Abstract
Hyaluronic acid, a glycosaminoglycan in the extracellular matrix, binds to CD44 and Toll-like receptor 4 (TLR4). We previously addressed the role of hyaluronic acid in small intestinal and colonic growth in mice. We addressed the role of exogenous hyaluronic acid by giving hyaluronic acid intraperitoneally and the role of endogenous hyaluronic acid by giving PEP-1, a peptide that blocks hyaluronic acid binding to its receptors. Exogenous hyaluronic acid increased epithelial proliferation but had no effect on intestinal length. PEP-1 resulted in a shortened small intestine and colon and diminished epithelial proliferation. In the current study, we sought to determine whether the effects of hyaluronic acid on growth were mediated by signaling through CD44 or TLR4 by giving exogenous hyaluronic acid or PEP-1 twice a week from 3–8 wk of age to wild-type, CD44−/−, and TLR4−/− mice. These studies demonstrated that signaling through both CD44 and TLR4 were important in mediating the effects of hyaluronic acid on growth in the small intestine and colon. Extending our studies to early postnatal life, we assessed the effects of exogenous hyaluronic acid and PEP-1 on Lgr5+ stem cell proliferation and crypt fission. Administration of PEP-1 to Lgr5+ reporter mice from postnatal day 7 to day 14 decreased Lgr5+ cell proliferation and decreased crypt fission. These studies indicate that endogenous hyaluronic acid increases Lgr5+ stem cell proliferation, crypt fission, and intestinal lengthening and that these effects are dependent on signaling through CD44 and TLR4.
Keywords: hyaluronic acid, CD44, Toll-like receptor 4, intestinal growth, crypt fission, Lgr5 stem cell proliferation
hyaluronic acid (HA), a glycosaminoglycan polymer with repeating units of disaccharides composed of d-glucuronic acid and N-acetyl-d-glucosamine, is an important constituent of the extracellular matrix. HA is secreted by many cell types; it is assembled at the plasma membrane by HA synthases (HASs) and extruded into the extracellular space. The HA chain can extend up to 2 × 105 disaccharides and up to 25 μm long.
HA expression is increased in many injury states, including Crohn's disease in humans, dextran sodium sulfate (DSS)-induced colitis in mice, and intestinal radiation injury (8, 16, 24). The angiogenic, inflammatory, and immunostimulatory effects of HA are mediated by binding to its receptors. The HA receptor CD44 is expressed on the plasma membrane of most cells, including fibroblasts, smooth muscle cells, epithelial cells, and immune cells (19). In addition to binding to CD44, HA also binds to Toll-like receptors 2 and 4 (TLR2 and TLR4), which are components of the innate immune system (7, 20, 24). TLR2 and TLR4 are widely distributed in the gastrointestinal tract and participate in mediating the host response to commensal and pathogenic bacteria.
Although LPS produced by Gram-negative bacteria is the ligand typically associated with TLR4, several host molecules including HA are also TLR4 ligands. HA binding to TLR4 promotes epithelial proliferation and homeostasis in the DSS colitis model and the radiation injury in mice (16, 24). In the DSS colitis model, HA binding to TLR4 increases cyclooxygenase-2 expression and prostaglandin synthesis. These downstream signaling events promote increased epithelial cell survival and proliferation. The expression of HA is induced in DSS colitis through increased expression of HAS2 and HAS3, enzymes involved in HA synthesis. Moreover, exogenous HA is protective in wild-type (WT) mice in DSS-induced colitis; intraperitoneal administration of HA before DSS results in reduced weight loss and improved histology compared with administration of DSS alone. HA expression is also induced in the intestine by irradiation, and intraperitoneal administration of HA before radiation blocks radiation-induced apoptosis.
Not only does HA promote epithelial protection and repair in the intestine and colon, it also regulates epithelial proliferation and small intestinal growth during normal development (15). The effects of exogenous HA on intestinal and colonic growth were addressed by administering HA intraperitoneally to mice twice a week from 3–8 wk of age. Treatment with exogenous HA resulted in increases in villus height and crypt depth in the intestine, crypt depth in the colon, and epithelial proliferation in the intestine and colon. Exogenous HA had no effect on intestinal or colonic elongation. The effects of endogenous HA on intestinal and colonic growth were addressed by the administration of PEP-1, a 12-mer peptide that blocks the binding of HA to its receptors (10). PEP-1 administration to mice from 3–8 wk of age resulted in a marked decrease in intestinal and colonic length along with decreases in villus height and crypt depth in the intestine, crypt depth in the colon, and epithelial proliferation in the intestine and colon. These data indicate that the binding of endogenous HA to its receptors is required for normal intestinal and colonic growth.
Having found that endogenous HA is required for the normal elongation of the small intestine and colon and that exogenous HA promotes epithelial proliferation in the small intestine and colon, we sought to determine whether these effects of HA on small intestinal growth and epithelial proliferation are mediated by signaling through CD44 or TLR4. In addition, we sought to determine whether the effects of endogenous HA, which we observed in adult mice, were also seen in early postnatal life when small intestinal and colonic lengthening are driven by the proliferation of Lgr5+ epithelial stem cells followed by crypt fission (4).
MATERIALS AND METHODS
Chemicals and reagents.
HA (750 kDa) was obtained from Sigma-Aldrich (St. Louis, MO). The HA-binding peptide PEP-1 (H2N-GAHWQFNALTVR-OH) and scrambled control peptide (H2N-WRHGEALTAVNQ-OH) were obtained from New England Peptide (Gardner, MA).
Animals and experimental procedures.
WT, CD44−/−, and TLR4−/− mice on a C57BL/6J background were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in house. WT Lgr5/enhanced green fluorescent protein (EGFP), CD44−/− Lgr5/EGFP, and TLR4−/− Lgr5/EGFP reporter mice were each generated by initially breeding nonreporter WT, CD44−/−, or TLR4−/− mice with WT Lgr5-EGFP-ires-creERT2 mice and by multiple generation selective breedings obtaining mice that were WT, CD44−/−, or TLR4−/− and heterozygous for the Lgr5-EGFP-ires-creERT2 reporter. Mice were maintained on a 12-h:12-h light/dark schedule in a temperature-controlled specific pathogen-free facility and fed standard laboratory mouse chow. Animal procedures were carried out in accordance with the Washington University School of Medicine Animal Studies Committee, which approved the protocols.
For long-term growth studies, intraperitoneal injections of HA (30 mg/kg) or PEP-1 (40 mg/kg) were administered to male WT, CD44−/−, and TLR4−/− mice starting at weaning (3 wk of age) and continuing twice a week for 5 wk. Control mice received 0.9% saline. At 8 wk of age, mice from all treatment groups were given an intraperitoneal injection of a mixture of 5-bromo-2′-deoxyuridine (BrdU, 120 mg/kg) and 5-fluoro-2′-deoxyuridine (FdU 12 mg/kg) 90 min before they were euthanized to label S-phase cells.
For intestinal stem cell proliferation and crypt fission studies in postnatal mice, intraperitoneal injections of HA (30 mg/kg) or PEP-1 (40 mg/kg) were administered to WT Lgr5/EGFP, CD44−/− Lgr5/EGFP, and TLR4−/− Lgr5/EGFP reporter mice starting at age 7 days and continuing every other day to age 14 days. Control mice received 0.9% saline. At age 14 days, mice received BrdU/FdU 90 min before they were euthanized.
For 8-wk-old and 14-day-old mice, small intestines and colons were removed and measured for length, flushed with cold Ca2Mg2-free PBS, and cut open longitudinally. Tissue strips were fixed in fresh 10% formalin (paraformaldehyde = 4%), pinned out on wax trays, and allowed to remain in fixative for 20 h. Fixed tissue strips were embedded in fresh 2% agar and then in paraffin for use in immunohistochemical studies.
Immunohistochemical analyses.
Formalin-fixed paraffin-embedded mouse proximal jejunum (PJ) and distal colon (DC) sections were used for all immunohistochemistry (IHC) and immunofluorescence (IF) analyses. Tissue sections were deparaffinized by warming on a 54°C slide warmer for 10 min followed by immersion in xylene at room temperature for 20 min. Rehydration of sections was by sequential bathing for 5 min each in ethanol solutions progressing from 100%, 95%, 70%, and finally in distilled water. Sections were washed in TNT [0.1 M Tris(hydroxymethyl) aminomethane, 0.15 M sodium chloride, 0.05% Tween-20, pH 7.5] wash buffer. For bright-field IHC, sections were incubated in 3% hydrogen peroxide at room temperature for 10 min, whereas tissues for IF did not receive this treatment. For heat-induced epitope retrieval (HIER), sections were placed in Rodent Decloaker Buffer (RD-913; BioCare Medical, Concord, CA) and heated at 99°C for 18 min in a pressurized decloaking chamber (BioCare Medical). At the end of heating, sections remained in the decloaking chamber as it depressurized for 10 min, were then removed and allowed to start cooling for 10 min on a bench, and finally placed under a stream of dH2O for 5 min to fully cool sections and displace the rodent decloaker buffer.
