Abstract
Terpenoids have an essential function in present-day cellular membranes, either as membrane reinforcers in Eucarya and Bacteria or as principal membrane constituents in Archaea. We have shown that some terpenoids, such as cholesterol and α, ω-dipolar carotenoids reinforce lipid membranes by measuring the water permeability of unilamellar vesicles. It was possible to arrange the known membrane terpenoids in a ‘phylogenetic’ sequence, and a retrograde analysis led us to conceive that single-chain polyprenyl phosphates might have been ‘primitive’ membrane constituents. By using an optical microscopy, we have observed that polyprenyl phosphates containing 15 to 30 C-atoms form giant vesicles in water in a wide pH range. The addition of 10 % molar of some polyprenols to polyprenyl phosphate vesicles have been shown to reduce the water permeability of membranes even more efficiently than the equimolecular addition of cholesterol. A ‘prebiotic’ synthesis of C10 and C15 prenols from C5 monoprenols was achieved in the presence of a montmorillonite clay. Hypothetical pathway from C1 or C2 units to ‘primitive’ membranes and that from ‘primitive’ membranes to archaeal lipids are presented.
Keywords: Early formation and evolution of membranes, Vesicles, Membrane reinforcement, Terpenoids, Isoprenoids, Cholesterol, Archaea, Polyprenyl phosphates, Polyprenols
Introduction
Life is cellular, and a boundary separates the living organism from the outside world. Yet most discussions on the molecular origins of life center around questions such as how amino acids, sugars and nucleotides were formed in interstellar space or on Earth, how proteins originated and how nucleic acids arose and became able to self-replicate. Here we consider a fourth question: how did membranes originate? (Deamer 1986; Morowitz et al. 1988).
Understanding the origin of membranes is central to an understanding of the origin of life. Life may have begun as a two-dimensional system limited by a mineral surface, but in a three-dimensional system in water, segregation of a separate compartment is achieved by a closed lipidic membrane or vesicle. Lipid bilayers have very low permeability to ions, polar solutes and biopolymers, and thus define what is inside as the living organism, what is around as the membrane, and what is outside as the rest of the world. This article will focus on the early formation and evolution of membranes.
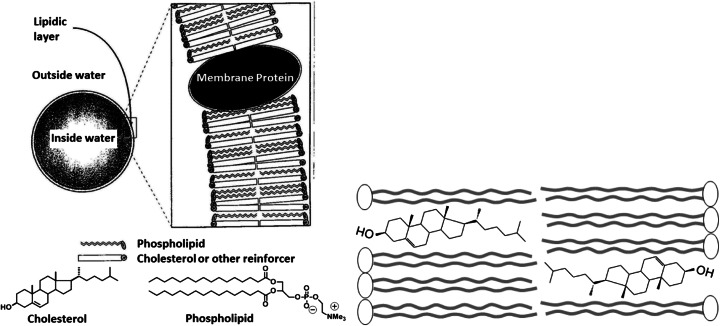
On the way of deducing the origins of membrane constituents, it is obvious to consider first how contemporary cell membranes are built. A high schematic representation - ignoring all the complexity of plasma membranes - is shown in Fig. 1 (Left). Cell membranes are composed of basically two main classes of molecules, lipids and proteins, independent on whether the cells belong to one of the three domains of living organisms: Eucarya, Bacteria and Archaea (Woese et al. 1990; Yamagishi et al. 1998). Eucaryotic membranes are formed by the self-assembly of amphiphilic di-acyl phospholipids, containing sterols like cholesterol. Bacterial membranes rarely contain cholesterol, but often contain hopanoids (Ourisson and Albrecht 1992; Ourisson and Rohmer 1992) or carotenoids (Liaane-Jensen 1979) (Fig. 2 Right). Archaeal membranes lack n-acyl lipids and instead consist of polyprenic diphytanylglyceryl or bis(diphytanylglyceryl) phospholipids (Fig. 3). Cholesterol, hopanoids, carotenoids, phytane and bisphytane are belonging to terpenoids (isoprenoids), which represent the most diverse family of natural products. These observations suggest that terpenoids are important for membrane function, either as membrane reinforcers in Eucarya and Bacteria or as principal membrane constituents in Archaea.
Fig. 1.
(Left, Ourisson and Nakatani 1994) Very simple model of eucaryotic membranes. Membranes separate ‘inside’ from ‘outside’. Eucaryotic membranes usually consist of a bilayer of di-acyl phospholipids and sterols like cholesterol. Amphipathic proteins are also present in the bilayer. (Right) Topography of cholesterol in lipid bilayers
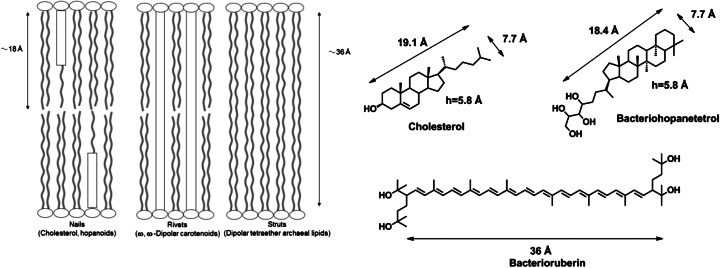
Fig. 2.
(Left, Ourisson and Nakatani 1994) Hypothetical mechanism of the reinforcement of lipidic membranes by cholesterol, hopanoids, α, ω-dipolar carotenoids, and bis(diphytanylglyceryl) phospholipids. (Right) Structure and molecular dimension of three membrane reinforcers: cholesterol, bacteriohopane tetrol, bacterioruberine
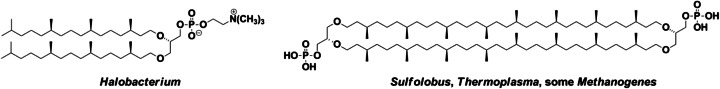
Fig. 3.
