Abstract
During the study repaglinide encapsulated floating microspheres were formulated and characterized for enhancing residence time of drug in git and thereby increasing its bioavailability. Floating microspheres of ethylcellulose (EC) and hydroxypropyl methyl cellulose (HPMC) (5 and 100 cps) were prepared by emulsion solvent diffusion technique. During process optimization various parameters were studied such as: drug: polymer ratio, polymer ratio, concentration of emulsifier and stirring speed. Selected optimized formulations were studied for SEM, entrapment, floating behavior, drug release and kinetics. In-vivo floating ability (X-ray) study and in-vivo antidiabetic activity were performed on alloxan induced diabetic rats. Microspheres prepared with different viscosity grade HPMC were spherical shaped with smooth surface. Size of microspheres was in the range of 181.1–248 μm. Good entrapment and buoyancy were observed for 12 h. X-ray image showed that optimized formulation remained buoyant for more than 6 h. Optimized formulation treated group shows significant (p < 0.01) reduction in blood glucose level as compared to pure drug treated group. Repaglinide loaded floating microspheres expected to give new choice for safe, economical and increased bioavailable formulation for effective management of NIDDM.
Abbreviations: EC, ethylcellulose; HPMC, hydroxypropyl methylcellulose; SEM, scanning electron microscopy; GRT, gastric residence time; ATP, adenosine tri phosphate; PVA, polyvinyl alcohol; PEG, polyethylene glycerol; UV, uv–visible
Keywords: Antidiabetic, Alloxan, Viscosity, Repaglinide, Significant
1. Introduction
Oral dosage form capable of having prolonged retention in stomach to extend drug delivery to a longer time has been receiving much attention nowadays (Moes, 1993). Gastric residence time (GRT) is one of the important factors affecting the bioavailability of drug in pharmaceutical dosage forms (Desai and Bolton, 1993). Variable and short gastric emptying time can result in incomplete drug release from delivery system above the absorption zone (stomach and upper part of small intestine), and thereby, abolishes the effectiveness of administered dose (Chueh et al., 1995). Drug bioavailability can be sufficiently increased by prolonging GRT through gastroretentive dosage form such as floating drug delivery system (FDDS) (Whitehead et al., 1998). Floating drug delivery remains buoyant over gastric and intestinal fluid owing to their lower density than aqueous medium. In floating dosage form both single and multiple systems have been developed. Multiple unit dosage form can be an attractive alternative than single unit form as they have been shown to reduce inter and intra variabilities in drug absorption as well as to lower the probability of accumulation of dose (Oth et al., 1992, Krogel and Bodmer, 1999, Bechgaard and Nielson, 1978).
Gastrointestinal tract targeting dosage forms are prepared to release the drug at gastrointestinal site. Several types of gastrointestinal target dosage forms including intragastric floating system (Rouge et al., 1998, Lee et al., 1999), high density system (Hwang et al., 1998), mucoadhesive system that gets adhere to gastric mucosal surface to extend GRT (Akiyama et al., 1995), magnetic system (Groning et al., 1998) and unfoldable extendible or swellable systems have been developed (Fix et al., 1993).
Floating drug delivery system is useful for several categories of drugs which act locally in the stomach, poorly soluble in alkaline pH, having narrow window of absorption, unstable in intestine or colonic environment and primarily absorbed in the stomach (Singh and Kim, 2000). Drugs having solubility in acidic medium and higher absorption in upper part of intestine can be used to deliver through floating system (Deshpande et al., 1997, Arora et al., 2005).
Repaglinide is an oral hypoglycemic agent and first member of meglitinide class, used to treat type-2 diabetes mellitus. It blocks the ATP dependent potassium channel to stimulate release of insulin by binding to specific site on pancreatic β-cells (Van Gall et al., 2001). Repaglinide requires frequent dosing before meals due to short half-life and there by imposing side effects such as skeletal muscles pain, headache and git effects (Fuhlendorff et al., 1998). Microspheres encapsulated with anti-diabetic drug, increase the effectiveness and release of drug in control manner from polymeric membrane and thereby, maintain its concentration for longer duration. Due to short lasting action, fast clearance, enzymatic stability and absorption throughout git make repaglinide a suitable target for developing gastroretentive dosage form. The aim of the study was to increase the bioavailability and reduce the mentioned side effects of repaglinide. Moreover, effect of different viscosity grade HPMC on drug loading and in-vitro drug release was examined and optimized formulation was subjected to in-vivo floating efficiency (X-ray) study.
