Abstract
Background
Diagnosis of pre-malignant and malignant lesions in the bile duct and the pancreas is sometimes cumbersome. This applies in particular to intraductal papillary mucinous neoplasia (IPMN) and bile duct strictures in primary sclerosing cholangitis (PSC).
Aims
To evaluate in a prospective cohort study the sensitivity and specificity of probe-based confocal laser microscopy (pCLE) during endoscopic retrograde cholangiopancreatography (ERCP).
Methods
We performed pCLE together with mother-baby endoscopy (SpyGlass) during 50 ERCP sessions in 45 patients. The Miami and Paris criteria were applied. Clinical diagnosis via imaging was compared to pCLE and the final pathological diagnosis from surgically-resected, biopsy, or cytology specimens. Patients were followed up for at least 1 year.
Results
We were able to perform pCLE in all patients. Prior to endoscopy, the diagnosis was benign in 23 patients and undetermined (suspicious) in 16 patients, while six patients had an unequivocal diagnosis of malignancy. Sensitivity was 91% and specificity 52%. The positive (PPV) and negative predictive value (NPV) was 82% and 100%, respectively. Apart from mild post-ERCP pancreatitis in two patients, no complications occurred.
Conclusions
Our study showed that pCLE is a safe, expert endoscopic method with high technical feasibility, high sensitivity and high NPV. It provided diagnostic information that can be helpful for decisions on patient management, especially in the case of IPMN and unclear pancreatic lesions, in individuals whom are at increased risk for pancreatic cancer.
Keywords: Bile ducts, cytology, diagnostics, endoscopy methods, endoscopic retrograde cholangiopancreatography, histology, intraductal papillary mucinous neoplasia, pancreatic cancer, pancreatic neoplasia, probe-based confocal laser endomicroscopy, SpyGlass
Introduction
Modern imaging has greatly improved the diagnosis of conditions in hidden organs, such as the bile ducts and pancreas. In line with this development, magnetic resonance cholangiopancreatography (MRCP) has substituted diagnostic endoscopic retrograde cholangiopancreatography (ERCP). The possibility to directly visualize lesions with cholangiopancreatoscopy has reserved this endoscopic technique a place as an expert method. Duodenoscope-assisted cholangiopancreatoscopy has become even more popular with the latest generation of steerable, single-operator mini-endoscopes, which also allow for the insertion of instruments such as for biopsy, or the intraductal electro-hydraulic lithotriptor (EHL) or laser lithotripter, in order to crush stones.1 This expert technique was mostly used in two indications that are difficult to address with conventional cross-sectional imaging: Biliary strictures of unknown nature, e.g. in primary sclerosing cholangitis (PSC), and lesions and cysts, in conjunction with the main pancreatic duct (MPD). Surgical interventions in this area, i.e. a major liver resection for suspected Klatskin tumors and pancreatoduodenectomy for malignant pancreatic lesions, comprise a significant amount of morbidity and mortality, even in specialized centers. Hence, a reliable (preoperative) diagnosis is crucial, for appropriate patient selection for this type of advanced surgery.
Direct duodenoscope-assisted cholangiopancreatoscopy has been increasingly used to diagnose suspected lesions by direct vision and biopsy, both within the biliary system and in the pancreas2; however, conventional methods such as intraductal biopsies, brush or fine needle aspiration (FNA) cytology often remain inconclusive, and are overall of low diagnostic accuracy.3 Cholangiopancreatoscopy performed with the SpyGlass™ Direct Visualization System (Boston Scientific, Natick, MA, USA) offers the possibility of introducing, through the working channel, a probe-based confocal laser endomicroscopy (pCLE) for real-time in vivo histological imaging.4 While this undoubtedly represents a major step forward, as it opens up new technical applications, it has become clear that interpretation may not be as straightforward as for confocal laser endomicroscopy (probe-based or with dedicated endoscopes) in other parts of the GI tract.5 Nevertheless, with the so-called Miami classification6 an attempt was made to agree on diagnostic criteria for the evaluation of pCLE images from the pancreatic and bile duct. This classification was recently extended to accommodate inflammatory lesions; however, only for the biliary tract, coined the Paris classification.7
The aim of this prospective study was to evaluate the sensitivity and specificity of pCLE regarding lesions in the pancreatic and biliary ducts, by using histology as the diagnostic gold standard.
Patients and methods
Patients
From October 2008 to December 2012, fifty investigations were performed on 45 patients at Gastrocentrum, Karolinska University Hospital in Stockholm, Sweden (Table 1). Three patients were investigated on two different occasions and one patient underwent three pCLE examinations. Prior to ERCP, imaging was performed with multiple detector computed tomography (MDCT) and/or magnetic resonance imaging (MRI) plus MRCP; selected cases underwent endoscopic ultrasound (EUS). All patients were assessed at the pancreatobiliary multidisciplinary team meeting, where management strategy was decided.8 Indications for pCLE were: undetermined biliary pathology (n = 24), pancreatic pathology (n = 16) or juxtapapillary pathology (n = 5) (Table 1). All patients gave their informed consent. Our study was approved by the local ethics committee (EPN 2011/824-31/2 and 2013/1658-31/2). Three additional patients, in whom pCLE was indicated and intended, could not undergo the procedure due to known contraindications to the administration of fluorescein (beta blocker therapy and arrhythmia).9
Table 1.
