Abstract
In able-bodied (AB) individuals, voluntary muscle activation progressively declines during sustained contractions. However, few data are available on voluntary muscle activation during sustained contractions in muscles weakened by spinal cord injury (SCI), where greater force declines may limit task performance. SCI-related impairment of muscle activation complicates interpretation of the interpolated twitch technique commonly used to assess muscle activation. We attempted to estimate and correct for the SCI-related-superimposed twitch. Seventeen participants, both AB and with SCI (American Spinal Injury Association Impairment Scale C/D) produced brief and sustained (2-min) maximal voluntary contractions (MVCs) with the first dorsal interosseous. Force and electromyography were recorded together with superimposed (doublet) twitches. MVCs of participants with SCI were weaker than those of AB participants (20.3 N, SD 7.1 vs. 37.9 N, SD 9.5; P < 0.001); MVC-superimposed twitches were larger in participants with SCI (SCI median 10.1%, range 2.0-63.2%; AB median 4.7%, range 0.0–18.4% rest twitch; P = 0.007). No difference was found after correction for the SCI-related-superimposed twitch (median 6.7%, 0.0–17.5% rest twitch, P = 0.402). Thus during brief contractions, the maximal corticofugal output that participants with SCI could exert was similar to that of AB participants. During the sustained contraction, force decline (SCI, 58.0%, SD 15.1; AB, 57.2% SD 13.3) was similar (P = 0.887) because participants with SCI developed less peripheral (P = 0.048) but more central fatigue than AB participants. The largest change occurred at the start of the sustained contraction when the (corrected) superimposed twitches increased more in participants with SCI (SCI, 16.3% rest twitch, SD 20.8; AB, 2.7%, SD 4.7; P = 0.01). The greater reduction in muscle activation after SCI may relate to a reduced capacity to overcome fast fatigue-related excitability changes at the spinal level.
Keywords: muscle activation, twitch interpolation, fatigue, doublet force, muscle atrophy
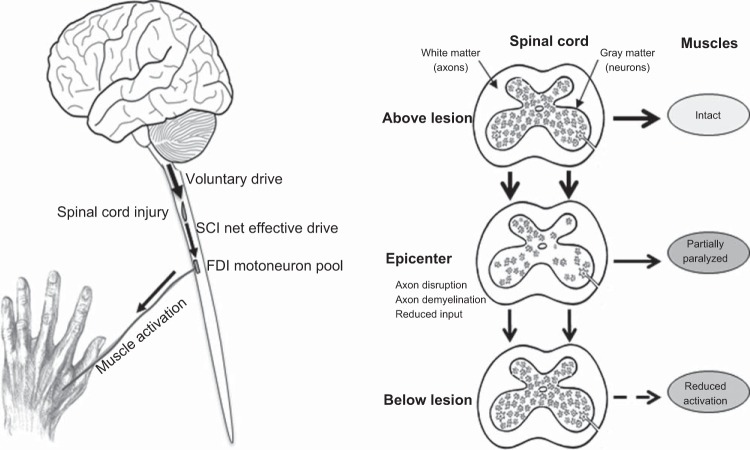
spinal cord injury (sci) can result in muscle weakness, an effect associated with reduced muscle activation, atrophy, and paralysis (35). At the spinal level, descending input to the motoneuron pools near and below the lesion is diminished (22), which can lead to reductions in motor unit recruitment and, in hand muscles, reductions in maximal firing rates (32, 42). After SCI, muscle activation (i.e., the net effective drive reaching muscle fibers, Fig. 1) is poorer in weaker muscles (35), but little is known about how muscle activation changes during sustained contractions. Signs of poor muscle activation during repeated submaximal contractions have been observed in individuals with SCI, but it has not been quantified (33). Repeated maximal voluntary contractions (MVCs) of the flexor carpi radialis has shown significantly lower muscle activation in individuals with SCI than in able-bodied (AB) individuals only at the start of the task (18).
Fig. 1.
Left: illustration of the terminology and the changes after spinal cord injury (SCI). The drive arising from supraspinal areas is defined as voluntary drive; the voluntary drive that passes the SCI and reaches the first dorsal interosseous (FDI) motoneuron pool is referred to as SCI net effective drive, and the drive reaching the muscle fibers is referred to as muscle activation. The difference between the voluntary drive and the SCI net effective drive equals the SCI-impaired drive. Right: above the SCI, neurons (gray matter) receive normal descending and spinal input (but less ascending input, not shown), and muscle function remains intact. At the epicenter, there is neuronal death, axon disruption, and demyelination (white matter) resulting in paralysis of some (but not all) muscle fibers. The net muscle force will depend on the number of motor units that are still under voluntary control. Motoneuron pools below the SCI receive reduced supraspinal input (SCI-effective drive) resulting in reduced muscle activation (reduced motor unit recruitment and less rate modulation).
In AB individuals, voluntary drive is estimated using twitch interpolation (2, 4, 11, 21). Peripheral nerve or muscle stimulation during MVC activates muscle fibers that are not maximally activated by the descending voluntary drive. The twitch evoked during contractions is inversely related to the voluntary drive (i.e., during maximal activation there is no superimposed twitch).
In muscles weakened by SCI, voluntary drive may or may not, or may only partially reach motoneurons (Fig. 1). Hence voluntary drive that arises from supraspinal motor areas may differ substantially from the net effective drive that eventually reaches motoneurons and the innervated muscle fibers. Stimulation of the peripheral nerve during a maximal contraction may therefore result in a superimposed twitch that involves 1) muscle fibers that are not maximally activated due to reduced voluntary drive (as can occur in AB individuals) plus (because of a SCI-specific reduction in net effective drive to motoneurons, Fig. 1) 2) contraction of paralyzed muscle fibers (SCI damage preventing motoneuron recruitment) and 3) muscle fibers not maximally activated due to SCI-related disruption of inputs that precludes maximal motoneuron firing rates (42). In this manuscript, the superimposed twitch-force related to the SCI (points 2 and 3) is referred to as the SCI-related-superimposed twitch. The drive that arises from supraspinal areas is defined as voluntary drive; the voluntary drive reaching the first dorsal interosseous (FDI) motoneuron pool is referred to as SCI-effective drive, and the drive that reaches the muscle fibers is referred to as muscle activation (Fig. 1). The difference between the voluntary drive and the SCI-effective drive equals the SCI-impaired drive.
