Abstract
Cannabinoids are involved in the regulation of neural stem cell biology and their receptors are expressed in the neurogenic niches of adult rodents. In the spinal cord of rats and mice, neural stem cells can be found in the ependymal region, surrounding the central canal, but there is evidence that this region is largely different in adult humans: lacks a patent canal and presents perivascular pseudorosettes, typically found in low grade ependymomas. Using Laser Capture Microdissection, Taqman gene expression assays and immunohistochemistry, we have studied the expression of endocannabinoid system components (receptors and enzymes) at the human spinal cord ependymal region. We observe that ependymal region is enriched in CB1 cannabinoid receptor, due to high CB1 expression in GFAP+ astrocytic domains. However, in human spinal cord levels that retain central canal patency we found ependymal cells with high CB1 expression, equivalent to the CB1HIGH cell subpopulation described in rodents. Our results support the existence of ependymal CB1HIGH cells across species, and may encourage further studies on this subpopulation, although only in cases when central canal is patent. In the adult human ependyma, which usually shows central canal absence, CB1 may play a different role by modulating astrocyte functions.
The Endocannabinoid System (ECBS) is formed by lipid ligands (endocannabinoids), the enzymatic machinery for their synthesis and degradation and their specific G-protein coupled CB1 and CB2 receptors. The most important endocannabinoids are 2-arachydonoylglycerol (2-AG) and anandamide (AEA)1. These compounds are involved in the control of neural stem cell biology2, and many of their effects are mediated by the cannabinoid receptor CB1. CB1 receptor is expressed in all neurogenic niches in rodents, including the ependymal region of the spinal cord (reviewed in3). In this region, that holds neural stem cell potential4,5, a subpopulation of cells expresses high levels of CB1 receptor (CB1HIGH cells), and proliferate after lesion or during postnatal development in rats6. However, the ependymal region of the adult human spinal cord is strikingly different from that of rodents and other primates: although it contains ependymal cells, lacks a patent central canal and shows perivascular pseudorosettes7,8,9. This means that observations made in rodents should be validated in human tissue to understand the composition and the regenerative potential of this niche. Here we have explored the presence of the ECBS and searched for an equivalent of rat and mice CB1HIGH cells in adult human spinal cord.
Results and Discussion
We found that human ependymal region consistently expresses CB1 cannabinoid receptor (CNR1 gene; Table 1). CB1 receptor could be the target of locally produced 2-AG, since we also found expression (although non enrichment) of enzymes related with 2-AG synthesis and degradation: diacylglycerol lipase α (DAGLA), diacylglycerol lipase β (DAGLB), monoacylglycerol lipase (MGLL) and abhydrolase domain-containing proteins – 6 (ABHD6) and –12 (ABHD12). On the contrary, we could not find consistent expression of enzymes related with direct anandamide synthesis or degradation (NAPE-phospholipase D and fatty acid amide hydrolase, respectively). However, it should be noted that alternative enzymatic routes have been described for AEA, involving glycerophosphodiester phosphodiesterase and N-acylethalnolamine-hydrolyzing acid amidase that have been not tested here2. We also did not find expression of CB2 cannabinoid receptor or the related GPR55 receptor. In previous works, we observed expression (but not enrichment) of PPAR-α, another cannabinoid-related receptor1, in human ependymal region9.
Table 1. Relative expression of endocannabinoid system related genes in the adult human ependymal region compared with ventral horn.
