Abstract
The purpose of this study was to evaluate cardiorespiratory fitness and reasons for exercise curtailment in a contemporary adult cystic fibrosis (CF) cohort with mild lung disease. Adults with mild CF (n = 19, forced expiratory volume in 1 s = 95 ± 17% predicted) were age-, sex-, ethnicity-, and body mass index-matched to healthy controls (n = 19) and underwent a detailed cardiopulmonary cycle exercise test. While CF subjects had a reduced peak oxygen uptake compared with controls, the values were normal when expressed as %predicted in 14/19 (74%) of subjects. Both groups demonstrated a normal cardiovascular limitation to exercise and stopped exercise primarily because of leg fatigue. Despite not being exercise-limited by respiratory factors, there was some evidence of ventilatory abnormalities as patients with mild CF had increased end-inspiratory lung volumes and reached an inflection/plateau in tidal volume relative to minute ventilation at lower exercise intensities compared with controls. Subjects with CF were not more likely to demonstrate expiratory flow limitation compared with controls and did not have evidence of dynamic hyperinflation during exercise. Despite increased end-inspiratory lung volumes and an earlier tidal volume inflection/plateau, CF subjects did not experience higher levels of dyspnea. In an exploratory analysis, a significant inverse correlation was observed between sweat chloride and peak work rate. Adult CF subjects with relatively well preserved spirometry have normal exercise performance relative to reference values and are primarily limited by nonrespiratory factors. However, ventilatory abnormalities were detected even in this mild CF cohort and should be evaluated in future therapeutic trials focused on disease-modifying therapies in mild CF.
Keywords: exercise limitation, dyspnea, ventilatory responses, expiratory flow limitation, dynamic hyperinflation
significant advances in health outcomes and survival have taken place in the field of cystic fibrosis (CF) over the past few decades (39). A more aggressive approach to the management of CF lung disease during the pediatric years has enabled patients to transition to adult clinics with normal or near normal lung function. Based on the 2013 US CF Foundation Patient Registry, the proportion of 18-year-olds in the normal/mild lung function category [i.e., forced expiratory volume in 1 s (FEV1) ≥ 70% predicted] has increased from 37% in 1988 to 72% in 2013 (6a).
Detection of physiological abnormalities in CF adults with relatively preserved pulmonary function can be challenging but may provide an opportunity to initiate disease-modifying treatments earlier. The FEV1 is considered the most robust measure of pulmonary function in CF and relates to multiple endpoints, including exacerbations and mortality, but is insensitive to early structural changes (42). Airways disease originates within the small peripheral airways in CF and therefore its extent is often underestimated based on FEV1 alone. Measurement of lung clearance index, an indication of ventilation inhomogeneity from small airways disease, is more sensitive than FEV1 and can precede changes in FEV1 by up to 2 to 3 yr (21) but its use is currently limited to the research setting. Cardiopulmonary exercise testing (CPET) is an alternative method that places stress on the cardiorespiratory system and may uncover early physiological abnormalities not otherwise detected with traditional spirometric parameters. Indeed, several recent studies have uncovered important physiological abnormalities in chronic obstructive pulmonary disease (COPD) patients with well-preserved FEV1 (5, 13, 16, 33).
To our knowledge, no prior studies have focused on the exercise performance and physiological reasons for exercise curtailment in adults with mild CF lung disease and compared their results to a group of carefully matched healthy controls. One small study which consisted of a mixed group of 10 adolescents and adults with mild CF lung disease demonstrated a slightly reduced peak oxygen uptake (V̇o2 peak) compared with age-matched healthy individuals (26). In contrast, another study found that V̇o2 peak was not reduced in six children with CF and normal lung function compared with controls (4). Accordingly, the purpose of this study was to evaluate cardiorespiratory fitness and to comprehensively characterize the ventilatory and perceptual responses to cycle exercise in CF patients with relatively well preserved spirometry. We hypothesized that adult CF patients with normal/mild lung disease based on FEV1 measurement would have reduced cardiorespiratory fitness, increased dyspnea, and greater mechanical ventilatory constraints compared with controls.
METHODS
Subjects.
This study included 19 subjects with mild CF (FEV1 > 70% predicted) (11) and 19 age-, sex-, ethnicity- and body mass index (BMI)-matched healthy controls. CF was confirmed based on abnormal sweat chloride testing and/or CF transmembrane conductance regulator (CFTR) genotyping according to published guidelines (10). Inclusion criteria were as follows: age between 19 to 50 yr, stable clinical status, and nonsmoker at the time of testing or past smoking history <20 pack-yrs. CF subjects were excluded if they had a disease other than CF that could contribute to dyspnea or exercise limitation, contraindications to exercise testing, and use of supplemental oxygen or desaturation <85% during exercise. Healthy controls were excluded if they had any respiratory, cardiovascular, neuromuscular, or musculoskeletal condition(s) that could contribute to dyspnea or exercise limitation.
Experimental overview.
This controlled, cross-sectional study received institutional ethical approval and all subjects provided written informed consent prior to participating. The study was conducted over one visit and included a detailed medical history, dyspnea evaluation, anthropometric measurements, pulmonary function testing, exercise familiarization, and a CPET. CF subjects were asked to refrain from using bronchodilator medications for between 6 and 12 h, depending on if the medication was short or long acting. All subjects were encouraged to avoid alcohol, caffeine, and heavy meals for at least 4 h, and to avoid strenuous exercise for at least 48 h before testing.
Pulmonary function.
Spirometry, plethysmography, diffusing capacity, maximum voluntary ventilation (MVV), and maximum respiratory pressures were performed according to previous recommendations (1, 24, 27, 44) by using a commercially available cardiopulmonary testing system (Vmax Encore 229, V62J Autobox; CareFusion, Yorba Linda, CA), and all measurements were expressed as %predicted. The “poorly communicating fraction” (PCF) of total lung capacity (TLC) was calculated as 1-(alveolar volume/TLC) and was expressed as a percentage as recently described (31).
