Abstract
Dopamine depletion in the putamen is associated with altered motor network functional connectivity in people with Parkinson's disease (PD), but the functional significance of these changes remains unclear, attributed to either pathological or compensatory mechanisms in different studies. Here, we examined the effects of PD on dorsal caudal putamen functional connectivity, off and on dopamine replacement therapy (DRT), using resting state fMRI. Motor performance was assessed with the Purdue pegboard task. Twenty-one patients with mild–moderate Parkinson's disease were studied twice, once after an overnight DRT washout and once after the administration of a standard dose of levodopa (Sinemet), and compared to 20 demographically-matched healthy control participants. PD patients off DRT showed increased putamen functional connectivity with both the cerebellum (lobule V) and primary motor cortex (M1), relative to healthy controls. Greater putamen–cerebellar functional connectivity was significantly correlated with better motor performance, whereas greater putamen–M1 functional connectivity was predictive of poorer motor performance. The administration of levodopa improved motor performance in the PD group, as expected, and reduced putamen–cerebellar connectivity to levels comparable to the healthy control group. The strength of putamen–cerebellar functional connectivity continued to predict motor performance in the PD group while on levodopa. These findings argue that increased putamen–M1 functional connectivity reflects a pathological change, deleterious to motor performance. In contrast, increased putamen–cerebellar connectivity reflects a compensatory mechanism.
Keywords: Dopamine, Putamen, Motor cortex, Cerebellum, Basal ganglia
Highlights
-
•
We examined the functional significance of altered motor networks in Parkinson's.
-
•
Patients showed greater putamen–cerebellar and –motor cortex connectivity.
-
•
Greater putamen–cerebellar connectivity correlated with better motor performance.
-
•
Greater putamen–motor cortex connectivity correlated with worse motor performance.
-
•
l-Dopa normalized putamen–cerebellar connectivity and improved motor performance.
1. Introduction
Parkinson's disease (PD) is associated with a progressive decline in motor control. Motor performance in PD is thought to reflect a balance between dysfunction of motor circuits, mainly due to dopamine denervation in the dorsal caudal putamen (Kordower et al., 2013), and compensatory processes, reflecting spontaneous adaptive adjustments in the interacting neural circuits that contribute to motor control (Lee et al., 2000, Nandhagopal et al., 2008, Palmer et al., 2009). Both dysfunction and compensation may be modulated by dopamine replacement therapy (DRT), the mainstay of PD treatment. The mechanisms underlying compensatory changes in PD remain unclear. It has been shown that patients may accomplish more difficult motor tasks by activating the same motor networks engaged in healthy controls, but to a greater degree and at easier stages of the task. Alternatively, or in addition, they may recruit other networks not typically recruited by healthy controls under the same conditions (Haslinger, 2001, Palmer et al., 2009, Samuel et al., 1997).
To date, investigations into the neural substrates of motor impairment in PD have mainly focused on alterations in cortico-striatal circuitry, detected with functional MRI or H2O15-PET during the performance of motor tasks. Contralateral caudal putamen and bilateral supplementary motor area (SMA) typically show diminished activation in PD patients off DRT, relative to healthy controls, during the performance of motor tasks (Wächter, 2013). This is widely considered a reflection of PD pathology and is explained by the model of basal ganglia dysfunction in PD in which dopamine deficiency in the putamen leads to excessive firing of the subthalamic nucleus and globus pallidus internal segment, resulting in decreased cortical excitation, and in turn, bradykinesia.
In contrast, several studies have found that activity is enhanced in primary motor cortex (M1) (Haslinger, 2001, Lewis et al., 2011, Sabatini et al., 2000, Wu et al., 2015, Yu et al., 2007) and cerebellum (Cerasa et al., 2006, Lewis et al., 2011, Palmer et al., 2009, Wu and Hallett, 2005, Yu et al., 2007) in PD patients off DRT during the performance of motor tasks. The relationship of these increased activations to motor performance remains unclear, in part because of variability in both task demands and PD patient performance across studies.
The role of enhanced task-related activity in cerebellum also remains unclear. Although the cerebellum is known to play a role in motor control, it has only recently been recognized as potentially important in motor function in PD in particular (Bell et al., 2015, Festini et al., 2015, Martinu and Monchi, 2013, Wu and Hallett, 2013). Recent anatomical work in non-human primates has found bidirectional, disynaptic subcortical communication between the basal ganglia and cerebellum: motor regions of the dentate nucleus project via the thalamus to the sensorimotor putamen, and motor regions of the subthalamic nucleus project via the pons to cerebellar cortex, region HVIIB (Bostan and Strick, 2010, Hoshi et al., 2005). These findings emphasize the need to better understand striatal–cerebellar interactions in PD, in addition to the more thoroughly studied cortico-striatal circuits.
Resting state fMRI offers a different perspective on neural network changes in PD, avoiding the confounds related to differential task performance that can arise when comparing clinical and healthy populations (Fox, 2010, Zhang and Raichle, 2010). This method has proved useful in better understanding cognitive function in PD (Baggio et al., 2015, Putcha et al., 2015). Here, we use this approach to study network changes in relation to motor function in PD, both off and on DRT.
Studies show the putamen and M1, and the putamen and cerebellum are functionally connected at rest in healthy adults (Bernard et al., 2013, Di Martino et al., 2008, Kelly et al., 2009). To date, the effects of PD on these resting state networks remain unclear. A consensus has yet to emerge likely because of differences in disease severity or medication status across studies, and because of the small number of studies to date. For instance, putamen–cerebellum functional connectivity has been reported as decreased (Hacker et al., 2012) or not different from healthy controls (Helmich et al., 2010). Likewise, putamen–M1 functional connectivity has been reported as decreased (Helmich et al., 2010), increased (Hacker et al., 2012) or not different from healthy controls (Kwak et al., 2010). Furthermore, the functional significance of alterations to these networks remains unknown: while DRT has been shown to relatively normalize (Wu et al., 2009a), fully normalize (Bell et al., 2015), or lead to differences in motor network functional connectivity in PD compared to controls (Festini et al., 2015), it is unclear how these DRT-induced changes in motor network connectivity in PD relate to motor performance. Moreover, changes in functional connectivity may be viewed as deleterious (disease related) or compensatory, depending on their relationship to motor performance.
Here, we aimed to address key questions about the functional significance of the effects of mild–moderate PD on motor network functional connectivity measured with resting state fMRI in a group of patients tested off and on DRT. Given the putamen's core role in motor networks, and that it is the site of the most severe dopamine depletion in PD, we focused on dorsal caudal putamen functional connectivity.