| GENE | Assay | ΔCt Ependymal Region vs18S (average) | ΔCt Ventral Horn vs18S (average) | RQ vs Ventral Horn | p |
|---|---|---|---|---|---|
| CNR1 | Hs01038522_s1 | 8,379754298 | 13,57232086 | 57,27 | 0,012* |
| CNR2 | Hs00361490_m1 | – | – | ND# | – |
| GPR55 | Hs00271662_s1 | 17,13233 | 18,721182 | NCD# | – |
| DAGLA | Hs00391374_ml | 11,7639845 | 13,04953063 | 2,34 | 0,8 |
| DAGLB | Hs00373700_ml | 11,1895161 | 8,199532 | 14,6 | 0,51 |
| MGLL | Hs00200752_ml | 10,5709992 | 9,519471 | 0,25 | 0,44 |
| ABHD6 | Hs00977889_m1 | 12,6129394 | 14,365685 | 1,48 | 0,75 |
| ABHD12 | Hs01018047_m1 | 12,6756672 | 12,6676875 | 4,7 | 0,095 |
| NAPEPLD | Hs00419593_m1 | 13,0519701 | 6,908164 | NCD# | – |
| FAAH | Hs01038660_m1 | 20,2593 | 19,962125 | NCD# | – |
*Significantly enriched in Ependymal region vs Ventral Horn (Student T-test).
#ND: Non detected in the Ependymal region of any individual; NCD: Non consistently detected (detected in less than 3 of the 4 individuals).
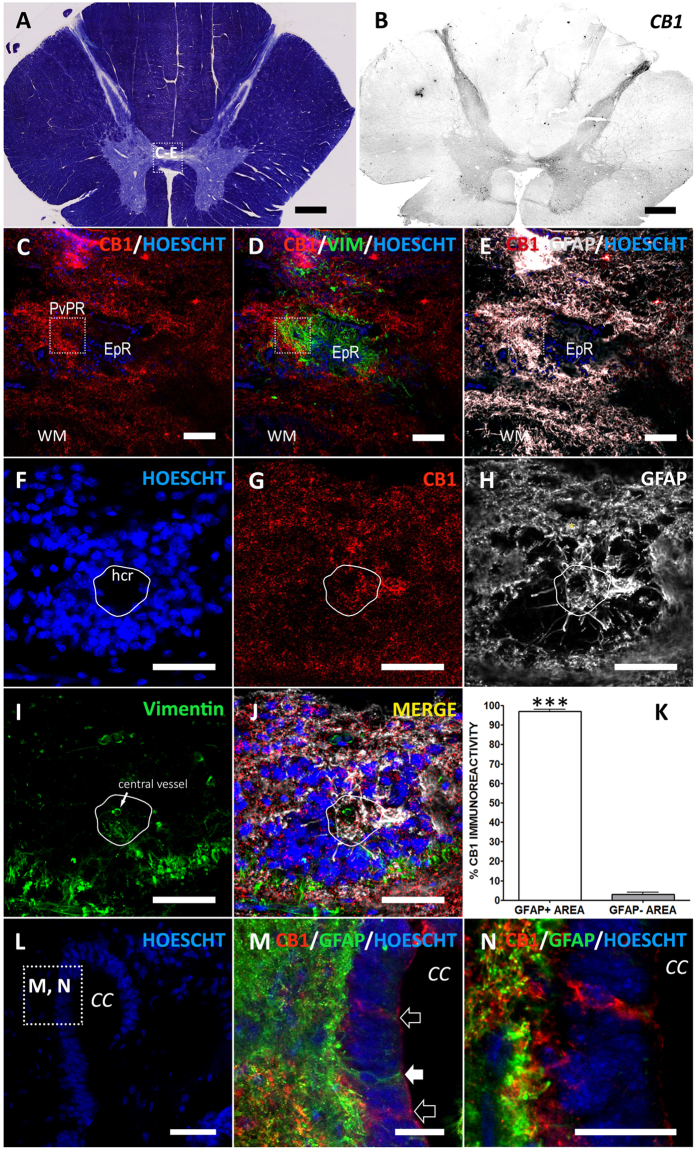
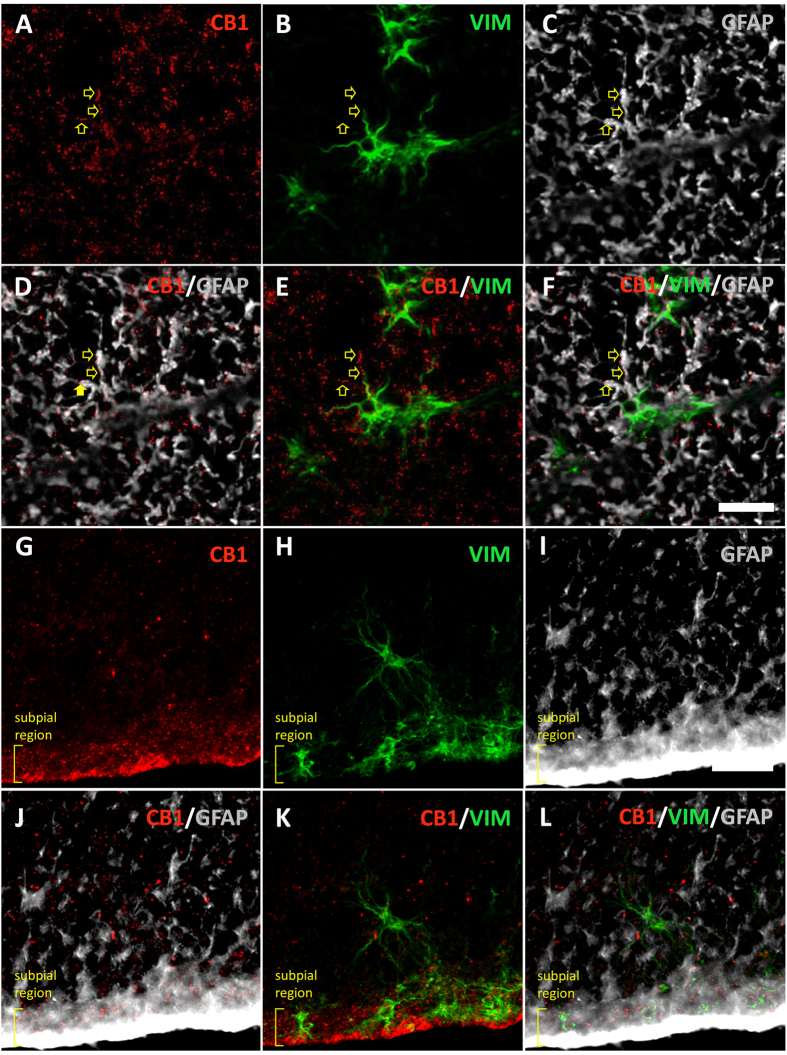
When compared with ventral horn, only CNR1 (CB1 receptor) was significantly enriched at the ependymal region (Table 1). Accordingly, we found a strong CB1 immunoreactivity in central gray matter by immunohistochemistry (Fig. 1B–J). But CB1 enrichment in adult humans ependyma is not equivalent to that found in rodents: In humans, CB1 is expressed by astrocytes, forming part of the gliosis that accompanies central canal closure (Fig. 1C–E) and in the GFAP+ hypocellular ribbon of perivascular pseudorosettes (Fig. 1F–K)9,10. CB1 receptor is also expressed in astrocytes from other spinal cord areas (Fig. 2), and its intensity is apparently related to high GFAP expression. Accordingly, a strong CB1 expression has been reported in reactive astrocytes of human pathologies like spinocerebellar ataxia11 or temporal lobe epilepsia12. The role of astrocytic CB1 could be multiple: protection13, metabolism increase14, control of inflammation15,16,17, inhibition of glutamate transporters18 or release of neurotransmitters such as glutamate19, ATP and D-serine20.
Figure 1. CB1 cannabinoid receptor in adult human spinal cord.
