Introduction
The group A streptococcus, Streptococcus pyogenes, and its link to autoimmunity and disease, has acquired a new level of understanding since the early reports of rheumatic fever and rheumatic heart disease (1–14). The Jones criteria which describe the onset and diagnosis of rheumatic fever include major manifestations of the heart, brain, joints and skin. Polymigrating arthritis, carditis associated with a heart murmur, erythema marginatum, a circinate skin rash, subcutaneous nodules, and the neurologic manifestation, Sydenham chorea are inflammatory features that may occur in acute rheumatic fever (4, 10). Most rheumatic fever follows pharyngitis or other mucosal infections and diagnosis requires the presence of elevated antibodies against S. pyogenes including anti-streptolysin O and/or anti-DNAse B antibody increases over normal levels or a positive throat culture or quick strep test for group A streptococci (10). Streptococcal infection of the throat and skin or mucosa precedes the immune mediated sequelae which can occur in acute rheumatic fever (15–17)
Streptococcal sequelae such as rheumatic fever (18–21) occur primarily in childhood and adolescence and. Rheumatic fever is a group A streptococcal induced global disease found in many regions of the world (15, 22–25), and a resurgence of rheumatic fever has been reported in the past 3 decades in the United States (26–29). Both rheumatic heart disease and Sydenham chorea and related brain sequelae and the possible autoimmune pathogenic mechanisms will be addressed in this review. Both rheumatic carditis and Sydenham chorea have been for some time correlated with autoantibodies against the heart and brain (16, 17, 30–34), but the pathogenic mechanisms of autoimmunity and inflammation in these streptococcal sequelae are continually under investigation. Both of these streptococcal sequelae may occur through autoimmune mechanisms related to molecular mimicry (35, 36). Molecular mimicry is part of the normal immune response including the response of the host to the group A streptococcus. Mimicry and production of crossreactive antibodies provide ‘survival of the fittest’ advantage to the host through immune recognition and response against pathogens and other microbes with the production of antibodies which recognize both host and microbial antigens. Studies have for some time supported the hypothesis that molecular mimicry between the group A streptococcus and heart was important in the immune responses in rheumatic fever (35, 37–41). In studies of molecular mimicry between the streptococcus and heart, the definition of crossreactive antibodies which could recognize several types of epitopes were defined (16, 37, 42–44). Other mechanisms may involve collagen or anti-collagen antibodies and has recently been reviewed (43, 45).
Although rheumatic heart disease of the valve is the most serious manifestation and has been the focus of research for decades (16, 17, 46–52), more recent studies of Sydenham chorea (53) and its related sequelae, pediatric autoimmune neurologic disorder associated with streptococci (PANDAS), has gained attention (54–59). The first 50 cases of PANDAS were described by Swedo and colleagues to present with tics or obsessive compulsive symptoms and often display in particular small pianoplaying choreiform movements of the fingers and toes (60, 61). The heterogeneous group of children with infections as well as acute and chronic tic and obsessive compulsive disorders has led to a climate of confusion in the literature about these behavioral disorders (62). However, evidence strongly supports a group of children with OCD/tics with small choreiform movements that is similar to Sydenham chorea and is called by the acronym PANDAS (55, 60, 63). The acronym PANDAS is based on the premise that the syndrome described is due to a prior streptococcal infection. However, acute onset tic and OCD symptoms can also follow infections other than group A streptococci and are considered as pediatric acute onset neuropsychiatric syndrome or PANS (64) in the absence of streptococcal infections. The rationale for alternative terms such as PANS were due situations where there was a lack of evidence that the syndrome was actually caused by streptococcal infection. Another clinical research group called for a broader concept of childhood acute neurologic symptoms or CANS (65). The PANDAS subgroup is known to have the small choreiform movements particularly of the fingers and toes which are usually not present in some of the other groups with acute or chronic tics and OCD which would be called PANS. Studies of anti-neuronal autoantibodies in Sydenham chorea and PANDAS with choreiform movements clearly identified a specific group of anti-neuronal antibodies present in both Sydenham chorea and PANDAS and identified specific antibody mediated neuronal cell signaling mechanisms which in part may lead to disease symptoms (53, 66–69).
Rheumatic carditis, Sydenham chorea and the new group of behavioral disorders called PANDAS will be reviewed with consideration of autoantibody and T cell responses and the role of molecular mimicry between the host and the group A streptococcus as well as how immune responses contribute to the pathogenic mechanisms of these diseases. The combination of autoimmunity and behavior is a relatively new concept linking the brain, behavior and neuropsychiatric disorders with streptococcal infections.
Rheumatic Carditis: Mimicry Between Group A Streptococci and Heart
Mimicry between group A streptococci and heart antigens is supported by evidence from previous studies (35, 40, 53, 70). Originally, mouse monoclonal antibodies (mAbs) produced against group A streptococci and heart reacted with striations in myocardium or mammalian muscle (50) as previously reported for human acute rheumatic fever sera or sera from animals immunized with group A streptococcal antigens (40, 41, 50, 71). Studies utilizing human and animal sera were complicated years ago and difficult to determine crossreactivity and molecular mimicry between the host and streptococcus. Both mouse and human mAbs led to the identification of cardiac myosin as one of the major proteins in heart which crossreacted with the group A carbohydrate or streptococcal M protein antigens (16, 35, 37). The human mAbs which reacted with myocardium and valve recognized primarily the group A carbohydrate epitope N-acetyl-beta-D-glucosamine which is the immunodominant epitope of the group A carbohydrate composed of a polyrhamnose backbone with side chains of N-acetyl-beta-D-glucosamine in the group A carbohydrate specificity (35).
The endothelium surrounding the valve must become inflamed to allow T cells to enter the valve and produce scarring. Human monoclonal autoantibodies from acute rheumatic fever were produced from disease and reacted against cardiac myosin and the streptococcus and reacted with both myocardium and valve endothelium. The target on the surface of the valve was laminin and specific laminin peptide epitopes (35), but the crossreactivity could also result from glycosylated proteins such as laminin or other extracellular proteins at the valve surface. The glycosylated proteins and carbohydrate epitopes on the valve were shown to crossreact with the group A carbohydrate (72) and later persistence of elevated antibody responses against the group A carbohydrate were found to correlate with poor prognosis of valvular heart disease (30). The evidence supports a correlation between rheumatic valvular heart disease and group A carbohydrate epitope. Many of the human mAbs produced from rheumatic fever recognized the group A carbohydrate epitope N-acetyl-beta-D-glucosamine (73). Targeting of antibodies or immune complexes to the valve surface would lead to the cellular infiltration and inflammation seen in the valve endothelium with upregulation of vascular cell adhesion molecule-1 (VCAM-1) as shown in valves from rheumatic heart disease (74). A model diagram showing these principles in rheumatic carditis has recently been reviewed (17). The model depicts the activation of the endothelium by antibodies against the group A carbohydrate with infiltration of T cells reactive with the streptococcal M protein. The valve is shown to be vulnerable to the attack by the immune system following the activation of the endothelium with subsequent cellular infiltration (17, 74).
Studies have also linked alpha-helical structures such as found in streptococcal M proteins, cardiac myosin, keratin, and laminin with crossreactivity against the group A carbohydrate epitope N-acetyl-beta-D glucosamine (16, 75, 76). Human mAbs which target the group A carbohydrate epitope GlcNAc also react with alpha-helical coiled-coil molecules and very well defined peptide epitopes that suggest hydrophobic and aromatic amino acids are important in the interaction with crossreactive antibody molecules (75). Peptides from alpha-helical coiled-coil molecules have been described which mimick the group A carbohydrate epitope (75). In addition, analysis of a crystallized group A streptococcal M1 protein fragment defines how the alpha-helical coiled-coil structures and epitopes are recognized in alpha helical proteins as a basis for molecular mimicry and crossreactivity between streptococcal M proteins and cardiac myosin (77). The alpha-helical coiled-coil streptococcal M protein structure is well known for its crossreactive properties with antibodies against cardiac myosin (16). The alpha helical structure in M1 protein was observed to exhibit substantial irregularities and instabilities of a non-idealized alpha helix (77) similar to that seen in cardiac myosin. The study showed that mutations in the M1 protein encoding an idealized alpha helix, stabilized the alpha-helical structure and diminished the cardiac myosin crossreactive properties of the streptococcal M1 protein (77).