Structure of archaeal lipids: diphytanylglyceryl phospholipids in Halobacterium and bis(diphytanylglyceryl) phospholipids in Sulfolobus, Thermoplasma, Methanogene. Both the diether phophatidylcholine (left hand) and the 72-membered macrocyclic tetraether di-phosphates (right hand) formed vesicles in water (Dannenmuller et al. 2000; Eguchi et al. 2000)
The Role of Cholesterol in Membranes: Membrane Reinforcer
Let us first consider the effect of cholesterol on eucaryotic phospholipid membranes; it is incorporated in the bilayer, its OH group forms a hydrogen bond with the head-group of a phospholipid (Rohmer et al. 1979; Nakatani et al. 1996). Its dimensions allow cooperative van der Waals attractive forces to elongate and order the neighboring lipidic chains by decreasing their ability to undergo trans-gauche isomeric rotations (Scott HL 1991) (Fig. 1 Right).
There are different types of membrane reinforcement (Fig. 2 Left). Cholesterol and hopanoids act as ‘nails’, inserting into one half of the lipid bilayer. α, ω-Dipolar carotenoids cross both halves of the bilayer like ‘rivets’ (Milon et al. 1986a). Some archaeal lipids (bola-amphiphilic type molecules: Fuhrhop JH and Wang T 2004) form both halves of the bilayer themselves and these membranes are automatically reinforced like ‘struts’.
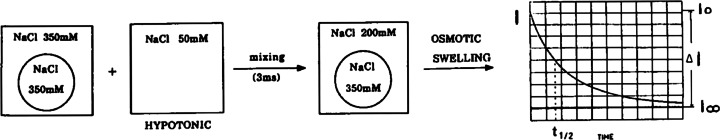
This reinforcement of membrane architecture has been shown by a wide variety of physical methods (Kwok and Evans 1981; Demel and De Kruyff 1976). We developed a new direct method to evaluate the mechanical properties of vesicles in the presence of cholesterol or its surrogates (Sun et al. 1986; Milon et al. 1986b). More precisely, we employed a stopped-flow/light scattering method to follow the swelling of unilamellar vesicles in response to osmotic pressure (Fig. 4).
Fig. 4.
Recording of scattered light versus time during vesicle swelling upon osmotic pressure. Analysis : I(t) – Io = ΔI [1 – e(−t/τ)], I: light scattering intensity; Apparatus : stopped-flow: t ½ : reaction half-time. After rapid mixing (3 ms) of a vesicle solution with the same volume of a hypotonic buffer, the scattered light decreases upon vesicle swelling as a result of the decreasing bilayer thickness (Milon et al. 1986b)
We first established that the kinetics of vesicle swelling is controlled by the water permeability. We then observed that cholesterol and α, ω-dipolar carotenoids lower the water permeability, showing that they reinforce the membrane (Milon et al. 1986a; Lazrak et al. 1987). The reinforcement of membranes by hopanoids was shown on monolayer studies (Kannenberg et al. 1983).
The amphiphilic molecules in primitive membranes must have been very different from the molecules in membranes of the present-day living organisms. As was mentioned above, there are striking differences in the membrane lipid structures found in Archaea (polyprenic phospholipids) and those in Eucarya (di-acyl phospholipids). The “fatty acid scenario” has been well discussed for the origin of di-acyl phospholipids (Gebicki and Hicks 1973; Hargreaves and Deamer 1978; Walde et al. 1994; Walde 2006; McCollom et al. 1999). Recently, it was shown by a stopped-flow method that single-chain fatty acid membranes are more permeable to ribose than di-acyl phospholipid membranes (Budin and Szostak 2011). We present here our hypothesis that polyprenyl phosphates could be the precursors of archaeal membrane lipids. This scenario would be an alternative of the former one.
Search for the Most ‘primitive’ Membranes
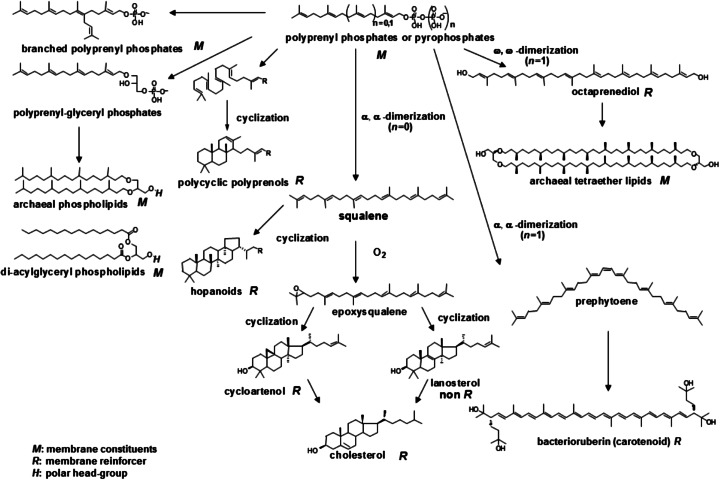
It was possible to arrange the known membrane terpenoids in a ‘phylogenetic’ sequence, and a retrograde analysis led us to conceive that polyprenyl phosphates might have been ‘primitive’ membrane constituents (Fig. 5).
Fig. 5.
(adapted from Ourisson and Nakatani 1994) Hypothetical evolution of membrane terpenoids (membrane components and reinforcers). Di-acylglyceryl phospholipids are shown for comparison
Starting from these polyprenyl phosphates, the step-wise recruitment of novel reactions, non-enzymatic at first, could have led to changes in the polar head-group, to cyclizations, dimerizations, and oxidations, and finally to archaeal lipids, carotenoids, hopanoids, and cholesterol.
‘Prebiotic’ Synthesis of Polyprenols
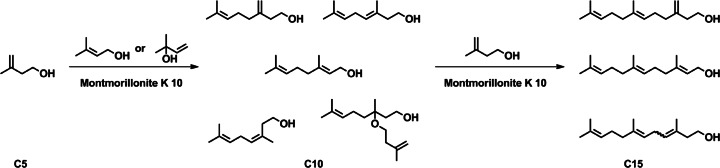
Polyprenyl chains of Archaea are biosynthesized by successive C5 increments of C5 isoprene units. The chemistry involved in these elongation steps is electrophilic alkylations of double bonds. The duplication of C5 units has been realized in 57 % yield (a mixture of isomeric C10 prenols) by simple treatment of monoprenols in the presence of montmorillonite clay (Fig. 6). These steps have been repeated, and led from geraniol (C10) to C15 prenols in 15 % yield (Désaubry et al. 2003). These steps should be tested with C5 and C10 prenylphosphates or pyrophosphates (Fig. 7).
Fig. 6.
