Abstract
The long noncoding (lnc) RNA-Ifng-AS1 plays an essential role in the transcription of the gene encoding IFN-γ by Th1 cells, and its human ortholog, IFNG-AS1, is expressed in human Th1 cells. However, IFNG-AS1 contributing to Th1 cells’ response in Hashimoto’s thyroiditis (HT) patients has not been reported. Twenty-eight HT patients and 20 healthy controls were enrolled in the study. The proportion of circulating Th1 cells and the level of T-bet, IFNG mRNA were increased in HT patients, the expression of IFNG-AS1 was upregulated and positively correlated with the proportion of circulating Th1 cells or T-bet, and IFNG expression, or serum level of anti-thyroglobulin antibody/thyroperoxidase antibody in HT patients. IFNG-AS1 regulated the expression of IFNG at both transcriptional and translational level in human CD4+ T cells. Furthermore, strong positive correlations between the increased transcript level of IFNG-AS1 and the increased transcript level of T-bet or IFNG were revealed in thyroid tissues from HT patients. Our results indicate that enhanced expression of lncRNA-IFNG-AS1 contributes to Th1 cell response in HT patients and may be involved in the pathogenesis of HT.
Hashimoto’s thyroiditis (HT), also named chronic lymphocytic thyroiditis, is a clinical, organ-specific autoimmune disease characterized by lymphocytic infiltration of the thyroid parenchyma, diffusely enlarged thyroid gland and elevated production of autoantibodies, mainly including anti-thyroglobulin antibody (TgAb) and thyroperoxidase antibody (TPOAb)1,2. It is now considered to be the most common autoimmune disease and is often associated with other autoimmune diseases, such as Graves’ disease, systemic lupus erythematosus and rheumatoid arthritis3,4,5. HT is a complex and ever-expanding disease caused by genetic susceptibility, environmental factors and immunopathogenesis6. Accumulated evidence over recent years has suggested that CD4+ T helper cells disorder might be involved in the pathogenesis of HT7,8.
CD4+ T helper cells can be classified into Th1, Th2, Th17 and follicular helper T cells, based on the cytokine production9. The Th1 cell lineage is an inflammatory CD4+ T cells subset that mainly produces interferon gamma (IFN-γ), an inflammatory cytokine that participates protectively against intracellular microbes, delayed type hypersensitivity and autoimmune diseases10,11. The development and differentiation of Th1 cells depends on the activation of JAK/STAT pathway components STAT1 and STAT4 in the presence of interleukin 1212,13. T-bet is the key transcription factor, contributing to the transcription of IFNG in Th1 cells14. Recent studies have shown that Th1 cells might be involved in the development of HT7,15,16. However, our understanding of increased Th1 cells in HT patients remains largely unknown.
Long noncoding RNAs (lncRNAs) are increasingly appreciated as key regulators of genome expression, and only some of their functions have been characterized17. In some well-studied cases, lncRNAs play critical roles in various biological processes and diseases18,19,20. However, only a few lncRNAs, such as lnc-DC, NRON, Gas5, have been described in the regulation of the immune system by regulating particular genes21,22,23. Ifng-AS1 (Ifng antisense RNA 1), also named NeST (nettoie Salmonella pas Theiler’s), or Tmevpg1 (Theiler’s murine encephalomyelitis virus persistence candidate gene 1), a lncRNA was initially identified as a candidate gene for the control of Theiler’s virus persistence24. Ifng-AS1 and its human ortholog are located adjacent to IFN-γ-encoding gene in both mouse and human. Recent studies have described that Tmevpg1 (Ifng-AS1, NeST) is recognized to be a key checkpoint that requires T-bet for active transcription and contributes to Ifng expression in Th1 cells25,26. However, it is not yet known whether IFNG-AS1 regulates Th1 cells response in HT patients. Therefore, we explore the role of IFNG-AS1 in the pathogenesis of HT.
In this study, we investigated whether the expression of IFNG-AS1 is dysregulated in HT patients. We found that the expression of IFNG-AS1 was upregulated, and positively correlated with the proportion of circulating Th1 cells in HT patients. These findings provide new insights in understanding the role of IFNG-AS1 in the pathogenesis of HT.
Materials and Methods
Subjects and samples
Twenty-eight patients with HT, including twenty-three females and five males were enrolled into the study. The main clinical characteristics of these patients are summarized in Table 1. All patients were diagnosed by clinical manifestation and auxiliary examination, including B-ultrasonic and laboratory criteria. The serum concentration of free triiodothyronine (FT3), free thyroxine (FT4), thyroid stimulating hormone (TSH), TgAb, and TPOAb were measured by LDX-800 (BECKMAN COULTER, California, USA), according to the manufacturer’s instructions. Ten HT patients with hypothyroidism had a high level of TSH and low level of FT4, other patients with euthyroid had a normal level of both TSH and FT4. All patients had a positive test for TgAb and TPOAb. Twenty age- and sex-matched healthy subjects were included as controls. All healthy subjects were free of thyroid-specific autoantibodies and had no history of thyroid disease or other autoimmune diseases. The number of peripheral leukocytes was within normal range. Peripheral blood samples were obtained from all patients and healthy controls.