Autoantibodies against collagen I are produced along with responses against cardiac myosin (78) which could be due to aggregation of collagen by certain streptococcal serotypes such as M3 protein (45, 79, 80), but also may be due to release of collagen from the damaged valve during rheumatic heart disease (17). The anti-cardiac myosin/anti-streptococcal antibody and T cell responses are crossreactive based on studies of human and mouse mAbs and human T cell clones, while the responses against collagen I are not crossreactive indicating that release of collagen from the valve could be an important source of exposure of collagen to the human immune system. In addition, streptococcal proteins with similarity to collagen have been reported (81, 82).
Although there is no cardiac myosin directly in the valve, the valve is attached in papillary muscle containing cardiac myosin and myocardium (45, 74). The link between cardiac myosin and the valve is related to the crossreactivity of the cardiac myosin found in the myocardium with laminin or other components on the valve surface. The valve is believed to be injured initially in acute rheumatic fever by the autoantibody response that is directed at the valve endothelium. The chordae tendinae which hold the valve in place are vulnerable to inflammatory attack and become elongated and stretched by edema and stress following the initial damage. Valve endothelium is an infiltration site for lymphocyte extravasation into the valve (74). In addition, rheumatic heart disease is characterized by involvement of all three layers of the heart, pericardium, myocardium and endocardium, but the valvular lesions are most likely to lead to chronic disease and heart failure.
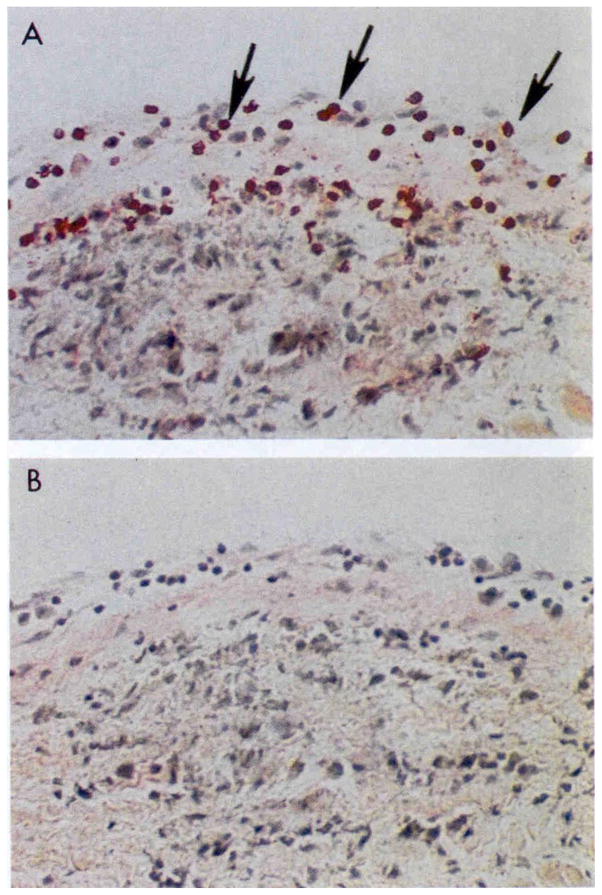
Both CD4+ and CD8+ T cells infiltrate the valves in rheumatic fever (74) but the CD4+ T cell subset predominates over the CD8+ T cell subset in the rheumatic valve (Figure 1). The granulomatous Th1 reaction is evident and the presence of gamma IFN has been reported in rheumatic valves (83). Although less is known about Th17 responses in rheumatic heart disease, they are probably present and may contribute toward the granulomatous reaction in the heart.
Figure 1.
Extravasation of CD4+ lymphocytes into valve above Aschoff’s body in the subendocardium of the left atrial appendage. Original magnification 200X Taken from Roberts et al (74).
As shown in Figure 1 in human rheumatic carditis, CD4+ T cell infiltrates extravasate directly through the valve endocardium into the valve as well as into the papillary muscle where valve is attached (74). Studies of T lymphocytes from both human and Lewis rat (84–86) indicate that there is strong crossreactivity between cardiac myosin and the streptococcal M protein (39) (83, 87–90). It should also be noted that in humans the T cells from peripheral blood reflect similar reactivities and specificities as that found in the heart valves (91). This makes sense as the infiltrating lymphocytes would extravasate directly into heart valves from the peripheral blood. It may be a misconception in some diseases that the peripheral blood has no value in studies of human organ specific autoimmune diseases. In the Lewis rat, the intact M protein, as well as peptides from the A, B and C repeat regions of the streptococcal M protein molecule have been investigated for their potential to cause valvular heart disease (84, 86, 92, 93).
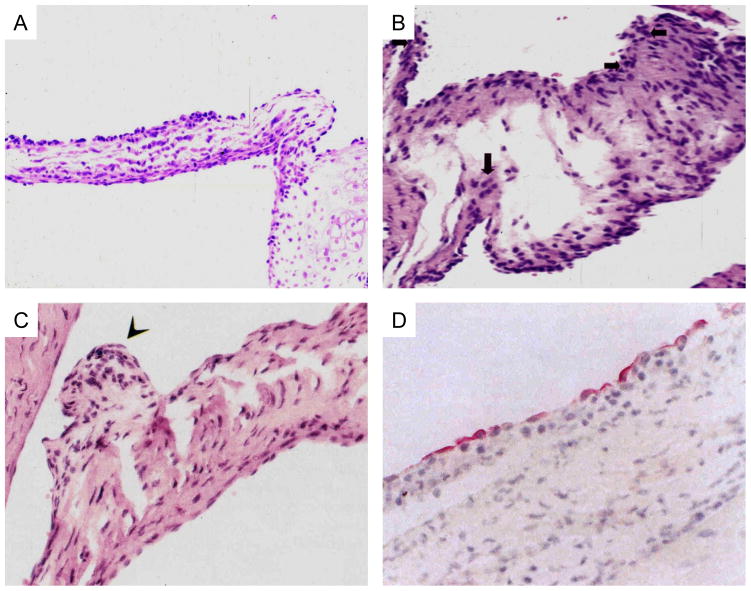
In recent studies, T cell lines from Lewis rats immunized with streptococcal M protein induced valvulitis and were strongly stimulated by specific M5 peptides (86). M protein specific Lewis rat T cell lines were capable of passively transferring valvulitis characterized by infiltration of CD4+ cells and upregulation of VCAM-1, while a control T cell line against different epitopes did not target the valve (86) in naïve rats. M protein-specific T cells may be important mediators of valvulitis in the Lewis rat model of rheumatic carditis (85) as shown in Figures 2A–C. Figure 2A shows the infiltrating mononuclear cells at the valve surface and inner valve in Lewis rats immunized with the group A streptococcal M5 serotype amino acid sequence residues 1–76 in the A repeat region (86). Figure 2B shows cellular infiltration and edema seen in valves from Lewis rats immunized with group A streptococcal M5 serotype amino acid sequences in residues 59–115 found in the latter fragment of the A repeat region of M protein (86). Figure 2C illustrates a verrucous type lesion which developed in Lewis rats immunized with recombinant M6 protein (85). Verrucae have been observed in acute rheumatic fever (1–14). Figure 2D illustrates the upregulation of VCAM-1 in human valves from rheumatic heart disease (74). VCAM-1 was observed on activated endothelium in the Lewis rat after administration of pathogenic T cell lines which targeted the valve and led directly to upregulation of VCAM-1 at the valve surface allowing penetration of the T cell lines after their passive transfer (86) (not shown). The upregulation of VCAM-1 on the Lewis rat valve was similar to that seen in humans as shown in Figure 2D. Only T cell lines against specific streptococcal M5 protein epitopes such as DKLKQQRDTLSTQKETLE (NT5/6 ~ M5 peptide amino acid sequence from A repeat region of M5 protein serotype of Streptococcus pyogenes) as defined in the human T cell studies by Guilherme and colleagues (83, 88) were found to target the valve in passive transfer studies (86). Other T cell lines recognizing other epitopes were not pathogenic and did not target the valve (86).
