Abstract
Long noncoding RNAs (lncRNAs) are emerging as key species-specific regulators of cellular and disease processes. To identify potential lncRNAs relevant to acute and chronic renal epithelial injury, we performed unbiased whole transcriptome profiling of human proximal tubular epithelial cells (PTECs) in hypoxic and inflammatory conditions. RNA sequencing revealed that the protein-coding and noncoding transcriptomic landscape differed between hypoxia-stimulated and cytokine-stimulated human PTECs. Hypoxia- and inflammation-modulated lncRNAs were prioritized for focused followup according to their degree of induction by these stress stimuli, their expression in human kidney tissue, and whether exposure of human PTECs to plasma of critically ill sepsis patients with acute kidney injury modulated their expression. For three lncRNAs (MIR210HG, linc-ATP13A4-8, and linc-KIAA1737-2) that fulfilled our criteria, we validated their expression patterns, examined their loci for conservation and synteny, and defined their associated epigenetic marks. The lncRNA landscape characterized here provides insights into novel transcriptomic variations in the renal epithelial cell response to hypoxic and inflammatory stress.
Keywords: noncoding RNA, RNA sequencing, hypoxia, inflammation, epithelial injury
thousands of noncoding RNAs are pervasively transcribed from the human genome (14). Although poorly understood, long noncoding RNAs (lncRNAs), transcripts of >200 nucleotides long and expressed at low levels in a tissue-specific manner, are emerging as key regulators in biological and pathological processes (7, 9, 29, 60). Indeed, dysregulation of lncRNA expression occurs in a variety of human diseases, highlighting the need for identifying disease-relevant lncRNAs and studying how their sequences contribute to biological function (21, 22, 29, 43). However, the role of lncRNAs in renal physiology and disease has not been extensively explored.
Recent studies have shown that lncRNAs participate in regulatory mechanisms such as chromatin reprogramming, cis regulation at enhancer regions, and post-mRNA processing (10, 23, 36, 53). Although lncRNAs may perform key regulatory actions that are usually expected to be conserved, lncRNA transcripts from syntenic noncoding loci [genomic regions flanked by homologous protein-coding genes (60)] are not well conserved among species and could undergo species-specific alternative splicing (26, 63). With these evolutionary characteristics, lncRNAs in human renal disease would thus require their discovery within human cells and tissue.
Previous studies using murine models of renal injury have led to important advances in our understanding of pathological mechanisms in acute kidney injury (AKI) and chronic kidney disease (CKD), emphasizing the roles of hypoxia and inflammation in both processes (2, 4, 6, 20, 24, 25, 27, 30, 34, 46, 49, 57). However, these studies were mostly limited to the protein-coding domains of the genome. Emerging evidence suggests that the human inflammatory response at the genomic level differs significantly from that seen in murine models despite a high degree of protein-coding conservation between the human and mouse (54). Although many factors may contribute to this phenomenon, lncRNAs, many of which are species -specific, may represent an important missing link between animal models of injury and human disease. For example, a lncRNA found to be highly upregulated in a mouse model of renal fibrosis is not expressed in humans (64), suggesting that renal injury in mice involves genomic regulatory pathways not necessarily seen in humans, and vice versa. Thousands of human lncRNAs have been discovered (7, 29), but their cell-specific roles have yet to be fully defined.
To characterize the human lncRNA landscape in a renal injury model system, we performed unbiased transcriptomic profiling through RNA sequencing to identify lncRNAs that have altered expression in renal proximal tubular epithelial cells (PTECs) under hypoxic and inflammatory conditions. To help prioritize lncRNAs with human translational potential for further study, we evaluated which hypoxia- and/or inflammation-modulated lncRNAs are present in human proximal tubule samples. We then determined whether expression of these lncRNAs in PTECs changes upon exposure to plasma of critically ill sepsis patients with AKI, since sepsis is characterized by both severe immune dysregulation and tissue hypoxia from septic shock. In the present study, we report the first comprehensive lncRNA profiles of human PTECs in stress models relevant to acute and chronic renal injury and examine their genomic features.
MATERIALS AND METHODS
Culture of human PTECs.
Immortalized HKC-8 cells (51) were cultured in DMEM-nutrient mixture F-12 supplemented with 5% FBS (GE Life Sciences), 1% insulin-transferrin-selenium (Invitrogen GIBCO), and 0.1% Primocin (Invivogen) and incubated at 37°C and 5% CO2. STR profiling confirmed that the HKC-8 line was not contaminated with other known cell lines (Promega, American Type Culture Collection), and the cell line was confirmed to be mycoplasma negative. For the hypoxia experiments, HKC-8 cells serum starved for 24 h at 85% confluence in six-well plates were placed in anaerobic chamber bags (Becton Dickinson) that induced hypoxic conditions at 0.1% O2 per the manufacturer's protocol, with hypoxia confirmed through immunoblot analysis for hypoxia-inducible factor (HIF)-1α (Novus). For the cytokine treatment experiments, serum-starved HKC-8 cells at 85% confluence were treated with a cytokine cocktail consisting of 50 ng/ml IL-6, 50 ng/ml TNF-α, and 20 ng/ml interferon-γ (Peprotech) to mimic systemic inflammation rather than induce one specific inflammatory pathway. Based on our initial dose-response experiments, the lowest concentrations that evoked increased mRNA expression of inflammatory genes without evidence of cell toxicity were chosen (data not shown). Cells treated for 12, 24, and 48 h underwent RNA isolation (see below). Hypoxia and cytokine treatment experiments were performed in triplicate at least three times; RNA sequencing was performed on unpooled samples collected from three separate experiments. Experiments in which HKC-8 cells were treated with human plasma used samples from patients with severe sepsis enrolled in the Molecular Epidemiology of Severe Sepsis in the ICU (MESSI) study, a single-center prospective observational cohort of individuals with severe sepsis (45, 52), or control plasma pooled from healthy volunteers recruited to the GENE study (18, 41). These cohorts were recruited under protocols approved by the Institutional Review Board of the University of Pennsylvania. HKC-8 cells serum starved for 24 h at 85% confluence in 12-well plates were washed and then incubated for 12 h in 10% unpooled plasma from AKI or non-AKI MESSI patients or pooled plasma from control GENE subjects. To prevent fibrin formation during plasma treatment of cells, 1 unit of heparin (Sigma-Aldrich) was added to each well. After treatment, cells were washed with PBS and collected for RNA isolation. Treatment of HKC-8 cells with human plasma samples was performed in three separate experiments revealing reproducible inflammatory effects [e.g., increased mRNA expression of IL-6 and TNF-α-induced protein 3 (TNFAIP3) by quantitative real-time PCR; data not shown]; quantitative real-time PCR for lncRNA expression was performed on cells collected from the third experiment.
RNA isolation, library preparation, and RNA sequencing.
Total RNA was isolated from HKC-8 cells using the miRNeasy kit and underwent on-column DNase treatment (Qiagen). RNA used for RNA sequencing had a minimum RNA integrity number of 9.5 (Agilent 2100 Bioanalyzer). Because a large portion of lncRNAs are polyadenylated (14, 41, 60), strand-specific barcoded libraries for RNA sequencing were generated from the polyadenylated fraction of RNA using the TruSeq Stranded mRNA Library Prep Kit (Illumina) according to the manufacturer's protocol with the following modifications: 1) the fragmentation time was decreased from 8 to 6 min to ensure libraries were >100 bp long and 2) PCR amplification was limited to 12 cycles to avoid bias from PCR “jackpot” mutations. Library length and concentration were evaluated with the Agilent 2100 Bioanalyzer and PCR quantification (KAPA) and pooled at 2 nM for massively parallel sequencing (2 × 100 bp) performed on the HiSeq 2500 (Illumina). On average, we obtained ∼32 million filtered reads per sample (see below) with >85% reads uniquely mapped to the human genome.
Transcriptional expression analysis.