Table 1. Clinical features of the HT patients and healthy controls included in the study.
| HT patients | Healthy controls | Range | P-values | |
|---|---|---|---|---|
| Number | 28 | 20 | ||
| Gender (M/F) | 5/23 | 3/17 | ||
| Age (year) | 42 ± 15 | 42 ± 8 | 0.98 | |
| FT3 (pmol/liter) | 4.87 ± 0.64 | 4.99 ± 0.56 | 3.1–6 | 0.51 |
| FT4 (pmol/liter) | 10.42 ± 2.36 | 10.72 ± 1.36 | 7.86–17.41 | 0.61 |
| TSH (uIU/ml) | 7.26 ± 11.26 | 1.98 ± 0.94 | 0.34–5.6 | <0.05 |
| TgAb (IU/ml) | 162.6 ± 349.9 | 0.3 ± 1.0 | 0–4 | <0.05 |
| TPOAb (IU/ml) | 570.3 ± 883.5 | 0.6 ± 0.5 | 0–9 | <0.01 |
Data correspond to the arithmetic mean ± SD and were compared using unpaired t-tests. M: male; F: female.
Fresh tissue samples from the thyroid gland of ten HT patients were collected from thyroidectomy and stored at −80 °C. Lymphocytic infiltration was detected in thyroid samples. Thyroid tissues from five patients with simple goiter were used as control thyroid samples.
The study conformed to the principles outlined in the Declaration of Helsinki and in accordance with the approved guidelines. Written informed consent was obtained from each participant prior to blood samples collection. All samples were taken in accordance with the regulations and approval of the Affiliated People’s Hospital of Jiangsu University.
Cell isolation and purification
Human peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation over Ficoll-Hypaque solution (Haoyang Biological Technology Co., Tianjin, China) and stored at −80 °C until use for quantitative real-time PCR (qRT-PCR). Human CD4+ T cells were purified from PBMCs by magnetic beads using CD4+ T cell Isolation Kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Human CD4+ T cells were cultured in RPMI-1640 medium (Gibco, California, USA) supplemented with 10% fetal bovine serum (Gibco, California, USA) for transfection.
Thyroid mononuclear cells (TMCs) were obtained from thyroid specimens, which were minced and digested with collagenase II (Sigma-Aldrich, St. Louis, MO) for 1–2 h at 37 °C and then isolated by density-gradient centrifugation over Ficoll-Hypaque solution. Cell viability was found to be more than 95%.
Flow cytometric analysis
Separated PBMCs were resuspended at 1 × 106/ml in RPMI-1640 medium containing 10% fetal bovine serum and stimulated with 50 ng/ml of phorbol myristate acetate (PMA; Sigma-Aldrich, California, USA) and 1 μg/ml of ionomycin (Sigma-Aldrich, California, USA) for 2 hours and then incubated for an additional 4 hours in the presence of 1 μg/ml of brefeldin-A (eBioscience, San Diego, USA) at 37 °C and 5% CO2. After the incubation, the suspended cells were stained with phycoerythrin-cyanin 5 (PE-Cy5) -conjugated anti-human CD3 mAb and fluorescein isothiocyanate (FITC) -conjugated anti-human CD8 mAb (eBioscience, San Diego, USA) against cell surface antigens for thirty minutes at 4 °C in the dark. Cells were then fixed and permeabilized using an intracellular staining kit (Invitrogen, Carlsbad, USA), followed by incubation for 45 minutes at 4 °C in the dark with PE-conjugated anti-human IFN-γ mAb (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The stained cells were analyzed with Accuri C6 (Becton Dickinson, San Jose, USA). To analyze the proportion of Th1 cells, the population of CD3+ CD8- IFN-γ+ cells was defined as Th1 cells.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated from PBMCs with TRIzol reagent (Invitrogen, California, USA) according to the manufacturer’s instructions. The cDNA was synthesized with random primer and ReverTraAca®qPCR RT kit (Toyobo, Osaka, Japan). Quantitative real-time PCR was performed in triplicate using Bio-Rad SYBR green super mix (Bio-Rad, Hercules, USA). Primer sequences were as follows: T-bet, sense, 5′-TTGAGGTGAACGACGGAGAG-3′, antisense, 5′-GGCATTCTGGTAGGCAGTCA-3′; IFNG, sense, 5′-GAGTGTGGAGACCATCAAGGA-3′, antisense, 5′-TGTATTGCTTTGCGTTGGAC-3′; IFNG-AS1, sense, 5′-GCTGATGATGGTGGTGGCAATCT-3′, antisense, 5′-TTAGCAGTTGGTGGGCTTCT-3′. β-actin, sense, 5′-GAGTGTGGAGACCATCAAGGA-3′, antisense, 5′-TGTATTGCTTTGCGTTGGAC-3′. The level of each gene was expressed as the ratio to β-actin transcript level. Data were analyzed with Bio-Rad CFX Manager software.
