Highlight
This review highlights current information on the various factors involved in the abscission of flowers and fruitlets, a highly regulated developmental process.
Key words: Carbon metabolism, climate change, environmental stress, flower/fruit abscission, hormonal balance.
Abstract
In plants, flowering is a crucial process for reproductive success and continuity of the species through time. Fruit production requires the perfect development of reproductive structures. Abscission, a natural process, can occur to facilitate shedding of no longer needed, infected, or damaged organs. If stress occurs during flower development, abscission can intervene at flower level, leading to reduced yield. Flower abscission is a highly regulated developmental process simultaneously influenced and activated in response to exogenous (changing environmental conditions, interactions with microorganisms) and endogenous (physiological modifications) stimuli. During climate change, plant communities will be more susceptible to environmental stresses, leading to increased flower and fruit abscission, and consequently a decrease in fruit yield. Understanding the impacts of stress on the reproductive phase is therefore critical for managing future agricultural productivity. Here, current knowledge on flower/fruit abscission is summarized by focusing specifically on effects of environmental stresses leading to this process in woody plants. Many of these stresses impair hormonal balance and/or carbohydrate metabolism, but the exact mechanisms are far from completely known. Hormones are the abscission effectors and the auxin/ethylene balance is of particular importance. The carbohydrate pathway is the result of complex regulatory processes involving the balance between photosynthesis and mobilization of reserves. Hormones and carbohydrates together participate in complex signal transduction systems, especially in response to stress. The available data are discussed in relation to reproductive organ development and the process of abscission.
Introduction
Throughout their development, plants are subject to a multiplicity of stresses, which lead to molecular, biochemical, physiological, anatomical, and morphological changes that may adversely affect their growth and productivity (Stopar, 1998). The abscission process has been developed by plants to facilitate the shedding of no longer needed, infected, damaged or senescent organs. This phenomenon can occur in both vegetative and reproductive organs (González-Carranza et al., 1998; Taylor and Whitelaw, 2001; Estornell et al., 2013).
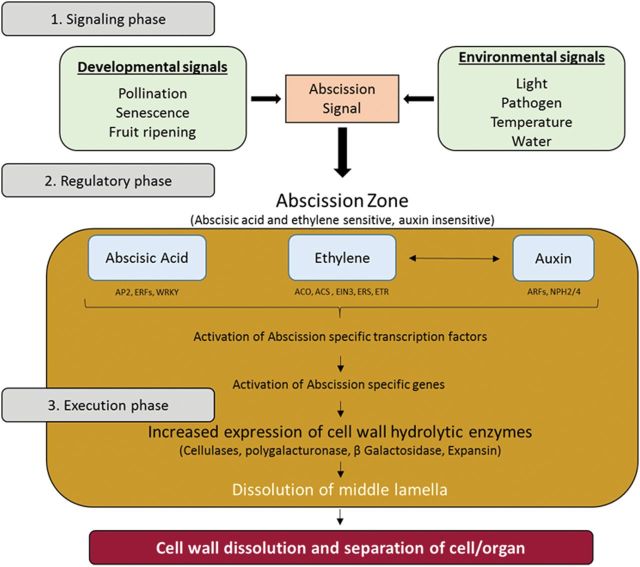
Abscission is an active physiological process that occurs through the dissolution of cell walls at predetermined positions, the abscission zones (AZs), often related to stress and senescence (Addicott, 1982; Taylor and Whitelaw, 2001). Secretion of hydrolytic enzymes, increased peroxidase activity, and loss of calcium and pectin from the wall of separation layer cells presumably lead to the dissolution of the pectin-rich middle lamella, weakening the cell wall and leading to disintegration of AZ tissues (Fig. 1; Addicott, 1982; Osborne, 1989; Tripathi et al., 2008). In flower, the AZs are located at the boundary between floral organs and the receptacle (González-Carranza et al., 2002; 2007; Lashbrook and Cai, 2008), but also within the flower pedicel (Zanchin et al., 1995; del Campillo and Bennett, 1996).
Fig. 1.
A model showing major events leading up to abscission [adapted from Tripathi et al. (2008)]. The complete abscission follows three phases. In the signalling phase, formation of the AZ takes place under various developmental and environmental conditions. In the regulatory phase, the AZ is able to perceive different stimuli generated by both external and internal factors and transduced by signals resulting in ethylene/ABA sensitivity and auxin insensitivity in the cell, activating several cascades and transcriptional regulators. In the execution phase, the initiation of abscission starts with the expression of several wall-loosening agents like cellulases, polygalacturonases, or expansin. The collective actions of all these agents accelerate the dissolution of middle lamella. Finally, cell wall dissolution takes place resulting in cell/organ separation. ACO, ACC oxidase; ACS, acyl CoA synthetase; ETR, ethylene response; ERS, ethylene response sensor; EIN3, ETHYLENE INSENSITIVE 3; AP2, ARF, ERF, NPH2/4, and WRKY are transcription factors.
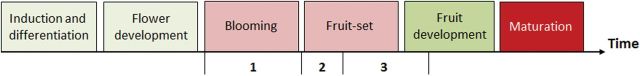
Three waves of abscission are recognized in fruit trees and can vary according to species (Fig. 2). Generally, the first wave occurs at blooming or shortly after, following pistil abortion, and consists largely of abnormal and unpollinated flowers. The second wave appears after failure of fertilization (Aloni et al., 1996; Rodrigo and Herrero, 2002; Acar and Kakani, 2010). The third wave, known as ‘June drop’, involves fruitlets and occurs following competition for nutrients (both among fruitlets and between fruitlets and vegetative shoots) and failure of embryo development (Goldschmidt and Koch, 1996; Yuan and Greene, 2000). As discussed later, abscission is a highly regulated developmental process that is simultaneously influenced and activated in response to exogenous (changing environmental conditions, interactions with microorganisms) and endogenous (physiological modifications) stimuli.