First, we investigated the relationships between the strength of putamen's functional connectivity with M1 and cerebellum, and motor performance, assessed outside the scanner with the Purdue pegboard task in PD patients off DRT, compared to healthy controls. Second, we examined how DRT, in the form of a standard dose of levodopa, affected the strength of functional connectivity between these regions, and, how it affected the relationships between connectivity and motor performance. We confirmed the specificity of the findings by carrying out the same analyses in a control region also affected in PD, the dorsal caudate.
2. Materials and methods
2.1. Participants
Twenty-one patients with mild–moderate idiopathic Parkinson's disease (mean age 67, S.D. 8.9) and 20 demographically matched healthy control subjects (mean age 65, S.D. 6.7) participated in this study. Patients were recruited from the McGill University Health Centre Movement Disorders clinic. Experienced movement disorder neurologists identified patients with idiopathic PD without dementia, based on the UK brain bank criteria (Hughes et al., 1992). All patients scored ≥ 24/30 on the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005), a screening test for cognitive impairment. Patients with other neurological diagnoses that might affect cognition, or overt clinical depression, were excluded. Healthy controls were recruited from the local community. Controls were excluded if they had a history of neurologic or psychiatric disease, head injury, or were taking psychoactive medication. The local research ethics committee approved the study.
2.2. Study procedure
All participants underwent two MRI scans in a single morning session. PD patients were scanned after an overnight (minimum 18 h) washout of their DRT (off DRT state) and again 45 min after receiving a single standard dose of Sinemet (100 mg l-dopa; 25 mg carbidopa) (on DRT state), timed to coincide with peak plasma concentrations (Olanow and Obeso, 2000). Patients continued all other medications as usual in the washout condition. Controls were not administered DRT but underwent two resting state fMRI scans, with procedures exactly the same as for the patients. The control group's first scan was compared to the PD group's first scan (off DRT), and the control group's second scan was compared to the PD group's second scan (on DRT).
2.3. Behavioral testing: motor performance
Patients completed the Purdue pegboard task (Lafayette Instruments, Lafayette, IN) immediately before both scans, in their DRT ‘off’ and ‘on’ states. The task requires using one hand to place as many pins as possible into the holes of a pegboard in 30 s. The score is the number of pins successfully placed. Two trials were performed with each hand (scores were averaged across trials for each hand). Higher scores signify better motor performance.
2.4. Image acquisition
Imaging was carried out with a 3 T Siemens Trio scanner equipped with a standard 12-channel head coil. Foam pads were used to fix the subject's head within the coil to minimize head motion. A high-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE) structural volume was acquired for registration purposes (TE = 2.98 ms, TR = 2300 ms, inversion time = 900 ms, flip angle = 9°, field of view = 256, voxel dimension = 1 mm isotropic). For the two resting state fMRI scans, participants were instructed to lie still with their eyes open, to think of nothing in particular and to not fall asleep. Whole-brain functional imaging was performed using a gradient echo echoplanar imaging sequence (176 volumes, TE = 30 ms, TR = 2160 ms, field of view = 256 mm, flip angle = 90°, matrix = 64 × 64, voxel dimension 4 mm isotropic, acquisition time = 6 min 22 s).
2.5. Image preprocessing
Images were preprocessed and analyzed using tools from the FMRIB Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Preprocessing included: slice time correction, motion correction (3D volume registration using least squares alignment of 3 translational and 3 rotational parameters), non-brain removal, spatial smoothing with a Gaussian 5 mm FWHM kernel, high-pass temporal filtering at 100 s and reslicing to 2 mm isotropic. Registration of high resolution structural images to the MNI152 (Montreal Neurological Institute) template was performed using FLIRT (Jenkinson and Smith, 2001, Jenkinson et al., 2002). Transformation to MNI152 standard space was then further refined using FNIRT nonlinear registration (Andersson et al., 2007).
The time series of eight nuisance variables were identified for inclusion: white matter, cerebrospinal fluid and six variable head motion parameters. To extract the covariate time series for white matter and cerebrospinal fluid, each individual's high-resolution structural image was segmented using FSL's FAST segmentation program. Segmented white matter images were thresholded at 0.95 to ensure tissue type probability. CSF and white matter images were then binarized and applied to each individual's 4D time series.
2.6. Functional connectivity
The sensorimotor putamen seed was an approximately spherical (radius = 1 voxel) region of interest (ROI) located in the dorsal caudal putamen (coordinates ± 28 1 3 mm in MNI152 space), consistent with previous studies (Bell et al., 2015, Di Martino et al., 2008, Kelly et al., 2009). A seed was also placed in the dorsal caudate, as a control for the specificity of the putamen findings, at coordinates ± 13 15 9, also in line with previous studies (Bell et al., 2015, Di Martino et al., 2008, Kelly et al., 2009).
We first extracted the ROI-associated BOLD time courses from each subject's 4D time series and then created subject-level whole brain maps of all voxels that were correlated with each of the ROI's time courses, using FEAT in FSL. Analyses of group-level functional connectivity (i.e., for HCTL runs 1 and 2, PD off, PD on) and of between group (i.e., PD vs. HCTL) and paired group comparisons (e.g., HCTL run 1 vs. run 2, PD off vs. on DRT,) were performed using FEAT's FLAME (FMRIB's Local Analysis of Mixed Effects) as implemented in FSL. Thresholded Z-score maps of putamen and caudate functional connectivity were created for each group. Direct voxelwise group/condition comparisons produced thresholded Z-score maps of those voxels showing significant differences in functional connectivity between groups or across ‘off’–‘on’ medication conditions. For all analyses, corrections for multiple comparisons were performed at the cluster level using Gaussian random field theory (min. Z > 2.3 cluster significance: p < 0.05, corrected).
We tested for correlations between the strength of putamen–M1 or putamen–cerebellar functional connectivity and Purdue pegboard performance in PD patients. We focused on functional connectivity in the motor-dominant contralateral (left) hemisphere for M1 and ipsilateral (right) cerebellum in this cohort of right-handed PD patients whose symptoms were bilateral in all but one case. We extracted the mean parameter estimate values from M1 and cerebellar ROI masks (1-voxel radius spheres) that were centered on the ‘PD off’ group level activation maxima, from the first level analysis. As the location of maximum activation may vary between conditions (off vs. on DRT), a dispersion of coordinates up to 3 mm as per Michely et al. (2015) was allowed between conditions to ensure within-subject consistency of anatomical areas in patients. Finally, to enable comparison of our findings to the literature, correlational analyses were performed between the strength of putamen–M1 or putamen–cerebellar functional connectivity and disease severity (UPDRS scores) or tremor scores.