Muscle fatigue, quantified as a reduction in force-generating capacity (7), reflects both changes in voluntary drive [i.e., central fatigue, reflected by increases in the superimposed twitch (12)] and alterations in peripheral neuromuscular processes [i.e., peripheral fatigue, shown by a decline in twitch force at rest after the fatiguing contraction (1)]. In AB individuals performing a sustained MVC, twitches at rest and during the contraction are used to quantify central (fatigue-related changes at or proximal to the motoneuron) and peripheral (fatigue-related changes distal to the motoneuron) aspects of muscle fatigue. However, in muscles weakened by SCI, the interpretation of twitches evoked during and after a sustained MVC is more complicated. Similar to brief MVCs, superimposed twitches during a sustained contraction reflect both the reduced voluntary drive (central fatigue) and the SCI-impaired drive. Furthermore, muscle fibers not accessible to voluntary drive (due to SCI) will not be affected by fatigue even if voluntary drive is maximal. Thus the decline in the twitch after the sustained contraction will be attenuated because the twitch is the resultant of both the voluntarily activated (fatigued) and nonvoluntarily activated (nonfatigued) muscle fibers. Consequently, fatigue-related changes in the muscle induced by voluntary contraction and measured by twitches at rest will be underestimated. This underestimation will also result in larger superimposed twitches in muscles weakened by SCI, making it essential to correct these superimposed twitches for both the SCI-related-superimposed twitch and peripheral fatigue.
Limitations of the interpolated twitch technique in individuals with SCI have been explored using transcranial magnetic stimulation (35). Because similar descending inputs can be activated voluntarily or with cortical stimulation, an absence of evoked force during MVCs demonstrated that individuals with SCI can exert maximal voluntary drive (35) (point 1 above). In the present manuscript we aimed to correct for the SCI-related-superimposed twitch (points 2 and 3 above) generated by peripheral nerve stimulation. Our aims were to assess 1) the effects of chronic cervical SCI on (voluntary and electrically evoked) force and electromyographic (EMG) recordings generated during brief and sustained MVCs of the FDI, and to explore how to estimate 2) voluntary and SCI net effective drive during brief contractions by correcting the superimposed twitch for the SCI-related-superimposed twitch and 3) reductions in voluntary drive during sustained contractions by adjusting for both the SCI-related-superimposed twitch and for peripheral fatigue. We expected to find that participants with SCI would be weaker than AB individuals and would show reduced muscle activation during voluntary contractions because of SCI-related impairment. After correcting for the SCI-related-superimposed twitch we expected to find similar superimposed twitches during the brief and sustained contractions for all participants.
METHODS
Study Population
We included 17 participants with chronic cervical SCI (age 29–63, 4 women; Table 1). Inclusion criteria included the presence of SCI for more than 1 yr, lesion level between C3 and C8, American Spinal Injury Association Impairment Scale (AIS) C or D, and an ability to abduct at least one index finger. If participants could abduct both index fingers, the FDI of the right hand was the target muscle (n = 14 participants). AB participants (n = 17) were age- and sex-matched to participants with SCI. Approval of the experimental procedures was provided by the medical ethical board of the University Medical Center Groningen. Written informed consent was obtained from all participants.
Table 1.
Demographics of participants with SCI
| Sex | Age | Neurological Level* | AIS Score† | Etiology |
|---|---|---|---|---|
| Male | 37 | C4 | D | Traumatic |
| Male | 40 | C4 | C | Traumatic/cervical stenosis |
| Male | 46 | C4 | D | Traumatic |
| Male | 55 | C4 | D | Traumatic |
| Male | 55 | C4 | D | Abscess |
| Male | 56 | C4 | D | Traumatic |
| Male | 57 | C4 | D | Traumatic |
| Male | 58 | C5 | D | Cervical stenosis |
| Male | 58 | C5 | C | Traumatic |
| Male | 61 | C5 | D | Cervical stenosis |
| Male | 61 | C4 | D | Traumatic |
| Male | 62 | C4 | D | Traumatic |
| Male | 63 | C4 | C | Traumatic |
| Female | 29 | C5 | D | Ependymoma |
| Female | 46 | C5 | D | Cervical hernia |
| Female | 48 | C4 | C | Traumatic |
| Female | 54 | C8 | D | Abscess |
C, cervical.
AIS, American Spinal Injury Association Impairment Scale (score C, <50% of the muscles below the SCI level with force grade 3–5; D, >50% of the muscles with force grade 3–5).
Force Recordings
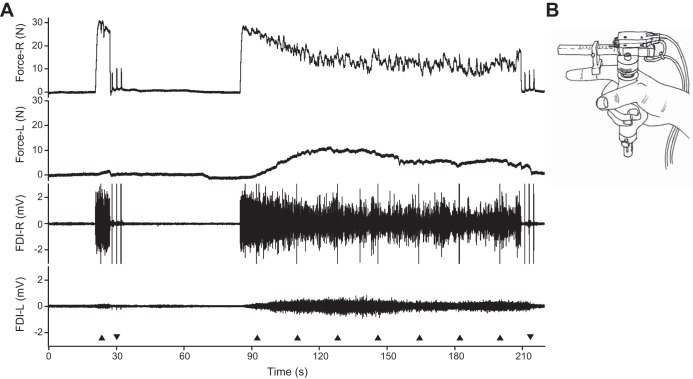
The abduction force of each FDI muscle was measured with hand-held force transducers (Fig. 2B). The horizontal bar of the transducer was aligned parallel to the index finger with the finger bracket placed over the proximal interphalangeal joint [for technical details see (37)]. To maintain the hand in the same position relative to the transducer throughout the experiment, the transducers were taped to participants' hands and index fingers. Force signals were sampled at 500 Hz and recorded on a computer using a 1401 interface and Spike 2 software (version 7.04, Cambridge Electronic Design, Cambridge, UK).
Fig. 2.
A: Force and electromyographic (EMG) data recorded from a participant with SCI during the sustained contraction showing the target force (R), associated nontarget force (L), EMG of target FDI (R), and nontarget FDI (L). Black triangles (base down) indicate one pair of stimuli (10-ms interval), triangles (base up) indicate three double pulses at rest. B: illustration of a hand holding the force transducer. During the experiment the thumb and fingers are taped to each other to prevent changes in hand position.
Electromygraphic Recordings
EMG activity was recorded from the FDI and abductor digiti minimi (ADM) muscles of both hands. After cleaning the skin with alcohol, one sintered silver/silver chloride electrode with conducting gel was positioned over the belly of each muscle. The second electrode was placed over the adjacent metacarpophalangeal joint (In Vivo Metric, Healdsburg, CA). The electrodes were secured in place with tape. A grounding electrode was taped over the right wrist. EMG signals were amplified (×200), filtered (10 Hz to 1 kHz), and sampled at 2 kHz.