2. Materials and methods
2.1. Materials
Repaglinide was procured as gift sample from Torrent Pharmaceuticals, Ahmadabad, India. Ethylcellulose, and hydroxypropyl methylcellulose (5 and 100 cps) were purchased from Himedia Chemicals, India. Analytical grade ethanol, dichloromethane, and polyvinyl alcohol (PVA) were purchased from SD fine chemicals Mumbai, India. All other chemicals used were of analytical grade.
2.2. Preparation of microspheres
Microspheres containing antidiabetic drug as a core material were prepared by solvent diffusion–evaporation technique with slight modification (Kawashima et al., 1992). Drug, polymers and 0.1% of PEG (as surfactant) were mixed in 1:1 mixture of ethanol and dichloromethane at room temperature. The slurry was slowly introduced into 80 ml of 0.46% w/v of polyvinyl alcohol as emulsifier. The system was stirred using propeller agitator for about 1 h for evaporation of organic phase. The prepared microspheres were washed 3–4 times with distilled water, dried for 1 h at room temperature and subsequently stored in desiccators over fused calcium chloride.
Various process variables such as polymer ratio, drug:polymer ratio, concentration of emulsifier and stirring speed were studied during optimization of formulation. On the basis of result of variables, optimized conditions for formulation were selected and recorded in Table 3.
Table 3.
Formula for microspheres with different grades of HPMC after optimization.
| Optimized parameters | Values |
|---|---|
| Polymer ratio | 1:2 |
| Drug:polymer ratio | 1:3 |
| Emulsifier concentration (% w/v) | 0.1 |
| Stirring speed (rpm) | 900 |
2.3. Characterization of floating microspheres
2.3.1. Surface morphology
The surface morphology was measured by scanning electron microscope (SEM) (Jeol JSM-1600, Tokyo, Japan).
2.3.2. Drug entrapment efficiency
The floating microspheres equivalent to 50 mg of repaglinide were weighed accurately and crushed. The powdered microspheres were placed in 10 ml of ethanol and kept for 12 h. The solution was then filtered through Whatman filter paper No. 44. The solution was diluted with fresh solvent and absorbance was measured at 247 nm using UV spectrophotometer (Shimadzu 1700) and the percent drug entrapped was calculated as follows:
2.3.3. In-vitro evaluation of floating ability
Floating microspheres (50 mg) were placed in simulated gastric fluid (pH 1.2, 100 ml) containing Tween 20 (0.02 w/v %) and stirred at 100 rpm using a magnetic stirrer. After 12 h, the layer of buoyant microparticles was pipette and separated from the settled microspheres by filtration. Particles of both the types were dried and weighed. The buoyancy of microspheres was calculated by using the formula given as
where Wf and WS are the respective weights of the floating and settled microparticles.
2.3.4. In-vitro drug release study
The drug release rate from floating microspheres was determined using paddle type six-station dissolution test apparatus (Veego, VDA-6DR, USP Std). A weighed amount of floating microspheres equivalent to 16 mg drug was kept in 0.1 N HCl (1.2 pH) having Tween 20 (0.02 w/v %) maintained at 37 ± 0.5 °C at a rotation speed of 100 rpm. Sink condition was maintained during the study. 1 ml sample was withdrawn at 30 min time interval, passed through 5 μm membrane filter and analyzed spectrophotometrically at 247 nm. The same process was repeated using pH 6.8 as dissolution medium. All experiments were performed in triplicate.
2.3.5. Release kinetics studies
Release data of optimized formulation were fitted to different mathematical models to reveal the release mechanism from the microspheres: Zero order (% cumulative drug release vs. time), first order (log % drug release vs. time), Higuchi model (% cumulative drug release vs. square root of time) and Peppas exponential equation (log % drug release vs. log time). All curve fitting, simulation and plotting were performed using commercially available Microsoft excel solver and regression coefficient (r2) values were calculated.
2.3.6. In-vivo floating behavior
Healthy albino rats, weighing 500–600 g were treated with optimized formulation and monitored through radiological method (Tanwar et al., 2007) with modification. The study was approved by Institutional Animal Ethics Committee, Shri Ram Institute of Technology Pharmacy, Jabalpur, Madhya Pradesh (Protocol No: SRITP/IAEC/2014/01). Animals were housed individually in polypropylene cages and maintained under standard conditions (12-h light and 12-h dark cycle; 25–30 °C). The animals were fasted for 12 h and at first X-ray was taken to ensure absence of radio opaque material in the stomach. During the study animals were not allowed to eat food but water was provided ad libitum. Radiopaque microspheres were prepared by incorporating 500 mg of barium sulfate into polymeric solution and similar procedure by which optimized formulation was prepared was followed. At varying time intervals X-ray photographs (Siregraph-B, Siemens, Karlsruhe, Germany) of gastric region were taken for monitoring the floating behavior of microspheres.