Synthesis of polyprenols under ‘prebiotic’ conditions (adapted from Désaubry et al. 2003)
Fig. 7.
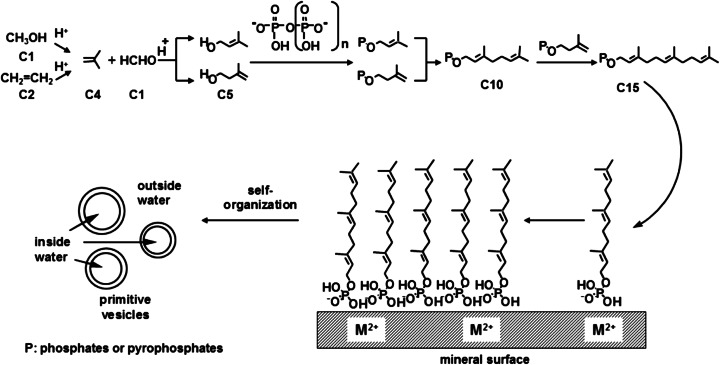
(adapted from Ourisson and Nakatani 1994) Hypothetical pathway from C1 or C2 units through C5 units to ‘primitive’ membranes. Farnesyl phosphate (C15) is synthesized and accumulated on the mineral surface
Search for the Origin of C5 Isoprene Units
What compounds might be the starting materials of C5 isoprene units in the prebiotic synthesis ? In present-day living organisms, the C5 unit is made either by the mevalonate pathway (Bloch 1992) or by the methylerythritol phosphate pathway (Rohmer 2003). Both are too complex to have close prebiotic analogues. However, these C5 units could be derived from simpler precursors (Fig. 7). Indeed, they (isopentenol and dimethylallyl alcohol) were synthesized by acid catalyzed Prins reaction from formaldehyde and isobutene (Blomquist and Verdol 1955). Isobutene itself was obtained by olefin homologation on zeolite, either from methanol (C1) or from ethylene (C2) in high temperature (Cui et al. 2008; Ilias and Bhan 2013; Oikawa et al. 2006). These abiotic conditions are potentially prebiotic, and isobutene, ethylene and formaldehyde do occur in volcanic gases (Capaccioni et al. 2004, 2011; Minissale et al. 2007; Tassi et al. 2009; Kiyosu and Asada 1995; Oro 1994). These steps should be tested experimentally in similar conditions on the prebiotic Earth (Martin et al. 2008; Mulkidjanian et al. 2012).
Hypothetical Pathway for the Formation of ‘primitive’ Membranes
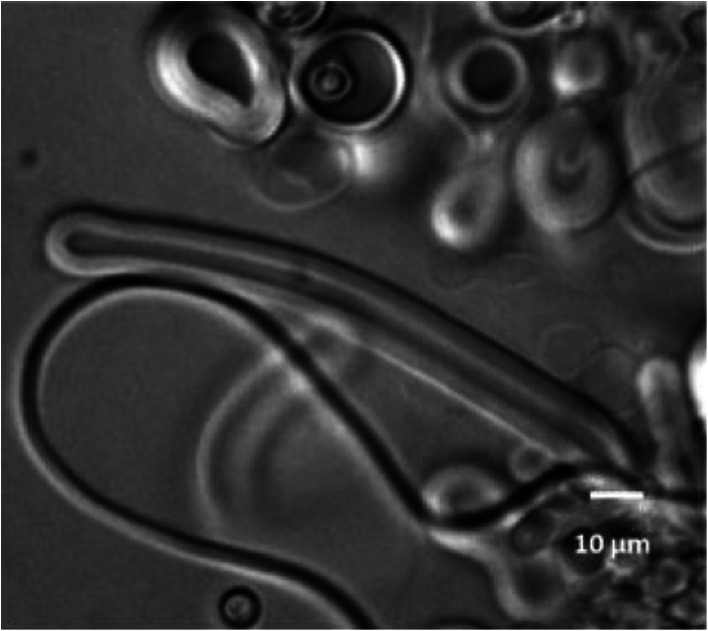
We postulated that the simplest possible polar head could be a phosphate and synthesized a series of polyprenyl phosphates. We have shown by optical and confocal microscopies that single-chain polyprenyl phosphates containing 15 C-atoms (three C5 units) to 30 C-atoms (six C5 units) form giant vesicles in a wide pH range of 2 to 9 (Pozzi et al. 1996; Streiff et al. 2007) (Fig. 8). Based on the above observations, we postulated a plausible scheme for the chain elongation of polyprenols leading to the formation of ‘primitive’ membranes (Fig. 7).
Fig. 8.
Phase contrast image of giant vesicles of farnesyl phosphates (C15) with spherical and discoid shapes at pH = 3.6 and T = 25 °C. Bar: 10 μm
The synthesis of polyprenyl phosphates (and pyrophosphates) with progressively longer chains (C5 increments) could be achieved by anchoring one end of the molecule to a solid mineral surface through a phosphate group. Once critical length and concentration have been achieved, polyprenyl phosphates would peel off the surface to form vesicles (Fig. 7). Note that the intermediates involved closely simulate those operating, with enzymes, in the biosynthesis of polyprenols (Porter and Spurgeon 1981). In contemporary organisms, the stepwise condensation of C5 phosphate units is achieved by specific prenyl-transferases (Liang et al. 2002). It is probably not a coincidence, but a reminiscence that the modern biosynthesis of polyprenols involves pyrophosphates at all stages.
Polyprenols as Reinforcers of Primitive Membranes
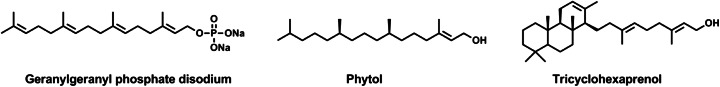
We have above observed that single-chain polyprenyl phosphates occupy a central position in the ‘phylogenetic’ sequence of membrane terpenoids (Fig. 5) and they form vesicles by themselves. Which components could have been reinforcers of polyprenyl phosphates ? We speculated that ‘primitive’ membranes might have been made up of a mixture of polyprenyl phosphates and polyprenyl alcohols (polyprenols), in much the same way, n-acyl carboxylates gave vesicles when mixed with equimolecular amounts of n-alcohols of the same chain length (Hargreaves and Deamer 1978; Apel et al. 2002). We have shown that the addition of the corresponding polyprenols increases the stability of vesicles of polyprenyl phosphates at higher pHs up to pH 12 (Streiff et al. 2007; Ribeiro et al. 2007), which is in good agreement with the ratio of hydrophilic and hydrophobic volume to predict the vesicle formation (Israelachivili et al. 1977). Using the stopped-flow/light scattering method, we demonstrated that the addition of 10 mol % of some polyprenols decreases the water permeability of polyprenyl phosphate vesicles (Table 1). This study thus provided a favorable argument that polyprenols might have been reinforcers of polyprenyl phosphate membranes. It may be noted that phytol and tricyclohexaprenol are as much as or better reinforcers than cholesterol in geranylgeranyl phosphate membrane (Fig. 9).