3. Results
3.1. Patient characteristics
The demographic and clinical data are shown in Table 1. All participants were right handed. There were no significant differences between patients and controls with regard to age, education or estimated IQ, as assessed with the WASI–II (t tests, ps > 0.05). PD patients had higher Beck Depression Inventory (BDI) scores (t(39) = 2.4, p = 0.01) compared to controls, although patients' scores were still well below the usual thresholds for depression on this scale. Hoehn and Yahr ratings (Hoehn and Yahr, 1967) ranged from 1.5 to 3. Motor signs were bilateral in all patients except one, whose symptoms were restricted to the right side. The severity of motor signs was rated using part III of the Unified Parkinson's Disease Rating Scale (UPDRS) (Fahn and Elton, 1987). Individual scores were significantly lower (fewer motor signs) on DRT compared to off (t(20) = 4.73, p < 0.001). Tremor scores were derived from the sum of UPDRS-III items 20 (tremor at rest) and 21 (action or postural tremor of hands) divided by 7 (the number of single body regions) as per Eggers et al. (2011). The tremor scores (S.D.) were 1.1 (0.8) off DRT, and 0.7 (0.7) on DRT, and were significantly lower on DRT compared to off (t(20) = 2.12, p < 0.05).
Table 1.
Characteristics of subjects (mean (S.D.)).
| PD | HCTL | |
|---|---|---|
| N | 21 | 20 |
| H & Y | 2.4 (0.6) | n/a |
| Education (years) | 16.1 (3.7) | 15.8 (2.9) |
| Disease duration (years) | 6.6 (3.3) | n/a |
| UPDRS | ||
| Off DRT | 15.3 (5.2)⁎⁎⁎ | n/a |
| On DRT | 11.0 (4.4) | n/a |
| LEDD (mg) | 592 (382) | n/a |
| DA LEDD (mg) | 170 (102) | n/a |
| BDI | 6.9 (3.7)⁎ | 2.7 (4.5) |
| MoCA | 26.2 (2.1) | 27.6 (1.3) |
| IQ | 118 (8) | 116 (4) |
BDI, Beck Depression Inventory; DA, dopamine agonist; DRT, dopamine replacement therapy; H & Y, Hoehn & Yahr rating; LEDD, levodopa equivalent daily dose; MoCA, Montreal Cognitive Assessment; IQ estimated with the Wechsler Abbreviated Scale of Intelligence (WASI–II); and UPDRS, Unified Parkinson's Disease Rating Scale.
p < 0.001, PD off DRT–on DRT.
p < 0.05, PD-HCTL.
Seventeen patients were taking l-dopa/carbidopa, and 4 were taking dopamine agonists (ropinirole or pramipexole) in isolation or in combination with l-dopa therapy. Six patients were taking a COMT inhibitor (entacapone), 8 were taking MAO B inhibitors (rasagiline or selegiline) and 4 were taking amantadine. Additional medications included venlaflaxine and amitriptyline (in 2 patients, neither of whom were depressed at the time of testing) and trihexyphenidyl in 2 patients. All patients were on stable medication for at least 3 months before the study. When data from patients taking antidepressants or trihexyphenidyl were removed from the analyses, the patterns of results remained the same, as reported below in the full sample. Levodopa equivalent daily dose (LEDD) and dopamine agonist equivalent daily dose (DA LEDD) calculated according to Pahwa et al. (1997) are shown in Table 1.
3.2. Purdue pegboard performance
With the right (dominant) hand, patients placed on average 8.9 (S.D. 2.7) pins when off DRT and 10.3 (S.D. 2.6) pins when on levodopa. With the left hand, patients placed on average 8.9 (S.D. 2.8) pins off DRT and 10.1 (S.D. 2.5) pins on levodopa. As expected, levodopa was associated with significantly improved performance for both right and left hands, within subject (paired t tests; ps < 0.05). The mean improvement in pegboard performance (on–off DRT, within subject) was 1.4 (S.D. 0.8), right hand and 1.2 (S.D. 0.8), left hand. Healthy control subjects did not perform the motor task.
3.3. Head movement
Total head displacement did not exceed 1.2 mm in the patient group, and 1.3 mm in the healthy control group.
3.4. Functional connectivity of dorsal caudal putamen
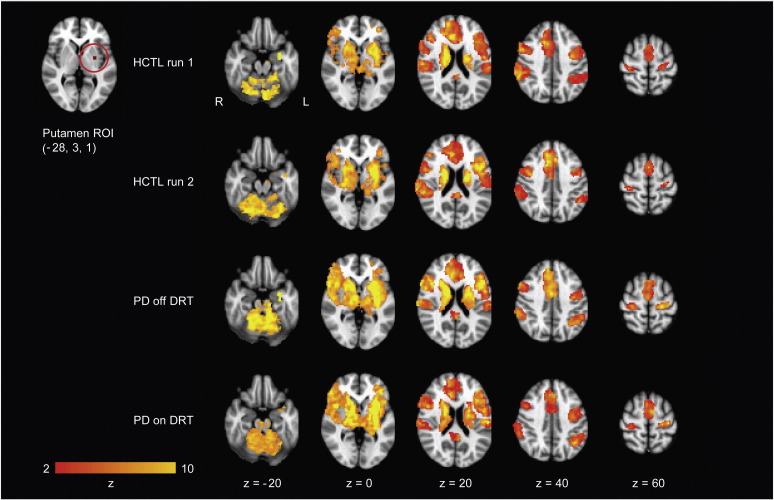
We assessed which brain regions were functionally connected to the dorsal caudal putamen in PD patients off and on DRT, and in healthy control subjects, over two runs, approximately 1 h apart. As expected, the putamen was functionally connected to primary and secondary motor cortical and subcortical regions, including M1, primary somatosensory cortex, and the cerebellum (lobules I–IV, V and VI), as well as prefrontal association areas (Fig. 1 and Supplementary Table 1). The connectivity patterns were broadly similar in PD patients and healthy controls. Results shown are for left putamen. Results for right putamen were similar, consistent with previous studies (Di Martino et al., 2008, Kelly et al., 2009, Kwak et al., 2010).
Fig. 1.