Nerve Stimulation
Muscle activation of the target FDI was assessed using twitch interpolation. The ulnar nerve of the right (n = 14) or left (n = 3) arm was stimulated using a constant-current stimulator (pulse width 200 μs, DS7A; Digitimer, UK) via a pair of self-adhesive electrodes taped over the ulnar nerve close to the wrist (cathode proximal to the anode). The current was increased in 5-mA steps to evoke a maximum EMG response (M-wave) from the FDI. Throughout the experiment we used a stimulus intensity of at least 130% of this value (36) delivered as a doublet (paired pulse, 10-ms interval) to improve the signal-to-noise ratio (13). For clarity, doublet-forces are referred to as twitches.
Motor Tasks
Each participant was seated behind a desk with their arms flexed at ∼90° on a wooden plateau on the desk. We adjusted the height of the desk so that participants were able to relax their shoulders and were seated comfortably. A monitor provided participants with a visual representation of the task (task-line) and feedback of the produced force in real time. The timing of the tasks was similar to an earlier study by our group (28). Participants were encouraged by the investigators to produce maximal efforts during the MVCs.
Maximal voluntary contractions.
To determine the MVC of the FDI, participants produced maximum force against the horizontal bar of the transducer by abducting their index finger. Participants generated seven MVCs (10-s duration, 50-s interval) alternating between the right and left FDI, starting with the target FDI. A superimposed twitch was evoked 3 to 5 s into the second, third, and fourth MVC of the target FDI when the force was close to maximum because the force increase was slow in some SCI participants. After the last MVC, three twitches were evoked at rest (2-s interval) to provide potentiated twitches. The largest MVC and largest potentiated twitch were used for normalization of voluntary and evoked forces, respectively.
Submaximal contractions.
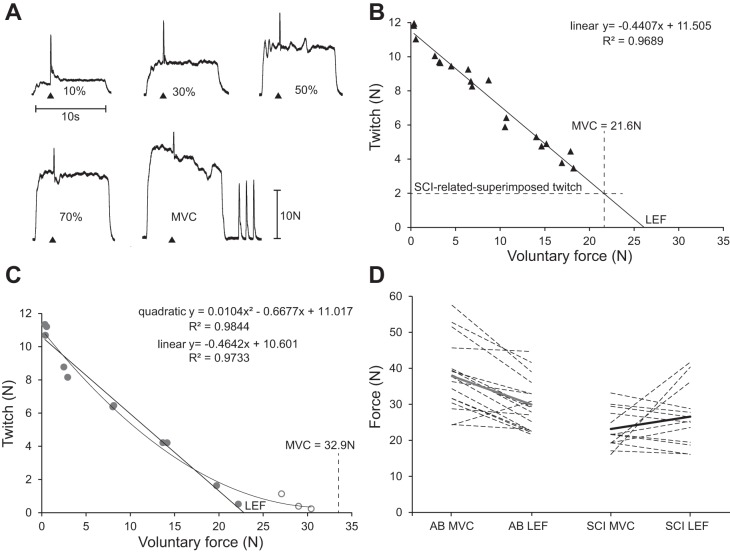
Participants performed blocks of submaximal contractions at 10, 30, 50, and 70% MVC (10-s duration, 40-s interval; Fig. 3). The order of contractions in each block was semirandomized. Six blocks were performed in total, alternating with the left and right FDI. Stimuli were given during the target FDI contractions (3 to 5 s after the start of the contraction) to determine the relationship between the voluntary force and superimposed twitch.
Fig. 3.
A: submaximal contractions (10, 30, 50, and 70%) and maximal voluntary contraction (MVC) showing interpolated twitches and twitches at rest for the same participant with SCI as in B. Black triangles (base down) indicate one pair of stimuli (10-ms interval). B: linear extrapolation of the voluntary force-twitch relationship in a participant with SCI used to determine the SCI-related-superimposed twitch and the linearly estimated FDI force (LEF; x-intercept). The SCI-related-superimposed twitch (2 N) is estimated on the basis of linear regression because no stimuli were delivered when the force was strongest during the MVC (21.6 N). C: force-twitch relationship in an AB participant to show that a quadratic function provides a better fit for the twitches evoked during strong voluntary forces (open symbols). LEF was determined by linear regression using only the superimposed twitches during the submaximal contractions and the twitches at rest (filled symbols). D: measured MVC and LEF for individual participants (dotted lines) and group means (gray and black lines). AB, able-bodied; SCI, spinal cord injury.
Sustained MVC.
Participants first produced a brief MVC with their target FDI (6-s duration, 50-s rest). A superimposed twitch was evoked during this contraction followed by three twitches at rest (the largest twitch was considered the prefatigue twitch). To induce muscle fatigue, participants generated a sustained MVC (124 s) with the target FDI (Fig. 2). Starting at 8 s, superimposed twitches were evoked seven times during the sustained contraction (18-s interval). Following the contraction, three twitches were evoked at rest (the largest twitch was considered the fatigued twitch).
Outcome Measures
We analyzed the data using Spike 2 software (version 7.04). The maximal force and maximal root-mean-square (RMS) of the EMG signal (RMS-EMG) (500 ms moving window) were determined from all of the brief MVCs. To enable comparison between participants, force and EMG data were expressed as a percentage of these maximum values. During MVCs, force of the contralateral FDI was also quantified and expressed as a percentage of the MVC.
Twitch amplitudes were manually measured. A cursor was positioned at the start of the twitch, close to the moment of stimulation, and a second cursor was placed at the maximal force. The force difference equaled the twitch amplitude. Superimposed twitches were expressed as a percentage of the largest potentiated twitch at rest. The RMS of the M-wave was determined over a 20-ms interval to determine the ratio between voluntary and stimulated EMG signals.
To calculate the SCI-related-superimposed twitch, linear regression was used to fit the rest and superimposed twitches (during brief MVCs and submaximal contractions) to the voluntary force (Fig. 3B). The regression was extrapolated to estimate the twitch that would be evoked at the strongest force attained during all brief MVCs (the voluntary force at the time of stimulation was often not the maximal force). If the estimated SCI-related superimposed twitch was greater than zero, it was subtracted from the pre- and postfatigue twitches at rest and the superimposed twitches to produce an SCI-corrected twitch (see Equations 2 and 3). If the resultant twitch was negative, a zero was included for this time point.
We also estimated the linearly estimated force (LEF) that could be attained if muscle activation had not been impaired by SCI. Linear regression between the twitches at rest and the superimposed twitches (during the submaximal contractions) vs. voluntary force was extrapolated to the x-intercept to provide the LEF (Fig. 3A; see Limitations of the Interpolated Twitch in the discussion). The reliability of estimating LEF was tested in AB participants (n = 5) by calculating the intraclass correlation coefficient (ICC) for measurements performed on three separate days, and in participants with SCI by analyzing the ICC of the LEF for each of the three sets of submaximal contractions.
