Abstract
Mass spectrometry has been widely used, particularly in pharmacokinetic investigations and for therapeutic drug monitoring purposes. Like any other analytical method some difficulties exist in employing mass spectrometry, mainly when it is used to test biological samples, such as to detect drug candidates in mammalian serum, which is rich in proteins, lipids and other contents that may interfere with the investigational drug. The complexity of the serum proteome presents challenges for efficient sample preparation and adequate sensitivity for mass spectrometry analysis of drugs. Enrichment procedures prior to the drug analysis are often needed and as a result, the study of serum or plasma components usually demands either methods of purification or depletion of one or more. Selection of the best combination of sample introduction method is a crucial determinant of the sensitivity and accuracy of mass spectrometry. The aim of this study was to determine the highest serum protein precipitation activity of five commonly used sample preparation methods and test their suitability for mass spectrometry. We spiked three small molecules into rabbit serum and applied different protein precipitation methods to determine their precipitation activity and applicability as a mass spectrometry introductory tool.
Keywords: Mass spectrometry, Protein extraction method, Serum sample, In-gel alkylation
Abbreviations: PP, protein precipitation; MS, mass spectrometry; LC, liquid chromatography
1. Introduction
Plasma is frequently used as a biological matrix as it is easy to collect (Olsen et al., 2004, Sjoholm et al., 1979). Typically, it is widely used in studies of analytical method development and validation, just prior to the animal trials. Indeed, appropriate sample preparation is essential for obtaining reliable and meaningful results. Consequently, sample preparation is still an area of high importance when a liquid chromatography and mass spectrometry (LC/MS/MS) method is developed to assay biological samples (Xu et al., 2005). It is predominantly used in the ‘optimisation’ of a sample for analysis with mass spectrometry (MS) techniques. The importance of sample preparation is to ensure that the analytical method maintains certain essential elements of robustness and consistency that are expected in any bioanalytical assay (Xu et al., 2005).
Generally, the two main sample preparation methods used for the MS analysis of blood, serum plasma and urine samples are liquid–liquid extraction or solid-phase extraction (SPE) (Bouzas et al., 2009). However, for drug discovery and pharmacokinetics, protein precipitation (PP)/extraction is the most common sample preparation procedure, which is the simplest approach that requires minimal method development and removes the majority of the protein from the sample (Xu et al., 2005). PP with miscible organic solvents (usually acetonitrile or methanol) is the most commonly used sample preparation method because of its low cost and minimal method development requirements (Ma et al., 2008). While, there are many PP solvents that are widely used including organic and inorganic solvents (Bouzas et al., 2009, Lawson, 1989), the selection predominantly depends on the investigational compound used. Usually, the use of methanol is especially valuable for support of preclinical pharmacokinetic studies conducted during the lead optimisation stages of drug discovery, where rapid development of assays for new compounds is essential (Henry et al., 2013, Ma et al., 2008). In an attempt to investigate the suitability of each of the solvents used for MS analysis of small molecules in pharmacokinetics studies, we performed PP using five different solvent systems and compared their ability to precipitate serum proteins and extract potential drug molecules for MS analysis.
2. Materials and methods
2.1. Serum samples
Blood was collected from a healthy rabbit housed at the Small Animal Facility of the CSIRO Australian Animal Health Laboratory. Serum was obtained by allowing the blood to clot at room temperature for 2 h. The clotted blood was then centrifuged for 10 min at 12,000g. Serum was then collected and stored at −20 °C.
2.2. Confirmation of compounds identity and purity using MS
Three potential antiviral compounds of small molecular weight (pending patent) were selected for this study and given different codes (AAHL 13, AAHL 18 and AAHL 42). The compounds were initially dissolved in methanol at a concentration of 0.5 mg/ml, then diluted in 50% methanol/0.2% formic acid to a final concentration of 10 μg/ml. Diluted samples were analysed by direct infusion at a rate of 10 μl/min into the electrospray ionisation source of an LCQ ion-trap mass spectrometer (Thermo, San Jose, CA, USA). Spectra were acquired and averaged over 50 consecutive scans. Full scans were acquired over the mass range m/z 50–500 to give an indication of sample purity. High resolution zoom scans were also performed that allowed determination of the mass/charge state of the selected ion and hence an accurate mass measurement of the selected ion.