Patterns of dorsal caudal putamen functional connectivity in PD patients off and on DRT and healthy control subjects over two runs, approximately 1 h apart. Maps are thresholded at a z-score > 2.3, cluster significance: p < 0.05, corrected for multiple comparisons. Images are displayed according to radiological convention (left is right). L, left; and R, right.
3.5. Functional connectivity of dorsal caudate
To test the specificity of the putamen findings, we carried out the identical analysis for a seed in the dorsal caudate. This seed did not show significant connectivity with motor cortex or motor lobules of the cerebellum in either group, under either condition. Instead, caudate showed connectivity with more anterior prefrontal regions, and different cerebellar regions (lobules VIIb and crus II) (Supplementary Table 2), consistent with previous studies (Di Martino et al., 2008, Kelly et al., 2009, Kwak et al., 2010). These patterns were broadly similar in PD patients and healthy controls. Results shown are for left caudate. Results for right caudate were similar.
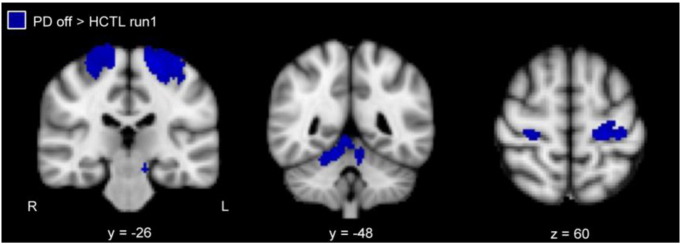
3.6. Functional connectivity in PD patients off DRT
We compared putamen functional connectivity between PD patients withdrawn from DRT and healthy control subjects (run 1). The putamen showed significantly increased functional connectivity bilaterally with the primary motor cortex and cerebellum (lobules I–IV, V), and, ipsilaterally with the somatosensory cortex, in PD patients compared to healthy controls (Fig. 2, Supplementary Table 3). There were also regions outside the motor system showing greater functional connectivity with the putamen, including the superior temporal lobe and angular gyrus, in the PD patients (Supplementary Table 3). None of these regions were identified in the same contrast with the caudate seed (not shown). The opposite contrast did not identify any regions that showed greater putamen functional connectivity in the control group.
Fig. 2.
Greater putamen functional connectivity in PD patients off DRT relative to healthy control subjects (min z > 2.3; cluster significance p < 0.05). L, left; and R, right.
3.7. Effects of levodopa
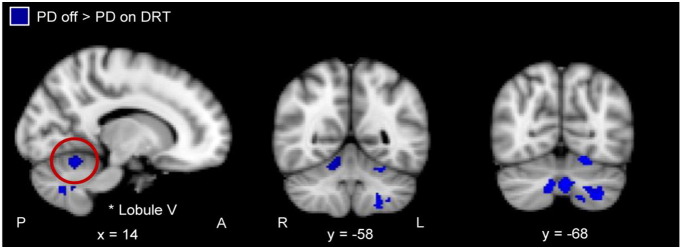
After a standard dose of levodopa, there were no longer any detectable differences in putamen functional connectivity between PD patients and healthy control subjects (run 2). There were no significant differences in putamen functional connectivity between run 1 and run 2 in healthy controls. PD patients showed significantly reduced functional connectivity between the putamen and cerebellum (lobules V, VI, VIIIa, VIIb) in the ‘on’ compared to ‘off DRT’ conditions, within subject. No significant effects were detected in the opposite contrast (i.e., on > off DRT), nor did we detect an effect of levodopa on putamen–M1 functional connectivity (Fig. 3 and Supplementary Table 4). The same contrasts for the caudate seed showed no significant functional connectivity changes with any of the regions identified for the putamen (not shown).
Fig. 3.
Regions showing significantly greater functional connectivity with putamen in PD patients off compared to on DRT (min z > 2.3; cluster significance p < 0.05). L, left; and R, right. A, anterior; and P, posterior.
3.8. Relationship between putamen functional connectivity and motor performance
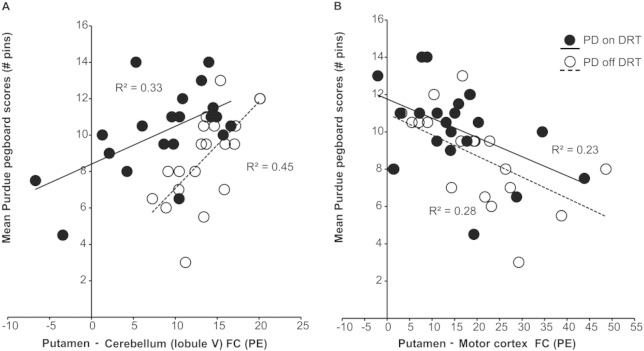
We asked whether the increased strength of putamen–cerebellar and putamen–M1 functional connectivity observed in PD patients off DRT predicted motor performance, to understand whether these differences are pathological or compensatory. Correlation analyses were performed between pegboard performance of the right (dominant) hand and functional connectivity between the areas within right cerebellum and left M1 that showed peak connectivity with the putamen in the off DRT condition (see PD ‘off’ Supplementary Table 1): cerebellum, lobule V (14, − 50, − 16), and primary motor cortex (− 24, − 26, 60).
Greater functional connectivity between putamen and cerebellum (lobule V) significantly predicted better motor performance (pegboard scores) in PD patients off DRT (r2 = 0.45, p = 0.001). In contrast, greater functional connectivity between putamen and primary motor cortex significantly predicted worse motor performance in the same cohort (r2 = 0.28, p = 0.01) (Fig. 4).
Fig. 4.
Scatter plots with best-fitting regression lines for the Purdue pegboard score as a function of (a) putamen–cerebellar functional connectivity and, (b) putamen–motor cortex functional connectivity. Data for PD off (empty circles; dashed line) and PD on (filled circles; solid line) are from the same cohort of subjects tested after overnight DRT washout (‘off’), and 45 min after a standard dose of Sinemet (‘on’).
3.9. Relationship between putamen functional connectivity and UPDRS scores
In keeping with the current literature, we also tested for correlations between the strength of putamen–M1 or putamen–cerebellar (lobule V) functional connectivity and disease severity (UPDRS scores) or tremor scores. We found that worse disease severity as assessed by the UPDRS score was significantly correlated with greater putamen–M1 functional connectivity in PD patients only off DRT (r2 = 0.27, p = 0.02), with a trend in the same direction on levodopa (r2 = 0.16, p = 0.07) but there was no relationship with putamen–cerebellar connectivity under either condition. Tremor scores did not predict putamen–M1 or putamen–cerebellar functional connectivity either off or on DRT (all ps > 0.05).
