Abstract
In healthy humans, tests of the hypothesis that lactic acid, PGE2, or ATP plays a role in evoking the exercise pressor reflex proved controversial. The findings in humans resembled ours in decerebrate rats that individual blockade of the receptors to lactic acid, PGE2, and ATP had only small effects on the exercise pressor reflex provided that the muscles were freely perfused. This similarity between humans and rats prompted us to test the hypothesis that in rats with freely perfused muscles combined receptor blockade is required to attenuate the exercise pressor reflex. We first compared the reflex before and after injecting either PPADS (10 mg/kg), a P2X receptor antagonist, APETx2 (100 μg/kg), an activating acid-sensing ion channel 3 (ASIC) channel antagonist, or L161982 (2 μg/kg), an EP4 receptor antagonist, into the arterial supply of the hindlimb of decerebrated rats. We then examined the effects of combined blockade of P2X receptors, ASIC3 channels, and EP4 receptors on the exercise pressor reflex using the same doses, intra-arterial route, and time course of antagonist injections as those used for individual blockade. We found that neither PPADS (n = 5), APETx2 (n = 6), nor L161982 (n = 6) attenuated the reflex. In contrast, combined blockade of these receptors (n = 7) attenuated the peak (↓27%, P < 0.019) and integrated (↓48%, P < 0.004) pressor components of the reflex. Combined blockade injected intravenously had no effect on the reflex. We conclude that combined blockade of P2X receptors, ASIC3 channels, and EP4 receptors on the endings of thin fiber muscle afferents is required to attenuate the exercise pressor reflex in rats with freely perfused hindlimbs.
Keywords: metaboreflex, chemoreflex, muscle afferents
contraction of limb muscles evokes the “exercise pressor reflex,” which is manifested by a constellation of autonomic effects including increases in arterial blood pressure, increases in heart rate, and increases in muscle and renal sympathetic nerve activity (5, 33, 37). Both mechanical and metabolic stimuli arising within the contracting muscles evoke the exercise pressor reflex, the sensory arm of which is comprised of group III (Aδ) and group IV (C) afferents (6, 33). Substantial evidence exists in animals and humans demonstrating that the exercise pressor reflex plays an important role in causing the cardiovascular adjustments to exercise (1, 2, 5, 13, 14, 33, 38, 41).
Both mechanical and metabolic stimuli appear to activate the group III and IV muscle afferents responsible for evoking the reflex (20, 21). Prime candidates for the metabolites responsible for evoking the reflex include lactic acid by activating acid-sensing ion channel 3 (ASIC3) channels, prostaglandin (PG) E2 by activating EP4 receptors, and adenosine triphosphate (ATP) by activating purinergic (P) 2X receptors. Each of these substances is produced by contraction, and each can be recovered from the muscles while they contract (3, 27, 34, 48, 55). The evidence is strong that during exercise the concentration of each of the substances increases within the muscle interstitium, which is the location of many of the endings supplying group III and IV afferent fibers.
In healthy humans, tests of the hypothesis that lactic acid, PGE2, or ATP plays a significant role in evoking the exercise pressor reflex have proved controversial. For example, some investigators have reported that lactic acid plays a role in evoking the reflex (11, 46), whereas others have reported that lactic acid plays no or little role in evoking the reflex (31, 57). Likewise, some have reported that prostaglandins play a role (8, 12), whereas others have reported that prostaglandins do not (4, 9, 10, 40). Finally, some investigators have reported that ATP evokes the exercise pressor reflex (7), whereas others have reported that ATP plays little or no role in evoking the reflex (15).
Examination of these reports suggested to us that even when a role was found for one of these metabolic by-products of contraction in evoking the exercise pressor reflex in humans that the effect was modest. In fact, when viewed together the findings in humans resembled our findings in decerebrate rats that blockade of the receptors to lactic acid, PGE2, and ATP, when initiated individually, had only a small, if any, effect on the exercise pressor reflex provided that the working muscles were freely perfused (54, 56, 60). This similarity between humans and rats resulted in our generating the hypothesis that the stimulating effects of individual metabolites on the thin fiber muscle afferents evoking the exercise pressor reflex were redundant. Specifically, we hypothesized that removing the input of one metabolite onto the thin fiber afferents evoking the reflex would be compensated for by the input of other metabolites, and as a consequence the magnitude of the exercise pressor reflex would be little changed. We tested this hypothesis in decerebrate unanesthetized rats whose contracting hindlimb muscles were freely perfused. We compared the effects of combined blockade of the receptors to lactic acid, PGE2, and ATP with the effects of individual blockades of these receptors on the magnitude of the exercise pressor reflex.
MATERIALS AND METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine. Adult male rats (Sprague-Dawley rats, n = 29, weighing 415 ± 11 g) were used in this study.