Figure 2.
2A) Induction of valvulitis and cellular infiltration in hematoxylin and-eosin-stained heart valves from Lewis rats immunized with group A streptococcal M5 peptides NT1-NT4/5 [AVTRGTINDPQRAKEALD amino acid (aa) residues 1–18; NT-2 KEALDKYELENHDLKTKN aa residues 14–31; NT-3 LKTKNEGLKTENEGLKTE aa residues 27–44; NT-4GLKTENEGLKTENEGLKTE aa residues 40–58; NT-4/5 GLKTEKKEHEAENDKLK aa residues 54–70 Kirvan, et al (86)
2B) Induction of valvulitis, edema and cellular infiltration in hematoxylin and-eosin-stained heart valves from Lewis rats immunized with group A streptococcal M5 peptides NT1-NT4/5 [AVTRGTINDPQRAKEALD amino acid (aa) residues 1–18; NT-2 KEALDKYELENHDLKTKN aa residues 14–31; NT-3 LKTKNEGLKTENEGLKTE aa residues 27–44; NT-4 GLKTENEGLKTENEGLKTE aa residues 40–58; NT-4/5 GLKTEKKEHEAENDKLK aa residues 54–70 Kirvan, et al (86).
2C) Verrucous nodule observed on valve after immunization with group A streptococcal rM6 protein. Taken from Quinn et al (85).
2D) Vascular Cell Adhesion Molecule-1 (VCAM-1) expressed on rheumatic valve. Taken from Roberts et al (74).
To summarize pathogenic mechanisms in the valve in rheumatic carditis, studies in the Lewis rat model suggest that the more potent T cell clones may actually lead to the activation of the VCAM-1 on the valve endothelium promoting the infiltration of particular clones (86). Antibodies which bind the valve may also affect the surface endothelium and lead to activation of the endothelium/endocardium on the valve. Antibodies against the valve would react with the streptococcal group A carbohydrate and glycosylated proteins or sequences in alpha helical matrix proteins in the laminar basement membrane such as laminin as has been shown for human mAbs derived from rheumatic carditis (35). Once the valve endothelium is activated and collagen exposed, the valve may continually be damaged by antibodies against collagen which are not crossreactive and are present in acute rheumatic fever (78). The anti-collagen antibodies may be generated either by collagen bound to M proteins or other collagen binding proteins on the group A streptococcus or collagen is released to the immune system once the valve is damaged initially (43, 45). The valve may be initially damaged by annular dilation and chordal elongation, which prevents adequate surface coaptation of the valve leaflets (94). Troponin levels are not elevated indicating that myocardial function is not compromised. Cardiac myosin is not present in the valve. However, antibodies or T cells specific to cardiac myosin react with the valve in rheumatic carditis because they cross-react with the valve proteins laminin and vimentin (35, 72, 95, 96). The similarity of cardiac myosin with proteins in the valve may be the basis of cross-reactivity with the valve. Mimicry may result in initial damage to the valve while release of collagen I from damaged valve may lead to the immune response observed against collagen I in rheumatic heart disease (78). Anti-streptococcal cross reactive anti-cardiac myosin antibodies initially may cause valve inflammation at the endothelium, leading to edema, cellular infiltration, and fibrinous vegetations in the rough zone of the anterior leaflet. Scarring of the leaflets appears after chordal elongation, which is the initial cause of mitral regurgitation. Chordae tendinae are the most susceptible cardiac structures to attack by crossreactive antibody.
Recurrent rheumatic fever attacks due to repetitive streptococcal infections in children lead to increased scar formation in the valve. After the initial attack of acute rheumatic fever and carditis, the valve scars and thus becomes neovascularized and open to perpetuated disease. If antibodies against the group A carbohydrate remain highly elevated, there is a poor prognosis of rheumatic carditis, and poor recovery until the diseased valve is replaced (30). Furthermore, disease activity in rheumatic heart disease is associated with responses against specific epitopes of human cardiac myosin heavy chain rod in the S2 region of the molecule (38). Disease progression in rheumatic heart disease and potentially monitoring of effective treatment can be followed by responses against human cardiac myosin disease specific peptides in the S2 fragment of human cardiac myosin (97). Similar epitopes in cardiac myosin were found in sera from children with rheumatic carditis from the US, India and Hawaii. The similarity of these epitopes suggest recognition of cardiac myosin by B cells which are allowed to proliferate and are not tolerized by deletion, receptor editing or anergy. The appearance of T cell clones with strong avidity to cardiac myosin would normally be deleted or rendered anergic by normal tolerance mechanisms. Human T cell clones showing strongest avidity to cardiac myosin compared to other host proteins were isolated from rheumatic carditis (39) and were stimulated strongly by peptides of streptococcal M protein and cardiac myosin. T cells isolated from human rheumatic valves were similar to those found in peripheral blood and also proliferated to peptides of streptococcal M protein and cardiac myosin (88).
Study of the human antibody responses against human cardiac myosin peptides have identified epitopes which are associated clinically with acute rheumatic carditis (38). Our studies have identified disease-specific epitopes of human cardiac myosin in the development of rheumatic carditis in humans. We found that immune responses to cardiac myosin were similar in rheumatic carditis among a small sample of worldwide populations, in which immunoglobulin G targeted human cardiac myosin epitopes in the S2 subfragment hinge region within S2 peptides containing human cardiac myosin amino acid residues 842–992 and 1164–1272. Specific cardiac myosin epitopes were recognized in rheumatic carditis which targeted the S2 hinge region of cardiac myosin. The epitopes were also found to be similar among populations with rheumatic carditis worldwide, regardless of the infecting group A streptococcal M serotype. In rheumatic carditis, repeated group A streptococcal throat infections with rheumatogenic streptococci containing cardiac myosin–like sequences in the M protein may be important in mimicry and breaking tolerance, inducing epitope spreading, and initiating disease in susceptible individuals. Streptococcal M protein serotypes induce human antibody responses with serotype specificity as well as antibodies that react with human cardiac myosin epitopes in the S2 region of cardiac myosin. Homologous epitopes shared among different rheumatogenic streptococcal M protein serotypes could prime the immune system against the heart during repeated streptococcal exposure and eventually lead to rheumatic heart disease in susceptible individuals. T cell clones from patients with rheumatic carditis respond to streptococcal M protein and cardiac myosin epitopes supporting the hypothesis that cardiac myosin and streptococcal M protein are important antigens in the development of rheumatic heart disease (39).
Sydenham Chorea and Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococci (PANDAS): Mimicry between Group A Streptococci and Brain
Sydenham chorea is the well established neurologic manifestation of acute rheumatic fever (98) and was originally characterized by antibodies that were found in the cytoplasm of neurons in the caudate and putamen regions of human brain (34). Little was known about the antibodies and how they affected the brain until human mAbs were derived from Sydenham chorea (36) and found to react by mimicry with the group A streptococcal carbohydrate epitope N-acetyl-beta-D-glucosamine and brain antigens lysoganglioside (36) and tubulin (99). The human chorea mAb evidence strongly supports autoantibody crossreactivity between streptococci and brain (36, 99, 100). Human mAbs as well as antibody in sera or cerebrospinal fluid from Sydenham chorea signaled human neuronal cells to activate calcium calmodulin dependent protein kinase II (CaMKII) (36) as well as led to an increase in dopamine release from the human neuronal cell line (100). Further study indicated that the chorea derived mAb (24.3.1) induced tyrosine hydroxylase activity in dopaminergic neurons after intrathecal transfer of purified human chorea derived mAb 24.3.1 (36) into Lewis rat brain (100). Removal of IgG from serum caused a loss of neuronal cell signaling activity in sera (36, 68) and plasmaphoresis has been found to lead to improvement of symptoms (101, 102). Antibody-mediated neuronal cell signaling was induced by IgG antibodies in serum or cerebrospinal fluid from Sydenham chorea and the presence of these signaling autoantibodies were associated with symptoms (36, 69). Antibody-mediated neuronal cell signaling in Sydenham chorea is a novel pathogenic mechanism which is important in the movement and neuropsychiatric disorder of acute rheumatic fever (36). Sydenham chorea may be a model for other movement and neuropsychiatric disorders associated with infections such as pediatric autoimmune neuropsychiatric disorder associated with streptococci (PANDAS) (61).
