Abstract
Apolipoprotein (apo) A-V is a protein synthesized only in the liver that dramatically modulates plasma triglyceride levels. Recent studies suggest a novel role for hepatic apoA-V in regulating the absorption of dietary triglycerides, but its mode of action on the gut remains unknown. The aim of this study was to test for apoA-V in bile and to determine whether its secretion is regulated by dietary lipids. After an overnight recovery, adult male Sprague-Dawley bile fistula rats indeed secreted apoA-V into bile at a constant rate under fasting conditions. An intraduodenal bolus of intralipid (n = 12) increased the biliary secretion of apoA-V but not of other apolipoproteins, such as A-I, A-IV, B, and E. The lipid-induced increase of biliary apoA-V was abolished under conditions of poor lymphatic lipid transport, suggesting that the stimulation is regulated by the magnitude of lipids associated with chylomicrons transported into lymph. We also studied the secretion of apoA-V into bile immediately following bile duct cannulation. Biliary apoA-V increased over time (∼6-fold increase at hour 16, n = 8) but the secretions of other apolipoproteins remained constant. Replenishing luminal phosphatidylcholine and taurocholate (n = 9) only enhanced apoA-V secretion in bile, suggesting that the increase was not due to depletion of phospholipids or bile salts. This is the first study to demonstrate that apoA-V is secreted into bile, introducing a potential route of delivery of hepatic apoA-V to the gut lumen. Our study also reveals the uniqueness of apoA-V secretion into bile that is regulated by mechanisms different from other apolipoproteins.
Keywords: bile fistula, fat absorption, negative feedback regulation, chylomicron, bile salts
secretion of bile is a unique function of the liver and serves many purposes: 1) bile salts enable the micellar solubilization and absorption of fat, 2) IgA in bile protects against infection, and 3) liver-derived metabolites, some toxic, are secreted into bile for excretion from the body. The composition of bile is complex, containing bile salts, lipids (namely phospholipids and cholesterol), and proteins (albumin, secretory component, IgA, apolipoproteins) and their secretions vary with the nutritional state of the individual (see reviews in Refs. 5, 38).
Generally known to be synthesized only by the liver (26, 39), apolipoprotein (apo) A-V profoundly modulates plasma triglyceride levels. Compared with wild-type mice, apoA-V knockout (KO) mice have drastically elevated plasma triglyceride levels while transgenic mice overexpressing apoA-V have profoundly reduced plasma triglyceride levels (26). In humans, apoA-V polymorphisms correlate with hypertriglyceridemia and cardiovascular disease (17, 20, 23, 26, 28). Intravenous injection of recombinant apoA-V protein can lower plasma triglyceride levels in hypertriglyceridemic apoA-V KO mice (33), demonstrating the therapeutic potential of apoA-V in hyperlipidemia.
Recent studies from our laboratory reveal a novel role for apoA-V in modulating the absorption of dietary lipids and subsequent production of chylomicrons by the gut. ApoA-V KO mice display a twofold increase in the transport rate of dietary lipids into the lymph, which was correlated with increased intestinal apoB secretion (43). Although previous studies have reported an effect of apoA-V on postprandial triglyceride metabolism (9, 21), no studies had examined the possibility that apoA-V may affect triglyceride handling by the gut, with the general assumption that apoA-V is not synthesized in the gut. Our previous study (43) was first to report a direct role of apoA-V in lipid absorption. However, an interesting question arises: how does hepatic apoA-V gain access to the small intestine? We hypothesize that the liver secretes apoA-V into bile to provide a luminal source of apoA-V that may regulate the formation and secretion of chylomicrons.
The aim of this study was to test for apoA-V secretion into bile and, if present, to study how its secretion into bile is regulated by dietary lipid absorption. We employed the bile fistula rat model because the rat lacks a gallbladder and thereby is a suitable animal model for measuring biliary secretion rates. Additionally, the bile fistula model allows for the study of bile secretion over a period of time rather than a single sample collected at one time point. In the present work, we show for the first time that apoA-V is indeed present in bile. Furthermore, we showed that its secretion increases in response to absorption of dietary lipid into lymph. We also observed that bile drainage stimulates the secretion of apoA-V into bile during the day of surgery, which occurs independently of the deprivation of luminal phospholipids and bile salts.
MATERIALS AND METHODS
Animal models.
Sprague-Dawley rats were purchased from Harlan Sprague Dawley (Indianapolis) and were allowed to acclimatize to the vivarium environment for at least 2 wk prior to the experiment. The rats were maintained on standard rodent chow (LM-485 Mouse/Rat Sterilizable Diet; Harlan Laboratories, Madison, WI) and were housed under conditions of controlled temperature, humidity and a normal 12:12-h light-dark cycle at the University of Cincinnati Laboratory Animal Medical Services. Only 3- to 4-mo-old males were used in the study. Prior to surgery, animals were fasted overnight with free access to water. The following morning, the animals were anesthetized with isoflurane. The surgical procedures are detailed below. After surgery, the animals were placed in Bollman restraining cages in an environment maintained at a temperature of 30°C to prevent hypothermia. Normally animals nestle together to keep warm but they cannot do so because of restriction of the Bollman cages. Despite restraints, the animals still had considerable freedom to move forward and backward and sideways. The animals were allowed to recover overnight from surgery while receiving a continuous intraduodenal infusion of 5% glucose-saline solution. All procedures were performed in accordance with the University of Cincinnati Internal Animal Care and Use Committee and in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Bile fistula surgery with duodenal cannulation.
