Abstract
The igf1 gene is alternatively spliced as IGF-IEa and IGF-IEc variants in humans. In fibrostenotic Crohn's disease, the fibrogenic cytokine TGF-β1 induces IGF-IEa expression and IGF-I production in intestinal smooth muscle and results in muscle hyperplasia and collagen I production that contribute to stricture formation. Mechano-growth factor (MGF) derived from IGF-IEc induces skeletal and cardiac muscle hypertrophy following stress. We hypothesized that increased IGF-IEc expression and MGF production mediated smooth muscle hypertrophy also characteristic of fibrostenotic Crohn's disease. IGF-IEc transcripts and MGF protein were increased in muscle cells isolated from fibrostenotic intestine under regulation by endogenous TGF-β1. Erk5 and MEF2C were phosphorylated in vivo in fibrostenotic muscle; both were phosphorylated and colocalized to nucleus in response to synthetic MGF in vitro. Smooth muscle-specific protein expression of α-smooth muscle actin, γ-smooth muscle actin, and smoothelin was increased in affected intestine. Erk5 inhibition or MEF2C siRNA blocked smooth muscle-specific gene expression and hypertrophy induced by synthetic MGF. Conditioned media of cultured fibrostenotic muscle induced muscle hypertrophy that was inhibited by immunoneutralization of endogenous MGF or pro-IGF-IEc. The results indicate that TGF-β1-dependent IGF-IEc expression and MGF production in patients with fibrostenotic Crohn's disease regulates smooth muscle cell hypertrophy a critical factor that contributes to intestinal stricture formation.
Keywords: insulin-like growth factor (IGF), muscle hypertrophy, extracellular-signal-regulated kinase (ERK), fibrosis, mechano growth factor
insulin-like growth factor -I (IGF-I) is a regulator of prenatal and postnatal cell growth, development, and survival. Targeted deletion of hepatic-derived IGF-I reduces circulating levels of IGF-I by 80% but does not significantly impair normal development (3). Observations in mice overexpressing igf1 are particularly enlightening because these animals develop hypertrophy of both visceral and vascular smooth muscles (30, 39). These observations demonstrate the importance of paracrine and autocrine sources of IGF-I and in particular that produced by smooth muscle.
Expression of the igf1 gene, particularly in the liver, is normally under the control of growth hormone (GH). In the setting of Crohn's disease a relative GH-insensitive state exists (26, 37). In Crohn's disease, expression of igf1 is instead regulated by products of inflammation: the fibrogenic cytokine TGF-β1 and inflammatory cytokines TNF-α and IL-6, all of which are increased in muscle of strictured intestine (14, 24, 34). The igf1 gene is alternatively spliced (Fig. 1) (15, 29). The mRNA and amino acid sequences of each pro-IGF-I isoform are identified (Table 2). The IGF-IEa splice variant encodes pro-IGF-IEa giving rise to mature IGF-I. The majority of circulating IGF-I is produced in the liver and is the same as that produced locally by smooth muscle cells of the intestine with regard to structure and function in cellular proliferation, survival, and inhibition of apoptosis. In intestinal smooth muscle cells, endogenous IGF-I regulates smooth muscle cell hyperplasia by concomitantly stimulating proliferation by activation of both Erk1/2 and PI3-kinase and by inhibiting apoptosis via Akt (1, 20, 21).
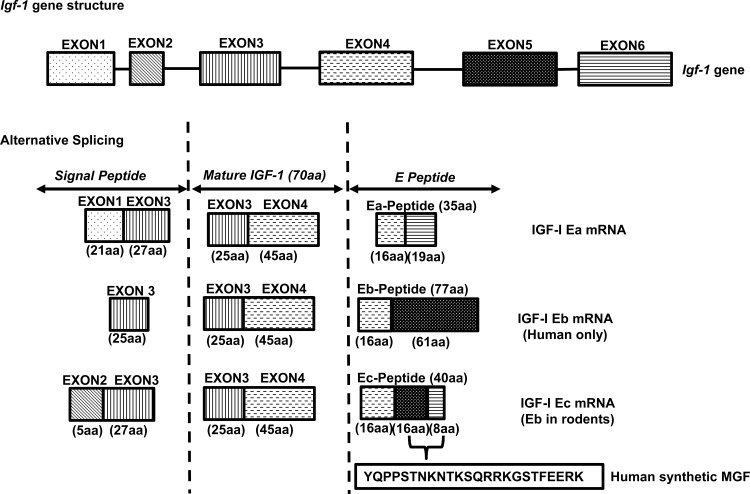
Fig. 1.
Gene structure, alternative splicing, and peptide products of the igf1 gene. The igf1 gene structure contains 6 exons. Alternative splicing of igf-1 pre-mRNA results in multiple mRNA isoforms (IGF-I Ea, Eb, and Ec mRNAs), depending on the combinations between leader sequence (signal peptide) and COOH-terminal exons. Heterogeneous signal peptides of different amino acid sequences are generated due to various transcription initiation sites that are present in exons 1 and 2. All the mRNA isoforms contain exons 3 and 4, which encode the mature IGF-I peptide with 70 amino acids (aa). mRNAs containing spliced exons 4 and 6 at the 3′ end of the mRNA are designated as IGF-I Ea, whereas those containing spliced exons 4, 5, and 6 are designated as IGF-I Ec in humans and IGF-I Eb in rodents. Translation of all these alternative spliced mRNA isoforms produces diverse pre-pro-IGF-Is, which are processed to generate the mature IGF-I peptide and diverse E peptides. IGF-I Ec in human (IGF-I Eb in rodents) is also termed as mechano-growth factor (MGF), which has a 49-base insert in the human in exon 5 that encodes 16 amino acid sequences. In the present study human synthetic MGF with 24-amino acid length is generated from spliced exons 5 (16aa) and 6 (8aa) within the Ec domain.
Table 2.
NCBI NM/NP numbers related to each pro-IGF-I isoform
| Name | NM | NP |
|---|---|---|
| Pro-IGF-I Ea | NM_001111284 | NP_001104754 |
| Pro-IGF-I Eb | NM_001111285 | NP_001104755 |
| Pro-IGF-I Ec | NM_001111283 | NP_001104753 |
The IGF-I gene also gives rise to the IGF-IEc mRNA splice variant (n.b. IGF-IEb in rodents) (Fig. 1). A 49-base pair insert between exons 5 and 6 (6, 28) results in a reading frame shift encoding pro-IGF-IEc and yields an Ec peptide termed “mechano-growth factor” (MGF). Expression of the IGF-IEc mRNA splice variant increases in skeletal muscle and cardiac muscle cells when subjected to stress or inflammation (6, 15, 16, 32). MGF activates satellite cells and cardiac muscle eliciting skeletal and cardiac muscle hypertrophy (6, 28). In neurons the IGF-IEc mRNA splice variant increases in the ischemic brain where E peptide is thought to exert neuroprotective effects (7). This evidence is largely based on the effects of incorporation of IGF-IEb cDNA or administration of a consensus COOH terminal 24 amino acid peptide, termed “synthetic MGF” (7). To date no direct evidence of an endogenous MGF exists and hence its putative effects have not been uniformly accepted (28). E peptides are thought by some to modulate the actions of IGF-I on its cognate receptor (4, 9). It is also not known whether the IGF-1Ec mRNA splice variant is expressed in human intestinal smooth muscle cells or whether its expression like that of the IGF-Ea splice variant is increased in Crohn's disease or whether it plays a role in mediating the hypertrophy of intestinal smooth muscle cells that is one characteristic of fibrostenosis in patients with Crohn's disease.
Three hallmark features of intestinal strictures in patients with Montreal B2 fibrostenotic Crohn's disease are smooth muscle cell hyperplasia, smooth muscle cell hypertrophy, and increased extracellular matrix production particularly of collagen I (23). Upregulation of autocrine TGF-β1 in mesenchymal cells in the gut including smooth muscle increases IGF-IEa expression resulting in increased IGF-I levels, smooth muscle hyperplasia, and collagen I production (12, 27, 34). The central role of the igf1 gene in the hyperplasia that characterizes stricturing Crohn's disease was evident in igf1 heterozygous (IGF-I+/−) mice. In contrast to their wild-type littermates, IGF-I+/− mice develop significantly less fibrosis in response to chronic TNBS-induced colitis, a model of fibrostenotic Crohn's disease (27). The mechanisms leading to the concomitant smooth muscle cell hypertrophy that occurs in fibrostenotic Crohn's disease have yet to be determined.