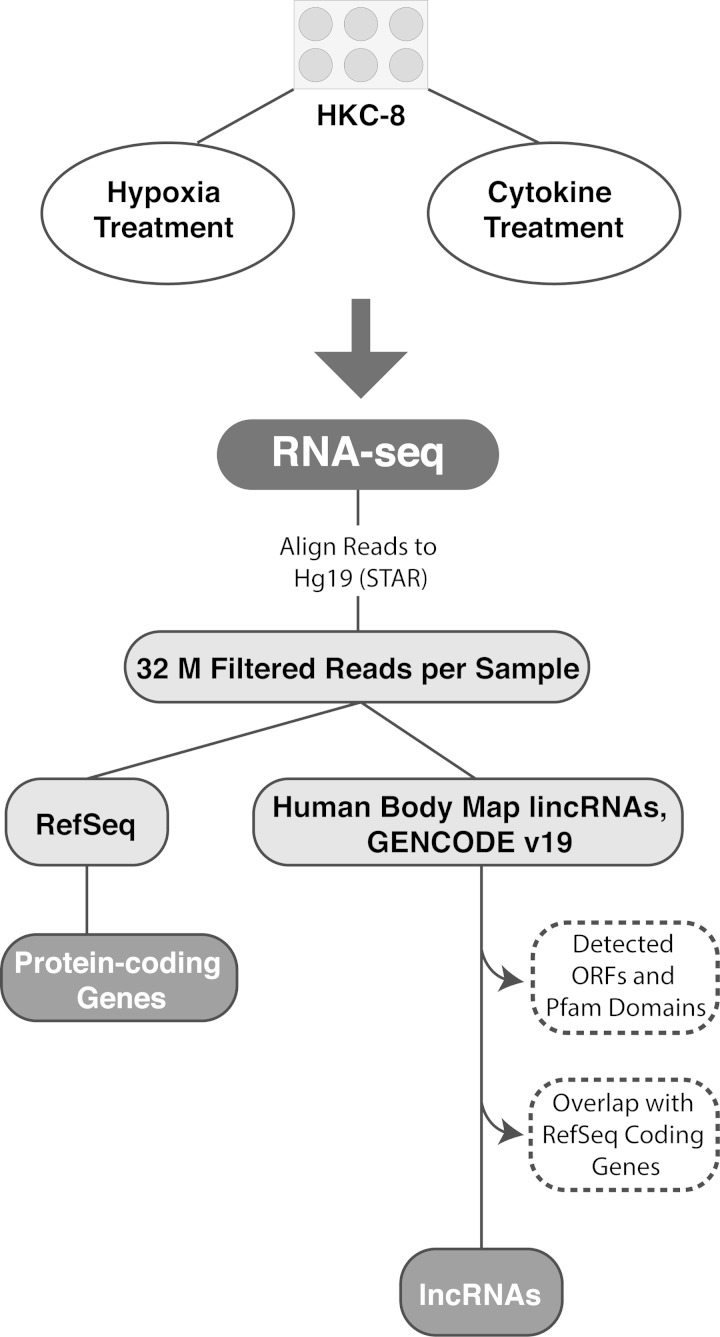
The overall workflow is shown in Fig. 1. Sequencing data were aligned to the hg19 reference genome using STAR 2.3.0e (15) under default options. The following filtering steps were applied: each read was uniquely mapped, and a pair of reads were mapped to the same chromosome with expected orientations and a mapping distance of <500,000 bp between the two reads. All subsequent analyses were performed on filtered alignment files. Transcripts were annotated using publicly available datasets from RefSeq (50) for protein-coding regions and GENCODE 19 (13) and the Human Body Map LincRNAs (7) annotation for long noncoding genes. Because the coding status of previously annotated lncRNAs can change based on updated publicly available data, annotated lncRNAs in our RNA sequencing data set underwent further filtering steps, including evaluation of coding potential with iSeeRNA and presence of a Pfam domain (19, 55). To increase confidence that a transcript was noncoding and not an extension of a novel protein-coding isoform, the 1-kb region flanking each candidate transcript was examined for the presence of ≥10 reads in >50% of samples; if such reads were present, the transcript was considered as protein coding and excluded from lncRNA analyses. The list of filtered lncRNAs was then manually curated to ensure that no protein-coding transcripts were annotated in the same locus on the same strand as a lncRNA. The abundance of each RNA transcript was measured in fragments per kilobase of exon per million reads mapped (FPKM) using Cufflinks 2.1.1 (58). Differential expression (DE) between control and stimulation conditions was tested with Cuffdiff (58). Genes and lncRNAs with a false discovery rate-adjusted P value of <0.05 and a fold change of >1.5 were considered as differentially expressed. Data were deposited in the GEO repository (GSE70544). Gene ontology (GO) analysis was performed using Ingenuity Pathway Analysis software (Qiagen).
Fig. 1.
Overview of the RNA sequencing (RNA-seq) workflow, including filtering and annotation of expressed transcripts. M, million; lincRNA, long intergenic noncoding RNA; lncRNA, long noncoding RNA. Please see methods for more details.
Conservation and synteny analysis.
Many functional lncRNAs are known to have synteny [genomic regions flanked by homologous protein-coding genes (60)] despite low sequence similarity across species (26, 47, 61). We examined the synteny of lncRNAs in the mouse using HomoloGene release 68 (http://www.ncbi.nlm.nih.gov/homologene). The neighboring genes of lncRNAs in human were identified, and the homologous genes were searched in HomoloGene. If homologous genes in the mouse were found for the two nearest neighboring genes in the human, we considered the lncRNA syntenic. For syntenic lncRNAs, we evaluated their sequence conservation using BLASTN (8). The human lncRNA sequence was queried against the mouse genome with an E-value cutoff of 1e−10. Any hits in the mouse within the syntenic region were then searched in human with the same E-value cutoff. Sequences that passed the reciprocal steps were conserved. These conserved sequences were then queried in the University of California-Santa Cruz Genome Browser (https://genome.ucsc.edu) using GENCODE M4 to assess whether they were publicly annotated mouse lncRNAs.
Quantitative real-time RT-PCR.
Total RNA was isolated as described above. Reverse transcription was performed from 1 μg total RNA per sample using the High Capacity RNA to cDNA Master Mix kit (Applied Biosystems, Life Technologies). Quantitative real-time RT-PCR was performed in a total volume of 10 μl on the QuantStudio 7 Flex Real-Time PCR System (Applied Biosystems, Life Technologies) using SYBR green PCR mix (Bio-Rad; primers were obtained from IDT and are shown in Supplemental Table S1 in the Supplemental Material).1 Unless otherwise indicated, each transcript's cycle threshold (Ct) value was normalized to the ACTB Ct value for each sample, and a transcript's relative expression was determined through the 2−(ΔΔCt) method.
Nuclear and cytoplasmic RNA fractionation.
For localization of lncRNAs prioritized for further study, unstimulated HKC-8 cells underwent subcellular fractionation using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific) with the addition of RNase inhibitor (Invitrogen) to the lysis buffers. Total RNA was then isolated from each subcellular fraction using the miRNeasy kit (Qiagen). Quantitative real-time RT-PCR was performed for the lncRNAs in each fraction, with normalization of each fraction to the mean Ct of U6 and GAPDH combined. To ensure each subcellular fraction had only limited cross-contamination, relative U6 and GAPDH levels were measured separately to confirm their abundance in the nuclear and cytoplasmic compartments, respectively.
Statistical analysis.
Nonsequencing data were analyzed with GraphPad Prism 6 (GraphPad Software) and are shown as means ± SD. Statistical differences between groups were determined in a pairwise fashion using a nonparametric Mann-Whitney U-test. The correlation coefficient of expression levels for genes differentially expressed in both hypoxia and cytokine treatments were assessed using Spearman's rank correlation.
RESULTS
Transcriptomic response of PTECs in hypoxic and inflammatory stress.
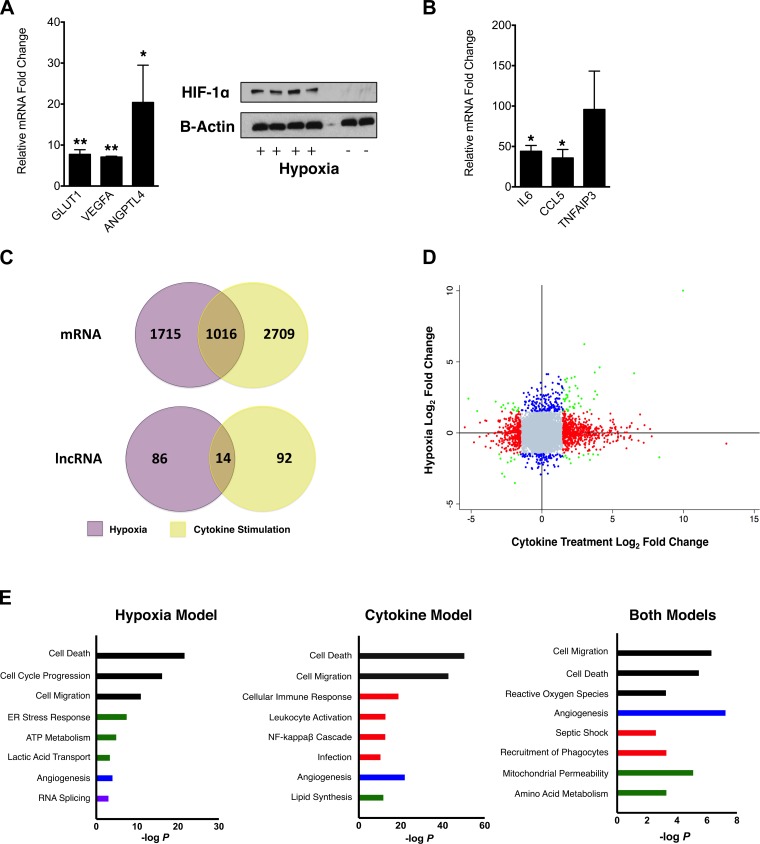
To assess the transcriptomic response of PTECs to stressors related to renal epithelial injury, we performed RNA sequencing on HKC-8 cells that were subjected to hypoxia, treatment with multiple cytokines associated with clinical AKI and/or in vitro PTEC injury (IL-6, TNF-α, and interferon-γ) (4, 11, 31–33, 40, 56), or control conditions. The hypoxic response to cell culture conditions was confirmed with translocation of the HIF-1α protein to the nucleus and induction of HIF-1α target genes evaluated by quantitative PCR, including solute carrier (SLC)2A1 (P < 0.001), VEGF-A (P < 0.001), and angiopoietin-like 4 (P = 0.02; Fig. 2A). Treatment of HKC-8 cells with the cytokine cocktail resulted in the induction of genes relevant to the innate immune response, including increased expression of IL-6 (P = 0.01), chemokine (C-C motif) ligand 5 (P = 0.04), and TNFAIP3 (P = 0.10; Fig. 2B).