2.3.7. Antidiabetic activity
In-vivo evaluation studies were performed on healthy male albino rats of average body weight of about 250–300 g. Four groups of five rats each in a group were housed properly in cages, maintained with standard conditions. Groups 1 and 2 contain normal and diabetic control rats provided with drinking water and groups 3 and 4 contain diabetic rats treated with pure repaglinide (4 mg/kg body weight) and optimized formulation of floating microspheres equivalent to the dose of drug respectively using intragastric tube. Diabetes was induced in overnight fasted animals by single iv injection of 120 mg/kg of alloxan (CDH). Plasma glucose level of rats was determined after 48 h of injection and rats above 250 mg/Dl were considered as diabetic to be used in experimental work. Animals were provided with water ad libitum during the course of study. From the tail vein of rats blood samples were taken and checked for plasma glucose level using Accu-check® active glucose strips in Accu-check active test meter.
2.3.8. Histopathological studies
At the end of the study on fifteenth day, animals were sacrificed and organs such as liver, pancreas, heart and kidney were isolated individually. These collected organs were excised quickly and fixed in 10% formalin for histopathological test. Tissues were fixed in paraffin blocks, sliced and placed onto glass slides. After staining slides were observed and photographs were taken using topical microscope.
3. Results and discussions
3.1. Formulation and optimization of floating microspheres
The floating microspheres were successfully prepared by solvent-diffusion evaporation technique using ethylcellulose and HPMC (5 and 100 cps). Formulations were prepared by varying concentration of HPMC with fixed ratio of EC to study the effect of increasing polymer concentration on various characterized parameters. Microspheres were formed by pouring solution of polymer and drug prepared in ethanol and dichloromethane, into aqueous solution of PVA. The ethanol rapidly distributed into external aqueous phase and layer of polymer get precipitated around dichloromethane droplets. Evaporation of entrapped dichloromethane leads to formation of cavities within microspheres (Jain et al., 2005). This forms smooth surfaced, spherical shaped microsphere that can float over gastric fluid.
Different viscosity grades of HPMC were used to demonstrate the effect of viscosity on particle size and drug release. Results shown in Table 1, Table 2 indicate that various formulation variables have significant effect on particle size, buoyancy and entrapment efficiency. The mean particle size of microspheres increases with increasing HPMC concentration and viscosity at the same ratio. The size of the particles was in the range of 181.1 ± 1.5–204.2 ± 3.5 μm and 221.2 ± 3.2–236 ± 6.1 μm for microspheres prepared by HPMC (5 cps) and HPMC (100 cps) respectively. Increase in HPMC concentration increases the size of particles and larger size microspheres with HPMC (100 cps) as compared to HPMC (5 cps) were formed. This is due to significant increase in viscosity in a fixed volume of solvent, thus causing increase in emulsion drop size and finally increases in size of particles (Fu et al., 2005). Formulations were optimized for drug: polymer ratio and size of particles were in the range of 179.6 ± 3.5–193.2 ± 7.2 μm and 220.1 ± 1.3–233.5 ± 1.8 μm for increasing grades of HPMC used during formulation.
Table 1.
Process variables for microspheres with HPMC (5 cps).
| Formulation variables | Particle size | % Entrapment efficiency | % Buoyancy |
|---|---|---|---|
| Polymer ratio | |||
| 1:1 | 181.1 ± 1.5 | 58.5 ± 4.6 | 70.3 ± 1.1 |
| 1:2 | 192.5 ± 2.4 | 62.5 ± 3.2 | 77.2 ± 2.3 |
| 1:3 | 204.2 ± 3.5 | 63.2 ± 1.3 | 80.3 ± 3.3 |
| Drug:polymer ratio | |||
| 1:1 | 179.6 ± 3.5 | 60.7 ± 2.3 | 70.4 ± 2.6 |
| 1:2 | 189.4 ± 4.5 | 64.8 ± 6.3 | 78.1 ± 5.8 |
| 1:3 | 193.2 ± 7.2 | 66.6 ± 4.9 | 81.3 ± 6.4 |
| Emulsifier concentration (% w/v) | |||
| 0.46 | 208.1 ± 4.2 | 67.2 ± 5.3 | 84.3 ± 8.0 |
| 0.66 | 194.2 ± 1.1 | 64.3 ± 7.2 | 80.2 ± 6.1 |
| 0.86 | 182.3 ± 3.2 | 58.6 ± 6.2 | 79.4 ± 0.1 |
| Stirring speed | |||
| 600 | 211.1 ± 0.3 | 65.2 ± 1.2 | 82.4 ± 4.2 |
| 900 | 201.2 ± 2.4 | 59.5 ± 2.6 | 78.1 ± 2.1 |
Table 2.
