Abstract
Background
Adrenal insufficiency (AI), also known as hepato-adrenal syndrome, is a well-known entity in cirrhotic patients. However, factors associated with AI and its effect on survival are still not clear. We determined the prevalence of AI in patients with cirrhosis who had no hemodynamic instability or any acute deterioration, and studied its influence on short-term survival.
Patients and methods
In consecutive cirrhotic patients, presence of AI was determined either by total serum cortisol <18 µg/dl, 60 minutes after 250 µg synacthen injection, or when the delta-fraction (post-synacthen serum cortisol minus basal serum cortisol) was <9 µg/dl.
Results
A total of 120 patients were included in the study (median age 50 years (range 27–73), males 87%). The median CTP and MELD scores were 10 (range 6–13) and 20 (range 6–40). The etiology of cirrhosis was alcohol (51%), cryptogenic (28%), viral (19%) and autoimmune (2%). Sixty-nine patients (58%) had AI and the remaining 51 (42%) had normal adrenal function. Serum bilirubin was significantly higher (p < 0.05) in the AI group, and total cholesterol, HDL, LDL and hemoglobin were significantly lower (p < 0.05) in the AI group. CTP score, MELD score, and basal cortisol levels were not different between those with and without AI (p = NS). By 120 days of follow-up, 41 patients had died. Thus, the 120-day survival was 66%, and this was higher in patients without AI than in patients with AI (78% vs 56%; p = 0.019). On multivariate analysis absence of AI, low WBC and low CTP score independently predicted 120-day survival.
Conclusions
AI is present in more than half of cirrhotic patients but does not parallel the severity scores of cirrhosis. Its presence predicts early mortality in these patients, and this prediction is independent of CTP or MELD scores.
Keywords: Adrenal insufficiency, cirrhosis, severity, mortality, MELD, CTP
Introduction
Cortisol hormone is released from the zona fasciculata of the adrenal gland in response to stress and activates anti-stress and anti-inflammatory pathways. During acute illness, such as septic shock, tissue corticosteroid levels are increased, which is an important protective response.1 Adrenal insufficiency (AI) is a condition in which the adrenal glands do not produce adequate cortisol. Critical illness-related corticosteroid insufficiency (CIRCI) is a form of AI in critically ill patients who have blood corticosteroid levels that are inadequate for the severe stress response they experience. Combined with decreased glucocorticoid receptor sensitivity and tissue response to corticosteroids, this AI constitutes a negative prognostic factor for intensive care patients.2
AI in patients with liver cirrhosis has been described for more than half a century.3,4 Both cirrhosis and septic shock share many hemodynamic abnormalities such as hyperdynamic circulatory failure, decreased peripheral vascular resistance, increased cardiac output, hypo-responsiveness to vasopressors, increased levels of proinflammatory cytokines (interleukin (IL)-1, IL-6, tumor necrosis factor-alpha) and it has, consequently, been reported that AI is common in critically ill cirrhotic patients.3 In fact, it has also been observed that patients with liver disease have increased mortality if they have concomitant adrenal disturbances.5,6 The term “hepato-adrenal syndrome” has been proposed to define an inadequate glucocorticoid activity with respect to the severity of illness in a patient with liver disease.7
Although AI has been clearly documented in critically ill patients with cirrhosis, especially those having hemodynamic instability, it is unclear whether the AI is related to critical illness per se or is a pre-existing condition.8 Hence, the aim of our study was to investigate the frequency of AI in patients with cirrhosis who did not have hemodynamic instability or sepsis, and to study its influence on short-term survival. We also assessed the risk factors associated with abnormal adrenal response in these patients.
Patients and methods
Patients
This prospective study was performed at Sir Ganga Ram Hospital, New Delhi.
Inclusion criteria
Consecutive patients with cirrhosis, aged between 18 to 75 years, admitted to the department of gastroenterology, were included in the study. Diagnosis of cirrhosis was based on clinical, laboratory and ultrasonographic data or histology if available.
Exclusion criteria
(i) Patients who had obvious evidence of infection (such as on chest x-ray, urine examination, systolic blood pressure (SBP)) or had any culture positivity or sepsis at the time of enrollment; (ii) patients with hemodynamic instability (defined by a mean arterial pressure less than 60 mm Hg or vasopressor dependency on the day of the tests); (iii) patients with acute flares (aspartate aminotransferase (AST)/alanine aminotransferase (ALT) >250 IU/ml), or those diagnosed as acute-on-chronic liver failure; (iv) patients with a history of hypothalamic-pituitary or adrenal disease; (v) current or recent history of corticosteroid or immunosuppressive therapy; and (vi) patients who did not give consent to participate in the study.
The study was approved by the local ethics committees and a written informed consent was obtained from all participants. The study conformed to the Helsinki declaration of 1975 as revised in 1983.