Small interfering RNA knockdown
Small interfering RNA (siRNA) (Ribobio, Guangzhou, China) was designed against the sequence of IFNG-AS1. Nonspecific scramble siRNA was used as negative control (NC). The purified human CD4+ T cells were transfected with the IFNG-AS1 siRNA or NC at 100 nM dose using the Entranster-R (Engreen Biosystem, Co Ltd, Beijing, China) according to the manufacturers’ instructions for 48 hours in the presence of 0.5 μg/ml functional anti-human CD3 mAb plus 2 μg/ml functional anti-human CD28 mAb (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). IFN-γ+ cells were measured by flow cytometric analysis.
Statistical analysis
Student’s unpaired t test was applied for two comparisons in accordance with the standard t test. Mann-Whitney U test was used to analyze the difference between the two groups. Correlation between variables was determined by Pearson’s correlation coefficient. p value < 0.05 was considerate as significant (*p < 0.05, **p < 0.01, ***p < 0.001). Data were analyzed with GrapPadPrism version 5 software (GraphPad Software, Inc., San Diego, USA).
Results
Increased circulating Th1 cells in HT patients
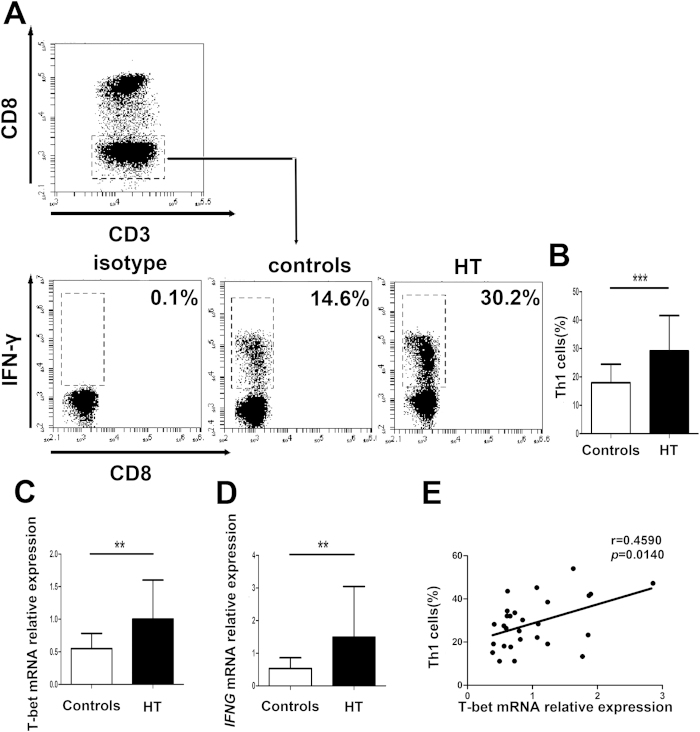
To quantify peripheral Th1 cells in patients with HT, we first gated on CD3+ CD8- cells as CD4+ T cells owing to the downregulated expression of surface membrane CD4 molecule on PBMCs after being stimulated with PMA and ionomycin27 and then identified IFN-γ+ cells to distinguish Th1 cells from activated PBMCs (Fig. 1A). The proportion of peripheral Th1 cells in PBMCs from patients with HT was significantly higher than that in healthy controls (Fig. 1B).
Figure 1. Increased circulating Th1 cells in peripheral blood from HT patients.
Peripheral blood was obtained from 28 HT patients and 20 healthy controls. (A) Representative flow cytometry dot plots of Th1 cells in HT patients and healthy controls are shown. Values in the upper left rectangular region correspond to the proportion of Th1 cells. We used isotype control to determine the positive cells, and all of the values were gated on CD3+ CD8- cells. (B) The percentages of Th1 cells were compared between HT patients and healthy controls. (C) The transcript level of T-bet mRNA in PBMCs from HT patients and healthy controls was determined by qRT-PCR. (D) The transcript level of IFNG mRNA in PBMCs from HT patients and healthy controls was determined by qRT-PCR. (E) The correlation between the transcript level of T-bet mRNA and the percentage of Th1 cells in peripheral blood from 28 HT patients. Each data point represents an individual subject, horizontal lines show the mean. *p < 0.05; **p < 0.01.
