression to a greater extent than placebo during recovery in human skeletal muscle. Our data point to a possible mechanism to explain the enhanced mitochondrial adaptation observed after chronic HIIT combined with NaHCO3 supplementation in rats (5). Contrary to our hypothesis, there was no difference between treatments in the phosphorylation of p38 MAPK or ACC, a marker of AMPK activity, despite the putative link of p38 MAPK and AMPK with PGC-1α signaling and mitochondrial biogenesis.
Blood and Muscle Metabolites
The supplementation protocol resulted in elevated blood pH and bicarbonate concentrations similar to those reported in previous studies (9, 15). The HIIT-induced decline in blood pH was also attenuated in the BICARB trial. Consistent with previous data on sprint (4, 7) and continuous (25, 45) exercise with NaHCO3 supplementation, a higher plasma lactate concentration was observed. The elevated lactate has been proposed to result from a greater concentration gradient across the sarcolemma that facilitates lactate and H+ cotransport out of the active muscle (4, 25). Despite the potentially greater efflux of lactate, NaHCO3 supplementation commonly results in the same or even elevated intramuscular lactate content (4, 7, 25, 45). Although muscle lactate was not determined in this study, Hollidge-Horvat et al. (25) previously reported that NaHCO3 supplementation resulted in greater lactate accumulation during cycling at 75% V̇o2 max without a change in pyruvate oxidation. This was indicative of a greater allosteric upregulation of glycogen phosphorylase and phosphofructokinase potentiating glycolytic flux while pyruvate dehydrogenase was already fully activated and unable to facilitate greater pyruvate oxidation (25). Consistent with this previous study, we observed 16% greater glycogen depletion with NaHCO3 in the present study. Hollidge-Horvat et al. (25) also noted altered cellular energetics because ADP and AMP concentrations were elevated, but these measurements were not included in the present study.
Messenger RNA Expression
Although the mechanisms that regulate adaptations to exercise are obviously complex, transient yet repeated increases in gene expression could lay the foundation for increases in mitochondrial protein content [reviewed in (15)]. Some empirical evidence for this notion stems from work by Perry et al. (35) who investigated the temporal changes associated with single and repeated bouts of HIIT. They found that, in general, transient increases in mRNA expression preceded changes in protein content and enzymatic activity (35). The ∼28% greater acute increase in PGC-1α mRNA expression during recovery from exercise after NaHCO3 ingestion is relatively modest; however, the difference could have physiological relevance if repeated over chronic training sessions, because it could lay the foundation for elevated PGC-1α protein content. PGC-1α is a well known transcriptional coactivator of a number of genes required for mitochondrial biogenesis. Therefore, an increase in PGC-1α protein content could augment the adaptations to subsequent training sessions. In contrast to observations by Little et al. (31), we did not find evidence of an increase in PGC-1α protein content in the nucleus following an acute bout of exercise. In the present study, the content of PGC-1α in the nuclear-enriched fraction was more variable than the values reported by Little et al., which could have masked an exercise-induced signal, if one had been present. Alternatively, the discrepancy might be a result of the different exercise intensities used in each study: four bouts of all-out sprints vs. 10 bouts of intense but submaximal exercise.
We also observed increases in the expression of peroxisome proliferator-activated receptor-delta (PPARD) and hypoxia-inducible factor 1-alpha (HIF1A) mRNA that were independent of the supplement. PPARD promotes oxidative metabolism, increases PGC-1α gene expression (20), and has been shown to increase following acute HIIT (35). HIF-1A regulates oxidative metabolism (43), and it was recently shown to be downregulated by endurance training (28). We did not observe elevations in other markers of mitochondrial gene expression (i.e., CS, TFAM, or COX IV mRNA) or PHD2, a negative regulator of HIF1A. Although some studies have reported increases in CS, TFAM, and COX IV gene expression 3 h after an acute bout of interval exercise (30, 40), others have found that the timing of expression occurs later into recovery (35, 37). Therefore, although the timing of the biopsy was selected to facilitate potential detection of a divergent PGC-1α response, it may not have been optimal for detecting potential temporal differences in the response of other genes.