General Surgery
Rats were initially anesthetized with a mixture of 4% isoflurane and 100% oxygen. The trachea was cannulated, after which the lungs were mechanically ventilated (Harvard Apparatus) with the anesthetic mixture. The right jugular vein and both carotid arteries were cannulated. Arterial blood pressure was measured by attaching the right carotid arterial catheter to a pressure transducer (model P23 XL; Statham); heart rate was calculated beat to beat from the arterial pressure pulse (Gould Biotach). The left superficial epigastric artery was cannulated with a PE-8 catheter whose tip was placed near the junction of the femoral and superficial epigastric arteries. A reversible snare (2-0 silk suture) was placed around the left iliac artery and vein (i.e., proximal to the location of the catheter placed in the superficial epigastric artery). Arterial blood gases and pH were measured using an automated blood-gas analyzer (model ABL 80 FLEX; Radiometer). Arterial Pco2 and pH were maintained within normal physiological range by either adjusting ventilation or intravenous administration of sodium bicarbonate (8.5%). Arterial Po2 varied between ∼95-115 mmHg. Core body temperature was monitored using a rectal temperature probe and maintained at 37–38°C using a heat lamp.
The rats were then secured in a Kopf-customized spinal frame by clamps placed on the rostral lumbar vertebrae and the pelvis. A precollicular decerebration was performed by sectioning the brain less than 1 mm anterior to the superior colliculi. Dexamethasone (0.2 mg) was injected intravenously before the decerebration procedure to minimize brain stem edema. All neural tissue rostral to the section was removed. To minimize bleeding, small pieces of oxidized regenerated cellulose (Ethicon; Johnson & Johnson) were placed on the internal skull surface and the cranial cavity was packed with gauze. Immediately after the precollicular transection, gas anesthesia was discontinued and the rats' lungs were ventilated mechanically with room air. Experiments were performed in decerebrate, unanesthetized rats because anesthesia has been shown to depress the exercise pressor reflex in this species (51). After decerebration, the rats were allowed to stabilize for at least 1 h before any experimental protocol was initiated. The left calcaneal bone was sectioned and was then attached to a force transducer (FT-10; Grass) to measure tension developed by the statically contracting triceps surae muscles. The left sciatic nerve in region of the popliteal fossa was surgically isolated by blunt dissection.
Experimental Protocols
Individual blockade.
We first examined the effects of individually blocking P2X receptors, ASIC3 channels, and EP4 receptors on the exercise pressor reflex. The left hindlimb muscles were statically contracted for 30 s by electrically stimulating (40 Hz; 0.01 ms) the sciatic nerve. The current of the individual pulses applied to the sciatic nerve never exceeded twice the threshold current needed to evoke a muscle twitch. After waiting 10 min, we infused (20 μl/min) PPADS (10 mg/kg), a P2X antagonist, into superficial epigastric artery while the snare around the iliac artery and vein was tightened. After an infusion period of 10 min, the snare was released and hindlimb muscles were freely perfused for another 10 min. Following that period, the leg was again statically contracted using the same parameters as those used for contraction before the drug infusion. This protocol is identical to that used previously by our laboratory (54). All infusions were done with a CMA microdialysis pump. In a different group of rats, we injected APETx2 (100 μg/kg; 0.1 ml), an ASIC3 antagonist, into the superficial epigastric artery while the snare around the iliac artery and vein was tightened. After 10 min the snare was released and the hindlimb was freely perfused for another 10 min. Following that period, the leg was again statically contracted using the same parameters as those used for contraction before the drug injection. This protocol is identical to that used previously by our laboratory (56). In yet another group of rats, we injected L161982 (2 μg/kg; 0.1 ml), an EP4 antagonist, into the superficial epigastric artery while the snare around the iliac artery and vein was tightened. After 5 min, the snare was released and the hindlimb was freely perfused for another 25 min. Following that period, the leg was again statically contracted using the same parameters as those used for contraction before the drug injection. This protocol is identical to that used previously by our laboratory (60).
Combined blockade.
We next examined the effects of combined blockade of ASIC3, PGE2, and P2X receptors on the exercise pressor reflex in rats with freely perfused hindlimbs. The rats used in the combined blockade experiments were not used in the individual blockade experiments. We used the same doses of drugs and time courses of drug injections as those mentioned above for individual blockade experiments. L161982 was injected ten min after the first contraction. Ten minutes later, APETx2 was injected which was immediately followed by the infusion of PPADS. The hindlimb muscles were contracted again 10 min after all drugs were injected or, in other words, 40 min after the first contraction. The doses of the blocking agents used were shown previously to attenuate markedly the pressor responses to femoral artery injections of their respective agonists (54, 56, 60). To control for the possibility that the drugs circulated to the spinal cord or brainstem to exert an attenuating effect on the exercises pressor reflex, we injected the same combination of drugs into the vena cava in a separate group of rats.