Subsequently, we determined the transcript level of T-bet and IFNG in PBMCs from HT patients and healthy controls by qRT-PCR and found that the transcript level of T-bet and IFNG were substantially greater in HT patients than in healthy controls (Fig. 1C,D). Moreover, a positive correlation between the transcript level of T-bet and the proportion of Th1 cells was found in HT patients (r = 0.4590; p = 0.0140) (Fig. 1E).
Positive correlations between elevated transcript level of IFNG-AS1 and increased circulating Th1 cells in HT patients
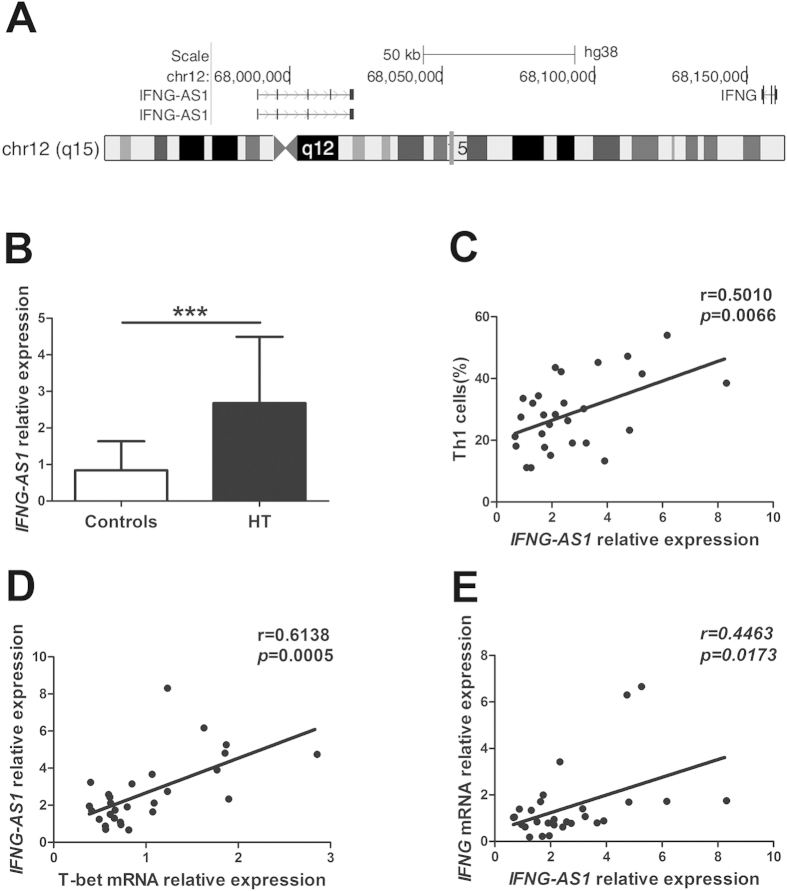
IFNG-AS1 is a long noncoding RNA that is comprised of four exons and located at Chromosome 12q15 on the opposing strand to IFNG (Fig. 2A). Ifng-AS1 (Tmevpg1, NeST) expression contributes to driving Th1 cell-dependent Ifng expression, and its human ortholog, IFNG-AS1 (TMEVPG1, NEST), is selectively expressed in Th1 cells25. To address the possibility that IFNG-AS1 contributes to increased Th1 cells in HT patients, the transcript level of IFNG-AS1 was determined by qRT-PCR. As shown in Fig. 2B, the transcript level of IFNG-AS1 from PBMCs was increased in HT patients compared with that in healthy controls. Moreover, positive correlations were observed between the transcript level of IFNG-AS1 and the percentage of Th1 cells (r = 0.5010; p = 0.0066) (Fig. 2C) or the transcript level of T-bet (r = 0.6138; p = 0.0005) (Fig. 2D) or the transcript level of IFNG (r = 0.4463; p = 0.0173) in HT patients (Fig. 2E). In contrast to the relationship we observed between the transcript level of IFNG-AS1 and the proportion of Th1 cells, there was no correlation between the transcript level of IFNG-AS1 and the proportion of CD8+ IFN-γ+ T cells (Supplemental Fig. 1A), which was significantly greater in HT patients than in healthy controls (Supplemental Fig. 1B).
Figure 2. The correlation between the transcript level of IFNG-AS1 and Th1 cells in HT patients.
(A) Genomic position of IFNG-AS1 on human chromosome. (B) The transcript level of IFNG-AS1 in PBMCs from 28 HT patients and 20 healthy controls was determined by qRT-PCR. (C) The correlation between the transcript level of IFNG-AS1 and the percentage of Th1 cells in 28 HT patients. (D) The correlation between the transcript level of IFNG-AS1 and the transcript level of T-bet mRNA in 28 HT patients. (E) The correlation between the transcript level of IFNG-AS1 and the transcript level of IFNG mRNA in 28 HT patients. Each data point represent an individual subject, horizontal lines show the mean. ***p < 0.001.
