Abstract
Purpose
Metastatic melanoma, a highly vascularized tumor with strong expression of vascular endothelial growth factor, has an overall poor prognosis. We conducted a placebo-controlled, double-blind phase II study of carboplatin plus paclitaxel with or without bevacizumab in patients with previously untreated metastatic melanoma.
Patients and Methods
Patients were randomly assigned in a two-to-one ratio to carboplatin (area under the curve, 5) plus paclitaxel (175 mg/m2) and bevacizumab (15 mg/kg; CPB) or placebo (CP) administered intravenously once every 3 weeks. Progression-free survival (PFS) was the primary end point. Secondary end points included overall survival (OS) and safety.
Results
Two hundred fourteen patients (73% with M1c disease) were randomly assigned. With a median follow-up of 13 months, median PFS was 4.2 months for the CP arm (n = 71) and 5.6 months for the CPB arm (n = 143; hazard ratio [HR], 0.78; P = .1414). Overall response rates were 16.4% and 25.5%, respectively (P = .1577). With 13-month follow-up, median OS was 8.6 months in the CP arm versus 12.3 months in the CPB arm (HR, 0.67; P = .0366), whereas in an evaluation 4 months later, it was 9.2 versus 12.3 months, respectively (HR, 0.79; P = .1916). In patients with elevated serum lactate dehydrogenase (n = 84), median PFS and OS were longer in the CPB arm (PFS: 4.4 v 2.7 months; HR, 0.62; OS: 8.5 v 7.5 months; HR, 0.52). No new safety signals were observed.
Conclusion
The study did not meet the primary objective of statistically significant improvement in PFS with the addition of bevacizumab to carboplatin plus paclitaxel. A larger phase III study will be necessary to determine whether there is benefit to the addition of bevacizumab to carboplatin plus paclitaxel in this disease setting.
INTRODUCTION
Metastatic melanoma is a devastating disease, with more than 8,600 deaths annually in the United States alone.1 Currently, dacarbazine, high-dose interleukin-2, and ipilimumab are approved for stage IV disease. In phase III studies with dacarbazine, median progression-free survival (PFS) ranged from 1.5 to 1.6 months, and overall survival (OS) ranged from 5.6 to 7.8 months.2–4 In two recent phase III studies in patients with previously treated advanced melanoma with carboplatin plus paclitaxel5 and ipilimumab,6 median OS was reported to be 9.8 and 10.0 months, respectively. Despite these modest advances in OS, the prognosis for these patients remains grave, and more effective treatment is urgently needed.
Malignant melanoma is a highly vascular tumor in which vascular endothelial growth factor (VEGF) is strongly expressed and seems to play an important role in disease progression.2–5,7–12 Moreover, increased serum or tumor VEGF levels correlate with worse outcome.7–11,13–16 These preclinical findings support the hypothesis that VEGF stimulates melanoma growth and progression in an autocrine and/or paracrine fashion and that blocking VEGF signaling may control growth of melanoma lesions. Bevacizumab is a monoclonal antibody that selectively binds to VEGF and blocks receptor binding. Several large randomized phase III trials in various indications have demonstrated that when combined with chemotherapy or targeted therapies, bevacizumab prolongs PFS and OS.17–19
We conducted a randomized phase II study in patients with previously untreated metastatic melanoma to characterize the efficacy and safety of bevacizumab when combined with carboplatin plus paclitaxel. Carboplatin plus paclitaxel was chosen as the cytotoxic regimen because of its well-characterized safety profile, preclinical data suggesting strong efficacy in combination with VEGF inhibition, convenience of dosing, and promising clinical activity in patients with metastatic melanoma.5,20–22
PATIENTS AND METHODS
Patient Selection
Eligible patients were required to have histologically confirmed stage IV malignant melanoma for which they had not received any systemic therapy (including cytokine treatment). Patients with metastatic melanoma of cutaneous, mucosal, or unknown primary origin—but not of uveal origin—were eligible. Patients had to be age 18 years or older and have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 with adequate organ function. Patients who had received prior radiation therapy must have had at least one evaluable metastatic lesion that had not been treated with or progressed after irradiation. A history of Bacillus Calmette-Guérin, granulocyte-macrophage colony-stimulating factor, or vaccine therapy after complete surgical resection or complete irradiation/radiotherapy ablation of stage IV disease before disease progression was also acceptable. Key exclusion criteria included prior therapy with any VEGF pathway–targeted therapy; known metastatic disease in the CNS; inadequately controlled hypertension; history of stroke or transient ischemic attack within 6 months prior or history of bleeding diathesis or significant coagulopathy; receiving warfarin; proteinuria with urine protein-to-creatinine ratio of 1.0 or greater; or any significant comorbid condition that was contraindicated.