CPET protocol.
An incremental exercise test was performed by using an electronically braked cycle ergometer (Ergoselect 200P; Ergoline, Bitz, Germany). The test consisted of a steady-state rest for 6 min, a 1-min warm-up of unloaded pedaling, and 20-W stepwise increases in work rate every 2 min until symptom limitation. All subjects were familiarized with the exercise testing procedures. This involved subjects performing unloaded cycling so that they could practice inspiratory capacity (IC) maneuvers and become familiar with the symptom scales and breathing apparatus.
CPET measurements.
Standard cardiorespiratory measures were recorded on a breath-by-breath basis and averaged over 30-s epochs (Vmax Encore 229; CareFusion). Heart rate, blood pressure, and arterial oxygen saturation were monitored by using 12-lead electrocardiography, manual sphygmomanometry, and pulse oximetry, respectively. The anaerobic threshold was calculated by using the V-slope method (3). Operating lung volumes (i.e., end-expiratory and end-inspiratory lung volumes) were derived from dynamic IC maneuvers as previously described (14). The ventilatory reserve was determined as the ratio between maximal minute ventilation and the measured MVV. The inflection in tidal volume (VT) relative to minute ventilation (V̇e) was determined for each participant during the exercise test by examining individual Hey plots (17). The presence and magnitude of expiratory flow limitation (EFL) was assessed as previously described (15). Briefly, subjects performed graded expiratory maneuvers from TLC to residual volume at varying efforts before and after exercise to account for both thoracic gas compression and exercise-induced bronchodilation while in the cycling position. Subjects received extensive practice on how to correctly perform these maneuvers. A representative maximum expiratory flow volume (MEFV) curve was constructed by taking the highest flows achieved for any given lung volume from all pre- and postexercise expiratory vital capacity maneuvers. This approach significantly reduces the false detection and overestimation of EFL (15). Multiple tidal breaths at rest and for each stage of exercise were ensemble averaged and then positioned within the MEFV curve according to the measured end-expiratory lung volume. The magnitude of EFL was calculated as the % overlap between the expiratory portion of the tidal breaths and the reconstructed MEFV curve. Subjects were considered flow limited if they experienced >5% EFL at any point during the exercise test. An estimate of the ventilatory capacity (V̇ecap) was determined as previously described (18). The %V̇ecap was determined by dividing V̇e by V̇ecap. Predicted values for peak V̇o2 and work rate are from Jones (20).
Dyspnea evaluation.
Dyspnea intensity (defined as “the sensation of labored or difficult breathing”) and perceived leg discomfort were evaluated at rest, every minute during exercise, and at peak exercise by using the modified 0-10 Borg scale. Upon exercise cessation, participants were asked to verbalize their main reason(s) for stopping exercise (i.e., breathing discomfort, leg discomfort, combination of breathing and legs, or some other reason).
Statistical analysis.
Between-group comparisons for descriptive characteristics and exercise responses at the VT/V̇e inflection and at peak exercise were compared by using unpaired t-tests. Comparisons at standardized submaximal cycle work rates were compared by using repeated measures ANOVA. To determine if group differences were present at various work rates, the interaction between group and work rate was tested, followed by Bonferroni-adjusted post hoc comparisons when results were significant. Spearman's and Pearson's correlation coefficients were used to examine the association between measured variables [FEV1, PCF, BMI, sweat chloride and peak V̇o2, and work rate (%predicted)]. An independent t-test was used to compare peak V̇o2 and work rate (%predicted) by chronic Pseudomonas aeruginosa infection status (yes/no). Reasons for stopping exercise and number of subjects with EFL were analyzed as frequency statistics and compared between control and CF participants by using the Fisher's exact test. Statistical significance was set at P < 0.05. Data are presented as means ± SD unless otherwise specified.
RESULTS
Subject characteristics.
Subject characteristics are summarized in Table 1. Both groups were well matched for sex, age, and BMI, and all but two participants were Caucasian. Compared with controls, subjects with mild CF had significantly higher PCF values and a lower FEV1/FVC, FEV1, and FEF25–75 (P < 0.05). The median age of diagnosis for this mild CF cohort was 5 yr (IQR 0.3 to 21 years) with a mean sweat chloride of 89 ± 20 mmol/l. For CFTR genotype, 32% of participants were F508del homozygous, 58% were F508del heterozygous, and the remaining 10% had two non-F508del mutations. Despite milder lung disease, 84% of participants were pancreatic insufficient (based on pancreatic enzyme use) and 47% had chronic colonization with Pseudomonas aeruginosa.
Table 1.
Subject characteristics
| Mild CF | Controls | |
|---|---|---|
| Sex, M:F | 12:7 | 12:7 |
| Age, yr | 30 ± 9 | 30 ± 8 |
| Height, cm | 170 ± 9* | 176 ± 8 |
| Mass, kg | 71 ± 17 | 74 ± 12 |
| BMI, kg/m2 | 24 ± 4 | 24 ± 3 |
| O2 cost diagram, mm | 89 ± 10 | 94 ± 8 |
| mMRC | 0.2 ± 0.4* | 0.0 ± 0.0 |
| FEV1/FVC, % | 75 ± 8* | 80 ± 6 |
| FVC, %predicted | 99 ± 13 | 104 ± 9 |
| FEV1, %predicted | 95 ± 17* | 106 ± 10 |
| FEF25–75, %predicted | 75 ± 33* | 93 ± 20 |
| TLC, %predicted | 102 ± 12 | 101 ± 8 |
| FRC, %predicted | 96 ± 24 | 97 ± 15 |
| PCF, % | 14 ± 6* | 7 ± 6 |
| sRaw, %predicted | 169 ± 47 | 158 ± 32 |
| DLCO, %predicted | 105 ± 18 | 113 ± 15 |
| MIP, cmH2O | 132 ± 38 | 119 ± 37 |
| MEP, cmH2O | 135 ± 54 | 150 ± 55 |
| MVV, l/min | 136 ± 33† | 161 ± 44 |
Values are means ± SD.