Table 1.
(adapted from Ribeiro et al. 2007) Water permeability of vesicles made of geranylgeranyl phosphate alone or with 10 mol% of polyprenols, measured at 15.0 ± 0.1 °C in a stopped-flow/light scattering apparatus
| Lipid composition of vesicles | Vesicle diameter a (nm) | t1/2 b (ms) |
|---|---|---|
| Geranylgeranyl phosphate (GGP) | 180 ± 16 | 20 ± 1 |
| GGP + 10 mol% phytol | 165 ± 24 | 234 ± 7 |
| GGP + 10 mol% tricyclohexaprenol | 170 ± 39 | 250 ± 20 |
| GGP + 10 mol% cholesterol | 162 ± 35 | 193 ± 8 |
aAverage diameter ± standard deviation (evaluated by photon correlation spectroscopy)
bAverage t1/2 ± standard deviation. t ½: reaction half-time
Fig. 9.
Structure of geranylgeranyl phosphate, phytol and tricyclohexaprenol
What is the actual advantage for a membrane to be“reinforced” by a sterol or a polyprenol ? One could propose several arguments to this issue. At a physical level, the membrane is subject to a number of physical stressing events during a cell’s life. Cells have to maintain the interior integrity, as well as transmembrane potential at all time. Thus it is essential for the membrane to resist to any harsh situation and the optimization of membrane reinforcers obviously has been nature’s strategy. It is routinely observed that hyperosmotic shocks on non-reinforced plain phospholipid bilayers lead to their disruption and leakage of their content. On an evolutionary point of view, it is amazing to observe how eucaryotic cells have been optimizing cholesterol structure to make it a perfect membrane reinforcer. The history of this optimization over millions of years can be traced in cholesterol metabolic pathway, and especially in the successive removal of methyl groups on its alpha face (Fig. 5) which lowered its membrane interactions (Bloch 1983). Our hypothesis here is that even before eucaryotic cells and cholesterol appeared on earth, at the level of procaryotic cells, this strategy of incorporating in membranes and optimizing membrane reinforcers was already in place.
Prevalence of Phosphates and Phosphorylation of Alcohols
Phosphates are present, in the form of an ester, in adenosine triphosphate (ATP), nucleic acids, and membrane lipids of all living organisms. The indispensable role of phosphates in many aspects of biochemistry is assigned to the specific properties of this group (Westheimer 1987). But, this ubiquitous role of phosphates is in sharp contrast with the low abundance of phosphorus in the Earth crust (0.12 %). There are, however, some scenarios in which phosphates could have been made available for prebiotic chemistry (Schwartz 2006). Pyrophosphate, triphosphate and tetraphosphate have been found in volcanic gases (Yamagata et al. 1991). The concentration of oligophosphate ions in layered minerals could be much increased resulting from the weathering of magmatic rocks (Arrhenius et al. 1997). Polyphosphates are also produced when orthophosphate salts are heated (Rabinowitz et al. 1968). The phosphorylation of polyprenols may have initially involved polyphosphoric acid (PPA), and phosphorylation of adenosine with polyphosphate salts in aqueous solution afforded a mixture of 5′adenosine monophosphate (5′AMP), 2′AMP and 3′AMP (Schwartz and Ponnamperuma 1968). Recently, PPA was employed for the synthesis of phosphoglycolamide from glycolamide (Weber et al. 2003; Krishnamurthy et al. 1999). Phosphorylation of adenosine into adenosine-5′-triphosphate was performed using trimetaphosphate (cyclotriphosphate) (Saffhill 1970; Etaix and Orgel 1978). Phosphorylation of some nucleosides with urea-inorganic phosphate mixtures gave nucleoside phosphates (Lohrmann and Orgel 1970). C5-C20 isoprenoid phosphates along with their pyrophosphates were simply obtained from the condensation of the corresponding alcohols with bis-triethylammonium phosphate (Keller and Thompson 1993) or tetrabutylammonium dihydrogen phosphate (Danilov et al. 1989). The conversion of alcohols into the corresponding phosphate esters was achieved using acetic anhydride activation (Dueymes et al. 2008) or imidoyl phosphate activation (Powner and Sutherland 2011) of inorganic phosphate. Phosphitylation of alcohols followed by oxidation could be an alternative pathway for the formation of phosphates (Yoza et al. 1992; De Graaf and Schwartz 2000; Bryant et al. 2010). Recently, it was proposed that meteorites would have provided the reduced iron-nickel phosphide schreibersite (Pasek et al. 2013). They have shown that schreibersite reacts with glycerol in an aqueous solution to give glyceryl phosphate. In spite of these observations, the origin of the phosphate in prebiotic system and the non-enzymatic synthesis of organophosphates continue to remain unresolved issues.