2.3. Detection of compounds in rabbit serum
Rabbit serum was spiked with three investigational compounds (AAHL 13, AAHL 18 or AAHL 42) at a concentration of 0.5 mg/ml. The spiked serum then underwent protein precipitation using the described methods. The supernatants from each treatment were collected and diluted 1:1 with 0.4% v/v formic acid to give a final solvent composition of 50% methanol/0.2% formic acid and analysed by MS.
2.4. Methanol extraction method
Briefly, 100 μl of serum was mixed with 900 μl of HPLC-grade methanol. Following centrifugation, aliquots of 100 μl of the supernatants were dried and then resuspended in electrophoresis sample buffer (MES) and analysed by electrophoresis, or aliquots were diluted in 50% methanol/0.2% formic acid for MS analysis.
2.5. Folch extraction method
A mixture of chloroform–methanol in the ratio of 2:1 by volume was prepared and 400 μl of this mixture was added to a 100 μl of serum. The upper phase of each sample was used for analysis, because the proteins were precipitated in the middle and lower phases.
2.6. Acetone extraction method
Briefly, 900 μl of acetone was added to 100 μl of serum. The supernatant only was used for analysis. For electrophoresis, samples were dried and then resuspended in sample buffer (MES) and for MS analysis samples were diluted in 50% methanol/0.2% formic acid.
2.7. Acetonitrile extraction method
A volume of 100 μl of serum was mixed with a volume of 300 μl of acetonitrile. The sample was centrifuged and supernatant of the mixture was collected and then analysed.
2.8. Proteinase K protein depletion method
The Proteinase K method was performed as per manufacturer’s recommendation. Briefly, serum samples were treated with 200 μg/ml of proteinase K for 18 h at 37 °C. In order to determine the most effective concentration of proteinase K, a number of different concentrations and incubation periods were trailed. The most effective concentrations were then used and compared to other extraction methods. Proteinase K treated samples were centrifuged and supernatants were collected for analysis.
2.9. Confirmation of protein precipitation by electrophoresis
Supernatants from protein precipitated serum samples from different extraction methods were obtained after centrifugation of treated samples that pelleted the precipitated proteins. Supernatants were then dried in a centrifugal vacuum concentrator (Savant Speedvac, Thermo). Dried samples were then diluted 1:100 in electrophoresis sample buffer. The diluted samples were then separated by SDS–PAGE and proteins were visualised by Coomassie blue or silver staining.
2.10. In-gel alkylation and digestion of proteins
Briefly, the Coomassie blue stained bands were cut from the SDS–PAGE gel, reduced and alkylated, in-gel digested using trypsin, extracted and analysed using LC–MS/MS to determine their identity.
3. Results
3.1. Confirmation of compounds identity and purity
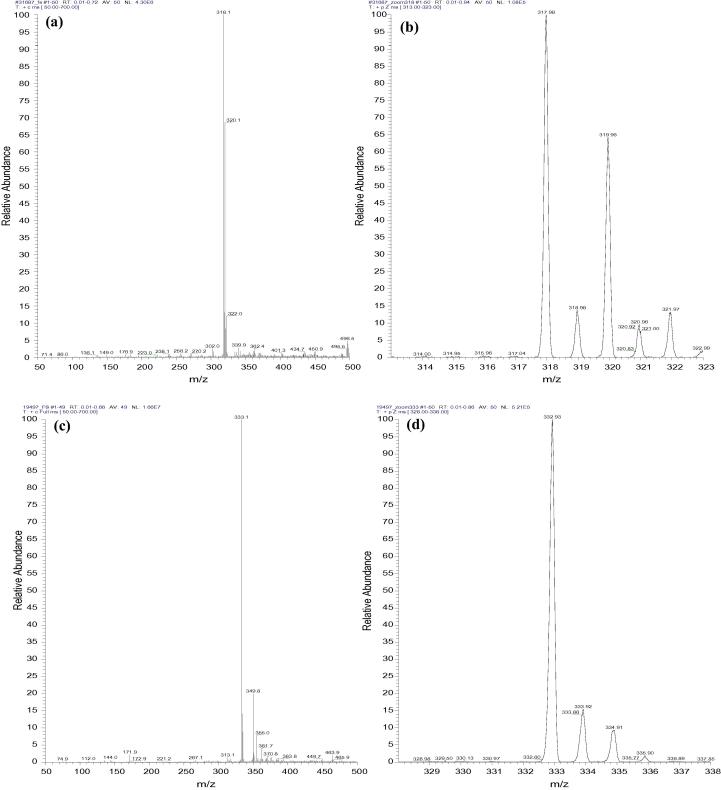