Process variables for microspheres with HPMC (100 cps).
| Formulation variables | Particle size | % Entrapment efficiency | % Buoyancy |
|---|---|---|---|
| Polymer ratio | |||
| 1:1 | 221.2 ± 3.2 | 61.2 ± 0.2 | 68.4 ± 2.1 |
| 1:2 | 232.8 ± 4.8 | 64.5 ± 0.8 | 76.5 ± 3.6 |
| 1:3 | 236.0 ± 6.1 | 65.5 ± 3.2 | 79.1 ± 2.2 |
| Drug:polymer ratio | |||
| 1:1 | 220.1 ± 1.3 | 61.8 ± 3.2 | 67.2 ± 8.1 |
| 1:2 | 229.1 ± 4.5 | 65.3 ± 1.2 | 78.8 ± 5.1 |
| 1:3 | 233.5 ± 1.8 | 68.6 ± 2.6 | 79.0 ± 6.3 |
| Emulsifier concentration (% w/v) | |||
| 0.46 | 251.5 ± 0.5 | 70.5 ± 3.1 | 80.2 ± 1.1 |
| 0.66 | 243.6 ± 4.2 | 66.2 ± 3.2 | 77.9 ± 2.1 |
| 0.86 | 229.4 ± 3.6 | 62.3 ± 6.9 | 75.3 ± 7.3 |
| Stirring speed | |||
| 600 | 248.2 ± 3.2 | 69.1 ± 4.2 | 81.2 ± 3.5 |
| 900 | 236.7 ± 3.6 | 62.4 ± 5.0 | 77.2 ± 2.3 |
Effect of increasing concentration of emulsifier on particle size was also studied. At low concentration of emulsifier particle size was larger which decreases with increasing emulsifier concentration. The amount of PVA as an emulsifier does not have much effect on percent buoyancy (decreases to lesser extent) but entrapment efficiency and particle size of microspheres decrease with increase in emulsifier concentration. This may be due to more PVA molecules overlay the surface of droplets, which will reduce protection of the droplets against coalescence resulting in formation of small emulsion droplets. As microspheres were formed from emulsion droplets after evaporation of solvent, their size was dependent on the size of emulsion droplets (Lakshmana et al., 2009). Effect of stirring rate was also studied and as it increases from 600 to 900 rpm there is decrease in size of particles in all batches. The results of optimization were consolidated and optimized formula was shown in Table 3.
3.2. Surface morphology
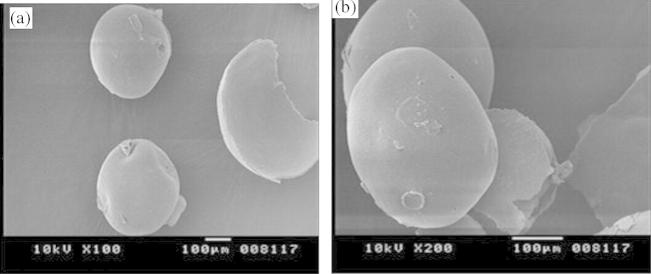
Scanning electron microscope was used to study surface morphology and Fig. 1 shows that prepared microspheres were spherical shaped with smooth surface.
Figure 1.
SEM photographs of microspheres prepared: (a) HPMC 5 cps and (b) 100 cps.
3.3. Percent buoyancy and entrapment efficiency
Percent buoyancy was studied for 12 h and was in the range of 70.3–84.3% and 67.4–81.2% for microspheres prepared with HPMC 5 and 100 cps respectively. Quantity of polymers, ratio of polymers and nature of solvent used during formulation affect the floating of microspheres (Streubel et al., 2006). Microspheres were remained floated in continuous manner and percent buoyancy was found to increase with increasing amount of polymer.