(A) Myelin staining of a representative thoracic spinal cord section. Square depicts the area shown in (C–E). (B) In low magnification a strong CB1 immunoreactivity can be found in dorsal horn, lamina X and ventral gray matter. (C–E) Higher magnification of central gray shows CB1 expression in GFAP+ areas surrounding the Vimentin+ cells at the ependymary region (EpR). Square highlights the location of a perivascular pseudorosette (PvPR). (F–J) Strong CB1 immunoreactivity is found at the GFAP+ domains around and inside perivascular pseudorosettes, including the GFAP ribbon at the hypocellular region of the pseudorosette (hcr, outlined in white). In PvPRs cells are arranged around a central vessel ((I) arrow). (K) Quantification supports qualitative observations: CB1 immunoreactivity is significantly accumulated in GFAP+ areas. (L) Detail of the dorsal aspect of an ependymal region with a patent central canal. Square depicts location of images M and N. (M) CB1HIGH ependymal cells (empty arrows) can be found intermingled with ependymocytes lining the central canal. GFAP+ cells contacting central canal lumen (arrowhead) are CB1−. (N) Detail of M, showing a CB1HIGH cell with a dim staining of GFAP in the apical region. ***T student, p < 0.001; CC, Central Canal; EpR, ependymal region; hcr, hypocellular region; PvPR, perivascular pseudorosette; WM, white matter. Scale bars: A,B = 1 mm; C-E: 100 μm; F-L = 50 μm; M,N = 25 μm.
Figure 2. CB1 immunoreactivity can be found in astrocytes of other regions in the spinal cord.
(A–F) CB1 is expressed in the processes of GFAP+ and Vim+ astrocytes (arrows) at the dorsolateral white matter. (G–I) A strong CB1 expression can be found in astrocytic processes at the subpial region. Scale bars: 25 μm.
Interestingly, we obtained some sections from adult individuals in which parts of the central canal were patent. In those sections, we found ependymal cells with high expression of CB1 receptors lining the canal (Fig. 1L–N), resembling those CB1HIGH cells described for rats and mice6. These cells were mostly GFAP-, except for a very dim expression at the apical pole (Fig. 1N), in contrast with strongly GFAP+ cells embeded in the ependymal layer (Fig. 1M).
Our results support the existence of ependymal CB1HIGH cells across species, and may encourage further studies on this subpopulation, although only in cases when there is central canal patency, i.e. childhood and upper cervical levels8,9. But in the majority of adult ependyma, CB1 is enriched in astrocyte domains, and cannabinoids may play a different role, that still might be relevant, in terms of homeostasis maintenance and response to injury.
Methods
Human tissue was obtained from the HUFA BioBank (Alcorcon, Spain) and the HUB-ICO-IDIBELL BioBank (Hospitalet de Llobregat, Spain). Samples were obtained from donor individuals deceased without clinical or histopathological involvement of the spinal cord (Table 2). Donation always included a written informed consent from donors while alive or from their families after death. Data from donors and handling of samples were carried out after approval by the Clinical Research Ethical Committee (CEIC) in Toledo (Spain), in accordance with the Spanish law and International Guidelines (LOPD 15/1999; RD 1720/2007; Helsinki declaration, 2008).
Table 2. Postmortem Spinal Cord tissue samples used for immunohistochemistry (IHC) and/or Laser Capture Microdissection (LCMD).