At the end of every experiment, rats were paralyzed with pancuronium bromide (1 mg/kg iv), and the sciatic nerve was stimulated with the same stimulation parameters as those used to induce muscle contraction. This was done to ensure that the pressor responses observed during contraction were not the result of electrical activation of the axons of thin fiber afferents in the sciatic nerve. Additionally, at the end of every experiment in which we injected antagonists into the superficial epigastric artery, blue dye was also injected into the superficial epigastric artery (0.1–0.2 ml) to ensure that the drugs did in fact travel to the triceps surae muscles.
Data analysis.
In all experiments, baseline as well as reflex changes in mean arterial pressure, heart rate, and developed tension were recorded continuously with a Spike 2 data acquisition system (CED; Cambridge) and stored on a computer hard drive (Dell). Two methods were used to analyze the data. In the first, the peak pressor and cardioaccelerator responses to static contraction, regardless of where they occurred during the 30-s contraction period, were compared before and after pharmacological blockade. In the second method, the time courses of the pressor responses were analyzed. Specifically, the mean pressor responses for each second of the 30-s static contraction period were plotted and then compared before and after pharmacological blockade. Mean arterial pressure is expressed in millimeters mercury and heart rate is in beats per minute. The tension-time index was calculated by integrating the area between the tension trace and the baseline level and is expressed in kilogram seconds.
All values are expressed as means ± SE. Statistical comparisons were performed with either a paired t-test for the first method or two-way repeated-measures ANOVA for the second method. If an overall F value was significant for the repeated-measures ANOVA, then post hoc tests were performed with the Holm-Sidak's tests between individual means. The criterion for statistical significance was set as P < 0.05.
RESULTS
In all experiments, electrical stimulation of the sciatic nerve in rats paralyzed by intravenous injection of pancuronium abolished the pressor response to static contraction that was evoked before paralysis. The currents, frequencies, and pulse durations used to stimulate the sciatic nerve after paralysis were the same as those used before paralysis. Likewise, in all experiments, blue dye injection into the superficial epigastric artery resulted in staining of the triceps surae muscles. These findings are consistent with the concept that the pressor responses to contraction were reflex in origin and were evoked by contraction of the triceps surae muscles. These findings are also consistent with the concept that injections into the superficial epigastric artery traveled to the triceps surae muscles.
Individual Blockade Experiments
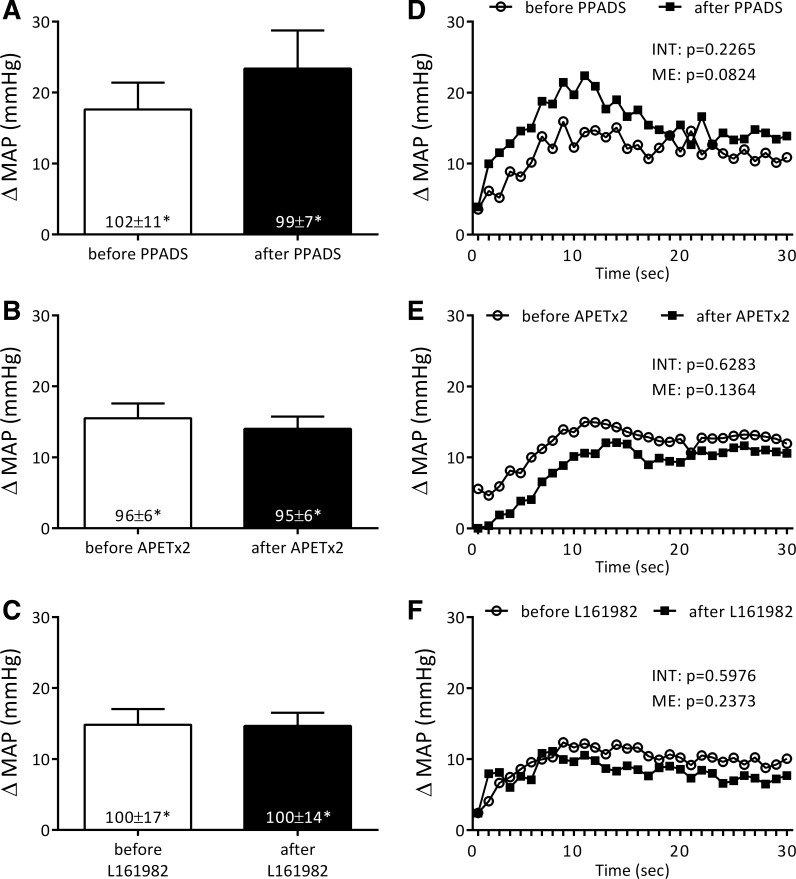
Neither PPADS (n = 5), APETx2 (n = 6), nor L161982 (n = 6) had any effect on the pressor or cardioaccelerator components of the exercise pressor reflex (Fig. 1; Table 1). To be specific, none of the three blocking agents had a significant effect on the peak pressor response to contraction. Likewise, neither of three blocking agents had a significant main (ME) or interactive (INT) effect on the second by second increases in the pressor responses evoked during the 30-s contraction period (Fig. 1). In addition, none of the three individual blockades had any effect on the small cardioaccelerator responses to static contraction (Table 1). The tension time indexes before and after injection of any of the three blocking agents, given individually, were not significantly different from each other (Table 1).