The identity and purity of each of the three compounds (Fig. 1a–f) were confirmed against the given masses (Table 1). Of note, a number of small peaks are shown in all the spectra (Fig. 1a–f), which represent the background readings of each sample. Thus, the zoomed spectra provide more accurate readings of the dominant peaks that can be used to confirm the identity and estimate the purity of the investigational compound. A noticeable peak at mass 320 is evident in the spectra of AAHL 42 and AAHL 18 (Fig. 1a and e, respectively). While the source of this peak is unknown, it is well known that precipitated serum samples contain high concentrations of salts (Huang et al., 2013, Cai et al., 2002); hence, it is possible that the 320 mass peak is one of the dominant salts present in the supernatant. Importantly, the mass values presented by the peaks are slightly different to those in Table 1. For instance the detected peaks for AAHL 42 are at 318.1, AAHL 13 are at 333.1 and AAHL 18 are detected at 402.9, while their reported values (Table 1) show AAHL 42 at 315.8, AAHL 13 at 332.36 and AAHL 18 at 402.16. The differences observed are due to the fact that the values in Table 1 are molecular weight values (isotopic average mass that might include the less abundant naturally occurring isotopes), which are the values used in the periodical table of elements (Grueiro Noche et al., 2013, Leigh et al., 1998) whereas, the peak values presented in the spectra are of the monoisotopic mass spectrum (a spectrum containing only ions made up of the principal isotopes of atoms making up the original molecule) (Selvadurai and Meyyanathan, 2011, McNaught and Wilkinson, 1997). Monoisotopic mass is the mass of the abundance isotopes of chemical elements as naturally found, which is also known as naturally abundance isotopes (Leigh et al., 1998).
Figure 1.
MS spectra of the 3 lead compounds. Electron ionisation mass spectrum of AAHL 42 (a) and a zoomed format of the spectrum (b), represent the well resolved high peak of the expected mass of the hit compound at approximately 318. Figures (c) and (d) represent the electron ionisation mass spectrum and its zoomed format for AAHL 13 respectively. The well-defined high peaks of 333.1 presented in the spectrum show the expected mass value of the hit compound. The spectrum for AAHL 18 hit compound is presented in figures (e) and (f) with highest peak of 402.86 representing the mass number for this compound (refer to Table 1 for compound masses).
Table 1.
Characterisation of the investigational compounds.
| Compound ID | Molecular weight | HPLC purity (%) | Method of confirming compound identity |
|---|---|---|---|
| AAHL 42 | 315.8 | 98.8 | ESI MS/1H NMRa |
| AAHL 13 | 323.36 | 99.8 | ESI MS/1H NMR |
| AAHL 18 | 402.16 | 99.5 | ESI MS/1H NMR |
The table includes the identity, molecular weight, HPLC purity and method of confirmation of purity.
ESI MS = [electrospray ionisation mass spectrometry]. 1H NMR = [Hydrogen-1 nuclear magnetic resonance].
3.2. Comparison of different extraction methods
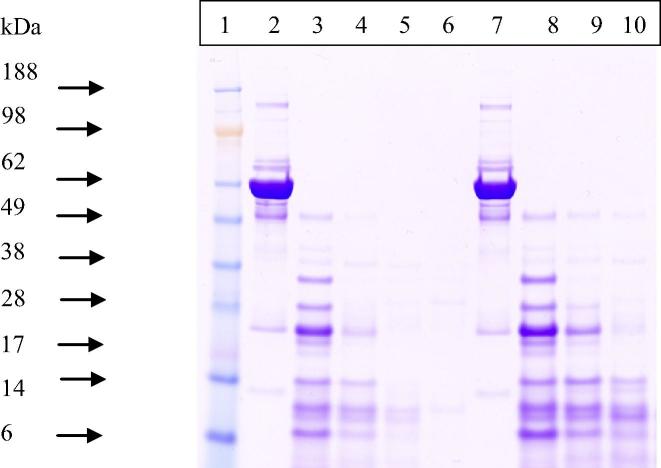
Different concentrations of proteinase K were used at different incubation times to determine the optimal concentration to use (see Fig. 2). Based on the current results we can conclude that at 18 h of incubation a 200 μg/ml of proteinase K has digested and removed most of the serum protein. After the determination of a suitable proteinase K concentration, a number of known protein extraction methods were compared using electrophoretic analysis.