Fig. 2.
A: for hypoxia-stimulated HKC8 cells, upregulation of hypoxia-inducible factor (HIF)-1α targets was confirmed by quantitative real-time PCR for the listed genes (*P < 0.05 and **P < 0.001). HIF-1α stabilization and translocation to the nucleus was confirmed by HIF-1α immunoblot analysis of nuclear fractions of HKC-8 cells exposed to hypoxic conditions. Immunoblot analysis for control β-actin was performed on whole cell lysates. GLUT, glucose transporter; ANGPTL4, angiopoietin-like 4. B: for cytokine-treated HKC8 cells, upregulation of inflammatory genes was confirmed by quantitative real-time PCR (*P < 0.05). CCL, chemokine (C-C motif) ligand; TNFAIP3, TNF-α-induced protein 3. C: hypoxia and cytokine treatment of HKC-8 cells induced many differentially expressed (DE) protein-coding genes and lncRNAs with only a small fraction of DE transcripts overlapping in both conditions. D: scatterplot of log2 fold changes in the expression of protein-coding genes expressed in each stimulation condition revealed that most transcripts were specifically DE in one condition or another. Blue dots represent transcripts DE in hypoxia only; red dots represent transcripts DE in cytokine treatment only; green dots represent transcripts DE in both conditions; gray dots are transcripts not DE in either condition. E: Gene Ontology (GO) analysis for each injury model or both revealed the induction of DE genes in pathways and functions listed on a −log(P value) scale. Pathways and processes are color coded according to groups of function: cellular viability and motility (black), inflammation (red), metabolism (green), vascular biology (blue), and transcriptional regulation (purple).
We found a total of 12,275 expressed protein-coding genes and lncRNAs. Hypoxic conditions led to the DE of 2,731 protein-coding genes, whereas cytokine treatment induced the DE of 3,725 protein-coding genes (Fig. 2C), with 1,016 (8.6% of all expressed protein-coding genes and 18.7% of DE protein-coding genes) DE in both hypoxic and inflammatory conditions (Fig. 2C). Among these, 79 had a more than threefold change of expression (Fig. 2D, green), with Spearman's ρ = 0.36 between expression levels induced by hypoxia and cytokine treatments (P = 0.001). Ingenuity pathway analysis of GO terms revealed that these 79 genes are involved in functions relevant to cell death, septic shock, the phagocytic response, mitochondrial integrity, and angiogenesis (Fig. 2E). However, GO analysis of the full sets of DE genes revealed distinct expression profiles for hypoxia-treated cells and for cytokine-treated cells. Hypoxia modulated genes related to anerobic metabolism not regulated in response to cytokine treatment, whereas cytokine treatment led to the DE of genes in certain immune response pathways not changed by hypoxia (Fig. 2E). In summary, hypoxia and cytokine treatment induced distinct transcriptomic responses, with a modest fraction (∼19%) of genes dysregulated in both conditions involved in pathways relevant to cellular injury.
Hypoxia and cytokine stimulation induce distinct lncRNA signatures.
After finding in our RNA sequencing experiments that the protein-coding transcriptomic landscape was mostly distinct for hypoxia-stimulated and cytokine-stimulated HKC-8 cells, we probed whether the lncRNA landscape also differed between these experimental stress conditions. Our RNA sequencing data revealed that 667 publicly annotated lncRNAs, including antisense transcripts and long intergenic noncoding RNAs (lincRNAs), are expressed in HKC-8 cells (Supplemental Tables S3 and S5). Although hypoxia and cytokine treatment of HKC-8 cells induced DE of approximately the same number of lncRNAs (Fig. 2C), only 14 (2% of total expressed lncRNAs and 7.2% of all DE lncRNAs) overlapped between conditions, with 5 of these DE in concordant directions (Table 1). The 100 lncRNAs modulated by hypoxia were located in loci adjacent to protein-coding genes involved in many pathways, including those for Wnt/β-catenin signaling, ATP metabolism, vitamin D receptor activation, and cellular differentiation (Table 2). In contrast, the 106 cytokine DE lncRNAs were located in loci adjacent to protein-coding genes involved in inflammation-specific pathways, including cytokine and innate immune signaling (Table 2). Thus, hypoxic and inflammatory stimulations induce DE of distinct sets of lncRNAs, with limited overlap (∼7%) of DE lncRNAs, located in loci specific and directly relevant to the experimental stress condition.
Table 1.
LncRNAs differentially expressed by both hypoxia and cytokine treatments
| Hypoxia |
Cytokine Treatment |
||||
|---|---|---|---|---|---|
| LncRNA Name | Locus | Fold change | q Value | Fold change | q Value |
| linc-PADI1-1 | chr1:17516277–17524112 | 1.56 | 0.010 | −2.46 | <0.001 |
| linc-WNT8B | chr10:102133332–102154988 | 2.09 | <0.001 | −1.95 | 0.003 |
| MIR210HG | chr11:565656–568457 | 5.69 | <0.001 | 2.35 | 0.022 |
| linc-IGHMBP2 | chr11:68638131–68642010 | −1.96 | 0.004 | 13.0 | <0.001 |
| RP11–169D4.1 | chr11:72281703–72284273 | −1.64 | 0.008 | −1.54 | 0.041 |
| AC156455.1 | chr12: 122501211–122506522 | 1.72 | 0.004 | −1.76 | 0.032 |
| linc-ZIC5 | chr13:100738208–100741196 | −4.04 | 0.001 | −4.05 | 0.001 |
| linc-CREBBP | chr16:3980004–4002465 | 2.74 | 0.017 | 2.85 | 0.032 |
| CTD-2319I12.1 | chr17: 58156669–58169246 | −2.87 | 0.004 | −3.77 | <0.001 |
| MIR22HG | chr17:1614797–1641893 | −8.78 | <0.001 | 3.95 | <0.001 |
| ERVK3-1 | chr19:58816714–58827023 | −1.67 | 0.008 | 1.72 | 0.008 |
| linc-CEBPB-1 | chr20:48788627–48793208 | 2.37 | <0.001 | −2.65 | 0.020 |
| linc-B3GAT2-4 | chr6:72117910–72130522 | −1.71 | 0.017 | −2.81 | <0.001 |
| AC083843.1 | chr8: 135804262–135810515 | 2.87 | <0.001 | −2.46 | <0.001 |
LncRNA, long noncoding RNA; lincRNA, long intergenic noncoding RNA.
The q value is the false discover rate-adjusted P value.
Table 2.
Pathways of protein-coding genes near modulated lncRNA loci
| Pathway | −Log(P Value) |
|---|---|
| Hypoxia | |
| Wnt/β-catenin signaling | 3.63 |
| P2Y purigenic receptor signaling | 1.91 |
| VDR/RXR activation | 1.69 |
| Methylmalonyl pathway | 1.51 |
| Stem cell pluripotency | 1.45 |
| Cytokine treatment | |
| IL-17A signaling | 2.45 |
| PPAR signaling | 2.07 |
| NF-κΒ signaling | 1.81 |
| IL-6 signaling | 1.75 |
| IL-10 signaling | 1.74 |
VDR, vitamin D receptor; RXR, retinoid X recepter; PPAR, peroxide proliferator-activated receptor.
Prioritization of LncRNAs relevant to human renal epithelial injury.
LncRNAs DE under hypoxic or inflammatory stimulation were prioritized for further study according to three separate criteria. First, to focus on noncoding RNAs most strongly modulated by hypoxia or cytokine treatment, we selected from our RNA sequencing data set the most abundant lncRNAs (FPKM values > 1 in either control or stimulation conditions) and prioritized for further study those with a greater fold change in expression (top 30th percentile) after either hypoxic or cytokine stimulation (Supplemental Tables S2 and S4). Next, to ensure that the candidate lncRNAs were indeed relevant in the human kidney, we examined whether they were expressed in human kidney tissue. Finally, to aid our prioritization of lncRNAs for further study, we evaluated whether exposure of human PTECs to plasma of critically ill sepsis patients with AKI, a method previously shown to induce PTEC injury (44), modulated their expression.