Muscle Signaling
It is well documented that ADP and AMP allosterically regulate AMPK by binding to the γ subunit and may maintain AMPK activation by preventing dephosphorylation (41). AMPK has also been proposed to be regulated by the sequestering of the β subunit by oligosaccharides such as glycogen (32). Conversely, under conditions of glycogen depletion (i.e., CHO restriction or NaHCO3 ingestion in this study), the β subunit may be liberated to facilitate AMPK activation and promote its downstream signaling. Despite the hypothesized elevation of ADP and AMP concentrations, as well as observed glycogen reductions with BICARB, we did not observe a difference in the activation of AMPK with supplementation. In fact, we surprisingly did not observe an effect of exercise on AMPK phosphorylation. It is not clear why AMPK phosphorylation was not observed with this protocol, though it was trending toward significance (P = 0.07). This may be due to the use of threonine 172 phosphorylation assessed through Western blotting as a surrogate measure of AMPK activity. However, no differences in ACC phosphorylation, a downstream target of AMPK, were observed, providing further support that AMPK activation was not altered between treatments. This implies that the greater PGC-1α expression in BICARB was unrelated to AMPK activity. Similar to our findings, recent data reported by Metcalfe et al. (33) demonstrated elevated ACC phosphorylation without an increase in AMPK phosphorylation following acute sprint interval exercise.
We sought to investigate whether p38 MAPK phosphorylation was influenced by NaHCO3 ingestion, owing to its acute activation with a number of HIIT protocols (2, 11, 30) and putative role in training-induced mitochondrial biogenesis (38). As with AMPK, we did not observe any differences between trials in acute p38 activation. It has been suggested that p38 activation may play a permissive role more so than being the direct signal itself. This is because p38 MAPK is phosphorylated to the same extent at both low- and high-intensity endurance exercise, in contrast to other kinases that are activated only at higher intensities and have been suggested to ultimately result in an augmented expression of PGC-1α (16).
Despite the lack of evidence for a mechanistic link between reduced glycogen and elevated PGC-1α mRNA expression in this study, speculation can be made regarding possible alternative mechanisms that were not examined. For example, Philp et al. (36) recently demonstrated in rats that glycogen content, in conjunction with contractile activity, regulates the activity of the transcription factor PPARD. Interestingly, there is some evidence that PPARD can regulate PGC-1α mRNA expression through a PPAR response element in the PGC-1α promoter (26). We did not observe an augmented induction of PPARD gene expression (Fig. 6C); however, it has been suggested that there is a disconnect between the protein activity and mRNA levels with the activity having more physiological relevance (36). Therefore, the increased PGC-1α gene expression observed with the BICARB trial may be mediated by the altered activity of transcription factors such as PPARD due to the greater glycogen utilization.
A number of pathways have been implicated in the expression of PGC-1α mRNA and subsequently mitochondrial biogenesis that were not investigated in this study. It is interesting to speculate on the implications of NaHCO3 effects on lactate production in response to exercise and its potential to directly mediate PGC-1α mRNA expression. For example, Hashimoto et al. (20) reported that lactate can act as a signaling molecule within L6 cells, specifically through an induction of reactive oxygen species, which signals transcription factors affecting mitochondrial biogenesis (i.e., PGC-1α). Although supporting evidence for this is scarce, others have noted that lactate plays other important regulatory roles, including the regulation of transcription through the signaling of histone remodeling (27), providing yet another link in which lactate may regulate PGC-1α mRNA expression and by extension skeletal muscle remodeling. In the current study we observed elevated plasma lactate concentrations immediately after HIIT (Fig. 2C) however, we did not measure intramuscular concentrations of lactate and therefore can only speculate on its role in augmenting PGC-1α mRNA expression. We can also speculate that other metabolic factors are implicated in the augmented PGC-1α expression. For example, cellular NAD/NADH ratios are monitored by the NAD+-dependent deacetylase, SIRT1, which has been shown to play a critical role in the regulation of PGC-1α activity (8). Amat et al. (1) have also demonstrated that PGC-1α gene expression is at least partially dependent on SIRT1 binding to the PGC-1α promoter. Another potential mechanism that may warrant further investigation is the effect of NaHCO3 on calcineurin, a protein phosphatase activated by Ca2+. Specifically, Yamaguchi et al. (47) demonstrated that NaHCO3 caused human myoblasts to undergo fast-to-slow fiber shifts. These shifts coincided with elevated PGC-1α mRNA expression that was calcineurin-dependent, providing in vitro evidence that NaHCO3 can stimulate PGC-1α transcription.
