Abstract
Levetiracetam (LEV) is an established second generation anti-epileptic drug and LEV associated severe cutaneous reactions are rare. Here we report the case of psoriasiform drug eruption in a patient with newly diagnosed epilepsy who had been treated with levetiracetam. To our knowledge this is the first report of a patient with a psoriasiform eruption that appeared after the administration of LEV.
Keywords: Levetiracetam, Epilepsy, Psoriasiform drug eruption
1. Introduction
Levetiracetam (LEV) is an established second generation anti-epileptic drug which is used as monotherapy in patients with new diagnosed partial onset or generalized tonic-clonic (GTC) seizures, adjunctive therapy in patients with refractory partial onset seizures and idiopathic generalized epilepsy with myoclonic or GTC seizures. LEV associated severe cutaneous reactions are rare (Lyseng-Williamson, 2011). Here we report the case of psoriasiform drug eruption in a patient with newly diagnosed epilepsy who had been treated with levetiracetam. To our knowledge this is the first report of a patient with a psoriasiform eruption that appeared after the administration of LEV.
2. Case report
In April 2013, a right handed 35 year old woman presented to our neurology department with generalized tonic-clonic convulsions 2 times a week. Both seizures lasted approximately for 2 min. This was her first seizure and there was no history of febrile seizure, head trauma, cerebrovascular disease, central nervous system infection and family history of epilepsy. Neurological examination findings were normal. Laboratory findings were normal except eosinophilia and brain magnetic resonance imaging examination result was normal. The patient underwent electroencephalography (EEG) monitoring and two episodes of 3 Hz sharp and wave discharges lasting 2–2, 5 s in frontal lobes of both hemispheres were noted.
She was diagnosed with epilepsy and started treatment with LEV 500 mg per day. The dose of LEV was gradually increased to 1000 mg per day. 10 days after antiepileptic therapy, the erythematous skin lesions developed. She had not taken any other medications and she had no personal or family history of psoriasis. Physical examination revealed that erythematous plaques with scales were presented on both knees and elbows. Her scalp, nails and palmar plantar regions were not affected. She had no fever and laboratory findings were normal except eosinophilia. Histological examination of the skin biopsy specimen taken from a lesion on the knee, revealed irregular acanthosis of epidermis, oedema in papillary dermis, dilated capillary congestion nearby basal membrane and perivascular infiltrate composed of mononuclear cells in superficial reticular dermis as shown in figure. The patient was diagnosed with psoriasiform drug eruption due to LEV. So LEV therapy was stopped and carbamazepine 400 mg per day was initiated. The skin eruptions began to disappear within few weeks after discontinuing LEV therapy. No further recurrence of skin lesions and epileptic seizures was observed in her follow up (see Fig. 1)
Figure 1.
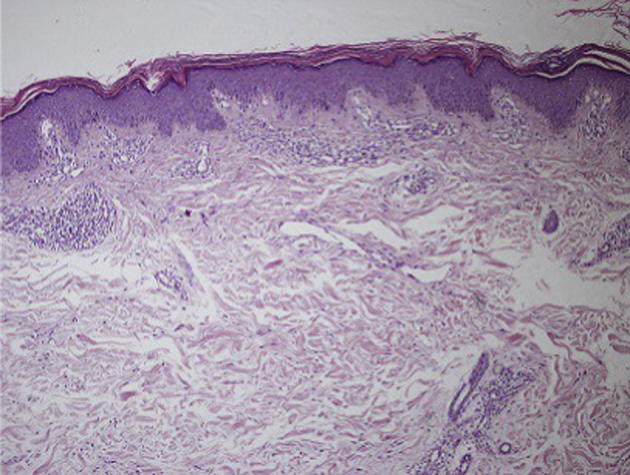
Histological features of the skin biopsy from the knee reveals irregular acanthosis of epidermis, oedema in papillary dermis, dilated capillary congestion nearby basal membrane and perivascular infiltrate composed of mononuclear cells in superficial reticular dermis. (Haematoxylin-eosin, original magnification 100×).
3. Discussion
Psoriasiform drug eruptions simulating psoriasis clinically and/or histologically can be induced by several drugs such as beta blockers, lithium, antimalarial drugs, antibiotics, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, interferons, terbinafine, benzodiazepines (Kim and Del Rosso, 2010, Sehgal et al., 2008). Although the histopathological features are similar to psoriasis, perivascular and interstitial infiltration of eosinophils in the upper dermis is more frequent in psoriasiform drug eruption (Justiniano et al., 2008). Psoriasiform drug eruption is divided into 2 categories. The first category is exacerbation of pre-existing psoriasis and development of psoriatic lesions on uninvolved skin in patients with psoriasis. The second category includes precipitation of psoriasis in patients with no predisposed individuals and family history of psoriasis (Yamamoto et al., 2008).
Skin reaction is a common side effect of antiepileptic drugs (AED) Alvestad et al., 2007, Wang et al., 2010. The most common type of adverse reactions is mild maculopapular rashes which disappeared within few days after discontinuation of the drug (Mockenhaupt et al., 2005, Chadwick et al., 1984). More serious AED-related adverse reactions can also occur as toxic epidermal necrolysis (TEN), Stevens–Johnson syndrome, Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), anticonvulsant hypersensitivity syndrome and angioedema (Mockenhaupt et al., 2005, Alkhotani and McLachlan, 2012, Pereira de Silva, 2011, Newell et al., 2009).