Based on these criteria, three hypoxia- or inflammation-modulated lncRNAs were prioritized: MIR210HG, linc-ATP13A4-8, and linc-KIAA1737-2. All three lncRNAs were present in human kidney tissue with detectable expression (mean FPKM values ranging from 0.61 to 4.38) in microdissected proximal tubules isolated from T0 biopsies of healthy kidney transplant allografts (GEO Accession No. GSE60119; Table 3) (39), indicating that their expression is not limited to the in vitro setting. As expected, known proximal tubular protein-coding marker genes, such as SLC36A2 (mean FPKM: 37.321), γ-glutamylcyclotransferase (mean FPKM: 27.030), and intestinal alkaline phosphatase (mean FPKM: 7.144) (3, 16, 48), are expressed at higher levels in these tissue samples. Linc-ATP13A4-8 is also annotated as kidney specific in healthy human kidney tissue (7).
Table 3.
LncRNA expression in human nicrodissected renal proximal tubules
| Donor 1, FPKM | Donor 2, FPKM | |
|---|---|---|
| MIR210HG | 0.741 | 0.817 |
| linc-ATP13A4-8 | 1.648 | 0.498 |
| linc-KIAA1737-2 | 0.065 | 1.163 |
Shown are expression levels of lncRNAs in human renal proximal tubules microdissected from healthy transplant kidney biopsies.
FPKM, fragments per kilobase of exon per million reads mapped.
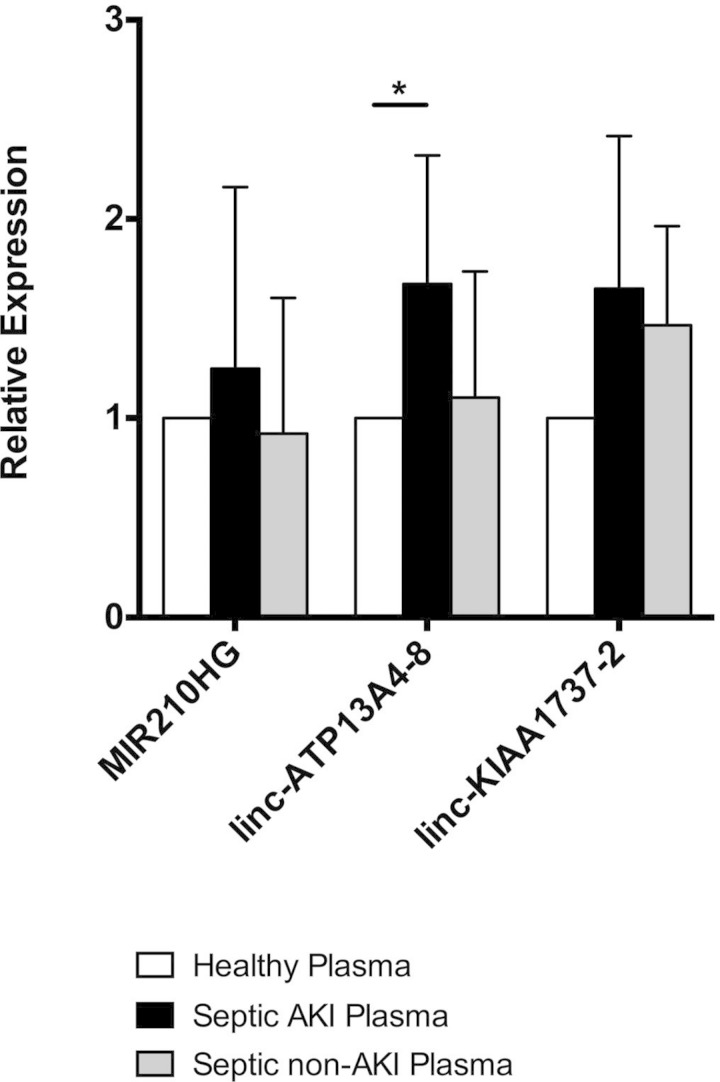
To probe which lncRNAs are potentially modulated by in vivo AKI-relevant stress, we treated PTECs with plasma collected from septic patients with AKI, septic patients without AKI (Table 4), and healthy human subjects, the characteristics of whom have been previously described (18). Culture of PTECs with plasma from critically ill AKI patients has been shown to induce more severe cellular injury than in vitro exposure to plasma from non-AKI patients (44). Thus, to prioritize further which lncRNAs may be most relevant to human renal epithelial injury, we examined which noncoding RNAs were induced by PTEC exposure to AKI plasma. Sepsis-associated AKI, rather than CKD, was selected as the most cogent disease process to test because more pronounced differences in circulating stressors of relevance to the pathophysiology of renal injury, including hypoxia and severe inflammation, were expected between disease and control plasma. Indeed, compared with cells treated with healthy plasma or septic non-AKI plasma, cells treated with plasma from septic patients with AKI showed a trend toward increased MIR210HG, linc-ATP13A4-8, and linc-KIAA1737-2 expression (Fig. 3), lncRNAs that were DE by hypoxia or inflammatory stress.
Table 4.
Clinical characteristics of sepsis cohort subjects
| Number of Subjects | Age, yr | African Ancestry, % | Male/Female, % | APACHE III | Serum Creatinine Rise, % | Death, % | |
|---|---|---|---|---|---|---|---|
| No acute kidney injury | 5 | 54.2 (26.2) | 40 | 80/20 | 56 (9.3) | 5.3 | 0 |
| Acute kidney injury | 5 | 61 (23.5) | 40 | 80/20 | 85 (29) | 58.3 | 80 |
Data are represented as means (SD) or as a proportion.
Fig. 3.
Quantitative real-time PCR evaluation of lncRNA expression in HKC-8 cells treated with human septic acute kidney injury (AKI) plasma, septic non-AKI plasma, or healthy plasma (*P < 0.05).
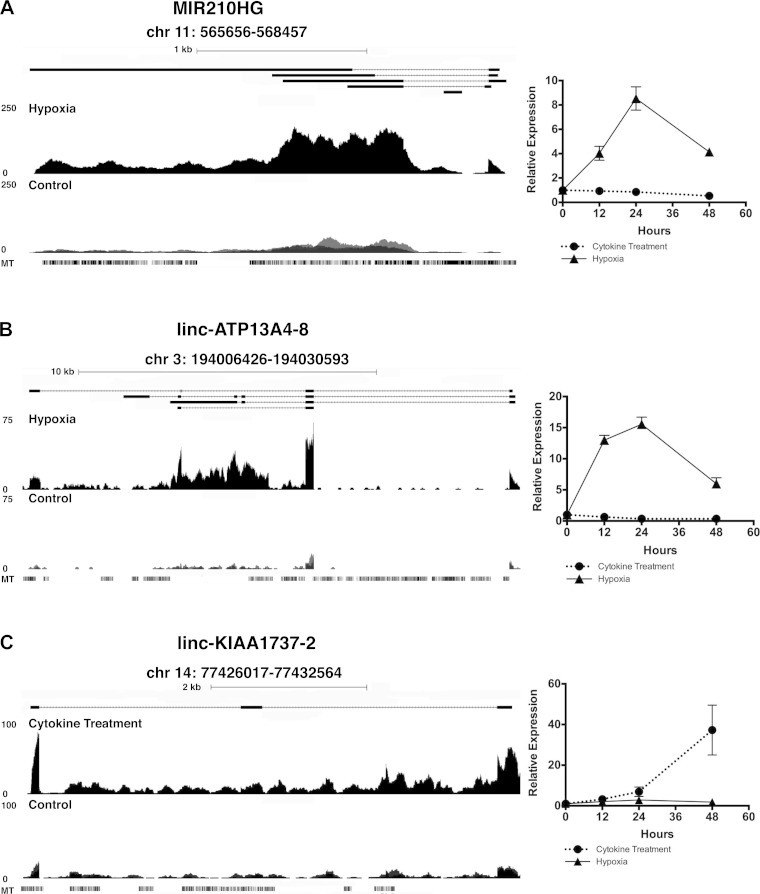
These three lncRNAs were markedly induced by either hypoxia (MIR210HG and linc-ATP13A4-8) or cytokine treatment (MIR210HG and linc-KIAA1737-2). Genome browser views of RNA sequencng reads for these three lncRNAs during hypoxia or cytokine treatment and respective controls are shown in Fig. 4. MIR210HG was induced by hypoxia, with its quantitative PCR-validated expression peaking at 24 h in a time-course experiment (Fig. 4A). The expression of microRNA-210, which is transcribed from the intronic region of MIR210HG, was also increased (>30-fold) by 48 h of hypoxia (data not shown). MIR210HG is not, however, an experimentally observed or predicted binding target of microRNA-210 (http://bioinfo.life.hust.edu.cn/lncRNASNP and Qiagen Ingenuity Pathway Analysis). In contrast to its response to hypoxia, expression of MIR210HG was barely modulated by cytokine treatment. Linc-ATP13A4-8, like MIR210HG, had its peak induced expression at 24 h after the onset of hypoxia (Fig. 4B). Linc-KIAA1737-2, induced only by cytokine treatment, continued to rise (>40-fold increase) after 48 h of cytokine stimulation (Fig. 4C). In summary, MIR210HG, linc-ATP14A4-8, and linc-KIAA1737-2 exhibit distinct induction patterns under hypoxic and inflammatory conditions, are confirmed to be present in human proximal tubules, and may also be modulated by plasma of AKI patients.