Patient characteristics, clinical diagnosis and outcome of SpyGlass and pCLE. (a) investigations in the biliary tract and (b) investigations in the pancreatic duct
| Case # | Sex | Age | Clincal diagnosis (imaging) | Paris | Miami | pCLE Dx | Histology/final diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | F | 78 | gallbladder cancer or cholangiocarcinoma | papillary | pos | adenom | inoperable |
| abnormal | pos | IPMN | IPMN (papillary, MUC5A/6/1+) | ||||
| 2 | F | 74 | gallbladder cancer or cholangicarcinoma | normal | indetermined | benign | bile duct cancer |
| 6 | F | 73 | retroperitoneal fibrosis | normal | pos | benign | endoscopically benign stenosis of the duodenum |
| 17 | F | 59 | inflammation | normal | neg | benign | na |
| 19 | M | 59 | dilation of intrahepatic bile duct | normal | indetermined | benign | na |
| 20 | F | 61 | distal cholangiocarcinoma | abnormal | pos | indetermined | chronic pancreatitis |
| 21 | F | 61 | strictures in bile duct | abnormal | pos | IPMN? | cholangiocarcinoma |
| 22 | F | 59 | normal | neg | benign | PSC | |
| 23 | F | 54 | diffuse attenuated changes in intrahepatic bile ducts | normal | neg | benign | F/U normal |
| 25 | M | 60 | suspected cholangiocarcinoma | abnormal | pos | indetermined | na |
| 26 | M | 60 | inflammatory strictures in intrahepatic duct | abnormal | pos | indetermined | na |
| 27 | F | 57 | biliary tree tumor | pit-pattern | pos | malignt | cholangiocarcinoma |
| 28 | M | 55 | suspicious distal bile duct strictures | abnormal | pos | indetermined | na |
| 32 | M | 52 | cystic structures; fatty liver | abnormal | pos | IPMN | mixed IPMN |
| 33 | M | 47 | choledocus stones and strictures | normal | indetermined | benign | secondary sclerosing cholangitis |
| 34 | M | 42 | PSC or cholangiocarcinoma | abnormal | pos | tumor | cholangiocarcinoma Bismuth 4 not resectable |
| 35 | F | 43 | liver atrophy | abnormal | pos | indetermined | follow up; clearly Miami+, single pathological vessels |
| 36 | M | 41 | PSC | borderline | pos | indetermined | PSC |
| 38 | M | 32 | multiple stenosis in intrahepatic bile ducts | abnormal | indetermined | benign | PSC |
| 39 | F | 26 | strictures in the hilus | abnormal | pos | indetermined | PSC |
| 40 | F | 23 | stenosis in extrahepatic bile ducts | abnormal | pos | indetermined | metastatic disease |
| abnormal | pos | indetermined | na | ||||
| 42 | M | 33 | intrahepatic strictures (PSC) | normal | neg | benign | na |
| 43 | M | 68 | uniform thickness of swollen pancreas, with caliber changes | normal | neg | benign | inflammation; autoimmune cholangitis |
| 44 | M | 65 | changes in choledocus; suspected cancer | borderline | indetermined | benign | inflammation caused by stone |
| 45 | M | 69 | intrahepatic bile duct dilation; pancreatic head lesion | normal | neg | benign | inflammation |
| (b) | |||||||
| Case # | Sex | Age | Clinical diagnosis (imaging) | Paris | Miami | pCLE Dx | Histology/final diagnosis |
| 3 | M | 75 | IPMN | abnormal | pos | tumor | PanIN1-2, chron. pancreatitis |
| 4 | M | 72 | IPMN | normal | indetermined | benign | branch duct IPMN |
| 5 | M | 70 | mixed type IPMN | abnormal | pos | IPMN? | IPMC = IPMN w/ high grade dysplasia = IPMN-carcinoma |
| 7 | M | 72 | mixed IPMN or microcystic adenoma | abnormal | pos | tumor | IPMN, MUC2/5 A/6 positive |
| 8 | F | 70 | normal | normal | n/a | benign | typical papillary adenoma |
| 9 | F | 70 | IPMN branch duct | borderline | pos | indetermined | locally circumscript, definitively Miami + |
| 10 | M | 74 | side asset IPMN or microcystic adenoma | normal | neg | benign | follow up |
| 11 | M | 70 | branch type IPMN | abnormal | pos | indetermined | follow up; definitively Miami+, single pathological vessels |
| 12 | M | 68 | IPMN | abnormal | pos | indetermined | mixed IPMN (papillary) |
| 13 | MPD stenosis | abnormal | pos | indetermined | IPMNC, PanIN 1+2B | ||
| 14 | F | 66 | normal | papillary | n/a | adenom | tubulovillous adenoma |
| 15 | F | 66 | chronic pancreatitis, and dilation and strictures of MPD | borderline | indetermined | benign | chronic pancreatitis |
| 16 | F | 64 | papillary adenoma | abnormal | n/a | adenom | tubulovillous adenoma with high-grade dysplasia |
| 18 | F | 62 | branch-type IPMN | abnormal | pos | papillary | invasive PDAC with PanIN III |
| abnormal | pos | indetermined | follow up | ||||
| abnormal | pos | papillary | IPMN | ||||
| 24 | M | 61 | mix type IPMN | abnormal | pos | indetermined | IPMNC |
| 29 | F | 57 | multicystic aspect with calcification (IPMN) | abnormal | pos | IPMN | IPMN, PanIN 1B, PDAC |
| 30 | M | 55 | chronic pancreatitis and confluent cysts | abnormal | indetermined | IPMN | mixed IPMN moderate dysplasia |
| 31 | F | 56 | dilated pancreatic duct; suspected IPMN | normal | pos | benign | na |
| 37 | M | 31 | duodenal adenoma and lesion in the pancreatic head | abnormal | indetermined | adenom | FAP with adenom in the pancreatic duct |
| 41 | M | 12 | suspected wall, duct changes | abnormal | pos | IPMN | na |