Baseline evaluation
In all included patients routine biochemistry, hematology and coagulation profiles were obtained on the day of enrollment. Etiology of cirrhosis was determined and those patients who did not have a history of significant alcohol intake and were negative for viral markers (hepatitis B surface antigen (HBsAg) and anti-hepatitis C virus (HCV)), autoimmune markers (antinuclear antibodies (ANA), anti-smooth muscle antibody (ASMA), liver kidney microsome (LKM), antimitochondrial antibodies (AMA)), Wilson’s disease work-up (serum ceruloplasmin, Kayser-Fleischer (KF) ring), and hemachromatosis work-up (iron profile) were labeled as cryptogenic. The severity of liver disease was graded by the Child-Turcotte-Pugh (CTP) and Model for End-Stage Liver Disease (MELD) scores.
Determination of AI
Adrenal function was assessed by performing the short synacthen test (SST). Synthetic adrenocorticotropic hormone (ACTH) was given intravenously at 10 a.m., after an overnight fast. Blood samples were obtained immediately before and 60 minutes after injection. Measurements of serum cortisol were performed with the use of a standard chemiluminescent immunoassay (CLIA). Basal cortisol was defined as morning cortisol concentration (before 10 a.m.), before synacthen injection. Peak cortisol was defined as the cortisol concentration at 60 minutes after synacthen injection. Delta fraction was defined as the difference between peak and basal cortisol. AI was defined by a total serum cortisol <18 µg/dl, 60 minutes after 250 µg synacthen injection or delta fraction (post-synacthen minus basal cortisol) <9 µg/dl.
Follow-up
All the patients were followed from the date of enrollment till discharge from the hospital and later by telephonic communications, hospital information system records and interview in the outpatient department (OPD) for a period of 120 days.
Statistical analysis
Quantitative data were expressed as median (range) and analyzed using Mann-Whitney U test. Qualitative data were expressed as number (%) analyzed by Fisher’s exact test or Pearson chi-square test. P value < 0.05 was considered significant. Survival analysis was performed by Kaplan-Meier curve and compared using the log-rank test. Univariate analysis of baseline factors predicting 120-day mortality was performed and factors found significant were entered into multivariate analysis by binary logistic regression using the backward conditional method. Nonparametric correlation by Spearman rank analysis for basal serum cortisol, post-cosyntropin cortisol and delta fraction with rest of the parameters was also performed. Statistical analysis was conducted using the SPSS 17.0 statistical package (SPSS Inc, Chicago, IL, USA).
Results
Patients
From July 2010 through July 2011 a total of 256 consecutive patients with cirrhosis were admitted and were screened for enrollment. One hundred and thirty-six patients were excluded for the following reasons: patients with any sign of infection or sepsis (n = 80), hemodynamic instability or on vasopressor support (n = 26); patients with acute flares (AST/ALT > 250 IU/ml), or those diagnosed as acute-on-chronic liver failure (ACLF) (n = 23); patients with a history of hypothalamic-pituitary or adrenal disease (n = 1), and patients on current or recent history of corticosteroid therapy (n = 4). Two patients refused to give consent to participate in the study. Hence, the remaining 120 patients were enrolled in this study. The majority were males (87%) and the median age was 50 years (range 27–73 years). The clinical characteristics of included patients are shown in Table 1. Thirty-four patients were admitted with a predominant complaint of variceal bleeding, 39 with a predominant complaint of hepatic encephalopathy, 34 with a predominant complaint of ascites, and the remaining 13 were admitted for evaluation of cirrhosis.
Table 1.
Baseline characteristics of included patients
| Parameter | Value (n = 120) | 95% Confidence interval of mean |
|---|---|---|
| Age, years | 50 (27–73) | 49 52 |
| Gender, n (%) | ||
| Male | 104 (87%) | |
| Female | 16 (13%) | |
| Etiology of cirrhosis, n (%) | ||
| Alcohol | 61 (51%) | |
| Cryptogenic | 34 (28%) | |
| Hepatitis B | 13 (11%) | |
| Hepatitis C | 9 (8%) | |
| Autoimmune | 3 (2%) | |
| Hemoglobin, g/dl | 9.6 (3.3–14.2) | 9.2 9.9 |
| Platelets, ×103/cumm | 96 (17–335) | 95 117 |
| WBC, ×103/cumm | 8.4 (1.6–31.2) | 8.4 10.7 |
| INR | 1.9 (0.9–6.3) | 2.0 2.3 |
| BUN, mg/dl | 16 (4–117) | 18 24 |
| Serum creatinine, mg/dl | 0.9 (0.4–7.0) | 1.0 1.4 |
| Serum sodium, mmoles/l | 135 (109–149) | 133 135 |
| Serum potassium, mmoles/l | 3.9 (2.3–5.7) | 3.8 4.1 |
| Total serum bilirubin, mg/dl | 3.1 (0.2–35.9) | 4.1 6.2 |
| AST, IU/l | 70 (25–213) | 72 87 |
| ALT, IU/l | 33 (11–163) | 36 46 |
| Serum alkaline phosphatase, IU/l | 110 (20–511) | 111 138 |
| GGT, IU/l | 62 (11–627) | 81 124 |
| Total serum proteins, g/dl | 6.5 (3.4–9.3) | 6.2 6.6 |
| Serum albumin, g/dl | 2.3 (1.4–4.2) | 2.3 2.4 |
| Total serum cholesterol, mg/dl | 88 (29–288) | 89 105 |
| HDL, mg/dl | 12 (1–93) | 13 17 |
| LDL, mg/dl | 42 (9–146) | 44 54 |
| VLDL, mg/dl | 28 (9–185) | 29 37 |
| Triglyceride, mg/dl | 78 (24–591) | 80 103 |
| MELD score | 20 (6–40) | 19 22 |
| CTP score | 10 (6–13) | 10 10 |
| History of variceal bleeding | 34 (28%) | |
| Ascites | 89 (74%) | |
| Hepatic encephalopathy | 50 (42%) | |
Continuous values are expressed as median (range) and discrete values are expressed as number (%). WBC: white blood cells; INR: international normalized ratio; BUN: blood, urea, nitrogen; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma-glutamyl transferase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very low-density lipoprotein; MELD: Model for End-Stage Renal Disease; CTP: Child-Turcotte-Pugh.