The common bile duct, just below the two hepatic ducts, was cannulated with polyvinylchloride tubing tipped with polyethylene tubing [0.20-mm inner diameter (ID); 0.50-mm outer diameter (OD), BIOCORP Aust, Victoria, Australia] and exteriorized through a stab wound in the animal's right flank, as previously described (37). The fundus of the stomach was incised to introduce a silicone feeding tube (0.5 mm ID; 0.8 mm OD) through the stomach, ∼2 cm into the duodenum. The tube was secured by a purse-string suture and the incision was sealed with a drop of tissue glue. After surgery, 5% glucose-saline was infused intraduodenally at 1.5 ml/h to replace fluid and electrolyte lost by bile diversion. Bile was collected hourly by gravity drainage into microcentrifuge tubes on ice for various times as indicated in results. In some experiments the glucose-saline infusion was replaced by saline or by 6.6 mM phosphatidylcholine for the phospholipid (PL) and 10 mM sodium taurocholate (NaTC) in saline to replenish the loss of these important components of bile through interruption of the enterohepatic circulation by bile drainage. Taurocholate was used because it is the primary bile salt in the rat (2) and used by previous investigators studying the rat (6, 31).
Lymph fistula surgery with duodenal cannulation.
To measure the absorption and transport of intestinal lipids, we used the lymph fistula model in a separate cohort of animals. The mesenteric lymph duct was also cannulated with polyvinylchloride tubing (0.20 mm ID; 0.50 mm OD), with the slight modification of original procedures (4) by securing the cannula with a drop of tissue glue instead of suture. As described above, a silicon feeding tube was introduced through the stomach into the duodenum for saline or lipid infusions. After surgery, 5% glucose-saline was infused intraduodenally at a rate of 3.0 ml/h to compensate for fluid and electrolyte loss due to lymphatic drainage. After overnight recovery, the animals were given a 3-ml intraduodenal bolus of Liposyn III 20% (Hospira, Lake Forest, IL), which contained 20% soybean oil, 1.2% egg phosphatides, and 2.5% glycerin in water. To avoid a volume overload in the small intestine, we waited 30 min before giving a continuous intraduodenal infusion of saline or 6.6 mM PL and 10 mM NaTC, as indicated in results. Lymph was collected by gravity drainage in microcentrifuge tubes on ice for 6 h.
Measurement of apolipoproteins.
Apolipoproteins A-I, A-IV, A-V, B, and E were quantitated by Western blotting, and 2 μl of bile samples were loaded onto 4–15% polyacrylamide gradient gels and transferred to polyvinylidene difluoride membranes. Following transfer, membranes were blocked with 5% nonfat milk in 0.1% Tween 20 in Tris-buffered saline (TBS) for 1 h. Membranes were incubated with antibodies raised against apoA-I, A-IV, A-V, B, and E (each 1:5,000) overnight. After a rinsing with TBS with 0.1% Tween 20, membranes were incubated with peroxidase-conjugated anti-goat secondary antibody at 1:10,000 for 30 min and developed with Immobilon Western Chemiluminescent horseradish peroxidase substrate (Millipore, Billerica, MA). Protein quantification was performed by densitometric analysis of films using Total Lab Quant Analysis Software (TL100, FOTODYNE, Hartland, WI).
Measurement of triglyceride, cholesterol, phospholipids, and bile salts.
Triglyceride concentration was measured by Total Triglyceride assay kits (Randox Laboratories, Kearneysville, WV). Cholesterol was measured by chemical assay using Infinity Cholesterol Liquid Stable Reagent (Fisher Diagnostics, Thermo Scientific, Middletown, VA). Phospholipid was measured by chemical assay using Phospholipids C reagent (Wako Diagnostics, Mountain View, CA) after dilution of samples 1:5 in saline. Bile acids were measured by enzymatic assay using Rat Total Bile Acids Kit (Crystal Chem, Downers Grove, IL) after bile samples were diluted 1:100 in 70% ethanol, as recommended by the manufacturer for bile. All assays were performed according to the manufacturer instructions.
Statistical analysis.
The data shown are means values ± SE. Two-way repeated-measures ANOVAs with Sidak multiple-comparisons posttest analysis were used. Statistical analyses were performed with GraphPad Prism v6. Data were considered significant if P was less than 0.05.
RESULTS
ApoA-V is present in bile and is secreted at a constant rate under fasting conditions.
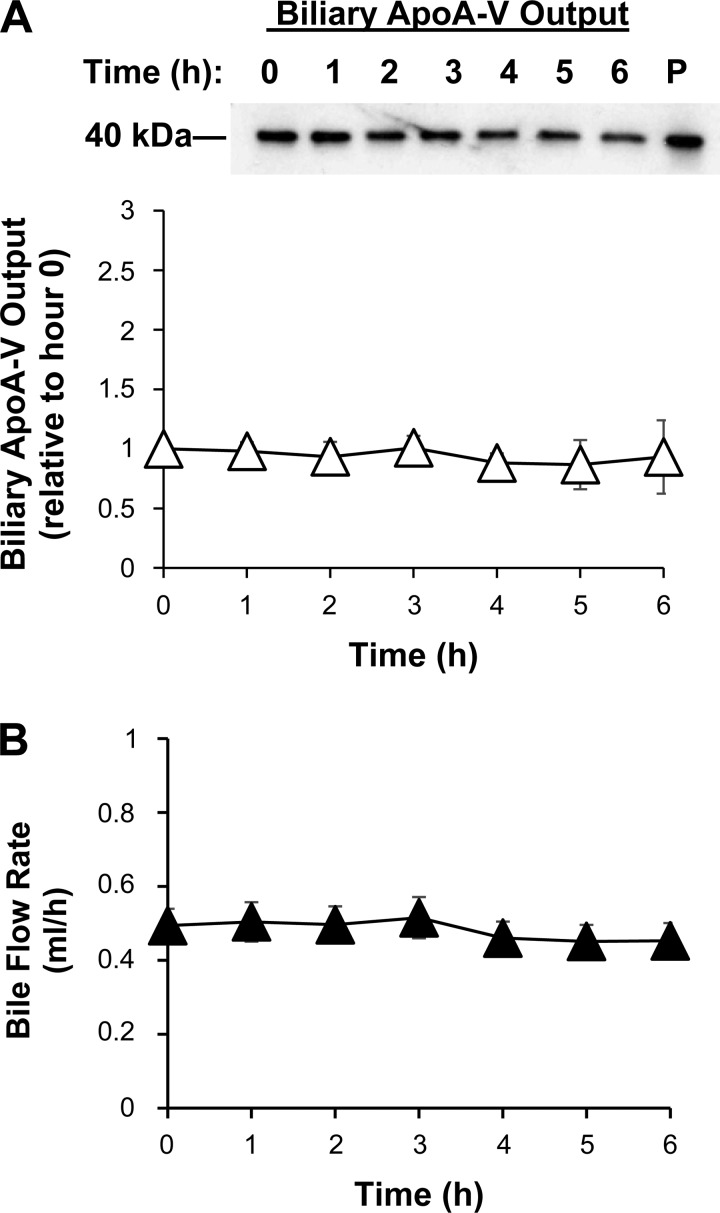
After a 24-h recovery from surgery, bile fistula rats received an intraduodenal infusion of saline at 1.5 ml/h while bile was collected hourly by gravity drainage. The presence of apoA-V in bile was quantitated by Western blot. ApoA-V was indeed present in bile and was secreted at a constant rate under fasting conditions (Fig. 1A). This is based on the fact that bile flow rate did not vary during the study (Fig. 1B). For the Western blot analysis, we used 2 μl of each hourly bile sample. Biliary secretion, or output, was determined by relative concentration × bile flow.