Influence of IFNG-AS1 on the transcription of IFNG in human CD4+ T cells
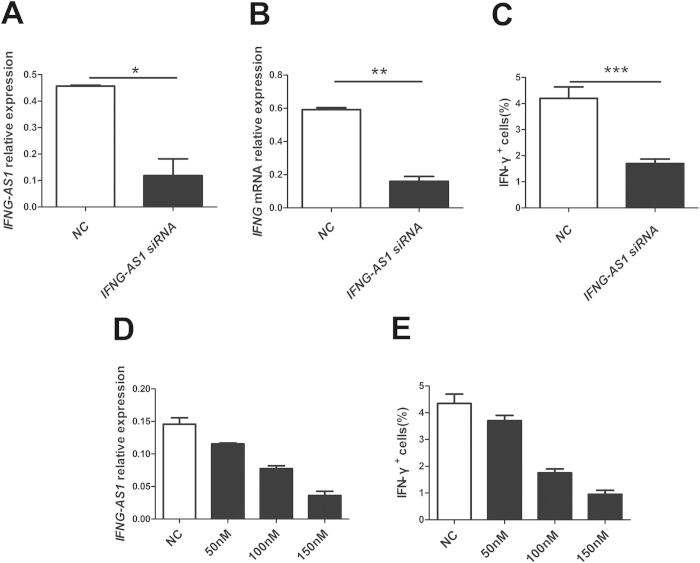
To determine whether IFNG-AS1 affects IFNG transcription from CD4+ T cells, human purified CD4+ T cells were transfected with IFNG-AS1-specific siRNA and negative control. Manipulation of IFNG-AS1-specific siRNA resulted in the reduction of the transcript level of IFNG-AS1 and IFNG compared with that of the negative control (Fig. 3A,B). Down-regulated expression of IFNG-AS1 with siRNA resulted in the reduction of the percentage of IFN-γ+ cells compared with that of the negative control (Fig. 3C). Moreover, IFNG-AS1-specific siRNA suppressed the percentage of IFN-γ+ cells in a dose-dependent manner (Fig. 3D,E). Together, these results indicate that IFNG-AS1 regulates the expression of IFNG in human CD4+ T cells.
Figure 3. Influence of IFNG-AS1 on the transcription of IFNG in vitro.
Human CD4+ T cells were purified from PBMCs by magnetic beads and transfected with IFNG-AS1 -specific siRNA and negative control (100 nM) in the presence of functional anti-human CD3 mAb and anti-human CD28 mAb for 24 h to determine the transcript level of IFNG, or 48 h before restimulation with PMA, ionomycin and brefeldin-A to determine the proportion of IFN-γ+ cells. (A) The transcript level of IFNG-AS1 was determined by qRT-PCR after transfection. (B) The transcript level of IFNG mRNA was determined by qRT-PCR after transfection. (C) The proportion of IFN-γ+ cells was analyzed by flow cytometric analysis. (D) The transcript level of IFNG-AS1 was detected by qRT-PCR after transfection after transfection with IFNG-AS1-specific siRNA in a dose-dependent manner. (E) The proportion of IFN-γ+ cells was analyzed by flow cytometric analysis after transfection with IFNG-AS1-specific siRNA in a dose-dependent manner. The results are indicated as the means ± SD of three independent experiments, horizontal lines show the mean. *p < 0.05; **p < 0.01; ***p < 0.001.
Increased expression of IFNG-AS1 with elevated production of autoantibodies in HT patients
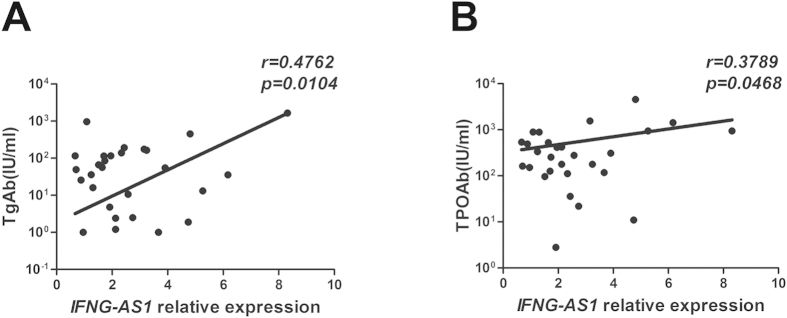
TgAb and TPOAb participate in thyroid destruction in the manner of antibody-dependent cell-mediated cytotoxicity and activation of complement. These autoantibodies are a critical diagnosis index of HT28. Our results indicated that there were positive correlations between the transcript level of IFNG-AS1 and the level of TgAb (r = 0.4762, p = 0.0104) or TPOAb (r = 0.3789, p = 0.0468) (Fig. 4A,B).