CF, cystic fibrosis; BMI, body mass index; mMRC, modified medical research council dyspnea scale; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEF25–75, forced expiratory flow between 25 and 75% of FVC; TLC, total lung capacity; FRC, functional residual capacity; PCF, poorly communicating fraction; sRaw, specific airways resistance; DLCO, diffusing capacity of the lung for carbon monoxide; MIP, maximal inspiratory pressure at residual volume; MEP; maximal expiratory pressure at TLC; MVV, maximum voluntary ventilation.
Significantly different from control subjects, P < 0.05;
P = 0.05.
Cardiorespiratory fitness and exercise performance.
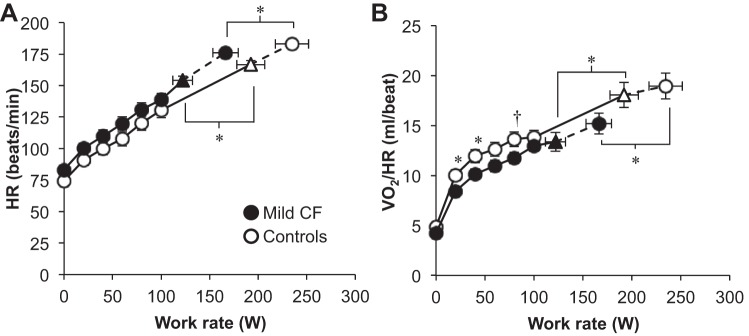
Subjects with CF had a lower peak work rate and V̇o2 when expressed in absolute terms and as %predicted compared with controls (Table 2). However, %predicted values for peak work rate and V̇o2 were, on average, “normal” [i.e., >84% predicted (2)] in the CF subjects. V̇o2peak (%predicted) was “normal” in 14 of 19 (74%) CF subjects. The anaerobic threshold occurred at a slightly lower percentage of V̇o2peak in the subjects with CF vs. controls but this did not reach statistical significance (67 ± 9 vs. 72 ± 9%, P = 0.09). Figure 1 shows the heart rate and O2 pulse responses to exercise. Both groups achieved maximal heart rates in excess of 90% predicted but CF subjects had a slightly lower absolute and %predicted maximal heart rate compared with controls (Table 2). The O2 pulse exceeded 100% predicted in both groups at peak exercise (Table 2). However, the O2 pulse response was lower in subjects with mild CF compared with controls throughout exercise (Fig. 1).
Table 2.
Exercise responses at the VT/V̇e inflection and at maximal exercise
| Mild CF |
Controls |
|||
|---|---|---|---|---|
| VT/V̇e inflection | Maximum | VT/V̇e inflection | Maximum | |
| Work rate, W | 122 ± 44* | 166 ± 57* | 192 ± 61 | 235 ± 74 |
| Work rate, %predicted | 63 ± 18* | 85 ± 24* | 86 ± 18 | 108 ± 22 |
| V̇o2, ml/kg/min | 29 ± 6* | 38 ± 8* | 40 ± 11 | 47 ± 12 |
| V̇o2, l/min | 2.05 ± 0.63* | 2.68 ± 0.82* | 3.00 ± 0.89 | 3.47 ± 1.05 |
| V̇o2, % predicted | 78 ± 17* | 102 ± 22* | 101 ± 17 | 119 ± 21 |
| V̇co2, l/min | 2.14 ± 0.66* | 2.92 ± 0.85* | 3.05 ± 0.86 | 3.75 ± 1.04 |
| RER | 1.06 ± 0.08 | 1.10 ± 0.07 | 1.02 ± 0.06 | 1.09 ± 0.06 |
| V̇e, l/min | 65 ± 21* | 95 ± 27* | 88 ± 24 | 125 ± 40 |
| VT, l | 2.34 ± 0.63 | 2.32 ± 0.51† | 2.76 ± 0.78 | 2.72 ± 0.70 |
| Fb, breaths/min | 28 ± 7† | 41 ± 9 | 33 ± 6 | 46 ± 8 |
| V̇e/V̇co2 | 30 ± 4 | 33 ± 4 | 29 ± 2 | 33 ± 3 |
| V̇e/V̇o2 | 32 ± 6 | 36 ± 6 | 30 ± 3 | 36 ± 3 |
| PetCO2, mmHg | 38 ± 6 | 35 ± 5 | 39 ± 2 | 35 ± 3 |
| Estimated VD/VT | 0.14 ± 0.05* | 0.14 ± 0.04* | 0.10 ± 0.05 | 0.11 ± 0.05 |
| ΔIC, l | 0.17 ± 0.43* | 0.02 ± 0.38* | 0.58 ± 0.35 | 0.39 ± 0.38 |
| EELV, %TLC | 49 ± 8 | 51 ± 8 | 46 ± 6 | 49 ± 5 |
| EILV, %TLC | 87 ± 5 | 89 ± 6 | 87 ± 6 | 90 ± 4 |
| IRV, l | 0.80 ± 0.36 | 0.67 ± 0.39 | 0.86 ± 0.36 | 0.66 ± 0.29 |
| VT/IC, % | 75 ± 9 | 78 ± 10 | 75 ± 11 | 80 ± 9 |
| V̇e/MVV, % | 48 ± 12 | 71 ± 14 | 55 ± 11 | 77 ± 12 |
| HR, beats/min | 154 ± 14* | 176 ± 11* | 167 ± 13 | 183 ± 9 |
| HR, %predicted | 81 ± 7* | 92 ± 6* | 87 ± 6 | 96 ± 4 |
| O2 Pulse, ml/beat | 13 ± 4* | 15 ± 4* | 18 ± 5 | 19 ± 6 |
| O2 Pulse, %predicted | 97 ± 18* | 110 ± 22† | 115 ± 20 | 124 ± 22 |
| SpO2, % | 97 ± 1 | 97 ± 1 | 97 ± 2 | 96 ± 4 |
| Breathing discomfort, Borg scale | 2.7 ± 2.0* | 6.0 ± 2.7 | 4.3 ± 2.7 | 7.1 ± 2.6 |
| Leg discomfort, Borg scale | 4.1 ± 2.6 | 8.1 ± 2.3 | 5.3 ± 2.3 | 8.5 ± 2.0 |
V̇o2, oxygen consumption; V̇co2, carbon dioxide production; RER, respiratory exchange ratio; V̇e, minute ventilation; VT, tidal volume; Fb, breathing frequency V̇e/V̇co2, ventilatory equivalent for carbon dioxide; V̇e/V̇o2, ventilatory equivalent for oxygen; PetCO2, partial pressure of end tidal carbon dioxide; VD/VT, dead space to tidal volume ratio; ΔIC, change in inspiratory capacity from rest; EELV, end-expiratory lung volume; EILV, end-inspiratory lung volume; TLC, total lung capacity; IRV, inspiratory reserve volume; MVV, maximal voluntary ventilation; HR, heart rate; SpO2, arterial oxygen saturation via pulse oximetry. Values are means ± SD,
Significantly different from control subjects, P < 0.05;
P = 0.05.