Study Design
BEAM (Bevacizumab Advanced Melanoma) was a phase II, multicenter, randomized, double-blind, placebo-controlled trial. The protocol was approved by the institutional review board at each participating institution. After providing informed consent, patients were randomly assigned in a ratio of two to one to either the carboplatin plus paclitaxel with bevacizumab (CPB) or carboplatin plus paclitaxel and placebo (CP) arm. Random assignment was performed using an interactive voice response system and stratified by ECOG performance status (0 or 1) and disease stage (stage IV M1a/b v IV M1c). Patients received bevacizumab (CPB arm, 15 mg/kg) or placebo (CP arm) with carboplatin (area under the curve, 5) plus paclitaxel (175 mg/m2), all by intravenous infusion. Patients received either bevacizumab or placebo until disease progression (PD), unacceptable toxicity, or patient withdrawal for a maximum of 102 weeks. Because of increased risk of hypersensitivity reaction with prolonged treatment,23 carboplatin administration was capped at 10 cycles. Paclitaxel alone could be continued with bevacizumab or placebo after carboplatin was discontinued. No bevacizumab dose reductions were allowed. Dose reductions for carboplatin and/or paclitaxel were allowed for grade 4 neutropenia lasting longer than 7 days, febrile neutropenia, platelet count of 50,000 μL or less, or grade 3 or 4 toxicity attributed to chemotherapy (except for grade 4 hypersensitivity reactions); additional dose modifications were allowed per institutional standards and guidelines. If study drug was held because of adverse events (AEs), dose remained unchanged once treatment resumed. If it was necessary to withhold the study drug for more than 60 days, the patient was removed from the study. During treatment, full supportive care, including hematopoietic growth factors, transfusions of blood and blood products, aspirin, antibiotics, and antiemetics, was permitted.
Study End Points
The primary efficacy end point was PFS based on investigator tumor assessments. Secondary efficacy end points included OS, objective response rate (ORR), duration of response, OS at 6 months, PFS at 24 weeks, and safety.
Study Evaluations
Tumor measurements were performed before treatment and every 6 weeks for the first 54 weeks and then every 12 weeks thereafter until PD. Response evaluations were determined using RECIST (Response Evaluation Criteria in Solid Tumors) guidelines.24 Safety assessments, performed at the beginning of each treatment cycle, included urine protein-to-creatinine ratio and measurement of protocol-specified vital signs (including blood pressure). Measurement of protocol-specified hematology, clinical chemistry, and urinalysis were conducted at screening, day 1 of cycle one, and termination. AE severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Statistical Analysis
For efficacy, the population analyzed included all randomly assigned patients. Median PFS, defined as time from random assignment to documented PD or death, was the primary end point of this study. PFS data for patients receiving nonprotocol cancer therapy before documented disease progression were censored at the time of last tumor assessment before initiation of the nonprotocol cancer therapy. OS was defined as time from random assignment to death from any cause. ORR was defined as the percentage of patients with measurable disease who achieved a complete or partial response confirmed 28 days or more after initial documentation of response. Duration of response was from first occurrence of response to date of first PD or death. Survival at 6 months was the proportion of patients surviving 6 months after random assignment. The 24-week landmark PFS was the proportion of patients without PD or death 24 weeks after random assignment. Exploratory analyses were performed to determine 1-year OS and effects of demographic and baseline prognostic characteristics on PFS and OS.
Data analyses were prespecified to occur 8 months after the last patient was enrolled; the number of expected PFS events was estimated to be 166. Primary analyses occurred with a data cutoff date of April 3, 2009, and included 169 PFS events. For this analysis, a stratified Cox proportional hazards model was used to obtain a point estimate of the hazard ratio (HR) for CPB regimen relative to CP regimen, along with a 95% CI for the HR for each time-to-event analysis. ECOG performance status and disease stage were included as stratification factors. Assuming median PFS of 4 months for the CP regimen and 6 months for the CPB regimen (yielding HR of 0.67 for 166 PFS events observed), one would expect 106 events in the CPB arm and 60 in the CP arm. One hundred sixty-six PFS events would provide approximately 68% power for a two-sided test conducted at α = 0.05 to conclude the superiority of the experimental arm; meeting this criteria could lead to further consideration of a phase III study evaluating this therapeutic combination. Time-to-event data were compared between treatment arms using a stratified log-rank test. The Kaplan-Meier method was used to estimate median PFS, median OS, one-year survival, and 24-week PFS. The 95% CIs for medians were computed using the Brookmeyer and Crowley method. The HRs for time-to-event data were estimated using a stratified Cox regression model. ORRs in patients with measurable disease at baseline were compared using the stratified Mantel-Haenszel χ2 test. Only patients with measurable disease at baseline who achieved a response (either partial or complete) per RECIST were included in the analysis of duration of response. For patients who achieved a response and had no documentation of PD, duration of response was censored at the time of the last tumor assessment.
The safety analysis population comprised all patients who received any amount of study treatment. The AE and serious AE reporting period began after study treatment was initiated and ended 30 days after the last administration of study treatment or study discontinuation/termination, whichever was earlier. After that period, only serious AEs attributed to study treatment were reported. Safety analyses were performed using descriptive statistics.