3.10. The effect of levodopa on the relationship between functional connectivity and motor performance in PD patients
We examined the relationship between motor performance and functional connectivity in the on levodopa condition, as described above for the ‘off’ condition. Correlations were assessed between right hand motor performance on levodopa and functional connectivity on levodopa between the putamen and regions in right cerebellum (lobule V; [12, − 50, − 16]) and left M1 (− 24, − 26, 62), which corresponded to those in the off DRT analyses (Supplementary Table 1).
After the administration of levodopa, although functional connectivity between putamen and cerebellum was reduced (indicated by significant leftward shift in functional connectivity from off to on DRT in Fig. 4a; asterisk in Fig. 3 off > on DRT indicates location in cerebellum), greater functional connectivity between putamen and cerebellum continued to significantly predict better motor performance. Similarly, as shown in Fig. 4b (PD on levodopa), after the administration of levodopa greater functional connectivity between the putamen and M1 continued to predict poorer motor performance (the absence of a significant left/rightward shift from off to on DRT in Fig. 4b reflects the absence of an effect of levodopa on putamen–M1 functional connectivity). There was no detectable relationship between the magnitude of the within-subject levodopa-related change in putamen–cerebellar functional connectivity and levodopa-related change in motor performance.
Finally, to further explore whether levodopa affected the relationship between putamen functional connectivity and motor performance, we ran a multiple linear regression, predicting motor performance from putamen functional connectivity, DRT condition, and their interaction. A significant interaction would indicate that functional connectivity could predict motor performance changes with DRT condition. I.e., Y = a + b(put–cerebellum) + c(put–M1) + d(DRT condition) + e(put–cerebellum ∗ DRT) + f(put–M1 ∗ DRT).
As expected, the strength of putamen–cerebellar and putamen–M1 functional connectivity, as well as DRT condition, significantly added to the prediction of motor performance (p < 0.05). There was a trend–level interaction effect between putamen–cerebellar functional connectivity and DRT (i.e., putamen–cerebellum ∗ DRT), indicating a trend for DRT to reduce the strength of the relationship between putamen–cerebellum connectivity and motor performance. The estimated coefficient was − 0.10 (S.E. 0.05; p = 0.075). There was no significant interaction between putamen–M1 and DRT condition.
4. Discussion
We examined the behavioral significance of changes in functional connectivity between the putamen and key motor control regions measured with resting state fMRI in a cohort of PD patients off and on DRT, relative to healthy controls. We focused our correlational analyses on two motor regions that we found were strongly connected to the dorsal caudal putamen in this cohort of patients off DRT, and that have been identified as having different motor-related activity or connectivity in PD relative to healthy controls: M1 and cerebellum. We were specifically interested in differentiating compensatory changes, which should correlate with better motor performance, from direct disease effects, which should show the opposite pattern. Motor performance was assessed with the Purdue pegboard task outside the scanner in patients off and on DRT. We chose the dorsal caudal putamen as the seed region for the connectivity analysis a priori, given this region has the greatest dopamine denervation even at the earliest stages of PD (Kish et al., 1988, Kordower et al., 2013) and is extensively connected with regions critical for movement.
First, we confirmed the dorsal caudal putamen is part of a functional motor network in PD patients and healthy older adults: it was functionally connected with supplementary motor area, M1 and anterior lobe of the cerebellum, replicating findings in healthy young adults (Di Martino et al., 2008, Kelly et al., 2009). This pattern of connectivity was specific to the putamen, and not observed for a seed placed in the nearby dorsal caudate, which instead demonstrated extensive connectivity with prefrontal and posterior cerebellar regions implicated in cognitive function. Then, we determined that functional connectivity between putamen and motor regions in the cerebellum (Stoodley and Schmahmann, 2009) was increased in PD off DRT, relative to healthy controls, and, found this predicted better motor performance amongst the patients. Connectivity between putamen and M1 was also increased in patients, relative to healthy controls, but that increased connectivity predicted worse motor performance. Finally, we showed a standard dose of levodopa normalized functional connectivity between the putamen and cerebellum, but not between putamen and M1.
Recent studies using task-based fMRI have pointed to a role for the cerebellum in PD, reporting increased BOLD signal in the cerebellum of patients off DRT during the performance of motor tasks (Cerasa et al., 2006, Jahanshahi et al., 2010, Palmer et al., 2009, Yu et al., 2007). Resting state fMRI studies have also implicated the cerebellum in PD, finding increased cerebellar regional homogeneity (Wu et al., 2009a) and increased connectivity of the cerebellum with the rest of the motor network more generally (Wu et al., 2009b). These fMRI findings, together with recent anatomical reports of disynaptic links between the striatum and cerebellum in non-human primates (Bostan and Strick, 2010, Hoshi et al., 2005), suggest connectivity between the putamen and cerebellum may be of particular importance in understanding motor function in PD.
To date, only a handful of studies have examined resting state putamen functional connectivity in PD (Bell et al., 2015, Hacker et al., 2012, Helmich et al., 2010, Kwak et al., 2010, Sharman et al., 2013); results with respect to the cerebellum have been inconsistent. Here, increased functional connectivity between putamen and cerebellum in patients off DRT was localized to the cerebellum's anterior lobe, where we detected both increases in correlation strength and a more diffuse pattern of connectivity in lobules I–IV and V that extended into lobule VI, consistent with the cerebellum's motor topography (Habas et al., 2009, O'Reilly et al., 2010, Stoodley and Schmahmann, 2009). In contrast, Hacker et al. (2012) reported decreased connectivity between the posterior putamen and anterior cerebellum in advanced, medicated patients (candidates for deep brain stimulation), relative to healthy controls. In other work, Helmich et al. (2010) detected no differences in putamen–cerebellar connectivity between patients who had never been medicated (de novo) or were off DRT and healthy controls. Our finding of enhanced connectivity in the mild–moderate disease stage, together with this prior literature, raise the possibility that putamen–cerebellar connectivity in PD increases in the earlier to moderate stages of the disease, ultimately diminishing in those with severe motor symptoms. Such a pattern would be consistent with a compensatory role, eventually overwhelmed by disease progression.