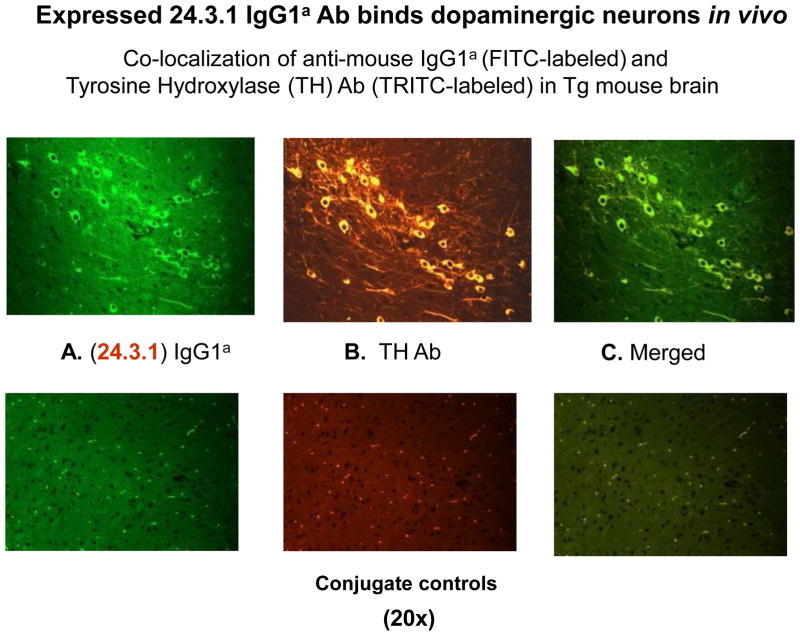
Expression of the V genes of Sydenham chorea mAb 24.3.1 (63) in transgenic (Tg) mice demonstrated that the Sydenham chorea antibody V gene expression in serum of Tg mice targeted dopaminergic tyrosine hydroxylase positive neurons in the basal ganglia of the Tg mice (63) as shown in Figure 3. These results were consistent with evidence seen in human Sydenham chorea (34). At the same time, the mAb 24.3.1 from Sydenham chorea was shown to react with and signal the human dopamine D2 receptor (63). Evidence using a flag tagged D2 receptor as well as signaling of the human D2 receptor in transfected cell lines demonstrated that the human mAb as well as human Sydenham chorea sera IgG targeted the dopamine D2 receptor (63). In addition, antibodies (IgG) were also present in serum against the human D1 receptor, and further studies suggested that the ratio of the anti-D1R/D2R antibodies correlated with symptoms (69). The studies also showed that anti-D1 receptor and anti-D2 receptor antibodies (IgG) were significantly elevated in serum from Sydenham chorea as well as PANDAS as described by Cox et al (63).
Figure 3.
Human Sydenham chorea 24.3.1 V gene expressed as human V gene-mouse IgG1a constant region in Transgenic (Tg) mice targets dopaminergic neurons. Chimeric Tg24.3.1 VH IgG1a Ab expressed in Tg mouse sera penetrated dopaminergic neurons in Tg mouse brain in vivo. Colocalization of Tg 24.3.1 IgG1a (anti-IgG1a Ab, green Left Panel) and Tyrosine Hydroxylase Antibody (anti-TH Ab, yellow Middle Panel). TH is a marker for dopaminergic neurons. Left panel shows IgG1a (FITC labeled), center panel shows TH Ab (TRITC labeled), and right panel is merged image (FITC-TRITC). Brain sections (basal ganglia) of VH24.3.1 Tg mouse (original magnification 320), showing FITC labeled anti-mouse IgG1a (A), TRITC-labeled anti-TH Ab (B), and merged image (C). Controls treated with secondary antibody are negative. Figure 3 is similar to figure shown in Cox et al (63).
PANDAS shares similar antibodies against the dopamine receptors as does Sydenham chorea (63). The symptoms of PANDAS as originally reported appear as small choreiform pianoplaying movements of the fingers and toes which were reported in the first 50 cases by Swedo, et al (61). PANDAS is characterized by tics and obsessive compulsive disorder (OCD) which in addition to the fine choreiform movements are not as obvious as those seen in Sydenham chorea (102, 103). The fine choreiform movements which may go unnoticed in PANDAS may lead the child to have poor handwriting skills associated with learning and behavioral regression, enuresis, separation anxiety and night-time fears and anorexia may appear in approximately 17 percent of cases (61). The appearance of PANDAS in a child is very disruptive because the onset is very sudden such as overnight behavioral changes leading to symptomatology.
For years the focus of Sydenham chorea was primarily on the chorea and involuntary movements with little attention given to the neuropsychiatric obsessive compulsive type symptoms which predate the chorea characterizing the neurologic manifestation of acute rheumatic fever (69). PANDAS may be seen in other types of infections and is then termed pediatric acute onset neuropsychiatric syndrome or PANS (64). There have been many questions about PANDAS/PANS which are in the process of being answered in current research investigations. Clearly, the confusion about the disease is not about the true PANDAS group which has many similarities with Sydenham chorea including the previous group A streptococcal infection. PANS or also more chronic types of tics and OCD are not associated with streptococcal infections but may be associated with Mycoplasma infections or Lyme disease and would be acute onset and termed PANS. More chronic tics and OCD may not display the small piano playing choreiform movements of the fingers and toes and are not similar to Sydenham chorea in their anti-neuronal antibody patterns of antibodies against the dopamine D2 receptor (Singer and Cunningham, unpublished data to be submitted). More chronic forms of tics and OCD do not have the IgG antibodies against the D2 receptor (Singer and Cunningham, unpublished data to be submitted). The PANDAS cases that have the small pianoplaying choreiform movements of the fingers and toes share the antibodies against both D1 and D2 receptors and have elevated antibodies against tubulin and lysoganglioside as well (63, 68, 69, 99).
Animal models of movement and obsessive symptoms have been investigated over the past 10 years with a mouse model and Lewis rat model both showing positive evidence that symptoms are associated with streptococcal antibodies. Immunization of a mouse model (104) with streptococcal components in Freund’s adjuvant led to behavioral alterations and compulsions, and a subset of the mice were found to have antibody deposits in several brain regions, including deep cerebellar nuclei (DCN), globus pallidus, and thalamus (104). Group A streptococcal immunized mice having increased deposits of IgG in the deep cerebellar nuclei exhibited increased rearing behavior compared with controls. These data suggested that immune responses against GABHS were associated with motoric and behavioral disturbances and suggested that anti-GABHS antibodies cross-reactive with brain components may lead to the symptomatology (104). Passive transfer of anti-streptococcal antibodies from the immunized mice into naïve mice led to autoantibody deposits in the brain as well as behavior changes (105)
Another animal model of Sydenham chorea was created in the Lewis rat (68) and demonstrated that exposure to group A streptococcal antigens during immunization leads to behaviors characteristic of Sydenham chorea and PANDAS. Rats following at least two immunizations were not able to hold a food pellet as well as control rats and also could not traverse a narrow beam as well as control rats (68). In addition, the rats demonstrated compulsive grooming behavior. Antibody IgG deposits were observed in the Lewis rat striatum, thalamus, and frontal cortex, and concomitant alterations in dopamine and glutamate levels in cortex and basal ganglia were observed, which are consistent with pathophysiology of Sydenham chorea and its related neuropsychiatric disorder. In the rat model, serum taken from group A streptococcal immunized rats activated CaMKII in SKNSH neuronal cells (68) similar to that observed for sera from acute Sydenham chorea (36). Expression of the Sydenham chorea mAb V genes in Tg mice demonstrate that the antibody in Sydenham chorea most likely targets the dopamine receptors on dopaminergic neurons since the antibody was observed in the cytoplasm of dopaminergic neurons in the basal ganglia (63) and was found to signal the dopamine D2 receptor as well as associate with the flag tagged D2 receptor on transfected cells (63).