RESULTS
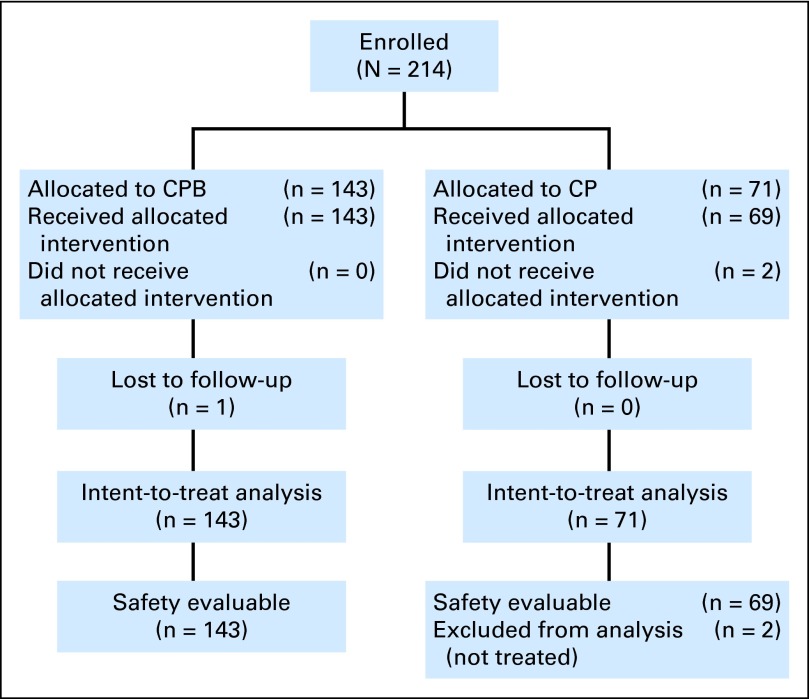
Between February 2007 and August 2008, a total of 214 patients with histologically or cytologically confirmed stage IV melanoma were enrolled and randomly assigned to the CBP (n = 143) or CP arm (n = 71; Fig 1). Eighteen patients (8.4%)—six (8.5%) in the CP arm and 12 (8.4%) in the CPB arm—did not meet at least one of the inclusion or exclusion criteria. The most common protocol deviation was use of adjuvant systemic therapy before stage IV disease within 4 months from random assignment (12 patients total; 5.6% in each arm).
Fig 1.
CONSORT diagram. CP, carboplatin plus paclitaxel and placebo; CPB, carboplatin plus paclitaxel and bevacizumab.
Patient Characteristics
Patient baseline characteristics were comparable between the two treatment groups (Table 1). Almost three quarters of patients (72.9%; 156 of 214) had M1c disease; 84 (39.3%) had elevated lactate dehydrogenase (LDH) levels.
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Characteristic | CP Arm (n = 71) |
CPB Arm (n = 143) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 60 | 60 | ||
| Range | 28-83 | 27-85 | ||
| ≤ 65 | 46 | 64.8 | 104 | 72.7 |
| > 65 | 25 | 35.2 | 39 | 27.3 |
| Sex | ||||
| Male | 50 | 70.4 | 98 | 68.5 |
| Female | 21 | 29.6 | 45 | 31.5 |
| ECOG performance status | ||||
| 0 | 48 | 67.6 | 100 | 69.9 |
| 1 | 23 | 32.4 | 43 | 30.1 |
| M classification | ||||
| M1a | 8 | 11.3 | 11 | 7.7 |
| M1b | 11 | 15.5 | 28 | 19.6 |
| M1c | 52 | 73.2 | 104 | 72.7 |
| Serum LDH at baseline | ||||
| Normal | 39 | 54.9 | 89 | 62.2 |
| Above normal | 31 | 43.7 | 53 | 37.1 |
| Unknown | 1 | 1.4 | 1 | 0.7 |
| Received prior adjuvant biologics therapy* | 18 | 25.4 | 44 | 30.8 |
| Measurable disease at baseline (RECIST) | 67 | 94.4 | 141 | 98.6 |
Abbreviations: CP, carboplatin plus paclitaxel and placebo; CPB, carboplatin plus paclitaxel and bevacizumab; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; RECIST, Response Evaluation Criteria in Solid Tumors.
Including vaccines and interferon.
Treatment
Among the 71 patients in the CP arm, 69 received treatment. Regarding the two patients who did not, one had rapid PD at time of random assignment, and the other withdrew from the study. Median number of treatment cycles was six for the CPB arm and five for the CP arm (Table 2). Across both arms, 70.1% of patients discontinued study drug treatment (bevacizumab or placebo) because of PD, and 8.9% of patients discontinued because of intolerable AEs in both arms (Table 2). Three patients (4.2%) in the CP arm and 18 patients (12.6%) in the CPB arm received nonprotocol treatments before PD or death.
Table 2.
Exposure to Study Treatment and Patient Disposition
| Exposure | CP Arm (n = 71) |
CPB Arm (n = 143) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Received any study treatment* | 69 | 97.2 | 143 | 100 |
| Cycles of bevacizumab or placebo | ||||
| Median | 5 | 6 | ||
| Range | 1-23 | 1-30 | ||
| Cycles of carboplatin | ||||
| Median | 5 | 6 | ||
| Range | 1-10 | 1-10 | ||
| Cycles of paclitaxel | ||||
| Median | 5 | 6 | ||
| Range | 1-17 | 1-30 | ||
| Still receiving study drug treatment† | 7 | 9.9 | 12 | 8.4 |
| Discontinued study drug treatment | 62 | 87.3 | 131 | 91.6 |
| Disease progression | 50 | 70.4 | 100 | 69.9 |
| Adverse events | 6 | 8.5 | 13 | 9.1 |
| Death‡ | 0 | 0 | 2 | 1.4 |
| Other§ | 6 | 8.5 | 16 | 11.2 |
Abbreviations: CP, carboplatin plus paclitaxel and placebo; CPB, carboplatin plus paclitaxel and bevacizumab.
Two patients randomly assigned to placebo arm never received study drug.
As of May 21, 2009.