Figure 2.
SDS PAGE analysis of serum proteins following treatment with different concentrations of proteinase K. Electrophoretic analysis of rabbit serum treated with different proteinase K concentrations. From left to right, first lane has the MW markers, lane 2 contains untreated serum diluted 1:200 in running buffer, lanes 3, 4 and 5 are samples that were digested for 18 h with 100 μg/ml, 200 μg/ml 300 μg/ml of proteinase K, respectively. While, lane 6 is empty, lanes 7 through to 10 contain serum samples digested with the same concentration and order of the previous lanes except these were treated for 1 h instead.
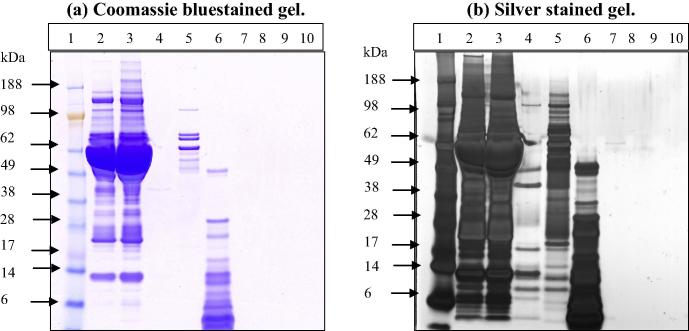
All of the concentrations and treatments were carried out as per manufacturer’s or literature recommendations. The results indicated that most of the solvents used produced significant reduction in the serum proteins (Fig. 3a and b). Methanol, acetone and acetonitrile extraction methods have almost completely removed all of the serum proteins. Based on the fact that the methanol extraction method was effective as well as the fact that investigational compounds are dissolved in methanol, gave the methanol extraction method some advantage over the other methods used.
Figure 3.
SDS PAGE comparison between supernatants from different extraction methods. Electrophoretic analysis of rabbit serum precipitated by different protein precipitation methods. From left to right, first lane contain MW markers, second and third lanes represent serum (1:100 in running buffer) and pellet from methanol precipitated serum respectively, acetone precipitation method (lane 4), chloroform–methanol method (lane 5). While lane 6 represents serum that was precipitated with 200 μg/ml proteinase K, and lane 7 and lane 8 represent acetonitrile and methanol precipitated serum, respectively. Serum precipitated samples were dried and then reconstituted in 100 μl MES, of which 15.6 μl were used per lane.
3.3. Recovery of investigational compounds as assessed by MS
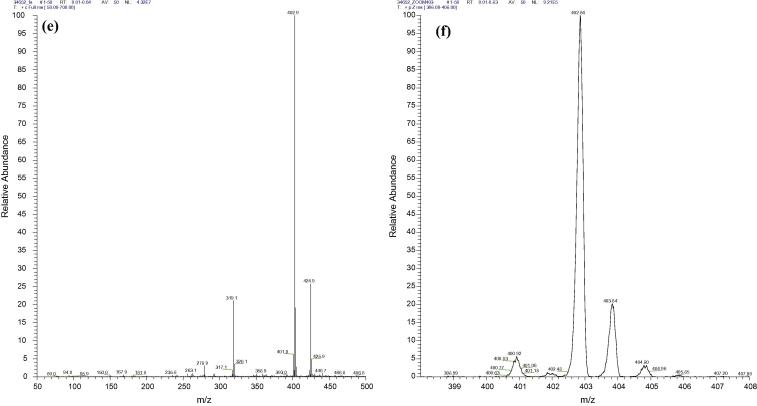
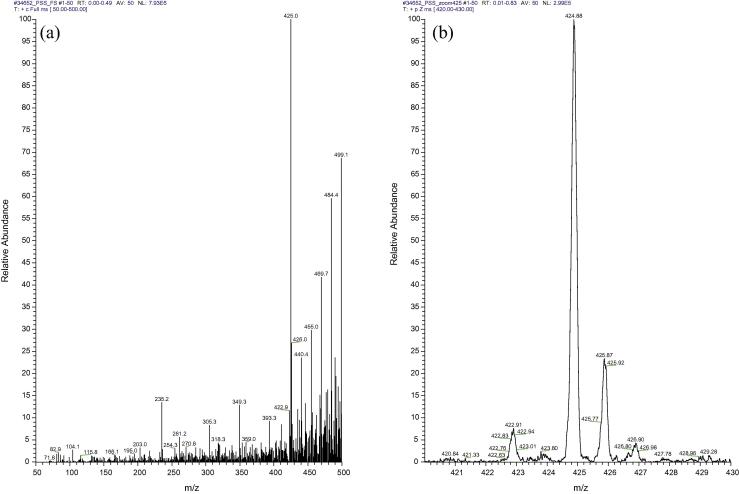
The MS analysis of samples from serum supernatants treated with acetone, acetonitrile, chloroform–methanol and proteinase K showed no recovery of any of the investigational compounds. However, one out of three investigational compounds from the methanol treated serum AAHL18 was detected by MS (Fig. 4). The compound was detected mostly as a sodiated adduct (M + Na) at m/z 425 (also seen in Fig. 1 e) with a minor amount of the non-sodiated at m/z 403.