Fig. 1.
Under fasting conditions, Apolipoprotein (apo) A-V is present in bile and secreted at a constant rate. A: ApoA-V output into bile with a representative Western blot shown (20 second outputs of bile loaded into each well). P, diluted plasma (1:5) as a comparative marker for apoA-V. B: bile flow rate. After a 24-h recovery from surgery, bile fistula rats were given a continuous intraduodenal infusion of saline. ApoA-V was measured in bile by Western blot and quantified by densitometry. Output was determined by concentration × bile flow. Values are means ± SE.
ApoA-V secretion into bile increases after lipid absorption.
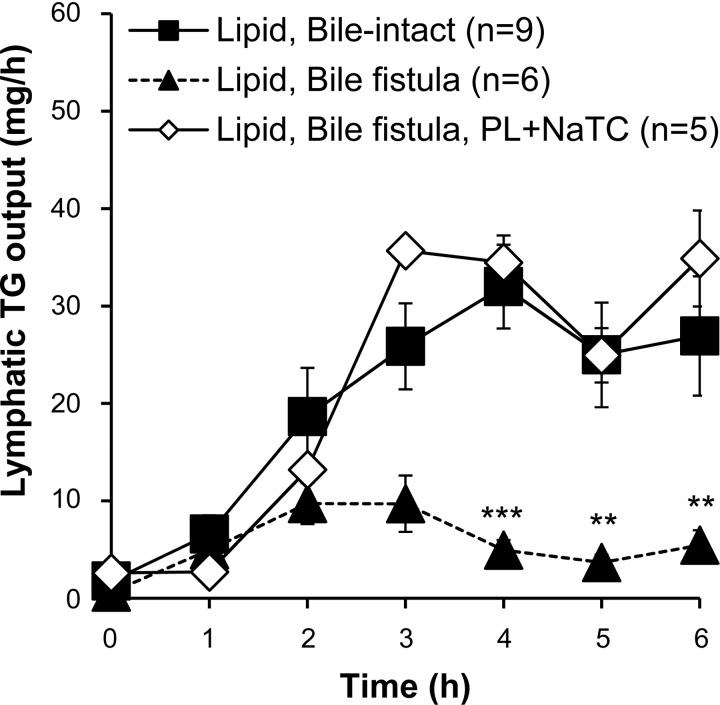
We next sought to determine whether apoA-V secretion into bile is regulated by dietary lipid absorption. However, lipid digestion and transport into lymph are compromised in bile fistula rats (29), likely because of the lack of luminal phosphatidylcholine for adequate coating of chylomicrons (36). The lack of bile salts in the lumen of the bile fistula rats impaired micellar solubilization of fatty acids monoacylglycerols for efficient uptake by the enterocytes (13) across the unstirred water layer that borders the luminal surface of intestinal cells (41). Therefore we infused 6.6 mM phosphatidylcholine and 10 mM sodium taurocholate (PL+NaTC) continuously into the duodenum at 1.5 ml/h for 2 h before administering the lipid bolus and for the remainder of the experiment. To confirm that lipid absorption in the bile fistula rats was restored by PL+NaTC infusion, a separate control experiment was performed with intestinal lymph collection in addition to bile diversion (see materials and methods). Figure 2 shows that our regimen of PL+NaTC infusion into bile fistula rats resulted in normal lymphatic fat transport, both in timing and extent, compared with bile-intact control rats. As expected, lymphatic fat transport was reduced in bile fistula rats without the PL+NaTC supplement (Fig. 2).
Fig. 2.
Supplementing bile fistula rats with a continuous intraduodenal infusion of phosphatidylcholine (PL) and taurocholate (NaTC) restored lymphatic triglyceride (TG) transport to the same level as bile intact rats. Bile intact or bile fistula rats were given an intraduodenal bolus of lipids, respectively. Another cohort of bile fistula rats was given an intraduodenal supplement of phospholipid-taurocholate showed the same lymphatic triglyceride output as bile-intact rats. Without the phospholipid-taurocholate supplemental infusion, bile fistula rats transported triglycerides poorly into lymph. All values are means ± SE. **P < 0.01, ***P < 0.001 is significance comparing Lipid, Bile fistula vs. Lipid, Bile-intact.