One patient died as result of unknown cause; relation to treatment cannot be ruled out. Other patient died as result of rapid disease progression, assessed by treating physician as possibly treatment related.
Lost to follow-up; investigator or patient decision to discontinue.
Efficacy
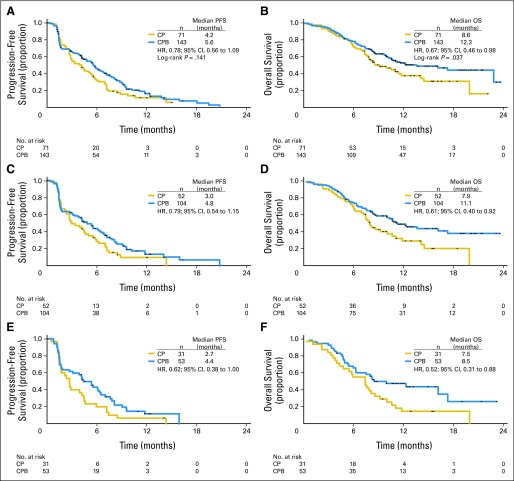
Median follow-up at the planned analysis in April 2009 was 13.3 months for the CP arm and 13.0 months for the CPB arm. At that time, 169 patients—59 (83%) in the CP arm and 110 (77%) in the CPB arm—had experienced PD or died. Among the intent-to-treat patients, combination with bevacizumab led to an approximately 20% reduction in risk of PD (HR, 0.78; 95% CI, 0.56 to 1.09; log-rank P = .1414; Fig 2A). In patients with elevated serum LDH (n = 84), median PFS and OS were longer in the CPB arm (PFS: 4.4 v 2.7 months; HR, 0.62; OS: 8.5 v 7.5 months; HR, 0.52). Median PFS duration was 4.2 months for the CP arm and 5.6 months for the CPB arm (Table 3). The 24-week PFS rate favored the CPB over CP arm (50.4% v 37.4%; P = .074).
Fig 2.
Kaplan-Meier estimated survival for all patients by treatment group: (A) progression-free survival (PFS) and (B) overall survival (OS) as of April 2009. Kaplan-Meier estimated survival for patients with poor prognosis by treatment group: (C) PFS and (D) OS for patients with M1c stage cancer; (E) PFS and (F) OS for patients with M1c stage cancer and elevated serum lactate dehydrogenase levels. CP, carboplatin plus paclitaxel and placebo; CPB, carboplatin plus paclitaxel and bevacizumab; HR, hazard ratio.
Table 3.
Efficacy End Points
| End Point | CP Arm (n = 71) |
CPB Arm (n = 143) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Median PFS, months | 4.2 | 5.6 | ||
| Hazard ratio | 0.78 | |||
| 95% CI | 0.56 to 1.09 | |||
| Log-rank P | .1414 | |||
| 24-week PFS rate, % | 37.4 | 50.4 | ||
| Median OS, months (protocol specified) | 8.6 | 12.3 | ||
| Hazard ratio | 0.67 | |||
| 95% CI | 0.46 to 0.98 | |||
| Log-rank P | .0366 | |||
| OS by landmark timepoints, % event free | ||||
| 6 months | 74.6 | 78.2 | ||
| 12 months | 36.6 | 51.9 | ||
| Patients with measurable disease at baseline | 67 | 94.4 | 141 | 98.6 |
| Complete response | 1 | 1.4 | 3 | 2.1 |
| Partial response | 10 | 14.1 | 33 | 23.1 |
| Overall response rate, % | 16.4 | 25.5 | ||
Abbreviations: CP, carboplatin plus paclitaxel and placebo; CPB, carboplatin plus paclitaxel and bevacizumab; OS, overall survival; PFS, progression-free survival.
At the time of the prespecified analysis of OS, a total of 114 patients (53%) had died: 44 (62%) in the CP arm and 70 (49%) in the CPB arm. Combination of carboplatin plus paclitaxel with bevacizumab led to a 33% reduction in risk of death (HR, 0.67; 95% CI, 0.46 to 0.98; log-rank P = .0366). Median OS duration was 8.6 months in the CP arm and 12.3 months in the CPB arm. ORR was higher in the CPB arm (25.5%; 95% CI, 18.3 to 32.7) compared with the CP arm (16.4%; 95% CI, 7.5 to 25.3; stratified P = .1577). Complete response was observed for one patient (1.5%) in the CP arm and three patients (2.1%) in the CPB arm. Median duration of response was shorter in the CPB arm compared with the CP arm (6.9 months; 95% CI, 4.86 to 8.90 v 7.7 months; 95% CI, 3.94 to 11.56). The 6-month OS rates were 74.6% for the CP arm and 78.2% for the CPB arm, a difference that was not statistically significant (Table 3).
On the basis of the encouraging OS results, a later exploratory analysis of OS was performed (data cutoff: August 14, 2009). At that time, median follow-up was 16.1 months for patients in the CP arm and 18.9 months for patients in the CPB arm; a total of 137 patients (64%) had died: 48 patients (68%) in the CP arm and 89 patients (62%) in the CPB arm. Median OS duration was 9.2 months in the CP arm and 12.3 months in the CPB arm (HR, 0.79; 95% CI, 0.55 to 1.13). The 12-month OS rate, another exploratory end point, was markedly lower in the CP arm compared with the CPB arm (36.6% v 51.9%; Table 3).