The present study tested whether the observed increased functional connectivity between putamen and cerebellum was compensatory or maladaptive, by relating it to a direct measure of motor performance. We found stronger putamen–cerebellar (lobule V) connectivity in patients off DRT correlated with better motor performance, supporting a compensatory role for this striatal–cerebellar network. The only other study to date relating motor performance to cerebellar functional connectivity in PD did not report any relationship between cerebellar (lobule V)–putamen functional connectivity and motor performance, either off or on DRT. However, the authors did find that stronger functional connectivity between the two cerebellar hemispheres (lobule V) (off DRT) was associated with better motor performance in PD (Festini et al., 2015). Other studies have provided indirect evidence for the same claim: greater cerebellar regional homogeneity and greater degrees of cerebellar–motor network functional connectivity in general have been associated with more severe motor symptoms in PD patients with mild–moderate disease (Wu et al., 2009a, Wu et al., 2009b).
The relationship between greater resting state putamen–cerebellar (lobule V) connectivity and better motor performance, together with this existing literature, suggest the cerebellum is increasingly engaged to carry out motor tasks made more difficult by the loss of dopamine innervation to the putamen, in line with the cerebellum's roles in optimizing motor control in healthy people. For instance, using positron emission tomography to measure cerebral blood flow (CBF) in healthy young adults during the early learning of timed motor sequences, Penhune and Doyon (2005) found that increased cerebellar CBF was linked with error correction to augment movement kinematics. Similarly, behavioral studies report patients with cerebellar lesions or degeneration display poorer motor adaptation to compensate for changing environments (Krakauer and Mazzoni, 2011), poorer coordination across joints during complex tasks (Bastian et al., 1996, Topka et al., 1998) and decreased accuracy in the timing of movements (Ivry et al., 1988) compared to healthy controls. This interpretation is also supported by task-related fMRI studies showing increased cerebellar activation in PD patients associated with normal task performance. Here, it is notable that functional connectivity between the putamen and cerebellum (lobule V) predicted motor performance even in the resting state. Similar observations have been made in patients recovering from stroke (Thiel and Vahdat, 2015).
We also found that putamen–M1 functional connectivity was increased in PD patients off DRT, relative to healthy controls. Only a few resting state fMRI studies have examined the effects of PD on functional connectivity between these regions, reporting inconsistent results. Similar to our findings, Hacker et al. (2012) detected increased posterior putamen–M1 connectivity in advanced, medicated PD patients. In contrast, Helmich et al. (2010) reported decreased putamen–M1 connectivity in de novo or off DRT patients, and Kwak et al. (2010) did not detect differences in putamen–M1 connectivity in mild–moderate PD patients off DRT, relative to healthy controls. Overall, it seems disease progression and exposure to DRT lead to increased connectivity between putamen and M1. Consistent with this model, task-related fMRI BOLD signal in M1 has been shown to be decreased in early stage, never medicated PD patients (Buhmann et al., 2003), and increased in previously treated PD patients (Yu et al., 2007), relative to healthy controls. DRT-induced cortical re-organization has been proposed to account for the increased signal observed in M1 of previously treated patients during task performance (Buhmann et al., 2003, Yu et al., 2007).
We found that stronger putamen–M1 connectivity was correlated with worse motor performance, whether indexed by pegboard score, or by UPDRS score, in patients off DRT, evidence that this connectivity difference reflects a pathological, maladaptive process. Similar relationships between M1 connectivity (e.g., increased regional homogeneity, increased degree of functional connectivity with the motor network in general and, increased functional connectivity with the subthalamic nucleus) and more severe disease have been reported (Baudrexel et al., 2011, Wu et al., 2009a, Wu et al., 2009b). Collectively, these observations provide support for the long-held notion of dysfunctional cortico-striatal circuitry underlying motor impairments in PD.
Finally, as expected, a standard dose of levodopa significantly improved motor performance in the PD group. Levodopa also reduced putamen–cerebellar functional connectivity in PD; connectivity values fell to levels indistinguishable from healthy controls. It had no detectable effect on the maladaptive, increased putamen–M1 connectivity. The extent of the levodopa-related decrease in putamen–cerebellar functional connectivity in PD did not predict the degree of improvement in motor performance. This may have been due to the limited range of performance change measurable from off to on levodopa here: a larger levodopa dose or more sensitive motor performance measure, or both, would better address this issue. Interestingly, the levodopa-related decrease in putamen–cerebellar functional connectivity followed a ‘left shift’ pattern. That is, although putamen–cerebellar connectivity dropped overall on levodopa, the positive relationship between putamen–cerebellar functional connectivity and better motor performance persisted, raising the possibility that dopamine only indirectly influenced this network through its effects on motor performance. Thus, although putamen–cerebellar functional connectivity is modulated by levodopa, the compensation reflected by increased putamen–cerebellum (lobule V) functional connectivity does not seem to be directly mediated by dopamine. Rather, this connectivity may be only indirectly influenced by changes in other aspects of the motor network, dynamically contributing to optimize motor performance when needed. This may also explain why there was a tendency for the positive relationship between putamen–cerebellar connectivity and motor performance to be weaker in the ‘on’ levodopa condition, when compensation requirements were presumably reduced.
In this study, functional connectivity between putamen and both superior temporal gyrus (STG) and angular gyrus was increased in PD patients off DRT, relative to healthy controls. Although outside of the core motor system, these regions have shown functional connectivity with the putamen in healthy young adults at rest (Kelly et al., 2009), and altered putamen–STG connectivity has been noted in PD. Helmich et al. (2010) previously reported decreased putamen–STG functional connectivity in de novo or off DRT patients, relative to healthy controls. This report, in conjunction with ours, suggest that disease progression leads to increased putamen–STG connectivity in PD, at least in the earlier to moderate stages of the disease. Future work could offer insight into the functional significance of these changes. The link between increased putamen–angular gyrus (BA 39) functional connectivity and PD is not immediately clear, although increased task-related fMRI BOLD signal in the angular has been linked to slower motor recovery post-stroke (Loubinoux, 2003), suggesting that it may reflect a maladaptive process.
This study has some limitations. First, we used a standard dose of levodopa, lower than the usual dose of most of the patients in this study, presumably yielding a relatively weak manipulation of dopamine-related effects. It is notable that this manipulation nonetheless was associated with a detectable change in putamen resting state connectivity, as well as in motor performance, albeit over a limited range. The absence of a similar effect on putamen–M1 connectivity does not exclude the possibility that a higher dose or a larger sample size could lead to detectable changes in this latter network. Second, for practical reasons the design of our study confounded the DRT manipulation with scan order. We mitigated this potential problem by also scanning the control group twice over the same time frame; in controls, the connectivity measures of interest did not change across scans. This, together with the agreement of the findings with other studies, makes it unlikely that the effects we attribute to levodopa were instead related to task order. Finally, a positive correlation between amplitude of PD tremor and resting state BOLD signal has been observed in motor cortex and cerebellum (Helmich et al., 2011). It is unlikely our findings reflect oscillatory tremor because increased putamen–cerebellar connectivity predicted better motor performance, not worse, and no correlation was detected between putamen–cerebellar functional connectivity and tremor scores. Future studies incorporating EMG measures of tremor could address this point definitively.