Entrapment efficiency was calculated for all the batches and was in the range of 58.5–70.5%. Increasing polymer concentration in the internal phase shows increase in drug loading. This may be due to increase in viscosity of internal phase which reduces the migration of drug in aqueous phase (Das and Das, 1998). Thus viscous polymer used during formulation shows more entrapment. But drug loading decreasing with increasing stirring rate from 600 rpm to 900, may be due to smaller size microspheres formed at higher speed of rotation. Also loss of drug from surface of small particles is more as compared to larger during washing of microspheres. Microspheres were optimized for drug:polymer ratio keeping other parameters constant. The drug entrapment initially increased from 60.7% to 66.6% and 61.8% to 68.6% for both the batches with decrease in drug polymer ratio up to 1:3 after which it decreases (data not shown). Entrapment efficiency and particle size are dependent on several factors such as concentration of emulsifier, stirring rate and time; increase in polymer concentration will disturb the equilibrium between these parameters. But as the polymer concentration increases there is decrease in drug release. Thus, optimized ratio selected for drug:polymer ratio was 1:3.
3.4. In-vitro drug release study
Release of repaglinide from microspheres was evaluated in SGF for 12 h. Release of drug shows no burst effect in any of the formulation, indicating homogenous drug distribution. The cumulative release of optimized formulation prepared by different viscosity grades HPMC was compared in Fig. 2 and found to decrease with increase in polymer concentration. Drug release is more (83.2%) for formulation constituting low viscosity HPMC (5 cps) as compared to HPMC (100 cps) (74.18%). The increased density of polymer at higher concentration results in an increased diffusional pathlength, which results in overall decrease in release of drug.
Figure 2.
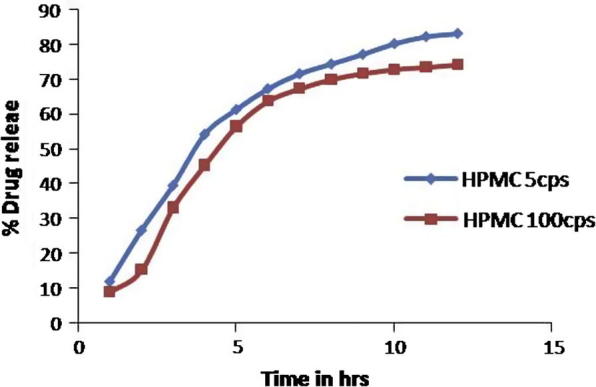
In-vitro release profile of optimized formulation of both the batches.
3.5. Release kinetics study
The best selected in-vitro release data of optimized formulation were fitted to various mathematical models such as zero order, first order, Higuchi and Peppas kinetic models. The highest regression (0.975) was obtained for first order kinetics followed by Higuchi (0.951) and Peppas (0.938) model. Release mechanism was studied by Peppas equation. Value of slope (n) was calculated and found to be less than 0.89 which indicates coupling of diffusion and erosion mechanism, indicating drug release is controlled by more than one process. First order plot of optimized formulation was shown in Fig. 3.
Figure 3.
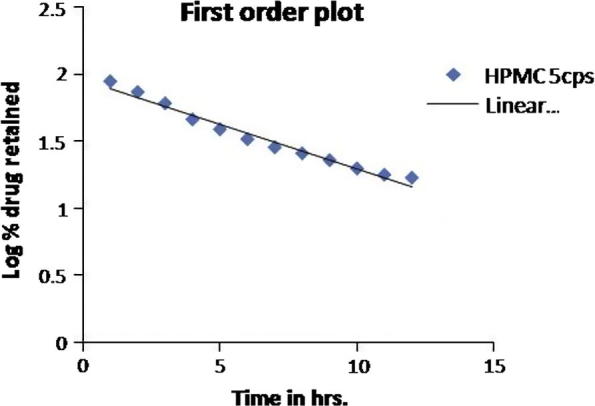
First order plot of repaglinide from EC:HPMC formulations (H1–H10).