From ‘primitive’ Membrane Lipids to Archaeal Lipids
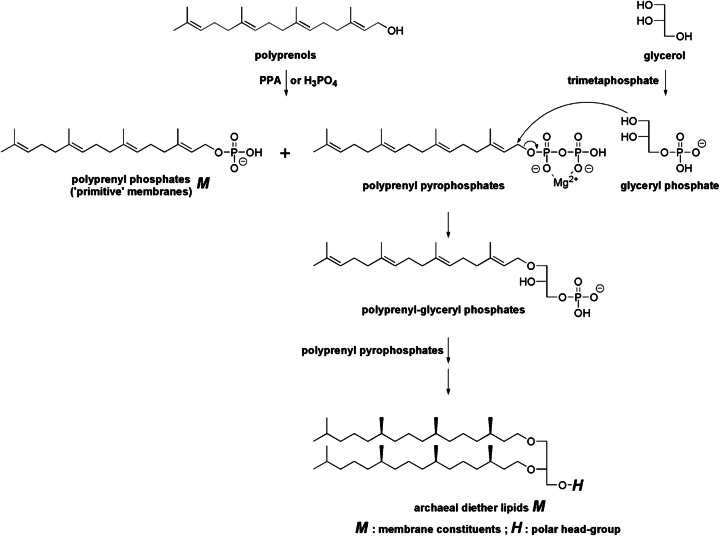
The next stage of elaboration of polyprenyl phosphates (‘primitive’ membrane lipids) could have been to change the head-group. There could be several plausible ways in which the polyprenyl phosphates might have been converted to di-polyprenyl-glyceryl phosphates, contemporary membrane constituents of Halobacterium. One hypothetical pathway could involve polyprenyl-glyceryl phosphates (Fig. 10). Starting compounds would be glycerol and a polyprenol. Glyceryl phosphate could be obtained by the phosphorylation of glycerol, for example, with amido-triphosphate derived from metatriphosphate in the presence of Mg 2+ (Mullen and Sutherland 2007). Polyprenyl phosphate and polyprenyl pyrophosphate could be obtained by phosphorylation by polyphosphate (Schwartz and Ponnamperuma 1968) or simply by phosphate (Keller and Thompson 1993; Danilov et al. 1989). The OH group of glyceryl phoshate is known to be a good nucleophile for the acylation (Ichihara et al. 2005). The nucleophilic attack on polyprenyl pyrophosphate (complexed with Mg 2+) by glyceryl phoshate could afford polyprenyl-glyceryl phosphates in a similarity with the biosynthesis of geranylgeranyl-glyceryl phosphate from geranylgeraniol and glyceryl phosphate (Zhang et al. 1990). The hydrophobic/hydrophilic balance in the structure of polyprenyl-glyceryl phosphates might not be favorable for the formation of vesicles (Tanaka et al. 2004). It is likely, however, that polyprenyl-glyceryl phosphates could also form vesicles, perhaps when combined with suitable amounts of polyprenols. These scenarios will have to be experimentally examined.
Fig. 10.
Hypothetical pathway from polyprenyl pyphosphates to archaeal lipids and mechanism for the formation of a glyceryl head-group. In the transition state for the reaction of polyprenyl pyrophosphate and glyceryl phosphate, the carbocation intermediate at C1-C3 carbons of polyprenyl pyrophosphate is formed and the negative charge developed on pyrophosphate group is stabilized by coordination with Mg 2+. The pyrophosphate complexed with Mg 2+ could serve as a good leaving group. PPA polyphosphoric acid
Conclusion
In contrast to the fatty acid ester motif found in Eucarya and Bacteria, archaeal lipids are composed of diphytanylglyceryl units bearing phosphates as polar head group. We present here our hypothesis that polyprenyl phosphates could be the precursors of archaeal membrane lipids. We first observed that single-chain polyprenyl phosphates occupy a central position in the ‘phylogenetic’ sequence of membrane terpenoids (Fig. 5). We then confirmed that polyprenyl phosphates form vesicles in water alone or with polyprenols. Polyprenols were shown to be good membrane reinforcers. These observations led us to hypothesize that primitive membranes might have been formed by polyprenyl phosphates and polyprenols. A hypothetical pathway from C1 or C2 units through C5 isoprene units to ‘primitive’ membrane was discussed. Finally, we proposed a hypothetical pathway from polyprenyl phosphates (‘primitive’ membrane lipids) to archaeal lipids. These questions are important and they should be examined in a significant manner when the issue of prebiotic reactions is clarified. Nobody knows which the first membrane-forming molecules were. But ‘polyprenyl phosphates scenario’ could be a good and reasonable alternative to the ‘fatty acid scenario’.
Furthermore, once vesicles are formed, a remarkable series of new properties are automatically brought into operation and make it possible to progressively complexify the system towards proto-cells (Walde et al. 1994; Ourisson and Nakatani 1999, 2006; Szostak et al. 2001; Luisi 2002; Takakura et al. 2003; Walde 2006; Luisi and Stano 2011).
Acknowledgments
We express our hearty thanks to Prof. Guy Ourisson, who invited us to join to the exciting search on the origin and molecular evolution of biomembranes. Y.N. is greatly indebted to Prof. Guy Ourisson and co-workers, listed in many publications from the laboratory. Y.N. thanks also Prof. Peter Walde, Prof. Patrick Pale, Prof. Tsutomu Hamada and Prof. Taro Toyota for their critical reading of the manuscript.