Because increased TGF-β1 stimulates IGF-I Ea transcription in these patients, we hypothesized that IGF-I Ec and the resultant IGF-I Ec peptide, MGF, may also increase and be responsible for the muscle cell hypertrophy seen in the development of fibrostenotic Crohn's disease (27, 34). In the present study we sought to provide evidence that human intestinal smooth muscle expresses the IGF-IEc splice variant and immunoreactive E peptide and that smooth muscle hypertrophy results, in part, from increased expression of muscle specific proteins: α-smooth muscle actin, γ-smooth muscle actin, smoothelin, and desmin. Since transcription of these genes is regulated by the myocyte enhancer factor 2 C (MEF2C) transcription factor we investigated whether MEF2C activity was regulated by MGF and played a role in smooth muscle cell hypertrophy in fibrostenotic Crohn's disease.
The present study provides evidence that human intestinal smooth muscle express the IGF-IEc splice variant and immunoreactive E peptide. The results indicate that increased TGF-β1-dependent IGF-IEc expression and MGF production in patients with fibrostenotic Montreal B2 phenotype Crohn's disease regulate the increased smooth muscle cell hypertrophy by an Erk5- and MEF2C-dependent pathway that contributes to fibrostenosis and stricture formation in Crohn's disease.
MATERIALS AND METHODS
Materials.
Intestinal muscle cells were isolated from human intestine that was obtained from patients undergoing ileal/ileal-colonic resection for each Montreal classification phenotype of Crohn's disease, B1, B2, and B3, and from patients undergoing surgery without Crohn's disease (27). Patients with mixed phenotypes were not analyzed in this paper. Subject demographics are presented in Table 1. Muscle cells were isolated by enzymatic digestion from the circular muscle layer of strictured intestine and from histologically normal intestinal resection margin as described previously (12, 25). Muscle cells from normal intestine in patients without Crohn's disease were also isolated for comparison. Isolated smooth muscle cells were used to prepare mRNA or whole cell lysates or placed into primary cell culture (11, 12). The smooth muscle phenotype and characteristics are retained in culture as reported and validated previously (36).
Table 1.
Subject demographics
| Age, yr | Patient No. (% of total) |
|---|---|
| Under 20 | 3 (10) |
| 20–29 | 11 (37) |
| 30–39 | 8 (27) |
| 40–49 | 5 (17) |
| 50–59 | 2 (7) |
| Over 60 | 1 (3) |
| Sex | |
| Male | 11 (37) |
| Female | 19 (63) |
| Race | |
| White | 17 (57) |
| Black or African | 12 (40) |
| Other/unknown | 1 (3) |
| Non-CD Subjects | 6 |
| CD Subjects Montreal Phenotype | |
| B1-nonstricturing, non penetrating | 6 (25) |
| B2-stricturing | 12 (50) |
| B3-penetrating | 6 (25) |
CD, Crohn's disease.
Ethical considerations.
Studies in human subjects were conducted under an approval by the Virginia Commonwealth University Institutional Review Board. All subjects provided written, informed consent prior to inclusion in this study.
Quantitative real-time PCR.
Quantitative real-time PCR (qRT-PCR) was used to measure RNA transcripts of the IGF-1Ea (5′-GTGGAGACAGGGGCTTTTATTTC-3′ and 5′-CTTGTTTCCTGCACTCCCTCTACT-3′) and IGF-IEc splice variants (5′-CGAAGTCTCAGAGAAGGAAAGG-3′ and 5′-ACAGGTAACTCGTGCAGAGC-3′) by use of a 2−ΔΔCt method based on GAPDH amplification that remained constant across subjects and regions (10, 12). qRT-PCR was also used to measure relative expression levels of human α-smooth muscle actin (ACTA2, TaqMan probe ID: Hs00426835_g1); gamma 2, smooth muscle actin (ACTG2, TaqMan probe ID: Hs01123712_m1); smoothelin (SMTH, TaqMan probe ID: Hs00426835_g1); desmin (DES, TaqMan probe ID: Hs00157258_m1); glyceraldehyde 3-phosphate dehydrogenase (GAPDH, TaqMan probe ID: Hs03929097_g1) (Life Technologies, Grand Island, NY). The regulation of IGF-IEc transcription by TGF-β1 (R&D Systems, Minneapolis, MN; cat. no. 240-B-002/CF) was measured by addition of 1–10 ng/ml rhTGF-β1 in the presence and absence of the Smad3 inhibitor SIS3 (6,7-dimethoxy-2-((2E)-3-(1-methyl-2-phenyl-1H-pyrrolo[2,3-b]pyridin-3-yl-prop-2-enoyl))-1,2,3,4-tetrahydroisoquinoline; Calbiochem; cat. no. 566405) or in the presence of a neutralizing antibody to TGF-β1 (R&D Systems, Minneapolis, MN; cat. no. MAB1835) (10, 18).
Immunological detection of MGF.
A polyclonal antibody was raised in rabbits against a peptide immunogen (CKKKEQRREIGSRNA; lot no. 27993-2) based on the predicted amino acid sequence of human MGF (GenScript, Piscataway, NJ). The antibody was used to detect Ec peptide by immunofluorescence and by ELISA. Ec peptide was identified in smooth muscle cells by immunofluorescence using frozen sections of human intestine and in cultured cells by using a 1:250 dilution of anti-human Ec antibody or preabsorbed antibody as control. Ec peptide levels were measured in whole cell lysates prepared from muscle cells isolated from strictures and normal resection margins by using ELISA in plates coated with a 1:5,000 dilution of anti-human MGF antibody. The threshold of detection was 7 pg/ml. No appreciable cross-reactivity with rhIGF-I was detected.
Immunoblot analysis.
Cell lysates were prepared as described previously either from freshly isolated smooth muscle cells from strictured intestine or normal proximal margin in the same patient or from primary cultures of muscle cells (10, 12). Phosphorylated signaling intermediates were measured in cultured muscle cells stimulated with a peptide corresponding to the COOH-terminal 25 amino acids of human MGF termed synthetic MGF (Phoenix Pharmaceuticals, Phoenix, AZ; cat. no. 033-35). Immunoblot analysis was used to measure phospho-Erk5(T218/Y220) and Erk5 (cat. nos. 3371 and 3372), phospho-Erk1/2(T202/Y204) and Erk1/2 (cat. nos. 4377 and 9102), and phospho-AKT(S473) and Akt (cat. nos. 9271 and 9272) (Cell Signaling Technologies, Danvers, MA) and phospho-MEF2C(S387) (cat. no. 13920-R), phospho-MEF2C(T300) (cat. no. 130201), and total MEF2C (cat. no. 13266) (Santa Cruz Biotechnologies, Santa Cruz, CA) levels. Results were normalized to either unphosphorylated protein or β-actin (Sigma-Aldrich. St. Louis, MO; cat. no. A5441) levels as appropriate. IGF-I receptor (cat. no. 3027) phosphorylation was measured in phospho-tyrosine (cat. no. 9411) (Cell Signaling Technologies) immunoblots as described previously (22).
Measurement of smooth muscle cell hypertrophy.