Figure 4. The correlation between the transcript level of IFNG-AS1 and serum autoantibodies in HT patients.
The correlation between the transcript level of IFNG-AS1 and serum concentrations of TgAb (A) and TPOAb (B) in 28 HT patients.
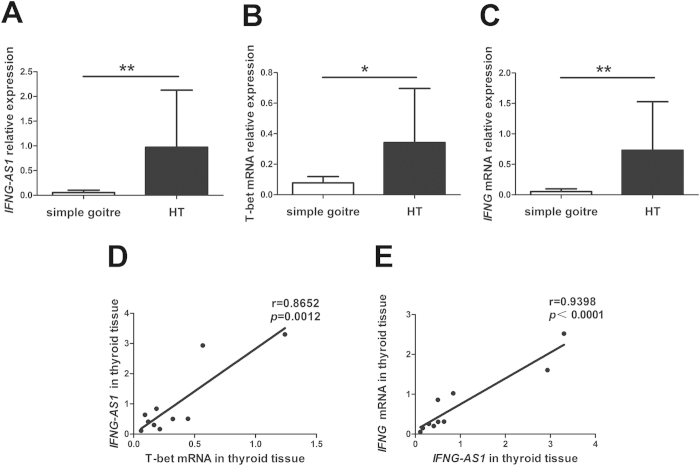
Upregulated expression of IFNG-AS1, T-bet and IFNG mRNA in thyroid tissues from HT patients
HT is an organ-specific autoimmune disease characterized by lymphoid infiltration and thyroid structure destruction. To determine whether IFNG-AS1, T-bet and IFNG mRNA were also expressed in local thyroid tissue, qRT-PCR analysis displayed enhanced expression of IFNG-AS1, T-bet and IFNG mRNA in TMCs from patients with HT compared to those from patients with simple goiter (Fig. 5A–C). Strong positive correlations were observed between the transcript level of IFNG-AS1 and T-bet mRNA (r = 0.8652, p = 0.0012) or IFNG mRNA (r = 0.9398, p < 0.0001) in patients with HT (Fig. 5D,E).
Figure 5. Upregulated expression of IFNG-AS1, T-bet and IFNG mRNA in thyroid tissues.
The transcript level of IFNG-AS1 (A), T-bet mRNA (B) and IFNG mRNA (C) in thyroid glands were determined by qRT-PCR from 10 patients with HT and 5 patients with simple goiter. Correlations between the transcript level of IFNG-AS1 and T-bet mRNA (D) or IFNG mRNA (E) in thyroid glands from 10 HT patients. Each data point represents an individual subject, horizontal lines show the mean. *p < 0.05; **p < 0.01.
Discussion
Lower conservative expression of lncRNAs sequences has prevented most of the sequences from being functionally characterized29. Presently, more and more data suggest lncRNAs are mainly divided into two functional categories that enhance or repress the transcription or translation of protein-coding genes. Previous studies have described T-bet may regulate the expression of Ifng via inducing active transcription of Ifng-AS1 (Tmevpg1, NeST), a Th1-specific lncRNA that contributes to the transcription of Ifng25,26. Transgenic mice models also have demonstrated that Ifng-AS1(NeST) plays a critical role in increasing Theiler’s virus persistence and resisting Salmonella enterica pathogenesis30. However, little is known regarding the role of IFNG-AS1 in the disease pathogenesis of HT. Based on this, we speculated that the expression of IFNG-AS1 might contribute to increased Th1 cells in HT patients. As expected, a higher transcript level of IFNG-AS1 was found in peripheral blood and thyroid tissues from HT patients. Furthermore, positive correlations were found between the transcript level of IFNG-AS1 and the proportion of Th1 cells, as well as transcript level of T-bet or IFNG in HT patients. Although a study has reported that Tmevpg1 promoted the transcription of Ifng from murine Th1 cells in vitro25, the influence of IFNG-AS1 on IFNG transcription in humans is still unknown. To investigate the role of IFNG-AS1 in human CD4+ T cells, we used IFNG-AS1-specific siRNA to knockdown IFNG-AS1 and observed the alteration of IFNG. IFNG-AS1 knockdown resulted in a considerable reduction of IFNG in gene expression and protein expression (IFN-γ). Interestingly, IFNG-AS1-specific siRNA downregulated the proportion of CD4+ IFN-γ+ cells in a dose-dependent manner. In the present study, we provide direct evidence that IFNG-AS1 regulates the transcription of IFNG from human Th1 cells in vitro. However, the epigenetic mechanism of Ifng-AS1 promotes Ifng expression in Th1 cells is poorly understood.