Adenom: adenoma; Dx: diagnosis; F: female gender; FAP: familiar adenoma polyposis; F/U: follow up; IPMN: intraductal papillary mucinous neoplasia; M: male gender; MPD: main pancreatic duct; MUC: mucin; na: not applicable; neg: negative; PanIN: pancreatic intra-epithelial neoplasia; pCLE: probe-based confocal laser endomicroscopy; pos: positive; PSC: primary sclerosing cholangitis
Endoscopy
All procedures were carried out under general anesthesia. Systemic antibiotic prophylaxis was administered to all patients (intravenous (i.v.) Tazocin). We obtained the pCLE images during ERCP procedures, using a 0.94 mm diameter probe (CholangioFlex™, Cellvizio™, Mauna Kea Technologies, Paris, France) in conjunction with a duodenoscope-assisted cholangiopancreaticoscope (SpyScope™, Boston Scientific, Natik, MA, USA). After successful duct cannulation, combined with sphincterotomy if needed (n = 38), a conventional cholangio-or pancreatogram was obtained. The SpyGlass™ probe was then carefully introduced, under direct vision and fluoroscopy, into the duct and then low-flow, low-pressure saline was used for ductal irrigation and clearance.2 For the endoscopic diagnosis of intraductal papillary mucinous neoplasias, we applied criteria that were previously described in the literature.10 After removal of the guide wire, the Cholangioflex™ probe was introduced into the working channel of the SpyScope and advanced, until the tip was visible in the duct with SpyGlass™. Images were obtained by placing the tip of the probe in contact with the mucosa after i.v. injection of 2.5 ml of 10% fluorescein.9 This was done under direct vision. In some cases, the CholangioFlex™ probe was advanced into subsegmental bile ducts or side-branch pancreatic ducts, under fluoroscopy monitoring of the radio-opaque tip of the probe.7,11 In two cases investigating biliary lesions, a Gastroflex™ probe (1 mm diameter probe with large view and higher resolution) was inserted in the bile duct under fluoroscopy, after removal of the Spyglass.18 All images were recorded and stored in a special database, on a dedicated computer equipped with the proprietary software (Cellvizio 1.5.0/1.6.0).
Evaluation with pCLE
The pCLE images were interpreted according to the Miami criteria, as: epithelial structures, thick white bands (>20 µm), thick dark bands (>40 µm) or dark clumps (Table 1 and Table 2).6,12 Interpretation was done in real-time by the endoscopists performing the investigation, immediately following the acquisition of the video sequences, or directly after completion of the endoscopic procedure. The on-site presumptive diagnosis was recorded in the patient record file.
Table 2.
| Criteria suggestive for malignancy | Criteria suggestive for benign lesions/strictures | Inflammatory conditions |
|---|---|---|
| Thick, dark bands (>40 µm) | Thin, dark bands (branching) | Vascular congestion |
| Thick, white bands (>20 µm) | Thin, white bands | Dark granular patterns with scales |
| Dark clumps | Increased interglandular space | |
| Epithelium (villi, glands) | Thickened reticular structure | |
| Fluorescein leakage |
pCLE: probe-based confocal laser endomicroscopy
Furthermore, two independent investigators (RL, SS), who were blinded to the index diagnosis and not present during the endoscopic procedure, reviewed the files at a later time point, with the proprietary software (CellvizioViewer 1.6.0) in the proprietary file format (.mkt), preserving all the information from the original investigation. All investigators completed a standard online training module, consisting of 20 pCLE videos of histopathologically-confirmed benign and malignant lesions of the pancreatobiliary system (http://www.cellvizio.net). Upon review, the extended pCLE criteria (Paris classification) were also applied (Table 2).7,11
Pathology
Whenever discrete, circumscribed areas containing finger-like protrusions, mucus or tumor vessels were identified during the Spyglass endoscopy, biopsy specimens were obtained from several different parts of the lesion, under direct vision and by using dedicated mini-forceps (SpyBite™, Boston Scientific, Natick, MA, USA).2 In the absence of such lesions, random samples from the duct epithelium were taken where Miami criteria were met, and then sent for examination by a specialized pathologist. In many of the patients, we also obtained brush cytology specimens; while in selected cases, we also collected for cytology the washings from the bile duct or MPD during Spyglass use.2 To corroborate a diagnosis of high-grade dysplasia, we performed fluorescence in-situ hybridization (FISH) and Desoxyribonucleic acid (DNA) flow cytometry whenever sufficient material was available (n = 38).13
The final diagnosis that permitted an assessment of the accuracy of the pCLE results was defined as a definitive histopathological or cytopathological diagnosis of cancer, obtained by any tissue sampling method, including surgical pathology. The final diagnosis was considered benign if all the tissue samplings were negative for neoplasia and the 1-year clinical follow-up in the outpatient clinic and with imaging did not demonstrate the presence of any tumor.