In conclusion, we demonstrate that mild–moderate PD is associated with enhanced putamen–M1 and putamen–cerebellar functional connectivity. The former was associated with worse motor performance, while the latter was associated with better performance, and was also reduced by administration of levodopa, consistent with engagement of a compensatory process. Together, these findings emphasize the importance of the cerebellum in offsetting the functional impact of PD-related striatal dysfunction, highlight the need to include the cerebellum in models of motor function in PD, and suggest potential novel therapeutic directions aiming to enhance cerebellar compensation.
Acknowledgments
We thank the clinicians in the McGill University Health Centre Movement Disorders clinic for referring patients, the Parkinson Society of Canada and CIHR (MOP 97821) for operating grant support, and the patients and their families for their participation.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.11.005.
Appendix A. Supplementary data
Supplementary tables.
References
- Andersson J., Jenkinson M., Smith S. FMRIB Technical Report (Technical Report No. TR07JA2) Oxford, FMRIB Center; Oxford, UK: 2007. Non-linear registration aka spatial normalisation. [Google Scholar]
- Baggio H.-C., Segura B., Sala-Llonch R., Marti M.-J., Valldeoriola F., Compta Y., Tolosa E., Junqué C. Cognitive impairment and resting-state network connectivity in Parkinson's disease: connectivity in Parkinson's disease. Hum. Brain Mapp. 2015;36:199–212. doi: 10.1002/hbm.22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian A.J., Martin T.A., Keating J.G., Thach W.T. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J. Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- Baudrexel S., Witte T., Seifried C., von Wegner F., Beissner F., Klein J.C., Steinmetz H., Deichmann R., Roeper J., Hilker R. Resting state fMRI reveals increased subthalamic nucleus–motor cortex connectivity in Parkinson's disease. NeuroImage. 2011;55:1728–1738. doi: 10.1016/j.neuroimage.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Bell P.T., Gilat M., O'Callaghan C., Copland D.A., Frank M.J., Lewis S.J.G., Shine J.M. Dopaminergic basis for impairments in functional connectivity across subdivisions of the striatum in Parkinson's disease: connectivity deficits in Parkinson's disease. Hum. Brain Mapp. 2015;36:1278–1291. doi: 10.1002/hbm.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.A., Peltier S.J., Wiggins J.L., Jaeggi S.M., Buschkuehl M., Fling B.W., Kwak Y., Jonides J., Monk C.S., Seidler R.D. Disrupted cortico-cerebellar connectivity in older adults. NeuroImage. 2013;83:103–119. doi: 10.1016/j.neuroimage.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan A.C., Strick P.L. The cerebellum and basal ganglia are interconnected. Neuropsychol. Rev. 2010;20:261–270. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhmann C., Glauche V., Stürenburg H.J., Oechsner M., Weiller C., Büchel C. Pharmacologically modulated fMRI–cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain J. Neurol. 2003;126:451–461. doi: 10.1093/brain/awg033. [DOI] [PubMed] [Google Scholar]
- Cerasa A., Hagberg G.E., Peppe A., Bianciardi M., Gioia M.C., Costa A., Castriota-Scanderbeg A., Caltagirone C., Sabatini U. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson's disease. Brain Res. Bull. 2006;71:259–269. doi: 10.1016/j.brainresbull.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D.S., Kelly A.M.C., Uddin L.Q., Shehzad Z., Biswal B., Walters J.R., Castellanos F.X., Milham M.P. Functional connectivity of human striatum: a resting state fMRI study. Cereb. Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Eggers C., Kahraman D., Fink G.R., Schmidt M., Timmermann L. Akinetic-rigid and tremor-dominant Parkinson's disease patients show different patterns of FP-CIT Single photon emission computed tomography. Mov. Disord. 2011;26:416–423. doi: 10.1002/mds.23468. [DOI] [PubMed] [Google Scholar]
- Fahn S., Elton R. Unified Parkinson's Disease Rating Scale. In: Fahn S., Masden C., Calne D., Goldstein M., editors. Recent Developments in Parkinson's Disease. Macmillan; Florham Park, New Jersey: 1987. pp. 153–163. [Google Scholar]
- Festini S.B., Bernard J.A., Kwak Y., Peltier S., Bohnen N.I., Müller M.L.T.M., Dayalu P., Seidler R.D. Altered cerebellar connectivity in Parkinson's patients ON and OFF L-DOPA medication. Front. Hum. Neurosci. 2015;9:214. doi: 10.3389/fnhum.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D. Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 2010 doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C., Kamdar N., Nguyen D., Prater K., Beckmann C.F., Menon V., Greicius M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker C.D., Perlmutter J.S., Criswell S.R., Ances B.M., Snyder A.Z. Resting state functional connectivity of the striatum in Parkinson's disease. Brain. 2012;135:3699–3711. doi: 10.1093/brain/aws281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger B. Event-related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain. 2001;124:558–570. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Helmich R.C., Derikx L.C., Bakker M., Scheeringa R., Bloem B.R., Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb. Cortex. 2010;20:1175–1186. doi: 10.1093/cercor/bhp178. [DOI] [PubMed] [Google Scholar]
- Helmich R.C., Janssen M.J.R., Oyen W.J.G., Bloem B.R., Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann. Neurol. 2011;69:269–281. doi: 10.1002/ana.22361. [DOI] [PubMed] [Google Scholar]
- Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hoshi E., Tremblay L., Féger J., Carras P.L., Strick P.L. The cerebellum communicates with the basal ganglia. Nat. Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry R.B., Keele S.W., Diener H.C. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp. Brain Res. 1988;73:167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M., Jones C.R.G., Zijlmans J., Katzenschlager R., Lee L., Quinn N., Frith C.D., Lees A.J. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson's disease. Brain. 2010;133:727–745. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kelly C., de Zubicaray G., Di Martino A., Copland D.A., Reiss P.T., Klein D.F., Castellanos F.X., Milham M.P., McMahon K. l-Dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J. Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish S.J., Shannak K., Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N. Engl. J. Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Kordower J.H., Olanow C.W., Dodiya H.B., Chu Y., Beach T.G., Adler C.H., Halliday G.M., Bartus R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer J.W., Mazzoni P. Human sensorimotor learning: adaptation, skill, and beyond. Curr. Opin. Neurobiol. 2011;21:636–644. doi: 10.1016/j.conb.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Kwak Y., Peltier S., Bohnen N.I., Müller M.L.T.M., Dayalu P., Seidler R.D. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front. Syst. Neurosci. 2010;4:143. doi: 10.3389/fnsys.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.S., Samii A., Sossi V., Ruth T.J., Schulzer M., Holden J.E., Wudel J., Pal P.K., de la Fuente-Fernandez R., Calne D.B., Stoessl A.J. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson's disease. Ann. Neurol. 2000;47:493–503. [PubMed] [Google Scholar]
- Lewis M.M., Du G., Sen S., Kawaguchi A., Truong Y., Lee S., Mailman R.B., Huang X. Differential involvement of striato– and cerebello–thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson's disease. Neuroscience. 2011;177:230–239. doi: 10.1016/j.neuroscience.2010.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubinoux I. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. NeuroImage. 2003;20:2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Martinu K., Monchi O. Cortico-basal ganglia and cortico-cerebellar circuits in Parkinson's disease: pathophysiology or compensation? Behav. Neurosci. 2013;127:222–236. doi: 10.1037/a0031226. [DOI] [PubMed] [Google Scholar]
- Michely J., Volz L.J., Barbe M.T., Hoffstaedter F., Viswanathan S., Timmermann L., Eickhoff S.B., Fink G.R., Grefkes C. Dopaminergic modulation of motor network dynamics in Parkinson's disease. Brain. 2015;138:664–678. doi: 10.1093/brain/awu381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandhagopal R., Mak E., Schulzer M., McKenzie J., McCormick S., Sossi V., Ruth T.J., Strongosky A., Farrer M.J., Wszolek Z.K., Stoessl A.J. Progression of dopaminergic dysfunction in a LRRK2 kindred: a multitracer PET study. Neurology. 2008;71:1790–1795. doi: 10.1212/01.wnl.0000335973.66333.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- O'Reilly J.X., Beckmann C.F., Tomassini V., Ramnani N., Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow C.W., Obeso J.A. Preventing levodopa-induced dyskinesias. Ann. Neurol. 2000;47:S167–S176. (discussion S176–178) [PubMed] [Google Scholar]
- Pahwa R., Wilkinson S., Smith D., Lyons K., Miyawaki E., Koller W.C. High-frequency stimulation of the globus pallidus for the treatment of Parkinson’s disease. Neurology. 1997;49:249–253. doi: 10.1212/wnl.49.1.249. [DOI] [PubMed] [Google Scholar]
- Palmer S.J., Ng B., Abugharbieh R., Eigenraam L., McKeown M.J. Motor reserve and novel area recruitment: amplitude and spatial characteristics of compensation in Parkinson's disease. Eur. J. Neurosci. 2009;29:2187–2196. doi: 10.1111/j.1460-9568.2009.06753.x. [DOI] [PubMed] [Google Scholar]
- Penhune V.B., Doyon J. Cerebellum and M1 interaction during early learning of timed motor sequences. NeuroImage. 2005;26:801–812. doi: 10.1016/j.neuroimage.2005.02.041. [DOI] [PubMed] [Google Scholar]
- Putcha D., Ross R.S., Cronin-Golomb A., Janes A.C., Stern C.E. Altered intrinsic functional coupling between core neurocognitive networks in Parkinson's disease. NeuroImage Clin. 2015;7:449–455. doi: 10.1016/j.nicl.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini U., Boulanouar K., Fabre N., Martin F., Carel C., Colonnese C., Bozzao L., Berry I., Montastruc J.L., Chollet F., Rascol O. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain J. Neurol. 2000;123(Pt 2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Samuel M., Ceballos-Baumann A.O., Blin J., Uema T., Boecker H., Passingham R.E., Brooks D.J. Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain J. Neurol. 1997;120(Pt 6):963–976. doi: 10.1093/brain/120.6.963. [DOI] [PubMed] [Google Scholar]
- Sharman M., Valabregue R., Perlbarg V., Marrakchi-Kacem L., Vidailhet M., Benali H., Brice A., Lehéricy S. Parkinson's disease patients show reduced cortical–subcortical sensorimotor connectivity: PD sensorimotor connectivity. Mov. Disord. 2013;28:447–454. doi: 10.1002/mds.25255. [DOI] [PubMed] [Google Scholar]
- Stoodley C., Schmahmann J. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Thiel A., Vahdat S. Structural and resting-state brain connectivity of motor networks after stroke. Stroke. 2015;46:296–301. doi: 10.1161/STROKEAHA.114.006307. [DOI] [PubMed] [Google Scholar]
- Topka H., Massaquoi S.G., Benda N., Hallett M. Motor skill learning in patients with cerebellar degeneration. J. Neurol. Sci. 1998;158:164–172. doi: 10.1016/s0022-510x(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Wächter T. Functional MRI of motor signs in Parkinson's Disease. In: Tuite P., Dagher Alain, editors. Magnetic Resonance Imaging in Movement Disorders: A Guide for Clinicians and Scientists. Cambridge University Press; New York, USA: 2013. pp. 57–71. [Google Scholar]
- Wu T., Hallett M. A functional MRI study of automatic movements in patients with Parkinson's disease. Brain J. Neurol. 2005;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Wu T., Hallett M. The cerebellum in Parkinson's disease. Brain. 2013;136:696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Long X., Zang Y., Wang L., Hallett M., Li K., Chan P. Regional homogeneity changes in patients with Parkinson's disease. Hum. Brain Mapp. 2009;30:1502–1510. doi: 10.1002/hbm.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Wang L., Chen Y., Zhao C., Li K., Chan P. Changes of functional connectivity of the motor network in the resting state in Parkinson's disease. Neurosci. Lett. 2009;460:6–10. doi: 10.1016/j.neulet.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Wu T., Hou Y., Hallett M., Zhang J., Chan P. Lateralization of brain activity pattern during unilateral movement in Parkinson's disease: motor lateralization in Parkinson's disease. Hum. Brain Mapp. 2015 doi: 10.1002/hbm.22743. (n/a–n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Sternad D., Corcos D.M., Vaillancourt D.E. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. NeuroImage. 2007;35:222–233. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Raichle M.E. Disease and the brain's dark energy. Nat. Rev. Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.