Fig. 2.
Schematic representation of the three waves of abscission (1, 2, and 3) occurring during the course of flower/fruit development [according to Goldschmidt and Monselise (1977)]. Major processes are indicated.
During climate change, stressed plant communities will be more susceptible to biotic and abiotic stress (Petoukhov and Semenov, 2010; IPCC, 2013), leading to flower and fruit abscission, and consequently to a decrease in fruit yield. During the activation phase of abscission, phytohormones are well known to be the principal transducers of genetic information that modulates the expression of abscission-related genes (Chandler, 2011; Sauer et al., 2013) (Fig. 1). Carbohydrates may also trigger the response of AZ cells to abscission signals.
This review reports on current information on various factors involved in the abscission of reproductive structures, especially in woody species. A special interest will be devoted to environmental, physiological and molecular changes, at hormone and carbohydrate levels, governing the abscission process in reproductive organs. We will focus on the signal transduction cascades affecting the abscission process in flowers and fruitlets (the abscission of mature fruits, which is better documented, is not discussed).
Impacts of biotic and abiotic stresses on abscission
As sessile organisms, plants are often exposed to unfavourable conditions due to biotic and/or abiotic stressses that can delay growth and development, reduce productivity and, in extreme cases, lead to death.
Biotic stresses
Following biotic stresses, the activation of the plant immune system, which allows a switch from growth and development into a defensive mode, leads to a lack of nutrients through changes in hormonal and/or carbohydrate content, inducing abscission. Although it is known that biotic stress induce abscission by developmental and physiological modifications (Bergey et al., 1999; Peres et al., 2008; Tripathi et al., 2008), only a few studies have been dedicated to specific effects of biotic stresses on flower abscission. For example, it was suggested that Citrus fruit drop induced by Colletotrichum acutatum might be due to an alteration of the balance between auxin and related indole compounds (Chung et al., 2003). Due to the limited information available on biotic stresses, these are not discussed further here.
Abiotic stresses
Abiotic stress factors have a huge impact on world agriculture by reducing average yields for most major crop plants (Wang et al., 2003). At blooming, temperatures (cold/hot), water availability, and light radiation (quality and quantity) are considered as the major causes of abscission.
Temperature stress
The ability of plants to cope with hostile temperatures is a complex process, depending not only on the temperature regime, but also on genetic traits, and this has been reported in various species (Bertamini et al., 2007; Ledesma et al., 2008; Acar and Kakani, 2010; Cottee et al., 2010; Greer and Weston, 2010). Many studies reported effects of harmful temperatures (cold/hot) on reproductive organs and subsequent fruit set (Table 1). Briefly, temperature stress can create asynchrony between male and female reproductive development, both of which are required for successful reproduction (Herrero, 2003; Hedhly et al., 2008). For example, warm conditions accelerate anthesis but not pistil development, resulting in flowers with a reduced pistil weight and a shorter style length in apricot (Rodrigo and Herrero, 2002).
Table 1.
Effects of temperature stress on reproductive organs in woody species
| Negative effect | Species | Temperature | References | |
|---|---|---|---|---|
| Fruit set | Fruit set decrease | Apricot | >25°C in the pre-blooming period | Rodrigo and Herrero, 2002 |
| Cherimoya | 30/25°C | Higuchi et al., 1998 | ||
| Cotton | 40°C | Reddy et al., 1992 | ||
| Peach | 32°C | Couto et al., 2007 | ||
| Pear | 17°C | Tromp and Borsboom, 1994 | ||
| Grapevine | 38/33°C from budbreak to after anthesis | Buttrose and Hale, 1973 | ||
| 19°C<night<35°C | Buttrose, 1974 | |||
| >25°C during bloom fruit set period | Kliewer, 1977 | |||
| 12/9°C one week near flowering | Ebadi et al., 1995, 1996 | |||
| 17/12°C 14/9°C |
Haeseler and Fleming, 1967; Buttrose and Hale, 1973 | |||
| Sweet cherry | 25°C before anthesis | Beppu et al., 2001 | ||
| >20°C | Hedhly et al., 2007 | |||
| Development of reproductive structures | Abortive ovule | Apple | <0°C | Simons, 1969 |
| Cherry | <0°C | Stösser and Anvari, 1982 | ||
| Plum | 20°C at onset of full bloom | Cerovic et al., 2000 | ||
| Sweet cherry | >20°C | Hedhly et al., 2007 | ||
| 25°C before anthesis | Beppu et al., 2001 | |||
| Flower abscission | Avocado | >28°C | Sedgley, 1977 | |
| Grapevine | 40/25°C | Greer and Weston, 2010 | ||
| Flower bud death | Apricot | 1h or 3h at –4°C in the dark at first or full bloom | Gunes, 2006 | |
| Between –2 and –9°C one night at first or full bloom | ||||
| Flower drop | Cherimoya | 30/25°C | Higuchi et al., 1998 | |
| Fruitlet abscission | Avocado | 33/28°C | Sedgley and Annells, 1981 | |
| Cotton | >30/20°C ≥32°C daily 36/28°C |
Reddy et al., 1991; Hodges et al., 1993; Zhao et al., 2005 |
Water stress
As a result of a decline in plant growth and vigour, water stress might promote organ abscission (Taylor and Whitelaw, 2001). For instance, in Satsuma mandarin, reduced flowering occurs under severe water deficit, and in olive water availability increases flowering and fruit set, and reduces fruit drop (Michelakis, 1989; Lavee et al., 1990). In apple and citrus, water stress during flowering affects the final fruit number per tree, significantly reducing the yield (George and Nissen, 1988; García-Tejero et al., 2010).
Light stress
Dark and low-light treatments increase flower and fruit abscission in various species, since light quality and quantity are critical for photomorphogenesis (Taylor and Whitelaw, 2001). In apple, cotton, grapevine, and pepper, shading (30–90%) during reproductive development dramatically increases inflorescence abscission and reduces fruit set (Aloni et al., 1996; Ferree et al., 2001; Marcelis et al., 2004; Zhu et al., 2011).