| Autopsy number | Cause of Death | Gender | Age | Coded as | Postmortem delay | Used for |
|---|---|---|---|---|---|---|
| BC01015 | Unknown. No significant neuropathological alterations in the spinal cord | Male | 60 | Control | Unknown | IHC |
| BC01684 | Acute Hypoxia-ischemia | Male | 27 | Control | Unknown | IHC |
| A07/00044 | Cardiopulmonary arrest | Male | 39 | Control | 3 h 30 min | IHC |
| A07/00067 | Refractary septic shock and cardiac arrest. Ischemic cardiopathy | Male | 47 | Control | 4 h 55 min | IHC |
| A10/00017 | Hepatic metastasis. Probable pancreatic neoplasia | Male | 52 | Control | 03 h | IHC |
| A07/00041 | Multiorganic failure. Gastric tumour | Male | 43 | Control | 5 h 55 min | IHC, LCMD |
| A07/00084 | Refractary septic shock | Male | 46 | Control | 15 h | IHC, LCMD |
| A10/00026 | Multiorganic failure. Severe broncopathy | Male | 61 | Control | 3 h 55 min | LCMD |
| A05/00134 | Carcinoma and metastasis. With brain but not spinal cord metastasis. | Female | 32 | Control | 11 h 45 m | LCMD |
| A11/00052 | Endocarditis. No neuropathological features | Male | 76 | Control | 06 h 30 m | LCMD |
| A12/00046 | Cardiac arrest. No neuropathological features | Female | 75 | Control | 06 h10 m | LCMD |
Gene expression in human ependymal region
All procedures were performed according to our published protocol9. Briefly, fresh frozen spinal cord blocks were cut in 25 μm thick sections and the ependymal region microdissected with a Laser Dissection Microscope. RNA extraction, amplification and reverse transcription were performed as previously described9. We also collected microdissected portions of ventral horn, which we used as a non-neurogenic, non-ependymal reference for gene expression.
Gene expression was studied with Taqman PCR Assays (Life Technologies, Madrid, Spain) either incorporated in Taqman Low Density Arrays (DAGLA, #Hs00391374_m1; DAGLB, #Hs00373700_m1; MGLL, #Hs00200752_m1; NAPEPLD, #Hs00419593_m1) or in individual assays (ABHD6, #Hs00977889_m1; ABHD12, #Hs01018047_m1; CNR1, #Hs01038522_s1; CNR2, #Hs00361490_m1; FAAH, #Hs01038660_m1; GPR55, #Hs00271662_s1). We used 18S gene as an endogenous control (18S, #Hs03003631_g1). For assays incorporated on TLDAs, we added 1.25 ng cDNA/well. For assays performed individually, we added 1.5 ng cDNA/well. Assays were run on an Applied Biosystems® 7900HT Fast Real-Time PCR System. Data were analysed as described9 using automatic detection of Ct, normalized with the endogenous gene (ΔCt vs 18S). Only genes expressed in at least three out of four samples were considered as consistently expressed and included in statistics. Enrichment was defined as higher and statistically significant expression in ependymal region vs ventral horn (Student’s t-test with ΔCts, p < 0.05). To obtain folds of enrichment, we used Relative quantity formula, RQ = 2^−ΔΔCt.
Immunohistochemistry
To improve signal to noise ratio and avoid autofluorescence, we amplified CB1 immunoreactivity using Tyramide Signal Amplification System (TSA Plus Cyanine 3 System #NEL744001KT, Perkin Elmer, USA). Free floating vibratome sections (40 μm) were rinsed on 0.1 M phosphate-buffered saline containing 0.5% bovine serum albumin +0.3% Triton X-100. Endogenous peroxidase inhibition and antigen demasking were performed as described9. Sections were then blocked with TSA Blocking Solution (45′) and incubated for 2 days with primary antibodies diluted in rinse solution +10% Normal Donkey Serum: guinea pig anti-CB1 (1:2000, #CB1-GP-Af530-1, FSI, Japan), rabbit anti-GFAP (1:2000, #Z0334, DAKO, Spain) and mouse anti-Vimentin (1:300, #M0725, DAKO, Spain). Immunoreactivity was visualized by incubating sections with Alexa 488-, Alexa 555- and Alexa 633- secondary antibodies (1:1000, Invitrogen, Spain) or horseradish peroxidase donkey anti-guinea pig antibody (1:300, Jackson Immunoresearch, UK) followed by Tyramide-Cy3 diluted in TSA Amplification Buffer (1:50). Samples were analyzed with a LEICA SP5 confocal microscope. We ruled out the interference of nonspecific staining by omitting primary antibodies. We set the confocal parameters at a point where no signal was observed in these primary antibody controls and those settings were used for all the image acquisitions (Supplementary Figure 1A–F). Furthermore, as discussed in several reports, there is a variety of antibodies against CB1 receptor, and some of them may show non-specific staining21,22,23. The specificity of CB1 antibody used for this report has been extensively validated by other laboratories and ourselves in previous works6,24,25. We show here an additional validation in the Supplementary Figure 1 by using immunohistochemistry and TSA amplification on wild type (C57BL/6N) and CB1 knockout mice tissue (kindly donated by Dr. Galve-Roperh26). Using restrictive confocal parameters (as we did for humans), we got rid out of autofluorescence, background staining and most of the non-specific staining observed in the knockout mice that, in these conditions, is limited to a dim intracellular neuronal staining, largely different from that observed in the wild type mice (Supplementary Figure 1L-Q). All post-capture image modifications were identically performed for controls, including cropping, noise reduction and minor adjustments to optimize contrast and brightness.