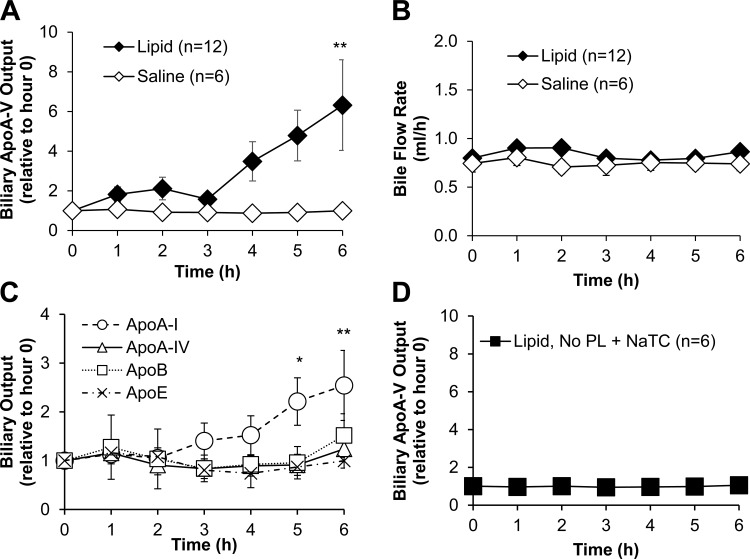
With these conditions, we proceeded to test the biliary response of apoA-V to dietary lipids. Figure 3A shows that apoA-V secretion into bile increased approximately sixfold in response to active dietary lipid absorption and did not increase in response to saline. The increase began 3 h after administration of the lipid bolus (P < 0.01 at hour 6). Compared with the results shown in Fig. 2, the increase of biliary apoA-V was well correlated with the maximal triglyceride delivery into lymph. The lipid-induced increase was abolished in animals given lipid but with no phospholipid/bile salt supplementation (Fig. 3), suggesting that biliary apoA-V stimulation required the lymphatic lipid flux associated with the production of triglyceride-enriched chylomicrons.
Fig. 3.
Dietary lipids stimulated the secretion of apoA-V into bile and required transport of lipids into lymph. A: biliary apoA-V output in rats given an intraduodenal bolus of dietary lipids or saline (with PL+NaTC supplemental infusion), **P < 0.01. B: biliary outputs of other apolipoproteins in response to dietary lipids, *P < 0.05, **P < 0.01 ApoA-I with lipid treatment vs. with saline treatment. C: bile flow between the 2 groups. D: biliary apoA-V output in rats given dietary lipid but no PL+NaTC supplementation (a condition of poor lymphatic triglyceride transport). Apolipoprotein concentration in bile was determined by Western blot and quantified by densitometry. Output was determined by concentration × bile flow. Experimental protocols described in materials and methods. All values are means ± SE.
To determine whether the lipid-induced increase was specific to apoA-V or a general response of the biliary secretion of apolipoproteins in response to lipid absorption, we measured the biliary outputs of the other apolipoproteins. Figure 3B shows that, except for a modest twofold increase of apoA-I, the biliary secretions of other apolipoproteins (apoA-IV, apoB, apoE) were not affected by active dietary lipid absorption. This indicated that the dramatic increase of biliary apoA-V output in response to dietary lipid was specific, suggesting a possible role for biliary apoA-V in dietary lipid absorption. Lipid treatment did not affect the rate of bile flow, which remained between 0.8 and 1.0 ml/h (Fig. 3C). Taken together, the data show that apoA-V secretion into bile is regulated by active absorption of dietary fat.
Dietary lipids do not significantly stimulate biliary secretions of lipids or bile salts.
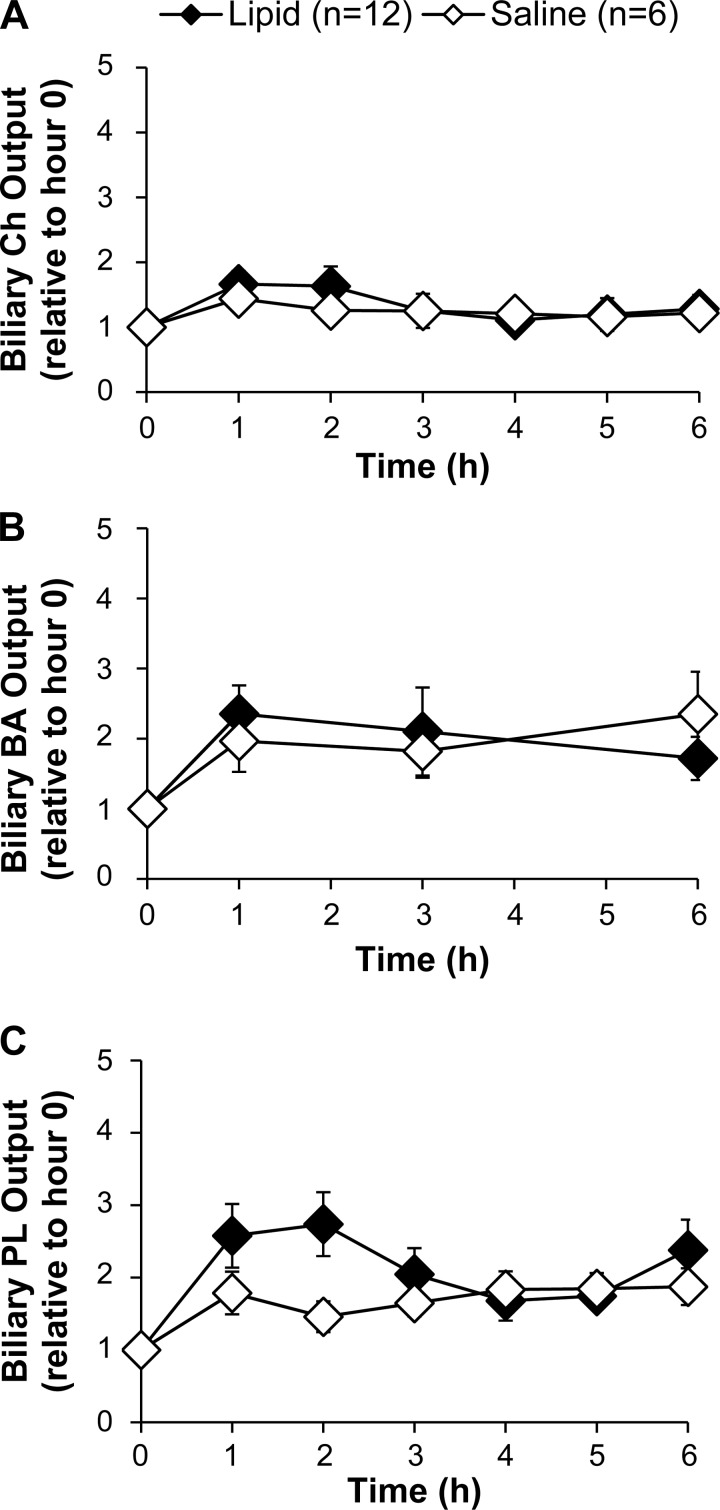
We next determined whether the increase in biliary apoA-V secretion was correlated with increased secretion of biliary lipids or bile salts. Lipid treatment did not significantly change the biliary outputs of cholesterol and bile salts compared with saline treatment (Fig. 4, A and B). Lipid treatment increased total biliary phospholipid output (P < 0.05 in treatment factor, two-way ANOVA) with the increase apparent at 1 and 2 h following administration of the lipid bolus, but there were no statistical differences between groups at individual time points (Fig. 4C). Taken together, we conclude that a single bolus of dietary lipid does not significantly impact the biliary secretion of cholesterol and bile salts, with the possible exception of phospholipid, thus reinforcing the unique response of biliary apoA-V secretion to dietary lipid.