Smooth muscle cell hypertrophy was measured in cells cultured on registered coverslip slides and microscope stage and loaded with Vybrant CFDA SE cell tracer (Life Technologies, Grand Island, NY). Medium was changed to Complete serum-free medium (Mediatech, Manassas, VA) on day 0. Either 100 nM synthetic MGF or 100 nM rhIGF-I was added daily for 3 additional days. Cell volume of the same groups of muscle cells, ∼25 cells per region, were imaged daily using confocal microscopy to develop Z-stacks and analyzed with Volocity software (Perkin-Elmer, Waltham, MA). Results were expressed as mean cell volume (μm3/cell). Hypertrophy was also measured from the ratio of total cellular protein, measured by the Bio-Rad protein assay (Bio-Rad, Hercules, CA; cat. no. 5000002) to cell number in cultured muscle cells treated in the same fashion with either 100 nM synthetic MGF or 100 nM rhIGF-I, and in the presence or absence of the selective Erk5 inhibitor BIX02188 (30 μM) (35) (Selleck Chemicals, Houston, TX; cat. no. S1530).
The role of MGF to regulate hypertrophy was also examined by incubation of muscle cells with synthetic MGF for a similar 4-day period followed by measurement of smooth muscle protein-specific genes including α-smooth muscle actin (ACTA), γ-smooth muscle actin (ACTG2), and smoothelin (SMTH) as well as desmin (DES) in cells transfected with MEF2C siRNA (Applied Biological Materials, Richmond, BC, Canada; cat. no. i029050a-d) or scrambled sense as control (Applied Biological Materials; cat. no. LV015-G).
The role of MGF to regulate hypertrophy in a paracrine manner was investigated by incubation of normal muscle cells with supernatants from strictured muscle cells that were pretreated by addition of individual neutralizing antibody for MGF or pro-IGF-IEc (GroPep Bioreagents, Thebarton, SA, Australia; cat. no. PAAS1) (2.5 and/or 10 μg/ml) compared with control IgG (10 μg/ml).
Immunofluorescent staining.
Immunofluorescent staining of MGF was performed in 7-μm cryostat tissue sections or primary cultured cells. Tissue sections were fixed overnight in 4% paraformaldehyde-sucrose (20% wt/vol) solution at 4°C prior to sectioning. Tissue sections or cultured cells were incubated with acetone for 5 min at −20°C and air dried for 10 min, followed by one wash in PBS. Sections or cells were then permeabilized for 15 min in PBS containing 0.3% Triton X-100 (PBST), washed three times with PBST at room temperature, and then blocked in PBS containing 5% normal donkey serum (NDS) for 1 h at room temperature. Sections or cells were incubated with primary antibody containing 3% NDS for overnight at 4°C. Slides were washed three times in PBST prior to incubation with secondary antibody (AlexaFluor 488 or 594 goat anti-rabbit or donkey anti-mouse; 1:800, Invitrogen, Molecular Probes; cat. no. A11034 and A21203) for 2 h at room temperature. After washing in PBS, sections were mounted with Duolink In Situ Mounting Medium with DAPI (Olink Bioscience, Uppsala, Sweden; cat. no. 82040-0005) for 15 min. Stained slides were analyzed with a Leica TCS-SP2 AOBS Confocal Laser Scanning Microscope (Leica, Buffalo Grove, IL).
Measurement of smooth muscle cell hyperplasia.
Muscle cells hyperplasia was measured from the incorporation of [3H]thymidine into quiescent smooth muscle cells in culture following the addition of 100 nM synthetic MGF peptide or 100 nM rhIGF-I as previously reported (21).
siRNA-mediated knockdown of MEF2C.
The participation of MEF2C in MGF-mediated hypertrophy was examined by siRNA-mediated MEF2C knockdown. Muscle cells growing in primary culture were transfected with a pool of siRNAs targeting MEF2C or scrambled antisense as negative control (Applied Biological Materials, BC, Canada). Transfection was performed by using X-treme transfection reagent (Roche, MA; product no. 04476093001) in serum-free medium. After 24 h cells were incubated with 100 nM synthetic MGF. We transfected 2 μg DNA (0.5 μg/μl) per well of a six-well plate when cell confluence reached 50–75%. After 24 h cells were incubated with 100 nM synthetic MGF for different time points.
Statistical analysis.
Values represent means ± SE of n experiments, where n represents the number of experiments on cells derived from separate subjects or animals. Statistical significance was tested by Student's t-test for either paired or unpaired data as appropriate. Comparison between multiple groups were made by ANOVA (>2 groups) with a Tukey's test for post hoc comparisons. Significance was assumed for P < 0.05.
RESULTS
IGF-IEc, Ec peptide, and muscle-specific gene expression increases in fibrostenotic Crohn's disease.
IGF-IEc transcript levels in muscle cells from normal intestine (patients undergoing resection of normal intestine and without Crohn's disease) were compared with IGF-IEc transcript levels in muscle cells isolated from the normal ileal resection margin and affected segments of patients with Montreal B1 (inflammatory), B2 (fibrostenotic) and B3 (penetrating) phenotype Crohn's disease (33). In muscle cells isolated from normal non-Crohn's intestine IGF-IEc transcript levels were low (Fig. 2A). In Montreal B1 and B3 disease, transcript levels in the affected segment of ileum were significantly lower than in the normal resection margin of the same patient. In contrast, IGF-IEc transcript levels were significantly higher in the strictured muscle cells compared with the normal resection margin in the same patient (Fig. 2A). These results could not be attributed to differences in inflammation (24). We have previously shown that in muscle cells of strictured intestine, IGF-IEa mRNA transcripts increase approximately fourfold compared with the identical levels of IGF-IEa transcript abundance measured in normal proximal resection margin and normal non-Crohn's intestinal muscle cells (12).
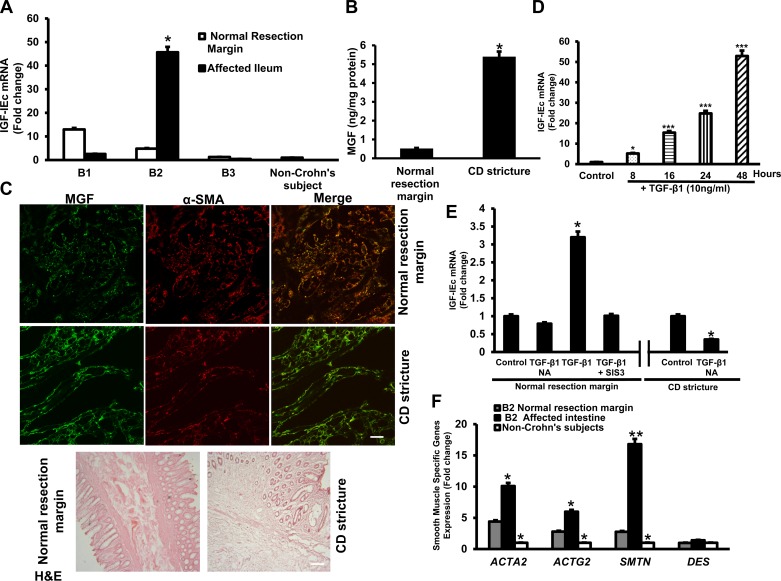
Fig. 2.
Increased IGF-IEc and E peptide (MGF) in smooth muscle cells of stricturing Crohn's disease. A: transcript levels of IGF-IEc were low in normal non-Crohn's disease intestinal muscle. IGF-IEc transcripts specifically increase in muscle cells of strictures in Montreal B2 Crohn's disease even compared with the normal resection margin of the same patient. The opposite pattern was seen in muscle cells of Montreal B1 and B3 phenotype Crohn's disease in which IGF-IEc transcripts are decreased in the affected segments. IGF-IEc transcripts were measured by quantitative RT-PCR (qRT-PCR) with the 2−ΔΔCt method. B: E peptide (MGF) protein levels increased in muscle cells of strictures in Crohn's disease compared with normal margin in the same patient. E peptide levels were measured by ELISA. C: representative immunofluorescent images of immunoreactive E peptide. Greater levels of immunoreactive E peptide were seen in the muscle cells of the muscularis propria of strictured intestine compared with normal resection margin in the same patient. D: exogenous TGF-β1 (10 ng/ml) elicited time-dependent increase in IGF-IEc transcripts in primary cultures of normal muscle cells. E: the increase in IGF-IEc expression after 8 h incubation with 10 ng/ml TGF-β1 in primary cultures of normal resection margin muscle cells is abolished by the Smad3 inhibitor SIS3 (10 μM). Immunoneutralization of endogenous TGF-β1 (1 μg/ml TGF-β1 antibody) in primary cultures of muscle cells from strictured intestine decreased basal IGF-IEc transcript levels compared with incubation with isotype control antibody. F: transcript levels of the smooth muscle-specific genes: SMTH (smoothelin), DES (desmin), ACTA (α-smooth muscle actin), and ACTG2 (γ-enteric muscle actin) were increased in strictured intestinal muscle compared with normal intestine in the same patient or to non-Crohn's patient intestine. Transcript levels were measured by quantitative RT-PCR using GAPDH as control. Results are means ± SE of 4–6 paired samples. *P < 0.05 vs. normal resection margin in the same patient or untreated cells. **P < 0.01 vs. control. ***P < 0.001 vs. control. CD, Crohn's disease; α-SMA, α-smooth muscle actin; H&E, hematoxylin and eosin; NA, neutralizing antibody.