The expression of Tmevpg1 was found in Th1 cells from both mice and human, but was not detected in effector CD8+ T cells under the same culture conditions, which also produced IFN-γ25. Our supplemental data also demonstrated that there was no correlation between the transcript level of IFNG-AS1 and increased proportion of CD8+ IFN-γ+ T cells in HT patients. One possible interpretation is the different mechanisms of IFN-γ production between Th1 cells and CD8+ T cells. T-bet is the master regulator of Ifng expression in Th1 cells14. However, IFN-γ production by effector CD8+ T cells is dependent of Eomesodermin (Eomes), a paralogue of T-bet, which plays key role in the differentiation and function of CD8+ T cells31. Another possible interpretation is the difference in regulating IFNG expression by IFNG-AS1 between Th1 cells and CD8+ T cells. Although one model is generally indicated that Ifng-AS1(NeST) was required for Ifng expression in response to infection with Salmonella, and Ifng-AS1(NeST) was found to bind WDR5 component of histone H3 lysine 4 methytransferase complex, and to modify H3K4me3 at the Ifng locus by CD8+ T cells30. The differences between these reported findings may be due to different animal models, and the further mechanisms will be investigated in future.
Accumulating studies have demonstrated that HT is a Th1-mediated autoimmune disease because there are abundant Th1 cells infiltrating and thyrocyte destruction in HT patients14,31,32. Our results also showed that the proportion of Th1 cells and related genes, such as T-bet and IFNG, were higher in peripheral blood and thyroid gland from HT patients. It is widely accepted that elevated serum concentrations of TgAb and TPOAb are the most common manifestations of HT. These autoantibodies could indicate the development of HT, and IFN-γ production by Th1 cells drives the generation of autoantibodies28. We analyzed the correlation between the transcript level of Ifng-AS1 and serum level of autoantibodies. Positive correlations were found between the transcript level of Ifng-AS1 and the level of TgAb or TPOAb. These data suggest that IFNG-AS1 expression could reflect disease severity of HT to some extent.
In summary, our results demonstrate that the lncRNA IFNG-AS1 is significantly increased and may contribute to the pathogenic role of Th1 cells response in HT patients. Further exploration of the mechanism of IFNG-AS1-driven Th1 cells response may lead to better understanding of the pathogenesis of HT.
Additional Information
How to cite this article: Peng, H. et al. The Long Noncoding RNA IFNG-AS1 Promotes T Helper Type 1 Cells Response in Patients with Hashimoto's Thyroiditis. Sci. Rep. 5, 17702; doi: 10.1038/srep17702 (2015).
Acknowledgments
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20133227110008), Health Department Foundation of Jiangsu Province (Grant No. Z201312), National Natural Science Foundation of China (Grant Nos. 31470881, 31270947), Natural Science Foundation of Jiangsu (Grant No. BK20150533), Specialized Project for Clinical Medicine of Jiangsu Province (Grant No. BL2014065), Science and Technology Support Program (Social Development) of Zhenjiang (Grant Nos. SH2015045, SH2013040), Jiangsu Province “333” Project (Grant No. BRA2015197), Summit of the Six Top Talents Program of Jiangsu Province (Grant No. 2015-WSN-116), Jiangsu University Initial Founding for Advanced Talents (Grant Nos. 15JDG070, 11JDG093), Jiangsu Key Laboratory of Laboratory Medicine Foundation (Grant No. JSKLM-2014-013), Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Author Contributions H.P., Y.L. and J.T. carried out experiments, analyzed data, and wrote the paper; J.M., X.T., K.R., X.T. and C.M. helped with experiments and analyzed data; L.L. and H.X. analyzed data; P.J. supervised all the work on this paper; S.W. planned experiments and supervised all the work on this paper. All authors discussed the results and commented on the manuscript.