Statistics
This was a prospective exploratory study with descriptive statistics. Sensitivity and specificity, as well as the positive predictive value (PPV) and negative predictive value (NPV), were calculated using histopathology as the gold standard.
Results
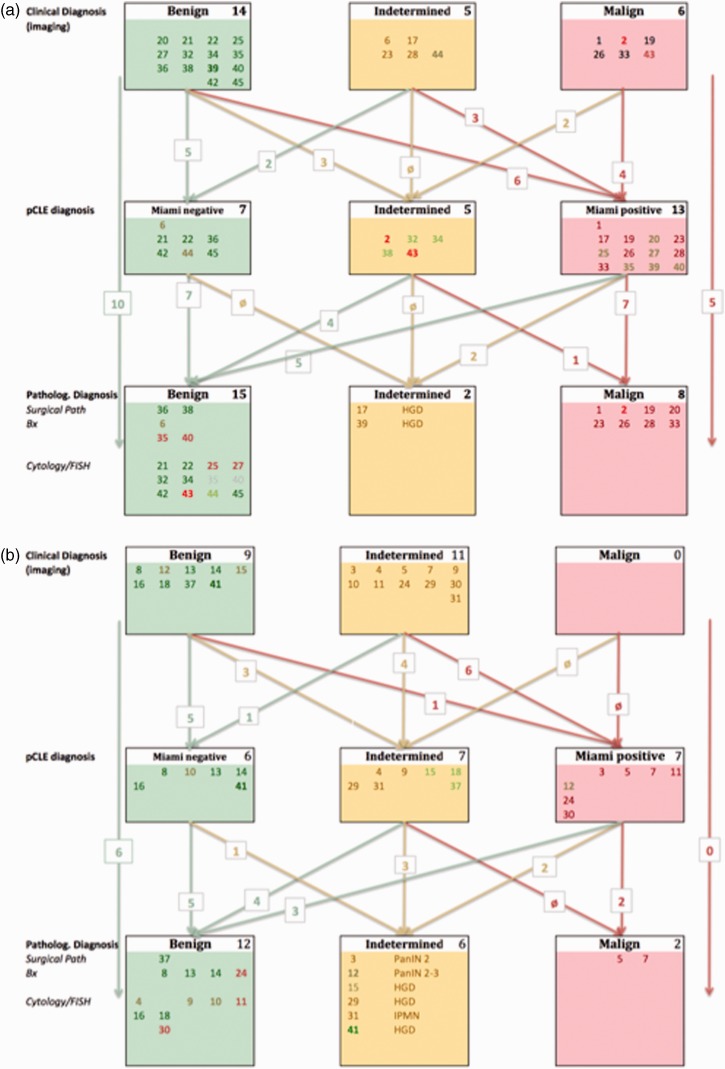
A total of 50 investigations in 45 patients were performed (24 male and 21 female patients; mean age 56.4 ± 15.5, median 60, range 12–75 years of age (Table 1)). Cholangiopancreatoscopy could technically be performed successfully in all the selected cases, with intention to treat. Of those cases, 30 had matching histopathological material available for comparison (Figure 1 and Table 1). The procedure was performed in all cases where cholangiopancreaticoscopy with the SpyGlass™ was possible (technical success rate, 100%). The primary indication was an indeterminate stricture in the pancreatobiliary system (n = 32) or a filling defect/cystic dilatation in the pancreatic duct (n = 13). We executed 25 investigations in the biliary tract and 20 in the pancreatic duct (Table 1 and Figure 1). Two patients subjected to our pancreatic investigations developed post-ERCP pancreatitis, with uneventful outcomes.
Figure 1.
Algorithm of initial diagnosis (clinical and imaging), findings during pCLE, and the final diagnosis based on the tissue pathology (from surgical observation, biopsy, cytology and FISH) and clinical follow-up. (a) Biliary pCLE investigations. (b) Pancreatic pCLE investigations. Individual patients were color-coded for better tracking throughout the three diagnostic levels, referring to the initial imaging diagnosis. Cases in bold had changed in more than one category. Patient #1 and #12 did receive two pCLE; and patient #17 underwent three pCLE. In all cases, the results were identical (Miami +). In the grey-shaded cases (#8, #35 and #40), both the biopsy and cytology/FISH were normal, thus non-malignant.