IHC.
Hematoxylin-eosin-stained (H and E) sections from 8-wk-old mice were used to obtain measurements of small intestinal villus heights and small intestinal and colonic crypt depths. Villus heights and crypt depths were measured from images taken at ×100 magnification and saved as Axiovision zvi files using the Scalings program (Carl Zeiss Imaging Systems, Maple Grove, MN). In each of the six mice per treatment group, 30 villi and 30 crypts were measured.
H and E sections from mice treated from age 7 days to age 14 days with HA (30 mg/kg) or PEP-1 (40 mg/kg) or saline (controls) were used to determine the frequency of crypt fission. Sections through fissioning crypts were identified according to Dehmer et al. (4) and Dekany et al. (5) as having a bifurcation creating two (and occasionally three) flask-shaped bases with a common crypt-villus junction. At least 100 hundred crypt sections were counted in each of at least 6 mice per treatment group. H and E sections were also used to assess apoptosis by morphological criteria as described by Pritchard et al. (13). Apoptosis was scored on a cell-positional basis by light microscopic analysis of 40 half-crypt sections per mouse with 6–10 mice per treatment group. All crypts chosen were at least 16 cells in height, with cell position 1 located at the crypt base.
IHC detection of BrdU localization for assessment of epithelial proliferation was carried out after HIER. Tissue sections were blocked for 30 min in Rodent Block M (RBM961; BioCare Medical) followed by incubation with goat anti-BrdU (1:2,000, a gift from Dr. Jeffery I. Gordon) overnight at 4°C. Sections were washed in TNT buffer and incubated for 15 min with goat probe, followed by 25 min in goat-on-rabbit horseradish peroxidase polymer (GHP516; BioCare Medical), to which was added XM Factor (XMF963; BioCare Medical) to reduce background staining. Detection of BrdU was with 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich, St. Louis, MO) and counterstaining with hematoxylin (Vector Laboratories, Burlingame, CA). For long-term studies in 8-wk-old mice, the numbers of BrdU-labeled crypt epithelial cells in PJ and DC crypts were scored, with 30 crypts counted in at least 6 mice per treatment group. Data are presented as number of BrdU-positive cells per crypt section. For 14-day-old mice, we generated a proliferation index for each treatment group. BrdU-labeled proliferating crypt epithelial cells were scored on a cell-positional basis by light microscope analysis of strips of intestinal tissues sections. One hundred well-oriented half-crypt sections per mouse were counted with at least six mice in each group. Cell position 1 was located at the crypt base, and position numbers increased up each side of the crypt section. Proliferation index data are presented as the percentage of crypt epithelial cells at each position along the crypt that are positive for BrdU staining.
IF.
IF detection of CD44, TLR4, BrdU, lysozyme, and Lgr5 cellular localizations were carried out after HIER. Sections were blocked for 1 h in normal donkey serum because all secondary antibodies were raised in donkey. After the blocking, sections were incubated sequentially in the first primary antibody overnight at 4°C, followed by 1.5 h in the first secondary antibody, and the procedure was repeated for the second primary and secondary antibodies. Primary antibodies were rat anti-mouse CD44 (1:50; cat. no. 550538; BD Biosciences, San Diego, CA), rabbit anti-mouse TLR4 (1:200; cat. no. NB100-56580; Novus, Littleton, CO), goat anti-BrdU (1:2,000), chicken anti-GFP (1:100; cat. no. 13970; Abcam, Cambridge, MA), and rabbit anti-lysozyme (1:1,000; cat. no. 108508; Abcam). Secondary antibodies purchased from Life Technologies (Carlsbad, CA) were AF594 donkey-rat IgG, AF594 donkey anti-rabbit IgG, and AF594 donkey-goat IgG and used at 1:200 dilution. AF488 donkey anti-chicken was purchased from Jackson ImmunoResearch (1:200; cat. no. 703-545-155, West Grove, PA). IF detection of HA was with HA-binding protein (1:800; cat. no. HKD-BC4; Cosmo Bio USA, Carlsbad, CA) overnight at 4°C, followed by 1.5 h in AF594 labeled streptavidin (1:1,000, Life Technologies).
Small intestinal stem cell proliferation in 14-day-old Lgr5+/EGFP reporter mice was assessed by IF. Lgr5+-expressing intestinal crypt base columnar stem cells in formalin-fixed paraffin-embedded tissues were detected by use of an antibody against GFP. Epithelial cells labeled with GFP (Lgr5), with BrdU, or with both GFP and BrdU were scored from images taken at ×200 magnification and saved as Axiovision zvi files using the Scalings program (Carl Zeiss Imaging Systems). Proliferating stem cells were identified by the colocalization of GFP (Lgr5) and BrdU, and data were reported as a percentage of the total number of epithelial cells per crypt section. Thirty small intestinal crypts were scored in each of six mice per treatment group. Census of Paneth cells in 14-day-old WT, CD44−/−, and TLR4−/− mice was obtained by IF using an antibody against lysozyme. At least 100 crypt sections were scored in each of 6 mice per treatment group.
Enteroid culture and assessments of proliferation activity.
Small intestinal enteroid cultures from WT C57BL/6 mice were established and maintained per published protocols (9, 22). Briefly, a 1-cm2 segment of ileal tissue was removed and washed in DMEM/F12 containing 10% FBS to inactivate endogenous proteases. The tissue was then minced with fine sterile scissors, incubated with collagenase for 20 min with vigorous pipetting every 5 min, filtered using a 40-μm cell strainer, and then washed once with DMEM/F12 in a 15-ml centrifuge tube. The pellet was resuspended in DMEM/F12 and centrifuged at 200 g for 5 min. The pellet was then resuspended in Matrigel. Matrigel (15 μl) containing the epithelial cells was placed in the center of each well of a 24-well plate and incubated in a tissue culture incubator for 10 min upside down to avoid the cells attaching to the bottom of the plate. After this incubation, 500 μl of 50% l-WRN (9) conditioned medium (containing Wnt3a, Noggin, and R-spondin) supplemented with 10 μM Y27632 and 10 μM SB431542 was added. Experiments were performed using purified epithelial spheroid populations.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay kit (ATCC, Manassas, VA) was used to measure cell growth as we have previously described with the following modifications (22). Cells were resuspended in unsupplemented Matrigel or Matrigel containing the described concentrations of HA or PEP-1. Equal numbers of cells per volume of Matrigel were plated in each well of a 96-well plate. A minimum of five wells were used in each treatment group. After 3 days of growth, the MTT assay was performed per the manufacturer's protocol. In a separate experiment, these same concentrations of PEP-1 and HA were added to the l-WRN for incubation with the enteroids. Similarly treated cells were harvested for RNA expression analysis of markers of proliferation and activation of Wnt signaling (Ki-67 and Cyclin-D). RNA was isolated using Nucleospin RNA II kit (Macherey-Nagel, Bethlehem, PA) per the manufacturer's protocol. Quantitative real-time PCR was performed as previously with the following primers (21): mCyclin D1-F: GCGTACCCTGACACCAATCTC, mCyclin D1-R: CTCCTCTTCGCACTTCTGCTC; mKi67-F: ATCATTGACCGCTCCTTTAGGT, mKi67-R: GCTCGCCTTGATGGTTCCT; GAPDH-F: TGACAACGAATTTGGCTACAGC, GAPDH-R: TGATGGTACATGACAAGGTGC. In a separate experiment, these same concentrations of PEP-1 and HA were added to the l-WRN for incubation with the enteroids.
RESULTS
Signaling through both CD44 and TLR4 is required for elongation of the small intestine and colon in response to endogenous HA.