Limitations
Our research provides novel insight into acute changes within muscle that may underpin the aforementioned adaptations to chronic NaHCO3 supplementation; however, we were unable to conclusively elucidate a mechanism causing greater PGC-1α mRNA expression. Adenosine nucleotides, redox status, pH, and ion concentrations (i.e., Ca2+) have all been implicated in adaptations to exercise [reviewed in (15)], and a number of these have also been suggested to be affected by NaHCO3 supplementation (25) but were not measured in the present study. Another practical consideration is that owing to the relatively high dose of NaHCO3 commonly consumed to elicit ergogenic and metabolic effects, great consideration has to be made regarding placebos. NaCl is a common choice to match the Na+ content because it is well known to affect plasma volume and water distribution in the body (4, 14, 15). However, it has been suggested that NaCl is not necessarily chemically inert due to its effects on ion redistribution and hyperchloremic acidosis (12, 29). Despite this argument, we chose NaCl to mimic the protocols showing enhanced adaptations with training (15).
Future Directions
Future research should focus on the acute mechanisms regulating the effects of NaHCO3 ingestion on skeletal muscle, especially in elite athletes, and whether these acute responses would translate to enhanced adaptations as well. Evidence suggests that at the same relative workload, gene expression is not different between trained and untrained individuals (34, 39); therefore, we may hypothesize that we would see similar results, at least acutely, in elite athletes.
Whether or not the findings of Edge et al. (15) have relevance to elite athletes has been questioned due to their already well-adapted lactic threshold and mitochondrial oxidative capacity. To investigate this hypothesis, Driller et al. (14) had elite-level rowers ingest NaHCO3 or placebo prior to a short block of HIIT over a 4-wk period. Both groups noted improvements in 2,000-m rowing performance without a difference between groups. On the basis of these findings, the authors concluded that there was no further advantage to chronically supplement elite athletes with NaHCO3 (14). It is interesting to note that although it is statistically nonsignificant, a 0.8-s greater enhancement was observed in athletes who had been supplemented with NaHCO3. Considering the study was limited in their statistical power (n = 6 per group) and consisted of only eight training sessions over 4 wk, more subjects, a longer intervention, or perhaps unmatched work throughout training may have led to greater adaptations, although this is only speculative. Therefore, evidence for NaHCO3 supplementation to be an effective intervention exists; however, the implications for the athletic community remain to be determined.
Conclusion
We found that ingestion of 0.4 g/kg body weight of NaHCO3, administered in two doses of 0.2 g/kg body weight before an acute session of HIIT augmented skeletal muscle glycogenolysis during exercise and enhanced the expression of PGC-1α mRNA during recovery. Thereby, we provide evidence of potential mechanisms mediating the enhanced adaptations previously observed with chronic NaHCO3 ingestion during training. Furthermore, the increased gene expression does not appear to be associated with greater activation of AMPK or p38 MAPK.
GRANTS
The project was supported by an operating grant to M.JG. from the Natural Science and Engineering Research Council (NSERC). M.E.P. was supported by an NSERC Canada Graduate Scholarship (Masters), J.B.G. held an NSERC Vanier Canada Graduate Scholarship (Doctoral), L.E.S. held an NSERC Canada Graduate Scholarship (Masters), and M.J.M. held an NSERC Postdoctoral Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.E.P. and M.J.G. conception and design of research; M.E.P., B.J.M., J.B.G., L.E.S., M.J.M., A.E.G., M.A.T., and M.J.G. performed experiments; M.E.P., B.J.M., J.B.G., L.E.S., M.J.M., and A.E.G. analyzed data; M.E.P., B.J.M., J.B.G., L.E.S., M.J.M., M.A.T., and M.J.G. interpreted results of experiments; M.E.P. prepared figures; M.E.P. drafted manuscript; M.E.P., B.J.M., J.B.G., L.E.S., M.J.M., A.E.G., M.A.T., and M.J.G. edited and revised manuscript; M.E.P., B.J.M., J.B.G., L.E.S., M.J.M., A.E.G., M.A.T., and M.J.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all the study participants for their time and effort. We also acknowledge Todd Prior and Sophie Joanisse for their technical assistance.