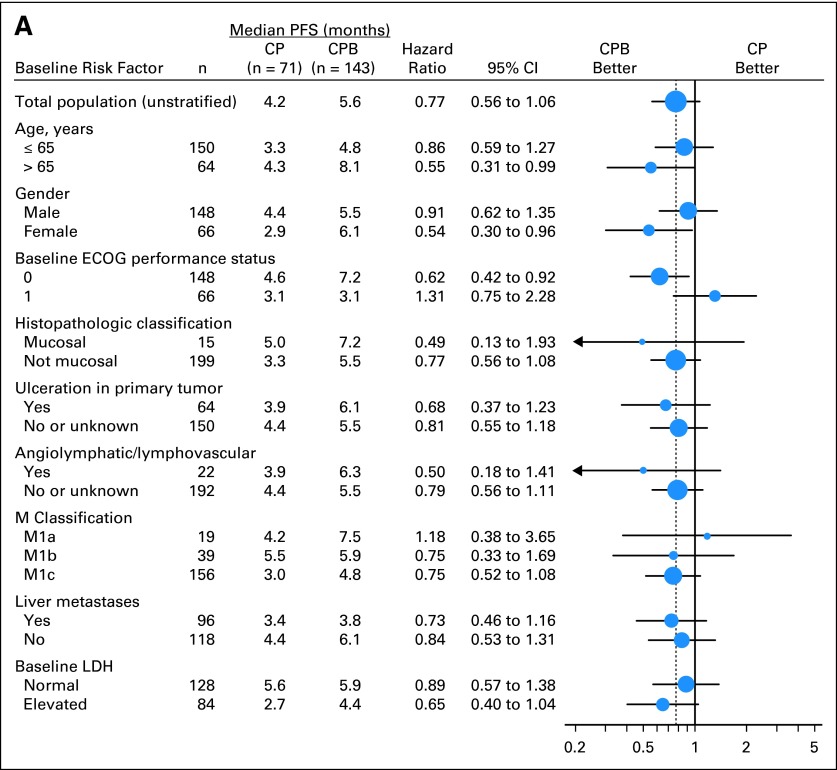
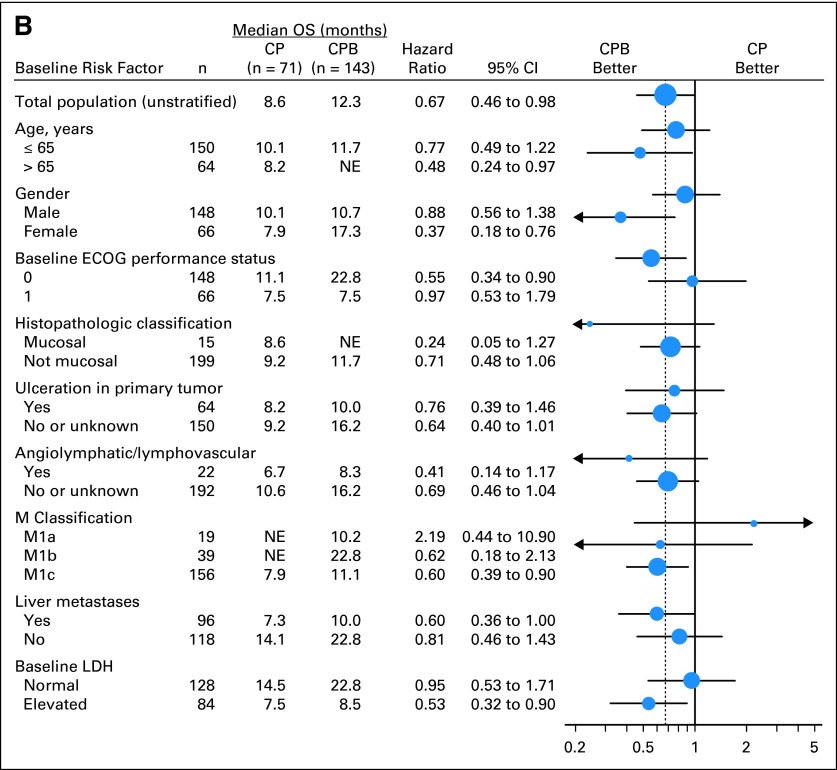
Unstratified exploratory subgroup analyses of both PFS and OS were performed (Fig 3). Among patients with M1c staging (n = 156), combination with bevacizumab led to a 25% reduction in risk of progression (HR, 0.75; 95% CI, 0.52 to 1.08) and 40% reduction in risk of death (HR, 0.60; 95% CI, 0.39 to 0.90; Fig 3). Among patients with both M1c staging and elevated baseline serum LDH level (n = 84), combination with bevacizumab led to a 35% reduction in risk of progression (HR, 0.65; 95% CI, 0.40 to 1.04). Median PFS was longer in patients in the CPB arm (4.4 v 2.7 months). Risk of death was decreased by 47% (HR, 0.53; 95% CI, 0.32 to 0.90; Fig 3), with longer median OS (8.5 months in CPB arm v 7.5 months in CP arm).
Fig 3.
Analysis of median survival by subgroups for all randomly assigned patients. (A) Progression-free survival (PFS); (B) overall survival (OS). CP, carboplatin plus paclitaxel and placebo; CPB, carboplatin plus paclitaxel and bevacizumab; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; NE, not estimable.