Fig. 1.
Pressor responses to static contraction before and after blockade of individual activating acid-sensing ion channel 3 (ASIC3), P2X, and EP4 receptors. Peak pressor responses to static contraction before and after PPADS (A; n = 5), APETx2 (B; n = 6), and L161982 (C; n = 6). Time courses, plotted second by second, of the changes in mean arterial pressure (MAP) averaged for each second of contraction before and after PPADS (D), APETx2 (E), and L161982 (F). For purpose of illustration, SE bars were omitted. *Increases in MAP were significantly greater than their respective baseline levels in A–C.
Table 1.
Peak changes in heart rate and overall developed tension during 30 s of static contraction of the hindlimb muscles before and after injecting P2X receptor, ASIC3 channel, and/or EP4 receptor inhibitors into the femoral artery
| ΔHR, beats/min |
TTI, kg · s |
||||
|---|---|---|---|---|---|
| Drug | Before | After | Before | After | n |
| PPADS (10 mg/kg) | 11.6 ± 3 (352 ± 14*) | 10.6 ± 3 (347 ± 19*) | 23.8 ± 3 | 23.6 ± 2 | 5 |
| APETx2 (100 μg/kg) | 9.7 ± 3 (375 ± 22*) | 6.5 ± 2 (368 ± 19*) | 22.1 ± 2 | 22.1 ± 2 | 6 |
| L161982 (2 μg/kg) | 6.2 ± 3 (351 ± 22) | 11.2 ± 5 (347± 24) | 19.0 ± 3 | 18.7 ± 3 | 6 |
| PPADS (10 mg/kg ia), APETx2 (100 μg/kg ia), and L161982 (2 μg/kg ia) | 11.3 ± 3 (348 ± 27*) | 7.6 ± 2 (326 ± 24*) | 21.0 ± 2 | 21.3 ± 2 | 7 |
| PPADS (10 mg/kg iv), APETx2 (100 μg/kg iv), and L161982 (2 μg/kg iv) | 22.6 ± 6 (363 ± 31*) | 17.8 ± 3 (372 ± 22) | 20.3 ± 3 | 21.9 ± 3 | 5 |
Baseline values (means ± SE) for mean arterial pressure and heart rate (HR) are reported in italics next to peak change. ASIC3, activating acid-sensing ion channel 3; TTI, tension-time index.
P < 0.05, HR increased significantly over baseline values.
Combined Blockade Experiments
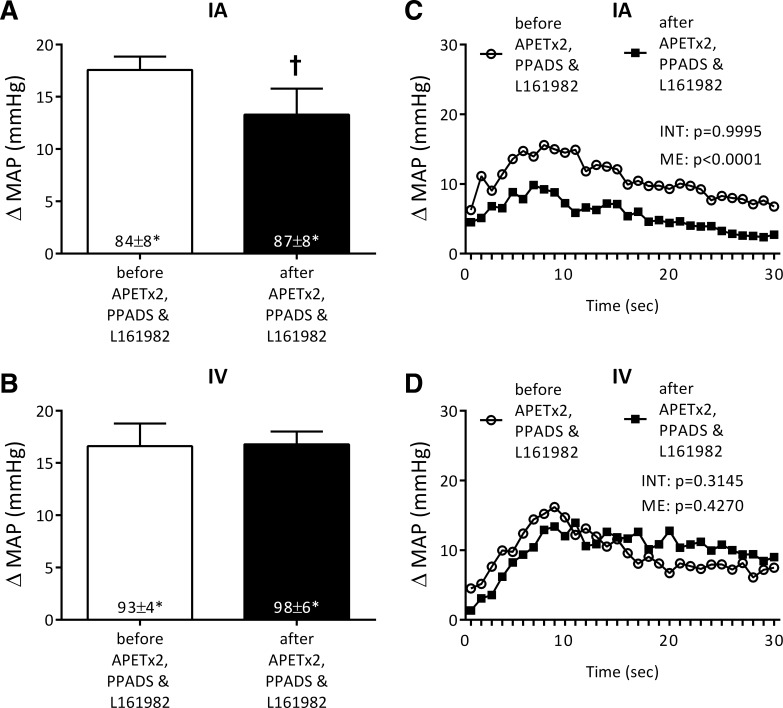
PPADS, APETx2, and L161982, injected in combination into the superficial epigastric artery, significantly reduced the peak pressor response to static contraction by 27 ± 11% (P = 0.019; Fig. 2A). Second-by-second analysis revealed that the attenuation of the pressor response to contraction by the combined blockade was highly significant (main effect: P < 0.0001; Fig. 2C). Moreover, integrating the area under the curve that plotted the increase in arterial pressure vs. time revealed that combined blockade decreased the overall pressor response to 30 s of contraction from 321 ± 24 to 166 ± 41 mmHg·s, values that represent a 48% reduction (n = 7; P < 0.004). Combined blockade had no effect on the peak cardioaccelerator response to static contraction (Table 1). Intravenous injection of PPADS, APETx2, and L161982 in the same doses as those injected into the superficial epigastric artery had no effect on the exercise pressor reflex (Fig. 2, B and D; Table 1); this finding indicates that the attenuation of the reflex caused by combined blockade was not attributable to circulation of the three antagonists to the spinal cord or brainstem. The tension time indexes before and after injection of any of the three blocking agents, given in combination, were not significantly different from each other (Table 1).