References
- Apel CL, Deamer DW, Mautner MN. Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim Biophys Acta. 2002;1559:1–9. doi: 10.1016/S0005-2736(01)00400-X. [DOI] [PubMed] [Google Scholar]
- Arrhenius GB, Sales BS, Mojzsis ST, Lee T. Entropy and charge in molecular evolution - the case of phosphate. J Theor Biol. 1997;187:503–522. doi: 10.1006/jtbi.1996.0385. [DOI] [PubMed] [Google Scholar]
- Bloch K. Sterol structure and membrane function. CRC Crit Rev Biochem. 1983;14:47–92. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- Bloch K. Sterol molecule: structure, biosynthesis and function. Steroids. 1992;57:378–383. doi: 10.1016/0039-128X(92)90081-J. [DOI] [PubMed] [Google Scholar]
- Blomquist AT, Verdol JA. The thermal isobutylene-formaldehyde condensation. J Am Chem Soc. 1955;77:77–80. [Google Scholar]
- Bryant DE, Marriott KER, Macgregor SA, Kilner C, Pasekc MA, Kee TP. On the prebiotic potential of reduced oxidation state phosphorus: the H-phosphinate-pyruvate system. Chem Commun. 2010;46:3726–3728. doi: 10.1039/c002689a. [DOI] [PubMed] [Google Scholar]
- Budin I, Szostak JW. Physical effects underlying the transition from primitive to modern cell membranes. Proc Natl Acad Sci U S A. 2011;108:5249–5254. doi: 10.1073/pnas.1100498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaccioni B, Taran Y, Tassi F, Vaselli O, Mangani G, Macias JL. Source conditions and degradation processes of light hydrocarbons in volcanic gases: an example from El Chichon volcano (Chiapas State, Mexico) Chem Geol. 2004;206:81–96. doi: 10.1016/j.chemgeo.2004.01.011. [DOI] [Google Scholar]
- Capaccioni B, Aguilera F, Tassi F, Darrah T, Poreda RJ, Vaselli O. Geochemical and isotopic evidences of magmatic inputs in the hydrothermal reservoir feeding the fumarolic discharges of Tacora volcano (northern Chile) J Volcanol Geotherm Res. 2011;208:77–85. doi: 10.1016/j.jvolgeores.2011.09.015. [DOI] [Google Scholar]
- Cui ZM, Liu Q, Ma Z, Bian SW, Song WG. Direct observation of olefin homologations on zeolite ZSM-22 and its implications to methanol to olefin conversion. J Catal. 2008;258:83–86. doi: 10.1016/j.jcat.2008.05.029. [DOI] [Google Scholar]
- Danilov LL, Druzhinina TN, Kalinchuk NA, Maltsev SD, Shibaev VN. Polyprenyl phosphates: synthesis and structure-activity relationship for a biosynthetic system of Salmonella anatum O-specific polysaccharide. Chem Phys Lipids. 1989;51:191–203. doi: 10.1016/0009-3084(89)90006-6. [DOI] [PubMed] [Google Scholar]
- Dannenmuller O, Arakawa K, Eguchi T, Kakinuma K, Blanc S, Albrecht AM, Schmutz M, Nakatani Y, Ourisson G. Membrane properties of archæal macrocyclic diether phospholipids. Chem Eur J. 2000;6:645–654. doi: 10.1002/(SICI)1521-3765(20000218)6:4<645::AID-CHEM645>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- De Graaf RM, Schwartz AW. Reduction and activation of phosphate on the primitive earth. Orig Life Evol Biosph. 2000;30:405–410. doi: 10.1023/A:1006700512902. [DOI] [PubMed] [Google Scholar]
- Deamer DW. Role of amphiphilic compounds in the evolution of membrane structure on the early Earth. Orig Life Evol Biosph. 1986;17:3–25. doi: 10.1007/BF01809809. [DOI] [PubMed] [Google Scholar]
- Demel RA, De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976;457:109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Désaubry L, Nakatani Y, Ourisson G. Toward higher polyprenols under ‘prebiotic’ conditions. Tetrahedron Lett. 2003;44:6959–6961. doi: 10.1016/S0040-4039(03)01624-1. [DOI] [Google Scholar]
- Dueymes C, Pirat C, Pascal R. Facile synthesis of simple mono-alkyl phosphates from phosphoric acid and alcohols. Tetrahedron Lett. 2008;49:5300–5301. doi: 10.1016/j.tetlet.2008.06.083. [DOI] [Google Scholar]
- Eguchi T, Arakawa K, Kakinuma K, Rapp G, Ghosh S, Nakatani Y, Ourisson G. Giant vesicles from 72-membered macrocyclic archæal phospholipid analogues. Initiation of vesicle formation by molecular recognition between membrane components. Chem Eur J. 2000;6:3351–3358. doi: 10.1002/1521-3765(20000915)6:18<3351::AID-CHEM3351>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Etaix E, Orgel LE. Phosphorylation of nucleosides in aqueous solution using trimetaphosphate: formation of nucleoside triphosphates. J Carbohydr Nucleosides Nucleotides. 1978;5:91–110. [Google Scholar]
- Fuhrhop JH, Wang T. Bolaamphiphiles. Chem Rev. 2004;104:2901–2938. doi: 10.1021/cr030602b. [DOI] [PubMed] [Google Scholar]
- Gebicki JM, Hicks M. Ufasomes are stable particles surrounded by unsaturated fatty acid membranes. Nature. 1973;243:232–234. doi: 10.1038/243232a0. [DOI] [PubMed] [Google Scholar]
- Hargreaves WR, Deamer DW. Liposomes from ionic, single-chain amphiphiles. Biochemistry. 1978;17:3759–3768. doi: 10.1021/bi00611a014. [DOI] [PubMed] [Google Scholar]
- Ichihara K, Iwasaki H, Ueda K, Takizawa R, Naito H, Tomosugi M. Synthesis of phosphatidylcholine: an improved method without using the cadmium chloride complex of sn-glycero-3-phosphocholine. Chem Phys Lipids. 2005;137:94–99. doi: 10.1016/j.chemphyslip.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Ilias S, Bhan A (2013) Mechanism of the catalytic conversion of methanol to hydrocarbons. ACS Catal 3: 18–31
- Israelachivili JN, Mitchell J, Ninham BW. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. Biochim Biophys Acta. 1977;470:185–202. doi: 10.1016/0005-2736(77)90099-2. [DOI] [PubMed] [Google Scholar]
- Kannenberg E, Blume A, Mc Elhaney RN, Poralla K. Monolayer and calorimetric studies of phosphatidylcholines containing branched-chain fatty acids and of their interactions with cholesterol and with a bacterial hopanoid in model membranes. Biochim Biophys Acta. 1983;733:111–116. doi: 10.1016/0005-2736(83)90096-2. [DOI] [Google Scholar]