Fig. 4.
Genome browser views of lncRNAs MIR210HG (A), linc-ATP13A4-8 (B), and linc-KIAA1737-2 (C) with quantitative real-time PCR evaluation of relative expression levels at 12, 24, and 48 h under each stimulation condition compared with control. MT, mouse track.
Conservation and synteny of MIR210HG, linc-ATP13A4-8, and linc-KIAA1737-2.
Because lncRNA transcripts tend to be species specific with a lower degree of conservation than protein-coding genes (26, 60), we evaluated the human specificity of MIR210HG, linc-ATP13A4-8, and linc-KIAA1737-2 by looking in the mouse for sequence conservation and synteny (i.e., homology of flanking protein-coding genes at noncoding loci). PhastCons scoring for each chromosomal position is represented by the conservation track (labeled MT in Fig. 4, A–C) in the genome browser views of each lncRNA. MIR210HG had 87.2% conservation of a specific 148-nt sequence in a syntenic region flanked by Ras-associated domain family (NH2-terminal) member 7 and PHD and ring finger domains 1. However, no known lncRNAs are publicly annotated in this syntenic region in the mouse genome. Linc-ATP13A4-8 had 80.6% conservation of a 206-nt region and is located in a syntenic region flanked by hairy and enhancer of split-1 and carboxypeptidase N polypeptide 2. This specific sequence also does not overlap any known mouse lncRNAs but is within 10 kb of noncoding transcript Gm26562. Linc-KIAA1737-2 did not have sequence conservation in the mouse but did, along with two other human lncRNAs, have synteny between chromosome 14 open reading frame 166 and interferon regulatory factor 2 binding protein-like, nearby protein-coding genes in the mouse. This syntenic region contains noncoding transcript Gm25888 in the mouse, although it is unclear which of the three human lncRNAs may share functional conservation with this mouse lncRNA. This degree of sequence conservation within syntenic regions with the mouse is greater than for the majority of human lncRNAs (26, 47), suggesting potential evolutionary conserved functions (60, 61) of the syntenic regions of these PTEC-expressed lncRNAs.
Localization and regulatory landscape of MIR210HG, linc-ATP13A4-8, and linc-KIAA1737-2.
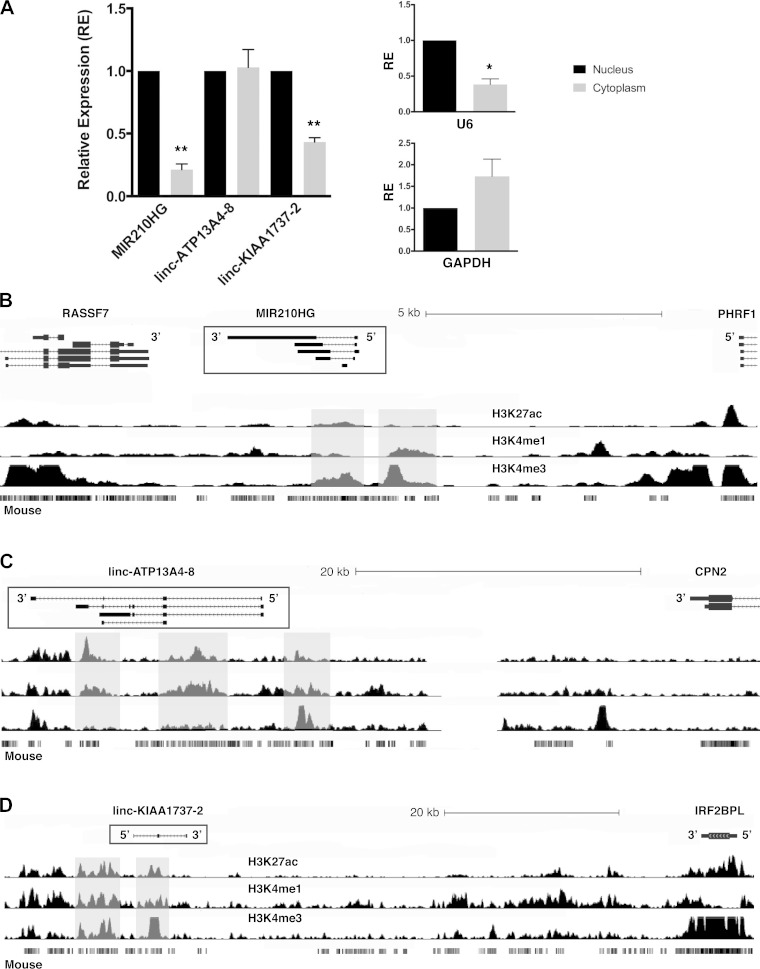
LncRNAs can play important roles via transcriptional or posttranscriptional regulation depending on their primary intracellular location. To determine whether these lncRNAs are enriched in the nucleus or cytoplasm, we evaluated their relative abundance in the nucleus versus cytoplasm by subcellular fractionation followed by quantitative PCR. MIR210HG and linc-KIAA1737-2 were located primarily in the nucleus, whereas linc-ATP13A4-8 was expressed with equal abundance in both compartments (Fig. 5A).
Fig. 5.
A: subcellular localization of each lncRNA. Relative U6 and GAPDH expression for each subcellular compartment confirmed separation of nuclear from cytoplasmic RNA. B–D: epigenetic landscapes of MIR210HG (B), linc-ATP13A4-8 (C), and linc-KIAA1737-2 (D) with chromatin histone modification tracks drawn from adult kidney tissue in the National Institutes of Health Epigenomics Roadmap.
We evaluated their genomic loci for epigenetic marks consistent with active transcription as well as for enhancer region marks that also might indicate whether these lncRNAs could be involved in transcriptional regulation. Using publicly available human adult kidney histone modification data (37), we found that MIR210HG has its own canonical promoter region with increased reads for H3K4me3 at the 5′-end (Fig. 5B). Its associated intronic microRNA-210 also overlapped with a region with higher H3K4me3 reads (Fig. 5B). Linc-ATP13A4-8 overlapped with multiple active transcription marks in the kidney, including relatively high H3K4me3 reads at its 5′-end and increased H3K4me1 and high H3K27ac reads in other regions consistent with enhancer marks (Fig. 5C). Linc-KIAA1737-2 also had active marks for transcription in or near its locus, with increased H3K4me3, H3K4me1, and H3K27ac reads (Fig. 5D). This epigenetic landscape is consistent with active transcription at all three lncRNA loci, with possible enhancer regions near or at the genomic loci for linc-ATP13A4-8 and linc-KIAA1737-2.
DISCUSSION
LncRNAs are emerging as important regulators of cellular function, and their dysregulation can contribute to human disease (9, 12, 21, 22, 38, 43, 59). However, their role in kidney disease has not been widely studied, with little known about human- and kidney-specific lncRNAs. To gain insights into the lncRNA landscape in renal epithelial injury, we used RNA sequencing to study the human PTEC response to hypoxia and cytokine treatments, which induce quite distinct transcriptomic signatures in proximal tubule cells. We identified 667 lncRNAs expressed in human PTECs, with 100 of these lncRNAs being DE by hypoxia, whereas 106 lncRNAs were DE during exposure to exogenous cytokines. To our knowledge, this is the first study to provide a comprehensive profile of the lncRNA landscape in human renal epithelial cells with a specific focus on those that may be most relevant to kidney injury.
Although both hypoxia and inflammation contribute to acute and chronic renal injury, the transcription-level changes these stressors induce appear to be mostly unique to each insult, with only 18.7% of DE protein-coding genes and 7.2% of DE lncRNAs overlapping between the two transcriptomic profiles. Interestingly, protein-coding genes that are DE in both hypoxic and inflammatory transcriptomes are enriched in functional pathways of cell death, septic shock, and mitochondrial permeability, suggesting that genes modulated by both stress conditions are involved in important final common pathways of cellular injury. However, the majority of protein-coding genes DE during hypoxia fall into pathways of hypoxia-specific functions, such as anerobic metabolism, whereas cytokine treatment induced its own specific intracellular inflammatory response pathways predominantly not activated by hypoxia. In this protein-coding gene transcriptional context, lncRNAs are also differentially expressed with distinct signatures for hypoxia and cytokine stresses. DE lncRNAs are flanked by protein-coding genes enriched for functions specific to each stimulus (e.g., cellular differentiation and metabolism genes for hypoxia compared with inflammatory signaling genes for cytokine treatment). These trends, found through a “guilt by association” approach (53), suggest that each stimulus has its own directly relevant lncRNA signature. Importantly, the presence of independent activating epigenetic marks at lncRNA loci both in our data set and in others (28, 38) suggests that the lncRNAs modulated by these stresses may play an active role in regulating nearby protein-coding genes rather than being mere transcriptional byproducts. Our results indicate that hypoxic and inflammatory conditions induce common pathways of cellular injury in addition to broadly distinct transcriptomic responses in PTECs, as expected based on published data in other cell systems (17). This finding reveals the complex coding and noncoding transcriptional networks involved in different types of cell stress. In vivo, both systemic inflammation and regional hypoxia likely contribute in an additive or synergistic manner to acute and chronic renal injury, and we may expect the polyadenylated RNA fraction of an AKI kidney or a CKD kidney to include more overlap of the hypoxic and inflammatory transcriptomic profiles. An important future step would be to characterize the transcriptome of human AKI or CKD tissue to examine more closely how these coding and noncoding transcriptional pathways may intersect or diverge in renal diseases.