Fig. 2.
Pressor responses to static contraction before and after simultaneous blockade of ASIC3, P2X, and EP4 receptors. Peak pressor responses to static contraction before and after the combination of PPADS, APETx2, and L161982 injected into the femoral artery (A) and vena cava (B; n = 7). Time courses, plotted second by second, of the changes in MAP averaged for each second of contraction before and after the combination of PPADS, APETx2, and L161982 injected into the femoral artery (C) and vena cava (D) (n = 5). For purpose of illustration, standard error bars were omitted. *P < 0.05, increases in MAP were significantly than their respective baseline levels in A and B. †P < 0.05, significant attenuation in the peak pressor response after drugs compared with that before drugs.
DISCUSSION
The exercise pressor reflex has been shown to play an important role in evoking the cardiovascular responses to exercise in both humans (1, 38) and animals (5, 22, 33, 41, 42). In our experiments in decerebrate rats, we found that individual blockade of ASIC3 receptors, EP4 receptors, or P2X receptors had minimal effects on the exercise pressor reflex. In contrast, when the three receptors were blocked simultaneously, the peak pressor response was reduced by 27% and the overall pressor component of the reflex was reduced by almost half (i.e., 48%). The doses of the blocking agents used in our experiments were unlikely to be “subthreshold” because these doses in previous studies from our laboratory attenuated by more than half the pressor responses to femoral arterial injections of large and presumably supraphysiological amounts of their respective agonists (54, 56, 60).
Our finding that individual blockade of ASIC3, EP4, or P2X receptors on the endings of group III and IV afferents had no effect on the exercise pressor reflex, whereas combined blockade of these receptors attenuated the reflex by almost half in decerebrated unanesthetized rats with freely perfused hindlimbs, is consistent with the concept of redundant mechanisms controlling the cardiovascular system during exercise. This concept offers a reasonable explanation for our finding that removing the influence of one receptor had no effect on the exercise pressor reflex because the remaining input from other receptors was sufficient to evoke the reflex at its preblockade magnitude. In contrast, simultaneously removing the influence of the three receptors reduced afferent input to the dorsal horn of the spinal cord to a level that was insufficient to maintain the expression of the reflex at its preblockade magnitude.
Although combined receptor blockade appreciably reduced the blood pressure response to contraction in our experiments, it had little effect on the cardioaccelerator response to contraction. One interpretation of this finding is that ASIC3, P2X, and EP4 receptors do not contribute to metaboreflex control of heart rate in rats. Alternatively, our finding that combined blockade did not decrease the cardioaccelerator response to contraction might be explained by the fact that the attenuated pressor response caused less baroreceptor stimulation than that before combined blockade. The reduced baroreflex, in turn, countered the effect on heart rate of a reduced exercise pressor reflex, resulting in the same cardioaccelerator response to contraction before and after combined blockade.
The concept of redundancy has been used to explain other findings concerning the cardiovascular responses to exercise. At first the concept was used to explain the fact that both the exercise pressor reflex and central command were capable of evoking the pressor and cardioaccelerator responses to static exercise (36). Subsequently, the concept was extended to other exercise-induced effects, such as redundant adrenergic and muscarinic mechanisms initiating the pressor response to static exercise (39) and redundant vasodilator mechanisms initiating the hyperemic response to rhythmic forearm exercise (50). In addition, the concept was extended to interpret the finding that the exercise pressor reflex and central command both reset the baroreceptor reflex (35, 43).