During the sustained contraction, mean force and RMS-EMG were calculated over 2-s intervals for both FDI muscles (intervals containing peripheral stimulation were excluded). Muscle fatigue was calculated using the following equation:
| (1) |
Peripheral fatigue (corrected for the SCI-related-superimposed twitch, TSCI) was calculated using the twitches (T) at rest obtained before (Tprefatigue) and after (Tfatigued) the sustained contraction:
| (2) |
Superimposed twitches obtained during the sustained MVC were corrected for both the SCI-related-superimposed twitch and the peripheral fatigue (26). A linear force decline for the prefatigue and fatigued twitches was used to correct the twitch (T at time t) for peripheral fatigue:
| (3) |
Muscle activation during the sustained contraction was calculated as the mean of all corrected superimposed twitches (n = 7).
Statistical Analysis
In the text, values are given as mean and SD unless stated otherwise. Data were analyzed using SPSS (IBM SPSS Statistics 22). We compared outcome measures (Table 2) between both groups of participants using univariate ANOVAs (sex was used as a covariate for the MVCs) and Mann-Whitney U-tests (for non-normally distributed data).
Table 2.
Primary outcomes for study participants
| Measurement Parameter | SCI* | AB† | P |
|---|---|---|---|
| Age, yr | 52.3 (29–63) | 50.7 (29–65) | 0.654 |
| FDI M-wave, mV | 16.1 (5.1) | 22.9 (4.3) | <0.001‡ |
| ADM M-wave, mV | 13.5 (5.7) | 18.0 (4.2) | 0.017‡ |
| Potentiated doublet force, N | 9.1 (2.6) | 10.1 (3.3) | 0.369 |
| Potentiated doublet force, % MVC | 44.0 (15.8) | 26.8 (5.9) | 0.001‡ |
| Brief MVC | |||
| MVC-L, N | 31.9 (10.4) | 47.2 (11.0) | <0.001‡ |
| MVC-R, N | 20.3 (7.1) | 37.9 (9.5) | <0.001‡ |
| Superimposed twitch, % potentiated-twitch | 10.1 (2.0–63.1) | 4.7 (0.0–18.4) | 0.007‡ |
| Superimposed twitch corrected, %potentiated-twitch | 6.7 (0.0–17.5) | 0.402§ | |
| RMS-EMG/RMS M-wave ratio | 0.19 (0.10) | 0.28 (0.05) | 0.005‡ |
| Sustained MVC | |||
| Force mean of last 6 s, %MVC | 30.0 (10.0, n = 14) | 32.8 (10.9) | 0.469 |
| EMG mean of last 6 s, %MVC | 45.3 (16.0, n = 14) | 44.7 (15.6) | 0.928 |
| Muscle fatigue, % | 58.0 (15.1, n = 14) | 57.2 (13.3) | 0.887 |
| Peripheral fatigue, % | 22.7 (19.0, n = 12) | 45.6 (21.6) | 0.007‡ |
| Corrected peripheral fatigue, % | 29.9 (17.7, n = 12) | 0.048§ | |
| Mean (corrected) twitch, % prefatigue-twitch | 27.7 (20.5, n = 12) | 12.4 (13.5) | <0.001‡ |
ADM, abductor digiti minimi; EMG, electromyographic; FDI, first dorsal interosseous muscle; MVC, maximum voluntary contraction; MVC-L, MVC of the left FDI; MVC-R, MVC of the right FDI; RMS-EMG, root-mean-square of the EMG signal.
Participants with spinal cord injury (SCI), n = 17.
Able-bodied (AB) participants, n = 17. Values are mean (SD) or median (range).
Values differ significantly between groups.
SCI compared with the value for AB participants in the previous row.
Force and EMG data from sustained MVCs were compared using a repeated measures ANOVA to test for within-subject (time) and between-subject (group) effects. The force and EMG data of the nontask-performing FDI were not normally distributed, so they were transformed by taking the square root of each data point. Superimposed twitches during the sustained contraction were analyzed using a multilevel linear model to assess the effects of time (covariate) and group (factor). First, the model was fitted with time, group, and the interaction between time and group as fixed parameters. The next step was to allow random intercepts, followed by a model that allowed random slopes. The model with the smallest log-likelihood score is presented.
Linear regression analyses were used to investigate the association between the superimposed MVC twitch vs. the MVC force and EMG data. To correct for possible sex and hand (left and right) differences in force, the MVCs of the participants with SCI were normalized to the mean able-bodied MVC of the corresponding group. Furthermore, associations between muscle fatigue vs. the mean superimposed twitch (during sustained MVC) and peripheral fatigue were analyzed with linear regression analysis in which group was added as a second-level variable.
RESULTS
Weaker Maximal Voluntary Contractions After SCI
Overall, the MVC of the left and right FDI was weaker in participants with SCI (left 32 N, SD 10.4; right 20 N, SD 7.1) than in AB participants (left 47 N, SD 11.0; right 38 N, SD 9.5; both P < 0.001; Table 2). The size of the potentiated (doublet) twitch was, however, similar for participants with SCI (9.1 N, SD 2.6) and AB participants (10.1 N, SD 3.3, P = 0.369; Table 2). Consequently, the potentiated twitch as a percentage of the MVC was larger for those with SCI (44%, SD 15.8) than for AB participants (27%, SD 5.9, P = 0.001). The M-wave amplitude was significantly lower in participants with SCI for both the FDI (SCI 16.1, SD 5.1 vs. AB 22.9 mV, SD 4.3, P < 0.001) and the ADM (SCI 13.5 mV, SD 5.7 vs. AB 18.0 mV, SD 4.2, P = 0.017).
Lower muscle activation in participants with SCI was indicated by larger superimposed MVC twitches (median 10.1% potentiated-twitch, range 2.0–63.1) than in AB participants (median 4.7% potentiated-twitch, range 0.0–18.4, P = 0.007; Table 2). Smaller superimposed twitches occurred in individuals with SCI with stronger MVCs (after correcting for sex and hand differences; R = −0.60, P = 0.013).
The RMS-EMG expressed as a ratio of the RMS M-wave was significantly decreased in participants with SCI (SCI 0.19, SD 0.10 vs. AB 0.28, SD 0.05, P = 0.005), another sign of decreased muscle activation. Both measures of muscle activation [i.e., the RMS-EMG/M-wave ratio and the superimposed twitch (high values represent lower activation)] showed a significant negative association (R = −0.46, P = 0.007).