The increase in IGF-IEc transcripts observed in Montreal B2 phenotype Crohn's disease was accompanied by a concomitant increase in MGF protein levels. In muscle cells isolated ex vivo from normal proximal resection margin, Ec protein levels were low, 0.52 ± 0.04 ng/mg protein (Fig. 2B) and increased to 5.4 ± 0.3 ng/mg protein in muscle cells from strictured intestine. By comparison, we have shown that levels of mature IGF-I are on the order of 2 ng/mg protein in normal muscle cells and increase twofold in muscle cells of strictured intestine (12).
Immunofluorescent staining of histological sections of patients with Crohn's disease demonstrated colocalization of MGF and α-smooth muscle actin in smooth muscle cells of the normal resection margin of proximal ileum. The intensity of MGF immunofluorescence was increased in the strictured ileum in these patients (Fig. 2C).
In human mesenchymal cells and murine intestinal smooth muscle cells, IGF-IEa expression is regulated by TGF-β1 (27, 34). We therefore investigated the ability of TGF-β1 to regulate IGF-IEc transcription. In normal human intestinal smooth muscle TGF-β1 (10 ng/ml) increased IGF-IEc transcripts over 48 h (Fig. 2D). Using a concentration of exogenous TGF-β1 that is comparable to that measured in intestinal muscle in Crohn's disease, 1 ng/ml, we found that the increase in IGF-IEc transcripts induced by TGF-β1 after 8 h was abolished in the presence of the Smad3 inhibitor, SIS3 (10 μM) (10, 25). In untreated muscle cells from strictured intestine of Crohn's disease, immunoneutralization of the constitutively upregulated TGF-β1 with a neutralizing antibody (1 μg/ml) resulted in a significant reduction in IGF-Ec transcripts indicating that endogenous TGF-β1 regulates IGF-IEc expression in these cells (Fig. 2E).
The expression of smooth muscle-specific genes, α-smooth muscle actin (ACTA), γ-smooth muscle actin (ACTG2), smoothelin (SMTH), and desmin (DES) was measured in muscle cells from non-Crohn's subjects and from the normal and affected segments of intestine in the same Crohn's patients with stricturing disease. Compared with muscle cells of non-Crohn's patients, and the normal intestine in Montreal B2 Crohn's disease, the level in the affected stricture was significantly increased for each gene examined except for desmin (Fig. 2F).
Synthetic MGF phosphorylates Erk5.
The signaling pathways activated by MGF in muscle cells were examined in primary cultures of human intestinal smooth muscle cells. Treatment of muscle cells with 100 nM synthetic MGF peptide for periods up to 240 min elicited no detectable phosphorylation of the IGF-I receptor (IGF-IR) compared with total IGF-IR protein level (Fig. 3A). In contrast, treatment of cells with 100 nM rhIGF-I elicited prompt IGF-I receptor phosphorylation compared with unchanged total IGF-IR protein level (Fig. 3A). Treatment of cultured human intestinal muscle cells with 100 nM synthetic MGF peptide also elicited prompt phosphorylation of Erk5(T218/Y220) and Erk1/2(T202/Y204) but only modest transient phosphorylation of Akt(S473), unlike that in response to treatment with IGF-I that elicited prompt phosphorylation of all three signaling intermediates within 2 min (Fig. 3B). Activation of phospho-Erk5(T218/Y220) by synthetic MGF was accompanied by translocation of Erk5 from the cytoplasm to the nucleus (Fig. 3C); translocation of pErk5(T218/Y220) did not occur in response to treatment with rhIGF-I. In cells incubated with the selective Erk5 inhibitor 30 μM BIX02188, both Erk5(T218/Y220) phosphorylation and its nuclear translocation were inhibited. Erk5 phosphorylation and translocation were not affected by the MEK1/2 inhibitor PD98059 (Fig. 3C).
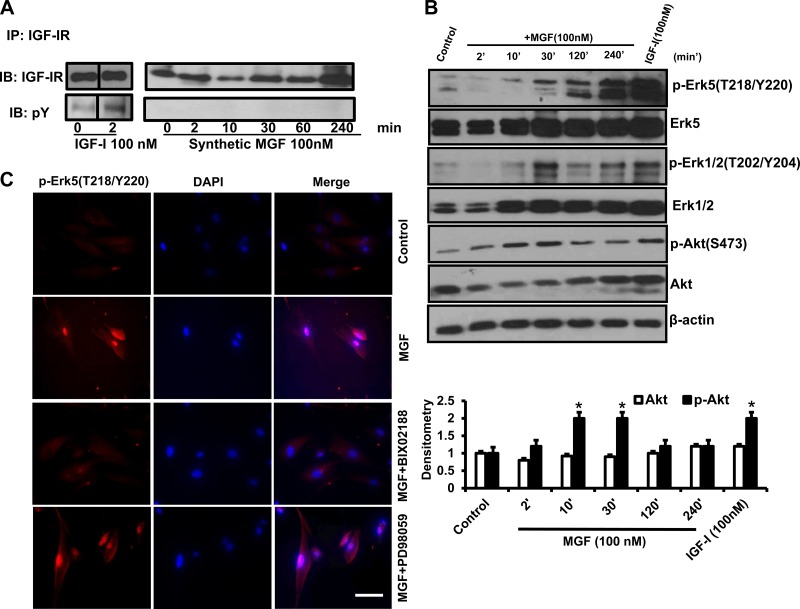
Fig. 3.
Synthetic MGF activates Erk-5 and induces Erk-5 nuclear translocation. A: rhIGF-I but not synthetic MGF induced IGF-I receptor phosphorylation. IGF-IR phosphorylation was measured in phosphotyrosine immunoblots of IGF-IR immunoprecipitates of human intestinal smooth muscle cells stimulated with synthetic MGF (100 nM) or rhIGF-I (100 nM). B: representative immunoblots showing that synthetic MGF (100 nM) elicited time-dependent phosphorylation of Erk5(T218/Y220) and Erk1/2(T202/Y204) but only modest transient phosphorylation of Akt(S473) in normal human intestinal muscle cells. In contrast, in addition to Erk5(T218/Y220) and Erk1/2(T202/Y204), rhIGF-I (100 nM, 2 min) also elicits Akt(S473) phosphorylation in primary cultures of normal human intestinal muscle. C: synthetic MGF (100 nM) elicited prompt, within 10 min, Erk-5(T218/Y220) phosphorylation and nuclear translocation that was inhibited by the selective Erk-5 inhibitor BIX02188 (30 μM). Cell nuclei were counterstained with DAPI. *P < 0.05 vs. control.
Nuclear translocation of activated Erk5 and MEF2C in response to synthetic MGF.
The transcription factor myocyte enhancer factor 2 C (MEF2C) has been reported to regulate muscle and cardiovascular development (38). It is involved in cardiac and skeletal muscle differentiation through transcriptional regulation of inducible gene expression during myocardia and skeletal muscle cell hypertrophy (38–39). In the present study, the ability of MGF to induce MEF2C activation and nuclear translocation was examined in two ways: by measurement of MEF2C(T300) and MEF2C(S387) phosphorylation in vivo, and by the ability of synthetic MGF to elicit Erk-5-dependent MEF2C activation and their nuclear cotranslocation.