Our findings in rats with ligated femoral arteries appear to parallel recent studies in humans in which blockade of either purinergic 2 receptors or cyclooxygenase attenuated the exercise pressor reflex in humans with either peripheral artery disease (40) or hypertension (15) but had minimal if any effect on their age matched healthy counterparts. Specifically, in rats with ligated femoral arteries, individual blockade of ASIC3, P2X, or EP4 receptors significantly attenuated the exercise pressor reflex, findings that contrast with those found in rats with freely perfused femoral arteries (54, 56, 60). The reasons for this difference remain to be determined, but an increase in receptor number is a strong possibility. For example, in rats whose femoral arteries were ligated for 3 days, the number of ASIC3 and P2X3 receptors innervating the hindlimb muscles was increased (29, 30). Another strong possibility is that femoral artery ligation causes a severe mismatch between blood/oxygen supply and demand in contracting muscles, thereby increasing production of the ischemic metabolites stimulating thin fiber muscle afferents.
We previously found in decerebrate cats with freely perfused hindlimb muscles that blocking individually the stimulating effects of ATP, lactic acid, or PGE2 on thin fiber muscle afferents significantly attenuated the magnitude of the exercise pressor reflex (17–19, 24, 47). In contrast, we now report that in decerebrate rats blocking individually the stimulating effects of these metabolites had no effect on the magnitude of the reflex. We can only speculate as to the reasons for the differences noted between each species but two possibilities come to mind. The first involves differences between receptor numbers on the endings of the thin fiber afferents, and the second involves differences in metabolite production during contraction. With respect to the first reason, ASIC3, P2X3, and EP4 receptors can be expected to be found on dorsal root ganglion cells of both rats and cats, but no comparison of their numbers has been made between the two species (49, 52, 58, 59). With respect to the second reason, muscle interstitial concentrations of lactic acid, ATP, and PGE2 have been measured in both cats and rats, but both the type and durations of the contractions differed greatly from that used in our experiments (26, 32, 58, 59), making any comparison difficult if not impossible. In our experiments we were not able to measure the interstitial concentration of these metabolites because the contraction period of 30 s was too short to allow for enough interstitial fluid to be collected. Whatever the explanation for this species difference, we speculate that our findings from decerebrate rats with freely perfused hindlimb muscles appear to be more representative of the healthy human condition than do our findings in decerebrate cats with freely perfused hindlimb muscles.
Two recent reports by Light and colleagues (28, 45) have provided evidence that muscle metabolites have a synergistic effect on thin fiber muscle afferents evoking the exercise pressor reflex or the sensations of either fatigue or pain. In the first report, separately exposing cultured murine dorsal root ganglion cells to either lactate, protons, or ATP, in the same concentrations as those found in exercising muscle, had little effect on intracellular concentrations of calcium, a measure that was used as an index of activation (28). In contrast, exposing murine dorsal root ganglion cells to a combination of the three metabolites, namely lactate, protons, and ATP, increased their intracellular calcium concentrations. In the second report, injecting lactate, protons, or ATP into the muscles of the human hand did not evoke reports of fatigue or pain, whereas combined injections did (45).
We performed experiments that might be considered the reverse of those performed by Light and colleagues. Specifically, we prevented muscle metabolites from stimulating thin fiber afferents innervating contracting muscles, whereas Light and colleagues added muscle metabolites in concentrations representative of contraction and then examined the responses of the thin fiber afferents or reports of pain and fatigue. We believe that our findings are best explained by the concept of redundancy, which postulates that removing one metabolite at a time would have no effect on the exercise pressor reflex because the loss of the stimulating effect of one metabolite would be compensated by the stimulating effects of other metabolites. In contrast, the findings of Light and colleagues are best explained by the concept of synergy, which postulates that removing one metabolite at a time would be sufficient to prevent the reflex from being expressed because all three (i.e., PGE2, lactic acid, and ATP) are needed to stimulate the thin fiber muscle afferents evoking the reflex. At first glance, our findings might appear to conflict with those of Light and colleagues. However, our approach was to remove the stimulating effect of metabolites on the afferents, whereas the approach of Light and colleagues was to add them. Because of the difference in approaches, no conclusion should be drawn at the present time.
In conclusion, we found that combined blockade of ASIC3, EP4, and P2X receptors reduced the peak and integrated pressor response to contraction by 27 and 48%, respectively. The remaining half could be due to the contraction-induced stimulation of other receptors by muscle metabolites. Two possible candidates are the BK2 receptor whose natural agonist is bradykinin (44, 53) and TRPA1 receptors whose natural agonist is also bradykinin as well as arachidonic acid and diprotonated phosphate (25). Alternatively, the remaining half of the exercise pressor reflex could be caused by mechanoreceptor stimulation (16, 23).