Fig. 1.
Cardiovascular responses to exercise. ▲ and △ represent the VT/V̇e inflection [n = 19 cystic fibrosis (CF) and 18 controls]. The highest equivalent work rate achieved by all subjects was 80 W. Data at 100 W include 17 CF subjects and 19 controls. VT, tidal volume; V̇e, minute ventilation; HR, heart rate; V̇o2/HR, oxygen pulse. Values are means ± SE. *P < 0.05, †P = 0.05.
Ventilatory responses.
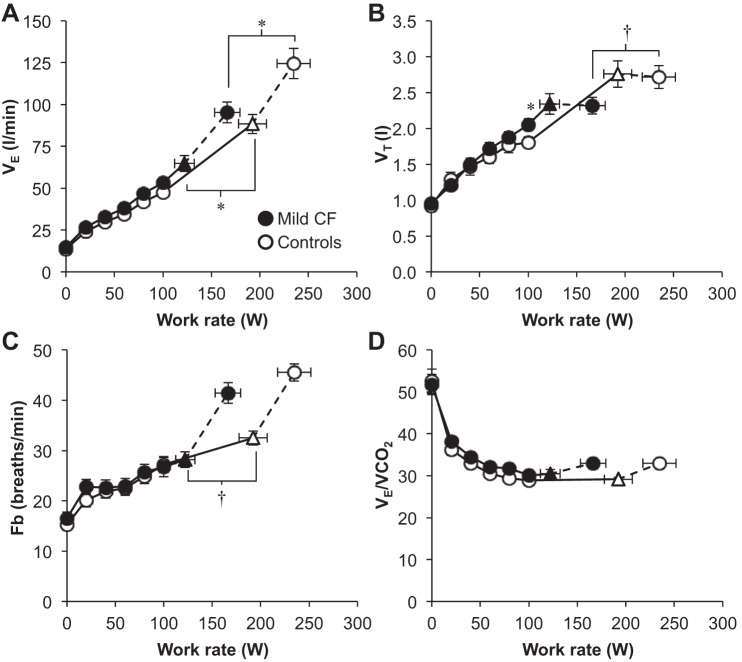
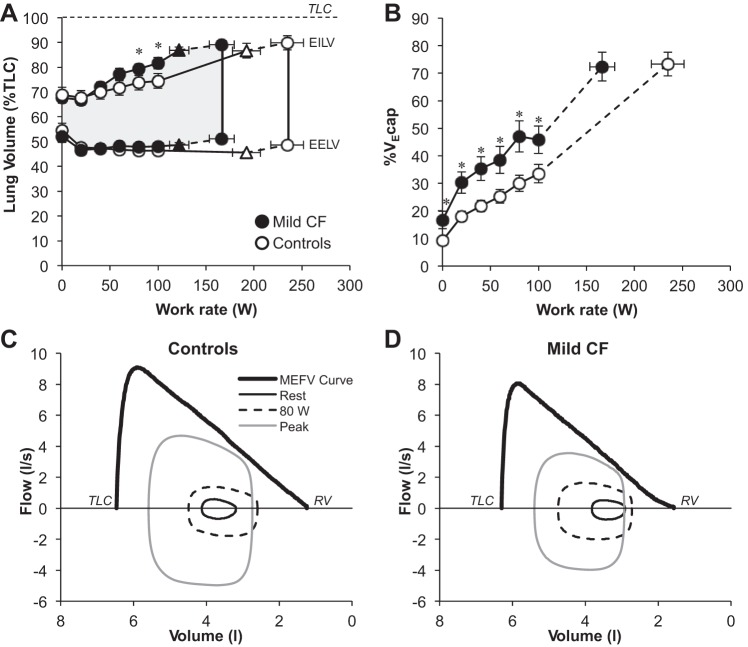
Maximum V̇e was significantly lower in subjects with CF and this was primarily driven by their lower VT (Table 2). Submaximal V̇e and breathing pattern responses were similar between groups (Fig. 2). There were no differences in the ventilatory equivalent for carbon dioxide throughout exercise. An inflection/plateau in VT relative to V̇e occurred at a significantly lower work rate, V̇o2, and V̇e in CF subjects compared with controls (Table 2 and Fig. 2). Operating lung volumes and %V̇ecap are shown in Fig. 3. End-inspiratory lung volume (EILV) was higher in CF subjects at submaximal work rates, but both groups achieved a similar EILV and inspiratory reserve volume at maximal exercise (Table 2). The %V̇e cap was significantly greater in CF subjects at rest and throughout all submaximal work rates with no differences at maximal exercise. EFL occurred in 58% of subjects with CF and 37% of controls (P > 0.05). Figure 3 shows group mean flow-volume loops at rest, the highest equivalent work rate (HEWR) achieved by all subjects (80 W), and peak exercise superimposed within the MEFV curve for both groups. CF subjects increased their end-expiratory lung volume (EELV) back toward resting levels at maximal exercise but did not increase EELV beyond resting values (i.e., no dynamic hyperinflation). In contrast, healthy controls maintained an EELV below resting values at maximal exercise.