To summarize, antineuronal antibodies which have been found in Sydenham chorea and in PANDAS with fine pianoplaying choreiform movements include anti-lysoganglioside (66), anti-tubulin (99), anti-dopamine D2 receptor (63, 68, 69), and anti-dopamine D1 receptor (69) antibodies. In Sydenham chorea, the ratio of the anti-dopamine D2 receptor/anti-dopamine D1 receptor antibodies correlated with the UFMG-Sydenham’s-Chorea-Rating-Scale (USCRS) clinical rating scale of neuropsychiatric symptoms (69). Most importantly, these antibodies in both Sydenham chorea and PANDAS signaled the SKNSH human neuronal cell line which is detected by antibody activation of calcium calmodulin protein kinase II (CaMKII) (36, 66) and may lead to excess dopamine release (100).
A model diagram has been shown in a recent review (17)
Mechanisms and effects of antineuronal antibodies on the brain include alterations in dopamine transmission including the release of excess dopamine from neuronal cells. Excess dopamine was released from the SKNSH cell line when treated with a human mAb from Sydenham chorea (100) and human mAb from PANDAS was found to cause alterations in the sensitivity of the receptors to dopamine (Zuccolo, et al, manuscript in preparation). Evidence in animal models and humans strongly suggest that antibodies mediate inflammatory consequences in Sydenham chorea, PANDAS and PANS (101). There may be other brain antigens targeted by autoantibodies in PANDAS/PANS and related autoimmune diseases that may affect memory and behavior (104–108).
Finally, molecular mimicry between the group A streptococcus and heart and brain is supported by evidence from studies of human mAbs and serum IgG antibodies from rheumatic fever (35, 36). The investigation of human mAbs from rheumatic carditis and Sydenham chorea has supported the hypothesis that antibodies against group A streptococcal carbohydrate epitope GlcNAc recognize crossreactive structures on the heart valve and on neuronal cells in the brain which may lead to the initiation of carditis and rheumatic heart disease and Sydenham chorea, respectively. T cells present in the rheumatic valve recognize cardiac myosin and streptococcal M protein epitopes and enter the valve through activated endothelium leading to a Th1 response in the valve. In the brain, antibody-mediated neuronal cell signaling of neuronal cells may be a mechanism of antibody pathogenesis in Sydenham chorea. The emerging theme in mimicry suggests that crossreactive autoantibodies target intracellular antigens but for disease pathogenesis, the antibodies must target the surface of neuronal cells or valve endothelial cells by signaling pathways in the neurons or by inflammatory effects on the endothelium of the valve. These mechanisms of molecular mimicry lead to the effects seen in acute rheumatic fever and related autoimmune sequelae associated with group A streptococcal infections.
Acknowledgments
Research supported by grants HL35280 and HL56267 from the National Heart Lung and Blood Institute and the National Institute of Mental Health Bench to Bedside grant and from the Oklahoma Center for the Advancement of Science and Technology.
Footnotes
Declaration of financial interest: MWC is chief scientific officer of Moleculera Labs, a company offering diagnostic testing for children with autoimmune movement and neuropsychiatric disorders.
References
- 1.Stollerman GH. Rheumatic and heritable connective tissue diseases of the cardiovascular system. In: Braunmald E, editor. Heart disease: a textbook of cardiovascular medicine. Philadelphia: W.B. Saunders; 1988. pp. 1706–34. [Google Scholar]
- 2.Stollerman GH. Rheumatic Fever. Lancet. 1997;349:935–42. doi: 10.1016/S0140-6736(96)06364-7. [DOI] [PubMed] [Google Scholar]
- 3.Wannamaker LW. The chain that links the throat to the heart. Circulation. 1973;48:9–18. doi: 10.1161/01.cir.48.1.9. [DOI] [PubMed] [Google Scholar]
- 4.Jones TD. The diagnosis of rheumatic fever. JAMA. 1944;126:481–4. [Google Scholar]
- 5.Dajani AS. Guidelines for the diagnosis of rheumatic fever (Jones criteria, 1992 update) JAMA. 1992;268:2069–73. [PubMed] [Google Scholar]
- 6.Dajani AS, Bisno AL, Chung KJ, Durack DT, Gerber MA, Kaplan EL, et al. Prevention of rheumatic fever: A statement for health professionals by the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, the American Heart Association. Pediatric Infectious Diseases. 1989;8:263–6. [PubMed] [Google Scholar]
- 7.Ayoub EM. Acute rheumatic fever. In: Adams FH, Emmanouilides GC, Riemenschneider TA, editors. Moss’ heart disease in infants, children and adolescents. 4. Baltimore: Williams and Wilkins; 1989. pp. 692–704. [Google Scholar]
- 8.Ayoub EM. Resurgence of rheumatic fever in the United States. Post Grad Med. 1992;92(3):133–42. doi: 10.1080/00325481.1992.11701445. [DOI] [PubMed] [Google Scholar]
- 9.Ayoub EM, Barrett DJ, Maclaren NK, Krischer JP. Association of class II human histocompatibility leukocyte antigens with rheumatic fever. J Clin Invest. 1986;77:2019–26. doi: 10.1172/JCI112531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber MA, Baltimore RS, Eaton CB, Gewitz M, Rowley AH, Shulman ST, et al. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis. A scientific statement from the American Heart Association. Rheumatic fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation. 2009 Feb; doi: 10.1161/CIRCULATIONAHA.109.191959. In Press. [DOI] [PubMed] [Google Scholar]
- 11.Wannamaker LW. Differences between streptococcal infections of the throat and of the skin. N Engl J Med. 1970;282:23–31. doi: 10.1056/NEJM197001012820106. [DOI] [PubMed] [Google Scholar]
- 12.Rammelkamp CH, Denny FW, Wannamaker LW. Studies on the epidemiology of rheumatic fever in the armed services. In: Thomas L, editor. Rheumatic Fever. Minneapolis: Univ. Minnesota Press; 1952. pp. 72–89. [Google Scholar]
- 13.Bisno AL. Non-Suppurative Poststreptococcal Sequelae: Rheumatic Fever and Glomerulonephritis. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. New York: Churchill Livingstone; 1995. pp. 1799–810. [Google Scholar]
- 14.Carapetis J, Steer A. Prevention of rheumatic fever. Pediatr Infect Dis J. 2009 Jan;29(1):91–2. doi: 10.1097/INF.0b013e3181bf53f3. author reply 2. [DOI] [PubMed] [Google Scholar]
- 15.Steer AC, Carapetis JR, Nolan TM, Shann F. Systematic review of rheumatic heart disease prevalence in children in developing countries: the role of environmental factors. J Paediatr Child Health. 2002 Jun;38(3):229–34. doi: 10.1046/j.1440-1754.2002.00772.x. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham MW. Pathogenesis of Group A Streptococcal Infections. Clinical Microbiology Reviews. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham MW. Streptococcus and rheumatic fever. Curr Opin Rheumatol. 2012 Jul;24(4):408–16. doi: 10.1097/BOR.0b013e32835461d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisno AL, Brito MO, Collins CM. Molecular basis of group A streptococcal virulence. Lancet Infect Dis. 2003 Apr;3(4):191–200. doi: 10.1016/s1473-3099(03)00576-0. [DOI] [PubMed] [Google Scholar]