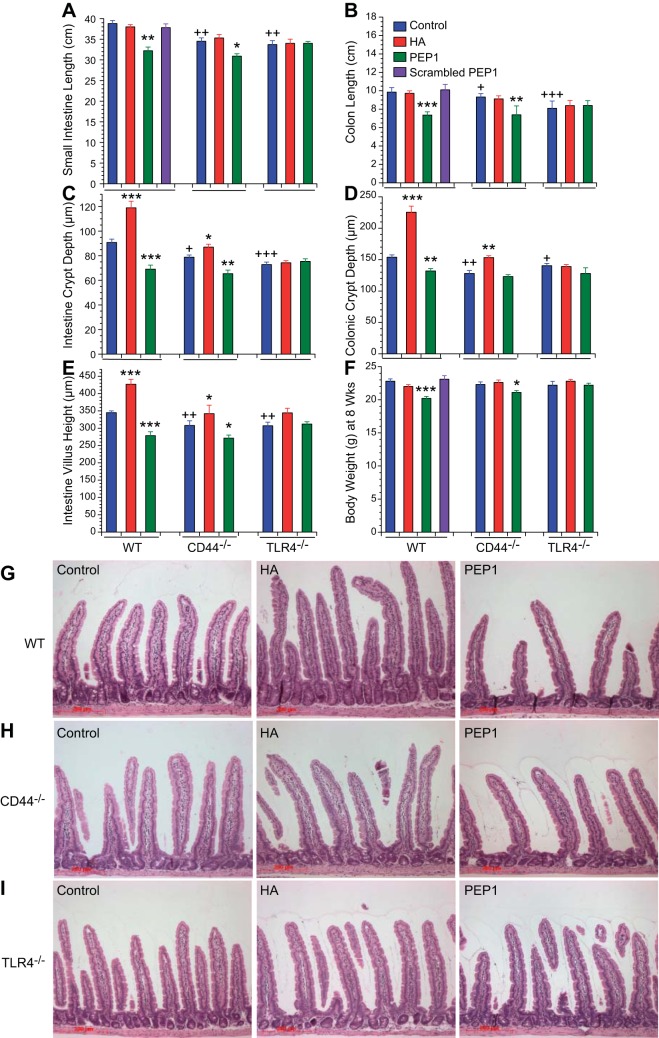
We have previously found that administration of PEP-1, a peptide that blocks HA binding to CD44 and TLR4, decreases small intestinal and colonic growth and decreases epithelial proliferation. Moreover, administration of exogenous HA induces increased proliferation and hyperplastic histological changes in the small intestine and colon. In this experiment, we sought to determine whether the effects of PEP-1 and exogenous HA on intestinal growth and epithelial proliferation require signaling through CD44 or TLR4 or both. WT, CD44−/−, and TLR4−/− mice were given intraperitoneal injections twice a week for 5 wk beginning at 21 days of age. These injections consisted of vehicle, exogenous HA, or PEP-1, a 12-mer peptide that blocks the binding of HA to both CD44 and TLR4, or scrambled PEP-1, which contains the same 12 amino acids as PEP-1 but in a different order. At 8 wk of age, the animals were weighed and killed, and the lengths of their small intestines and colons was measured. In the vehicle-treated animals, the lengths of the small intestine and colon were diminished in both the CD44−/− and TLR4−/− mice compared with WT mice (Fig. 1, A and B). Administration of exogenous HA had no effect on the lengths of the small intestines and colons in any of the genotypes studied. Blocking the binding of endogenous HA to its receptors with PEP-1 resulted in diminished length of the small intestine and colon in WT and CD44−/− mice but not in the TLR4−/− mice. At 8 wk of age, body weights were similar in WT, CD44−/−, and TLR4−/− mice (Fig. 1F). Administration of HA had no effect on body weight in any genotype. Administration of PEP-1 resulted in minimal decreases in body weight in WT and CD44−/− mice but not in TLR4−/− mice. The effects of PEP-1 on body weight were less striking than its effects on small intestinal and colonic length.
Fig. 1.
Effect of genotype and treatment with hyaluronic acid (HA) or a peptide that blocks HA binding to its receptors (PEP-1) on gross histology. The data from the wild-type (WT), CD44−/− and Toll-like receptor 4 (TLR4)−/− mice were collected contemporaneously. The WT data were reported previously (13) and are included here for statistical comparison. For the effect of genotype, we measured the following. A and B: average lengths of small intestines and colons in 8-wk-old CD44−/− and TLR4−/− control mice were shorter compared with 8-wk-old WT controls. C–E: average heights of intestinal villi and average depths of intestinal and colonic crypts were shorter in 8-wk-old CD44−/− and TLR4−/− mice compared with 8-wk-old WT controls. F: average total body weights of 8-wk-old CD44−/− and TLR4−/− mice were the same as 8-wk-old WT mice. +P < 0.02, ++P < 0.003, +++P < 0.0002 compared with WT control. For the effect of treatment, mice were given vehicle or HA or PEP-1 or scrambled PEP-1 intraperitoneally twice a week for 5 wk beginning at 3 wk of age. A and B: treatment with HA had no effect on length of small intestine or colon in WT, CD44−/−, or TLR4−/− mice. Treatment with PEP-1 resulted in a significant decrease in length of small intestine and colon in WT and CD44−/− mice but not in TLR4−/− mice. Values are means ± SE for 20 WT, 10 CD44−/−, and 10 TLR4−/− mice per treatment group. C–E: treatment with HA resulted in significant increases in intestinal villus height and intestinal and colonic crypt depth in WT mice and to a lesser degree in CD44−/− mice compared with their respective controls but had no effect in TLR4−/− mice. Treatment with PEP-1 resulted in significant decreases in intestinal villus height and intestinal and colonic crypt depth in WT mice and to a lesser degree in CD44−/− mice compared with their respective controls but had no effect in TLR4−/− mice. Values are means ± SE for 6 WT, 6 CD44−/−, and 6 TLR4−/− mice per treatment group. F: treatment with HA had no effect on average total body weight in WT, CD44−/−, or TLR4−/− mice. PEP-1 administration resulted in average weights that were 10% lower in WT and 8% lower in CD44−/− mice compared with controls, but there was no effect in TLR4−/− mice. Values are means ± SE for 20 WT, 10 CD44−/−, and 10 TLR4−/− mice per treatment group. *P < 0.003, **P < 0.0001, ***P < 0.00001 vs. control of the same genotype. G–I: hematoxylin and eosin (H and E) stained histologic sections of 8-wk-old WT, CD44−/−, and TLR4−/− mouse small intestines. Small intestines (G) and colons (not shown) of WT mice treated with exogenous HA for 5 wk had increased epithelial proliferation and developed the appearance of hyperplasia. Treatment with PEP-1 for 5 wk resulted in reduced small intestine and colonic epithelial proliferation and the appearance of hypoplasia. The effects of HA and PEP-1 on small intestine and colon histology were less in CD44−/− mice (H) compared with WT mice, and there was no effect in TLR4−/− mice (I). scale bar = 200 μm, original magnification ×100.
In vehicle-treated mice, jejunal villus height, jejunal crypt depth, and colonic crypt depth were all diminished in CD44−/− and TLR4−/− mice compared with WT mice (Fig. 1, C–E and G–I). Administration of exogenous HA from week 3 to week 8 resulted in increases in the jejunal villus height, jejunal crypt depth, and colonic crypt depth in the WT and CD44−/− mice but not in the TLR4−/− mice. Administration of the HA-blocking peptide PEP-1 resulted in diminished jejunal villus height, diminished jejunal crypt depth, and diminished colonic crypt depth in the WT and CD44−/− mice but not in TLR4−/− mice. The small intestines of WT and CD44−/− mice treated with exogenous HA had a histological picture consistent with hyperplasia with longer villi and deeper crypts (Fig. 1, G and H). In contrast, the small intestines of WT and CD44−/− mice treated with PEP-1 had a hypoplastic or atrophic appearance with shortened crypts and villi. Administration of HA or PEP-1 to TLR4−/− mice had little effect on the histological appearance of the small intestine (Fig. 1I).
Administration of exogenous HA results in increased epithelial proliferation in the intestine and colon through CD44 and TLR4 signaling.
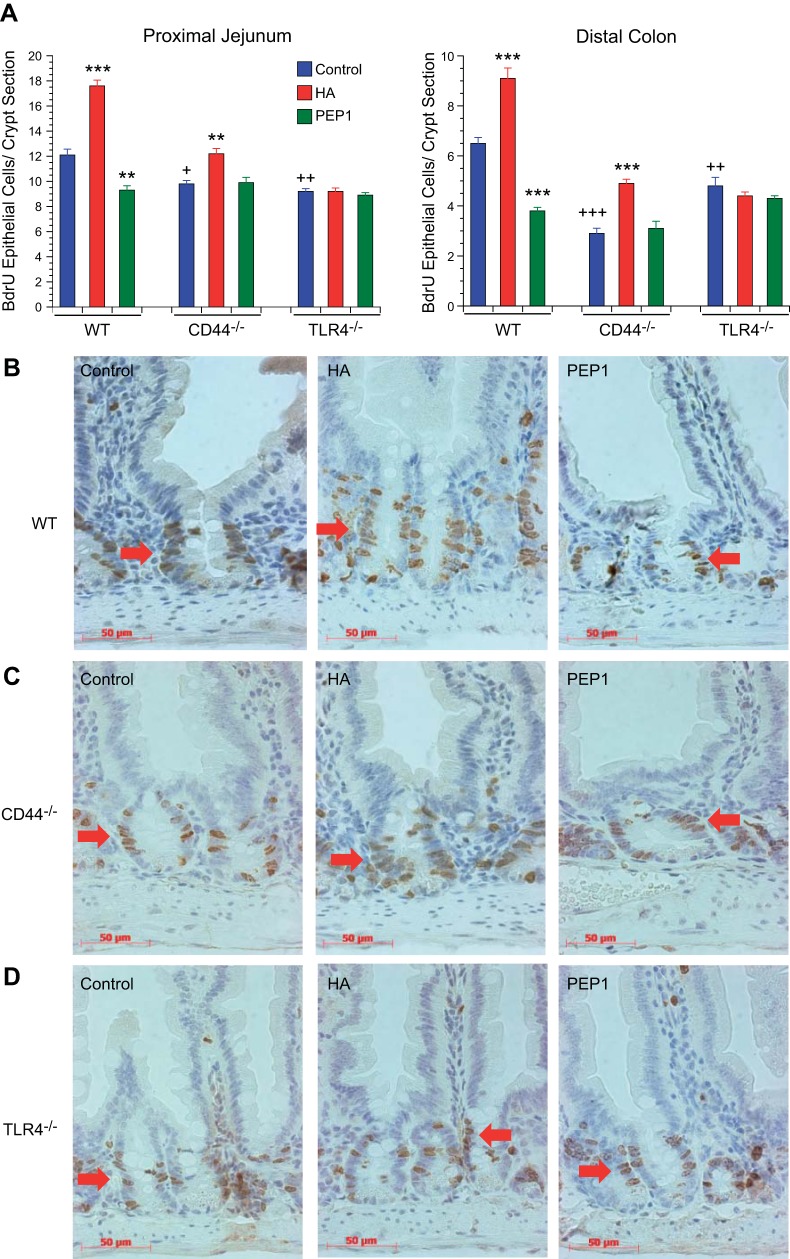
We next sought to determine whether either endogenous or exogenous HA affected epithelial proliferation in the small intestine and colon. Epithelial proliferation, as assessed by the number of BrdU+ cells per crypt section, was diminished in the small intestines and colons of CD44−/− and TLR4−/− mice compared with WT mice (Fig. 2, A–D). Administration of exogenous HA increased the number of BrdU+ cells in the WT and CD44−/− mouse small intestines and colons but not in the TLR4−/− mouse small intestines and colons. Blocking HA binding to its receptors with PEP-1 resulted in diminished proliferation in WT intestines and colons but not in the small intestines and colons of CD44−/− or TLR4−/− mice. The histological appearance of the small intestines of WT and CD44−/− mice treated with HA showing increased proliferation in the BrdU studies (Fig. 2, B and C) is consistent with the hyperplastic appearance of these small intestines in Fig. 1, G and H. Similarly, the diminished proliferation in the small intestines of WT and CD44−/− mice treated with PEP-1 is consistent with the hyperplastic or atrophic appearance of these small intestines in Fig. 1, G and H.