Impacts of hormonal balance on abscission
Abiotic stresses trigger many biochemical, molecular, and physiological changes and responses that influence various aspects of cellular and plant metabolism, leading to important signalling modifications for coping with these unfavourable conditions. Hormones and sugars are particularly important, interconnected molecules, and lead to abscission under stress conditions. The impact of hormonal balance and carbon metabolism on the abscission process will be detailed in the following sections.
In the overall process of abscission, regulatory effects of plant hormones are of major relevance since they mediate responses of plant organs to stress (Peleg and Blumwald, 2011; Estornell et al., 2013; Smékalová et al., 2013). Depending on their concentration in different tissues, the concentrations and affinities of their receptors, their homeostasis, their transport, or their interactions with each other, hormones can act as accelerating or inhibiting signals affecting abscission, and responses are complex. Several hormones, including ethylene, abscisic acid (ABA) and, in specific circumstances, cytokinins, act as abscission-accelerating signals (Sipes and Einset, 1983; Taylor and Whitelaw, 2001; Dal Cin et al., 2007), while auxin, gibberellins (GA), and polyamines are considered as abscission inhibitors (Ben-Cheikh et al., 1997; Taylor and Whitelaw, 2001; Aziz, 2003). Since plant hormones are involved in whole-plant biology, a large number of genes regulating abscission are also part of the hormone biosynthetic and signalling pathways or influence their metabolism.
Though the role of the many hormone families remains ambiguous, ethylene, auxin/ethylene balance and, more recently, ABA have been shown to trigger abscission. Later in this review, it is shown that crosstalk between these molecules is crucial in this process.
Ethylene
Ethylene biosynthesis increases before abscission in many shedding organs, including reproductive organs (Reid, 1985; Taylor and Whitelaw, 2001; Zhu et al., 2010). In woody plants, a role for ethylene in abscission has largely been confirmed by application of exogenous ethylene (ethephon) and its precursors (Table 2). Application promotes abscission, while different inhibitors of ethylene biosynthesis reduce it (Williams and Flook, 1980; Bessis et al., 2000; Zhu et al., 2010) (Fig. 3). In apple fruitlets, the induction of abscission with chemical thinner allows a stimulation of ethylene biosynthesis in parallel with the upregulation of key regulatory genes, which lead to the synthesis of 1-aminocyclopropane-1-carboxylic acid (ACC, an ethylene precursor) (Dal Cin et al., 2005, 2007, 2009). This regulation was also reported in grapevine during flower and fruit abscission (Hilt and Bessis, 2003). Moreover, increased expression of ACO genes (which encode the enzyme converting ACC to ethylene) as well as ACO activity have been reported in organs which undergo abscission (Ruperti et al., 2001; Dal Cin et al., 2005, 2007, 2009). Thus, ethylene biosynthetic and signalling pathways may be involved in abscission.
Table 2.
ABA, auxin and ethylene involvement in flower and fruit abscission
| Effect | Species | References | |
|---|---|---|---|
| Ethylene and precursors | Stylar abscission | Lemon | Sipes and Einset, 1982 |
| Flower abscission | Grapevine | Bessis et al., 2000 | |
| Fruitlet abscission | Cotton | Lipe and Morgan, 1973 | |
| Citrus | Goren, 1993 | ||
| Apple |
Dal Cin et al., 2005, 2007, 2009; Yuan and Carbaugh, 2007; Zhu et al., 2010 |
||
| Grapevine | Bessis et al., 2000 | ||
| Peach | Rasori et al., 2002 | ||
| Mango | Malik et al., 2003 | ||
| Auxins and related | Reduced stylar abscission | Cherry, plum, citrus | Addicott and Lynch, 1955; Einset et al., 1980 |
| Reduced fruitlet and fruit drop | Lychee | Stern et al., 2000; Peng et al., 2013 | |
| Citrus | Agustí et al., 2002 | ||
| Apple | Drazeta et al., 2004; Yuan and Carbaugh 2007 | ||
| Cherry | Else et al., 2004 | ||
| ABA | Flower and fruitlet abscission | Grapevine | Weaver and Pool, 1969 |
| Cotton | Guinn, 1982 | ||
| Citrus | Sagee and Erner, 1991; Zacarías et al., 1995 | ||
| Apple | Vernieri et al., 1992 |
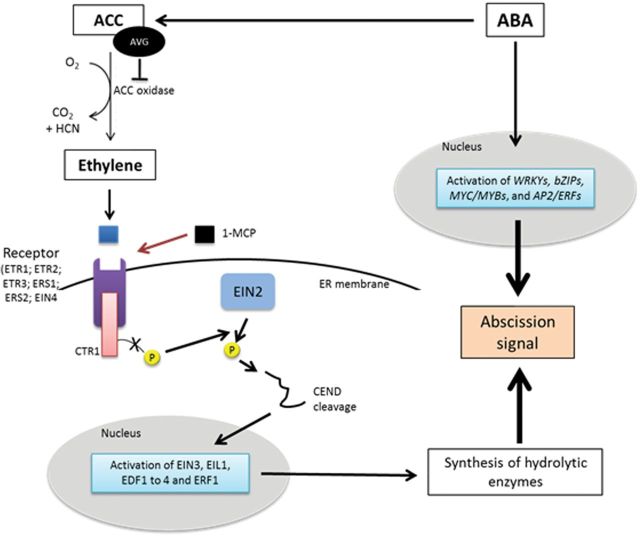
Fig. 3.