Determination of AI
The median basal total serum cortisol was 12.6 µg/dl (range 0.8 to 53.0 µg/dl). Sixty minutes after administration of 250 µg synacthen, the median cortisol level in the whole group rose to 21.3 µg/dl (range 0.8 to 53.7 µg/dl) and the median delta fraction was 8.2 µg/dl (range 0.4 to 28.5 µg/dl). In 69 (58%) patients the post-synacthen cortisol level was <18 µg/dl or the delta fraction <9 µg/dl, thus indicating AI. The remaining 51 (42%) patients had no AI. The median delta fraction in patients with no AI was 12.8 (range 9.0 to 28.5) while the delta fraction in patients with AI was only 5.5 (range 0.4 to 10.9).
Correlation of AI with other parameters
Various baseline parameters were correlated with presence of AI (Table 2). Adrenal response to SST did not parallel with severity of cirrhosis assessed by MELD score and CTP score. Serum albumin, platelet count, INR, AST, ALT, alkaline phosphatase, serum creatinine, CTP score and MELD score were similar between those with and without AI. Serum bilirubin was significantly higher in patients with AI and total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and hemoglobin values were significantly less in patients with AI (Table 2).
Table 2.
Comparison of various baseline characteristics in patients with and without AI
| Patients without AI (n = 51) | Patients with AI (n = 69) | p value | |
|---|---|---|---|
| Age, years | 52 (30–73) | 49 (27–68) | NS |
| Gender, n (%) | NS | ||
| Male | 44 (86%) | 60 (87%) | |
| Female | 7 (14%) | 9 (13%) | |
| Etiology of cirrhosis, n (%) | NS | ||
| Alcohol | 27 (53%) | 34 (49%) | |
| Cryptogenic | 17 (33%) | 17 (25%) | |
| Hepatitis B | 4 (8%) | 9 (13%) | |
| Hepatitis C | 2 (4%) | 7 (10%) | |
| Autoimmune | 1 (2%) | 2 (3%) | |
| Hemoglobin, g/dl | 8.8 (3.0–14.0) | 10.0 (4.0–14.0) | 0.023 |
| Platelets, ×103/cumm | 100 (32–332) | 93 (17–335) | NS |
| WBC, ×103/cumm | 7.6 (2.5–25.6) | 9.0 (1.6–31.2) | NS |
| INR | 1.8 (0.9–6.3) | 2.0 (1.1–4.6) | NS |
| BUN, mg/dl | 16 (4–75) | 16 (4–117) | NS |
| Serum creatinine, mg/dl | 1.0 (0.5–6.3) | 0.9 (0.4–7.0) | NS |
| Serum sodium, mmoles/l | 135 (114–147) | 134 (109–149) | NS |
| Serum potassium , mmoles/l | 3.9 (2.3–5.6) | 3.9 (2.5–5.7) | NS |
| Total serum bilirubin, mg/dl | 2.3 (0.5–18.1) | 3.9 (0.2–35.9) | 0.031 |
| AST, IU/l | 62 (25–197) | 76 (28–213) | NS |
| ALT, IU/l | 32 (14–93) | 35 (11–163) | NS |
| Serum alkaline phosphatase, IU/L | 105 (38–360) | 119 (20–511) | NS |
| GGT, IU/l | 61 (11–627) | 67 (11–518) | NS |
| Total serum proteins, g/dl | 6.6 (3.6–8.7) | 6.4 (3.4–9.3) | NS |
| Serum albumin, g/dl | 2.4 (1.6–4.2) | 2.3 (1.4–3.6) | NS |
| Total serum cholesterol, mg/dl | 103 (35–288) | 77 (29–256) | 0.019 |
| HDL, mg/dl | 15 (5–93) | 11 (1–40) | <0.001 |
| LDL, mg/dl | 52 (13–146) | 40 (9–122) | 0.013 |
| VLDL, mg/dl | 27 (9–104) | 28 (9–185) | NS |
| Triglyceride, mg/dl | 74 (31–591) | 80 (24–193) | NS |
| MELD | 18 (6–48) | 20 (9–53) | NS |
| CTP score | 10 (7–13) | 11 (6–13) | NS |
| History of variceal bleeding | 20 (39%) | 14 (20%) | 0.026 |
| Ascites | 34 (67%) | 55 (80%) | NS |
| Hepatic encephalopathy | 23 (45%) | 27 (39%) | NS |
| Basal serum cortisol, µg/dl | 13.0 (3.3–30.5) | 11.8 (0.8–53.0) | NS |
| Post-cosyntropin cortisol, µg/dl | 26.7 (14.9–47.0) | 17.7 (0.8–53.7) | <0.001 |
Continuous values are expressed as median (range) and discrete values are expressed as number (%). AI: adrenal insufficiency; WBC: white blood cells; INR: international normalized ratio; BUN: blood, urea, nitrogen; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma-glutamyl transferase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very low-density lipoprotein; MELD: Model for End-Stage Renal Disease; CTP: Child-Turcotte-Pugh.