Fig. 2.
Effect of genotype and treatment with HA or PEP-1 on epithelial proliferation. To measure the effect of genotype, the data from the WT, CD44−/−, and TLR4−/− mice were collected contemporaneously. The WT data were reported previously (13) and are included here for statistical comparison. A: baseline intestinal and colonic crypt epithelial cell proliferation in 8-wk-old control CD44−/− and TLR4−/− mice was significantly reduced compared with 8-wk-old WT controls. +P < 0.01, ++P < 0.001, +++P < 0.0001 compared with WT control. To measure the effect of treatment, treatment with HA as in Fig. 1 resulted in significantly increased crypt epithelial cell proliferation in small intestine and colon in WT mice and to a lesser degree in CD44−/− mice compared with their respective controls but had no effect on epithelial proliferation in TLR4−/− mice. Treatment with PEP-1 using the same protocol resulted in significant reduction in intestinal and colonic crypt epithelial cell proliferation in WT mice but had no effect on the already reduced levels of crypt epithelial cell proliferation in CD44−/− and TLR4−/− mice. Values are means ± SE for 4–8 mice per treatment group. **P < 0.001, ***P < 0.0001 compared with control of the same genotype. B–D: immunohistochemistry shows 5-bromo-2′-deoxyuridine (BrdU)-labeled proliferating small intestinal crypt epithelial cells (arrows) in 8-wk-old WT, CD44−/−, and TLR4−/− mice. Scale bar = 50 μm, original magnification ×400.
Administration of exogenous HA from age 7 days to 14 days results in increased distribution of HA.
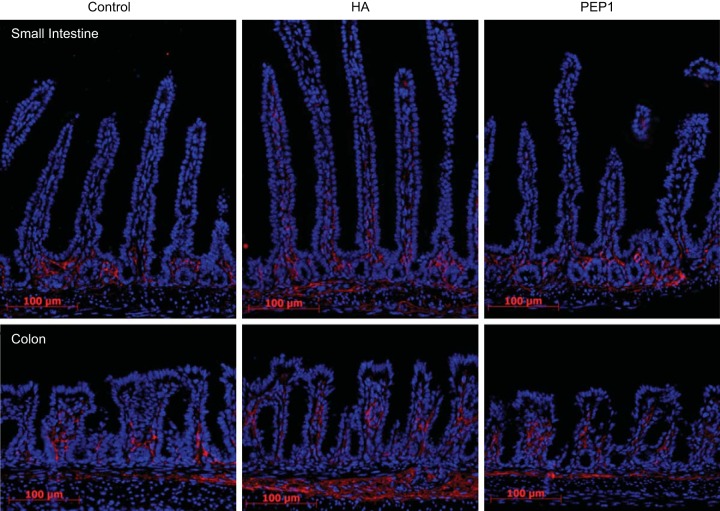
The period of most rapid small intestinal and colonic lengthening is early in postnatal life before 3 wk of age. To determine the effects of endogenous and exogenous HA on growth and the distribution of HA, mice were given intraperitoneal injections of vehicle, HA, or PEP-1 every other day from age 7 days to 14 days. The distribution of HA was assessed using an IF antibody for HA-binding protein. In the small intestine of WT animals, HA was found in the extracellular space adjacent to crypt epithelial cells (Fig. 3). Administration of exogenous HA was associated with extension of the distribution of HA up onto the villi. There was also increased expression in the muscularis mucosa. The distribution of HA in PEP-1-treated mice was similar to that in vehicle-treated animals. In the colon at 14 days of age, HA surrounds the epithelial cells at the base of the crypt. Administration of exogenous HA resulted in extension of the distribution of HA higher up in the colonic crypt and also in increased HA in the muscularis mucosa. As in the small intestine, administration of PEP-1 had little effect on the distribution of HA in the colon.
Fig. 3.
HA expression in 14-day-old mice. Immunofluorescence for HA-binding protein (red) in postnatal day 14 WT mouse small intestine and colon was measured; at baseline, in the small intestine endogenous HA was found primarily in the crypt zone lamina propria and in the colon surrounding the lower half of the crypts. Intraperitoneal administration of exogenous HA from age 7 days to age 14 days resulted in extension of HA distribution up into the villi in the intestine and higher up around colonic crypts, as well as increased distribution in the muscularis mucosa in the intestine and colon. Blocking endogenous HA with intraperitoneal administration of PEP-1 using the same regimen did not change the distribution of HA in intestine or colon compared with controls. Scale bar = 100 μm, original magnification ×200.
Inhibition of endogenous HA binding to its receptors early in postnatal life results in diminished crypt fission and diminished Lgr5+ epithelial stem cell proliferation.
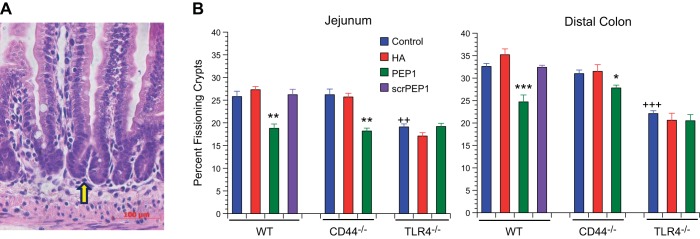
Having addressed the effect of endogenous and exogenous HA on small intestinal growth later in life, we next sought to determine their effects early in life at the time of maximal small intestinal elongation. Lengthening of the small intestine and colon occurs by crypt fission. In crypt fission, there is an increase in the number of epithelial cells in a crypt followed by an indentation at the base of the crypt, which extends upward until the single crypt is divided into two crypts (Fig. 4A). Mice received intraperitoneal injections with vehicle, exogenous HA, PEP-1, or scrambled PEP-1 every other day from age 7 days to 14 days. At 14 days of age, in vehicle-treated mice, 26% of the small intestinal crypts were undergoing fission in the WT and CD44−/− mice, whereas 20% were undergoing fission in the TLR4−/− mice (Fig. 4B). Administration of exogenous HA had no effect on crypt fission. Administration of PEP-1 resulted in a significant decrease in crypt fission in the WT and CD44−/− mice but not in the TLR4−/− mice. In vehicle-treated TLR4−/− mice, about 20% of the crypts were undergoing fission, which was similar to what was seen in the WT or CD44−/− mice that had received PEP-1. The pattern of crypt fission and the responsiveness to HA and PEP-1 was similar in the colon and small intestine.
Fig. 4.