Fig. 4.
Effects of dietary lipids on the biliary outputs of cholesterol (A), bile acids (B), and phospholipids (C). Dietary lipids significantly affected total biliary phospholipid output (P < 0.05 for treatment factor, 2-way ANOVA), but there was no statistical significance between groups at individual time points by multiple comparisons posttest analysis. Biliary concentrations were measured by chemical assays described in materials and methods. Output was determined by concentration × bile flow. All values are means ± SE. Ch, cholesterol; BS, bile salts; PL, phospholipids.
Acute response of biliary ApoA-V to bile fistula surgery.
In the previous experiments, we allowed the animals to recover from surgery for 24 h before proceeding to our lipid studies. Although this experimental design is consistent with previous studies (1, 16, 37), it diverts bile and interrupts the enterohepatic circulation. Because bile salts and farnesoid X receptor (FXR) ligands were found to stimulate hepatic apoA-V promoter activity in vitro (27), we wondered whether the biliary output of apoA-V responded to bile drainage per se during the initial period after surgery. We expected that apoA-V secretion into bile would decrease with bile drainage, which is expected for bile flow and the biliary outputs of other lipids (35). Interestingly, the biliary output of apoA-V increased over time during the 16-h study (P < 0.01 at hour 16 relative to hour 1 after bile duct cannulation) (Fig. 5A), while the outputs of other apolipoproteins remained constant (Fig. 5B), suggesting that bile diversion uniquely upregulated the secretion of apoA-V into bile.
Fig. 5.
Biliary output of apoA-V in bile increased dramatically after bile fistula surgery while the outputs of other apolipoproteins remained constant. A: biliary apoA-V output with representative Western blot (20-s output of bile loaded into each well). B: biliary outputs of apoA-I, apoA-IV, apoB, and apoE. Apolipoprotein concentration was determined by Western blot and quantified using densitometry. Output was determined by concentration × bile flow. All values are means ± SE. **P < 0.01 hour 16 vs. hour 1.
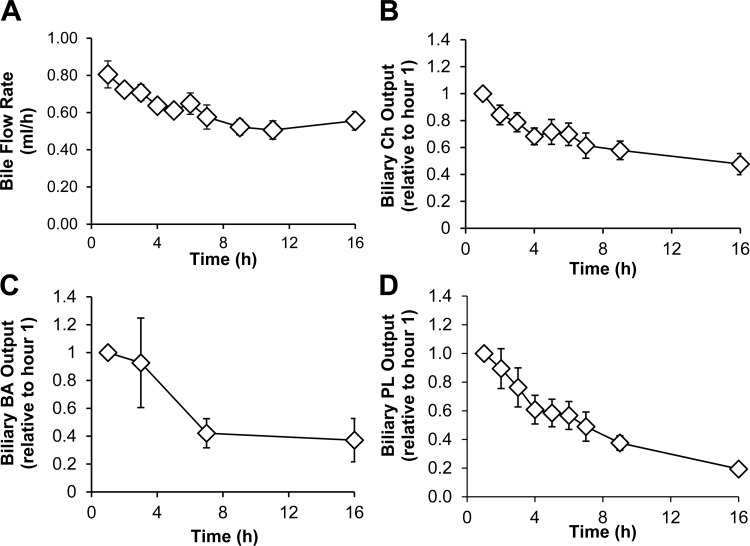
After bile fistula surgery, bile flow decreased from 0.80 ml/h (initial flow rate) to 0.60 ml/h during the 16-h period that we studied (Fig. 6A). Additionally, the biliary outputs of cholesterol (Fig. 6B), bile acids (Fig. 6C), and phospholipids (Fig. 6D) decreased ∼50, 60, and 80%, respectively. Taken together, the data show that while bile flow and biliary lipid and bile salt outputs decreased, the biliary output of apoA-V increased with bile drainage after bile fistula surgery.
Fig. 6.
After bile fistula surgery, bile flow and biliary lipid and bile salt outputs decrease. Bile flow rate (A) and biliary outputs of cholesterol (B), bile acids (C), and phospholipids (D). Bile fistula rats were given a continuous intraduodenal infusion of 5% glucose-saline immediately after surgery. Biliary concentrations of lipids were measured by chemical assays. Output is determined by concentration × bile flow. All values are means ± SE.
Biliary ApoA-V in response to luminal phospholipids and bile salts.
To test whether the increased output of apoA-V into bile was due to diversion of biliary phosphatidylcholine and bile salts from the enterohepatic circulation, we gave an intraduodenal infusion of phosphatidylcholine and taurocholate solution at physiological concentrations (matched to the phospholipid and bile salt concentration immediately after surgery) to restore these components of the enterohepatic circulation. Interestingly, instead of preventing the increase of biliary apoA-V output, the phospholipid-taurocholate infusion further enhanced biliary apoA-V output dramatically after 16 h compared with the saline group (statistically significance P < 0.01 after 24 h) (Fig. 7A).
Fig. 7.