Fig. 2.
Ventilatory responses to exercise. ▲ and △ represent the VT/V̇e inflection (n = 19 CF and 18 Controls). The highest equivalent work rate achieved by all subjects was 80 W. Data at 100 W include 17 CF subjects and 19 controls. Fb, breathing frequency; V̇e/V̇co2, ventilatory equivalent for carbon dioxide. Values are means ± SE. *P < 0.05, †P = 0.05.
Fig. 3.
Operating lung volumes, %ventilatory capacity, and flow-volume loops during exercise. ▲ and △ represent the VT/V̇e inflection (n = 19 CF and 18 Controls). The highest equivalent work rate achieved by all subjects was 80 W. Data at 100 W include 17 CF subjects and 19 controls. EILV, end-inspiratory lung volume; EELV, end-expiratory lung volume; V̇ecap, ventilatory capacity; TLC, total lung capacity; RV, residual volume; MEFV, maximal expiratory flow volume. Values are means ± SE. *P < 0.05.
Sensory responses.
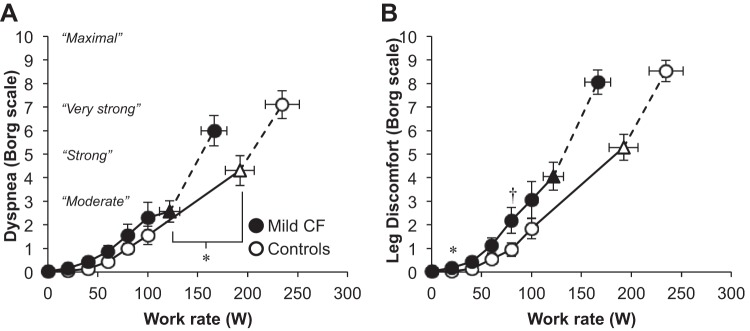
Figure 4 shows the primary reasons for stopping exercise. The majority of controls (58%) and CF subjects (63%) stopped because of leg discomfort alone. Five percent of CF subjects stopped because of dyspnea alone, compared with 16% of controls (P > 0.05). Thirty-two percent of CF subjects stopped because of dyspnea alone or in combination with leg discomfort, compared with 26% of controls (P > 0.05). Dyspnea intensity ratings were similar throughout submaximal and maximal exercise, but dyspnea ratings were higher in controls at the VT/V̇e inflection (Fig. 5). Leg discomfort ratings were similar between groups but tended to be greater in CF subjects at the HEWR of 80 W (P = 0.05).
Fig. 4.
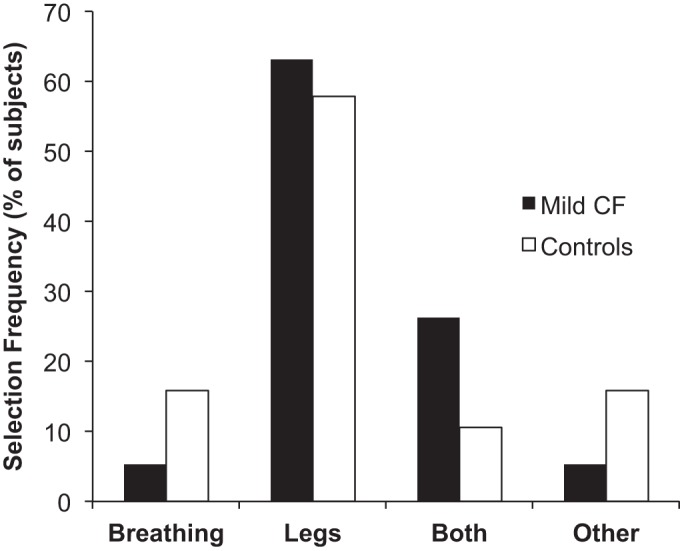
Reasons for stopping exercise.
Fig. 5.
Sensory responses to exercise. ▲ and △ represent the VT/V̇e inflection (n = 19 CF and 18 Controls). The highest equivalent work rate achieved by all subjects was 80 W. Data at 100 W include 17 CF subjects and 19 controls. Values are means ± SE. *P < 0.05, †P = 0.05.
Correlations.
There was a significant inverse relationship between sweat chloride and peak work rate (%predicted) (n = 17, r = −0.50; P = 0.04) and a trend toward a significant inverse association between sweat chloride and V̇o2 peak (%predicted) (n = 17, r = −0.45; P = 0.07). There was no significant correlation between FEV1 (%predicted), PCF, BMI, and peak V̇o2 (%predicted) or work rate (%predicted) (all P > 0.05). Subjects with chronic Pseudomonas aeruginosa infection had a lower V̇o2 peak (90 ± 24 vs. 112 ± 14% predicted; P = 0.02) and peak work rate (71 ± 23 vs. 98 ± 18% predicted; P = 0.01) compared with those without chronic Pseudomonas aeruginosa infection.