In moving from unbiased discovery in vitro to future profiling of the noncoding transcriptome in vivo, it is important to consider two hallmarks of lncRNAs (tissue specificity and species specificity) that bring to light the potentially pivotal importance of lncRNAs in human disease. Although >75% of the protein-coding genome is conserved between human and mouse, less than half of the noncoding transcriptome maintains this interspecies sequence conservation (26, 47, 61). While prior traditional viewpoints have dismissed nonconserved transcripts as nonfunctional, lncRNAs with little sequence conservation but transcribed from syntenic regions (loci flanked by homologous protein-coding genes) have been found to play a role in critical processes such as embryonic development (61). The limited level of lncRNA sequence conservation between the human and mouse may in part explain why responses to systemic inflammation, potentially regulated by lncRNAs (21, 28, 38, 53), have species-specific features (54). Therefore, the human specificity of many lncRNAs highlights the need for unbiased discovery and further study of noncoding transcripts specifically in human cells and tissue. In our kidney injury-focused analyses, three lncRNAs identified as cytokine or hypoxia regulated in human PTECs showed a higher degree of expression in PTECs exposed to human septic AKI plasma rather than septic non-AKI or control plasma. This finding suggests the possible relevance of these three lncRNAs to PTEC injury. Although these differences in expression did not reach statistical significance, this interrogation was only one of several criteria used to prioritize these transcripts with human translational potential for further study. Notably, all three lncRNAs were expressed in human microdissected proximal tubules. While two of these three lncRNAs share sequence conservation in a small portion of the syntenic genomic region, these sequences do not overlap with the loci of known annotated mouse lncRNAs. If RNA sequencing had been performed on mouse cells or tissue instead of on human cells, these regions are unlikely to have been annotated as transcripts at all using current algorithms and standards.
Of the three lncRNAs highlighted in this study, all are expressed in the kidney, but only linc-ATP13A4-8 is annotated as kidney specific in Human Body Map LincRNAs (7). However, MIR210HG (excluded from the Human Body Map annotation as it did not meet criteria as a strictly “intervening” lincRNA) may have renal-specific function as microRNA-210, housed in its intron, is associated with AKI when abundant in the circulation (1, 42). Although linc-KIAA1737-2 may not have had kidney-specific expression, its induction in PTECs by inflammatory stress supports a role in the kidney injury response; to date, its response to inflammatory stimuli has not been published for other human cell or tissues. However, consistent with potential kidney specificity of its response to stress, these lncRNAs are very lowly expressed in other cells and tissues. They were not induced in our own data sets of deeply sequenced adipose tissue and peripheral blood mononuclear cells obtained from healthy volunteers, who were subjected to experimental endotoxemia (data not shown) (41).
Forming conclusions about the functions of these lncRNAs in human proximal tubules is beyond the scope of this study, but their expression patterns do suggest directions for further investigation. For example, MIR210HG initially follows a similar expression pattern to microRNA-210 but peaks at 24 h, suggesting that it may be part of and modulate the acute phase response during hypoxia. MicroRNA-210, a negative regulator of HIF-1α (62), continues to rise in expression at 48 h, consistent with its putative role in negative feedback on hypoxia-induced pathways. Interestingly, microRNA-210 does not have a binding site on MIR210HG, and both transcripts have independent promoter marks in the human adult kidney, suggesting that although induced by the same stimulus, they may have independent roles in hypoxia and modulation of hypoxia-related injury. Future study of the nuclear protein interactions of MIR210HG will inform if it has a specific role in transcriptional regulation of HIF-1α target genes.
Like MIR210HG, linc-ATP13A4-8 (also annotated as lnc-CPN2-1) is induced by hypoxia. Furthermore, its expression is more abundant in human renal cell carcinoma tissue than in healthy renal tissue (5), consistent with a possible involvement in HIF-1α pathway modulation of tumor physiology through hypoxia and cell cycle dysregulation (35). The mechanism of action of linc-ATP13A4-8 remains to be elucidated, but its overlap with strong enhancer marks in adult kidney chromatin immunoprecipation sequencing (37) suggests that it might play a regulatory role in the transcription of hypoxia-modulated target genes in renal cells. Although little is known of the function of linc-KIAA1737-2, its overlap with epigenetic marks in the kidney that are consistent with enhancer regions suggests its modulated expression and function in the renal epithelial inflammatory response. Our PTEC fractionation data suggest that MIR210HG and linc-KIAA1737-2 are located primarily in the nucleus, whereas linc-ATP13A4-8 is found in both the nucleus and cytoplasm. Thus, further nuclear focus and studies to probe these interactions of lncRNAs with human chromatin- and kidney stress-relevant transcription factors may provide insights on their role in the epigenetics of renal epithelial injury.
This study has several strengths. First, we provide novel unbiased data on the transcriptomic landscape of hypoxic and inflammatory stimulation of human renal epithelial cells not available through prior sequencing in mouse-focused injury models or in unstimulated noncancerous human renal epithelial cells. Our approach thus reveals hundreds of human kidney-relevant noncoding elements, including those that are not highly conserved between species but may be functional in the human (26, 47, 61). Second, we demonstrate that our renal epithelial cell lncRNAs prioritized for further validation are present in human proximal tubules and may be relevant to human disease and, thus, are poised for future human translational studies. Third, we performed careful evaluation of conservation and synteny of the prioritized lncRNAs to assess human specificity. Finally, we explored the human adult kidney epigenetic landscape of the prioritized lncRNA loci and determined that they are indeed located in regions of active transcription and regulatory activity in the human kidney.
Our study also has limitations. Our RNA sequencing “discovery” used in vitro cell models of injury that may not capture the full transcriptomic response of human PTECs in vivo. However, our data suggest that RNA sequencing of multiple cells, tissues, and models of relevance to human kidney physiology and disease is worth pursuing as it is likely to reveal a great wealth of information regarding kidney lncRNAs and their potential regulatory roles in renal disease. In this study, we leveraged publicly available annotations of lncRNAs; although this has the advantage of focusing on an existing set of rigorously defined lncRNAs, performing our own de novo assembly may capture additional previously unrecognized kidney-specific lncRNAs. Finally, this study does not define the molecular functions of lncRNAs in PTECs and their possible mechanisms in kidney disease. Rather, our work provides the context and framework from which lncRNAs can be prioritized and future mechanistic studies can be pursued.
In summary, this study characterized the lncRNA landscape of human PTECs subjected to two separate stress conditions central to acute and chronic renal injury: hypoxia and inflammation. The lncRNA response echoes whole transcriptome patterns of distinct transcriptional signatures of each type of stimulation. With poor interspecies conservation, however, lncRNAs warrant specific study within human model systems of injury as their presence and potential regulatory roles in the kidney cell response to injury may be human specific. Future studies of lncRNAs in renal injury may provide a more complete understanding of the integrated genomic and transcriptomic processes that dictate human acute and chronic kidney diseases.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-HL-113147 (to M. P. Reilly and M. Li), R01-GM-108600 (to M. Li), and K24-HL-107643 (to M. P. Reilly). M. G. S. Shashaty is supported by NIH Grant K23-DK-097307. N. Meyer is supported by NIH Grant K23-HL-0225404. J. Lin and A. Grazioli are supported by NIH Grant T32-DK-007006. This work was also supported by NIH Pilot Grant UL1-TR-000003 (to J. Lin).
DISCLAIMERS
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L., X.Z., B.D.G., M.L., and M.P.R. conception and design of research; J.L., X.Z., H.Z., S.G., A.G., C.H., J.C., and W.L. performed experiments; J.L., C.X., M.G.S., and N.J.M. analyzed data; J.L., C.X., and M.P.R. interpreted results of experiments; J.L. prepared figures; J.L. drafted manuscript; J.L. and M.P.R. approved final version of manuscript; X.Z., C.X., H.Z., M.G.S., S.G., A.G., C.H., K.S., M.L., and M.P.R. edited and revised manuscript.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the University of Pennsylvania's Next Generation Sequencing Core for the technical assistance in performing RNA sequencing.