FISH: fluorescence in-situ hybridization; HGD: high-grade dysplasia; IPMN: intraductal papillary mucinous neoplasia; PanIN: pancreatic intra-epithelial neoplasia; pCLE: probe-based confocal laser endomicroscopy.
Of the 45 patients enrolled for ERCP with pCLE, 23 had a clinical and imaging diagnosis of a benign lesion, 16 were deemed suspicious for malignancy, and six had a definitive diagnosis of malignant disease (Figure 1 and Table 1). The diagnosis prior to ERCP was biliary stricture (n = 16), PSC (n = 4), cholangiocarcinoma (n = 6), intraductal papillary mucinous neoplasia (IPMN) (n = 13) and stricture of the pancreatic duct (n = 6) (Figure 1).
Use of pCLE changed the diagnosis in 24 patients (13 from the benign group, 12 from the indeterminate group, and two from the malignant group; Figure 1). There was a patient with a pre-diagnosed malign pancreatic condition. Diagnosis was changed from benign to indeterminate in six patients and to malignant, in seven patients. Three patients with clinically suspected malignancy did not demonstrate malignant criteria following the Miami criteria, during pCLE. Nine lesions (three biliary and six pancreatic) that had been deemed indeterminate were diagnosed as malignant with pCLE, while three lesions were found to be negative or normal (two biliary and one pancreatic) (Figure 1).
Comparing the final diagnosis based on histology and clinical follow-up (outpatient clinic visit and imaging; median 2.5 years; range 1–5 years) with the results from confocal laser microscopy, we found that 27 were normal/benign (+ 16 from pCLE), eight were high-grade dysplasia (– 7) and 10 were malignant (Figure 1). The original diagnosis of benign was maintained in 10 of the 14 biliary and six of the nine pancreatic investigations. This corresponds with an overall sensitivity of 91% for pCLE, which exceeded that of all other imaging modalities; however, its specificity was as low as for MDCT, i.e. 52% (Table 3). The PPV was 82% and the NPV was 100%.
Table 3.
Sensitivity and specificity, as well as PPV and NPV, of the different diagnostic procedures
| CT | MRI | CT+MRI | ERCP | pCLE | |
|---|---|---|---|---|---|
| Sensitivity (%) | 50 | 30 | 46 | 58 | 91 |
| Specificity (%) | 57 | 88 | 71 | 88 | 52 |
| PPV | 82 | ||||
| NPV | 100 |
CT: computed tomography; ERCP: endoscopic retrograde cholangiopancreatography; MRI: magnetic resonance imaging; NPV: negative predictive value; pCLE: probe-based confocal laser endomicroscopy; PPV: positive predictive value
Seven cases that were initially diagnosed as benign lesions, clearly demonstrated on pCLE to have more than one Miami-positive finding, although only one of these was eventually proven to be high-grade dysplasia of a bile duct. Of the nine lesions diagnosed as indeterminate/suspicious (one Miami-positive finding), seven eventually turned out to be benign, including one case that had been diagnosed clinically as being unequivocally malignant. These cases had been reviewed several times, and were found to show features that are now declared as inflammatory, according to the Paris classification: ‘increased interglandular space’, ‘dark granular patterns’, ‘vascular congestion’, and in particular, ‘thickened reticular structure’. Of those seven, two were in the pancreatic duct. The criteria put forward by the Paris classification, particularly the features ‘increased interglandular space’ and ‘thickened reticular structure’ (Table 2), could be identified in a total of 14 pancreatic duct lesions as well, together with clearly positive Miami criteria (Table 1); however, it is worth mentioning one of the patients’ diagnostic and imaging journeys, to illustrate the value of pCLE.