In vivo smooth muscle cells of strictured intestine have increased levels of p-MEF2C(S387) but not p-MEF2C(T300) compared with the normal resection margin in the same patient (Fig. 4A). In primary cultured normal muscle cells synthetic MGF (100 nM) increased MEF2C(S387) phosphorylation by 150 ± 7.8% above basal (Fig. 4B). Incubation of muscle cells with 100 nM synthetic MGF elicited phosphorylation of Erk5(Y218/S220) and MEF2C(S387) that was inhibited in the presence of the Erk5 inhibitor BIX-02188 (Fig. 4C), whereas MGF-elicited phosphorylation of Erk1/2 (T202/Y204) was decreased marginally by BIX02188.
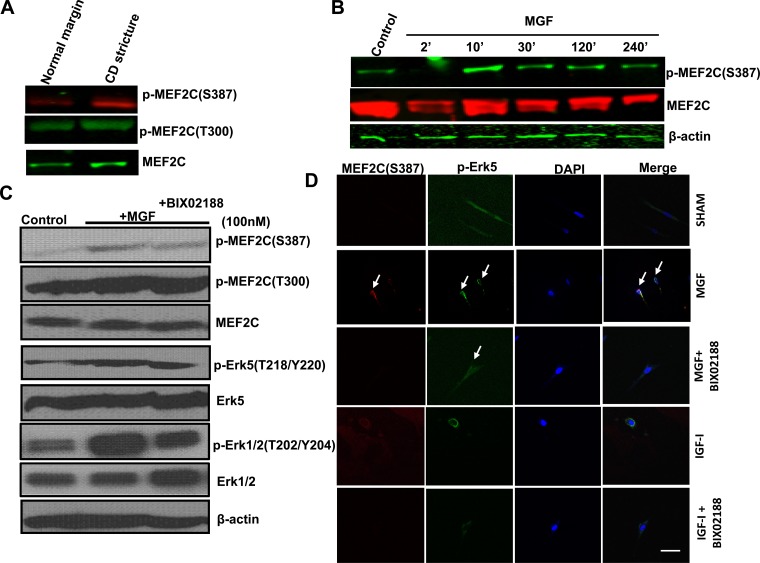
Fig. 4.
MEF2C activity is increased in muscle cells of stricturing Crohn's disease and increased in normal muscle cells by synthetic MGF. A: phosphorylation of MEF2C(S387) but not MEF2C(T300) is increased in muscle cells isolated from strictured intestine compared with normal resection margin in the same patient with stricturing Crohn's disease. B: synthetic MGF (100 nM) elicits time-dependent MEF2C(S387) phosphorylation in normal human intestinal muscle cells. MEF2C phosphorylation was measured by Western blot analysis. C: synthetic MGF (100 nM)-induced phosphorylation of MEF2C(S387) and Erk5(T218/Y220) was decreased in the presence of the selective Erk5 inhibitor BIX02188 in muscle cells isolated from normal resection margin. D: synthetic MGF-induced phosphorylation of Erk5(T218/Y220), colocalization with MEF2C and (indicated by white arrow) that was lost in the presence of the selective Erk-5 inhibitor BIX02188. In contrast, rhIGF-I (100 nM) had no effect on colocalization or nuclear translocation of Erk5 and MEF2C.
Synthetic MGF (100 nM) stimulated the translocation and colocalization of p-Erk5(T218/Y220) and p-MEF2C(S287) from the cytosol to colocalize in the primary cultured normal human intestinal smooth muscle cells (Fig. 4D). In the presence of the selective Erk5 inhibitor BIX02188 (30 μM), translocation and nuclear colocalization of Erk5 and MEF2C were lost (Fig. 4D). In contrast, although 100 nM IGF-I elicited Erk5(T218/Y220) phosphorylation, it did not elicit phosphorylation of MEF2C, suggesting this was a crucial step in the hypertrophy induced by MGF but not by IGF-I.
Synthetic MGF elicits hypertrophy but not hyperplasia of human intestinal smooth muscle cells.
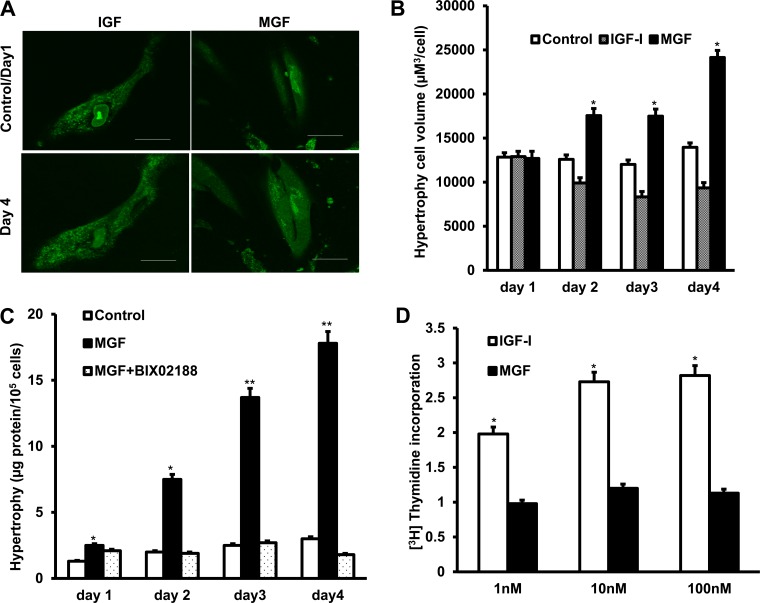
Cell hypertrophy was measured over the course of a 4-day period in two ways: by measurement of cell volume by confocal microscopy in Z-stacks and by the relative amount of protein per cell.
Synthetic MGF (100 nM) elicited a significant time-dependent increase in basal cell volume (12,840 ± 515 μm3/cell) to 188 ± 3% above basal volume after 4 days (Fig. 5, A and B). In contrast, 100 nM rhIGF-I elicited a time-dependent decrease in cell volume to 45 ± 11% of basal volume after 3 days (Fig. 5, A and B) (12).
Fig. 5.
Hypertrophy of human intestinal muscle cells is induced by synthetic MGF by an Erk-5-dependent mechanism. A: representative images of muscle cell hypertrophy induced by synthetic MGF but cell division induced by rhIGF-I. Hypertrophy was measured in the same groups of cells by confocal imaging of Vybrant CFDA SE cell tracer-loaded cells treated for 4 days with either synthetic MGF (100 nM) or rhIGF-I (100 nM). B: Z-stack analysis showing increased mean cell volume in cells treated with synthetic MGF (100 nM) but not rhIGF-I (100 nM). C: smooth muscle cell hypertrophy induced by synthetic MGF is inhibited by a selective Erk5 inhibitor. Hypertrophy was measured in cells incubated with synthetic MGF (100 nM) for 4 days in the presence or absence of BIX02188 (30 μM) from total cell protein per 104 cells. D: smooth muscle cell proliferation is induced by rhIGF-I but not synthetic MGF. Proliferation was measured by [3H]thymidine incorporation into quiescent muscle cells incubated for 24 h with either synthetic MGF (1–100 nM) or rhIGF-I (1–100 nM). Results are means ± SE of 5–6 separate experiments. *P < 0.05 vs. control.
In cultured normal human intestinal smooth muscle cells, incubation with synthetic MGF (100 nM) increased total protein from 2.11 to 19.23 μg protein/105 cells after 4 days (Fig. 5C). In the presence of the Erk5 inhibitor BIX 02188, the ability of synthetic MGF to stimulate muscle cell hypertrophy was lost and total cell protein decreased to 1.05 μg/105 cells after 4 days (Fig. 5C).
In contrast to the effects of synthetic MGF on hypertrophy, incubation of cultured muscle cells with 100 nM synthetic MGF for 24 h did not stimulate proliferation as measured by [3H]thymidine incorporation, whereas incubation for 24 h with 100 nM rhIGF-I did elicit a concentration-dependent increase in [3H]thymidine (Fig. 5D) (Fig. 5D).