Proposed model leading to activation of the abscission process involving ABA and/or ethylene. Ethylene is recognized by an ethylene receptor, leading to transcriptional activation of genes encoding hydrolytic enzymes. The synthesis of hydrolytic enzymes leads to an abscission signal and finally to cell wall dissolution in the AZ. AVG blocks ethylene synthesis and 1-methylcyclopropene (1-MCP) prevents ethylene recognition by the receptor. In each case, these ethylene inhibitors reduce the abscission signal. ABA acts as the modulator of ACC levels and therefore stimulates ethylene synthesis. Newly synthesized ABA as a result of the perception of environmental stress can also induce the activation of some specific ABA-signalling genes, leading to activation of the abscission signal. AdoMet, S-adenoysl-methionine; CEND: carboxyl end of EIN2; ER, endoplasmic reticulum; CTR1, CONSTITUTIVE TRIPLE RESPONSE 1; EDF1 to 4, ENDOTHELIAL DIFFERENTIATION-RELATED FACTOR 1 to 4; EIL1, ETHYLENE INSENSITIVE-LIKE1; EIN2 to 4, ETHYLENE INSENSITIVE2 to 4; ERS1, ETHYLENE RESPONSE SENSOR 1; ETR1 to 3, ETHYLENE RESPONSE 1 to 3; ERF1, WRKYs, bZIPs, MYC/MYBs, and AP2/ERFs are transcription factors.
Ethylene is often characterized as the final effector in the abscission process, triggering the final steps and activating the transcription of genes encoding hydrolytic enzymes and their secretion, responsible for cell wall dissolution in the AZ (Goren, 1993; Bonghi et al., 2000; Zhu et al., 2010). Finally, inhibition of ethylene biosynthesis/action, decreases particularly the ethylene-induced, and more generally stress-induced abscission (Reid, 1985). Nevertheless, the specificity of the AZ in the abscission response also depends on its ability to sense ethylene. Indeed, in some cases, abscission occurs without a rise in ethylene production.
Auxin
Auxin mediates diverse developmental responses including the control of senescence and organ abscission (Ellis et al., 2005). As for ethylene, the involvement of auxin in abscission was studied using exogenous spraying of auxin or auxin analogues. In 1955, Addicott and Lynch noted that application of indole-3-acetic acid (IAA), able to fulfil most auxin actions (Sauer et al., 2013), retarded abscission of reproductive structures in various species. Investigations in woody species have also reported a decrease of abscission with application of auxin and auxin analogues (Table 2). The currently accepted model for abscission implies that auxin is produced by the subtending organ and is then transported through the AZ, thereby delaying its activation by reducing the sensitivity of the AZ to ethylene (Dhanalakshmi et al., 2003; Blanusa et al., 2005; Meir et al., 2006, 2010). For instance, a decrease in polar auxin transport (PAT) through sweet cherry pedicels, caused by inhibitors of PAT, triggers fruit abscission by increasing the sensitivity of AZ cells to ethylene (Blanusa et al., 2005). In addition, in Mirabilis jalapa, some transcripts encoding Aux/IAA proteins, polygalacturonase inhibitor, β-expansin, and β-tubulin, are downregulated by auxin depletion (Meir et al., 2006). Recently, it was reported that 2,4-D sprayed on the canopy of lychee trees leads to a decline in the mRNA level of LcPG1, coinciding with a reduced fruitlet abscission rate (Peng et al., 2013). Moreover, in Arabidopsis, Basu et al. (2013) showed that auxin regulates the timing of organ abscission and that a functional IAA signalling pathway is required for setting up abscission. These results underline the relationship between auxin depletion/presence and up- and downregulation of the amount of polygalacturonase mRNA in the AZ, indicating that a critical threshold level of free IAA has to be supplied continuously to the AZ cells for effective and continuous expression of Aux/IAA genes.
Auxin can also be used as a thinning molecule to reduce the competition among fruitlets in many tree species (Table 2). Naphthaleneacetic acid (NAA) application induces ethylene evolution from spurs leading to fruitlet abscission (Currv, 1991; McArtney, 2002), and this is through ethylene biosynthesis and signalling (Zhu et al. 2010). Ethylene production might be a consequence of the impaired photosynthetic activity in leaves due to auxin application. Indeed, after treatment with NAA, Weinbaum and Simons (1974) and Schneider (1975) have reported reduced transport of exogenously applied, labelled sucrose to developing fruitlets in parallel with an increase of ethylene production in fruits. As a consequence, photosynthate availability or transport in fruitlets is reduced, leading to ethylene production and inhibition of PAT through the AZ, and then to abscission (Agustí et al., 2007a ; Mesejo et al., 2012). Last, Agustí et al. (2007a) have reported that application of 3,5,6-trichloro-2-pyridyloxyacetic acid (3,5,6-TPA) to whole trees, at the onset of the cell enlargement stage, significantly increased fruitlet abscission; and that this was dependent on the concentration applied, probably due to the higher level of ethylene produced in 3,5,6-TPA-treated fruits. In summary, the auxin status, as well as any factor that affects the supply of auxin to the AZ, control sensitivity of the AZ to ethylene.
ABA
Although a high level of ABA in the AZ, prior to abscission, has been reported in flowers and fruitlets of many species (Sagee and Erner, 1991; Vernieri et al., 1992; Zacarías et al., 1995), the direct involvement of ABA in the abscission process remains unclear. In Citrus, exogenous ABA does not promote abscission in intact plants. However, in aged or injured detached tissues, high amounts of ABA stimulate ethylene synthesis and promote abscission (Goren, 1993). ABA appears to act as the modulator of ACC levels, and therefore of ethylene, leading to increased abscission (Guinn, 1982; Talon et al., 1997; Gómez-Cadenas et al., 2000) (Fig. 3). For instance, nutritional stress in apple fruitlets leads to the activation of some specific ABA-signalling genes during the early phases of abscission (Botton et al., 2011; Eccher et al., 2013) (Fig. 3). Considering these findings, ABA newly synthesized after nutritional stress may be biologically active, acting either directly or indirectly on the abscission process.