During MVCs of the target FDI, the force in the nontarget (“resting”) hand was higher in participants with SCI (median left 9.6% MVC, range 2.0–26.4; median right 7.8% MVC, range 3.2–31.4) than in AB participants (median left 4.1% MVC, range 0.7–30.4; median right 3.6% MVC, range 0.8–31.5; both P < 0.05).
Twitch Correction and Estimated Maximal Contraction
The superimposed MVC twitch equaled 6.7% of the potentiated twitch after correction for the SCI-related-superimposed twitch and was not significantly different from that of AB participants (median 4.7% potentiated-twitch, Table 2, P = 0.402), suggesting that voluntary drive (i.e., corticofugal drive) was equally effective in both groups. The twitch correction was larger for participants with SCI who produced less force (R = −0.54, P = 0.031).
The linearly estimated force (LEF) was not significantly different between participants with SCI (26.5 N, 8.5 SD) and AB participants (29.9 N, 7.1 SD, P = 0.249). However, for many participants, the LEF was weaker than the actual MVC (Fig. 3D; see Limitations of the Interpolated Twitch Technique in the discussion). The ICC (absolute agreement) for the repeated measures in AB participants was 0.96 (P < 0.001) for average values and 0.89 (P < 0.001) for single values. The ICC (absolute agreement) for the calculation of the LEF on the basis of the three sets of submaximal contractions in participants with SCI was 0.91 (P < 0.001) for the average values and 0.77 (P < 0.001) for the single values.
Sustained MVC
At the end of the sustained contraction there was no group (SCI vs. AB) difference in the force or EMG decline (Fig. 4A, B; Table 2). Over time, mean force (F = 129.76, P < 0.001) and EMG (F = 21.31, P < 0.001) declined significantly (Table 2), but there was no effect of group or any interaction effect of group by time (Fig. 4). Data from three participants with SCI (two were left-handed) were excluded because of failure to adequately perform the task (one participant stopped repeatedly; two participants started at 50% MVC).
Fig. 4.
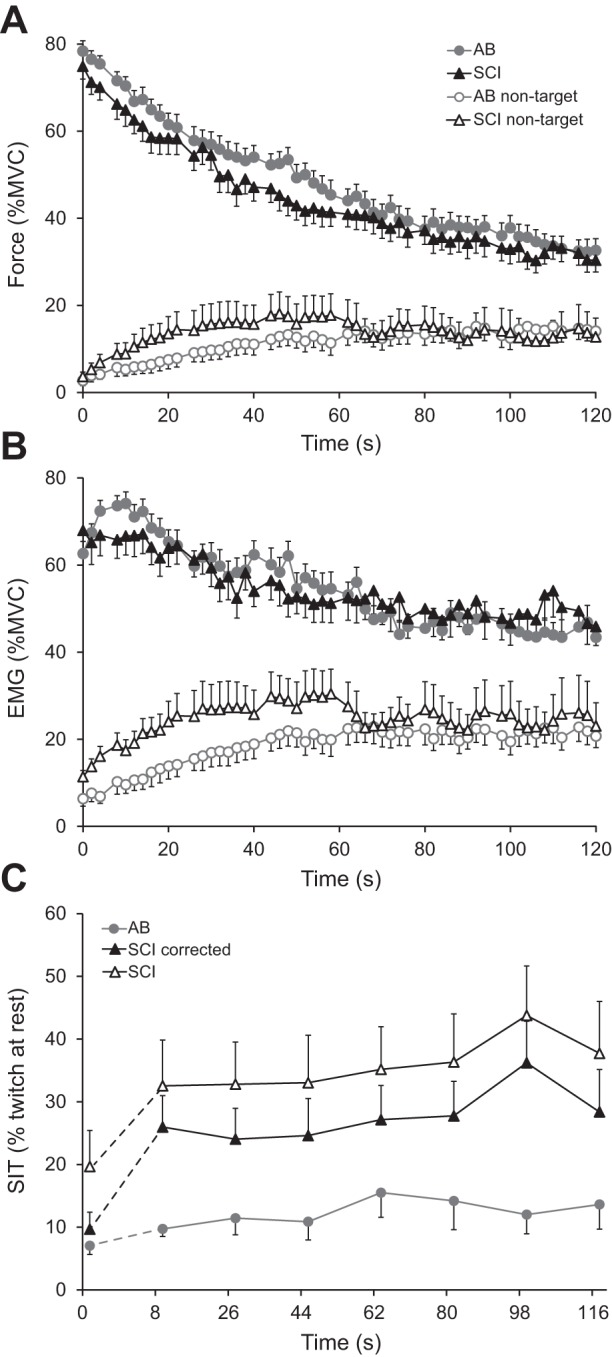
Mean (SE) force (A) and root-mean-square (RMS) of the electromyographic signal (B) (each 2-s epochs) during the sustained MVC for the target (filled symbols) and nontarget (open symbols) FDI of the SCI (black triangles) and AB group (gray circles). C: mean (SE) superimposed twitches during the brief MVC (0 s) and the sustained MVC (n = 7) for AB (gray) and SCI participants (black). Filled symbols represent twitches corrected for both the SCI-related-superimposed twitch and peripheral fatigue (see Equation 3). The increase in the (corrected) twitch between from 0 to 8 s is significantly larger in participants with SCI than in AB participants.
The relationship between the superimposed twitch (corrected for SCI-related-superimposed twitch and peripheral fatigue) and time was best fitted using the multilevel model that allowed for random intercepts and slopes. The model that included the twitch during the brief prefatigue MVC revealed larger superimposed twitches for the group of participants with SCI (F = 7.666, P = 0.010) and over time (F = 10.696, P = 0.003), but no interaction between group and time during the sustained contraction (Fig. 4C). Comparison between the twitch during the brief MVC and the first twitch during the sustained MVC showed a significant increase in twitch force (F = 12.320, P = 0.002), which was larger for the participants with SCI (delta 16.3% of the unfatigued twitch, SD 20.8 vs. 2.7%, SD 4.7 for AB participants, F = 6.342, P = 0.01; Fig. 4C). Thus at the start of the sustained contraction, the net effective drive to the muscle declined more in participants with SCI. Due to a technical problem with the electrical stimulation, twitch data from two participants with SCI were excluded (n = 12).
Less peripheral fatigue (corrected for SCI-related-superimposed twitch) occurred in the participants with SCI, which was indicated by a smaller decline in the twitch after the sustained contraction (29.9%) than for AB participants (45.6%, P = 0.048, Table 2). For all participants, peripheral fatigue (corrected) was greater when the force declined more (R = 0.38; P = 0.040, n = 29). Poorer muscle activation, determined by the mean superimposed (corrected) twitch was associated with less (corrected) peripheral fatigue (R = −0.78; P < 0.001) and with less force decline (R = −0.37, P = 0.05; Fig. 5). No significant effect of group on these associations were found (P > 0.2).