Toxicity
Two hundred twelve patients received at least one dose of study drug. Incidence of grade 3 to 5 AEs was higher in the CPB arm (57.4%) than in the CP arm (44.9%; Table 4). No new safety signals were detected on addition of bevacizumab to CP in this study. In the CPB arm, grade 3 or greater neutropenia, peripheral neuropathy, febrile neutropenia, arterial thromboembolic events, and hypertension were the most common AEs. No instances of hypertension greater than grade 3 were reported. There was no difference in the rate of serious AEs between arms (CP arm, 29.0%; CPB arm; 28.0%). There were two deaths on study, both in the CPB arm (1.4%). One patient died as a result of an unknown cause, and the other died as a result of rapid PD, assessed by the investigator as possibly related to treatment.
Table 4.
AEs
| Event | CP Arm (n = 69) |
CPB Arm (n = 143) |
||||||
|---|---|---|---|---|---|---|---|---|
| All |
Grade ≥ 3 |
All |
Grade ≥ 3 |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Any | 69 | 100 | 31 | 44.9 | 141 | 98.6 | 82 | 57.4 |
| Leading to death | 0 | 0 | 0 | 0 | 2 | 1.4 | 2 | 1.4 |
| Leading to discontinuation of bevacizumab | 6 | 8.7 | NA | NA | 16 | 11.2 | NA | NA |
| Serious | 20 | 29.0 | NA | NA | 40 | 28.0 | NA | NA |
| Selected AE (grade ≥ 3)* | CP Arm (n = 69) |
CPB Arm (n = 143) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Neutropenia | 13 | 18.8 | 34 | 23.8 |
| Febrile | 1† | 1.4 | 7 | 4.9 |
| Peripheral neuropathy | 0 | 0 | 13 | 9.1 |
| Arterial thromboembolic event | 1 | 1.4 | 3 | 2.1 |
| Hypertension | 0 | 0 | 5 | 3.5 |
| Hemorrhage | ||||
| Pulmonary | 1 | 1.4 | 2 | 1.4 |
| Not CNS or pulmonary | 4 | 5.8 | 0 | 0 |
| CNS | 0 | 0 | 0 | 0 |
| Proteinuria | 0 | 0 | 0 | 0 |
| GI perforation | 0 | 0 | 0 | 0 |
| RPLS | 0 | 0 | 0 | 0 |
NOTE. AEs occurring during planned treatment period graded according to National Cancer Institute Common Terminology Criteria for Adverse Events. For patients experiencing multiple occurrences of specific AE, AE counted only once at highest grade of occurrence.
Abbreviations: AE, adverse event; CP, carboplatin plus paclitaxel and placebo; CPB, carboplatin plus paclitaxel and bevacizumab; NA, not analyzed; RPLS, reversible posterior leukoencephalopathy syndrome.
All selected AEs were grade ≥ 3 except for one grade 2 arterial thromboembolic event and one grade 2 hemorrhage.
Coded as grade 2 but based on definition of febrile neutropenia, it must be grade 3.
DISCUSSION
Metastatic melanoma is a highly aggressive disease with few treatment options and relatively short survival. In this phase II, randomized, placebo-controlled study of 214 patients, which compared the combination of bevacizumab with carboplatin plus paclitaxel with carboplatin plus paclitaxel alone, PFS for the CP and CPB arms was 4.2 and 5.6 months, respectively. This difference was not statistically significant (HR, 0.78; log-rank P = .1414), and therefore, this study did not meet its primary end point.
The addition of bevacizumab to what is considered to be a first-line chemotherapy regimen was associated with an ORR of 25.5%, compared with 16.4% in the CP arm; however, this difference was not statistically different. Although the later exploratory analysis of OS demonstrated a 21% reduction in hazard of death (HR, 0.79; 95% CI, 0.55 to 1.13), this result was not significantly different between the CP and CPB arms. Therefore, the present study should not be used to support of use of bevacizumab in combination with carboplatin plus paclitaxel chemotherapy in advanced melanoma at the present time.
Of interest was the finding that patients with melanoma with the poorest prognosis (ie, those with stage IV M1c melanoma and elevated baseline serum LDH levels [n = 84]25–27) demonstrated improvements in both PFS and OS (PFS: HR, 0.62; OS: HR, 0.52). Elevated serum LDH has been postulated to result from hypoxic tumor tissue conditions in tumors, the same conditions that lead to upregulation of VEGF via the hypoxia-inducible factor pathway.28,29 Thus, in this setting, elevated serum LDH is possibly a marker of hypoxic tumor tissues that are reliant on VEGF for their maintenance and/or progression and may therefore also be more affected by the highly specific anti-VEGF activity of bevacizumab. This hypothesis could be addressed in subsequent studies by specifically enrolling large numbers of patients with elevated LDH levels.
In terms of OS, PFS, and ORR, results from BEAM, combined with those from ECOG2603, a randomized phase III study of carboplatin plus paclitaxel with or without sorafenib,5 confirm the benefit of the combination of carboplatin plus paclitaxel in patients with metastatic melanoma.2,4 Also of note, the control arm in BEAM behaved similarly to the control arm in ECOG2603, suggesting that the apparent benefit observed from the addition of bevacizumab to carboplatin plus paclitaxel would not likely have resulted from the control arm responding more poorly than expected.