Effect of genotype and treatment with HA or PEP-1 on crypt fission in 14-day-old mice. A: H and E-stained histologic section of 14-day-old WT mouse small intestine showing a fissioning crypt (arrow). Scale bar = 100 μm, original magnification ×200. B: effect of genotype was measured; TLR4−/− genotype in postnatal mice affects baseline crypt fission. 14-day-old TLR4−/− control mice had a baseline frequency of crypt fission that was significantly reduced in the small intestine (20%) and colon (22%) compared with 14-day-old WT control intestine (26%) and colon (33%). In 14-day-old CD44−/− mice, baseline frequencies of crypt fission in the intestine (26%) and colon (31%) were the same as in 14-day-old WT controls. ++P < 0.001, +++P < 0.0001 compared with WT control. Effect of treatment was measured; intraperitoneal administration of exogenous HA from age 7 days to age 14 days had no effect on the frequency of crypt fission in small intestine or colon in WT, CD44−/−, or TLR4−/− mice. Blocking endogenous HA by intraperitoneal administration of PEP-1 using the same regimen resulted in a significant reduction of intestinal and colonic crypt fission in WT and CD44−/− mice but had no effect on crypt fission in TLR4−/− mice. Values are means ± SE for 9 mice per treatment group. *P < 0.01, **P < 0.001, ***P < 0.0001 compared with control of the same genotype.
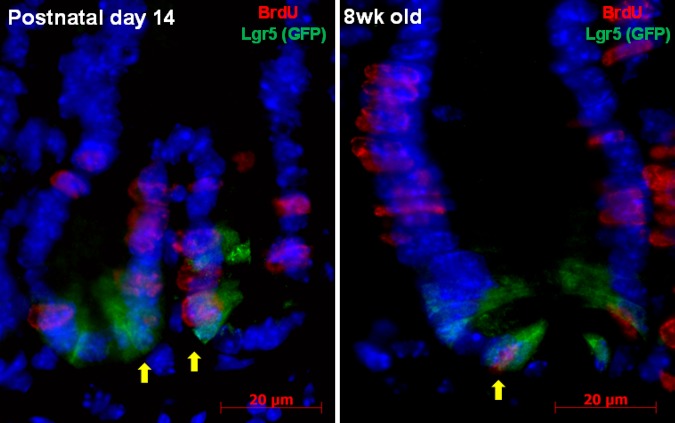
Under homeostatic conditions, epithelial cell proliferation in the small intestine and colon is regulated by the proliferation of Lgr5+ epithelial stem cells (4). Using Lgr5+ reporter mice given BrdU, we assessed BrdU and Lgr5+ expression in the small intestine of 14-day-old and 8-wk-old mice. In the 8-wk-old mice, the Lgr5+ stem cells are narrow and are separated by larger, more cuboidal Paneth cells (Fig. 5). Moreover, in the 8-wk-old mice, there are more BrdU-expressing epithelial cells higher up the crypt in the transit-amplifying zone. In contrast, the 14-day-old mice have fewer Paneth cells and more cuboidal Lgr5+ stem cells. In the 14-day-old mice, the BrdU-positive cells are largely in the base of the crypt, and there are more cells that coexpress BrdU and Lgr5.
Fig. 5.
Localization of BrdU (red) and Lgr5 (green fluorescent protein, GFP, green) in small intestinal crypt epithelial cells of WT mouse at age 14 days and at age 8 wk. Colocalization of BrdU and Lgr5+ (arrows) identifies proliferating crypt epithelial stem cells. Scale bar = 20 μm, original magnification ×1,000.
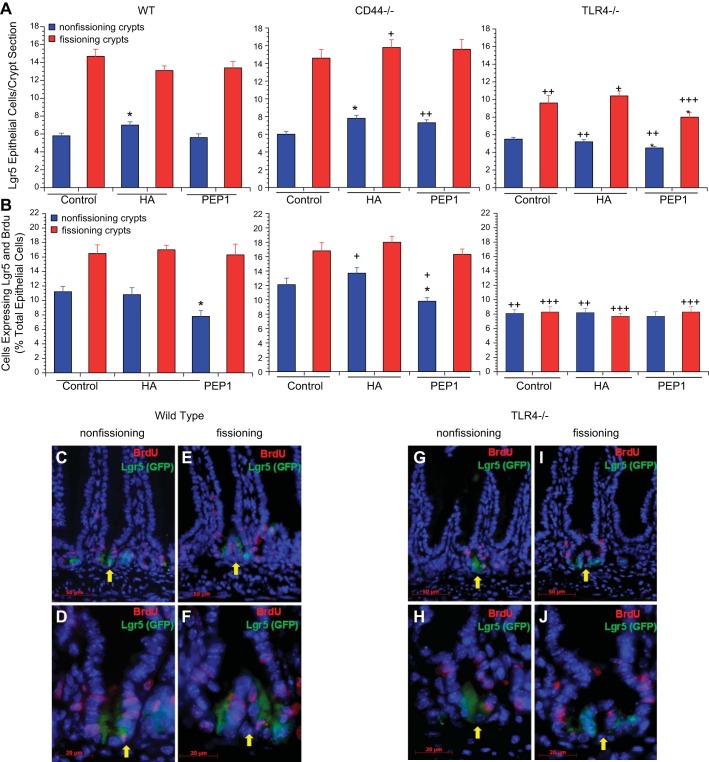
We next assessed the number of Lgr5+ cells and the percentage of epithelial cells that were both Lgr5+ and BrdU+ in 14-day-old mice treated with HA or PEP-1. The number of Lgr5+ cells in crypts undergoing fission was always greater than in nonfissioning crypts; as a result, we assessed the number of Lgr5+ cells in fissioning and nonfissioning crypts separately (Fig. 6A). The number of Lgr5+ cells in the nonfissioning crypts was similar in the WT, CD44−/−, and TLR4−/− mice with about six Lgr5+ epithelial cells per crypt section. In the fissioning crypts of WT and CD44−/− mice, there were about 14 Lgr5+ cells per crypt section; however, in the TLR4−/− mice, the number of Lgr5+ epithelial cells per crypt section was markedly diminished at 10. Administration of exogenous HA or PEP-1 had little effect on the number of Lgr5+ cells per crypt section although there was some increase in the number of Lgr5+ cells in nonfissioning crypts with HA in the WT and CD44−/− mice and a decrease with PEP-1 in the TLR4−/− mice.
Fig. 6.
Effect of genotype and treatment with HA or PEP-1 on Lgr5+ cell number and proliferation. A: Lgr5+ cell number. Effect of genotype was measured; 14-day-old WT and CD44−/− mice had similar numbers of Lgr5+ crypt epithelial cells per section in nonfissioning and fissioning crypts. TLR4−/− mice also had a similar number of Lgr5+ epithelial cells at baseline in nonfissioning crypts but had significantly fewer Lgr5+ epithelial cells in fissioning crypts compared with WT or CD44−/− mice. Overall Lgr5+ cell numbers were significantly diminished in 14-day-old TLR4−/− mice compared with WT and CD44−/− mice, especially in fissioning crypts. +P < 0.01, ++P < 0.001, +++P < 0.0001 compared with WT mice given the same treatment. Effect of treatment was measured; postnatal WT and CD44−/− mice showed a small increase in Lgr5+ cell numbers in nonfissioning crypts after treatment with exogenous HA from age 7 days to age 14 days, but there was no increase in TLR4−/− mice. Blocking endogenous HA by intraperitoneal administration of PEP-1 from age 7 days to age 14 days had no effect on Lgr5+ cell numbers in WT or CD44−/− mice, but there was a small further diminishment of Lgr5+ cell numbers in TLR4−/− mice. Values are means ± SE for 6 mice per treatment group. *P < 0.01 compared with control of the same genotype. B: Lgr5+ intestinal crypt epithelial cell proliferation. Effect of genotype was measured, and 14-day-old WT and CD44−/− mice had similar baseline rates of Lgr5+ intestinal crypt epithelial cell proliferation, with higher percentages of Lgr5+ epithelial cells proliferating in fissioning than in nonfissioning crypts. 14-day-old TLR4−/− mice had significantly lower rates of Lgr5+ epithelial cell proliferation compared with WT and CD44−/− mice. TLR4−/− mice also differed from WT and CD44−/− mice in having a rate of Lgr5+ epithelial proliferation that was no greater in fissioning crypts than in the nonfissioning crypts. +P < 0.01, ++P < 0.001, +++P < 0.0001 compared with WT mice given the same treatment. Effect of treatment was measured, and mice were treated with HA or PEP-1 as in Fig. 4. Treatment with HA had no effect on Lgr5+ epithelial cell proliferation in WT, CD44−/−, or TLR4−/− mice. Administration of PEP-1 resulted in a significant decrease in Lgr5+ cell proliferation in nonfissioning intestinal crypts in WT and CD44−/− mice, while not affecting Lgr5+ cell proliferation in fissioning crypts. PEP-1 had no effect on Lgr5+ epithelial cell proliferation in TLR4−/− mice. Values are means ± SE for 6 mice per treatment group. *P < 0.01 compared with control of the same genotype. C–J: immunofluorescence for BrdU (red) + Lgr5 (GFP, green) showing BrdU-labeled and Lgr5+ epithelial cells in nonfissioning and fissioning small intestinal crypts of postnatal day 14 WT (C–F) and TLR4−/− (G–J) mice. Scale bar for C, E, G, I = 50 μm, original magnification ×400. Scale bar for D, F, H, J = 20 μm, original magnification ×1,000.