GRANTS
This work was supported by National Institutes of Health Grants HL-096570 and AR-059397.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.J.S. and M.P.K. conception and design of research; A.J.S., S.W.C., and J.S.K. performed experiments; A.J.S., J.S.K., and M.P.K. analyzed data; A.J.S., S.W.C., and M.P.K. interpreted results of experiments; A.J.S. prepared figures; A.J.S. and M.P.K. drafted manuscript; A.J.S., S.W.C., and M.P.K. edited and revised manuscript; A.J.S., S.W.C., J.S.K., and M.P.K. approved final version of manuscript.
REFERENCES
- 1.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol 495: 587–596, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey DP, Joyner MJ. Prostaglandins do not contribute to the nitric oxide-mediated compensatory vasodilation in hypoperfused exercising muscle. Am J Physiol Heart Circ Physiol 301: H261–H268, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coote JH, Perez-Gonzalez JF. The response of some sympathetic neurones to volleys in various afferent nerves. J Physiol 208: 261–278, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J, Leuenberger UA, Blaha C, King NC, Sinoway LI. Effect of P2 receptor blockade with pyridoxine on sympathetic response to exercise pressor reflex in humans. J Physiol 589: 685–695, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui J, McQuillan P, Momen A, Blaha C, Moradkhan R, Mascarenhas V, Hogeman C, Krishnan A, Sinoway LI. The role of the cyclooxygenase products in evoking sympathetic activation in exercise. Am J Physiol Heart Circ Physiol 293: H1861–H1868, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davy KP, Herbert WG, Williams JH. Effect of indomethacin on the pressor responses to sustained handgrip contraction in humans. J Appl Physiol 75: 273–278, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Doerzbacher KJ, Ray CA. Muscle sympathetic nerve responses to physiological changes in prostglandin production in humans. J Appl Physiol 90: 624–629, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Fadel PJ, Wang Z, Tuncel M, Watanabe H, Abbas A, Arbique D, Vongpatanasin W, Haley RW, Victor RG, Thomas GD. Reflex sympathetic activation during static exercise is severely impaired in patients with myophosphorylase deficiency. J Physiol 548: 983–993, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana GA, Pantaleo T, Bongianni F, Gresci F, Lavorini F, TostiGuerra C, Panuccio P. Prostaglandin synthesis blockade by ketoprofen attenuates the respiratory and cardiovascular responses to static handgrip. J Appl Physiol 78: 449–530, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Friedman DB, Brennum J, Sztuk F, Hansen OB, Clifford PS, Bach FW, Arendt-Nielsen L, Mitchell JH, Secher NH. The effect of epidural anaesthesia with 1% lidocaine on the pressor response to dynamic exercise in man. J Physiol 470: 681–691, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman DB, Peel C, Mitchell JH. Cardiovascular responses to voluntary and nonvoluntary static exercise in humans. J Appl Physiol 73: 1982–1985, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Greaney JL, Matthews EL, Boggs ME, Edwards DG, Duncan RL, Farquhar WB. Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: role of purinergic receptors. Am J Physiol Heart Circ Physiol 306: H132–H141, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol 280: H2153–H2161, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol 581: 1271–2323, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol 290: H2239–H2246, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Hayes SG, McCord JL, Rainier J, Liu Z, Kaufman MP. Role played by acid-sensitive ion channels in evoking the exercise pressor reflex. Am J Physiol Heart Circ Physiol 295: H1720–H1725, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984. [DOI] [PubMed] [Google Scholar]
- 22.Kaur J, Spranger MD, Hammond RL, Krishnan AC, Alvarez A, Augustyniak RA, O'Leary DS. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in beta2-mediated vasodilation. Am J Physiol Heart Circ Physiol 308: H524–H529, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JK, Hayes SG, Kindig AE, Kaufman MP. Thin-fiber mechanoreceptors reflexly increase renal sympathetic nerve activity during static contraction. Am J Physiol Heart Circ Physiol 292: H866–H873, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Kindig AE, Hayes SG, Kaufman MP. Blockade of purinergic 2 receptors attenuates the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 293: H2995–H3000, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Koba S, Hayes SG, Sinoway LI. Transient receptor potential A1 channel contributes to activation of the muscle reflex. Am J Physiol Heart Circ Physiol 300: H201–H213, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Gao Z, Kehoe V, Xing J, King N, Sinoway L. Interstitial adenosine triphosphate modulates muscle afferent nerve-mediated pressor reflex. Muscle Nerve 38: 972–977, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol 95: 577–583, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X and TRPV1. J Neurophysiol 100: 1184–1201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299: H1357–H1364, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Li JD, Lu J, Xing J, Li J. Contribution of nerve growth factor to upregulation of P2X3 expression in DRG neurons of rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H1070–H1079, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K+, lactate, and phosphate determined with MSNA during exercise in humans. Am J Physiol Regul Integr Comp Physiol 278: R563–R571, 2000. [DOI] [PubMed] [Google Scholar]