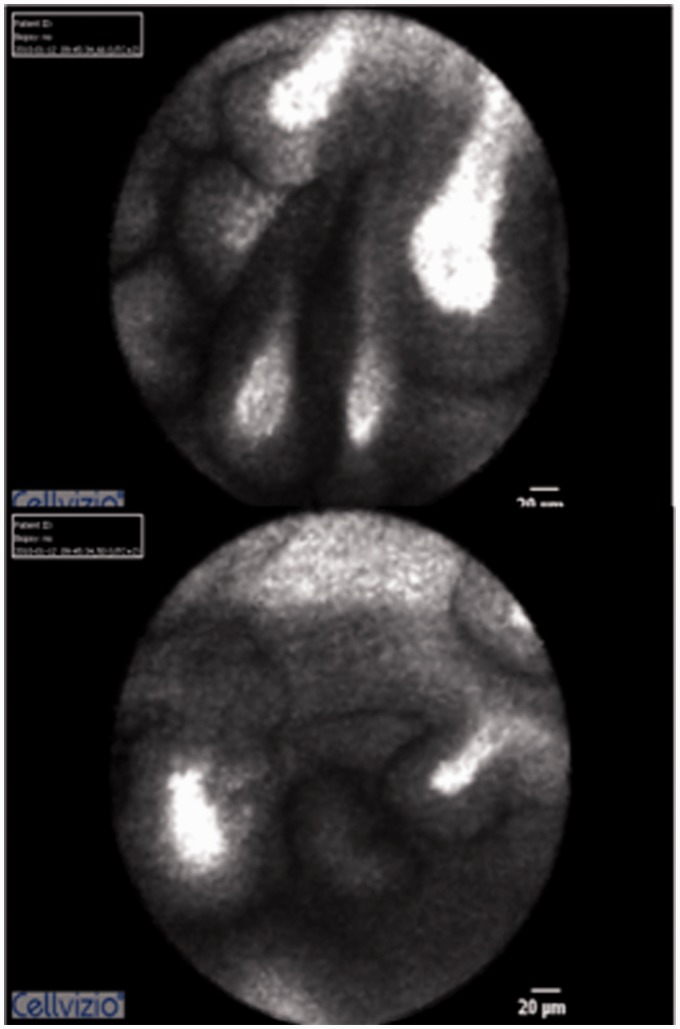
We investigated a 62-year-old female patient (#17) with chronic hereditary pancreatitis and a family history of pancreatic cancer. Being a heavy smoker (50 pack-years (PY)), the patient clearly had a high risk for developing a malignant lesion in the pancreas. During surveillance, a small side-branch irregularity had been detected, which could not be further characterized due to the underlying chronic pancreatitis (Cambridge 4). EUS was inconclusive. We then conducted ERCP with pCLE, which clearly revealed Miami-positive signs in a first-degree pancreatic duct that could be accessed only by the pCLE probe (Figure 3). All other tests (cytology/FISH) were initially negative. The findings were confirmed by two subsequent endoscopic controls, and on one occasion, the FISH analysis was positive (high proportion of aneuploid cells). The patient was then lost to follow-up, during 2 years. When she returned, a pancreatic cancer had developed, which was surgically resected and histologically confirmed.
Figure 3.
Patient #17 with a 50 pack-year history of smoking, who had chronic hereditary pancreatitis and a positive family history for pancreatic cancer. (a) and (b) Broad, papillary structures with blunt, exaggerated tips and contrast pooling in the tips. We performed the pCLE in a first-degree side branch of the main pancreatic duct that was not accessible with SpyGlass.
pCLE: probe-based confocal laser endomicroscopy; PY: patient-years.
Discussion
The newer endoscopic imaging modality of pCLE has kindled an interest in the field of advanced imaging, offering real-time in vivo histopathological evaluation of the pancreatobiliary system. It has strengthened and extended the realm of the gastroenterologist from that of therapeutic endoscopy to endopathology; however, this is not without problems, as this newly acquired expertise is certainly not common to all endoscopists involved in ERCP and the like.
The novel use of this technique is of particular significance for the two indications addressed in this prospective study: The diagnosis of indeterminate biliary strictures and of cystic lesions, filling defects or strictures of the pancreatic duct.4,7,12 This is the largest single-center study to date (50 investigations of 45 patients with pancreatobiliary disease). In this study, which was conducted prospectively and included a second, blinded evaluation of the pCLE images by two independent investigators, the overall sensitivity and specificity was 91% and 52%, respectively. The observed sensitivity is in line with that published previously (97%), whereas the specificity is significantly better, compared to the original study (33%);12 however, in none of the cases was the management of the patient changed based on an isolated positive finding from pCLE, although there was at least one such patient, whom underwent three pCLE investigations, all of which revealed abnormalities. As the diagnosis from brush cytology and endoscopic biopsy (SpyByte™) is difficult,2 the real benchmark is histopathology from surgical resections or real, true-cut biopsies.
At present the use of diagnostic ERCP is minimized, due to safer and less-invasive methods such as MDCT, MRI with MRCP, and EUS. Patients with solid or cystic masses in the pancreas are routinely subjected to EUS-guided biopsy with needle-based confocal laser microscopy as the newest addition.14 Patients with extra-hepatic strictures of unclear nature are a recurrent dilemma. They still represent a group of patients where the decision to undergo ERCP is rather easily made. There is only one study, which recruited about 100 patients from several centers, of whom 89 were investigated.11 That study reports a sensitivity of 98% and specificity of 67%; however, 40 patients in their series were already proven to have a cancer diagnosis. Furthermore, follow-up was significantly shorter than the 1 year in the current study.