GA
Application of GA significantly delays flower drop and increases fruit set (Mahouachi et al., 2009). Unfortunately no more data are available on the impact of GA on abscission.
Cytokinins
Cytokinins are mainly known as abscission-accelerating hormones. Indeed, using a stylar abscission bioassay, the timing of explant abscission was hastened when cytokinins were added to the medium (Sipes and Einset, 1983). Studing the possible relationships between cytokinins, ethylene, and abscission, these authors concluded that cytokinins can stimulate Citrus abscission in vitro. Nevertheless, cytokinins have also been reported as reducers of flower and fruitlet drop, although they are not the most efficient compared to other hormones (Trueman, 2010). Cytokinins were recently used to shed light on the signalling pathways mediating the induction of apple fruitlet abscission. Based on transcriptomic and metabolic data, a hypothetical model has been proposed that suggests a strong link between abscission induction, magnified by the cytokinin treatment, and the nutritional stress within the tree (Botton et al., 2011; Eccher et al., 2013).
Brassinosteroids
In Calamondin, Iwahori et al., (1990) have reported that brassinosteroids delay the abscission of fruitlets more strongly than IAA does, probably by increasing the availability of assimilates (Hayat et al., 2000; Gomes et al., 2006).
Polyamines
The link between polyamines and fruit abscission has been scrutinized particularly closely. Their effects vary according to the polyamine type, concentration, and time of application (Aziz, 2003; Malik and Singh, 2003, 2006; Khezri et al., 2010). For instance, in mango, spermine is probably the most critical polyamine for abscission (Malik and Singh, 2003), while in grapevine inflorescences, an increased percentage of abscission has been correlated with free polyamine levels, mainly spermidine (Aziz et al., 2001; Aziz, 2003). Further, application of specific inhibitors of polyamine pathways induce abscission (Aziz et al., 2001; Malik and Singh, 2003). It has been suggested that spermidine metabolism may influence sucrose synthesis or its accumulation in sink organs, with fruitlet abscission correlating with a low level of polyamine and sugars (Aziz, 2003).
Crosstalk between hormone families
Reproductive processes are strongly affected by plant growth regulators, indicating that the regulatory mechanism controlling abscission may involve a pivotal hormonal component (Gaspar et al., 2003; Chandler, 2009). For instance, in citrus fruitlets, GA deficiency is associated with a rise in ABA, release of ethylene, and ovary abscission (Zacarias et al., 1995; Iglesias et al., 2007). It has also been shown that GA application accelerates IAA metabolism in citrus, which might explain a reduction in fruitlet drop (Liao et al., 2006). In cotton, it seems that ABA, IAA, and GAs interact to influence both development and abscission of fruitlets (Smith, 1969). So, appropriate changes in auxin levels could either amplify or counteract abscission by accelerating the influence of moderate levels of ABA (Addicott, 1970). In addition, changed ABA content may result from disturbance of PAT (Bangerth, 2000).
Interaction between environmental stresses and hormonal status
Plant hormones are major signalling molecules under stress leading to adaptation to suboptimal environmental conditions (Santner and Estelle, 2009). However, there is only a little information available on their direct involvment in the abscission of reproductive structures of woody species following environmental stresses. In cotton, water stress increases ethylene and ABA content in young bolls, while it decreases the concentration of free IAA, resulting in boll abscission (Guinn et al., 1990). In conditions of severe water stress in citrus plants, a rise in ABA promotes the synthesis and accumulation of ACC through the stimulation of ACC synthase activity (Tudela and Primo-Millo, 1992). After plant rehydration, ACC is transported into the xylem stream from roots to aerial organs, where it is metabolized to ethylene; shortly thereafter the abscission process occurs. Thus, water stress-promoted ABA synthesis in roots triggers ethylene production in aerial parts of the plant and causes their abscission.
Carbohydrates
Under optimal growth conditions, the rate of flower abscission has been correlated with the pathway of both male and female organ development and with the amount of carbohydrates in the flowers (Herrero and Hormaza, 1996; Yu et al., 2000). For instance, abscission can be induced by treatments that reduce or block nutrient supply to the apple fruit AZ (Berüter and Droz, 1991). Further, only fruitlets where the glucose content in the pedicel is below a critical level abscise (Berüter and Droz, 1991), suggesting the presence of a glucose gradient in the AZ similar to auxin. More recently, Peng et al. (2013) have shown that carbohydrate shortage leads to dramatically accelerated lychee fruitlet abscission.
In plants, both photosynthetic rate and management of carbohydrate reserves reflect carbon metabolism. Pepper cultivars with distinct susceptibilities to flower abscission might differ in their capacity to produce sucrose and accumulate starch during the light (Aloni et al., 1996; Marcelis et al., 2004). If accumulation of starch during the day is lower, night respiration might deplete a large part of the flower carbohydrate reserves and, thereby, trigger abscission (Preiss, 1982). For instance, in grapevine, ‘abscission-sensitive’ Gewurztraminer exhibits stronger vegetative growth than ‘abscission-tolerant’ Pinot Noir, suggesting that flower abscission sensitivity is related to lower sugar availability for flower development (Duchêne et al., 2003).
An alteration of photosynthesis may disturb the whole-plant carbon balance, affecting both reserve restitution (Cruz-Castillo et al., 2010) and carbon nutrition in leaves, flowers, and fruitlets (Berüter and Droz, 1991; Gómez-Cadenas et al., 2000), leading to abscission. Flower and fruit abscission rates appear to be modified when incident radiation (Berüter and Droz, 1991; Aloni et al., 1996; Ferree et al., 2001), leaf area (Gómez-Cadenas et al., 2000; Iglesias et al., 2003; Marcelis et al., 2004), or competition between vegetative and reproductive organs are changed (Smithyman et al., 1998; Vasconcelos and Castagnoli, 2000). These reports suggest that fruit set is linked to assimilate supply (source strength). Further, differences in the ratio of fruitlet abscission between cultivars were highly correlated with the source strength of each cultivar. The importance of leaf photosynthates in fruitlet abscission has also been shown (Gómez-Cadenas et al., 2000). Indeed, in apple, the extent of fruitlet abscission is inversely correlated with the number of leaves on the shoot at the base of the cluster in the spur (Iwanami et al., 2012) and fruit (Atkinson et al., 2002).