Fig. 5.
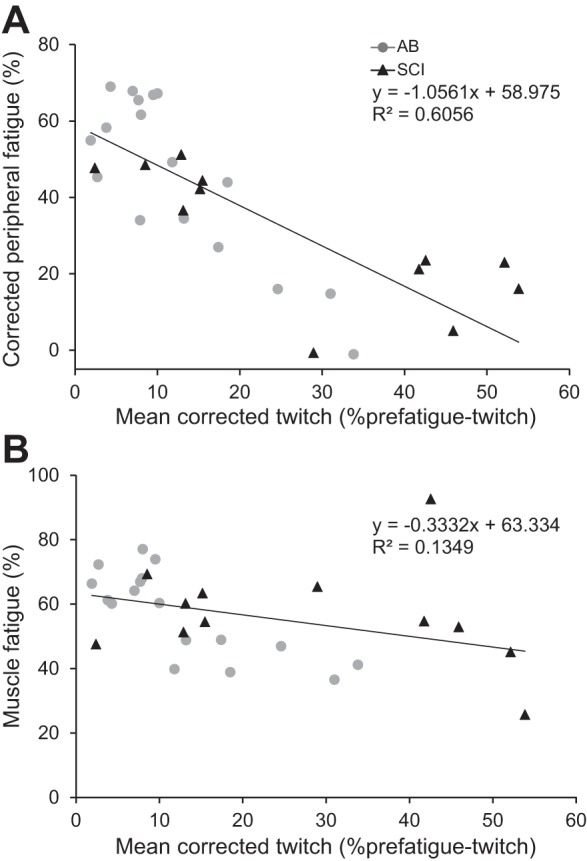
Association between peripheral fatigue (%) (A) and muscle fatigue (%) (B) and the mean corrected twitch during the sustained MVC. For participants with SCI, the mean twitch and peripheral fatigue have been corrected for the SCI-related-superimposed twitch.
The force and EMG of the nontarget (contralateral) FDI increased significantly over time in both groups (force F = 7.445, P < 0.001; EMG F = 6.846, P < 0.001; Fig. 4, A and B). No main effect of group was found. An interaction with time was observed for the EMG (F = 1.652, P = 0.003) but not for force (P = 0.082). The EMG of four participants with SCI were excluded due to grounding-related noise.
DISCUSSION
The present data suggest that during brief contractions, impaired muscle activation due to SCI was more important for explaining weak voluntary contractions than submaximal voluntary drive or muscle atrophy. Second, similar force declines occurred for AB participants and those with SCI during sustained contractions, but greater central fatigue in participants with SCI (larger superimposed twitches even after correcting for the SCI-related-superimposed twitch) was offset by less peripheral fatigue as a result of lower muscle activation.
Is Weak MVC Due Mainly to Reduced SCI-Effective Drive?
Weak maximal voluntary forces in participants with SCI could be the result of 1) reduced voluntary drive, 2) paralyzed muscle fibers (SCI damage preventing motoneuron recruitment), or 3) reduced net effective drive (SCI-related disruption of inputs), all of which may result in muscle atrophy. The first point reflects a decline in cortical output as is also observed in AB participants (2, 30), whereas the latter two points are specific SCI-related impairments (35). Our force recordings suggest no indication for weakness in the muscle (i.e., atrophy). First, similar doublet forces were evoked using stimulation at rest. Second, no difference in the linearly estimated FDI force was found between AB participants and those with SCI. The M-wave amplitude of the FDI was 30% lower in participants with SCI, however. Reduced M-wave amplitude (23, 31), atrophy, and weakness are common in muscles innervated from spinal segments at the lesion epicenter (27, 35) or in paralyzed muscles (5, 9, 25, 38). This is mainly the result of chronic denervation due to motoneuron death, or altered muscle use, or both. However, the relatively high neurological level of the lesion in most of the current participants (predominantly at cervical level 4 or 5) makes paralysis and muscle atrophy from death of FDI motoneurons less probable (39). Additionally, the strength of paralyzed muscle fibers may be preserved in muscles still under some voluntary control due to the contraction of surrounding muscle fibers; biomechanical factors such as stress on the muscle fibers and/or chemicals released by surrounding active muscle fibers and at muscle fiber endplates could help maintain intrinsic muscle fiber properties (15, 16). Nevertheless, it is possible that atrophy was underestimated due to an increase in the doublet/tetanus ratio after SCI (14), although these changes are not expected to affect the estimated force (2).
With respect to reduced voluntary drive (point 1), participants with SCI had weaker voluntary forces, but the similar superimposed (SCI-corrected) twitches for AB participants and those with SCI suggests an equally effective voluntary drive across the groups. Furthermore, the activity of the nontarget FDI during the brief MVCs was higher for the individuals with SCI [contrary to that reported by Bunday and Perez (8)]. Because activity in the contralateral nontarget muscle is related to increased effort (19, 24, 40), this increased nontarget FDI activity provides additional evidence to suggest that participants with SCI do provide near maximal effort during voluntary contractions.
Thus we suggest that weaker voluntary forces after SCI are more likely to result from reduced net effective drive to the motoneuron pool (point 3) than paralyzed muscle fibers, which was reflected by the twofold larger superimposed (uncorrected) twitch in participants with SCI during the brief MVCs. However, even though the deficits in MVC force were substantial in participants with SCI, it is still possible that most motor units were under some voluntary control. The weaker force may be caused by reduced maximal motor unit firing rates, as has been shown in human thenar muscle weakened by SCI (42). Ongoing muscle use would also explain the limited evidence for muscle atrophy.
Reduced Muscle Activation in Participants with SCI Results in Less Peripheral Fatigue But Similar Muscle Fatigue in AB Participants
No differences in relative force or EMG declines were observed during the sustained contraction performed by AB participants and those with SCI despite larger superimposed (corrected) twitches in participants with SCI. Interestingly, the reduction in activation was greatest at the start of the sustained MVC and could mean that participants with SCI have difficulty maintaining a strong effective drive for more than a few seconds (the first stimuli were delivered after >6 s into the contraction) (35). It is known that motoneuron excitability decreases drastically during sustained contractions (20) and that AB individuals (at least partially) compensate for this decline by increasing their voluntary drive (20, 24). All participants increased associated activation of the nontarget FDI during the sustained MVC (Fig. 4, A and B, and during brief MVCs, Fig. 2A) similar to earlier studies involving high-force or fatiguing contractions (19, 24, 40). These results support the assumption that the effort produced by participants with SCI is similar to the effort of AB participants. The question is whether this increased drive actually reaches the muscle fibers in those with SCI.