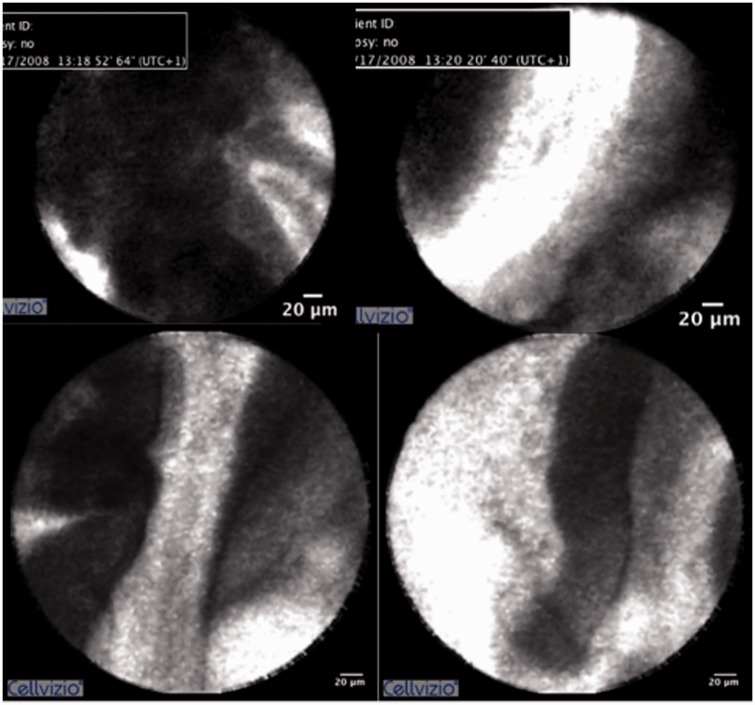
Besides the excellent sensitivity, both studies share the observation that the diagnostic criteria may not be sufficient, at least not for lesions in the pancreas. This may be explained by the lack of pathological expertise among endoscopists to evaluate any findings other than the rather coarse Miami criteria, i.e. thick dark and white bands or villous structures (Table 2 and Figure 2). The entire group of severe inflammatory changes that may mimic high-grade dysplasia/carcinoma or IPMN have not yet found a distinct correlate on pCLE imaging, but the Paris classification offers criteria for benign inflammatory conditions.7 While we could confirm that these can be easily applied specifically to lesions of the bile duct, we found that it was difficult to use these in the MPD.
Figure 2.
Examples of pCLE with the Cholangioflex probe in several patients, demonstrating the principal findings according to the Miami criteria. (a) Papillary structures with contrast enhancement along the tips. (b) Broad white bands within papillary structures. (c) Rather waisted tips, with contrast enhancement. (d) Broad dark bands.
pCLE: probe-based confocal laser endomicroscopy.
As imaging-based diagnoses of premalignant lesions are not free of error, especially not in the pancreas,15 any diagnostic measure that facilitates the decision-making process is welcome. This applies to the malignant transformation of PSC to bile duct cancer, and that of IPMN, to ductal adenocarcinoma of the pancreas. From the one patient who was lost from the surveillance program, we learned that the patient’s pCLE was clearly positive 3 years prior to the detection of a pancreatic cancer. Indeed, pCLE was the only and earliest modality that showed pathological findings; therefore, we suggest that in the group of at-risk patients, pCLE should be considered to investigate clinically indeterminate or suspicious lesions.
At present, there are no data available on the cost-effectiveness of pCLE on top of ERCP with Spyglass; and there are no data either for the role of ERCP alone or diagnostic tests, for suspected biliary or pancreatic malignancies. Model calculations for different parameters such as quality-adjusted life years (QALY) and health-adjusted life expectancy (HALE) are available for colorectal cancer; though here, intensive diagnostics such as screening colonoscopy had only a marginal gain over fecal occult blood test (FOBT).16
In summary, probe-based confocal laser endomicroscopy is a valuable addition to the diagnostic instrumentarium, with excellent sensitivity and satisfactory specificity. Nevertheless, as an expert technique, it should be used as the last step in a diagnostic step-up process, being the most invasive and also the most expensive method (Figure 4). Furthermore, the chronically inflamed epithelial linings and very early intraductal lesions, the pancreatic intra-epithelial neoplasia (PanIN), need to be studied in more detail, even in conjunction with histology, to learn more about their appearance in confocal microscopy.17
Figure 4.
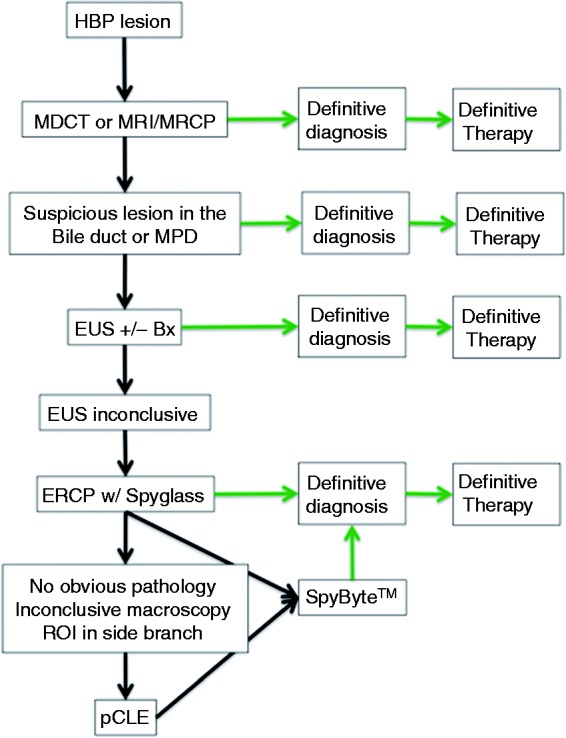
Diagnostic algorithm for the work-up of suspicious lesions of the bile ducts and the pancreas.