Taken together these results indicate that MGF causes cellular hypertrophy, whereas IGF-I stimulates proliferation (i.e., cell division and decreased cell volume) in these cells (19).
Synthetic MGF-induced intestinal smooth muscle hypertrophy requires MEF2C.
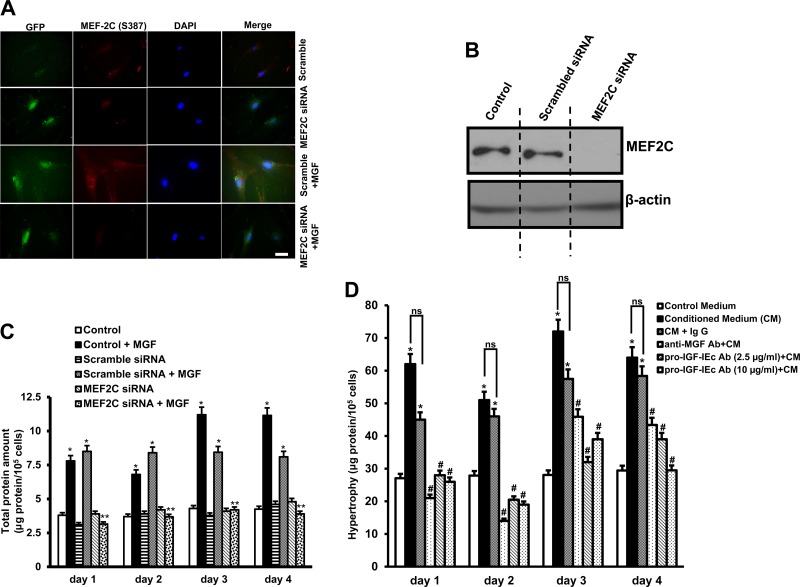
The role of the muscle-specific transcription factor MEF2C to regulate synthetic MGF-dependent hypertrophy of intestinal smooth muscle cells was examined in cells where MEF2C expression was knocked down by use of MEF2C siRNA. Transfection efficiency at 4 days was ∼75% as measured by immunofluorescent staining of cells transfected with GFP-tagged scrambled sense or GFP-tagged pooled MEF2C siRNA (Fig. 6A). Immunoblot analysis confirmed that transfection of scrambled sense siRNA did not affect MEF2C protein levels, whereas MEF2C protein levels were decreased 167 ± 8.5% of MEF2C by siRNA (Fig. 6B).
Fig. 6.
Synthetic MGF-induced hypertrophy of human intestinal smooth muscle cells is MEF2C dependent. A: synthetic MGF-induced MEF2C(S387) phosphorylation is inhibited in human intestinal muscle cells transfected with MEF2C siRNA but is not affected by transfection of scrambled sense siRNA. B: immunoblot analysis of MEF2C protein levels in human intestinal smooth muscle cells transfected with MEF2C siRNA. β-Actin was used as a loading control. C: hypertrophy of human intestinal muscle cells induced by incubation for 4 days with synthetic MGF (100 nM) was abolished in cells transfected with MEF2C siRNA but was not affected by transfection of scrambled sense siRNA. Hypertrophy was measured in cells incubated with synthetic MGF (100 nM) or rhIGF-I (100 nM) for 4 days with or without transfection of MEF2C siRNA. Hypertrophy was measured from total cell protein amount per 105 cells. D: smooth muscle hypertrophy is induced by incubation of normal muscle cells with conditioned medium of strictured muscle cells. Hypertrophy induced by conditioned medium is inhibited by immunoneutralization of MGF or pro-IGF-IEc but not affected by negative control IgG. Results are means ± SE of 5–6 separate experiments. Results are means ± SE of 5–6 separate experiments. *P < 0.05 vs. control. **P < 0.05 vs. scrambled sense siRNA transfection. #P < 0.05 vs. negative control IgG. ns, Not significant. Microscopy scale bar = 100 μm
In cells transfected with MEF2C siRNA, but not scrambled siRNA, the ability of synthetic MGF to elicit MEF2C activation and nuclear translocation was lost. In these cells transfected with MEF2C siRNA, hypertrophy of muscle cells induced by synthetic MGF (100 nM) was inhibited by 53 ± 2.65% after 4 days of transfection compared with hypertrophy of muscle following transfection of scrambled sense siRNA (Fig. 6C).
Smooth muscle hypertrophy is induced by autocrine MGF.
We next examined the ability of autocrine MGF to stimulate smooth muscle hypertrophy by treating primary cultured smooth muscle cells with media conditioned by primary cultured muscle cells from strictured intestine (with constitutively increased MGF production). Incubation of muscle cells with conditioned media elicited an increase in the total protein per cell over 4 days compared with treatment with fresh culture media (Fig. 6D). The increase in muscle cells hypertrophy stimulated by conditioned medium was inhibited by the addition of an anti-MGF antibody (10 μg/ml) but not affected by incubation with control rabbit IgG. Muscle cell hypertrophy stimulated by conditioned medium was also inhibited by an anti-pro-IGF-IEc (2.5 and 10 μg/ml) (Fig. 6D).
Synthetic MGF-induced smooth muscle-specific protein expression is mediated by Erk5 and MEF2C.
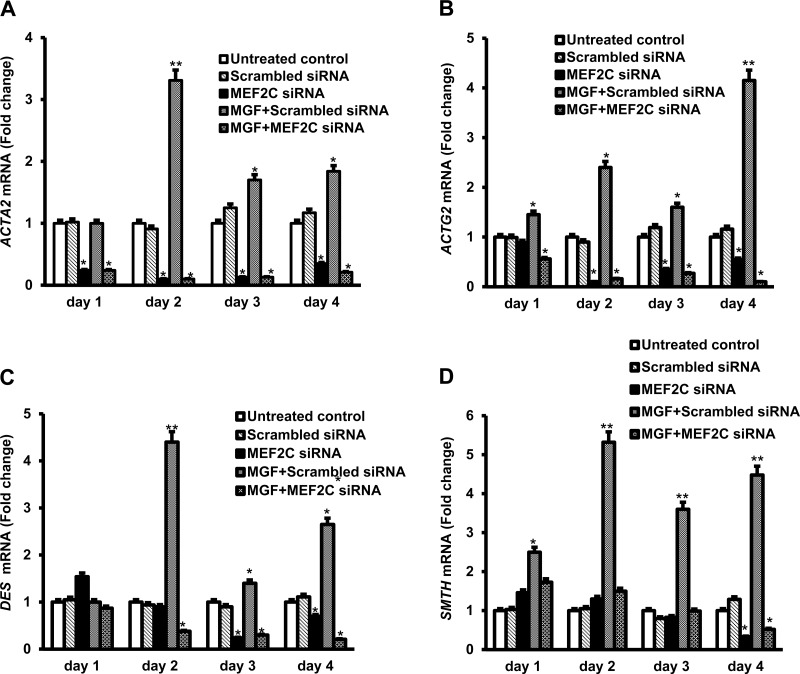
Because expression of genes encoding a number of smooth muscle-specific proteins was increased in muscle cells of strictured intestine, we next examined the possibility the increased expression could be the result of increased IGF-IEc expression and MGF production in these cells. Primary cultured muscle cells isolated from normal intestine in patients susceptible to fibrostenotic disease were treated with synthetic MGF for 4 days and gene expression was measured by qRT-PCR. Synthetic MGF (100 nM) elicited time-dependent increased expression of all four smooth muscle-specific genes: ACTA, ACTG2, DES, and SMTH. Compared with control cells or cells transfected with scrambled sense siRNA, in cells transfected with MEF2C pooled siRNA the ability of synthetic MGF to increase smooth muscle-specific protein expression was lost (Fig. 7, A–D). It is noteworthy that in the untreated control cells MEF2C silencing decreased basal expression of the smooth muscle-specific genes indicating that they are normally under transcriptional regulation by MEF2C.
Fig. 7.