The greater decline in fatigued twitches after the sustained contraction in AB participants suggests more peripheral fatigue, even after correcting for the SCI-related-superimposed twitch in those with SCI. The significant inverse association between peripheral fatigue and the mean superimposed twitch supports the idea that reduced peripheral fatigue in participants with SCI was mainly due to reduced muscle activation. These observations are consistent with earlier findings in which muscles that work at lower intensities show less peripheral and thus lower muscle fatigue (6). Similar muscle fatigue for AB participants and those with SCI occurred because less peripheral fatigue in those with SCI was accompanied by greater reductions in voluntary drive (i.e., more central fatigue). One study (18) investigating muscle fatigue during maximum volitional contractions found only indications of central fatigue but no peripheral fatigue in flexor carpi radialis muscles weakened by cervical SCI. A second study (33) investigating repeated submaximal contractions in triceps brachii muscles weakened by SCI also found less peripheral fatigue in individuals with SCI, and signs of central fatigue.
Limitations of the Interpolated Twitch Technique
In participants with SCI, we attempted to distinguish between reduced muscle activation as a result of SCI (SCI-related-superimposed twitch) and reduced voluntary drive because both factors will increase the twitches superimposed on voluntary contractions. Even though the corrected superimposed twitches were similar for AB participants and those with SCI during brief MVCs, the differences during the sustained MVC were large (Fig. 4C). We realize that the assumption that the voluntary activation was near maximal could result in an overestimation of the voluntary activation and therefore to an overestimation of the SCI-related-superimposed twitch. We were therefore surprised that after the subtraction of the (maybe overestimated) SCI-related-superimposed twitch the superimposed twitch was still so much larger during the sustained contraction.
Another technique to control for the SCI-related-superimposed twitch during fatiguing contractions would be to use transcranial magnetic stimulation of the primary motor cortex to evoke twitches and thereby estimate changes in voluntary drive over time in both AB individuals and those with SCI (30, 35). The absence of evoked force during MVCs has demonstrated that individuals with SCI can exert maximal voluntary drive during brief contractions (35) because similar descending inputs can be activated both voluntarily and with cortical stimulation. Systematic comparison of twitches evoked by cortical and peripheral nerve stimulation during the same fatiguing contraction would be an interesting path to follow for comparing the contributions of central and peripheral fatigue to force declines in both groups of participants.
Furthermore, we used linear regression analysis to estimate FDI force (10, 29) although a quadratic polynomial provides a slightly better fit for twitches evoked during strong voluntary forces generated by AB participants (Fig. 3C). We favored a linear function because some participants with SCI were incapable of producing strong voluntary forces (Fig. 3B), during which superimposed twitches would be expected to be small (17), and the use of a quadratic polynomial would systematically underestimate the force in these participants. We therefore included only submaximal contractions in the model because for these submaximal contractions, a linear model proves a better fit than a polynomial function for both groups. This model underestimates the estimated force for both groups of participants equally (see Fig. 3D) (4, 11).
A number of other methodological issues may affect extrapolation of the evoked-voluntary force relationship in the FDI. One factor that could not be controlled was activation of antagonist muscles by stimulation of the ulnar nerve (palmar interosseous, index finger adductor) (41). The contribution of the palmar interosseous to the superimposed twitch was kept as small as possible by having the index finger at zero abduction and the thumb wrapped around fingers II-III (Fig. 2B), thus favoring the index finger abductor over the index finger adductor as the prime contributor to the evoked force (3). Pilot experiments in AB participants (n = 5) demonstrated that estimation of the maximal FDI force using linear regression was similar (intraclass correlation coefficient 0.96, P < 0.001) when twitches were evoked by ulnar nerve stimulation vs. FDI (muscle) stimulation. This result suggests that the force contribution of the first palmar interosseous in the present hand position is relatively small compared to the contribution of the first dorsal interosseous.
Concluding Remarks
Voluntary drive is reduced during sustained contractions but not during brief maximal voluntary contractions in FDI muscles weakened by SCI. The decline in voluntary drive is accompanied by less peripheral fatigue, which provides an explanation for the similar force decline for AB participants and those with SCI during a sustained contraction. It would be interesting to learn whether the reduced voluntary drive during the sustained contraction results from a relative decline in the net effective drive reaching the motoneuron pool or from a reduced voluntary output. In other words, did participants with SCI increase their voluntary drive similarly to that of AB participants or did the SCI itself result in reduced voluntary drive?
In addition, our data suggest that in this group of SCI participants, reduced muscle activation, and not atrophy, was responsible for weak voluntary force. From a practical perspective, individuals with SCI who have some voluntary control of hand muscles innervated from spinal segments below the lesion epicenter (AIS C or D) should be encouraged to use these muscles because the exercise may decrease atrophy and weakness, even in paralyzed muscle fibers.
Our method of correcting twitches superimposed on MVCs for SCI-related impairment gives an indication of the voluntary drive that does not reach the motoneuron pool, which will reduce muscle activation. This method makes it possible to study changes in muscle activation over time and our data suggest that sustained force production by individuals with SCI could be hampered by fatigue-related processes at spinal or supraspinal levels, as well as neuromuscular processes. Furthermore, this method is relevant to any central nervous system disorder or trauma that physically disrupts inputs to spinal motoneurons, or when there is central conduction block, because this damage will introduce a mismatch between the number of motoneurons that can be voluntarily activated and the number of motoneurons that can be excited by peripheral nerve stimulation (34). Use of this approach could also be used to quantify changes in net effective drive to motoneurons following rehabilitation or interventions that aim to promote plasticity.
GRANTS
Support for this study was provided by a Junior Scientific Masterclass grant from the University Medical Center Groningen; by National Institute of Neurological Disorders and Stroke Grant N01-NS-3-2351; and by The Miami Project to Cure Paralysis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.K.T., M.T., and I.Z. conception and design of research; R.F.P., M.D., and I.Z. performed experiments; R.F.P., M.D., and I.Z. analyzed data; R.F.P., M.D., C.K.T., M.T., and I.Z. interpreted results of experiments; R.F.P. prepared figures; R.F.P., M.D., and I.Z. drafted manuscript; R.F.P., M.D., C.K.T., M.T., and I.Z. edited and revised manuscript; R.F.P., M.D., C.K.T., M.T., and I.Z. approved final version of manuscript.