DISCUSSION
To our knowledge, this is the first study focused on exercise performance and physiologic reasons for exercise curtailment in a contemporary adult CF cohort with mild lung disease. Our study cohort was unique as it focused exclusively on adults with normal to mildly impaired lung function (FEV1 = 95 ± 17% predicted). Previous exercise studies evaluating “mild” CF have been limited to ≤10 participants (4, 8, 26) or included patients with more substantial lung disease with a mean FEV1 ranging from 75 to 82% predicted (8, 34). Furthermore, the prior small studies are not directly comparable to our cohort as one study focused exclusively on children (≤13 years old) (4), one study focused on the noninvasive measurement of anaerobic threshold in adults (FVC > 70% predicted) without characterization of the ventilatory or perceptual responses to exercise (26), and the other study examined dead space loading in patients with more significant airflow obstruction (FEV1 range 63–82% predicted) (8). The largest study performed to date by Pastre et al. (34) combined patients with mild and moderate lung disease (FEV1 > 50% predicted), thus limiting comparisons with our mild cohort.
Both CF and healthy control groups experienced a normal cardiovascular limitation to exercise as reflected by their peak HR and O2 pulse values, both of which exceeded 90% predicted. Adult subjects with mild CF had reduced peak V̇o2 and maximal cycle work rates compared with controls, but most subjects had values that were in the normal range based on predicted values. The majority of CF subjects stopped exercise because of intolerable leg discomfort rather than dyspnea, a finding that was consistent with the healthy controls. Thus patients with mild CF appear to have reasonably well preserved cardiorespiratory fitness and exercise performance and are limited by nonrespiratory factors, similar to the findings of previous studies (6, 8, 22, 25). While exercise capacity measurements fell within the normal range for the majority of CF subjects, the impact of slightly reduced exercise capacity based on %predicted values should not be understated as mildly reduced exercise capacity (75–100% predicted vs. > 100% predicted) has been reported to be an independent predictor of mortality among healthy males (30).
The reduced O2 pulse and anaerobic threshold observed in our mild CF group likely reflects lower conditioning relative to the healthy controls. However, one cannot exclude pulmonary vascular disease or an abnormality in skeletal muscle O2 extraction as potential explanations. A prior study focused on mild CF (FEV1 56–80% predicted) suggested that reduced stroke volume recruitment might be a result of impaired right ventricular systolic function due to the impact of gas trapping and dynamic hyperinflation on pulmonary vascular resistance (35). We do not believe this to be the case in our mild CF group as there was no evidence of dynamic hyperinflation. Peripheral skeletal muscle dysfunction has been reported in mild CF and exists independent of lung function, nutritional status (36), muscle mass (28), muscle conditioning (36), and systemic inflammation (9). It is suspected to result from metabolic derangements intrinsic to the muscle itself (7), such as inefficient mitochondrial oxidative metabolism (7, 28, 38). Selvadurai et al. (36) demonstrated that CFTR dysfunction might affect oxidative and anaerobic metabolism in skeletal muscle, as patients with milder CFTR mutations (classes III–IV) demonstrate better exercise performance than patients with more severe mutations (classes I–II) despite similar lung function (37). CFTR has intrinsic ATPase activity (12), and therefore defective ATP hydrolysis could provide a potential mechanism for the inefficient oxidative and anaerobic metabolism observed in prior studies in CF. In an exploratory analysis, we found an inverse correlation between sweat chloride (a marker of cellular CFTR function) and peak work rate.
Ventilatory limitations during exercise are typically based on crude measures of ventilatory reserve. V̇e/MVV values exceeding ∼85% are generally considered evidence of a ventilatory limitation (2, 41). Both groups had adequate ventilatory reserve according to the V̇e/MVV (Table 2). However, this approach has well-established limitations and provides little insight into the nature or source of a ventilatory constraint (14, 19). Accordingly, we performed a comprehensive evaluation of ventilatory responses by using several approaches, including the assessment of the VT/V̇e inflection, EFL, operating lung volumes, and %V̇ecap. This is the first study to perform such a detailed assessment of ventilatory responses in CF patients with relatively well preserved spirometry. These data provide some evidence of ventilatory limitations in mild CF. For example, patients with mild CF reached an inflection/plateau in VT relative to V̇e at a lower absolute ventilation, V̇o2, and work rate. The VT/V̇e inflection is thought to represent a critical mechanical event during exercise in COPD and is associated with both the intensity and qualitative dimensions of dyspnea (23, 32). While dyspnea intensity increased sharply following attainment of the VT/V̇e inflection in both groups, individuals with CF experienced significantly less dyspnea compared with controls at the inflection point. This may suggest that individuals with CF might have a higher threshold to report dyspnea relative to healthy individuals. However, this remains speculative and warrants further investigation.
The EILV and %V̇ecap were elevated in mild CF relative to controls at submaximal work rates. This suggests that patients with mild CF tend to breathe closer to TLC and use a larger fraction of their maximum available flows to perform the same standardized work rates relative to healthy individuals. Surprisingly, these ventilatory abnormalities were not associated with a significant increase in dyspnea intensity compared with controls. Our mild CF cohort was not more likely to develop EFL or dynamic hyperinflation during exercise. This observation is consistent with recent studies in CF demonstrating that EFL does not occur in children with mild lung disease (4), and dynamic hyperinflation is more likely to develop in patients with a lower FEV1 (40).
We observed an inverse association between chronic Pseudomonas aeruginosa infection status and exercise capacity. While Pseudomonas aeruginosa status is likely a marker of disease severity as opposed to being causal in this relationship, one cannot exclude the potential role of Pseudomonas aeruginosa on systemic inflammation and its potential downstream adverse effect on skeletal muscle function and exercise capacity. A prior study has reported an inverse relationship between chronic inflammation (i.e., IgG) and chronic Pseudomonas aeruginosa infection status and maximal oxygen uptake, associations that remained significant following adjustment for several confounders such as age, lung function, and CFTR genotype (43).