Although improvements in PFS typically accompany OS benefits in clinical trials, we believe the BEAM results are of great enough promise to pursue a phase III multicenter trial powered for OS as the primary end point. Recently, a phase II study of single-agent bevacizumab with or without low-dose interferon alfa-2b reported promising clinical activity in metastatic melanoma,30 and several phase I and II studies are evaluating the use of other antiangiogenic agents as single agents as well as in combination with chemotherapy in this setting, such as sunitinib31–34 and E7080.35–37 In addition to antiangiogenic therapies, there have also been several other developments in the treatment of melanoma; for example, the anti-CTLA4 antibody ipilimumab, an immune activating therapy, was shown to significantly extend survival in a randomized phase III trial (HR, 0.66; 95% CI, 0.51 to 0.87; P = .0026). In addition, new therapies targeting genomic aberrations in BRAF kinase, present in 30% to 70% of melanomas (predominantly at codon 600 in exon 15 of BRAF gene),38,39 have validated this therapeutic target. In a phase I study of PLX4032, a selective inhibitor of mutated BRAF, in patients with the V600E mutation, a response rate of 81% was observed.40 That finding was subsequently confirmed in a larger and more rigorous phase II trial.41 Unlike BRAF-targeted treatments, the VEGF-targeting, antiangiogenic approach of bevacizumab is applicable to patients regardless of the mutation status of their tumor. The challenge of this approach is to identify predictive biomarkers for response to optimize patient care. Despite intensive preclinical and clinical research, finding such biomarkers has remained elusive.
This study failed to meet the primary objective of statistically significant improvement in PFS with the addition of bevacizumab to carboplatin plus paclitaxel. A larger phase III study will be necessary to determine whether there is benefit from the addition of bevacizumab to carboplatin plus paclitaxel in this disease setting.
Supplementary Material
Acknowledgment
We thank all the patients who participated in this study and their families, all of the BEAM (Bevacizumab Advanced Melanoma) investigators, and Asha Das, MD, for helpful discussions. This article was written by the authors with support from a medical writer at Genentech.
Footnotes
See accompanying editorial on page 6
Supported by Genentech, South San Francisco, CA.
Presented in part at the 15th European CanCer Organisation and 34th European Society of Medical Oncology Multidisciplinary Congress, September 20-24, 2009, Berlin, Germany; and the 2009 Perspectives in Melanoma XIII, October 8-10, 2009, Baltimore, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00434252.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Hoa Nguyen, Genentech, Roche (C); Peter Cheverton, Roche (C); Daniel Chen, Genentech, Roche (C); Amy C. Peterson, Genentech, Roche (C) Consultant or Advisory Role: Kevin B. Kim, Roche (C); Jeffrey A. Sosman, Genentech (C); John P. Fruehauf, Genentech (C); David F. McDermott, Roche (C); Jeffrey S. Weber, Roche (C); William E. Carson III, Genentech (C); Steven J. O'Day, Roche (C) Stock Ownership:Daniel Chen, Genentech, Roche; Amy C. Peterson, Roche Honoraria:Kevin B. Kim, Genentech, Roche; Jeffrey A. Sosman, Genentech; Gerald P. Linette, Genentech, GlaxoSmithKline; Steven J. O'Day, Roche Research Funding:Kevin B. Kim, Roche, Genentech; Jeffrey A. Sosman, Genentech; Steven J. O'Day, Roche Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Hoa Nguyen, Daniel Chen, William E. Carson III
Administrative support: Daniel Chen
Provision of study materials or patients: Kevin B. Kim, Jeffrey A. Sosman, John P. Fruehauf, David F. McDermott, Jeffrey S. Weber, Steven J. O'Day
Collection and assembly of data: Kevin B. Kim, Jeffrey A. Sosman, John P. Fruehauf, Gerald P. Linette, Svetomir N. Markovic, David F. McDermott, Jeffrey S. Weber, Daniel Chen, Steven J. O'Day
Data analysis and interpretation: Kevin B. Kim, Jeffrey A. Sosman, David F. McDermott, Hoa Nguyen, Peter Cheverton, Daniel Chen, Amy C. Peterson, Steven J. O'Day, Steven J. O'Day
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: The Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 3.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 4.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: A phase III study. J Clin Oncol. 2004;22:1118–1125. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 5.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 6.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birck A, Kirkin AF, Zeuthen J, et al. Expression of basic fibroblast growth factor and vascular endothelial growth factor in primary and metastatic melanoma from the same patients. Melanoma Res. 1999;9:375–381. doi: 10.1097/00008390-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Gorski DH, Leal AD, Goydos JS. Differential expression of vascular endothelial growth factor-A isoforms at different stages of melanoma progression. J Am Coll Surg. 2003;197:408–418. doi: 10.1016/S1072-7515(03)00388-0. [DOI] [PubMed] [Google Scholar]
- 9.Lacal PM, Failla CM, Pagani E, et al. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. J Invest Dermatol. 2000;115:1000–1007. doi: 10.1046/j.1523-1747.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- 10.Marcoval J, Moreno A, Graells J, et al. Angiogenesis and malignant melanoma. Angiogenesis is related to the development of vertical (tumorigenic) growth phase. J Cutan Pathol. 1997;24:212–218. doi: 10.1111/j.1600-0560.1997.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 11.Ugurel S, Rappl G, Tilgen W, et al. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 12.Nagengast WB, Lub-de Hooge MN, Hospers GA, et al. Towards clinical VEGF imaging using the anti-VEGF antibody bevacizumab and Fab-fragment ranibizumab. Presented at the 44th Annual Meeting of the American Society of Clinical Oncology; May 30-June 3, 2008; Chicago, IL. [Google Scholar]