Biliary apoA-V output remained increased after bile fistula surgery even after phospholipid-taurocholate treatment. A: biliary apoA-V output. B: bile flow. Immediately after bile fistula surgery, rats were given either a continuous intraduodenal infusion of 5% glucose-saline or a physiological concentration of phospholipid-taurocholate for 27 h. Biliary apoA-V concentration was determined by Western blot and quantified by densitometry. Output was determined by concentration × bile flow. All values are means ± SE. **P < 0.01, ***P < 0.001
The phospholipid-taurocholate infusion did not change bile flow for the first 12 h of infusion (which dropped from 0.8 to 0.5 ml/h for both groups) but significantly increased bile flow after 16 h, from 0.5 to 1.0 ml/h (P < 0.001) (Fig. 7B). This shows that phospholipid-taurocholate stimulated the secretion of apoA-V into bile, in addition to stimulating bile flow. Importantly, the data indicate that loss of biliary phospholipids and bile salts with bile drainage was not responsible for the increased biliary output of apoA-V after bile fistula surgery.
DISCUSSION
ApoA-V is an apolipoprotein made only in the liver that dramatically modulates plasma triglyceride levels, both fasting (26) and postprandially (9, 21), through mechanisms related to lipolysis, hepatic uptake, or hepatic secretion of lipoproteins that have been so ably reviewed by Forte et al. (8). We have recently reported a novel role for apoA-V in the absorption of dietary lipids by modulating the uptake and secretion of lipids packaged as chylomicrons into the lymph (43). However, the mode of how hepatic apoA-V is delivered to the gut is unknown.
While hepatocytes secrete triglycerides, cholesterol, cholesterol ester, and phospholipids basolaterally into plasma as very-low-density and high-density lipoprotein particles, hepatocytes also secrete bile salts as monomers as well as phospholipid and cholesterol as vesicles into bile. Apolipoproteins A-I, A-II, C-II, C-III, and B are also secreted into bile (30), either directly by hepatocytes or following uptake of lipoproteins from plasma (18). The functions of these biliary apolipoproteins are not well understood but they may be involved in the solubilization of biliary lipids. ApoA-I and apoA-II have been demonstrated to prolong the nucleation time of solid cholesterol monohydrate crystals as studied in model bile (19). Biliary apoA-I and the apolipoprotein anionic peptide factor has been shown to regulate dietary cholesterol absorption (14).
Our finding that apoA-V is present in bile introduces a second route, the first being via the circulation, by which this unique hepatic apolipoprotein is presented to and affects the function of enterocytes. From the circulation, the effect of apoA-V would be mediated through the basolateral membrane of the hepatocyte, whereas the biliary route presents the possibility that apoA-V may function apically: either through a luminal effect in the gut or intracellularly if it is taken up apically by enterocytes. Although an intracellular function for this protein has been described in the liver (3, 32), its function in enterocytes has not been explored and will no doubt be actively investigated in other laboratories as well as ours. A more fundamental question is whether biliary apoA-V escapes hydrolysis in the small intestinal lumen and, if so, is it taken up by the enterocytes? Siddiqi and colleagues (34) have recently examined this question in an initial report. Very interestingly, they found that apoA-V in the intestinal lumen can escape proteolysis, can remain intact, and is taken up by enterocytes by a mechanism that involves interaction with the fatty acid transporter CD36 and with caveolin-1, the main scaffolding protein in caveolar membranes. Although apoA-V is generally established to be expressed exclusively in the liver, it should be pointed out that extremely low mRNA expression levels of apoA-V have been observed in intestinal tissues of C57BL/6J mice (1,000 times lower than liver mRNA expression) and in intestinal polyA+mRNA samples of humans (32,000 times lower than liver expression) (11). However, in that study, Western blotting analyses of colon and colon+jejunum tissues showed that apoA-V protein from intestinal lysates was one-sixth of that of liver lysates. Extrapolated from our study, the protein product recovered from tissue lysates may not necessarily come from intestinal synthesis but rather may represent sources such as the bile. Thus whether the small intestine synthesizes apoA-V remains a possibility; however, the contribution from bile cannot and should not be ignored. On the basis of our data, the contribution of apoA-V from bile to enterocytes seems to significantly outweigh that produced endogenously by the gut.
If apoA-V is indeed absorbed through a system involving CD36 and caveolin-1, then an additional consideration than its effect as an absorbed molecule is whether its action might be mediated through CD36. CD36 is abundantly expressed in the gastrointestinal tract, with its expression highest in the duodenum and jejunum (24), and has been shown to affect chylomicron production (7, 25) partially by facilitating the uptake of fatty acids (24). One potential mechanism of increased fatty acid uptake is enhanced intracellular esterification without catalyzing the translocation of fatty acid across the plasma membrane (40, 42). With CD36 being central to fatty acid uptake via its effects on intracellular metabolism, it is plausible that biliary apoA-V may be affecting fatty acid transport through CD36. Additionally, we cannot rule out the contributive effects of other proteins involved in apical fatty acid transport and metabolism, beside CD36, such as caveolin(s), other fatty acid transport proteins, intracellular fatty acid binding proteins, and enzymes involved in the conversion of fatty acids to esters.
Our finding that the secretion of apoA-V into bile increases in response to dietary lipids is consistent with our previous report that apoA-V modulates chylomicron production (43). The increase of biliary apoA-V occurred 4 h after lipid administration, concomitant with maximum lymphatic output of triglyceride, which strongly suggests a link between lipid flux (as chylomicrons) from the small intestine and the stimulation of hepatic apoA-V secretion into bile. Furthermore, our finding that the increase in biliary apoA-V secretion was abolished when lymphatic fat transport was poor further reinforces the notion that a lipid flux is required to stimulate hepatic apoA-V secretion into bile. Whether the hepatic uptake of chylomicron remnants is the signal is a possibility, and this question is currently being explored in our laboratory. It should be pointed out that this research undertaking is complex, potentially involving numerous mechanisms, and requires the appropriate controls.