Footnotes
Supplemental material for this article is available at the American Journal of Physiology-Renal Physiology website.
REFERENCES
- 1.Aguado-Fraile E, Ramos E, Conde E, Rodríguez M, Martín-Gómez L, Lietor A, Candela Á, Ponte B, Liaño F, García-Bermejo ML. A pilot study identifying a set of microRNAs as precise diagnostic biomarkers of acute kidney injury. PLoS One 10: e0127175, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja N, Andres-Hernando A, Altmann C, Bhargava R, Bacalja J, Webb RG, He Z, Edelstein CL, Faubel S. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. Am J Physiol Renal Physiol 303: F864–F872, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amano T, Eishi Y, Yamada T, Uchida K, Minegishi K, Tamura T, Kobayashi D, Hiroshi K, Suzuki T, Board PG. Widespread expression of γ-glutamyl cyclotransferase suggests it is not a general tumor marker. J Histochem Cytochem 60: 76–86, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhargava R, Altmann CJ, Andres-Hernando A, Webb RG, Okamura K, Yang Y, Falk S, Schmidt EP, Faubel S. Acute lung injury and acute kidney injury are established by four hours in experimental sepsis and are improved with pre, but not post, sepsis administration of TNF-α antibodies. PLoS ONE 8: e79037, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blondeau J, Deng M, Syring I, Schrödter S, Schmidt D, Perner S, Müller SC, Ellinger J. Identification of novel long non-coding RNAs in clear cell renal cell carcinoma. Clin Epigenetics 7: 10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14: 55S–61, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, Rinn JL. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev 25: 1915–1927, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics 10: 421, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter S, Aiello D, Atianand MK, Ricci EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M, Lawrence JB, O'Neill LAJ, Moore MJ, Caffrey DR, Fitzgerald KA. A long noncoding RNA mediates both activation and repression of immune response genes. Science 341: 789–792, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cazalla D, Yario T, Steitz JA, Steitz J. Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 328: 1563–1566, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chawla LS, Seneff MG, Nelson DR, Williams M, Levy H, Kimmel PL, Macias WL. Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J Am Soc Nephrol 2: 22–30, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Cunnington MS, Santibanez Koref M, Mayosi BM, Burn J, Keavney B. Chromosome 9p21 SNPs associated with multiple disease phenotypes correlate with ANRIL expression. PLoS Genet 6: e1000899, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigo R. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22: 1775–1789, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Röder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigó R, Gingeras TR. Landscape of transcription in human cells. Nature 488: 101–108, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards N, Anderson CMH, Gatfield KM, Jevons MP, Ganapathy V, Thwaites DT. Amino acid derivatives are substrates or non-transported inhibitors of the amino acid transporter PAT2 (slc36a2). Biochim Biophys Acta 1808: 260–270, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 13: 852–869, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson JF, Patel PN, Shah RY, Mulvey CK, Gadi R, Nijjar PS, Usman HM, Mehta NN, Shah R, Master SR, Propert KJ, Reilly MP. Race and gender variation in response to evoked inflammation. J Transl Med 11: 63, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, Heger A, Hetherington K, Holm L, Mistry J, Sonnhammer EL, Tate J, Punta M. Pfam: the protein families database. Nucleic Acids Res 42: D222–D230, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentle ME, Shi S, Daehn I, Zhang T, Qi H, Yu L, D'Agati VD, Schlondorff DO, Bottinger EP. Epithelial cell TGF signaling induces acute tubular injury and interstitial inflammation. J Am Soc Nephrol 24: 787–799, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatinstate to promote cancer metastasis. Nature 464: 1071–1076, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han P, Li W, Lin CH, Yang J, Shang C, Nuernberg ST, Jin KK, Xu W, Lin CY, Lin CJ, Xiong Y, Chien HC, Bin Zhou Ashley E, Bernstein D, Chen PS, Chen HSV, Quertermous T, Chang CP. A long noncoding RNA protects the heart from pathological hypertrophy. Nature 514: 102–106, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature 495: 384–388, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Hashikata A, Yamashita A, Suzuki S, Nagayasu S, Shinjo T, Taniguchi A, Fukushima M, Nakai Y, Nin K, Watanabe N, Asano T, Abiko Y, Kushiyama A, Nagasaka S, Nishimura F. The inflammation-lipocalin 2 axis may contribute to the development of chronic kidney disease. Nephrol Dial Transplant 29: 611–618, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Heyman SN, Evans RG, Rosen S, Rosenberger C. Cellular adaptive changes in AKI: mitigating renal hypoxic injury. Nephrol Dial Transplant 27: 1721–1728, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Hezroni H, Koppstein D, Schwartz MG, Avrutin A, Bartel DP, Ulitsky I. Principles of long noncoding RNA evolution derived from direct comparison of transcriptomes in 17 species. Cell Rep 11: 1110–1122, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998. [DOI] [PubMed] [Google Scholar]
- 28.IIott NE, Heward JA, Roux B, Tsitsiou E, Fenwick PS, Lenzi L, Goodhead I, Hertz-Fowler C, Heger A, Hall N, Donnelly LE, Sims D, Lindsay MA. Long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat Commum 5: 3979, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, Poliakov A, Cao X, Dhanasekaran SM, Wu YM, Robinson DR, Beer DG, Feng FY, Iyer HK, Chinnaiyan AM. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet 47: 199–208, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang X, Konkalmatt P, Yang Y, Gildea J, Jones JE, Cuevas S, Felder RA, Jose PA, Armando I. Single-nucleotide polymorphisms of the dopamine D2 receptor increase inflammation and fibrosis in human renal proximal tubule cells. Hypertension 63: e74–e80, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Justo P, Sanz AB, Sanchez-Niño MD, Winkles JA, Lorz C, Egido J, Ortiz A. Cytokine cooperation in renal tubular cell injury: the role of TWEAK. Kidney Int 70: 1750–1758, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Kaminska D, Tyran B, Mazanowska O, Rabczynski J, Szyber P, Patrzalek D, Chudoba P, Polak WG, Klinger M. Cytokine gene expression in kidney allograft biopsies after donor brain death and ischemia-reperfusion injury using in situ reverse-transcription polymerase chain reaction analysis. Transplantation 84: 1118–1124, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol 109: e102–e107, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinsey GR, Sharma R, Okusa MD. Regulatory T cells in AKI. J Am Soc Nephrol 24: 1720–1726, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klatte T, Seligson DB, Riggs SB, Leppert JT, Berkman MK, Kleid MD, Yu H, Kabbinavar FF, Pantuck AJ, Belldegrun AS. Hypoxia-inducible factor 1 in clear cell renal cell carcinoma. Clin Cancer Res 13: 7388–7393, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Kretz M, Siprashvili Z, Chu C, Webster DE, Zehnder A, Qu K, Lee CS, Flockhart RJ, Groff AF, Chow J, Johnston D, Kim GE, Spitale RC, Flynn RA, Zheng GXY, Aiyer S, Raj A, Rinn JL, Chang HY, Khavari PA. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature 493: 231–235, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, Ziller MJ, Amin V, Whitaker JW, Schultz MD, Ward LD, Sarkar A, Quon G, Sandstrom RS, Eaton ML, Wu YC, Pfenning A, Wang X, ClaussnitzerYaping Liu M, Coarfa C, Alan Harris R, Shoresh N, Epstein CB, Gjoneska E, Leung D, Xie W, David Hawkins R, Lister R, Hong C, Gascard P, Mungall AJ, Moore R, Chuah E, Tam A, Canfield TK, Scott Hansen R, Kaul R, Sabo PJ, Bansal MS, Carles A, Dixon JR, Farh KH, Feizi S, Karlic R, Kim AR, Kulkarni A, Li D, Lowdon R, Elliott G, Mercer TR, Neph SJ, Onuchic V, Polak P, Rajagopal N, Ray P, Sallari RC, Siebenthall KT, Sinnott-Armstrong NA, Stevens M, Thurman RE, Wu J, Zhang B, Zhou X, Abdennur N, Adli M, Akerman M, Barrera L, Antosiewicz-Bourget J, Ballinger T, Barnes MJ, Bates D, Bell RJA, Bennett DA, Bianco K, Bock C, Boyle P, Brinchmann J, Caballero-Campo P, Camahort R, Carrasco-Alfonso MJ, Charnecki T, Chen H, Chen Z, Cheng JB, Cho S, Chu A, Chung WY, Cowan C, Athena Deng Q, Deshpande V, Diegel M, Ding B, Durham T, Echipare L, Edsall L, Flowers D, Genbacev-Krtolica O, Gifford C, Gillespie S, Giste E, Glass IA, Gnirke A, Gormley M, Gu H, Gu J, Hafler DA, Hangauer MJ, Hariharan M, Hatan M, Haugen E, He Y, Heimfeld S, Herlofsen S, Hou Z, Humbert R, Issner R, Jackson AR, Jia H, Jiang P, Johnson AK, Kadlecek T, Kamoh B, Kapidzic M, Kent J, Kim A, Kleinewietfeld M, Klugman S, Krishnan J, Kuan S, Kutyavin T, Lee AY, Lee K, Li J, Li N, Li Y, Ligon KL, Lin S, Lin Y, Liu J, Liu Y, Luckey CJ, Ma YP, Maire C, Marson A, Mattick JS, Mayo M, McMaster M, Metsky H, Mikkelsen T, Miller D, Miri M, Mukame E, Nagarajan RP, Neri F, Nery J, Nguyen T, O'Geen H, Paithankar S, Papayannopoulou T, Pelizzola M, Plettner P, Propson NE, Raghuraman S, Raney BJ, Raubitschek A, Reynolds AP, Richards H, Riehle K, Rinaudo P, Robinson JF, Rockweiler NB, Rosen E, Rynes E, Schein J, Sears R, Sejnowski T, Shafer A, Shen L, Shoemaker R, Sigaroudinia M, Slukvin I, Stehling-Sun S, Stewart R, Subramanian SL, Suknuntha K, Swanson S, Tian S, Tilden H, Tsai L, Urich M, Vaughn I, Vierstra J, Vong S, Wagner U, Wang H, Wang T, Wang Y, Weiss A, Whitton H, Wildberg A, Witt H, Won KJ, Xie M, Xing X, Xu I, Xuan Z, Ye Z, Yen CA, Yu P, Zhang X, Zhang X, Zhao J, Zhou Y, Zhu J, Zhu Y, Ziegler S, Beaudet AE, Boyer LA, De Jager PL, Farnham PJ, Fisher SJ, Haussler D, Jones SJM, Li W, Marra MA, McManus MT, Sunyaev S, Thomson JA, Tlsty TD, Tsai LH, Wang W, Waterland RA, Zhang MQ, Chadwick LH, Bernstein BE, Costello JF, Ecker JR, Hirst M, Meissner A, Milosavljevic A, Ren B, Stamatoyannopoulos JA, Wang T, Kellis M, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, Ziller MJ, Amin V, Whitaker JW, Schultz MD, Ward LD, Sarkar A, Quon G, Sandstrom RS, Eaton ML, Wu YC, Pfenning AR, Wang X, Claussnitzer M, Liu Y, Coarfa C, Harris RA, Shoresh N, Epstein CB, Gjoneska E, Leung D, Xie W, Hawkins RD, Lister R, Hong C, Gascard P, Mungall AJ, Moore R, Chuah E, Tam A, Canfield TK, Hansen RS, Kaul R, Sabo PJ, Bansal MS, Carles A, Dixon JR, Farh KH, Feizi S, Karlic R, Kim AR, Kulkarni A, Li D, Lowdon R, Elliott G, Mercer TR, Neph SJ, Onuchic V, Polak P, Rajagopal N, Ray P, Sallari RC, Siebenthall KT, Sinnott-Armstrong NA, Stevens M, Thurman RE, Wu J, Zhang B, Zhou X, Beaudet AE, Boyer LA, De Jager PL, Farnham PJ, Fisher SJ, Haussler D, Jones SJM, Li W, Marra MA, McManus MT, Sunyaev S, Thomson JA, Tlsty TD, Tsai LH, Wang W, Waterland RA, Zhang MQ, Chadwick LH, Bernstein BE, Costello JF, Ecker JR, Hirst M, Meissner A, Milosavljevic A, Ren B, Stamatoyannopoulos JA, Wang T, Kellis M. Integrative analysis of 111 reference human epigenomes. Nature 518: 317–330, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam MTY, Li W, Rosenfeld MG, Glass CK. Enhancer RNAs and regulated transcriptional programs. Trends Biochem Sci 39: 170–182, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ledo N, Ko YA, Park ASD, Kang HM, Han SY, Choi P, Susztak K. Functional genomic annotation of genetic risk loci highlights inflammation and epithelial biology networks in CKD. J Am Soc Nephrol 26: 692–714, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu KD, Altmann C, Smits G, Krawczeski CD, Edelstein CL, Devarajan P, Faubel S. Serum Interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care 13: R104, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Ferguson JF, Xue C, Ballantyne RL, Silverman IM, Gosai SJ, Serfecz J, Morley MP, Gregory BD, Li M, Reilly MP. Tissue-specific RNA-seq in human evoked inflammation identifies blood and adipose lincRNA signatures of cardiometabolic diseases. Arterioscler Thromb Vasc Biol 34: 902–912, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorenzen JM, Kielstein JT, Hafer C, Gupta SK, Kumpers P, Faulhaber-Walter R, Haller H, Fliser D, Thum T. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 6: 1540–1546, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Lorenzen JM, Schauerte C, Kielstein JT, Hubner A, Martino F, Fiedler J, Gupta SK, Faulhaber-Walter R, Kumarswamy R, Hafer C, Haller H, Fliser D, Thum T. Circulating long noncoding RNA TapSAKI is a predictor of mortality in critically ill patients with acute kidney injury. Clin Chem 61: 191–201, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Mariano F, Cantaluppi V, Stella M, Romanazzi G, Assenzio B, Cairo M, Biancone L, Triolo G, Ranieri VM, Camussi G. Circulating plasma factors induce tubular and glomerular alterations in septic burns patients. Crit Care 12: R42, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikkelsen ME, Shah CV, Meyer NJ, Gaieski DF, Lyon S, Miltiades AN, Goyal M, Fuchs BD, Bellamy SL, Christie JD. The epidemiology of acute respiratory distress syndrome in patients presenting to the emergency department with severe sepsis. Shock 40: 375–381, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Necsulea A, Soumillon M, Warnefors M, Liechti A, Daish T, Zeller U, Baker JC, Grützner F, Kaessmann H. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature 505: 635–640, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Oda K, Makino S, Masuda C, Yoshiki T, Kitamura Y, Takata K, Yanagisawa D, Taniguchi T, Tooyama I. The mRNA distribution of C7orf24, a γ-glutamyl cyclotransferase, in rat tissues. J Histochem Cytochem 57: 1121–1126, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 46: 1153–1160, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Pruitt KD, Brown GR, Hiatt SM, Thibaud-Nissen F, Astashyn A, Ermolaeva O, Farrell CM, Hart J, Landrum MJ, McGarvey KM, Murphy MR, O'Leary NA, Pujar S, Rajput B, Rangwala SH, Riddick LD, Shkeda A, Sun H, Tamez P, Tully RE, Wallin C, Webb D, Weber J, Wu W, DiCuccio M, Kitts P, Maglott DR, Murphy TD, Ostell JM. RefSeq: an update on mammalian reference sequences. Nucleic Acids Res 42: D756–D763, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Racusen LC, Monteil C, Sgrignoli A, Lucskay M, Marouillat S, Rhim JG, Morin JP. Cell lines with extended in vitro growth potential from human renal proximal tubule: characterization, response to inducers, and comparison with established cell lines. J Lab Clin Med 129: 318–329, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Reilly JP, Meyer NJ, Shashaty MG, Feng R, Lanken PN, Gallop R, Kaplan S, Herlim M, Oz NL, Hiciano I, Campbell A, Holena DN, Reilly MP, Christie JD. ABO blood type A is associated with increased risk of acute respiratory distress syndrome in caucasians following both major trauma and severe sepsis. Chest 145: 753–761, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem 81: 145–166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG; Inflammation and Host Response to Injury Large-Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun K, Chen X, Jiang P, Song X, Wang H, Sun H. iSeeRNA: identification of long intergenicnon-coding RNA transcripts fromtranscriptome sequencing data. BMC Genomics 14: S7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Susantitaphong P, Perianayagam MC, Tighiouart H, Liangos O, Bonventre JV, Jaber BL. Tumor necrosis factor α promoter polymorphism and severity of acute kidney injury. Nephron Clin Pract 123: 67–73, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol 31: 46–53, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tripathi V, Shen Z, Chakraborty A, Giri S, Freier SM, Wu X, Zhang Y, Gorospe M, Prasanth SG, Lal A, Prasanth KV. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet 9: e1003368, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell 154: 26–46, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulitsky I, Shkumatava A, Jan CH, Sive H, Bartel DP. Conserved function of lincRNAsin vertebrate embryonic development despite rapid sequence evolution. Cell 147: 1537–1550, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Flach H, Onizawa M, Wei L, McManus MT, Weiss A. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol 15: 393–401, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang XO, Yin QF, Wang HB, Zhang Y, Chen T, Zheng P, Lu X, Chen LL, Yang L. Species-specific alternative splicing leads to unique expression of sno-lncRNAs. BMC Genomics 15: 1–15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Q, Huang XR, Yu J, Yu X, Lan HY. Long noncoding RNA Arid2-IR is a novel therapeutic target for renal inflammation. Mol Ther 23: 1034–1043, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.