References
- Bindra A. & Braunstein G. D. Thyroiditis. Am Fam Physician 73, 1769–1776 (2006). [PubMed] [Google Scholar]
- Zhu C. et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab 97, 943–950 (2012). [DOI] [PubMed] [Google Scholar]
- Cardenas-Roldan J., Rojas-Villarraga A. & Anaya J. M. How do autoimmune diseases cluster in families? A systematic review and meta-analysis. BMC Med 11, 73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caturegli P., De Remigis A. & Rose N. R. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev 13, 391–397 (2014). [DOI] [PubMed] [Google Scholar]
- Chen J. et al. MiR-346 regulates CD4(+)CXCR5 (+) T cells in the pathogenesis of Graves’ disease. Endocrine 49, 752–760 (2015). [DOI] [PubMed] [Google Scholar]
- Antonelli A., Ferrari S. M., Corrado A., Di Domenicantonio A. & Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev 14, 174–180 (2015). [DOI] [PubMed] [Google Scholar]
- Colin I. M., Isaac J., Dupret P., Ledant T. & D’Hautcourt J. L. Functional lymphocyte subset assessment of the Th1/Th2 profile in patients with autoimmune thyroiditis by flowcytometric analysis of peripheral lymphocytes. J Biol Regul Homeost Agents 18, 72–76 (2004). [PubMed] [Google Scholar]
- Li D. et al. Th17 cell plays a role in the pathogenesis of Hashimoto’s thyroiditis in patients. Clin Immunol 149, 411–420 (2013). [DOI] [PubMed] [Google Scholar]
- Cosmi L., Maggi L., Santarlasci V., Liotta F. & Annunziato F. T helper cells plasticity in inflammation. Cytometry A 85, 36–42 (2014). [DOI] [PubMed] [Google Scholar]
- Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol 80, 225–235 (1996). [DOI] [PubMed] [Google Scholar]
- Cencioni M. T. et al. FAS-ligand regulates differential activation-induced cell death of human T-helper 1 and 17 cells in healthy donors and multiple sclerosis patients. Cell Death Dis 6, e1741 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Thieu V. T. & Kaplan M. H. Stat4 limits DNA methyltransferase recruitment and DNA methylation of the IL-18Ralpha gene during Th1 differentiation. EMBO J 26, 2052–2060 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. & Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol 7, 179–190 (2007). [DOI] [PubMed] [Google Scholar]
- Szabo S. J. et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100, 655–669 (2000). [DOI] [PubMed] [Google Scholar]
- Nanba T., Watanabe M., Inoue N. & Iwatani Y. Increases of the Th1/Th2 cell ratio in severe Hashimoto’s disease and in the proportion of Th17 cells in intractable Graves’ disease. Thyroid 19, 495–501 (2009). [DOI] [PubMed] [Google Scholar]
- Marique L. et al. The expression of dual oxidase, thyroid peroxidase, and caveolin-1 differs according to the type of immune response (TH1/TH2) involved in thyroid autoimmune disorders. J Clin Endocrinol Metab 99, 1722–1732 (2014). [DOI] [PubMed] [Google Scholar]
- Guttman M. et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458, 223–227 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjari M. & Salavaty A. HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol Med 12, 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiyomaru T. et al. Long non-coding RNA HOTAIR is targeted and regulated by miR-141 in human cancer cells. J Biol Chem 289, 12550–12565 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. Upregulation of long noncoding RNA TMEVPG1 enhances T helper type 1 cell response in patients with Sjögren syndrome. Immunol Res, 10.1007/s12026-015-8715-4 (2015). [DOI] [PubMed] [Google Scholar]
- Wang P. et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 344, 310–313 (2014). [DOI] [PubMed] [Google Scholar]
- Sharma S. et al. Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc Natl Acad Sci USA 108, 11381–11386 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourtada-Maarabouni M., Hasan A. M., Farzaneh F. & Williams G. T. Inhibition of human T-cell proliferation by mammalian target of rapamycin (mTOR) antagonists requires noncoding RNA growth-arrest-specific transcript 5 (GAS5). Mol Pharmacol 78, 19–28 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau S., Rohrlich P. S., Brahic M. & Bureau J. F. Tmevpg1, a candidate gene for the control of Theiler’s virus persistence, could be implicated in the regulation of gamma interferon. J Virol 77, 5632–5638 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S. P., Collins P. L., Williams C. L., Boothby M. R. & Aune T. M. Cutting edge: influence of Tmevpg1, a long intergenic noncoding RNA, on the expression of Ifng by Th1 cells. J Immunol 189, 2084–2088 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S. P., Henderson M. A., Tossberg J. T. & Aune T. M. Regulation of the Th1 genomic locus from Ifng through Tmevpg1 by T-bet. J Immunol 193, 3959–3965 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. M., Christensen E. I., Andresen B. S. & Moller B. K. Internalization, lysosomal degradation and new synthesis of surface membrane CD4 in phorbol ester-activated T-lymphocytes and U-937 cells. Exp Cell Res 201, 160–173 (1992). [DOI] [PubMed] [Google Scholar]
- Rapoport B. & McLachlan S. M. Graves’ hyperthyroidism is antibody-mediated but is predominantly a Th1-type cytokine disease. J Clin Endocrinol Metab 99, 4060–4061 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K. A. & Caffrey D. R. Long noncoding RNAs in innate and adaptive immunity. Curr Opin Immunol 26, 140–146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez J. A. et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell 152, 743–754 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E. L. et al. Control of effector CD8+T cell function by the transcription factor Eomesodermin. Science 302, 1041–1043 (2003). [DOI] [PubMed] [Google Scholar]
- Qin Q. et al. The increased but non-predominant expression of Th17- and Th1-specific cytokines in Hashimoto’s thyroiditis but not in Graves’ disease. Braz J Med Biol Res 45, 1202–1208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]