Synthetic MGF-induced expression of smooth muscle-specific genes in human intestinal smooth muscle cells is MEF2C dependent. Synthetic MGF elicited time-dependent increase in expression of ACTA2 (A), ACTG2 (B), DES (C), and SMTH (D) over a 4-day period. Increased gene expression was inhibited by transfection of MEF2C siRNA but not affected by scrambled sense siRNA. Cultured smooth muscle cells incubated with synthetic MGF (100 nM) for 4 days following transfection of MEF2C pooled siRNA or scrambled sense siRNA. Gene expression was measured from the relative transcript levels by using qRT-PCR with GAPDH as control. Results are means ± SE of 3 separate experiments. *P < 0.05 vs. control. **P < 0.05 vs. scrambled sense siRNA transfection.
DISCUSSION
Intestinal mesenchymal cells including smooth muscle cells are critical players in three features that characterize fibrostenotic Crohn's disease: cell hypertrophy, cell proliferation, and increased net extracellular matrix production particularly of collagen I (13, 23). Our previous work and that of others has shown that IGF-IEa gene expression and IGF-I levels are increased in the muscle cells of strictured intestine in patients with Montreal B2 phenotype Crohn's disease (12). Autocrine production of IGF-I by intestinal smooth muscle stimulates muscle cell hyperplasia and contributes to excess collagen I production in conjunction with that regulated by TGF-β1 and IGF binding protein (IGFBP)-3 and IGFBP-5 (10, 11, 27, 40). The present study shows that in stricturing Crohn's disease expression of the alternative igf1 splice variant, IGF-IEc and an Ec peptide (MGF) are also increased in response to autocrine TGF-β1 uniquely in fibrostenotic Crohn's disease. This Ec peptide, like synthetic MGF, stimulates smooth muscle cell hypertrophy through activation of Erk5 and the muscle-specific transcription factor, MEF2C, which in turn regulates smooth muscle-specific gene expression.
On the basis of their predicted sequence and structure, neither an Ec peptide nor MGF should activate the cognate IGF-I receptor (28). An MGF-specific receptor has yet to be identified or characterized. Available evidence, however, suggests that although mature IGF-I peptide activates both Erk pathways and Akt pathways, synthetic MGF activates predominantly Erk pathways (32). In both cardiac and skeletal muscle cells, activation of Erk5 by other factors activates MEF2C and transcriptional regulation of muscle-specific protein genes, leading to muscle hypertrophy (8). Unlike IGF-I, which activates the well-characterized IGF-I receptor, a specific cell surface receptor has yet to be identified that directly mediates the effects of MGF (28). Notably, the interaction of E peptides with the IGF-I receptor has been shown in the C2C12 skeletal muscle cell line (4, 9). Although E peptides did not elicit IGF-I receptor phosphorylation, blockade of IGF-I receptor with a tyrosine kinase inhibitor abolished their ability to elicit Erk1/2 signaling and skeletal muscle proliferation, migration, and differentiation (4). Our results confirm that synthetic MGF does not elicit IGF-I receptor tyrosine phosphorylation in intestinal smooth muscle cells but does elicit preferential Erk signaling, including Erk5, and subsequent MEF2C activation but not constant Akt activation. These findings suggest that a cytosolic signaling cascade is involved. It is also noteworthy that, in contrast to IGF-I that activates Erk signaling, including Erk5, MGF also activates MEF2C, which controls transcription of smooth muscle-specific genes that are key participants in the hypertrophic response of muscle.
In contrast to our findings and those in cardiac myocytes (24), Peng et al. (31) provided evidence in osteoblasts that the consensus nuclear localization signal sequence, “RRRK” or “K-K/R-X-K,” found within the E domain may mediate direct nuclear effects of E peptide. The notion that E peptide may exert predominantly nuclear effects is supported by the relative distribution of intracellular E peptide being higher in the nucleus than in the cytoplasm, where it may interact with transcription factors through an IGF-I-independent mechanism.
The synthetic MGF used in our studies (albeit on a background of autocrine IGF-I production in these cells) did not alter IGF-I receptor tyrosine phosphorylation and proliferation of muscle cells (19). Synthetic MGF, but not IGF-I, elicited muscle cell hypertrophy, indicating that the effects of Ec peptides may be cell type specific and their effects depend on the relevant cellular programing (5–7, 15). Our data using synthetic MGF and immunoneutralization of Ec peptides should be interpreted in light of the finding that immunoneutralization of pro-IGF-IEc to also inhibit hypertrophy induced by conditioned media (Fig. 6D). Our Ec peptide antibody may immunoneutralize pro-IGF-IEc and our results are attributable to actions of pro-IGF-Ec since pro-IGF-IEc also contains the Ec peptide domain thus MGF. In either case, an IGF-IEc-derived Ec peptide elicits hypertrophy of human intestinal smooth muscle.
MEF2C is a muscle-specific DNA binding protein that recognizes the consensus DNA sequence YTA(A/T)4TAR. MEF2C-homodimers or MEF-2-heterodimers bind DNA via the 57-amino acid MADS-box domain in the promoter regions of numerous muscle-specific genes (2, 17). MEF2C is implicated in the regulation of cardiac, skeletal, and smooth muscle hypertrophy as well as in hypertrophy of chondrocytes. In these tissues, and shown in this paper, hypertrophied human intestinal smooth muscle cells have increased phosphorylation of MEF2C(S387). Several mechanisms can account for the ability of MEF2C activation to elicit smooth muscle cell hypertrophy. Consensus MEF2C DNA binding sites are located in the 5′-untranslated region (5′-UTR) of several genes encoding key intestinal smooth muscle proteins. Increased α-smooth muscle actin and γ-enteric actin are characteristic features of activated mesenchymal cells including smooth muscle cells in Crohn's disease (3). Interestingly, ACTG1/2 (γ-enteric actins 1 and 2) do not possess consensus MEF2C DNA binding sites. But other Erk5 activated transcription factors, including CREB, do have consensus binding sites in the 5′-UTR of ACTG (γ-actin) including CREB. MEF2C also regulates transcription of intestinal smooth muscle-specific genes including serum response factor which elicits a switch from α-myosin heavy chain to β-myosin heavy chain production, reorganization of contractile proteins, and muscle cell hypertrophy (38).
In summary, the present study adds to our understanding of the development of fibrostenosis in the susceptible patient with Montreal B2 fibrostenotic Crohn's disease and actions of the autocrine IGF peptides IGF-I and MGF in this process. MGF derived from the IGF-Ec splice variant and IGF-I derived from the IGF-IEa splice variant are specifically increased in the affected segment of strictured intestine and characterize this phenotype of Crohn's disease (12). The increase in autocrine Ec peptide results in smooth muscle hypertrophy at least in part through MEF2C and its effect on expression of smooth muscle-specific protein genes. The increase in autocrine IGF-I concurrently results in smooth muscle hyperplasia and contributes to excess collagen I production. Thus all three features of fibrostenosis that characterize Montreal B2 fibrostenotic Crohn's disease are regulated by alternative splicing products of the igf1 gene. It is noteworthy that transcription of both the IGF-IEa and IGF-IEc splice variants are regulated by the locally increased levels of active TGF-β1 in intestinal mesenchymal cells in affected regions of fibrostenotic Crohn's disease (27, 34). The changes observed were not due to increased inflammation since our previous work has shown a level of inflammation in patients with fibrostenotic Crohn's disease that is similar to that in non-Crohn's disease control subjects (24). Once a mesenchymal cell is activated, this autocrine TGF-β1-dependent response increases IGF-IEa and IGF-IEc expression. This mechanism is self-perpetuating and can progress to fibrosis and bowel obstruction in the susceptible patient once initiated.
GRANTS
This study was supported by a grant DK49691 from NIH: National Institutes for Diabetes, Digestive and Kidney Diseases (JFK).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.L., K.H., and J.F.K. conception and design of research; C.L., K.V., and K.H. performed experiments; C.L. analyzed data; C.L. interpreted results of experiments; C.L. prepared figures; C.L. and J.F.K. drafted manuscript; C.L. and J.F.K. edited and revised manuscript; C.L. and J.F.K. approved final version of manuscript.