- 32.MacLean DA, La Noue KF, Gray KS, Sinoway LI. Effects of hindlimb contraction on pressor and muscle interstitital metabolite responses in the cat. J Appl Physiol 85: 1583–1592, 1998. [DOI] [PubMed] [Google Scholar]
- 33.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCord JL, Hayes SG, Kaufman MP. PPADS does not block contraction-induced prostaglandin E2 synthesis in cat skeletal muscle. Am J Physiol Heart Circ Physiol 295: H2043–H2045, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIlveen SA, Hayes SG, Kaufman MP. Both central command and exercise pressor reflex reset carotid sinus baroreflex. Am J Physiol Heart Circ Physiol 280: H1454–H1463, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell JH. Cardiovascular control during exercise: central and reflex neural mechanisms. Am J Cardiol 55: 34D–41D, 1985. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell JH, Reeves DR, Rogers HB, Secher NH. Epidural anesthesia and cardiovascular responses to static exercise in man. J Physiol 417: 13–24, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell JH, Reeves JDR, Rogers HB, Secher NH, Victor RG. Autonomic Blockade and cardiovascular responses to static exercise in partially curarized man. J Physiol 413: 433–445, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller MD, Drew RC, Ross AJ, Blaha CA, Cauffman AE, Kaufman MP, Sinoway LI. Inhibition of cyclooxygenase attenuates the blood pressure response to plantar flexion exercise in peripheral arterial disease. Am J Physiol Heart Circ Physiol 309: H523–H528, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999. [DOI] [PubMed] [Google Scholar]
- 42.O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Ogoh S, Wasmund WL, Keller DM, O-Yurvati A, Gallagher KM, Mitchell JH, Raven PB. Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol 543: 349–364, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan HL, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol 75: 2061–2068, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, White AT, Light KC, Schweinhardt P, Amann M, Light AR. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol 99: 368–380, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pryor SL, Lewis SF, Haller RG, Bertocci LA, Victor RG. Impairment of sympathetic activation during static exercise in patients with muscle phosphorylase deficiency (McArdle's Disease). J Clin Invest 85: 1444–1449, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates the responses of group IV muscle afferents to static contraction. Am J Physiol Heart Circ Physiol 259: H745–H750, 1990. [DOI] [PubMed] [Google Scholar]
- 48.Rotto DM, Massey KD, Burton KP, Kaufman MP. Static contraction increases arachidonic acid levels in gastrocnemius muscles of cats. J Appl Physiol 66: 2721–2724, 1989. [DOI] [PubMed] [Google Scholar]
- 49.Ruan HZ, Birder LA, de Groat WC, Tai C, Roppolo J, Buffington CA, Burnstock G. Localization of P2X and P2Y receptors in dorsal root ganglia of the cat. J Histochem Cytochem 53: 1273–1282, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Southall MD, Vasko MR. Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J Biol Chem 276: 16083–16091, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Stebbins CL, Carretero OA, Mindroiu T, Longhurst JC. Bradykinin release from contracting skeletal muscle of the cat. J Appl Physiol 69: 1225–1230, 1990. [DOI] [PubMed] [Google Scholar]
- 54.Stone AJ, Yamauchi K, Kaufman MP. Purinergic 2X receptors play a role in evoking the exercise pressor reflex in rats with peripheral artery insufficiency. Am J Physiol Heart Circ Physiol 306: H396–H404, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Symons JD, Theodossy SJ, Longhurst JC, Stebbins CL. Intramuscular accumulation of prostaglandins during static contraction of the cat triceps surae. J Appl Physiol 71: 1837–1842, 1991. [DOI] [PubMed] [Google Scholar]
- 56.Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J Physiol 589: 6173–6189, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vissing J, MacLean D, Vissing SF, Sander M, Saltin B, Haller RG. The exercise metaboreflex is maintained in the absence of muscle acidosis: insights from muscle microdialysis in humans with McArdle's disease. J Physiol 537: 641–649, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xing J, Lu J, Li J. Acid-sensing ion channel subtype 3 function and immunolabelling increases in skeletal muscle sensory neurons following femoral artery occlusion. J Physiol 590: 1261–1272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xing J, Lu J, Li J. Augmented P2X response and immunolabeling in dorsal root ganglion neurons innervating skeletal muscle following femoral artery occlusion. J Neurophysiol 109: 2161–2168, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamauchi K, Kim JS, Stone AJ, Ruiz-Velasco V, Kaufman MP. Endoperoxide 4 receptors play a role in evoking the exercise pressor reflex in rats with simulated peripheral artery disease. J Physiol 591: 2949–2962, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]