Bx: biopsy; ERCP: endoscopic retrograde cholangiopancreatography; EUS: endoscopic ultrasound; HBP: hepatobiliopancreatic; MDCT: multi-detector computer tomography; MPD: main pancreatic duct; MRCP: magnetic resonance cholangiopancreatogram; MRI: magnetic resonance imaging; pCLE: probe-based confocal laser endomicroscopy; ROI: region of interest.
Acknowledgements
We thank Margery Herrington for valuable discussions and constructive comments in the preparation of the manuscript. We thank our endoscopy nurses Inger Sahlgren, Susanne Stenbäck, Ann-Helen Stensröd, Sari Kallijärvi and Manuel Jimenez for their skillful assistance.
Funding
This work was supported by the region of Stockholm (SLL grant number ALF 20130512) and Levercancerfonden (both to JML); and by Boston Scientific International (some SpyGlass™ procedures and equipment received via an unrestricted educational grant to UA).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Yasuda I, Itoi T. Recent advances in endoscopic management of difficult bile duct stones. Dig Endosc 2013; 25: 376–385. [DOI] [PubMed] [Google Scholar]
- 2.Arnelo U, Siiki A, Swahn F, et al. Single-operator pancreatoscopy is helpful in the evaluation of suspected intraductal papillary mucinous neoplasms (IPMN). Pancreatology 2014; 14: 510–514. [DOI] [PubMed] [Google Scholar]
- 3.Heinzow HS, Woestmeyer C, Domschke W, et al. Endoscopic transpapillary biopsies are of limited value in the diagnostics of bile duct strictures of unknown etiology: Results of a histopathologically-controlled study in 312 patients. Hepatogastroenterology 2013; 60: 1569–1572. [PubMed] [Google Scholar]
- 4.Peter S, Bang JY, Monkemuller K, et al. Endomicroscopy of the pancreaticobiliary system. Diagn Ther Endosc 2013; 2013: 310105–310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canto MI, Anandasabapathy S, Brugge W, et al. In vivo endomicroscopy improves detection of Barrett's esophagus-related neoplasia: A multicenter international randomized controlled trial. Gastrointest Endosc 2014; 79: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meining A, Shah RJ, Slivka A, et al. Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary strictures. Endoscopy 2012; 44: 251–257. [DOI] [PubMed] [Google Scholar]
- 7.Caillol F, Filoche B, Gaidhane M, et al. Refined probe-based confocal laser endomicroscopy classification for biliary strictures: The Paris Classification. Dig Dis Sci 2013; 58: 1784–1789. [DOI] [PubMed] [Google Scholar]
- 8.Permert J, Löhr M. Organisation of a modern chain-of-care in pancreatology. In: Lohr JM, Andren-Sandberg A. (eds). Chronic Pancreatitis - Diagnosis and Therapy, Bremen: UniMed, 2011, pp. 245–247. [Google Scholar]
- 9.Wallace MB, Meining A, Canto MI, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Alimentary Pharmacol Therapeut 2010; 31: 548–552. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa T, Klöppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: A consensus study. Virchows Arch 2005; 447: 794–799. [DOI] [PubMed] [Google Scholar]
- 11.Caillol F, Bories E, Poizat F, et al. Endomicroscopy in bile duct: Inflammation interferes with pCLE applied in the bile duct. A prospective study of 54 patients. United Eur Gastroenterol J 2013; 1: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meining A, Chen YK, Pleskow D, et al. Direct visualization of indeterminate pancreatico-biliary strictures with probe-based confocal laser endomicroscopy: A multicenter experience. Gastrointest Endosc 2011; 74: 961–968. [DOI] [PubMed] [Google Scholar]
- 13.Ryan ME, Baldauf MC. Comparison of flow cytometry for DNA content and brush cytology for detection of malignancy in pancreaticobiliary strictures. Gastrointest Endosc 1994; 40: 133–139. [DOI] [PubMed] [Google Scholar]
- 14.Konda VJ, Aslanian HR, Wallace MB, et al. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas. Gastrointest Endosc 2011; 74: 1049–1060. [DOI] [PubMed] [Google Scholar]
- 15.Del Chiaro M, Segersvärd R, Pozzi Mucelli R, et al. Comparison of preoperative conference-based diagnosis with histology of cystic tumors of the pancreas. Ann Surg Oncol 2014; 21: 1539–1544. [DOI] [PubMed] [Google Scholar]
- 16.Hoed den CM, Isendoorn K, Klinkhamer W, et al. The societal gain of medical development and innovation in gastroenterology. United Eur Gastroenterol J 2013; 1: 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly KA, Bardeesy N, Anbazhagan R, et al. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med 2008; 5: e85–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shieh FK, Drumm H, Nathanson MH, et al.High-definition confocal endomicroscopy of the common bile duct. J Clin Gastroenterol 2012; 46: 401--406. [DOI] [PMC free article] [PubMed]