REFERENCES
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 1: 287–332, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Allen GM, Gandevia SC, McKenzie DK. Reliability of measurements of muscle strength and voluntary activation using twitch interpolation. Muscle Nerve 18: 6: 593–600, 1995. [DOI] [PubMed] [Google Scholar]
- 3.An KN, Ueba Y, Chao EY, Cooney WP, Linscheid RL. Tendon excursion and moment arm of index finger muscles. J Biomech 16: 6: 419–425, 1983. [DOI] [PubMed] [Google Scholar]
- 4.Behm DG, St-Pierre DM, Perez D. Muscle inactivation: assessment of interpolated twitch technique. J Appl Physiol 81: 2267–2273, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Biering-Sorensen B, Kristensen IB, Kjaer M, Biering-Sorensen F. Muscle after spinal cord injury. Muscle Nerve 40: 499–519, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Bigland-Ritchie B, Cafarelli E, Vollestad NK. Fatigue of submaximal static contractions. Acta Physiol Scand 128, Suppl 556: 137–148, 1986. [PubMed] [Google Scholar]
- 7.Bigland-Ritchie B, Johansson R, Lippold OC, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol 50: 1: 313–324, 1983. [DOI] [PubMed] [Google Scholar]
- 8.Bunday KL, Perez MA. Impaired crossed facilitation of the corticospinal pathway after cervical spinal cord injury. J Neurophysiol 107: 2901–2911, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro MJ, Apple DF Jr, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol 86: 350–358, 1999. [DOI] [PubMed] [Google Scholar]
- 10.de Haan A, Gerrits KH, de Ruiter CJ. Counterpoint: the interpolated twitch does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol 107: 355–357; discussion 357–358, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Folland JP, Williams AG. Methodological issues with the interpolated twitch technique. J Electromyogr Kinesiol 17: 317–327, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Gandevia SC, McKenzie DK. Activation of human muscles at short muscle lengths during maximal static efforts. J Physiol 407: 599–613, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin L, Godfrey S, Thomas CK. Stimulation pattern that maximizes force in paralyzed and control whole thenar muscles. J Neurophysiol 87: 2271–2278, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Houle JD, Morris K, Skinner RD, Garcia-Rill E, Peterson CA. Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve 22: 846–856, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Kernell D. The Motoneurone and its Muscle Fibres. New York: Oxford University Press, 2006. [Google Scholar]
- 17.Kooistra RD, de Ruiter CJ, de Haan A. Conventionally assessed voluntary activation does not represent relative voluntary torque production. Eur J Appl Physiol 100: 309–320, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin KH, Chen YC, Luh JJ, Wang CH, Chang YJ. H-reflex, muscle voluntary activation level, and fatigue index of flexor carpi radialis in individuals with incomplete cervical cord injury. Neurorehabil Neural Repair 26: 68–75, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Liu JZ, Shan ZY, Zhang LD, Sahgal V, Brown RW, Yue GH. Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: an FMRI study. J Neurophysiol 90: 300–312, 2003. [DOI] [PubMed] [Google Scholar]
- 20.McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol 589, Pt 14: 3533–3544, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merton PA. Voluntary strength and fatigue. J Physiol 123: 553–564, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol 590, Pt 16: 3647–3663, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelletier CA, Hicks AL. The length-tension relationship of human dorsiflexor and plantarflexor muscles after spinal cord injury. Spinal Cord 48: 202–206, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Post M, Steens A, Renken R, Maurits NM, Zijdewind I. Voluntary activation and cortical activity during a sustained maximal contraction: an fMRI study. Hum Brain Mapp 30: 1014–1027, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Round JM, Barr FM, Moffat B, Jones DA. Fibre areas and histochemical fibre types in the quadriceps muscle of paraplegic subjects. J Neurol Sci 116: 207–211, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Schillings ML, Stegeman DF, Zwarts MJ. Determining central activation failure and peripheral fatigue in the course of sustained maximal voluntary contractions: a model-based approach. J Appl Physiol 98: 2292–2297, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Shah PK, Stevens JE, Gregory CM, Pathare NC, Jayaraman A, Bickel SC, Bowden M, Behrman AL, Walter GA, Dudley GA, Vandenborne K. Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil 87: 772–778, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Steens A, de Vries A, Hemmen J, Heersema T, Heerings M, Maurits N, Zijdewind I. Fatigue perceived by multiple sclerosis patients is associated with muscle fatigue. Neurorehabil Neural Repair 26: 48–57, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Taylor JL. Point: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol 107: 354–355, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Taylor JL, Gandevia SC. Transcranial magnetic stimulation and human muscle fatigue. Muscle Nerve 24: 18–29, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Thomas CK. Contractile properties of human thenar muscles paralyzed by spinal cord injury. Muscle Nerve 20: 788–799, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Thomas CK, Bakels R, Klein CS, Zijdewind I. Human spinal cord injury: motor unit properties and behaviour. Acta Physiol (Oxf) 210: 5–19, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Thomas CK, del Valle A. The role of motor unit rate modulation versus recruitment in repeated submaximal voluntary contractions performed by control and spinal cord injured subjects. J Electromyogr Kinesiol 11: 217–229, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Thomas CK, Dididze M, Zijdewind I. Fatigue and neuromuscular diseases. In: Human muscle fatigue, edited by Williams C, Ratel S. New York: Routledge, 2009, p. 245–284. [Google Scholar]
- 35.Thomas CK, Zaidner EY, Calancie B, Broton JG, Bigland-Ritchie BR. Muscle weakness, paralysis, and atrophy after human cervical spinal cord injury. Exp Neurol 148: 414–423, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Vagg R, Mogyoros I, Kiernan MC, Burke D. Activity-dependent hyperpolarization of human motor axons produced by natural activity. J Physiol 507: 919–925, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Duinen H, Post M, Vaartjes K, Hoogduin H, Zijdewind I. MR compatible strain gauge based force transducer. J Neurosci Methods 164: 247–254, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Wu GA, Bogie KM. Not just quantity: gluteus maximus muscle characteristics in able-bodied and SCI individuals–implications for tissue viability. J Tissue Viability 22: 74–82, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Yang JF, Stein RB, Jhamandas J, Gordon T. Motor unit numbers and contractile properties after spinal cord injury. Ann Neurol 28: 496–502, 1990. [DOI] [PubMed] [Google Scholar]
- 40.Zijdewind I, Kernell D. Bilateral interactions during contractions of intrinsic hand muscles. J Neurophysiol 85: 1907–1913, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Zijdewind I, Kernell D. Index finger position and force of the human first dorsal interosseus and its ulnar nerve antagonist. J Appl Physiol 77: 987–997, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Zijdewind I, Thomas CK. Motor unit firing during and after voluntary contractions of human thenar muscles weakened by spinal cord injury. J Neurophysiol 89: 2065–2071, 2003. [DOI] [PubMed] [Google Scholar]