3.6. In-vivo floating behavior
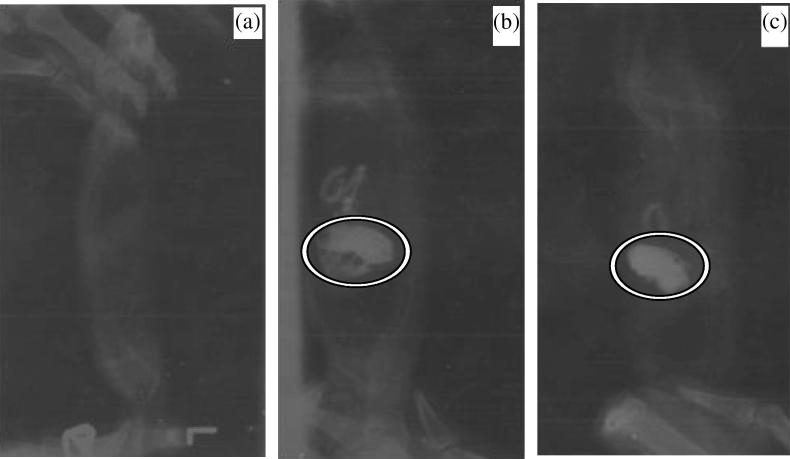
The optimized floating microspheres prepared with EC:HPMC (5 cps) 1:2 ratio show good in-vitro floating ability and enhanced drug release therefore was finally selected for studying in-vivo floating efficiency by radiological method. The radiographs obtained at 0, 2 and 4 h are shown in Fig. 4, which indicates uniform distribution of formulation over the stomach fluid and buoyancy for more than 6 h.
Figure 4.
X-ray images of formulation in the gastric region of rat: (a) before dosing, (b) 2 h after dosing, and (c) 4 h after dosing.
3.7. Antidiabetic activity
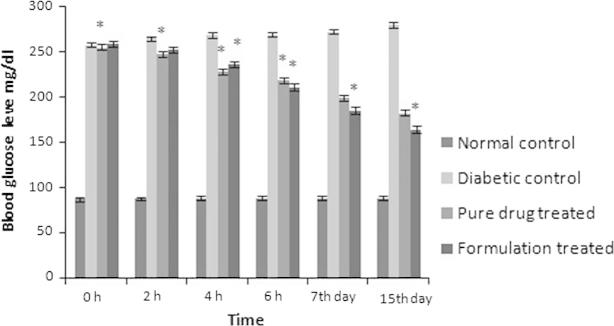
Biological studies were performed on healthy albino rats categorized in different groups. Alloxan induced diabetic rats were utilized to study antidiabetic effect of selected formulation. Treatment with formulation caused significant (p < 0.01) reduction in blood glucose level as compared to pure drug treated group of animals. Administration of formulation for a period of 15 days leads to persistent significant (p < 0.01) maintenance of glucose level as evident from Fig. 5.
Figure 5.
Comparative blood glucose level of different groups of animals.
3.8. Histopathological studies
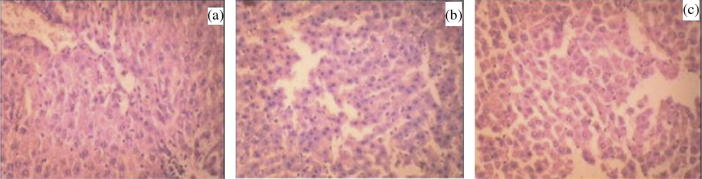
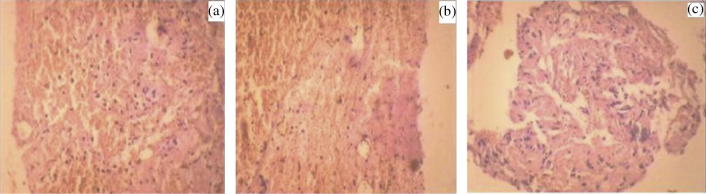
Histopathological slides of formulation treated group show no apparent change in normal cellular structure as compared to drug treated group. Histopathology of liver in control group of animals shows that hepatocytes were distinct and normal; there was absence of any sort of fatty changes or necrosis (Fig. 6a). Liver of repaglinide treated animal’s revealed presence of very slight dilation of blood vessels and mild inflammatory changes (Fig. 6b). Such features were completely absent on animals treated with formulation as shown in (Fig. 6c). In the images of pancreas, it was observed that control rat showed normal pancreatic histo-architecture islet with no abnormality (Fig. 7a). Whereas repaglinide treated rats demonstrated a slight degeneration of serous acini and the numbers of islet and islet cells, pancreas of formulation treated rats revealed virtually no anomaly (Fig. 7b and c).
Figure 6.
Histopathological view of liver showing protective effect of formulation in rats, (a) normal control, (b) pure drug treated, and (c) optimized drug loaded formulation treated group.
Figure 7.
Histopathological view of pancreas showing protective effect of formulation in rats, (a) normal control, (b) pure drug treated, and (c) optimized drug loaded formulation treated group.