- 13.Salven P, Heikkila P, Joensuu H. Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br J Cancer. 1997;76:930–934. doi: 10.1038/bjc.1997.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonetti O, Lucarini G, Brancorsini D, et al. Immunohistochemical expression of vascular endothelial growth factor, matrix metalloproteinase 2, and matrix metalloproteinase 9 in cutaneous melanocytic lesions. Cancer. 2002;95:1963–1970. doi: 10.1002/cncr.10888. [DOI] [PubMed] [Google Scholar]
- 15.Straume O, Akslen LA. Expresson of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol. 2001;159:223–235. doi: 10.1016/S0002-9440(10)61688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlaykova T, Laurila P, Muhonen T, et al. Prognostic value of tumour vascularity in metastatic melanoma and association of blood vessel density with vascular endothelial growth factor expression. Melanoma Res. 1999;9:59–68. doi: 10.1097/00008390-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 18.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 19.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 20.Rao RD, Holtan SG, Ingle JN, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006;106:375–382. doi: 10.1002/cncr.21611. [DOI] [PubMed] [Google Scholar]
- 21.Perez DG, Suman VJ, Fitch TR, et al. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: A North Central Cancer Treatment Group study, N047A. Cancer. 2009;115:119–127. doi: 10.1002/cncr.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimpfer-Rechner C, Hofmann U, Figl R, et al. Randomized phase II study of weekly paclitaxel versus paclitaxel and carboplatin as second-line therapy in disseminated melanoma: A multicentre trial of the Dermatologic Co-operative Oncology Group (DeCOG) Melanoma Res. 2003;13:531–536. doi: 10.1097/00008390-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Markman M, Kennedy A, Webster K, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141. doi: 10.1200/JCO.1999.17.4.1141. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Bedikian AY, Johnson MM, Warneke CL, et al. Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest. 2008;26:624–633. doi: 10.1080/07357900802027073. [DOI] [PubMed] [Google Scholar]
- 26.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 27.Hersey P, Bastholt L, Chiarion-Sileni V, et al. Small molecules and targeted therapies in distant metastatic disease. Ann Oncol. 2009;20(suppl 6):vi35–vi40. doi: 10.1093/annonc/mdp254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koukourakis MI, Giatromanolaki A, Winter S, et al. Lactate dehydrogenase 5 expression in squamous cell head and neck cancer relates to prognosis following radical or postoperative radiotherapy. Oncology. 2009;77:285–292. doi: 10.1159/000259260. [DOI] [PubMed] [Google Scholar]
- 29.Koukourakis MI, Kontomanolis E, Giatromanolaki A, et al. Serum and tissue LDH levels in patients with breast/gynaecological cancer and benign diseases. Gynecol Obstet Invest. 2009;67:162–168. doi: 10.1159/000183250. [DOI] [PubMed] [Google Scholar]
- 30.Varker KA, Biber JE, Kefauver C, et al. A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann Surg Oncol. 2007;14:2367–2376. doi: 10.1245/s10434-007-9389-5. [DOI] [PubMed] [Google Scholar]
- 31.Combination of temozolomide and sunitinib in treatment of patients with metastatic and unresectable malignant melanoma. http://www.clinicaltrials.gov/ct2/show/NCT00859326.
- 32.Clinical trial of sutent to treat metastatic melanoma. http://www.clinicaltrials.gov/ct2/show/NCT00631618.
- 33.Trial of single agent sunitinib for patients with chemo-refractory metastatic melanoma. http://www.clinicaltrials.gov/ct2/show/NCT01216657.
- 34.Temozolomide and sunitinib malate in treating patients with stage III or stage IV malignant melanoma. http://www.clinicaltrials.gov/ct2/show/NCT01005472.
- 35.A phase I/Ib, multicenter, open-label, dose escalation study of E7080 in patients with solid tumors and in combination with temozolomide in patients with advanced and/or metastatic melanoma. http://www.clinicaltrials.gov/ct2/show/NCT00121680.
- 36.An open-label, 2-cohort, multicenter, study of E7080 in previously treated subjects with unresectable stage III or stage IV melanoma. http://www.clinicaltrials.gov/ct2/show/NCT01136967.
- 37.E7080 in combination with dacarbazine versus dacarbazine alone as first line therapy in patients with stage IV melanoma. http://www.clinicaltrials.gov/ct2/show/NCT01133977.
- 38.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 39.Kumar R, Angelini S, Hemminki K. Activating BRAF and N-Ras mutations in sporadic primary melanomas: An inverse association with allelic loss on chromosome 9. Oncogene. 2003;22:9217–9224. doi: 10.1038/sj.onc.1206909. [DOI] [PubMed] [Google Scholar]
- 40.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sosman J, Kim K, Schuchter L. An open-label, multicenter phase II study of continuous oral dosing of RG7204 (PLX4032) in previously treated patients with BRAF V600E mutation-positive metastatic melanoma. Presented at the Society for Melanoma Research 7th International Melanoma Research Congress; November 4-7, 2010; Sydney, New South Wales, Australia. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.