Follow-up
The patients were followed up to 120 days from the date of admission. Forty-one patients had died and 79 were still surviving without orthotopic liver transplantation (OLT) (Figure 1). The causes of death in the 41 patients were as follows: progressive liver failure (n = 24), sepsis and SBP (n = 10), gastrointestinal bleeding (n = 3), hepatorenal syndrome (n = 3) and hepatocellular carcinoma (n = 1). Thus the overall 120-day survival was 66% (79/120), which was higher in patients without AI (78% (40/51)) than in patients with AI (56% (39/69); p = 0.019) (Figure 2). There was no significant difference in causes of death between patients with or without AI. Various factors analyzed to predict 120-day survival are shown in Table 3. On multivariate analysis by binary logistic regression, presence of AI (odds ratio (OR) 3.6 (95% confidence interval (CI) 1.5–8.3); p = 0.003) independently predicted 120-day survival, apart from high white blood cells (WBC) (OR 1.0 (95% CI 1.0–1.0); p = 0.009), and high CTP score (OR 1.1 (95% CI 1.0–1.3); p = 0.049).
Figure 1.
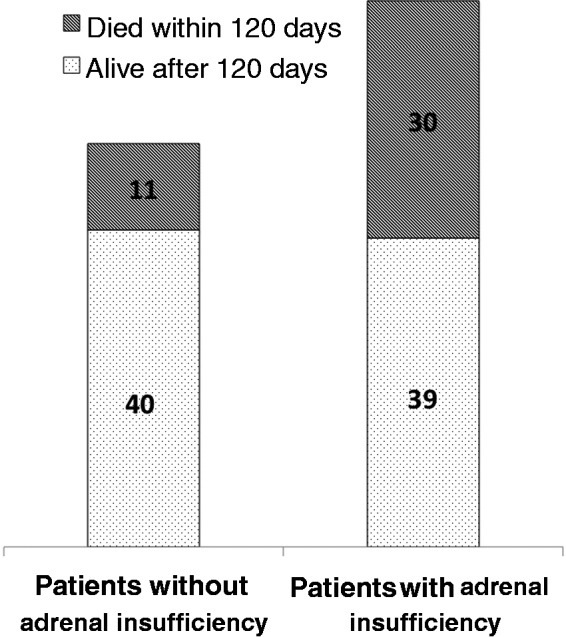
Outcome of patients with and without adrenal insufficiency.
Figure 2.
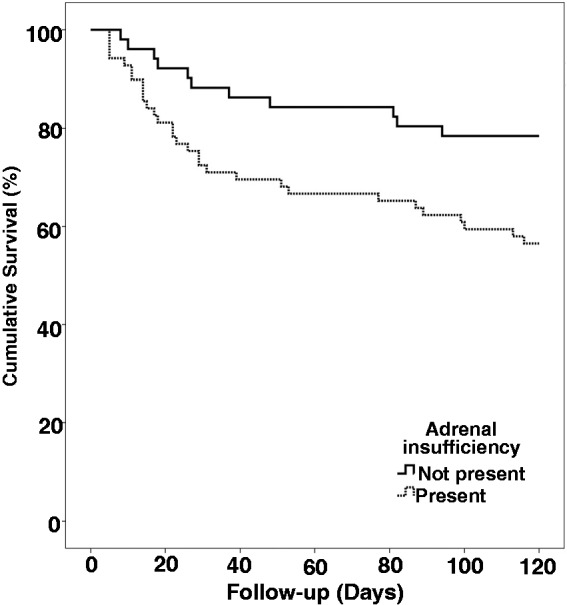
Kaplan-Meier curve for showing survival of patients with and without adrenal insufficiency and comparison using the log-rank test.