Interaction between carbohydrates and hormones
Carbohydrates and hormones participate in a complex signal transduction system (Gómez-Cadenas et al., 2000). For instance, defoliation treatments at anthesis in citrus induce fruitlet abscission due to a shortage of carbohydrate and a rise in hormones controlling abscission (Gómez-Cadenas et al., 2000; Iglesias et al., 2003). Defoliation reduces sugar concentrations by up to 98%, and raise ABA and ACC levels in fruitlets before their abscission. It was also observed that only the full defoliation treatment reduces endogenous GAs; exogenous application of GAs had no effect on abscission. These results have confirmed the hypothesis that carbon shortage reduces hormonal stimulators of growth (GAs) and increases stress-sensitive signals (ABA and ACC levels) as suggested by Talon et al. (1997), who indicated that fruitlet abscission is regulated by ABA and ACC originating in the fruits, while GAs are implicated in maintaining growth. ABA seems to act as a sensor of the intensity of nutrient shortage (higher ABA levels with full defoliation treatment) that modulates the levels of ACC and ethylene, which would be the hormonal effector (Gómez-Cadenas et al., 1996, 1998). ABA is an important signal of water stress; however, in citrus, ABA may be operating as a mediator between the adverse environment and abscission. More recently, Kuang et al. (2012) have reported that girdling plus defoliation reduce the endogenous IAA concentration concomitantly with increased fruitlet abscission, highlighting a key role of this hormone in fruit retention.
In tree species, the role of ethylene on the regulation of abscissionas has been widely illustrated, with this hormone considered as the last hormonal effector of abscission that is induced by different stress conditions (Tudela and Primo-Millo, 1992). The relationship between carbohydrate and ethylene levels in the process of fruitlet abscission has been investigated in citrus (Iglesias et al., 2006). It was reported that ACC treatments, combined or not with sucrose, always increase ethylene production; in contrast, aminoethoxyvinylglycine (AVG) and sucrose reduce the ethylene and ACC. Moreover, branch girdling treatment increases the carbohydrate content and decreases ethylene production, finally decreasing abscission rates (Iglesias et al., 2006; Sun et al., 2010). Finally, a reduction in abscission rate is preceded by elevated concentrations of hexose and starch, but also GAs, in developing ovaries and fruitlets in Satsuma mandarin, suggesting that carbohydrates and GA levels determine fruit set (Mahouachi et al., 2009).
ABA and ethylene appear to act as messengers of auxin in the signalling pathway leading to fruitlet abscission (Agustí et al., 2007b ). Reduced supply of auxin to the AZ concurrently with a likely depolarization of its transport would enhance its sensitivity to ethylene and the consequent activation of cell wall-degrading enzymes (Schröder et al., 2013). A transcriptomic analysis has also shown overexpression of a trehalose-6-phosphate synthase gene in abscising fruitlets, which may regulate ABA signalling in Arabidopsis (Avonce et al., 2004).
As auxin plays a pivotal role in plant organ abscission, it is increasingly interesting to investigate the role of genes involved in auxin signal transduction pathways in this process as recently done by Kuang et al. in litchi (2012). They reported that under a treatment of girdling with defoliation, expression patterns of many auxin-related genes differ during activation of the fruit AZ.
Eccher et al. (2013) have shown a strong correlation in fruitlets between isoprene emission and their abscission potential. Isoprenoid emissions have often been associated with low carbon supply under stress conditions (Loreto and Delfine, 2000; Brilli et al., 2007). Because the oxidative balance in abscising fruitlets is disrupted by high levels of ROS, isoprene might act directly to ROS accumulated in the fruit cortex (Velikova et al., 2004; Vickers et al., 2009; Botton et al., 2011). Therefore, the fruit stimulated to abscise may exploit isoprene emissions to recover a noncytotoxic oxidative status using a nonenzymatic ROS-scavenging system (Eccher et al., 2013). Exogenous application of ABA to apple is able to magnify fruitlet abscission but not to affect isoprene emission (Eccher et al., 2013). Thus, the involvement of ABA seems to be upstream of abscission induction and not a side effect of this process. Multiple networks of interaction between hormones (mainly ABA and ethylene) and other signalling molecules (i.e. ROS) orchestrate the abscission process at the cortex level. During induction of abscission, the production of both isoprene and ABA appears to be temporally coordinated, with a potential isoprene involvement in the ROS detoxification and activation of still-unknown secondary signalling pathways.
By adding together the results reported by Botton et al. (2011) and Eccher et al. (2013), an updated model of the induction of abscission under nutritional stress has been proposed (Fig. 4). Although this hypothetical model must be validated, we may hypothesize that under abiotic stresses, which lead to alterations of carbohydrate metabolism and therefore to nutritional stress, the same processes might occur, leading to abscission. This model may also have future applications, notably for the characterization of flower abscission, since abiotic stresses lead to alterations of flower development, pollination, and fertilization, which are also highly regulated by hormones.
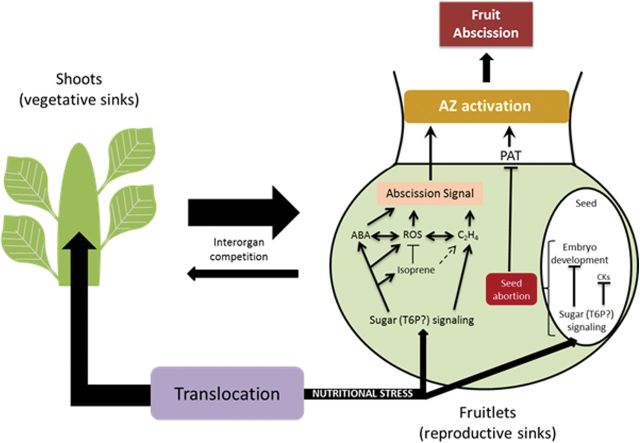
Fig. 4.