To assess the number of Lgr5+ cells undergoing proliferation, we counted the number of cells that were both Lgr5+ and BrdU+ and expressed that number as a percentage of the total epithelial cell population. At 14 days of age in WT and CD44−/− mice, 12% of epithelial cells were both Lgr5+ and BrdU+ in nonfissioning crypts and 16% in fissioning crypts (Fig. 6B). In both the WT and CD44−/− mice, administration of PEP-1 diminished the percentage of cells expressing BrdU and Lgr5 in the nonfissioning crypts but not in fissioning crypts. In the TLR4−/− mice, the percentage of cells expressing both Lgr5 and BrdU was markedly reduced to 8% in both the fissioning and nonfissioning crypts, and administration of HA or PEP-1 had no effect on the percentage of cells that expressed both BrdU and Lgr5.
Lgr5+ crypt epithelial cells express both CD44 and TLR4.
Our data suggest that signaling through CD44 and particularly through TLR4 is involved in mediating the effects of endogenous HA on Lgr5+ cell proliferation. It is not clear whether the effects of endogenous HA on the proliferation of Lgr5+ cells are stem cell autonomous or whether they are mediated by CD44 and TLR4 signaling in other cell types. To begin to address this issue, we used IF to assess the expression of CD44 and TLR4 in Lgr5 reporter mice. In the 14-day-old mouse small intestine, there is coexpression of CD44 and Lgr5 in epithelial cells at the base of the crypts (Fig. 7, A and B). CD44 is not expressed in epithelial cells higher up the crypt but is expressed in scattered lamina propria cells. In the Lgr5+ cells, CD44 is expressed on the basolateral plasma membrane. There is also coexpression of Lgr5+ and TLR4 in crypt epithelial cells but not in epithelial cells higher up the crypt (Fig. 7, E and F). In the Lgr5+ cells, TLR4 is cytoplasmic.
Fig. 7.
Expression of CD44 and TLR4 in the small intestine. Immunofluorescence for A–D: CD44 (red) + Lgr5 (GFP, green). Immunofluorescence for E–H: TLR4 (red) + Lgr5 (GFP, green) in small intestinal crypt sections of postnatal day 14 mice. Some Lgr5+ crypt epithelial cells in WT mice also expressed CD44 (arrow) (A and B) and TLR4 (arrow) (E and F). Intestinal crypt sections of CD44−/− and TLR4−/− mice were used as negative controls for CD44 and TLR4 immunofluorescence, respectively. Scale bar for A, C, E, G = 50 μm, original magnification ×400. Scale bar for B, D, F, H = 20 μm, original magnification ×1,000.
Positional proliferation and positional apoptosis along the crypt villus axis are affected by HA signaling through TLR4 and CD44.
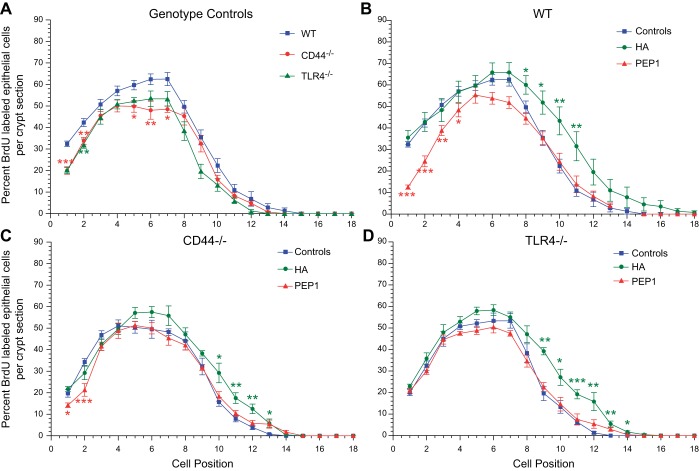
In the experiments presented in Fig. 2, we assessed the effects of endogenous and exogenous HA on proliferation in the small intestine and colon of the adult mouse. We found that CD44 and TLR4 signaling mediated the effects of endogenous and exogenous HA on epithelial proliferation. We next sought to assess the role of CD44 signaling and TLR4 signaling in the effects of endogenous and exogenous HA on positional proliferation and apoptosis in the small intestine of the 14-day-old mouse. WT, CD44−/−, and TLR4−/− mice were treated with intraperitoneal vehicle, HA, or PEP-1 from 7–14 days of age. The mice were given BrdU 90 min before death to label the proliferating epithelial cells. The data are presented as the percentage of epithelial cells that are BrdU+ at each position along the crypt villus axis (Fig. 8). The cells at the base of the crypt are defined as being at position 1; epithelial cells higher up the crypt are assigned progressively higher numbers. In WT mice, proliferation increases from position 1 to position 7 and then falls off higher up the crypt. Compared with WT mice, positional proliferation is diminished in both the CD44−/− and TLR4−/− mice from positions 1–7. At positions 7–10, proliferation is diminished in the TLR4−/− mice but not in the CD44−/− mice. Treatment with exogenous HA results in increased positional proliferation at positions 8–12 in WT, CD44−/−, and TLR4−/− mice, but treatment with HA had no effect on proliferation at the base of the crypts in any of the genotypes. Administration of PEP-1 resulted in a decrease in positional proliferation from positions 1–4 in the WT and CD44−/− mice but had no effect on proliferation in the TLR4−/− mice.
Fig. 8.
Positional distribution of BrdU-labeled proliferating small intestinal crypt epithelial cells in postnatal day 14 WT, CD44−/−, and TLR4−/− mice. In all 3 genotypes and in all treatment groups, the highest rate of epithelial cell proliferation was in the range of position 4 to position 7, with gradually decreasing proliferation at positions higher in the crypt. Effect of genotype was measured. A: at baseline positional proliferation in 14-day-old CD44−/− and TLR4−/− mice was significantly lower in the crypt base (positions 1–4) compared with WT mice. In CD44−/− mice, this significantly reduced proliferation extended up to position 7. Effect of treatment was measured. B–D: treatment with intraperitoneal HA from age 7 days to age 14 days resulted in significant increases in positional epithelial cell proliferation in the upper half of the intestinal crypt (positions 8–12) in WT, CD44−/−, and TLR4−/− mice but had no effect in the crypt base in any of these genotypes. Administration of PEP-1 using the same regimen resulted in decreased positional epithelial cell proliferation in the crypt base in WT and CD44−/− mice, but PEP-1 had no effect on positional epithelial cell proliferation in TLR4−/− mice. Values are means ± SE for 10–15 WT, 6 CD44−/−, and 6 TLR4−/− mice per treatment group. *P < 0.03, **P < 0.005, ***P < 0.0001 compared with control.
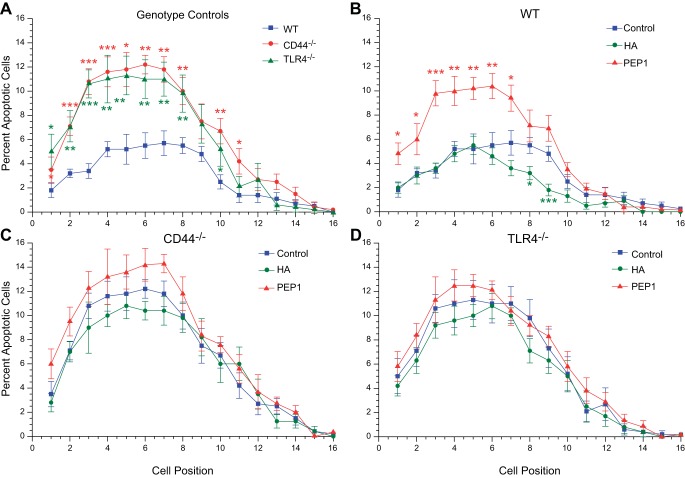
We previously demonstrated that endogenous HA affects the normal growth and elongation of the small intestine and colon. We correlated these effects on growth and elongation with parallel effects on epithelial proliferation. However, growth and elongation may be affected by apoptosis as well as proliferation. We next sought to assess the effects of HA on apoptosis. Positional apoptosis studies were done in a manner similar to the positional proliferation studies. The rate of spontaneous apoptosis in the epithelial cells of the WT small intestine is quite low. The percentage of epithelial cells undergoing spontaneous apoptosis varies from 1–4% depending on position with the peak level of 4% of cells undergoing apoptosis occurring between positions 4 and 9 (Fig. 9). Both CD44−/− and TLR4−/− mice have increased levels of spontaneous apoptosis peaking out at 10–12% at positions 4–9. In WT mice, administration of PEP-1 results in increased apoptosis at positions 4–9, achieving levels that are similar to what was seen in CD44−/− and TLR4−/− mice at baseline. In WT mice, administration of exogenous HA results in a slight decrease in spontaneous apoptosis at positions 8 and 9. In CD44−/− and TLR4−/− mice, administration of an exogenous HA or PEP-1 has little effect on positional apoptosis.
Fig. 9.