Table 3.
Univariate analysis of baseline factors predicting 120-day mortality
| Alive at 120 days (n = 79) | Died within 120 days (n = 41) | p value | |
|---|---|---|---|
| Age, years | 53 (33–73) | 50 (27–65) | NS |
| Gender, n (%) | NS | ||
| Male | 66 (84%) | 38 (93%) | |
| Female | 13 (16%) | 3 (7%) | |
| Etiology of cirrhosis, n (%) | NS | ||
| Alcohol | 35 (44%) | 26 (63%) | |
| Cryptogenic | 25 (32%) | 9 (22%) | |
| Hepatitis B | 10 (13%) | 3 (7%) | |
| Hepatitis C | 7 (9%) | 2 (5%) | |
| Autoimmune | 2 (2%) | 1 (3%) | |
| Hemoglobin, g/dl | 9.4 (4.7–14.2) | 9.9 (3.3–13.6) | NS |
| Platelets, ×103/cumm | 96 (17–301) | 93 (24–335) | NS |
| WBC, ×103/cumm | 6.0 (1.6–25.6) | 11.0 (2.8–31.2) | < 0.01 |
| INR | 1.8 (0.9–3.3) | 2.4 (1.3–6.3) | 0.054 |
| BUN, mg/dl | 15 (4–74) | 19 (4–117) | NS |
| Serum creatinine, mg/dl | 0.9 (0.4–3.1) | 1.0 (0.4–7.0) | NS |
| Serum sodium, mmoles/l | 135 (120–146) | 134 (109–149) | NS |
| Serum potassium , mmoles/l | 3.9 (2.3–5.7) | 3.9 (2.5–5.6) | NS |
| Total serum bilirubin, mg/dl | 2.3 (0.2–20.8) | 6.5 (0.7–35.9) | <0.01 |
| AST, IU/l | 65 (25–213) | 78 (28–201) | NS |
| ALT, IU/l | 33 (14–163) | 33 (11–106) | NS |
| Serum alkaline phosphatase, IU/l | 116 (20–360) | 108 (45–511) | NS |
| GGT, IU/l | 54 (11–627) | 76 (11–627) | NS |
| Total serum proteins, g/dl | 6.5 (3.6–9.3) | 6.5 (3.4–9.0) | NS |
| Serum albumin, g/dl | 2.4 (1.5–4.2) | 2.2 (1.4–3.8) | 0.021 |
| Total serum cholesterol, mg/dl | 94 (29–288) | 74 (39–206) | NS |
| HDL, mg/dl | 15 (1–93) | 11 (3–37) | 0.021 |
| LDL, mg/dl | 44 (9–146) | 33 (14–131) | NS |
| VLDL, mg/dl | 27 (9–185) | 31 (9–87) | NS |
| Triglyceride, mg/dl | 78 (24–591) | 78 (26–240) | NS |
| MELD | 18 (6–31) | 26 (12–40) | 0.001 |
| CTP score | 9 (6–13) | 11 (7–13) | 0.002 |
| History of variceal bleeding | 27 (34%) | 7 (17%) | 0.056 |
| Ascites | 55 (70%) | 34 (83%) | NS |
| Hepatic encephalopathy | 28 (35%) | 22 (54%) | 0.078 |
| Basal serum cortisol, µg/dl | 12.5 (1.0–25.4) | 12.6 (0.8–53.0) | NS |
| Post-cosyntropin cortisol, µg/dl | 22.0 (6.2–47.0) | 18.8 (0.8–53.7) | NS |
| Adrenal insufficiency | 0.019 | ||
| Present | 39 (49%) | 30 (73%) | |
| Absent | 40 (51%) | 11 (27%) |
Continuous values are expressed as median (range) and discrete values are expressed as number (%).WBC: white blood cells; INR: international normalized ratio; BUN: blood, urea, nitrogen; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma-glutamyl transferase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very low-density lipoprotein; MELD: Model for End-Stage Renal Disease; CTP: Child-Turcotte-Pugh.
Table 4 shows the correlation by Spearman rank analysis for basal serum cortisol, post-cosyntropin cortisol, and delta fraction versus the rest of the parameters.
Table 4.