- 19.Bisno AL. Acute pharyngitis. N Engl J Med. 2001 Jan 18;344(3):205–11. doi: 10.1056/NEJM200101183440308. [DOI] [PubMed] [Google Scholar]
- 20.Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–5. doi: 10.1056/NEJM199601253340407. [DOI] [PubMed] [Google Scholar]
- 21.Bisno AL, Pearce IA, Wall HP, Stollerman GS. Contrasting epidemiology of acute rheumatic fever and acute glomerulonephritis: nature of the antecedent streptococcal infection. N Engl J Med. 1970;283:561–5. doi: 10.1056/NEJM197009102831103. [DOI] [PubMed] [Google Scholar]
- 22.McDonald M, Currie BJ, Carapetis JR. Acute rheumatic fever: a chink in the chain that links the heart to the throat? Lancet Infect Dis. 2004 Apr;4(4):240–5. doi: 10.1016/S1473-3099(04)00975-2. [DOI] [PubMed] [Google Scholar]
- 23.Carapetis J, Robins-Browne R, Martin D, Shelby-James T, Hogg G. Increasing severity of invasive group A streptococcal disease in Australia: clinical and molecular epidemiological features and identification of a new virulent M-nontypeable clone. Clinical Infectious Diseases. 1995;21:1220–7. doi: 10.1093/clinids/21.5.1220. [DOI] [PubMed] [Google Scholar]
- 24.Carapetis JR, Currie BJ, Good MF. Towards understanding the pathogenesis of rheumatic fever [editorial] Scand J Rheumatol. 1996;25(3):127–31. doi: 10.3109/03009749609080000. [DOI] [PubMed] [Google Scholar]
- 25.Carapetis JR, Kilburn CJ, MacDonald KT, Walker AR, Currie BJ. Ten-year follow up of a cohort with rheumatic heart disease (RHD) Aust N Z J Med. 1997 Dec;27(6):691–7. doi: 10.1111/j.1445-5994.1997.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan MH, Johnson DR, Cleary PP. Group A streptococcal serotypes isolated from patients and sibling contacts during the resurgence of rheumatic fever in the United states in the mid-1980’s. J Infect Dis. 1989;159:101–3. doi: 10.1093/infdis/159.1.101. [DOI] [PubMed] [Google Scholar]
- 27.Veasy LG, Tani LY, Hill HR. Persistence of acute rheumatic fever in the intermountain area of the United States. J Pediatrics. 1994;124:9–16. doi: 10.1016/s0022-3476(94)70247-0. [DOI] [PubMed] [Google Scholar]
- 28.Veasy LG, Wiedmeier SE, Orsmond GS. Resurgence of acute rheumatic fever in the intermountain area of the United States. N Engl J Med. 1987;316(8):421–7. doi: 10.1056/NEJM198702193160801. [DOI] [PubMed] [Google Scholar]
- 29.Veasy LG, Tani LY, Daly JA, Korgenski K, Miner L, Bale J, et al. Temporal association of the appearance of mucoid strains of Streptococcus pyogenes with a continuing high incidence of rheumatic fever in Utah. Pediatrics. 2004 Mar;113(3 Pt 1):e168–72. doi: 10.1542/peds.113.3.e168. [DOI] [PubMed] [Google Scholar]
- 30.Dudding BA, Ayoub EM. Persistence of streptococcal group A antibody in patients with rheumatic valvular disease. J Exp Med. 1968;128:1081. doi: 10.1084/jem.128.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan MH, Bolande R, Rakita L, Blair J. Presence of bound immunoglobulins and complement in the myocardium in acute rheumatic fever. Association with cardiac failure. N Engl J Med. 1964;271:637–45. doi: 10.1056/NEJM196409242711301. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan MH, Frengley JD. Autoimmunity to the heart in cardiac disease: current concepts of the relation of autoimmunity to rheumatic fever, postcardiotomy and post infarction syndromes and cardiomyopathies. Am J Cardiol. 1969;24:459–73. doi: 10.1016/0002-9149(69)90489-5. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan MH, Dallenbach FD. Immunologic studies of heart tissue. II. Occurrence of bound gamma-globulin in auricular appendages from rheumatic hearts. Relationship to certain histopathologic features of rheumatic heart disease. J Exp Med. 1961;113:1–16. doi: 10.1084/jem.113.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husby G, van de Rijn I, Zabriskie JB, Abdin ZH, Williams RC. Antibodies reacting with cytoplasm of subthalamic and caudate nuclei neurons in chorea and acute rheumatic fever. J Exp Med. 1976;144:1094–110. doi: 10.1084/jem.144.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galvin JE, Hemric ME, Ward K, Cunningham MW. Cytotoxic monoclonal antibody from rheumatic carditis reacts with human endothelium: implications in rheumatic heart disease. J Clin Invest. 2000;106:217–24. doi: 10.1172/JCI7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003 Jul;9(7):914–20. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- 37.Krisher K, Cunningham MW. Myosin: a link between streptococci and heart. Science. 1985;227(4685):413–5. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- 38.Ellis NMJ, Kurahara D, Vohra H, Mascaro-Blanco A, Erdem G, Adderson EE, et al. Priming the immune system for heart disease: a perspective on group A streptococci. J Infect Dis. 2010;202:1059. doi: 10.1086/656214. [DOI] [PubMed] [Google Scholar]
- 39.Ellis NMJ, Li Y, Hildebrand W, Fischetti VA, Cunningham MW. T cell mimicry and epitope specificity of crossreactive T cell clones from rheumatic heart disease. J Immunol. 2005;175:5448–56. doi: 10.4049/jimmunol.175.8.5448. [DOI] [PubMed] [Google Scholar]
- 40.Zabriskie JB. Mimetic relationships between group A streptococci and mammalian tissues. Adv Immunol. 1967;7:147–88. doi: 10.1016/s0065-2776(08)60128-5. [DOI] [PubMed] [Google Scholar]
- 41.Zabriskie JB, Freimer EH. An immunological relationship between the group A streptococcus and mammalian muscle. J Exp Med. 1966;124:661–78. doi: 10.1084/jem.124.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham MW. Autoimmunity and molecular mimicry in the pathogenesis of post-streptococcal heart disease. Front Biosci. 2003 May 1;8:s533–43. doi: 10.2741/1067. [DOI] [PubMed] [Google Scholar]
- 43.Cunningham MW. Rheumatic fever revisited. Nature Reviews Cardiology. 2013 doi: 10.1038/nrcardio.2012.197-c1. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunningham MW, Antone SM, Gulizia JM, McManus BM, Fischetti VA, Gauntt CJ. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci USA. 1992;89:1320–4. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tandon R, Sharma M, Chandrashekhar Y, Kotb M, Yacoub MH, Narula J. Revisiting the pathogenesis of rheumatic fever and carditis. Nature Reviews Cardiology. 2013;10:171–7. doi: 10.1038/nrcardio.2012.197. [DOI] [PubMed] [Google Scholar]
- 46.Zabriskie JB. Rheumatic fever: the interplay between host, genetics and microbe. Circulation. 1985;71:1077–86. doi: 10.1161/01.cir.71.6.1077. [DOI] [PubMed] [Google Scholar]
- 47.Veasy LG. Rheumatic fever. Lancet Infect Dis. 2004 Nov;4(11):661. doi: 10.1016/S1473-3099(04)01180-6. [DOI] [PubMed] [Google Scholar]
- 48.Veasy LG. Rheumatic fever-T. Duckett Jones and the rest of the story. Cardiology in the Young. 1995;5:293–301. [Google Scholar]
- 49.Veasy LG. Time to take soundings in acute rheumatic fever. Lancet. 2001 Jun 23;357(9273):1994–5. doi: 10.1016/S0140-6736(00)05134-5. [DOI] [PubMed] [Google Scholar]
- 50.Zabriskie JB, Hsu KC, Seegal BC. Heart-reactive antibody associated with rheumatic fever: characterization and diagnostic significance. Clin Exp Immunol. 1970;7:147–59. [PMC free article] [PubMed] [Google Scholar]