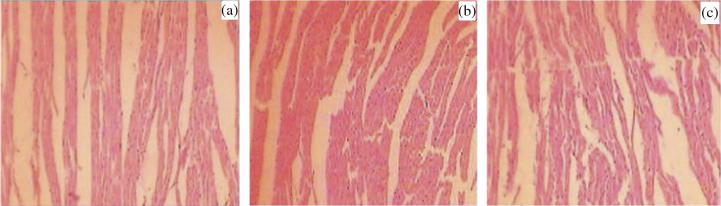
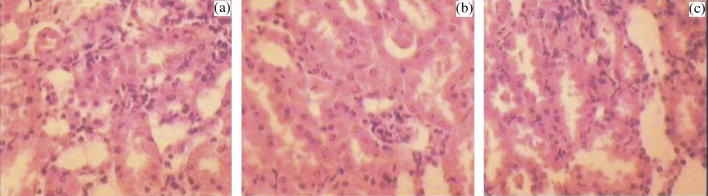
In histopathology, normal control rats showed normal anatomical features of cardiac tissues whereas, drug administration caused slight cellular infiltration along with partial fragmentation of muscle fibers (Fig. 8a and b). No such effect was observed on repaglinide microspheres administration. In the image of kidneys from animals of control group, normal renal anatomy was observed (Fig. 9a). A slight inflammation in glomerular and tubular area was observed in rats administered with drug, whereas, rats treated with formulation ameliorated renal histological inflammation, with no harmful effects (Fig. 9b and c).
Figure 8.
Histopathological view of heart showing protective effect of formulation in rats, (a) normal control, (b) pure drug treated, and (c) optimized drug loaded formulation treated group.
Figure 9.
Histopathological view of kidney showing protective effect of formulation in rats, (a) normal control, (b) pure drug treated, and (c) optimized drug loaded formulation treated group.
4. Conclusion
The formulation of repaglinide loaded EC and HPMC microspheres was successfully formulated. Different investigations on formulation, characterization, in-vitro release study and in-vivo evaluations were carried out and performance of the formulation was evaluated. The proposed optimized formulation depicts an effective way to prolong drug release. The in-vivo floating efficiency of optimized formulation was excellent and microspheres were retained in rat stomach for longer period of time. The histopathological results obtained after administration of microspheres to healthy rats were satisfactory. The developed microspheres are safe and are the need of pharmaceutical industry as an alternate for effective management of NIDDM.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akiyama Y., Nagahara N., Kashihara T., Hirai S., Toguchi H. In-vitro and in-vivo evaluation of mucoadhesive microspheres prepared for gastrointestinal tract by polyglycerol ester of fatty acids and (acrylic acid) derivatives. Pharm. Res. 1995;12:397–405. doi: 10.1023/a:1016208703380. [DOI] [PubMed] [Google Scholar]
- Arora S., Ali J., Ahuja A., Khar R.K., Baboota S. Floating drug delivery systems: a review. AAPS PharmSciTech. 2005;19:E372–E390. doi: 10.1208/pt060347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechgaard H., Nielson G.H. Controlled release multiple units and single unit doses. Drug Dev. Ind. Pharm. 1978;4:53–67. [Google Scholar]
- Chueh H.R., Zia H., Rhodes C.T. Optimization of sotalol floating and bioadhesive extended release tablet formulation. Drug Dev. Ind. Pharm. 1995;21:1725–1747. [Google Scholar]
- Das S.K., Das N.G. Preparation and in-vitro dissolution profile of dual polymer (Eudragit® RS 100 and RL 100) microparticles of diltiazem hydrochloride. J. Microencapsul. 1998;15:445–452. doi: 10.3109/02652049809006871. [DOI] [PubMed] [Google Scholar]
- Desai S., Bolton S. A floating controlled-release drug delivery system: invitro–invivo evaluation. Pharm. Res. 1993;10:1321–1325. doi: 10.1023/a:1018921830385. [DOI] [PubMed] [Google Scholar]
- Deshpande A.A., Shah N.H., Rhodes C.T., Malick W. Development of a novel controlled-release system for gastric retention. Pharm. Res. 1997;14:815–819. doi: 10.1023/a:1012171010492. [DOI] [PubMed] [Google Scholar]
- Fix J.A., Cargill R., Engle K. Controlled gastric emptying. Part 3. Gastric residence time of a non-disintegrating geometric shapes in human volunteers. Pharm. Res. 1993;10:1087–1089. doi: 10.1023/a:1018939512213. [DOI] [PubMed] [Google Scholar]