To quantitatively support CB1 enrichment in the astrocytic area, we calculated the fraction of CB1 found in GFAP+ vs GFAP− areas on confocal planes (image size 190 μm × 190 μm) using Fiji (http://pacific.mpi-cbg.de). For this, we outlined GFAP borders using manual Threshold with Otsu Filter and used this ROI on the CB1 image corresponding to the same confocal plane. We measured CB1+ Area inside and outside the selection (GFAP+ and GFAP− areas, respectively) and expressed them as % of total CB1 staining (Fig. 1K). We used Student T-test for statistical comparisons.
Additional Information
How to cite this article: Paniagua-Torija, B. et al. CB1 cannabinoid receptor enrichment in the ependymal region of the adult human spinal cord. Sci. Rep. 5, 17745; doi: 10.1038/srep17745 (2015).
Supplementary Material
Acknowledgments
This work was funded by Wings For Life Foundation (Salzburg, Austria) and Fundacion Mutua Madrileña (Madrid, Spain). Laboratory of Neuroinflammation is also supported by Instituto de Salud Carlos III (Ministry of Economy and Competitiveness of Spain). We are grateful to Concepción Sanchez-Caro for technical help and Javier Mazarío and José Ángel Rodriguez-Alfaro at the Microscopy Facilities at the National Hospital for Paraplegics.
Footnotes
Author Contributions B.P.-T. and D.G.-O. Collection and assembly of data; IF: Provision of study material or patients; A.A.-M., E.M.-H. and D.G.-O. Conception and design, data analysis and interpretation; E.M.-H. and D.G.-O. Financial support; D.G.-O. Manuscript writing. All authors gave their final approval to the manuscript.
References
- Pertwee R. G. et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev 62, 588–631, 10.1124/pr.110.00300462/4/588 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M., Guzman M., Mackie K., Doherty P. & Harkany T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci 15, 786–801, 10.1038/nrn3846nrn3846 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ovejero D. et al. Neuroimmmune interactions of cannabinoids in neurogenesis: focus on interleukin-1beta (IL-1beta) signalling. Biochem Soc Trans 41, 1577–1582, 10.1042/BST20130198BST20130198 (2013). [DOI] [PubMed] [Google Scholar]
- Sabourin J.-C. et al. A Mesenchymal-Like ZEB1+Niche Harbors Dorsal Radial Glial Fibrillary Acidic Protein-Positive Stem Cells in the Spinal Cord. Stem Cells 27, 2722–2733, 10.1002/stem.226 (2009). [DOI] [PubMed] [Google Scholar]
- Weiss S. et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 16, 7599–7609 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ovejero D., Arevalo-Martin A., Paniagua-Torija B., Sierra-Palomares Y. & Molina-Holgado E. A cell population that strongly expresses the CB1 cannabinoid receptor in the ependyma of the rat spinal cord. J Comp Neurol 521, 233–251, 10.1002/cne.23184 (2013). [DOI] [PubMed] [Google Scholar]
- Milhorat T. H., Kotzen R. M. & Anzil A. P. Stenosis of central canal of spinal cord in man: incidence and pathological findings in 232 autopsy cases. J Neurosurg 80, 716–722, 10.3171/jns.1994.80.4.0716 (1994). [DOI] [PubMed] [Google Scholar]
- Yasui K., Hashizume Y., Yoshida M., Kameyama T. & Sobue G. Age-related morphologic changes of the central canal of the human spinal cord. Acta Neuropathol 97, 253–259 (1999). [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D. et al. The ependymal region of the adult human spinal cord differs from other species and shows ependymoma-like features. Brain 138, 1583–1597, 10.1093/brain/awv089awv089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro-Cervello C. et al. The adult macaque spinal cord central canal zone contains proliferative cells and closely resembles the human. J Comp Neurol 522, 1800–1817, 10.1002/cne.23501 (2014). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cueto C. et al. Changes in CB(1) and CB(2) receptors in the post-mortem cerebellum of humans affected by spinocerebellar ataxias. Br J Pharmacol 171, 1472–1489, 10.1111/bph.12283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. D. et al. Astrocytic expression of cannabinoid type 1 receptor in rat and human sclerotic hippocampi. Int J Clin Exp Pathol 7, 2825–2837 (2014). [PMC free article] [PubMed] [Google Scholar]
- Carracedo A. et al. Ceramide sensitizes astrocytes to oxidative stress: protective role of cannabinoids. Biochem J 380, 435–440, 10.1042/BJ20031714BJ20031714 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C., Galve-Roperh I., Rueda D. & Guzman M. Involvement of sphingomyelin hydrolysis and the mitogen-activated protein kinase cascade in the Delta9-tetrahydrocannabinol-induced stimulation of glucose metabolism in primary astrocytes. Mol Pharmacol 54, 834–843 (1998). [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F., Molina-Holgado E. & Guaza C. The endogenous cannabinoid anandamide potentiates interleukin-6 production by astrocytes infected with Theiler’s murine encephalomyelitis virus by a receptor-mediated pathway. FEBS Lett 433, 139–142, S0014-5793(98)00851-5 (1998). [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F., Molina-Holgado E., Guaza C. & Rothwell N. J. Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocyte cultures. J Neurosci Res 67, 829–836, 10.1002/jnr.10165 (2002). [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F. et al. Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J Neurosci 23, 6470–6474, 23/16/6470 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivachar A. C. Cannabinoids inhibit sodium-dependent, high-affinity excitatory amino acid transport in cultured rat cortical astrocytes. Biochem Pharmacol 73, 2004–2011, S0006-2952(07)00185-2 (2007). [DOI] [PubMed] [Google Scholar]
- Navarrete M. & Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126, 10.1016/j.neuron.2010.08.043S0896-6273(10)00686-0 (2010). [DOI] [PubMed] [Google Scholar]
- Rasooli-Nejad S., Palygin O., Lalo U. & Pankratov Y. Cannabinoid receptors contribute to astroglial Ca(2)(+)-signalling and control of synaptic plasticity in the neocortex. Philos Trans R Soc Lond B Biol Sci 369, 20140077, 10.1098/rstb.2014.007720140077 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsey N. L. et al. Specific detection of CB1 receptors; cannabinoid CB1 receptor antibodies are not all created equal! J Neurosci Methods 171, 78–86, 10.1016/j.jneumeth.2008.02.014S0165-0270(08)00114-3 (2008). [DOI] [PubMed] [Google Scholar]
- Morozov Y. M. et al. Antibodies to cannabinoid type 1 receptor co-react with stomatin-like protein 2 in mouse brain mitochondria. Eur J Neurosci 38, 2341–2348, 10.1111/ejn.12237 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert-Chatelain E. et al. Cannabinoid control of brain bioenergetics: Exploring the subcellular localization of the CB1 receptor. Mol Metab 3, 495–504, 10.1016/j.molmet.2014.03.007S2212-8778(14)00074-X (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M. et al. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci 27, 3663–3676, 27/14/3663 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. et al. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci 26, 4740–4751, 26/18/4740 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado T. et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci 26, 1551–1561, 26/5/1551 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.