- 51.Narula J, Kaplan EL. Echocardiographic diagnosis of rheumatic fever. Lancet. 2001 Dec 8;358(9297):2000. doi: 10.1016/S0140-6736(01)06993-8. [DOI] [PubMed] [Google Scholar]
- 52.Reddy KS, Narula J, Bhatia R, Shailendri K, Koicha M, Taneja V, et al. Immunologic and immunogenetic studies in rheumatic fever and rheumatic heart disease. Indian J Pediatr. 1990 Sep-Oct;57(5):693–700. doi: 10.1007/BF02728716. [DOI] [PubMed] [Google Scholar]
- 53.Kirvan CA, Swedo SE, Heuser S, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nature Medicine. 2003;9:914–20. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- 54.Snider LA, Swedo SE. PANDAS: current status and directions for research. Mol Psychiatry. 2004 Oct;9(10):900–7. doi: 10.1038/sj.mp.4001542. [DOI] [PubMed] [Google Scholar]
- 55.Swedo SE. Sydenham’s chorea: A model for childhood autoimmune neuropsychiatric disorders. JAMA. 1994;272:1788–91. doi: 10.1001/jama.272.22.1788. [DOI] [PubMed] [Google Scholar]
- 56.Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264–71. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- 57.Swedo SE, Leonard HL, Mittleman BB, Allen AJ, Rapoport JL, Dow SP, et al. Identification of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections by a marker associated with rheumatic fever. Amer J Psych. 1997;154:110–2. doi: 10.1176/ajp.154.1.110. [DOI] [PubMed] [Google Scholar]
- 58.Murphy TK, Snider LA, Mutch PJ, Harden E, Zaytoun A, Edge PJ, et al. Relationship of movements and behaviors to Group A Streptococcus infections in elementary school children. Biol Psychiatry. 2007 Feb 1;61(3):279–84. doi: 10.1016/j.biopsych.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 59.Murphy TK, Storch EA, Lewin AB, Edge PJ, Goodman WK. Clinical factors associated with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Pediatr. 2011 Feb;160(2):314–9. doi: 10.1016/j.jpeds.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swedo SE. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) Mol Psychiatry. 2002;7( Suppl 2):S24–5. doi: 10.1038/sj.mp.4001170. [DOI] [PubMed] [Google Scholar]
- 61.Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998 Feb;155(2):264–71. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- 62.Singer HS, Gause C, Morris C, Lopez P. Serial immune markers do not correlate with clinical exacerbations in pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Pediatrics. 2008 Jun;121(6):1198–205. doi: 10.1542/peds.2007-2658. [DOI] [PubMed] [Google Scholar]
- 63.Cox CJ, Sharma M, Leckman JF, Zuccolo J, Zuccolo A, Kovoor A, et al. Brain human monoclonal autoantibody from sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine d2 receptor: implications in human disease. J Immunol. 2013 Dec 1;191(11):5524–41. doi: 10.4049/jimmunol.1102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome) Pediatrics and Therapeutics. 2012;2:113. http://dx.doi.org/10.4172/2161-0665.1000113. [Google Scholar]
- 65.Singer HS, Gilbert DL, Wolf DS, Mink JW, Kurlan R. Moving from PANDAS to CANS. J Pediatr. 2011 doi: 10.1016/j.jpeds.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 66.Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006 Oct;179(1–2):173–9. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 67.Kirvan CA, Cox CJ, Swedo SE, Cunningham MW. Tubulin is a neuronal target of autoantibodies in Sydenham’s chorea. J Immunol. 2007;178:7412–21. doi: 10.4049/jimmunol.178.11.7412. [DOI] [PubMed] [Google Scholar]
- 68.Brimberg L, Benhar I, Mascaro-Blanco A, Alvarez K, Lotan D, Winter C, et al. Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology. 2012 Aug;37(9):2076–87. doi: 10.1038/npp.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ben-Pazi H, Stoner JA, Cunningham MW. Dopamine Receptor Autoantibodies Correlate with Symptoms in Sydenham’s Chorea. PLoS One. 2013;8(9):e73516. doi: 10.1371/journal.pone.0073516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaplan MH. Immunologic relation of streptococcal and tissue antigens. I. Properties of an antigen in certain strains of group A streptococci exhibiting an immunologic cross reaction with human heart tissue. J Immunol. 1963;90:595–606. [PubMed] [Google Scholar]
- 71.Kaplan MH, Bolande R, Ratika L, Blair J. Presence of bound immunoglobulins and complement in the myocardium in acute rheumatic fever. The New England Journal of Medicine. 1964;271:637. doi: 10.1056/NEJM196409242711301. [DOI] [PubMed] [Google Scholar]
- 72.Goldstein I, Halpern B, Robert L. Immunological relationship between streptococcus A polysaccharide and the structural glycoproteins of heart valve. Nature. 1967;213:44–7. [Google Scholar]
- 73.Adderson EE, Shikhman AR, Ward KE, Cunningham MW. Molecular analysis of polyreactive monoclonal antibodies from rheumatic carditis: human anti-N-acetyl-glucosamine/anti-myosin antibody V region genes. J Immunol. 1998;161:2020–31. [PubMed] [Google Scholar]
- 74.Roberts S, Kosanke S, Dunn ST, Jankelow D, Duran CMG, Cunningham MW. Immune mechanisms in rheumatic carditis: Focus on valvular endothelium. J Infect Dis. 2001;183:507–11. doi: 10.1086/318076. [DOI] [PubMed] [Google Scholar]
- 75.Shikhman AR, Greenspan NS, Cunningham MW. Cytokeratin peptide SFGSGFGGGY mimics N-acetyl-beta-D-glucosamine in reaction with antibodies and lectins, and induces in vivo anti-carbohydrate antibody response. J Immunol. 1994;153(12):5593–606. [PubMed] [Google Scholar]
- 76.Shikhman AR, Cunningham MW. Immunological mimicry between N-acetyl-beta-D-glucosamine and cytokeratin peptides. Evidence for a microbially driven anti-keratin antibody response. J Immunol. 1994;152(9):4375–87. [PubMed] [Google Scholar]
- 77.McNamara C, Zinkernagel AS, Macheboeuf P, Cunningham MW, Nizet V, Ghosh P. Coiled-coil irregularities and instabilities in group A streptococcus M1 are required for virulence. Science. 2008;319:1405–8. doi: 10.1126/science.1154470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martins TB, Hoffman JL, Augustine NH, Phansalkar AR, Fischetti VA, Zabriskie JB, et al. Comprehensive analysis of antibody responses to streptococcal and tissue antigens in patients with acute rheumatic fever. Int Immunol. 2008 Mar;20(3):445–52. doi: 10.1093/intimm/dxn004. [DOI] [PubMed] [Google Scholar]
- 79.Dinkla K, Rohde M, Jansen WT, Carapetis JR, Chhatwal GS, Talay SR. Streptococcus pyogenes recruits collagen via surface-bound fibronectin: a novel colonization and immune evasion mechanism. Mol Microbiol. 2003 Feb;47(3):861–9. doi: 10.1046/j.1365-2958.2003.03352.x. [DOI] [PubMed] [Google Scholar]