REFERENCES
- 1.Amat R, Planavila A, Chen SL, Iglesias R, Giralt M, Villarroya F. SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD. J Biol Chem 284: 21872–21880, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett JD, Hwa Joo C, Jeong TS, Louhelainen J, Cochran AJ, Gibala MJ, Gregson W, Close GL, Drust B, Morton JP. Matched work high-intensity interval and continuous running induce similar increases in PGC-1a mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J Appl Physiol 112: 1135–1143, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JD, Louhelainen J, Iqbal Z, Cochran AJ, Gibala MJ, Gregson W, Close GL, Drust B, Morton JP. Reduced carbohydrate availability enhances exercise-induced p53 signaling in human skeletal muscle: implications for mitochondrial biogenesis. Am J Physiol Regul Integr Comp Physiol 304: R450–R458, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Bishop DJ, Edge J, Davis C, Goodman C. Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability. Med Sci Sports Exerc 36: 807–813, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bishop DJ, Thomas C, Moore-Morris T, Tonkonogi M, Sahlin K, Mercier J. Sodium bicarbonate ingestion prior to training improves mitochondrial adaptations in rats. Am J Physiol Endocrinol Metab 299: E225–E233, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health 16: 55–58, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Bouissou P, Defer G, Guezennec CY, Estrade PY, Serrurier B. Metabolic and blood catecholamine responses to exercise during alkalosis. Med Sci Sports Exerc 20: 228–232, 1988. [DOI] [PubMed] [Google Scholar]
- 8.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr AJ, Hopkins WG, Gore CJ. Effects of acute alkalosis and acidosis on performance. Sports Med 41: 801–814, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Carr AJ, Slater GJ, Gore CJ, Dawson B, Burke LM. Effect of sodium bicarbonate on [HCO3−], pH, and gastrointestinal symptoms. Int J Sport Nutr Exerc Metab 21: 189–194, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Cochran AJ, Little JP, Tarnopolsky MA, Gibala MJ. Carbohydrate feeding during recovery alters the skeletal muscle metabolic response to repeated sessions of high-intensity interval exercise in humans. J Appl Physiol 108: 628–636, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Constable PD. Hyperchloremic acidosis: the classic example of strong ion acidosis. Anesth Analg 96: 919–922, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Crouter SE, Churilla JR, Bassett DR. Accuracy of the Actiheart for the assessment of energy expenditure in adults. Eur J Clin Nutr 62: 704–711, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Driller MW, Gregory JR, Williams AD, Fell JW. The effects of chronic sodium bicarbonate ingestion and interval training in highly trained rowers. Int J Sport Nutr Exerc Metab 23: 40–47, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Edge J, Bishop DJ, Goodman C. Effects of chronic NaHCO3 ingestion during interval training on changes to muscle buffer capacity, metabolism, and short-term endurance performance. J Appl Physiol 101: 918–925, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O'Gorman DJ. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol 588: 1779–1790, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14: 101–102, 1982. [PubMed] [Google Scholar]
- 19.Gibala MJ, Gillen JB, Percival ME. Physiological and health-related adaptations to low-volume interval training: influences of nutrition and sex. Sports Med 44, Suppl 2: 127–137, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 21: 2602–2612, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Hawley JA, Burke LM, Phillips SM, Spriet LL. Nutritional modulation of training-induced skeletal muscle adaptations. J Appl Physiol 110: 834–845, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Hawley JA, Burke LM. Carbohydrate availability and training adaptation: effects on cell metabolism. Exerc Sport Sci Rev 38: 152–160, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Hawley JA. Nutritional strategies to modulate the adaptive response to endurance training. Nestle Nutr Inst Workshop Ser 75: 1–14, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 71: 177–203, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Hollidge-Horvat MG, Parolin ML, Wong D, Jones NL, Heigenhauser GJ. Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am J Physiol Endocrinol Metab 278: E316–E329, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Hondares E, Pineda-Torra I, Iglesias R, Staels B, Villarroya F, Giralt M. PPARdelta, but not PPARalpha, activates PGC-1alpha gene transcription in muscle. Biochem Biophys Res Commun 354: 1021–1027, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Latham T, Mackay L, Sproul D, Karim M, Culley J, Harrison DJ, Hayward L, Langridge-Smith P, Gilbert N, Ramsahoye BH. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res 40: 4794–4803, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindholm ME, Fischer H, Poellinger L, Johnson RS, Gustafsson T, Sundberg CJ, Rundqvist H. Negative regulation of HIF in skeletal muscle of elite endurance athletes: a tentative mechanism promoting oxidative metabolism. Am J Physiol Regul Integr Comp Physiol 307: R248–R255, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindinger MI, Kowalchuk JM, Heigenhauser GJ. Applying physicochemical principles to skeletal muscle acid-base status. Am J Physiol Regul Integr Comp Physiol 289: R891–R894, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Little JP, Safdar A, Bishop DJ, Tarnopolsky MA, Gibala MJ. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300: R1303–R1310, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ. Acute endurance exercise increases the nuclear abundance of PGC-1α in trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 298: R912–R917, 2010. [DOI] [PubMed] [Google Scholar]
- 32.McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab 9: 23–34, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metcalfe RS, Koumanov F, Ruffino JS, Stokes KA, Holman GD, Thompson D, Vollaard NB. Physiological and molecular responses to an acute bout of reduced-exertion high-intensity interval training (REHIT). Eur J Appl Physiol. First published July 9, 2015; doi: 10.1007/s00421-015-3217-6. [DOI] [PubMed] [Google Scholar]
- 34.Nordsborg NB, Lundby C, Leick L, Pilegaard H. Relative workload determines exercise-induced increases in PGC-1alpha mRNA. Med Sci Sports Exerc 42: 1477–1484, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philp A, MacKenzie MG, Belew MY, Towler MC, Corstorphine A, Papalamprou A, Hardie DG, Baar K. Glycogen content regulates peroxisome proliferator activated receptor-∂ (PPAR-∂) activity in rat skeletal muscle. PLoS One 8: e77200, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol 546: 851–858, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pogozelski AR, Geng T, Li P, Yin X, Lira VA, Zhang M, Chi JT, Yan Z. p38gamma mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS One 4: e7934, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popov DV, Zinovkin RA, Karger EM, Tarasova OS, Vinogradova OL. The effect of aerobic exercise on the expression of genes in skeletal muscles of trained and untrained men. Hum Physiol 39: 190–195, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Psilander N, Niklas P, Wang L, Li W, Westergren J, Jens W, Tonkonogi M, Michail T, Sahlin K, Kent S. Mitochondrial gene expression in elite cyclists: effects of high-intensity interval exercise. Eur J Appl Physiol 110: 597–606, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403: 139–148, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 15: 551–578, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Skelly LE, Andrews PC, Gillen JB, Martin BJ, Percival ME, Gibala MJ. High-intensity interval exercise induces 24-h energy expenditure similar to traditional endurance exercise despite reduced time commitment. Appl Physiol Nutr Metab 4: 1–4, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Stephens TJ, McKenna MJ, Canny BJ, Snow RJ, McConell GK. Effect of sodium bicarbonate on muscle metabolism during intense endurance cycling. Med Sci Sports Exerc 34: 614–621, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Thomas S, Reading J, Shephard R. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci 17: 338–345, 1992. [PubMed] [Google Scholar]
- 47.Yamaguchi T, Omori M, Tanaka N, Fukui N. Distinct and additive effects of sodium bicarbonate and continuous mild heat stress on fiber type shift via calcineurin/NFAT pathway in human skeletal myoblasts. Am J Physiol Cell Physiol 305: C323–C333, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Yeo WK, McGee SL, Carey AL, Paton CD, Garnham AP, Hargreaves M, Hawley JA. Acute signalling responses to intense endurance training commenced with low or normal muscle glycogen. Exp Physiol 95: 351–358, 2010. [DOI] [PubMed] [Google Scholar]