- Fu X., Ping Q., Gao Y. Effects of formulation factors on encapsulation efficiency and release behaviour in vitro of huperzine A-PLGA microspheres. J. Microencapsul. 2005;22:57–66. doi: 10.1080/02652040400026509. [DOI] [PubMed] [Google Scholar]
- Fuhlendorff J., Rorsman P., Kofod H., Brand C.L., Rolin B., Mackay P., Shymko R., Carr R.D. Stimulation of insulin release by repaglinide and glibenclamide involves both common and distinct processes. Diabetes. 1998;47:345–351. doi: 10.2337/diabetes.47.3.345. [DOI] [PubMed] [Google Scholar]
- Groning R., Berntgen M., Georgarakis M. Acyclovir serum concentration following preoral administration of magnetic depot tablets and the influence of extracorporal magnets to control gastrointestinal transit. Eur. J. Pharm. Biopharm. 1998;46:285–291. doi: 10.1016/s0939-6411(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Hwang S.J., Park H., Park K. Gastric retentive drug-delivery system. Crit. Rev. Ther. Drug Carrier Syst. 1998;15:243–284. [PubMed] [Google Scholar]
- Jain S.K., Awasthi A.M., Jain N.K., Agrawal G.P. Calcium silicate based microspheres of repaglinide for gastroretentive floating drug delivery: preparation and in-vitro characterization. J. Controlled Release. 2005;107:300–309. doi: 10.1016/j.jconrel.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Kawashima Y., Niwa T., Takeuchi H., Hino T., Itoh Y. Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J. Pharm. Sci. 1992;81:135–140. doi: 10.1002/jps.2600810207. [DOI] [PubMed] [Google Scholar]
- Krogel I., Bodmer R. Development of a multifunctional matrix drug delivery system surrounded by an impermeable cylinder. J. Controlled Release. 1999;61:43–50. doi: 10.1016/s0168-3659(99)00096-6. [DOI] [PubMed] [Google Scholar]
- Lakshmana P.S., Shirwaikar A.A., Shirwaikar A., Kumar A. Formulation and evaluation of sustained release microspheres of rosin containing aceclofenac. Ars Pharm. 2009;50:51–62. [Google Scholar]
- Lee J.H., Park T.G., Choi H.K. Development of oral drug delivery system using floating microspheres. J. Microencapsul. 1999;16:715–729. doi: 10.1080/026520499288663. [DOI] [PubMed] [Google Scholar]
- Moes A.J. Gastroretentive dosage form. Crit. Rev. Ther. Drug Carrier Syst. 1993;10:143–195. [PubMed] [Google Scholar]
- Oth M., Franz M., Timmermans J., Moes A. The bilayer floating capsule: a stomach directed drug delivery system for misoprostile. Pharm. Res. 1992;9:292–302. doi: 10.1023/a:1015870314340. [DOI] [PubMed] [Google Scholar]
- Rouge N., Cole E.T., Doelkar E., Buri P. Buoyancy and drug release patterns of floating minitablets containing piretanides and atenolol as model drugs. Pharm. Dev. Technol. 1998;3:73–84. doi: 10.3109/10837459809028481. [DOI] [PubMed] [Google Scholar]
- Singh B.M., Kim K.H. Floating drug delivery system: an approach to oral controlled drug delivery via gastric retention. J. Controlled Release. 2000;63:235–259. doi: 10.1016/s0168-3659(99)00204-7. [DOI] [PubMed] [Google Scholar]
- Streubel A., Siepmann J., Bodmeier R. Gastroretentive drug delivery system. Expert Opin. Drug Delivery. 2006;3:217–233. doi: 10.1517/17425247.3.2.217. [DOI] [PubMed] [Google Scholar]
- Tanwar Y.S., Naruka P.S., Ojha G.R. Development and evaluation of floating microspheres of verapramil hydrochloride. Braz. J. Pharm. Sci. 2007;43:529–534. [Google Scholar]
- Van G.L.F., Van A.K.L., De L.I.H. Repaglinide improves blood glucose control in sulphonylurea-naive type 2 diabetes. Diabetes Res. Clin. Pract. 2001;53:141–148. doi: 10.1016/s0168-8227(01)00253-4. [DOI] [PubMed] [Google Scholar]
- Whitehead L., Fell J.T., Collett J.H., Sharma H.L., Smith A.M. Floating dosage forms: an in-vivo study demonstrating prolonged gastric retention. J. Controlled Release. 1998;55:3–12. doi: 10.1016/s0168-3659(97)00266-6. [DOI] [PubMed] [Google Scholar]