Positional distribution of apoptotic epithelial cells in small intestine of postnatal day 14 WT, CD44−/−, and TLR4−/− mice. A: in all 3 genotype controls, the highest rate of apoptosis was in the range of positions 4–7, with double the rate in CD44−/− and TLR4−/− compared with WT mice. B: intraperitoneal administration of exogenous HA to WT mice from age 7 days to age 14 days reduced apoptosis in positions 7–9 but had no effect below this region of the crypt. Treatment with PEP-1 using the same regimen resulted in a 2-fold increase in apoptosis in the lower half of the crypt (positions 1–7). C and D: exogenous HA and PEP-1 had little to no effect on apoptosis in postnatal CD44−/− and TLR4−/− mice. *P < 0.03, **P < 0.005, ***P < 0.0001 compared with control.
HA and PEP-1 do not directly promote growth of isolated epithelial cells.
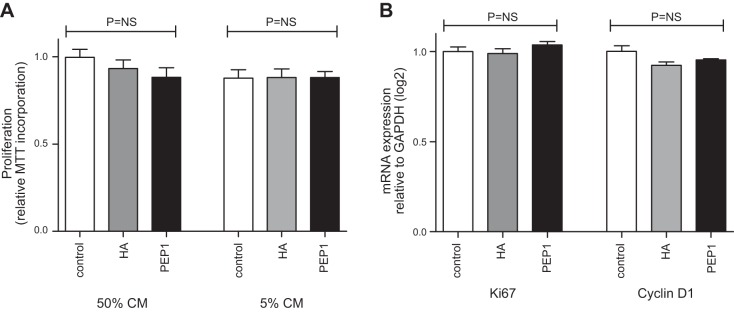
Enteroid cultures of primary small intestinal epithelial cells were used to determine whether the growth effects of HA or PEP-1 were epithelial cell autonomous. WT-derived enteroids were incubated in control Matrigel or Matrigel supplemented directly with HA or PEP-1. There was no difference in growth between groups at 72 h as measured by MTT incorporation either with the cells in their stem cell state (50% conditioned media, CM) or in conditions that supported differentiation into mature epithelial lineages (5% CM) (Fig. 10A). Similarly, no difference was identified in transcriptional markers of proliferation (Ki-67) or Wnt activation (Cyclin-D1) as shown in Fig. 10B. Experimental results were similar when the HA or PEP-1 was added to the culture media, rather than directly to the Matrigel (data not shown). These findings suggest that growth effects of HA and PEP-1 are not via direct signaling through epithelial cells but likely involve another intermediary cell in the stem cell niche.
Fig. 10.
HA does not directly promote epithelial cell proliferation. Small intestinal epithelial enteroids developed from WT C57BL/6 mice were used to assess the direct effect of HA or PEP-1 on cell proliferation. Enteroid cultures were examined in the stem cell state [50% l-WRN conditioned media (CM)] or in conditions supportive of terminal differentiation (5% l-WRN CM). HA (100 μg/ml) and PEP-1 (125 μg/ml) were added to Matrigel before the enteroids were resuspended. A: enteroid growth as measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) proliferation assay at 72 h in 50% or 5% l-WRN CM. B: RNA expression of Ki-67 and Cyclin-D1 at 72 h expressed as fold change from control enteroids incubated in unsupplemented Matrigel and normalized to GAPDH. Experimental controls include ≥5 wells per condition and were repeated twice. Statistical comparison by unpaired Student's t-test. NS, nonsignificant with P > 0.05.
Paneth cell homeostasis requires HA signaling through CD44 and TLR4.
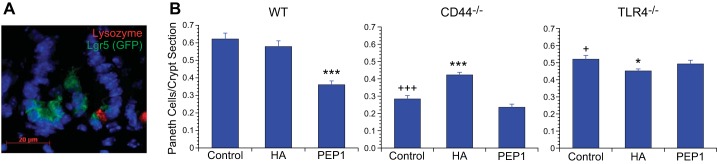
In the study of the effects of HA on intestinal growth, we found that exogenous HA increases the proliferation of enterocytes to a greater extent than secretory cells, including Paneth cells in the small intestinal epithelium. The effect of PEP-1 on the epithelial cell lineages was less than that of exogenous HA. PEP-1 resulted in a modest increase in enterocytes and a decrease in goblet cells in the crypts without affecting lineages in the villi. We are particularly interested in the potential effects of HA and PEP-1 on the number of Paneth cells in the small intestinal crypt in early life because of the important role of Paneth cells in supporting the proliferation of Lgr5+ stem cells. At birth, there are few or no Paneth cells seen in the small intestine of the mouse. By 14 days of age, 0.6 Paneth cells are seen per crypt section in WT mice (Fig. 11). At 14 days of age, there are fewer Paneth cells than in the adult. The number of Paneth cells per crypt section is significantly diminished in CD44−/− and TLR4−/− mice compared with WT mice. Administration of exogenous HA to WT mice had no effect on Paneth cell number; however, administration of PEP-1 resulted in a significant decrease in Paneth cell number. In CD44−/− mice, administration of exogenous HA increased Paneth cell number, whereas PEP-1 had no effect. In TLR4−/− mice, there was no effect on Paneth cell number with either HA or PEP-1.
Fig. 11.
Effect of genotype and treatment with HA or PEP-1 on Paneth cell number. A: immunofluorescence for lysozyme (red) identifies Paneth cells, and Lgr5 (GFP, green) identifies intestinal crypt epithelial stem cells in 14-day-old WT mouse small intestine. Scale bar = 20 μm, original magnification ×1,000. B: census of Paneth cells in postnatal day 14 WT, CD44−/−, and TLR4−/− mouse small intestines. The number of Paneth cells per crypt section was significantly lower in 14-day-old CD44−/− and to a lesser degree in TLR4−/− compared with WT mice. Intraperitoneal administration of exogenous HA from age 7 days to age 14 days had no effect on Paneth cell number in WT or TLR4−/− mice but resulted in an increase in CD44−/− mice. Blocking endogenous HA by intraperitoneal administration of PEP-1 using the same regimen resulted in a significant decrease in Paneth cell number in WT mice, but PEP-1 had no effect on Paneth cell number in CD44−/− or TLR4−/− mice. Values are means ± SE for 6 mice per treatment group. +P < 0.01, +++P < 0.0001 compared with WT control. *P < 0.02, ***P < 0.0001 compared with control of the same genotype.
DISCUSSION
In this study, we found that the effects of endogenous HA in promoting normal small intestinal and colonic elongation require signaling through both CD44 and TLR4. The hyperproliferative effects of exogenous HA were mediated primarily through TLR4. In extending these studies of the effects of HA on intestinal and colonic growth to the early postnatal period, we found that endogenous HA operating through both CD44 and TLR4 promotes the proliferation of Lgr5+ stem cells and promotes crypt fission, which is the basis of small intestinal and colonic elongation.
The requirement for endogenous HA signaling through both TLR4 and CD44 is demonstrated by the shorter small intestines and colons in TLR4−/− and CD44−/− mice. Endogenous HA signals through both CD44 and TLR4 as demonstrated by decreased proliferation in CD44−/− and TLR4−/− mice at positions 1–8 at the base of the crypt, the positions of epithelial cells exposed to endogenous HA. Administration of PEP-1 reduces small intestinal and colonic length in WT and CD44−/− mice but not in TLR4−/− mice, suggesting that the binding of endogenous HA to TLR4 is especially important in promoting growth. A role for HA signaling through TLR4 and CD44 in normal growth appears to be specific to the gastrointestinal tract in that body weights are similar in WT, CD44−/−, and TLR4−/− mice; administration of PEP-1 has only a minimal effect on body weight.
In the small intestine, positional proliferation studies show that the decrease in epithelial proliferation induced by PEP-1 is consistent with the distribution of endogenous HA. PEP-1 decreases proliferation in the epithelial cells at positions 1–8 but not in epithelial cells higher up the crypt. HA is found in the extracellular space surrounding the epithelial cells at the base of the crypt corresponding to positions 1–8.
The role of CD44 signaling in mediating the effects of HA on the epithelium is consistent with the distribution of CD44. In the small intestine, epithelial cell expression of CD44 is confined to the base of the crypt (23). In CD44-deficient mice, epithelial cell proliferation is diminished only in epithelial cells at the base of the crypt. Whether endogenous HA binds separately to CD44 and TLR4 or binds to a CD44/TLR4 complex is not clear. Taylor et al. (20) described HA binding to a CD44/MD2/TLR4 complex in macrophages.
The effects of endogenous HA on Lgr5+ stem cell proliferation and crypt fission in the postnatal mouse closely paralleled the effects of endogenous HA on small intestinal and colonic elongation in the adult mouse (15). Administration of PEP-1 decreases Lgr5+ cell proliferation and crypt fission in postnatal mice and decreases intestinal and colonic elongation in adult mice. These data support the suggestion that intestinal and colonic elongation is driven by the proliferation of Lgr5+ stem cells, which leads to an increase in epithelial cell number, which in turn leads to crypt fission and small intestinal and colonic elongation (4). PEP-1 only affects Lgr5+ cell proliferation in nonfissioning crypts, suggesting that endogenous HA promotes the initiation of crypt fission by stimulating Lgr5+ cell proliferation but once crypt fission has begun HA input is no longer important in promoting Lgr5+ stem cell proliferation.