- Keller RK, Thompson R. Rapid synthesis of isoprenoid diphosphates and their isolation in one step using either thin layer or flash chromatography. J Chromatogr. 1993;645:161–167. doi: 10.1016/0021-9673(93)80630-Q. [DOI] [PubMed] [Google Scholar]
- Kiyosu Y, Asada N. Light hydrocarbons in volcanic gases from the Japanese island arc. Geochem J. 1995;29:231–241. doi: 10.2343/geochemj.29.231. [DOI] [Google Scholar]
- Krishnamurthy R, Arrhenius G, Eschenmoser A. Formation of glycolaldehyde phosphate from glycolaldehyde in aqueous solution. Orig Life Evol Biosph. 1999;29:333–354. doi: 10.1023/A:1006698208873. [DOI] [PubMed] [Google Scholar]
- Kwok, Evans Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981;35:637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazrak T, Milon A, Wolff G, Albrecht A, Miehé M, Ourisson G, Nakatani Y. Comparison of the effects of inserted C40- and C50-terminally dihydroxylated carotenoids on the mechanical properties of various phospholipid vesicles. Biochim Biophys Acta. 1987;903:132–141. doi: 10.1016/0005-2736(87)90163-5. [DOI] [PubMed] [Google Scholar]
- Liaane-Jensen S. Carotenoids—a chemosystematic approach. Pure Appl Chem. 1979;51:661–675. [Google Scholar]
- Liang PH, Ko TP, Wang AHJ. Structure, mechanism and function of prenyltransferases. Eur J Biochem. 2002;269:3339–3354. doi: 10.1046/j.1432-1033.2002.03014.x. [DOI] [PubMed] [Google Scholar]
- Lohrmann R, Orgel Urea-inorganic phosphate mixtures as prebiotic phosphorylating agents. Science. 1970;171:492–493. doi: 10.1126/science.171.3970.490. [DOI] [PubMed] [Google Scholar]
- Luisi PL. Toward the engineering of minimal living cells. Anat Rec. 2002;268:208–214. doi: 10.1002/ar.10155. [DOI] [PubMed] [Google Scholar]
- Luisi PL, Stano P. Synthetic biology: minimal cell mimicy. Nat Chem. 2011;3:755–756. doi: 10.1038/nchem.1156. [DOI] [PubMed] [Google Scholar]
- Martin W, Baross J, Kelley D, Russell MJ (2008) Hydrothermal vents and the origin of life. Nat Rev Microbiol 6:805–814 [DOI] [PubMed]
- McCollom TM, Ritter G, Simoneit BR. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig Life Evol Biosph. 1999;29:153–166. doi: 10.1023/A:1006592502746. [DOI] [PubMed] [Google Scholar]
- Milon A, Wolff G, Ourisson G, Nakatani Y. Organization of carotenoid-phospholipid bilayer systems. Incorporation of zeaxanthin, astanxanthin and their C50 homologues into dimyristoylphosphatidylcholine vesicles. Helv Chim Acta. 1986;69:12–24. doi: 10.1002/hlca.19860690104. [DOI] [Google Scholar]
- Milon A, Lazrak T, Albrecht AM, Wolff G, Weill G, Ourisson G, Nakatani Y. Osmotic swelling of unilamellar vesicles by the stopped-flow light scattering method. Influence of vesicle size, solute, temperature, cholesterol and three α, ω-dihydroxycarotenoids. Biochim Biophys Acta. 1986;859:1–9. doi: 10.1016/0005-2736(86)90311-1. [DOI] [Google Scholar]
- Minissale A, Mattash MA, Vaselli O, Tassi F, Al-Ganad IN, Selmo E, Shawki NM, Tedesco D, Poreda R, Ad-Dukhain AM, Hazzae MK. Thermal springs, fumaroles and gas vents of continental Yemen: their relation with active tectonics, regional hydrology and the country’s geothermal potential. Appl Geochem. 2007;22:799–820. doi: 10.1016/j.apgeochem.2006.11.009. [DOI] [Google Scholar]
- Morowitz HJ, Heinz D, Deamer DW. The chemical logic of a minimum protocell. Orig Life Evol Biosph. 1988;18:281–287. doi: 10.1007/BF01804674. [DOI] [PubMed] [Google Scholar]
- Mulkidjanian AY, Bychkov AY, Dibrova DV, Galperin MY, Koonin EV. Origin of first cells at terrestrial, anoxic geothermal fields. Proc Natl Acad Sci U S A. 2012;109:E821–E830. doi: 10.1073/pnas.1117774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen LB, Sutherland JD. Formation of potentially prebiotic amphiphiles by reaction of beta-hydroxy-n-alkylamines with cyclotriphosphate. Angew Chem Int Ed Engl. 2007;46:4166–4168. doi: 10.1002/anie.200700394. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Yamamoto M, Diyizou Y, Warnock W, Dollé V, Hahn W, Milon A, Ourisson G. Studies on the topography of biomembranes: regioselective photolabelling in vesicles with the tandem use of cholesterol and a photoactivable transmembrane phospholipidic probe. Chem Eur J. 1996;2:129–138. doi: 10.1002/chem.19960020204. [DOI] [Google Scholar]
- Oikawa H, Shibata Y, Inazu K, Iwase Y, Murai K, Hyodo S, Kobayashi G, Baba T. Highly selective conversion of ethylene to propene over SAPO-34 as a solid acid catalyst. App Catal A: Gen. 2006;312:181–185. doi: 10.1016/j.apcata.2006.06.045. [DOI] [Google Scholar]
- Oro J. Early life on earth. In: Bengtson S, editor. Nobel symposium N° 84. New York: Columbia UP; 1994. pp. 48–59. [Google Scholar]
- Ourisson G, Albrecht P. Hopanoids. 1. Geohopanoids: the most abundant natural products on earth? Acc Chem Res. 1992;25:398–402. doi: 10.1021/ar00021a003. [DOI] [Google Scholar]
- Ourisson G, Nakatani Y. The terpenoid theory of the origin of cellular life: the evolution of terpenoids to cholesterol. Chem Biol. 1994;1:11–23. doi: 10.1016/1074-5521(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Ourisson G, Nakatani Y. Origins of cellular life: molecular foundations and new approaches. Tetrahedron. 1999;55:3283–3190. doi: 10.1016/S0040-4020(99)00116-7. [DOI] [Google Scholar]
- Ourisson G, Nakatani Y (2006) A rational approach to the origin of life: from amphiphilic molecules to protocells. Some plausible solutions, and some real problems. In: Gargaud M, Barbier B, Martin H, Reisse J (eds), Lectures in astrobiology, Vol. 1: Part 2, Springer, Berlin, Heidelberg, 29–48
- Ourisson G, Rohmer M. Hopanoids. 2. Biohopanoids: a novel class of bacterial lipids. Acc Chem Res. 1992;25:403–408. doi: 10.1021/ar00021a004. [DOI] [Google Scholar]