Several factors are associated with the development of skin reactions. Ageing and female gender seem to increase the risk of skin rashes (Blaszczyk et al., 2013). The pathogenesis of cutaneous adverse reactions is related to both metabolic and immunological mechanisms that can be caused by hapten hypothesis of drug hypersensitivity. Reactive metabolites of the drug occur as a result of imbalance between metabolic bio activation and detoxification. These metabolites may bind irreversibly to endogenous proteins and the interaction of T-cell lymphocyte clones and both drug and these drug modified proteins can cause delayed immune responses (Alvestad et al., 2007).
As noted in previous studies, AEDs with aromatic ring structures such as phenytoin and carbamazepine had relatively high incidences of cutaneous adverse reactions (Handoko et al., 2008). Sodium valproate, LEV, vigabatrin and topiramate are rarely associated with these reactions. LEV which is an established second generation antiepileptic drug, has a novel structure and is associated with rapid, complete absorption and high oral bioavailability. Renal clearance is the major way of elimination. Previous studies have reported that the incidence of LEV related adverse events was similar to placebo group. Severe hypersensitivity cutaneous reactions were not associated with LEV (Lyseng-Williamson, 2011).
Here we report a psoriasiform drug eruption related to levetiracetam. To our knowledge, this is the first case report of this type of cutaneous adverse reaction due to levetiracetam. So according to this, we must be prepared to any side effect of well tolerated and safely used antiepileptic drugs.
Source(s) of support
None.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alkhotani A., McLachlan R.S. Levetiracetam induced angioedema in a patient with previous anticonvulsant hypersensitivity reaction to phenytoin and lamotrigine. Seizure. 2012;21:407–408. doi: 10.1016/j.seizure.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Alvestad S., Lydersen S., Brodtkorb E. Rash from antiepileptic drugs: influence by gender, age, and learning disability. Epilepsia. 2007;48(7):1360–1365. doi: 10.1111/j.1528-1167.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- Blaszczyk B., Szpringer M., Czuczwar S.J., Lasoń W. Single centre 20 year survey of antiepileptic drug-induced hypersensitivity reactions. Pharmacol. Rep. 2013;65:399–409. doi: 10.1016/s1734-1140(13)71015-6. [DOI] [PubMed] [Google Scholar]
- Chadwick D., Shaw M.D., Foy P., Rawlins M.D., Turnbull D.M. Serum anticonvulsant concentrations and the risk of drug induced skin eruptions. J. Neurol. Neurosurg. Psychiatry. 1984;47(6):642–644. doi: 10.1136/jnnp.47.6.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handoko K.B., van Puijenbroek E.P., Bijl A.H., Hermens W.A., Zwart-van Rijkom J.E., Hekster Y.A., Egberts T.C. Influence of chemical structure on hypersensitivity reactions induced by antiepileptic drugs: the role of the aromatic ring. Drug Saf. 2008;31:695–702. doi: 10.2165/00002018-200831080-00006. [DOI] [PubMed] [Google Scholar]
- Justiniano H., Berlingeri-Ramos A.C., Sánchez J.L. Pattern analysis of drug-induced skin diseases. Am. J. Dermatopathol. 2008;30:352–369. doi: 10.1097/DAD.0b013e3181722ef4. [DOI] [PubMed] [Google Scholar]
- Kim G.K., Del Rosso J.Q. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J. Clin. Aesthet. Dermatol. 2010;3(1):32–38. [PMC free article] [PubMed] [Google Scholar]
- Lyseng-Williamson K.A. Levetiracetam: a review of its use in epilepsy. Drugs. 2011;71(4):489–514. doi: 10.2165/11204490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Mockenhaupt M., Messenheimer J., Tennis P., Schlingmann J. Risk of Stevens–Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics. Neurology. 2005;64:1134–1138. doi: 10.1212/01.WNL.0000156354.20227.F0. [DOI] [PubMed] [Google Scholar]
- Newell B.D., Moinfar M., Mancini A.J., Nopper A.J. Retrospective analysis of 32 pediatric patients with anticonvulsant hypersensitivity syndrome (ACHSS) Pediatr. Dermatol. 2009;26:536–546. doi: 10.1111/j.1525-1470.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Pereira de Silva N., Piquioni P., Kochen S., Saidon P. Risk factors associated with DRESS syndrome produced by aromatic and non-aromatic antiepileptic drugs. Eur. J. Clin. Pharmacol. 2011;67:463–470. doi: 10.1007/s00228-011-1005-8. [DOI] [PubMed] [Google Scholar]
- Sehgal V., Dogra S., Srivastava G., Aggarwal A.K. Psoriasiform dermatoses. Indian J. Dermatol. Venereol. Leprol. 2008;74:94–99. doi: 10.4103/0378-6323.39688. [DOI] [PubMed] [Google Scholar]
- Wang X.Q., Lang S.Y., Shi X.B., Tian H.J., Wang R.F., Yang F. Cross-reactivity of skin rashes with current antiepileptic drugs in Chinese population. Seizure. 2010;19:562–566. doi: 10.1016/j.seizure.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Ikeda M., Kodama H., Sano S. Transition of psoriasiform drug eruption to psoriasis de novo evidenced by histopathology. J. Dermatol. 2008;35:732–736. doi: 10.1111/j.1346-8138.2008.00558.x. [DOI] [PubMed] [Google Scholar]