There were some limitations to this study. First, we did not measure baseline physical activity levels, as differences in overall conditioning could have explained at least part of the difference in exercise performance observed between groups. Second, exercise testing was performed on just one occasion for each patient (i.e., cross-sectional) but airway obstruction and hence exercise performance can be affected by daily changes in mucus accumulation and adherence to medications (e.g., mucolytics). To minimize any day-to-day fluctuation, we focused on individuals with stable disease, and subjects refrained from using their bronchodilators prior to testing. Lastly, like most exercise studies, individuals with higher fitness levels might be more likely to participate resulting in selection bias. As a result, we may have underestimated the true extent of exercise limitation observed in mild CF.
In summary, the results of this study demonstrate that patients with mild CF lung disease have reasonably well preserved cardiorespiratory fitness and exercise performance. However, there was evidence of ventilatory abnormalities in our patients with mild CF relative to healthy controls, but this did not result in a corresponding increase in dyspnea intensity or exercise curtailment. These ventilatory abnormalities should be evaluated in future therapeutic trials focused on disease-modifying treatments in individuals with mild CF (i.e., residual function mutations), as changes in standard clinical endpoints such as FEV1 and dyspnea scores are unlikely to be sensitive enough to evaluate therapeutic responses in mild CF (29). Nonrespiratory factors likely limit exercise in adults with mild CF and therefore should be the focus of future research studies examining interventions to optimize exercise performance in this segment of the CF population. Future studies are also required to clarify the role of defects in CFTR on skeletal muscle function and whether exercise testing can be used to assess response to CFTR modulators.
GRANTS
This research was supported by infrastructure funding from the Canada Foundation for Innovation, British Columbia Knowledge Development Fund, and British Columbia Lung Association. Operating funds were provided by the Providence Health Care Research Institute and St. Paul's Hospital Foundation. B. Quon was supported by a Cystic Fibrosis Canada/University of British Columbia Clinician-Scientist Award. M. Schaeffer was supported by fellowships from the University of British Columbia and British Columbia Lung Association. Y. Molgat-Seon was supported by a Postgraduate Scholarship from the Natural Sciences and Engineering Research Council of Canada and a Fellowship from the University of British Columbia. J. Guenette was supported by a Scholar Award from the Michael Smith Foundation for Health Research and a New Investigator Award from the Providence Health Care Research Institute and St. Paul's Hospital Foundation.
DISCLOSURES
B.S.Q., S.S.W., Y.M.-S., M.R.S., A.H.R., and J.A.G. do not have any conflicts of interest to report relevant to this manuscript. P.G.W. has been a site principal investigator on multicentre clinical trials sponsored by Vertex Pharmaceuticals.
AUTHOR CONTRIBUTIONS
B.S.Q., P.G.W., and J.A.G. conception and design of research; B.S.Q., S.S.W., M.R.S., and A.H.R. performed experiments; B.S.Q., S.S.W., Y.M.-S., M.R.S., and J.A.G. analyzed data; B.S.Q., Y.M.-S., and J.A.G. interpreted results of experiments; B.S.Q. and J.A.G. drafted manuscript; B.S.Q., S.S.W., Y.M.-S., M.R.S., A.H.R., P.G.W., and J.A.G. edited and revised manuscript; B.S.Q., S.S.W., Y.M.-S., M.R.S., A.H.R., P.G.W., and J.A.G. approved final version of manuscript; Y.M.-S. and J.A.G. prepared figures.
ACKNOWLEDGMENTS
We thank our subjects for their enthusiastic participation.
REFERENCES
- 1.American Thoracic Society, European Respiratory Society. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med 166: 518–624, 2002. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society, American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167: 211–277, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 60: 2020–2027, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Borel B, Leclair E, Thevenet D, Beghin L, Gottrand F, Fabre C. Mechanical ventilatory constraints during incremental exercise in healthy and cystic fibrosis children. Pediatr Pulmonol 49: 221–229, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Chin RC, Guenette JA, Cheng S, Raghavan N, Amornputtisathaporn N, Cortes-Telles A, Webb KA, O'Donnell DE. Does the respiratory system limit exercise in mild chronic obstructive pulmonary disease? Am J Respir Crit Care Med 187: 1315–1323, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Coates AL, Boyce P, Muller D, Mearns M, Godfrey S. The role of nutritional status, airway obstruction, hypoxia, and abnormalities in serum lipid composition in limiting exercise tolerance in children with cystic fibrosis. Acta Paediatr Scand 69: 353–358, 1980. [DOI] [PubMed] [Google Scholar]
- 6a.Cystic Fibrosis Foundation. Patient Registry: 2013 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation, 2013. [Google Scholar]
- 7.de Meer K, Jeneson JA, Gulmans VA, van der Laag J, Berger R. Efficiency of oxidative work performance of skeletal muscle in patients with cystic fibrosis. Thorax 50: 980–983, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd JD, Barry SC, Gallagher CG. Respiratory factors do not limit maximal symptom-limited exercise in patients with mild cystic fibrosis lung disease. Respir Physiol Neurobiol 152: 176–185, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Dufresne V, Knoop C, Van Muylem A, Malfroot A, Lamotte M, Opdekamp C, Deboeck G, Cassart M, Stallenberg B, Casimir G, Duchateau J, Estenne M. Effect of systemic inflammation on inspiratory and limb muscle strength and bulk in cystic fibrosis. Am J Respir Crit Care Med 180: 153–158, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, Rock MJ, Campbell PW 3rd. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 153: S4–S14, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ Jr, Willey-Courand DB, Bujan J, Finder J, Lester M, Quittell L, Rosenblatt R, Vender RL, Hazle L, Sabadosa K, Marshall B. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 176: 957–969, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Gadsby DC, Dousmanis AG, Nairn AC. ATP hydrolysis cycles and the gating of CFTR Cl- channels. Acta Physiol Scand Suppl 643: 247–256, 1998. [PubMed] [Google Scholar]