REFERENCES
- 1.Berg KM, Bowers JG, Kuemmerle JF. The IGF-IEa (IGF-I) and IGF-IEc (MGF) splice variants of the IGF-I gene mediate hypertrophy and hyperplasia of human intestinal smooth muscle. Gastroenterology 132: A238, 2007. [Google Scholar]
- 2.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol 14: 167–196, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Blennerhassett MG, Bovell FM, Lourenssen S, McHugh KM. Characteristics of inflammation-induced hypertrophy of rat intestinal smooth muscle cell. Dig Dis Sci 44: 1265–1272, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Brisson BK, Barton ER. Insulin-like growth factor-I E-peptide activity is dependent on the IGF-I receptor. PLoS One 7: e45588, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheema U, Brown R, Mudera V, Yang SY, McGrouther G, Goldspink G. Mechanical signals and IGF-I gene splicing in vitro in relation to development of skeletal muscle. J Cell Physiol 202: 67–75, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Dai Z, Wu F, Yeung EW, Li Y. IGF-IEc expression, regulation and biological function in different tissues. Growth Horm IGF Res 20: 275–281, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Dluzniewska J, Sarnowska A, Beresewicz M, Johnson I, Srai SK, Ramesh B, Goldspink G, Gorecki DC, Zablocka B. A strong neuroprotective effect of the autonomous C-terminal peptide of IGF-1 Ec (MGF) in brain ischemia. FASEB J 19: 1896–1898, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Drew BA, Burow ME, Beckman BS. MEK5/ERK5 pathway: the first fifteen years. Biochim Biophys Acta 1825: 37–48, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durzyńska J, Philippou A, Brisson BK, Nguyen-McCarty M, Barton ER. The pro-forms of insulin-like growth factor I (IGF-I) are predominant in skeletal muscle and alter IGF-I receptor activation. Endocrinology 154: 1215–1224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn RS, Mahavadi S, Murthy KS, Grider JR, Kellum JM, Akbari H, Kuemmerle JF. Endogenous IGFBP-3 regulates excess collagen expression in intestinal smooth muscle cells of Crohn's disease strictures. Inflamm Bowel Dis 17: 193–201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn RS, Mahavadi S, Murthy KS, Kellum JM, Kuemmerle JF. Insulin-like growth factor-binding protein-5 stimulates growth of human intestinal muscle cells by activation of Gαi3. Am J Physiol Gastrointest Liver Physiol 297: G1232–G1238, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn RS, Murthy KS, Grider JR, Kellum JM, Kuemmerle JF. Endogenous IGF-I and αVβ3 integrin ligands regulate increased smooth muscle hyperplasia in stricturing Crohn's disease. Gastroenterology 138: 285–293, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelbmann CM, Mestermann S, Gross V, Kollinger M, Scholmerich J, Falk W. Strictures in Crohn's disease are characterised by an accumulation of mast cells colocalised with laminin but not with fibronectin or vitronectin. Gut 45: 210–217, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentilucci UV, Caviglia R, Picardi A, Carotti S, Ribolsi M, Galati G, Petitti T, Afeltra A, Cicala M. Infliximab reverses growth hormone resistance associated with inflammatory bowel disease. Aliment Pharmacol Ther 21: 1063–1071, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Goldspink G. Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology (Bethesda) 20: 232–238, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Hill M, Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549: 409–418, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infantino V, Convertini P, Menga A, Iacobazzi V. MEF2C exon α: role in gene activation and differentiation. Gene 531: 355–362, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-β-induced extracellular matrix expression. Mol Pharmacol 69: 597–607, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kuemmerle JF. Autocrine regulation of growth in cultured human intestinal muscle by growth factors. Gastroenterology 113: 817–824, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Kuemmerle JF. Endogenous IGF-I protects human intestinal smooth muscle cells from apoptosis by regulation of GSK-3β activity. Am J Physiol Gastrointest Liver Physiol 288: G101–G110, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kuemmerle JF. IGF-I elicits growth of human intestinal smooth muscle cells by activation of PI3K, PDK-1, and p70S6 kinase. Am J Physiol Gastrointest Liver Physiol 284: G411–G422, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Kuemmerle JF, Murthy KS. Coupling of the insulin-like growth factor-I receptor tyrosine kinase to Gi2 in human intestinal smooth muscle: Gβγ-dependent mitogen-activated protein kinase activation and growth. J Biol Chem 276: 7187–7194, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Lee EY, Stenson WF, DeSchryver-Kecskemeti K. Thickening of muscularis mucosae in Crohn's disease. Mod Pathol 4: 87–90, 1991. [PubMed] [Google Scholar]
- 24.Li C, Iness A, Yoon J, Grider JR, Murthy KS, Kellum JM, Kuemmerle JF. Noncanonical STAT3 activation regulates excess TGF-β1 and collagen I expression in muscle of stricturing Crohn's disease. J Immunol 194: 3422–3431, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Flynn RS, Grider JR, Murthy KS, Kellum JM, Akbari HM, Kuemmerle JF. Increased activation of latent TGF-β1 by αVβ3 in human Crohn's disease and fibrosis in TNBS colitis can be prevented by cilengitide. Inflamm Bowel Dis 19: 2829–2839, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund PK, Zimmermann EM. Insulin-like growth factors and inflammatory bowel disease. Baillieres Clin Gastroenterol 10: 83–96, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Mahavadi S, Flynn RS, Grider JR, Qiao LY, Murthy KS, Hazelgrove KB, Kuemmerle JF. Amelioration of excess collagen IαI, fibrosis, and smooth muscle growth in TNBS-induced colitis in IGF-I(+/−) mice. Inflamm Bowel Dis 17: 711–719, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matheny RW Jr, Nindl BC, Adamo ML. Minireview: Mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology 151: 865–875, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B, Goldspink G. Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol 516: 583–592, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohneda K, Ulshen MH, Fuller CR, D'Ercole AJ, Lund PK. Enhanced growth of small bowel in transgenic mice expressing human insulin-like growth factor I. Gastroenterology 112: 444–454, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Peng Q, Qiu J, Sun J, Yang L, Zhang B, Wang Y. The nuclear localization of MGF receptor in osteoblasts under mechanical stimulation. Mol Cell Biochem 369: 147–156, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Philippou A, Papageorgiou E, Bogdanis G, Halapas A, Sourla A, Maridaki M, Pissimissis N, Koutsilieris M. Expression of IGF-1 isoforms after exercise-induced muscle damage in humans: characterization of the MGF E peptide actions in vitro. In Vivo 23: 567–575, 2009. [PubMed] [Google Scholar]
- 33.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 55: 749–753, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmons JG, Pucilowska JB, Keku TO, Lund PK. IGF-I and TGF-β1 have distinct effects on phenotype and proliferation of intestinal fibroblasts. Am J Physiol Gastrointest Liver Physiol 283: G809–G818, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Tatake RJ, O'Neill MM, Kennedy CA, Wayne AL, Jakes S, Wu D, Kugler SZ Jr, Kashem MA, Kaplita P, Snow RJ. Identification of pharmacological inhibitors of the MEK5/ERK5 pathway. Biochem Biophys Res Commun 377: 120–125, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Teng B, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 275: G342–G351, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Theiss AL, Fuller CR, Simmons JG, Liu B, Sartor RB, Lund PK. Growth hormone reduces the severity of fibrosis associated with chronic intestinal inflammation. Gastroenterology 129: 204–219, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Vyas DR, McCarthy JJ, Tsika RW. Nuclear protein binding at the β-myosin heavy chain A/T-rich element is enriched following increased skeletal muscle activity. J Biol Chem 274: 30832–30842, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Niu W, Nikiforov Y, Naito S, Chernausek S, Witte D, LeRoith D, Strauch A, Fagin JA. Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest 100: 1425–1439, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann EM, Li L, Hou YT, Mohapatra NK, Pucilowska JB. Insulin-like growth factor I and insulin-like growth factor binding protein 5 in Crohn's disease. Am J Physiol Gastrointest Liver Physiol 280: G1022–G1029, 2001. [DOI] [PubMed] [Google Scholar]