Figure 4.
Spectrum of the AAHL18 compound recovered from the supernatant of methanol treated serum. A mass spectrum of AAHL 18 compound recovered from spiked serum (a) and its zoomed format (b). The recovery rate is fairly low as compared to positive control (Fig. 1e) with a number of other high peaks present in the spectrum. While the expected peak of 402 is almost missing, a high peak 425.0 represents the sodiated form of AAHL 18 compound.
The poor recovery of the other two compounds might be due to a range of different reasons. The most likely biochemistry related reasons that might provide answers to this are the instability of the investigational compounds (insentience reaction) and the possibility of the presence of reactive serum components remaining in the supernatant after PP, both of which require further investigation.
3.4. Determination of investigational compounds disappearance
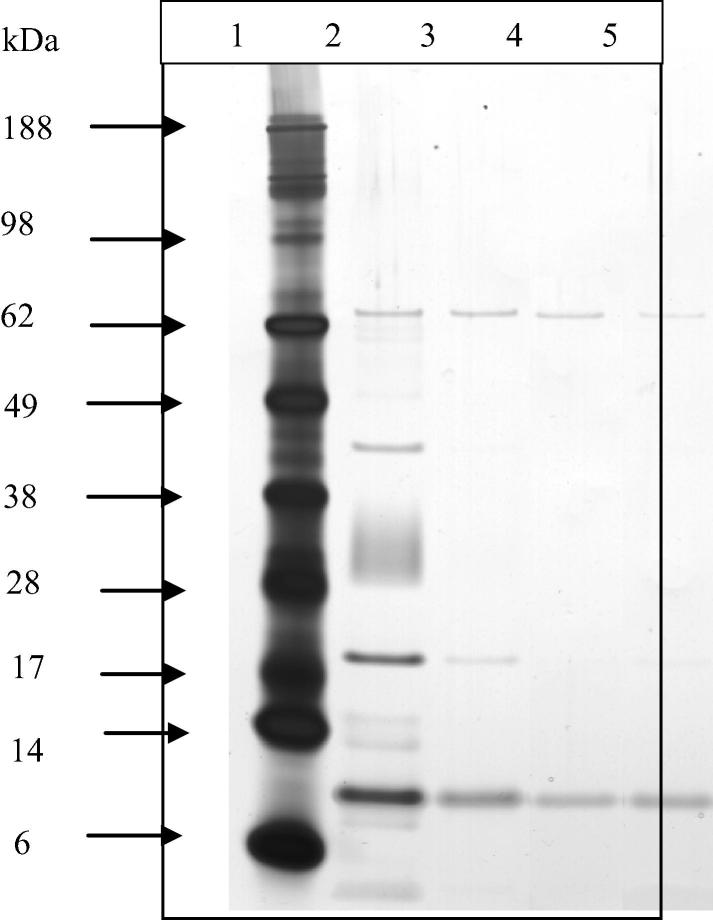
Potential reasons for the poor recovery of the investigational compounds from serum were investigated. One possibility that could explain the low recovery rate of the compounds is the stability of these compounds, which was considered unlikely based on the given data that their chemical structures appear to be stable, these compounds were prepared almost a decade ago and their chemical compositions were rechecked and confirmed repeatedly (data no shown). The second possibility may be the presence of reactive components in serum remaining after methanol-based PP. Dried supernatants from methanol extracted serum samples were reconstituted in different volumes of MES buffer, analysed by electrophoresis and then visualised by silver nitrate staining (Fig. 5). Unexpectedly, the samples were shown to contain a number of unknown proteins, which might, as speculated, have reacted with or modified the compounds and prevented their recovery.