The fact that the biliary output of apoA-V increased significantly while the outputs of other apolipoproteins remained constant indicates that dietary lipid regulation of apoA-V is unique and is not a marker of generalized hepatic response to a physiological challenge of diet or surgery. For instance, it has been demonstrated that the intestinal secretion of apoA-IV increases approximately twofold during active fat absorption (12, 15, 43). Yet our data show that active fat absorption did not modify the biliary secretion of apoA-IV. Additionally, hepatic secretion of biliary lipids and bile salts in response to the fat load did not change significantly as expected (5, 10), with the possible exception of phospholipids, which is also in agreement with the response of apoA-V being a unique phenomenon.
Independent of the lipid stimulus, we found that apoA-V secretion into bile increased after bile fistula surgery while the secretion of other apolipoproteins remained constant, suggesting that bile drainage specifically affected apoA-V. This effect is also recently seen in chow-fed male C57BL/6J mice (Wang DQH, unpublished observations). Since the apoA-V promoter contains a FXR response element and its activity is stimulated by bile salts in vitro (27), we wondered whether drainage of bile salts affected the secretion of apoA-V into bile. Unexpectedly, the secretion of apoA-V into bile increased steadily with time. However, when intestinal phospholipids and bile salts were restored by intraduodenal infusion, apoA-V secretion into bile increased severalfold higher than with only saline infusion. This latter result, at least, is consistent with the idea of Prieur et al. (27) that bile salts can stimulate hepatic apoA-V expression. Taken together, these results suggest that increased biliary apoA-V secretion after bile fistula surgery did not result from depletion of phospholipids and bile salts, two major components of bile.
This is the first report of the presence of apoA-V in bile and we conclude that hepatic apoA-V secretion into bile is regulated by active intestinal lipid absorption, although the mechanisms remain to be determined. Our present findings, combined with those reported previously (43), are consistent with a physiological role for biliary apoA-V in modulating lipid absorption in the intestinal lumen. Additionally, apoA-V was reported as a factor in early liver regeneration in 2001 when it was discovered by van der Vliet and colleagues (39), who suggested that upregulation of apoA-V served as a protective response by the liver to inhibit hepatic lipid uptake, to protect a hepatectomized liver from a lipid overload. Given the evidence that apoA-V inhibits triglyceride secretion in apoB-containing lipoproteins (3), one might also surmise that its upregulation in response to partial hepatectomy may be a means to retain lipids for new membrane assembly or for energy reserves, since regenerating liver hepatocytes accumulate fat microdroplets within a day of partial hepatectomy (22). We propose that increased apoA-V secretion into bile in response to intestinal lipid absorption occurs irrespective of bile drainage and constitutes a physiological mechanism to regulate the intestinal formation and secretion of chylomicrons. As such, apoA-V-dependent attenuation of chylomicron assembly and secretion may protect the liver from chylomicron remnant lipid flux overload. Furthermore, we believe that apoA-V also serves as a link between the liver and the gut in coordinating lipid metabolism during physiological states, e.g., fasting, and during active lipid absorption.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK092138 and DK059630 and a predoctoral fellowship from the American Heart Association 13PRE17140013.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.S.Z. and P.T. conception and design of research; L.S.Z., H.S., and Q.Y. performed experiments; L.S.Z. analyzed data; L.S.Z., H.S., R.O.R., D.Q..-H.W., P.N.H., and P.T. interpreted results of experiments; L.S.Z. prepared figures; L.S.Z. drafted manuscript; L.S.Z., R.O.R., D.Q..-H.W., P.N.H., and P.T. edited and revised manuscript; L.S.Z., H.S., Q.Y., R.O.R., D.Q..-H.W., P.N.H., and P.T. approved final version of manuscript.
REFERENCES
- 1.Bearnot HR, Glickman RM, Weinberg L, Green PH. Effect of biliary diversion on rat mesenteric lymph apolipoprotein-I and high density lipoprotein. J Clin Invest 69: 210–217, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergstrom S, Norman A. Metabolic products of cholesterol in bile and feces of rat; steroids and bile acids. Proc Soc Exp Biol Med 83: 71–74, 1953. [DOI] [PubMed] [Google Scholar]
- 3.Blade AM, Fabritius MA, Hou L, Weinberg RB, Shelness GS. Biogenesis of apolipoprotein A-V and its impact on VLDL triglyceride secretion. J Lipid Res 52: 237–244, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine or thoracic duct of the rat. J Lab Clin Med 33: 1349–1352, 1948. [PubMed] [Google Scholar]
- 5.Corring T, Juste C, Lhoste EF. Nutritional regulation of pancreatic and biliary secretions. Nutr Res Rev 2: 161–180, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Davis RA, Hyde PM, McNeal MM, Schexnayder JA. In vivo demonstration that bile acids do not directly induce biosynthetic feedback regulation. Arteriosclerosis 3: 490a, 1983. [Google Scholar]
- 7.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest 115: 1290–1297, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forte TM, Shu X, Ryan RO. The ins (cell) and outs (plasma) of apolipoprotein A-V. J Lipid Res 50: S150–S155, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fruchart-Najib J, Bauge E, Loredan-Stefan N, Pham T, Thomas B, Rommens C, Majd Z, Brewer B, Pennacchio LA, Fruchart JC. Mechanism of triglyceride lowering in mice expressing human apolipoprotein A5. Biochem Biophys Res Commun 319: 397–404, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Metzger AL. A physiological method for estimation of hepatic secretion of biliary lipids in man. Gastroenterology 62: 1200–1217, 1972. [PubMed] [Google Scholar]
- 11.Guardiola M, Alvaro A, Vallve JC, Rosales R, Sola R, Girona J, Serra N, Duran P, Esteve E, Masana L, Ribalta J. APOA5 gene expression in the human intestinal tissue and its response to in vitro exposure to fatty acid and fibrate. Nutr Metab Cardiovasc Dis 22: 756–762, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D, Tso P. Transport of lipid and apolipoprotein A-I and A-IV in intestinal lymph of the rat. J Lipid Res 31: 1613–1625, 1990. [PubMed] [Google Scholar]