Hypothetical model for fruitlet abscission under nutritional stress [according to Botton et al. (2011) and Eccher et al. (2013)]. The nutritional stress, enhancing the competition for assimilates between vegetative and reproductive sinks, is translated at both the fruit and seed levels through crosstalk between signalling pathways involving mainly sugars, ABA, and ethylene. Sugar signalling includes processes of sugar starvation, growth inhibition, sucrose accumulation, and trehalose-6-phosphate (T6P) signalling as described by Botton et al. (2011). In the fruit, the sugar signalling (through T6P) induces ABA and ethylene synthesis, which can increase ROS content and lead to an abscission signal. The abscission signal is then transmitted to the AZ, leading to fruit fall. When the seed perceives sugar depletion, a block of embryo development and cytokinin (CK) signalling occurs, leading to seed abortion. This crucial step would determine the depolarization of auxin transport, leading to the enhancement of AZ sensitivity to ethylene and its activation. The thickness of the arrows related to interorgan competition and storage partitioning is proportional to the strength of the organ as a sink.
Conclusions
Environmental stresses, leading to physiological perturbation in plants, extensively increase abscission and lead to important yield decreases. Hormones mediate the response of organs (flowers or fruits) to stress and finally trigger abscission. Hormones are the abscission effectors since they modulate AZ activation within a complex signalling system based on synthesis, catabolism, and transport of hormones. The auxin/ethylene balance is of a particular importance due to its action on AZ sensitivity and activation. Carbohydrates also participate with hormones in a complex signal transduction system in response to stress, leading to abscission.
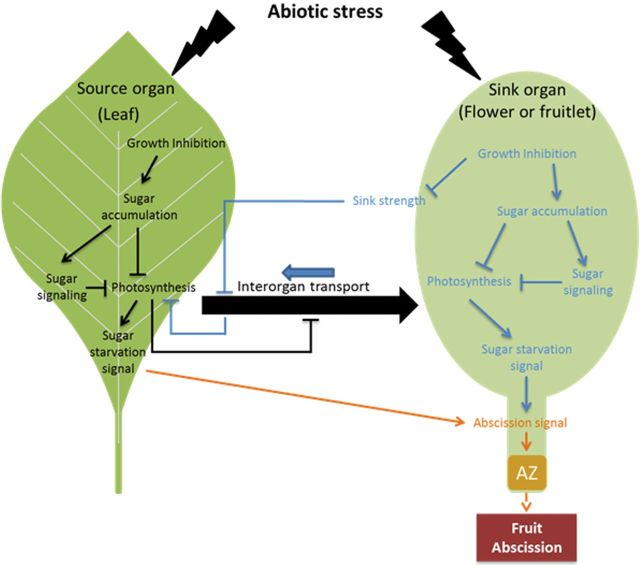
Since abiotic stresses lead to an imbalance of carbon between source and sink organs (Fig. 5) and therefore alter the nutritional equilibrium at the whole-plant level, future experiments should target the relationships between the source and sink organs using photosynthesis inhibitors. Understanding how distinct reproductive organs cope with stress might offer the potential to identify new traits that could be manipulated to improve their stress tolerance. Elaboration of models should allow the development of new strategies to improve stress tolerance, notably with the use of polyamines and brassinosteroids, implied to be involved in the abiotic stress response by stimulation of the carbon status. Transcriptomic, metabolic, and hormonometer analysis should be developed in woody species during flower and fruit development in order to strengthen models in different crop systems to increase resilience to environmental stresses while preserving productivity and quality.
Fig. 5.
Schematic representation of flower/fruitlet abscission following exposure of leaf or flower to abiotic stress. In the leaf, to abiotic stress inhibits development and decreases photosynthesis, leading to sugar starvation, perceived as unrecoverable by the fruit, which aborts. In flowers or fruitlets, the stress affects growth and photosynthesis. Consequently, the sugar balance between leaf and flower is altered and nutritional stress occurs. The sugar depletion induces an abscission signal leading to flower/fruit fall. Black arrows, leaf process; blue arrows, flower/fruit process; orange arrows, common process.