Figure 5.
SDS–PAGE analysis of unprecipitated proteins from methanol treated serum. Electrophoretic separation of proteins derived from supernatant of methanol protein precipitated rabbit serum. Lanes: (1) molecular weight markers lanes 2, 3, 4 and 5 dried supernatants reconstituted in 20, 40, 80 and 100 μl running buffer, respectively. Total volumes of 20 μl per lane of each sample were loaded (15.6 μl of reconstituted samples plus loading buffers making a final volume of 20 μl). Gels were stained with silver nitrate.
3.5. Identification of the proteins from rabbit serum by ‘in-gel’ protein alkylation and digestion
To determine the identity of the unknown proteins, four lanes from each of the upper and lower bands from the Coomassie blue stained gel were excised and treated as above and in-gel protease digested with trypsin. Generally, the Coomassie blue stain is less sensitive than the silver stain; thus, only the upper bands and lower bands (62 and 12 kDa, respectively) were stained by silver nitrate stain (Fig. 5). Hence, only these bands were sent for LC–MS/MS analysis.
Analysis of the raw LC–MS/MS data for the trypsin-digested unknown upper band (62 kDa) searched against the NCBI non-redundant protein database revealed a match to rabbit serum albumin. Thirteen peptides were identified that met the cross-correlation search criteria (see in bold peptides in Fig. 6) and these peptides represented 31% coverage of the rabbit serum albumin sequence. No identifications were obtained for the unknown trypsin digested lower band (∼12 kDa) (see Fig. 7).
Figure 6.
Sequence of rabbit serum albumin. Enbolded sequences represent peptides identified by MS analysis. The results were compared to that of NCBI non-redundant protein databases, of which thirteen peptides from the ∼62 kDa protein matched rabbit serum albumin.
Figure 7.
Mass spectrometry analysis of unprecipitated protein. Mass spectrometry analysis of the ∼62 kDa (a) and ∼12 kDa (b) proteins from methanol precipitated serum. Bands were cut from the gel and then undergone in gel protein alkylation and digestion. Digested proteins were then analysed by LC/MS/MS.
4. Discussion
Rapidity and reliability of the high throughput bioanalysis of drug candidates in plasma samples are essential for pharmacokinetics, pharmacodynamic and toxicokinetic studies (Bouzas et al., 2009, Ma et al., 2008). Mass spectrometry analysis has become the technique of choice for analysis (Grueiro Noche et al., 2013, Leigh et al., 1998) and it is the most widely used bioanalytical method in the drug discovery arena. The selected method is anticipated to be used to analyse the investigational compounds from serum samples. Therefore, due to the complexity of the matrix, in most cases an extraction step for sample clean-up and pre-concentration, such as protein precipitation, is required before analysis in order to achieve the required sensitivity (Moreno-Bondi et al., 2009). The importance of sample preparation for bioanalytical methods cannot be over emphasised. The sample preparation step before the MS analysis is intended to facilitate the determination of components of the drug candidate that involve pharmacokinetics and metabolic stability (Huang et al., 2013, Lee, 2002).
The current study describes the initial stage of pharmacokinetic analysis, namely analytical method validation using three investigational compounds (AAHL 13, AAHL 18 and AAHL 42). The analytical method for drug detection is a significant determinant factor in the conduct of any animal study. The primary objective of pharmacokinetic study is to determine the fate of an investigational compound following its administration to an experimental animal. This can only be achieved by the use of reliable analytical methods that can provide reliable and interpretable results. It is deemed unacceptable to conduct animal experimentation without the use of reliable and sensitive analytical methods. The objective of this study was to validate a sample preparation method for mass spectrometry analysis for pharmacokinetic studies. Accordingly, the precipitation abilities of five different protein extraction methods were compared using electrophoresis analysis. Plasma sample preparation is a key consideration in detection system reliability (Li et al., 2012, Ma et al., 2008). The comparison between the protein extraction abilities of each of the different methods showed significant differences among the tested methods with the methanol precipitation method being shown to have precipitated most of the serum proteins (Fig. 3a and b). The solubility of investigational compounds is an important factor in method selection, and in this study the investigational compounds are methanol soluble; hence, methanol