Correlation by Spearman rank analysis for basal serum cortisol basal, post-cosyntropin cortisol, and delta fraction versus rest of the parameters
| Basal serum cortisol | Post-cosyntropin cortisol | Delta fraction | ||
|---|---|---|---|---|
| Age | Correlation coefficient | 0.021 | 0.096 | 0.134 |
| Sig. (two tailed) | NS | NS | NS | |
| Hemoglobin | Correlation coefficient | 0.068 | −0.101 | −0.210 |
| Sig. (two tailed) | NS | NS | 0.021 | |
| Platelets | Correlation coefficient | 0.269 | 0.241 | 0.069 |
| Sig. (two tailed) | 0.003 | 0.008 | NS | |
| WBC | Correlation coefficient | 0.299 | 0.230 | −0.132 |
| Sig. (two tailed) | 0.001 | 0.012 | NS | |
| INR | Correlation coefficient | 0.002 | −0.015 | −0.125 |
| Sig. (two tailed) | NS | NS | NS | |
| BUN | Correlation coefficient | 0.142 | 0.124 | 0.009 |
| Sig. (two tailed) | NS | NS | NS | |
| Serum creatinine | Correlation coefficient | 0.129 | 0.127 | −0.007 |
| Sig. (two tailed) | NS | NS | NS | |
| Serum sodium | Correlation coefficient | −0.141 | −0.001 | 0.189 |
| Sig. (two tailed) | NS | NS | 0.039 | |
| Serum potassium | Correlation coefficient | −0.078 | −0.082 | −0.018 |
| Sig. (two tailed) | NS | NS | NS | |
| Total serum bilirubin | Correlation coefficient | 0.188 | 0.042 | −0.219 |
| Sig. (two tailed) | 0.040 | NS | 0.016 | |
| AST | Correlation coefficient | 0.030 | −0.035 | −0.118 |
| Sig. (two tailed) | NS | NS | NS | |
| ALT | Correlation coefficient | −0.206 | −0.205 | −0.031 |
| Sig. (two tailed) | 0.024 | 0.025 | NS | |
| Serum alkaline phosphatase | Correlation coefficient | −0.211 | −0.244 | −0.082 |
| Sig. (two tailed) | 0.020 | 0.007 | NS | |
| GGT | Correlation coefficient | 0.158 | 0.079 | −0.045 |
| Sig. (two tailed) | NS | NS | NS | |
| Total serum proteins | Correlation coefficient | 0.013 | −0.021 | v0.030 |
| Sig. (two tailed) | NS | NS | NS | |
| Serum albumin | Correlation coefficient | −0.037 | 0.007 | 0.148 |
| Sig. (two tailed) | NS | NS | NS | |
| Total serum cholesterol | Correlation coefficient | −0.026 | 0.117 | 0.191 |
| Sig. (two tailed) | NS | NS | .037 | |
| HDL | Correlation coefficient | −0.176 | 0.083 | 0.352 |
| Sig. (two tailed) | 0.055 | NS | <0.001 | |
| LDL | Correlation coefficient | 0.064 | 0.163 | 0.175 |
| Sig. (two tailed) | NS | NS | 0.055 | |
| VLDL | Correlation coefficient | −0.012 | 0.044 | 0.005 |
| Sig. (two tailed) | NS | NS | NS | |
| Triglyceride | Correlation coefficient | 0.166 | 0.149 | −0.005 |
| Sig. (two tailed) | NS | NS | NS | |
| MELD | Correlation coefficient | 0.150 | 0.052 | −0.193 |
| Sig. (two tailed) | NS | NS | 0.034 | |
| CTP score | Correlation coefficient | 0.113 | 0.035 | −0.175 |
| Sig. (two tailed) | NS | NS | 0.056 | |
| Survival | Correlation coefficient | −0.137 | 0.012 | 0.251 |
| Sig. (two tailed) | NS | NS | 0.006 |
WBC: white blood cells; INR: international normalized ratio; BUN: blood, urea, nitrogen; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma-glutamyl transferase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very low-density lipoprotein; MELD: Model for End-Stage Renal Disease; CTP: Child-Turcotte-Pugh.
To test whether basal serum cortisol alone can predict survival, a separate analysis was carried out. However, basal serum cortisol was not significantly different between survivors and nonsurvivors (median 12.5 (range 1.0–25.4) versus 12.6 (range 0.8–53.0) µg/dl), and hence could not be used.
Discussion
Our study showed that AI is present in 58% patients with cirrhosis who had no hemodynamic instability, sepsis, acute flare, or ACLF. Moreover, the presence of AI did not parallel the severity scores of cirrhosis. The presence of AI predicted early mortality in these patients, and this prediction was independent of CTP or MELD scores.
Unlike most other investigators, we investigated the presence of AI in patients with cirrhosis who were not critically ill at the time of the study. We found that not only is the prevalence of AI high, but it also is a predictor of early mortality. Most other investigators have usually studied the presence of AI in critically ill patients with various liver diseases. Harry et al.6 found adrenal response abnormalities in acute liver failure independently of the presence of sepsis. It was more frequent in those with severe liver disease and correlated with severity of illness. The authors concluded that adrenal dysfunction may contribute to hemodynamic instability and mortality in patients with acute hepatic dysfunction. Marik et al.7 studied the prevalence of AI in patients with liver disease admitted to a liver transplant intensive care unit and reported the prevalence as 72%. The prevalence of AI varied between groups: 33% patients with fulminant hepatic failure, 66% patients with chronic liver disease, 61% patients with a remote history of liver transplantation, and 92% patients who had undergone liver transplantation under steroid-free immunosuppression were diagnosed with AI. In cirrhotics the association of AI has been studied in critically ill patients with sepsis and septic shock. Tsai et al.5 were the first to study the association of AI in cirrhotics, and found it in 51% of patients with chronic liver disease and sepsis. Fernandez et al.9 found the prevalence of AI to be 68% in cirrhotics with septic shock.