- 13.Guenette JA, Chin RC, Cheng S, Dominelli PB, Raghavan N, Webb KA, Neder JA, O'Donnell DE. Mechanisms of exercise intolerance in global initiative for chronic obstructive lung disease grade 1 COPD. Eur Respir J 44: 1177–1187, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Guenette JA, Chin RC, Cory JM, Webb KA, O'Donnell DE. Inspiratory capacity during Exercise: measurement, analysis, and interpretation. Pulm Med 2013: 956081, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guenette JA, Dominelli PB, Reeve SS, Durkin CM, Eves ND, Sheel AW. Effect of thoracic gas compression and bronchodilation on the assessment of expiratory flow limitation during exercise in healthy humans. Respir Physiol Neurobiol 170: 279–286, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Guenette JA, Jensen D, Webb KA, Ofir D, Raghavan N, O'Donnell DE. Sex differences in exertional dyspnea in patients with mild COPD: physiological mechanisms. Respir Physiol Neurobiol 177: 218–227, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Hey EN, Lloyd BB, Cunningham DJC, Jukes MGM, Bolton DPG. Effects of various respiratory stimuli on the depth and frequency of breathing in man. Respir Physiol 1: 193–205, 1966. [DOI] [PubMed] [Google Scholar]
- 18.Johnson BD, Scanlon PD, Beck KC. Regulation of ventilatory capacity during exercise in asthmatics. J Appl Physiol (1985) 79: 892–901, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Johnson BD, Weisman IM, Zeballos RJ, Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest 116: 488–503, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Jones NL. Clinical Exercise Testing. Philadelphia, PA: W. B. Saunders, 1988. [Google Scholar]
- 21.Kraemer R, Blum A, Schibler A, Ammann RA, Gallati S. Ventilation inhomogeneities in relation to standard lung function in patients with cystic fibrosis. Am J Respir Crit Care Med 171: 371–378, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Lands LC, Heigenhauser GJ, Jones NL. Analysis of factors limiting maximal exercise performance in cystic fibrosis. Clin Sci (Lond) 83: 391–397, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Laveneziana P, Webb KA, Ora J, Wadell K, O'Donnell DE. Evolution of dyspnea during exercise in chronic obstructive pulmonary disease: impact of critical volume constraints. Am J Respir Crit Care Med 184: 1367–1373, 2011. [DOI] [PubMed] [Google Scholar]
- 24.MacIntyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 26: 720–735, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Marcotte JE, Grisdale RK, Levison H, Coates AL, Canny GJ. Multiple factors limit exercise capacity in cystic fibrosis. Pediatr Pulmonol 2: 274–281, 1986. [DOI] [PubMed] [Google Scholar]
- 26.McLoughlin P, McKeogh D, Byrne P, Finlay G, Hayes J, FitzGerald MX. Assessment of fitness in patients with cystic fibrosis and mild lung disease. Thorax 52: 425–430, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Moser C, Tirakitsoontorn P, Nussbaum E, Newcomb R, Cooper DM. Muscle size and cardiorespiratory response to exercise in cystic fibrosis. Am J Respir Crit Care Med 162: 1823–1827, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Moss RB, Flume PA, Elborn JS, Cooke J, Rowe SM, McColley SA, Rubenstein RC, Higgins M. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir Med 3: 524–533, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Neder JA, O'Donnell CD, Cory J, Langer D, Ciavaglia CE, Ling Y, Webb KA, O'Donnell DE. Ventilation distribution heterogeneity at rest as a marker of exercise impairment in mild-to-advanced COPD. COPD 12: 249–256, 2014. [DOI] [PubMed] [Google Scholar]
- 32.O'Donnell DE, Guenette JA, Maltais F, Webb KA. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest 141: 753–762, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Ofir D, Laveneziana P, Webb KA, Lam YM, O'Donnell DE. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177: 622–629, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Pastre J, Prevotat A, Tardif C, Langlois C, Duhamel A, Wallaert B. Determinants of exercise capacity in cystic fibrosis patients with mild-to-moderate lung disease. BMC Pulm Med 14: 74, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pianosi P, Pelech A. Stroke volume during exercise in cystic fibrosis. Am J Respir Crit Care Med 153: 1105–1109, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Selvadurai HC, Allen J, Sachinwalla T, Macauley J, Blimkie CJ, Van Asperen PP. Muscle function and resting energy expenditure in female athletes with cystic fibrosis. Am J Respir Crit Care Med 168: 1476–1480, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Selvadurai HC, McKay KO, Blimkie CJ, Cooper PJ, Mellis CM, Van Asperen PP. The relationship between genotype and exercise tolerance in children with cystic fibrosis. Am J Respir Crit Care Med 165: 762–765, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro BL. Evidence for a mitochondrial lesion in cystic fibrosis. Life Sci 44: 1327–1334, 1989. [DOI] [PubMed] [Google Scholar]
- 39.Stephenson AL, Tom M, Berthiaume Y, Singer LG, Aaron SD, Whitmore GA, Stanojevic S. A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. Eur Respir J 45: 670–679, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Stevens D, Stephenson A, Faughnan ME, Leek E, Tullis E. Prognostic relevance of dynamic hyperinflation during cardiopulmonary exercise testing in adult patients with cystic fibrosis. J Cyst Fibros 12: 655–661, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Stickland MK, Butcher SJ, Marciniuk DD, Bhutani M. Assessing exercise limitation using cardiopulmonary exercise testing. Pulm Med 2012: 824091, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiddens HA, Puderbach M, Venegas JG, Ratjen F, Donaldson SH, Davis SD, Rowe SM, Sagel SD, Higgins M, Waltz DA. Novel outcome measures for clinical trials in cystic fibrosis. Pediatr Pulmonol 50: 302–315, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Weert-van Leeuwen PB, Slieker MG, Hulzebos HJ, Kruitwagen CL, van der Ent CK, Arets HG. Chronic infection and inflammation affect exercise capacity in cystic fibrosis. Eur Respir J 39: 893–898, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J 26: 511–522, 2005. [DOI] [PubMed] [Google Scholar]