was selected as the most suitable serum precipitation method. Surprisingly, an extremely low recovery rate of the investigational compounds was observed in the methanol extracts. Following the failure to detect the investigational compounds from serum samples, other methods were then separately used to investigate whether the compound loss was methanol related. At this stage, the magnitude of the differences between the serum precipitation abilities observed earlier appeared to be unimportant. The detection rate of the investigational compounds using the other extraction methods remained low, suggesting that the inability of detecting the investigational compounds in serum samples might not be related to sample preparation methods used. In order to test this theory, supernatants from methanol precipitated serum samples were spiked with the investigational compounds. The MS analysis of the spiked supernatants only showed a low detection rate of one of the three tested compounds (AAHL 18) (Fig. 4), which was significantly below the detection limit. The low detection rate suggests the presence of serum component(s) in the supernatant, which might be interfering with the investigational compounds. Interestingly, Coomassie blue and silver stain analysis of the supernatant from methanol precipitated serum, clearly showed two protein bands (Fig. 5). The LC–MS/MS analysis of these bands revealed that the upper band (67 kDa) is albumin, but the lower band (12 kDa) did not match any of the databases. It is therefore, possible that the detected proteins might have interfered with the investigational compounds. For instance, albumin is the most abundant protein in blood plasma (Zammataro et al., 2011, Olsen et al., 2004) and has a high drug binding affinity (Wang et al., 2012, Sjoholm et al., 1979). Other unprecipitated proteins could have also affected the compounds. Theoretically, supernatant from precipitated serum samples is protein free, but in actual fact, at least 10% of serum proteins, mostly less than 20 kDa remain unprecipitated (Alpert and Shukla, 2003). The possibility of serum protein interference with the investigational compounds could potentially be confirmed by the use of rabbit serum dialysis; however, such confirmation would not have made a substantial contribution to their recovery by MS.
The inability to detect the compounds from serum samples might be due to multiple factors, one of which is the interference of serum components. It is possible that these components degraded or instantaneously adsorbed the spiked compounds. It might also be possible that the investigational compounds were precipitated with serum proteins. While the precipitates were not analysed, the failure to detect the compounds after spiking the supernatants from methanol extracted serum, which supposedly does not contain any proteins, ruled out the co-precipitation possibility. This has also ruled out the possibility of instant metabolism of the compounds by serum components. Despite the fact that these compounds were indicated to be relatively chemically stable, their stability in serum was not determined and thus, compound instability in serum might well be a possible factor that contributed to the low recovery rate. Of note, the compounds were shown to be stable in methanol both at room temperature and at 4 °C where they were stored for months. Thus, methanol would not be considered as a possible factor for the low recovery. The low detection of these compounds from serum could perhaps be a result of a combination of reasons that lead to small residual quantities that are not detectable by MS. However, the exact mechanism of how these compounds were lost is still unclear and unless determined, the possibilities would merely be speculations.
Sample preparation is an important part of MS for serum sample analyses. There seem to be significant differences between the protein precipitation ability of the five tested methods, with methanol extraction showed to have the highest precipitation activity amongst all. The inability of completely precipitating all serum proteins warrants further investigation into possible method modification to possibly enhance protein precipitation activity.
Acknowledgements
M.A. is supported by National Health and Medical Research Council of Australia (Peter Doherty Biomedical Fellowship #GNT1037092). The author would like to thank Mr. Brian Shield for his valuable assistant with mass spectrometry.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alpert, A.J., Shukla A.K., 2003. Precipitation of large, high-abundance proteins from serum with organic solvents. The Association of Biomolecular Resource Facilities (ABRF). No. P111-W, Denver, USA.