- Pasek MA, Harnmeijer JP, Buick R, Gull M, Atlas Z. Evidence for reactive reduced phosphorus species in the early Archean ocean. Proc Natl Acad Sci U S A. 2013;110:10089–10094. doi: 10.1073/pnas.1303904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JW, Spurgeon SL. Biosynthesis of isoprenoid compounds. New York: Wiley; 1981. [Google Scholar]
- Powner MW, Sutherland JD. Prebiotic chemistry: a new modus operandi. Phil Trans R Soc B. 2011;366:2870–2877. doi: 10.1098/rstb.2011.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi G, Birault V, Werner B, Dannenmuller O, Nakatani Y, Ourisson G, Terakawa S. Single-chain polyterpenyl phosphates form primitive membranes. Angew Chem Int Ed Engl. 1996;35:177–180. doi: 10.1002/anie.199601771. [DOI] [Google Scholar]
- Rabinowitz J, Chang S, Ponnamperuma C. Phosphorylation on the primitive earth. Nature. 1968;218:442–443. doi: 10.1038/218442a0. [DOI] [PubMed] [Google Scholar]
- Ribeiro N, Streiff S, Heissler D, Elhabiri M, Albrecht-Gary AM, Atumi M, Gotoh M, Desaubry L, Nakatani Y, Ourisson G. Reinforcing effect of bi- and tri-cyclopolyprenols on ‘primitive’ membranes made of polyprenyl phosphates. Tetrahedron. 2007;63:3395–3407. doi: 10.1016/j.tet.2007.01.076. [DOI] [Google Scholar]
- Rohmer M. Mevalonate-independent methylerythritol phosphate pathway for isoprenoid biosynthesis. Elucidation and distribution. Pure Appl Chem. 2003;75:375–387. doi: 10.1351/pac200375020375. [DOI] [Google Scholar]
- Rohmer M, Bouvier P, Ourisson G. Molecular evolution of biomembranes: structural equivalents and phylogenetic precursors of sterols. Proc Natl Acad Sci U S A. 1979;76:847–851. doi: 10.1073/pnas.76.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffhill R. Selective phosphorylation of the cis-2′, 3′-diol of unprotected ribonucleosides with trimetaphosphate in aqueous solution. J Org Chem. 1970;35:2881–2883. doi: 10.1021/jo00834a004. [DOI] [PubMed] [Google Scholar]
- Schwartz AW. Phosphorus in prebiotic chemistry. Phi Trans R Soc B. 2006;361:1743–1749. doi: 10.1098/rstb.2006.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Ponnamperuma C. Phosphorylation of adenosine with linear polyphosphate salts in aqueous solution. Nature. 1968;218:443. doi: 10.1038/218443a0. [DOI] [PubMed] [Google Scholar]
- Scott HL. Lipid-cholesterol interactions. Monte Carlo simulations and theory. Biophys J. 1991;59:445–455. doi: 10.1016/S0006-3495(91)82238-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streiff S, Ribeiro N, Wu Z, Gumienna-Kontecka E, Elhabiri M, Albrecht-Gary AM, Ourisson G, Nakatani Y. Primitive membrane from polyprenyl phosphates and polyprenyl alcohols. Chem Biol. 2007;14:313–319. doi: 10.1016/j.chembiol.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Sun ST, Milon A, Tanaka T, Ourisson G, Nakatani Y. Osmotic swelling of unilamellar vesicles by the stopped-flow light scaterring method. Elastic properties of vesicles. Biochim Biophys Acta. 1986;860:525–530. doi: 10.1016/0005-2736(86)90549-3. [DOI] [Google Scholar]
- Szostak JW, Bartel DP, Luisi PL. Synthesizing Life. Nature. 2001;409:387–390. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- Takakura K, Toyota T, Sugawara T. A novel system of self-reproducing giant vesicles. J Am Chem Soc. 2003;125:8134–8140. doi: 10.1021/ja029379a. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Sano R, Yamashita Y, Yamazaki M. Shape changes and vesicle fission of giant unilamellar vesicles of liquid-ordered phase membrane induced by lysophosphatidylcholine. Langmuir. 2004;20:9526–9534. doi: 10.1021/la049481g. [DOI] [PubMed] [Google Scholar]
- Tassi F, Aguilera F, Vasselli O, Medina E, Tedesco D, Delgado Huertas A, Poreda R, Kojima S. The magmatic- and hydrothermal-dominated fumarolic system at the active crater of Lascar volcano, northern Chile. Bull Volcanol. 2009;71:171–183. doi: 10.1007/s00445-008-0216-z. [DOI] [Google Scholar]
- Walde P. Surfactant assemblies and their various possible roles for the origin(s) of life. Orig Life Evol Biosph. 2006;36:109–150. doi: 10.1007/s11084-005-9004-3. [DOI] [PubMed] [Google Scholar]
- Walde P, Wick R, Fresta M, Mangone A, Luisi PL. Autopoietic self-reproduction of fatty acid vesicles. J Am Chem Soc. 1994;116:11649–11654. doi: 10.1021/ja00105a004. [DOI] [Google Scholar]
- Weber P, Fonvielle M, Therisod M. New facile synthesis of phosphoglycolohydroxamic acid and other phosphoglycolic acid derivatives. Tetrahedron Lett. 2003;44:9047–9049. doi: 10.1016/j.tetlet.2003.09.210. [DOI] [Google Scholar]
- Westheimer FH. Why nature chose phosphates. Science. 1987;235:1173–1178. doi: 10.1126/science.2434996. [DOI] [PubMed] [Google Scholar]
- Woese C, Kandler O, Wheelis M. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata Y, Watanabe H, Saitoh M, Namba T. Volcanic production of polyphosphate under primitive Earth conditions. Nature. 1991;204:516–519. doi: 10.1038/352516a0. [DOI] [PubMed] [Google Scholar]
- Yamagishi A, Kon T, Takahashi G, Oshima T. From the common ancestor of all living organisms to protoeukaryotic cell. In: Wiegel J, Adams MWW, editors. Thermophiles: the keys to molecular evolution and the origin of Life ? London: Taylor & Francis; 1998. pp. 287–295. [Google Scholar]
- Yoza N, Ueda N, Tokushige N, Miyajima T, Baba Y, Tsuhako M, Tateda A. High-performance liquid chromatographic and 31P NMR spectroscopic evidence for novel analogue of triphosphate formed by phosphonylation of orthophosphate with diphosphonate. Inorg Chim Acta. 1992;202:237–239. doi: 10.1016/S0020-1693(00)86840-1. [DOI] [Google Scholar]
- Zhang DL, Daniels L, Poulter CD. Biosynthesis of archaeal membranes. Formation of isoprene ethers by a prenyl transfer reaction. J Am Chem Soc. 1990;112:1264–1265. doi: 10.1021/ja00159a067. [DOI] [Google Scholar]