- 80.Dinkla K, Rohde M, Jansen WT, Kaplan EL, Chhatwal GS, Talay SR. Rheumatic fever-associated Streptococcus pyogenes isolates aggregate collagen. J Clin Invest. 2003 Jun;111(12):1905–12. doi: 10.1172/JCI17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lukomski S, Nakashima K, Abdi I, Cipriano VJ, Ireland RM, Reid SD, et al. Identification and characterization of the scl gene encoding a group A Streptococcus extracellular protein virulence factor with similarity to human collagen. Infect Immun. 2000 Dec;68(12):6542–53. doi: 10.1128/iai.68.12.6542-6553.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lukomski S, Nakashima K, Abdi I, Cipriano VJ, Shelvin BJ, Graviss EA, et al. Identification and characterization of a second extracellular collagen-like protein made by group A Streptococcus: control of production at the level of translation. Infect Immun. 2001 Mar;69(3):1729–38. doi: 10.1128/IAI.69.3.1729-1738.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guilherme L, Cury P, Demarchi LMF, Abel L, Lopez AP, Oshiro SE, et al. Rheumatic heart disease: pro-inflammatory cytokines play a role in the progression and maintenance of valvular lesions. Am J Pathol. 2004;165:1583–91. doi: 10.1016/S0002-9440(10)63415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lymbury RS, Olive C, Powell KA, Good MF, Hirst RG, LaBrooy JT, et al. Induction of autoimmune valvulitis in Lewis rats following immunization with peptides from the conserved region of group A streptococcal M protein. J Autoimmun. 2003 May;20(3):211–7. doi: 10.1016/s0896-8411(03)00026-x. [DOI] [PubMed] [Google Scholar]
- 85.Quinn A, Kosanke S, Fischetti VA, Factor SM, Cunningham MW. Induction of autoimmune valvular heart disease by recombinant streptococcal M protein. Infect Immun. 2001;69(6):4072–8. doi: 10.1128/IAI.69.6.4072-4078.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kirvan CA, Galvin JE, Hilt S, Kosanke S, Cunningham MW. Identification of Streptococcal M-Protein Cardiopathogenic Epitopes in Experimental Autoimmune Valvulitis. Journal of Cardiovascular Translational Research. 2013 doi: 10.1007/s12265-013-9526-4. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fae K, Kalil J, Toubert A, Guilherme L. Heart infiltrating T cell clones from a rheumatic heart disease patient display a common TCR usage and a degenerate antigen recognition pattern. Mol Immunol. 2004 Feb;40(14–15):1129–35. doi: 10.1016/j.molimm.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 88.Fae KC, da Silva DD, Oshiro SE, Tanaka AC, Pomerantzeff PM, Douay C, et al. Mimicry in recognition of cardiac myosin peptides by heart-intralesional T cell clones from rheumatic heart disease. J Immunol. 2006 May 1;176(9):5662–70. doi: 10.4049/jimmunol.176.9.5662. [DOI] [PubMed] [Google Scholar]
- 89.Guilherme L, Weidenbach W, Kiss MH, Snitcowdky R, Kalil J. Association of human leukocyte class II antigens with rheumatic fever or rheumatic heart disease in a Brazilian population. Circulation. 1991;83:1995–8. doi: 10.1161/01.cir.83.6.1995. [DOI] [PubMed] [Google Scholar]
- 90.Guilherme L, Dulphy N, Douay C, Coelho V, Cunha-Neto E, Oshiro SE, et al. Molecular evidence for antigen-driven immune responses in cardiac lesions of rheumatic heart disease patients. Int Immunol. 2000 Jul;12(7):1063–74. doi: 10.1093/intimm/12.7.1063. [DOI] [PubMed] [Google Scholar]
- 91.Guilherme L, Oshiro SE, Fae KC, Cunha-Neto E, Renesto G, Goldberg AC, et al. T-cell reactivity against streptococcal antigens in the periphery mirrors reactivity of heart-infiltrating T lymphocytes in rheumatic heart disease patients. Infect Immun. 2001 Sep;69(9):5345–51. doi: 10.1128/IAI.69.9.5345-5351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gorton D, Blyth S, Gorton JG, Govan B, Ketheesan N. An alternative technique for the induction of autoimmune valvulitis in a rat model of rheumatic heart disease. J Immunol Methods. 2010 Apr 15;355(1–2):80–5. doi: 10.1016/j.jim.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 93.Gorton D, Govan B, Olive C, Ketheesan N. B- and T-cell responses in group a streptococcus M-protein- or Peptide-induced experimental carditis. Infect Immun. 2009 May;77(5):2177–83. doi: 10.1128/IAI.01514-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Veasy LG, Tani LY. A new look at acute rheumatic mitral regurgitation. Cardiology in the Young. 2005 Dec;15(6):568–77. doi: 10.1017/S104795110500171X. [DOI] [PubMed] [Google Scholar]
- 95.Galvin JE, Hemric ME, Kosanke SD, Factor SM, Quinn A, Cunningham MW. Induction of myocarditis and valvulitis in Lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditis. Amer J Pathol. 2001;160:297–306. doi: 10.1016/S0002-9440(10)64373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gulizia JM, Cunningham MW, McManus BM. Anti-streptococcal monoclonal antibodies recognize multiple epitopes in human heart valves: cardiac myosin, vimentin, and elastin as potential valvular autoantigens. In: Gustav, Fisher, Verlag, editors. New perspectives on streptococci and streptococcal infections Proceedings of the XI Lancefield International Symposium; New York: Zbl Bakt Suppl; 1992. pp. 267–9. [Google Scholar]
- 97.Gorton DE, Govan BL, Ketheesan N, Sive AA, Norton RE, Currie BJ, et al. Cardiac myosin epitopes for monitoring progression of rheumatic fever. Pediatr Infect Dis J. 2011 Nov;30(11):1015–6. doi: 10.1097/INF.0b013e31823058dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taranta A, Stollerman GH. The relationship of Sydenham’s chorea to infection with group A streptococci. Am J Med. 1956 Feb;20(2):170–5. doi: 10.1016/0002-9343(56)90186-3. [DOI] [PubMed] [Google Scholar]
- 99.Kirvan CA, Cox CJ, Swedo SE, Cunningham MW. Tubulin is a neuronal target of autoantibodies in Sydenham’s chorea. J Immunol. 2007 Jun 1;178(11):7412–21. doi: 10.4049/jimmunol.178.11.7412. [DOI] [PubMed] [Google Scholar]
- 100.Kirvan CA, Swedo SE, Kurahara D, Cunningham MW. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham’s chorea. Autoimmunity. 2006 Feb;39(1):21–9. doi: 10.1080/08916930500484757. [DOI] [PubMed] [Google Scholar]
- 101.Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet. 1999 Oct 2;354(9185):1153–8. doi: 10.1016/S0140-6736(98)12297-3. [DOI] [PubMed] [Google Scholar]
- 102.Garvey MA, Snider LA, Leitman SF, Werden R, Swedo SE. Treatment of Sydenham’s chorea with intravenous immunoglobulin, plasma exchange, or prednisone. J Child Neurol. 2005 May;20(5):424–9. doi: 10.1177/08830738050200050601. [DOI] [PubMed] [Google Scholar]
- 103.Garvey MA, Swedo SE. Sydenham’s chorea. Clinical and therapeutic update. Adv Exp Med Biol. 1997;418:115–20. doi: 10.1007/978-1-4899-1825-3_28. [DOI] [PubMed] [Google Scholar]
- 104.Hoffman KL, Hornig M, Yaddanapudi K, Jabado O, Lipkin WI. A murine model for neuropsychiatric disorders associated with group A beta-hemolytic streptococcal infection. J Neurosci. 2004 Feb 18;24(7):1780–91. doi: 10.1523/JNEUROSCI.0887-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yaddanapudi K, Hornig M, Serge R, De Miranda J, Baghban A, Villar G, et al. Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol Psychiatry. Jul;15(7):712–26. doi: 10.1038/mp.2009.77. [DOI] [PubMed] [Google Scholar]
- 106.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc Nat Acad Sci (USA) 2006;103:678–83. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kowal C, DiGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, et al. Cognition and immunity: antibody impairs memory. Immunity. 2004;21:179–88. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 108.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nature Medicine. 2001;7:1189–93. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]