In our cohort of patients, using SST, we found that 58% of patients with cirrhosis, without hemodynamic instability, had an abnormal adrenal reserve (peak TC less than 18 µg/dl and/or delta fraction <9 µ/dl, 60 minutes post-stimulation) diagnostic of AI. In a similar study, Fede et al.10 also investigated the prevalence of AI in cirrhosis without infection or hemodynamic instability. They evaluated adrenal function using the SST in 101 patients with end-stage cirrhosis, in whom adrenal insufficiency was diagnosed in 38%. However, the prevalence of AI in our study was more than the prevalence in the study by Fede et al.10 This difference could be due to the use of a different dose of ACTH and different definitions of AI used in both studies.
We found no relationship between the severity of liver disease and prevalence of AI. Adrenal response to SST did not parallel severity of cirrhosis assessed by MELD score and CTP score. Thus, our study suggests that AI may be pre-existent in cirrhotics rather than developing in relation to increasing severity of cirrhosis. This finding was similar to that shown in a study by Marik et al.,7 but it contrasted with the findings of the study by Fede et al.,10 who showed in their cohort of 101 patients that CTP and MELD scores were significantly different between those with and without AI. In our study, serum albumin, platelet count, international normalized ratio (INR), AST, ALT, alkaline phosphatase, serum creatinine, CTP score and MELD score were similar between those with and without AI. Serum bilirubin was significantly higher in patients with AI, and TC, HDL, LDL and hemoglobin values were significantly less in patients with AI.
There are no established diagnostic criteria for AI using the SST and several threshold values of post-stimulation cortisol have been proposed.7,11–14 We considered a normal response to SST to be a serum cortisol concentration of at least 18 lg/dl (497 nmol/l) at 60 minutes after the synacthen injection, as described in several studies.11,12 Some authors suggest that tissue glucocorticoid activity rather than circulating glucocorticoid concentrations may be a potentially more useful index of functional adrenal status.15 This concept needs to be further explored.
During the condition of stress such as infections or other acute illness, adrenal activity is inadequate with respect to the severity of the patient’s illness, and becomes manifest, defined as “critical illness-related corticosteroid insufficiency.”2 The concept of glucocorticoid resistance is another issue recently discussed, mostly in the setting of critical illness.16,17 Liver disease, through different mechanisms, may cause a progressive impairment of adrenocortical reserve, with deficient production of glucocorticoids resulting in adrenocortical insufficiency. Currently the mechanisms by which liver disease leads to adrenal dysfunction are not sufficiently documented. Cholesterol is an essential precursor for steroid biosynthesis in adrenal glands, and the proportion of cholesterol bound to HDL is the most important substrate for steroidogenesis.18 A decrease in HDL concentrations is shown in cirrhosis, and is related to the severity of liver disease.19 An association between low HDL concentrations and adrenal dysfunction in patients with liver disease, in an acute setting (liver transplant intensive care unit), has been shown,20 suggesting that the lack of substrates can lead to a progressive exhaustion of adrenal reserve (“adrenal-exhaustion syndrome”). In our study too, we found that TC, serum HDL and serum LDL cholesterol were significantly reduced in patients with AI, thus suggesting the same adrenal-exhaustion syndrome operating causing AI.
What is the clinical relevance of AI? In our study we studied the effect of AI on short-term mortality and it was seen that AI was associated with a statistically significant increase in mortality in patients with AI as compared to patients without AI. We found that the 120-day survival was 66%, and this was higher in patients without AI (78%) than in patients with AI.5,6 Other factors associated with 120-day mortality on multivariated analysis were high WBC count and high CTP score. Thus we suggest that AI must be tested in all patients with cirrhosis, and its detection should prompt an increased vigilance for any impending deterioration. Whether corticosteroid replacement would be beneficial needs to be further studied.
It has been debated that free cortisol represents the biologically active component that may not be proportional to total cortisol in a variety of settings, including hypoalbuminemia. Although the biologically active free cortisol fraction depends on binding proteins, total cortisol correlates well to free cortisol in treatment-insensitive hypotension during critical illness. In fact in sepsis, albumin is not an important binding molecule. Subnormal increments in total cortisol upon ACTH suffice in assessing relative AI, particularly in sepsis.21 However, Tan et al. have advised caution in interpretation of adrenal function testing using total cortisol measurements in stable patients with severe liver disease because a significant discrepancy may exist between the rates of diagnosis of AI using the total and free cortisol criteria.22
We did not measure free cortisol in our study, which may be a limitation. Further studies need to be conducted measuring free cortisol and cortisol sensitivity in tissues to determine the presence of AI in specific tissues. Other limitations of the study may be inadvertent inclusion of patients with infections that were not picked up by routine investigations.