- 13.Hofmann AF, Borgstrom B. The intraluminal phase of fat digestion in man: the lipid content of the micellar and oil phases of intestinal content obtained during fat digestion and absorption. J Clin Invest 43: 247–257, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jourdheuil-Rahmani D, Charbonnier M, Domingo N, Luccioni F, Lafont H, Lairon D. Biliary anionic peptide fraction and ApoA-I regulate intestinal cholesterol uptake. Biochem Biophys Res Commun 292: 390–395, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Kalogeris TJ, Fukagawa K, Tso P. Synthesis and lymphatic transport of intestinal apolipoprotein A-IV in response to graded doses of triglyceride. J Lipid Res 35: 1141–1151, 1994. [PubMed] [Google Scholar]
- 16.Kalogeris TJ, Fukagawa K, Tsuchiya T, Qin X, Tso P. Intestinal synthesis and lymphatic secretion of apolipoprotein A-IV after cessation of duodenal fat infusion: mediation by bile. Biochim Biophys Acta 1436: 451–466, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Kao J, Wen H, Chien K, Hsu H, Li S. A novel genetic variant in the apolipoprotein A5 gene is associated with hypertriglyceridemia. Hum Mol Genet 12: 2533–2539, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto T, Mao SJ, LaRusso NF. Biliary excretion of apolipoprotein B by the isolated perfused rat liver. Relationship to receptor-mediated uptake of human low density lipoprotein and biliary lipid secretion. Gastroenterology 92: 1236–1242, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Kibe A, Holzbach RT, LaRusso NF, Mao SJ. Inhibition of cholesterol crystal formation by apolipoproteins in supersaturated model bile. Science 225: 514–516, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Lai C, Tai E, Tan CE, Cutter J, Chew SK, Zhu Y, Adiconis X, Ordovas JM. The APOA5 locus is a strong determinant of plasma triglyceride concentrations across ethnic groups in Singapore. J Lipid Res 44: 2365–2373, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA, Laatsch A, Heeren J. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem 280: 21553–21560, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Michalopoulos GK. Liver regeneration. J Cell Physiol 213: 286–300, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nabika T, Nasreen S, Kobayashi S, Masuda J. The genetic effect of the apoprotein AV gene on the serum triglyceride level in Japanese. Atherosclerosis 165: 201–204, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem 282: 19493–19501, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 131: 1197–1207, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennacchio LA, Olivier M, Hubacek JA, Krauss RM, Rubin EM, Cohen JC. Two independent apolipoprotein A5 haplotypes influence human plasma triglyceride levels. Hum Mol Genet 11: 3031–3038, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Prieur X, Coste H, Rodriguez JC. The human apolipoprotein AV gene is regulated by peroxisome proliferator-activated receptor-α and contains a novel farnesoid X-activated receptor response element. J Biol Chem 278: 25468–25680, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Ribalta J, Figuera L, Fernandez-Ballart J, Vilella E, Cabezas MC, Masana L, Joven J. Newly identified apolipoprotein AV gene predisposes to high plasma triglycerides in familial combined hyperlipidemia. Clin Chem 48: 1597–1600, 2002. [PubMed] [Google Scholar]
- 29.Saunders DR, Dawson AM. The absorption of oleic acid in the bile fistula rat. Gut 4: 254–260, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sewell RB, Mao SJT, Kawamoto T, LaRusso NF. Apolipoproteins of high, low, and very low density lipoproteins in human bile. J Lipid Res 24: 391–401, 1983. [PubMed] [Google Scholar]
- 31.Shefer S, Hauser S, Bekersky I, Mosbach EH. Feedback regulation of bile acid biosynthesis in the rat. J Lipid Res 10: 646–655, 1969. [PubMed] [Google Scholar]
- 32.Shu X, Nelbach L, Ryan RO, Forte TM. Apolipoprotein A-V associates with intrahepatic lipid droplets and influences triglyceride accumulation. Biochim Biophys Acta 1801: 605–608, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu X, Nelbach L, Weinstein MM, Burgess BL, Beckstead JA, Young SG, Ryan RO, Forte TM. Intravenous injection of apolipoprotein A-V reconstituted high-density lipoprotein decreases hypertriglyceridemia in apoav-/- mice and requires glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1. Arterioscler Thromb Vasc Biol 30: 2504–2509, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqi S, Polly S, Siddiqi T, Mansbach CM. Apolipoprotein-AV resists proteolysis in the intestinal tract and is absorbed intact, controlled by dietary phosphatidylcholine (Abstract). Gastroenterology 148: S880–S881, 2015. [Google Scholar]
- 35.Smit MJ, Temmerman AM, Havinga R, Kuipers F, Vonk RJ. Short- and long-term effects of biliary drainage on hepatic cholesterol metabolism in the rat. Biochem J 269: 781–788, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tso P, Balint JA, Simmonds WJ. Role of biliary lecithin in lymphatic transport of fat. Gastroenterology 73: 1362–1367, 1977. [PubMed] [Google Scholar]
- 37.Tsuchiya T, Kalogeris TJ, Tso P. Ileal transposition into the upper jejunum affects lipid and bile salt absorption in rats. Am J Physiol Gastrointest Liver Physiol 271: G681–G691, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Tuchweber B, Yousef IM, Ferland G, Perea A. Nutrition and bile formation. Nutr Res 16: 1041–1080, 1996. [Google Scholar]
- 39.Van der Vliet HN, Sammels MG, Leegwater AC, Levels JH, Reitsma PH, Boers W, Chamuleau RA. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J Biol Chem 276: 44512–44520, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Wang TY, Liu M, Portincasa P, Wang DQ. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur J Clin Invest 43: 1203–1223, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westergaard H, Dietschy JM. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake into the intestinal mucosal cell. J Clin Invest 58: 97–108, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu S, Jay A, Brunaldi K, Huang N, Hamilton JA. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry 52: 7254–7261, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Zhang LS, Xu M, Yang Q, Ryan RO, Howles PN, Tso P. Apolipoprotein A-V deficiency enhances chylomicron production in lymph fistula mice. Am J Physiol Gastrointest Liver Physiol 308: G634–G642, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]