Although signaling through both TLR4 and CD44 mediates the effects of endogenous HA on Lgr5+ stem cell proliferation, crypt fission, and intestinal elongation, the cellular location of the TLR4 and CD44 that mediates these effects is not clear. Lgr5+ stem cells express TLR4 and CD44, but other cells including macrophages and stromal cells also express these receptors. Neither HA nor PEP-1 affected proliferation in mouse small intestinal organoids, suggesting that the effects of HA on Lgr5+ stem cell proliferation are mediated through a nonepithelial cell.
There is a marked decrease in Lgr5+ stem cell number and proliferation in TLR4−/− mice. This is consistent with the observation that overexpression of TLR4 in intestinal epithelial cells results in increases in Lgr5+ cell number and proliferation (17). Although the role of TLR4 activation in normal small intestinal growth has not been previously addressed, TLR4 signaling is required for the enhanced colonic epithelial response seen in the repair of DSS colitis (2, 6, 14). Here we describe the effects of TLR4 activation by HA although TLR4 signaling is usually associated with activation by LPS. TLR4 activation by HA and LPS have different effects in the small intestinal epithelium. Using in situ hybridization and flow cytometry, Neal et al. (11) found coexpression of TLR4 and Lgr5+ in adult mouse crypt epithelial cells. In that study, administration of LPS in vivo or incubation of mouse colonic organoids with LPS resulted in markedly increased apoptosis and diminished proliferation in colon epithelial cells. Although both endogenous HA and exogenous LPS signal through TLR4, there are several significant distinctions. LPS signals through a TLR4/CD14/MD2 complex (3), whereas HA signals through a TLR4/MD2/CD44 complex (20). TLR4 signaling occurs through either MyD88, which results in NF-κB activation, or through TIR-domain-containing adapter-inducing interferon-β (TRIF), which leads to interferon regulatory factor 3 activation. Parenteral LPS affects small intestinal crypt cells through the TRIF pathway (10), whereas HA affects epithelial cell proliferation through MyD88 (24). In addition, parenteral LPS binds to TLR4 on all cells, whereas endogenous HA binds only to TLR4 on cells that are adjacent to extracellular HA.
Endogenous HA signaling through CD44 and TLR4 not only promotes epithelial cell proliferation, but also inhibits spontaneous apoptosis. In the positional proliferation and apoptosis studies, endogenous HA enhanced proliferation and diminished apoptosis in epithelial cells at positions 2–8, which includes the Lgr5+ stem cells. Although endogenous HA affects both proliferation and apoptosis, the effects on proliferation are numerically much greater, and thus the effects of endogenous HA on crypt fission and intestinal elongation are primarily the product of effects on proliferation.
We have previously demonstrated that in adult mice administration of exogenous HA results in the synthesis of additional HA through the induction of HAS1 and HAS3, enzymes involved in HA synthesis (12, 24). In the current study, we found that administration of exogenous HA from 7 to 14 days of life resulted in an expansion of the zone of HA expression to encompass areas further up the crypt in the intestine and colon. Thus the effects of exogenous HA on growth appear to be mediated through a feed-forward loop in which exogenous HA induces HA synthesis with expansion of the zone in which HA is expressed.
In adult mice, signaling from Paneth cells is thought to be important in Lgr5+ stem cell homeostasis and proliferation (18). In newborn mice, there are few or no Paneth cells in the small intestine. Treatment of WT mice with PEP-1 results in a decrease in Paneth cell number at 14 days of age, suggesting that HA signaling is required for the presence of a normal complement of Paneth cells. Paneth cell numbers are also diminished in CD44-deficient and TLR4-deficient mice, suggesting that the endogenous HA that supports Paneth cell number signals through both CD44 and TLR4.
In this study, we have demonstrated that Lgr5+ stem cell proliferation, crypt fission, and small intestinal elongation are all dependent on HA signaling through CD44 and TLR4. The most surprising aspect of this study is the role of TLR4 signaling in the normal growth of the small intestine and colon. Toll receptors play a role in growth and development in Drosophila (1), but this is the first demonstration of a TLR mediating normal growth in mammals.
GRANTS
This work supported by R37-DK33165 (W. Stenson), 5UO1AI095776 (M. Ciorba), DK100737 (M. Ciorba), and by DDRCC NIDDK P30DK052574.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.E.R., M.C., and W.F.S. conception and design of research; T.E.R., S.S., and L.F. performed experiments; T.E.R., S.S., M.C., and W.F.S. analyzed data; T.E.R. and W.F.S. interpreted results of experiments; T.E.R. and S.S. prepared figures; T.E.R., M.C., and W.F.S. drafted manuscript; T.E.R., S.S., L.F., M.C., and W.F.S. approved final version of manuscript; S.S., M.C., and W.F.S. edited and revised manuscript.
REFERENCES
- 1.Belvin MP, Anderson KV. A conserved signaling pathway: the Drosophila Toll-dorsal pathway. Annu Rev Cell Dev Biol 12: 393–416, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Brown SL, Riehl TE, Walker M, Geske M, Doherty J, Stenson WF, Stappenbeck T. MyD88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest 117: 258–269, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. J Biol Chem 276: 21129–21135, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Dehmer JJ, Garrison AP, Speck KE, Dekaney CM, Van Landeghem L, Sun X, Henning SJ, Helmrath MA. Expansion of intestinal stem cells during murine development. PLos One 6: e27070, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekaney CM, Gulati AS, Garrison AP, Helmrath MA, Henning SJ. Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest Liver Physiol 297: G461–G470, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan A, Thomas L, Xu R, Inoue H, Arditi M, Dannenberg A, Abreu M. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology 131: 862–877, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang D, Liang J, Fan J. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Majors AK, Austin RC, de la Motte CA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem 78: 47223–47231, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc 8: 2471–2482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mummert M, Mohamadzadeh M, Mummert D. Development of peptide inhibitor of hyaluronan-mediated leukocyte trafficking. J Exp Med 192: 769–779, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neal MD, Sodhi CP, Jia H, Dyer M, Egan CE, Yazji I, Good M, Afrazi A, Marino R, Slagle D, Ma C, Branca MF, Prindle T, Grant Z, Ozolek J, Hackam DJ. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem 287: 37296–37308, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neill LA. A feed-forward loop involving hyaluronic acid and Toll-like receptor-4 as a treatment for colitis? Gastroenterology 137: 1889–1890, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Pritchard DM, Potten CS, Korsmeyer SJ, Roberts S, Hickman JA. Damage-induced apoptosis in intestinal epithelia from Bcl-2-null and Bax-null mice: investigations of the mechanistic determinants of epithelial apoptosis in vivo. Oncogene 18: 7287–7293, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by TLRs is required for intestinal homeostasis. Cell 118: 229–241, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Riehl TE, Ee X, Stenson WF. Hyaluronic acid regulates normal intestinal and colonic growth in mice. Am J Physiol Gastrointest Liver Physiol 303: G377–G388, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riehl TE, Foster L, Stenson WF. Hyaluronic acid is radioprotective in the intestine through a TLR4 and COX-2 mediated mechanism. Am J Physiol Gastrointest Liver Physiol 302: G309–G316, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santaolalla R, Sussman DA, Ruiz JR, Davies JM, Pastorini C, Espana CL, Sotolongo J, Burlingame O, Bejarano PA, Philip S, Ahmed MM, Ko J, Dirisina R, Barrett TA, Shang L, Lira SA, Fukata M, Abreu MT. TLR4 activates the β-catenin pathway to cause intestinal neoplasia. PLoS One 8: e63298, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman L, Sleeman J, Herrlich PL. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol 6: 726–733, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem 282: 18265–18275, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Thaker AI, Rao MS, Bishnupuri KS, Kerr TA, Foster L, Marinshaw JM, Newberry RD, Stenson WF, Ciorba MA. IDO1 metabolites activate beta-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology 145: 416–425; e1–e4, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, Ciorba MA, Stappenbeck TS. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64: 911–920, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, Scoville D, He XC, Mahe MM, Box A, Perry JM, Smith NR, Lei NY, Davies PS, Fuller MK, Haug JS, McClain M, Gracz AD, Ding S, Stelzner M, Dunn JC, Magness ST, Wong MH, Martin MG, Helmrath M, Li L. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 145: 383–395, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfs T, Derikx J, Hodin C, Vanderlocht J, Driessen A, de Bruine AP, Bevins CL, Lasitschka F, Gassler N, van Gemert WG, Burman WA. Localization of the lipopolysaccharide recognition complex in the human healthy and inflamed premature and adult gut. Inflamm Bowel Dis 16: 68–75, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Zheng L, Riehl TE, Stenson WF. Regulation of colonic epithelial repair in mice by Toll-like receptors and hyaluronic acid. Gastroenterology 137: 2041–2051, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]