In summary, our study indicates that AI defined by an abnormal SST is a common feature in patients with cirrhosis without evidence of sepsis, hemodynamic derangement or acute deterioration and the presence of AI is not related to severity of liver disease. It can predict the mortality irrespective of severity of liver disease. Thus AI should be actively sought in patients of cirrhosis, and these patients may benefit from early transplantation.
This paper was presented at the oral session (OP 378) of the United European Gastroenterology Week (UEGW) 2012 October 24, 2012 in Amsterdam.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Aron D, Findling J, Tyrrell J. Glucocorticoids and adrenal androgens. In: Gardner D, Shoback D. (eds). Greenspan’s basic & clinical endocrinology, 8th ed New York: McGraw-Hill, 2007, 2007, pp. 356–363. [Google Scholar]
- 2.Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: Consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008; 36: 1937–1949. [DOI] [PubMed] [Google Scholar]
- 3.Trifan A, Chiriac S, Stanciu C. Update on adrenal insufficiency in patients with liver cirrhosis. World J Gastroenterol 2013; 19: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson RE. Adrenocortical steroid metabolism and adrenal cortical function in liver disease. J Clin Invest 1960; 39: 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai MH, Peng YS, Chen YC, et al. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology 2006; 43: 673–681. [DOI] [PubMed] [Google Scholar]
- 6.Harry R, Auzinger G, Wendon J. The clinical importance of adrenal insufficiency in acute hepatic dysfunction. Hepatology 2002; 36: 395–402. [DOI] [PubMed] [Google Scholar]
- 7.Marik PE, Gayowski T, Starzl TE. The hepatoadrenal syndrome: A common yet unrecognized clinical condition. Crit Care Med 2005; 33: 1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Beirne J, Holmes M, Agarwal B, et al. Adrenal insufficiency in liver disease—what is the evidence? J Hepatol 2007; 47: 418–423. [DOI] [PubMed] [Google Scholar]
- 9.Fernández J, Escorsell A, Zabalza M, et al. Adrenal insufficiency in patients with cirrhosis and septic shock: Effect of treatment with hydrocortisone on survival. Hepatology 2006; 44: 1288–1295. [DOI] [PubMed] [Google Scholar]
- 10.Fede G, Spadaro L, Tomaselli T, et al. Assessment of adrenocortical reserve in stable patients with cirrhosis. J Hepatol 2011; 54: 243–250. [DOI] [PubMed] [Google Scholar]
- 11.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med 2004; 350: 1629–1638. [DOI] [PubMed] [Google Scholar]
- 12.Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab 1994; 79: 923–931. [DOI] [PubMed] [Google Scholar]
- 13.May ME, Vaughn ED, Carey RM. Adrenocortical insufficiency—clinical aspects. In: Vaughan ED Jr, Carey RM. (eds). Adrenal disorders, New York: Thieme Medical, 1989, 1989, pp. 171–189. [Google Scholar]
- 14.Abdu TA, Elhadd TA, Neary R, et al. Comparison of the low dose short synacthen test (1 microg), the conventional dose short synacthen test (250 microg), and the insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab 1999; 84: 838–843. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J, Venkatesh B. Assessment of tissue cortisol activity. Crit Care Resusc 2009; 11: 287–289. [PubMed] [Google Scholar]
- 16.Ledderose C, Möhnle P, Limbeck E, et al. Corticosteroid resistance in sepsis is influenced by microRNA-124-induced downregulation of glucocorticoid receptor-α. Crit Care Med 2012; 40: 2745–2753. [DOI] [PubMed] [Google Scholar]
- 17.Yang N, Ray DW, Matthews LC. Current concepts in glucocorticoid resistance. Steroids 2012; 77: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 18.Yaguchi H, Tsutsumi K, Shimono K, et al. Involvement of high density lipoprotein as substrate cholesterol for steroidogenesis by bovine adrenal fasciculo-reticularis cells. Life Sci 1998; 62: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 19.Cicognani C, Malavolti M, Morselli-Labate AM, et al. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med 1997; 157: 792–796. [PubMed] [Google Scholar]
- 20.Marik PE. Adrenal-exhaustion syndrome in patients with liver disease. Intensive Care Med 2006; 32: 275–280. [DOI] [PubMed] [Google Scholar]
- 21.Molenaar N, Johan Groeneveld AB, Dijstelbloem HM, et al. Assessing adrenal insufficiency of corticosteroid secretion using free versus total cortisol levels in critical illness. Intensive Care Med 2011; 37: 1986–1993. [DOI] [PubMed] [Google Scholar]
- 22.Tan T, Chang L, Woodward A, et al. Characterising adrenal function using directly measured plasma free cortisol in stable severe liver disease. J Hepatol 2010; 53: 841–848. [DOI] [PubMed] [Google Scholar]