- Bouzas N.F., Dresen S., Munz B., Weinmann W. Determination of basic drugs of abuse in human serum by online extraction and LC-MS/MS. Anal. Bioanal. Chem. 2009;395(8):2499–2507. doi: 10.1007/s00216-009-3036-x. [DOI] [PubMed] [Google Scholar]
- Cai K., Miller J.L., Stenland C.J., Gilligan K.J., Hartwell R.C., Terry J.C., Evans-Storms R.B., Rubenstein R., Petteway S.R., Jr., Lee D.C. Solvent-dependent precipitation of prion protein. Biochim. Biophys. Acta. 2002;1597(1):28–35. doi: 10.1016/s0167-4838(02)00282-0. [DOI] [PubMed] [Google Scholar]
- Grueiro Noche G., Fernández Laespada M.E., Pérez Pavón J.L., Moreno Cordero B., Muniategui Lorenzo S. Determination of chlorobenzenes in water samples based on fully automated microextraction by packed sorbent coupled with programmed temperature vaporization-gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2013 doi: 10.1007/s00216-013-7112-x. June 19. [DOI] [PubMed] [Google Scholar]
- Henry M., Kowalczyk M., Maldini M., Piacente S., Stochmal A., Oleszek W. Saponin inventory from Argania spinosa kernel cakes by liquid chromatography and mass spectrometry. Phytochem. Anal. 2013 doi: 10.1002/pca.2440. June 18. [DOI] [PubMed] [Google Scholar]
- Huang X., Chen L., Yuan D. Development of monolith-based stir bar sorptive extraction and liquid chromatography tandem mass spectrometry method for sensitive determination of ten sulfonamides in pork and chicken samples. Anal. Bioanal. Chem. 2013 doi: 10.1007/s00216-013-7124-6. June 19. [DOI] [PubMed] [Google Scholar]
- Lawson A. In: Mass Spectrometry. Lawson A.M., editor. Walter de Gruyter; 1989. pp. 435–450. [Google Scholar]
- Lee M.S. John Wiley & Sons, Inc.; New York: 2002. LC/MS Application in Drug Development. [Google Scholar]
- Leigh G.J., Favre H.A., Metanomski W.V. Blackwell Science; 1998. Principles of Chemical Nomenclature: A Guide to IUPAC Recommendations. pp. 50–71, ISBN 0-86542-6856. [Google Scholar]
- Li H., Wen X.S., Di W. A simple LC-MS/MS method for determination of magnolol in rat blood and its application in a pharmacokinetic study. Arzneimittelforschung. 2012;62(2):83–87. doi: 10.1055/s-0031-1295485. Epub 2012 February 16. [DOI] [PubMed] [Google Scholar]
- Ma J., Shi J., Le H., Cho R., Huang J.C., Miao S., Wong B.K. A fully automated plasma protein precipitation sample preparation method for LC-MS/MS bioanalysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008;862(1–2):219–226. doi: 10.1016/j.jchromb.2007.12.012. [DOI] [PubMed] [Google Scholar]
- McNaught A.D., Wilkinson A. Compendium of Chemical Terminology. second ed. Blackwell Science; 1997. pp. 1307–1375. (The Gold Book). ISBN: 0-86542-6848. [Google Scholar]
- Moreno-Bondi M.C., Marazuela M.D., Herranz S., Rodriguez E. An overview of sample preparation procedures for LC-MS multiclass antibiotic determination in environmental and food samples. Anal. Bioanal. Chem. 2009;395(4):921–946. doi: 10.1007/s00216-009-2920-8. [DOI] [PubMed] [Google Scholar]
- Olsen H., Andersen A., Nordbo A., Kongsgaard U.E., Bormer O.P. Pharmaceutical-grade albumin: impaired drug-binding capacity in vitro. BMC Clin. Pharmacol. 2004;4:4. doi: 10.1186/1472-6904-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvadurai M., Meyyanathan S.N. Determination of deflazacort in human plasma by liquid chromatography-mass spectrometry after liquid-liquid extraction and its application in human pharmacokinetics studies. Pharm. Methods. 2011;2(2):106–111. doi: 10.4103/2229-4708.84450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoholm I., Ekman B., Kober A., Ljungstedt-Pahlman I., Seiving B., Sjodin T. Binding of drugs to human serum albumin: XI. The specificity of three binding sites as studied with albumin immobilized in microparticles. Mol. Pharmacol. 1979;16(3):767–777. [PubMed] [Google Scholar]
- Wang D., Li F., Li P., Zhang J., Liu L., Xu P., Zhou L., Liu X. Validated LC-MS/MS assay for the quantitative determination of clematichinenoside AR in rat plasma and its application to a pharmacokinetic study. Biomed. Chromatogr. 2012;26(10):1282–1285. doi: 10.1002/bmc.2691. Epub 2012 February 16. [DOI] [PubMed] [Google Scholar]
- Xu X., Lan J., Korfmacher W.A. Rapid LC/MS/MS method development for drug discovery. Anal. Chem. 2005;77(19):389A–394A. [PubMed] [Google Scholar]
- Zammataro A., Civiale C., Saletti R., Foti S. Development and validation of a liquid chromatography/electrospray ionization tandem mass spectrometry method for the quantification of latanoprost free acid in rabbit aqueous humor and ciliary body. J. Mass Spectrom. 2011;46(11):1168–1174. doi: 10.1002/jms.2004. [DOI] [PubMed] [Google Scholar]