References
- Acar I, Kakani VG. 2010. The effects of temperature on in vitro pollen germination and pollen tube growth of Pistacia spp. Scientia Horticulturae 125, 569–572. [Google Scholar]
- Addicott FT. 1970. Plant hormones in the control of abscission. Biological Reviews 45, 485–524. [Google Scholar]
- Addicott FT. 1982. Abscission. Berkeley: University of California Press. [Google Scholar]
- Addicott FT, Lynch RS. 1955. Physiology of abscission. Annual Review of Plant Physiology 6, 211–238. [Google Scholar]
- Agustí M, Juan M, Almela V. 2007. a . Response of ‘Clausellina’ Satsuma mandarin to 3,5,6-trichloro-2-pirydiloxyacetic acid and fruitlet abscission. Plant Growth Regulation 53, 129–135. [Google Scholar]
- Agustí J, Zapater M, Iglesias DJ, Cercós M, Tadeo FR, Talón M. 2007. b . Differential expression of putative 9-cis-epoxycarotenoid dioxygenases and abscisic acid accumulation in water stressed vegetative and reproductive tissues of citrus. Plant Science 172, 85–94. [Google Scholar]
- Agustí M, Zaragoza S, Iglesias DJ, Almela V, Primo-Millo E, Talón M. 2002. The synthetic auxin 3, 5, 6-TPA stimulates carbohydrate accumulation and growth in citrus fruit. Plant Growth Regulation 36, 141–147. [Google Scholar]
- Aloni B, Karni L, Zaidman Z, Schaffer AA. 1996. Changes of carbohydrates in pepper (Capsicum annuum L.) flowers in relation to their abscission under different shading regimes. Annals of Botany 78, 163–168. [Google Scholar]
- Atkinson CJ, Else MA, Stankiewicz A, Webster AD. 2002. The effects of phloem girdling on the abscission of Prunus avium L. fruits. Journal of Horticultural Science and Biotechnology 77, 22–27. [Google Scholar]
- Avonce N, Leyman B, Mascorro-Gallardo JO, Dijck PV, Thevelein JM, Iturriaga G. 2004. The Arabidopsis Trehalose-6-P Synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiology 136, 3649–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz A. 2003. Spermidine and related‐metabolic inhibitors modulate sugar and amino acid levels in Vitis vinifera L.: possible relationships with initial fruitlet abscission. Journal of Experimental Botany 54, 355–363. [DOI] [PubMed] [Google Scholar]
- Aziz A, Brun O, Audran JC. 2001. Involvement of polyamines in the control of fruitlet physiological abscission in grapevine (Vitis vinifera). Physiologia Plantarum 113, 50–58. [Google Scholar]
- Bangerth F. 2000. Abscission and thinning of young fruit and their regulation by plant hormones and bioregulators. Plant Growth Regulation 31, 43–59. [Google Scholar]
- Basu MM, González-Carranza ZH, Azam-Ali S, Tang S, Shahid AA, Roberts JA. 2013. The manipulation of auxin in the abscission zone cells of Arabidopsis flowers reveals that indoleacetic acid signaling is a prerequisite for organ shedding. Plant Physiology 162, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Cheikh W, Perez-Botella J, Tadeo FR, Talon M, Primo-Millo E. 1997. Pollination increases gibberellin levels in developing ovaries of seeded varieties of citrus. Plant Physiology 114, 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beppu K, Suehara T, Kataoka I. 2001. Embryo sac development and fruit set of ‘Satohnishiki’ sweet cherry as affected by temperature, GA3 and paclobutrazol. Journal of the Japanese Society for Horticultural Science 70, 157–162. [Google Scholar]
- Bergey DR, Orozco-Cardenas M, De Moura DS, Ryan CA. 1999. A wound-and systemin-inducible polygalacturonase in tomato leaves. Proceedings of the National Academy of Sciences, USA 96, 1756–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertamini M, Zulini L, Muthuchelian K, Nedunchezhian N. 2007. Low night temperature effects on photosynthetic performance on two grapevine genotypes. Biologia Plantarum 51, 381–385. [Google Scholar]
- Berüter J, Droz P. 1991. Studies on locating the signal for fruit abscission in the apple tree. Scientia Horticulturae 46, 201–214. [Google Scholar]
- Bessis R, Charpentier N, Hilt C, Fournioux JC. 2000. Grapevine fruit set: Physiology of the abscission zone. Australian Journal of Grape and Wine Research 6, 125–130. [Google Scholar]
- Blanusa T, Else MA, Atkinson CJ, Davies WJ. 2005. The regulation of sweet cherry fruit abscission by polar auxin transport. Plant Growth Regulation 45, 189–198. [Google Scholar]
- Bonghi C, Tonutti P, Ramina A. 2000. Biochemical and molecular aspects of fruitlet abscission. Plant Growth Regulation 31, 35–42. [Google Scholar]
- Botton A, Eccher G, Forcato C, Ferrarini A, Begheldo MZ, Moscatello S, Battistelli A, Velasco R, Ruperti B, Ramina A. 2011. Signaling pathways mediating the induction of apple fruitlet abscission. Plant Physiology 155, 185–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilli F, Barta C, Fortunati A, Lerdau M, Loreto F, Centritto M. 2007. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytologist 175, 244–254. [DOI] [PubMed] [Google Scholar]
- Buttrose MS. 1974. Climatic factors and fruitfulness in grapevines. Horticultural Abstracts 44, 319–326. [Google Scholar]
- Buttrose MS, Hale CR. 1973. Effect of temperature on development of the grapevine inflorescence after bud burst. American Journal of Enology and Viticulture 24, 14–16. [Google Scholar]
- Cerovic R, Ruzic D, Micic N. 2000. Viability of plum ovules at different temperatures. Annals of Applied Biology 137, 53–59. [Google Scholar]
- Chandler JW. 2009. Auxin as compère in plant hormone crosstalk. Planta 231, 1–12. [DOI] [PubMed] [Google Scholar]
- Chandler JW. 2011. The hormonal regulation of flower development. Journal of Plant Growth Regulation 30, 242–254. [Google Scholar]
- Chung KR, Shilts T, Ertürk Ü, Timmer LW, Ueng PP. 2003. Indole derivatives produced by the fungus Colletotrichum acutatum causing lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiology Letters 226, 23–30. [DOI] [PubMed] [Google Scholar]
- Cottee NS, Tan DKY, Bange MP, Cothren JT, Campbell LC. 2010. Multi-level determination of heat tolerance in Cotton (Gossypium hirsutum L.) under field conditions. Crop Science 50, 2553–2564. [Google Scholar]
- Couto M, Raseira MCB, Herter FG, Silva JB. 2007. Influence of high temperatures at blooming time on pollen production and fruit set of peach ‘Maciel’ and ‘Granada’. VIII International Symposium on Temperate Zone Fruits in the Tropics and Subtropics 872, 225–230. [Google Scholar]
- Cruz-Castillo JG, Woolley DJ, Famiani F. 2010. Effects of defoliation on fruit growth, carbohydrate reserves and subsequent flowering of ‘Hayward’ kiwifruit vines. Scientia Horticulturae 125, 579–583. [Google Scholar]
- Currv EA. 1991. NAA-induced ethylene and ACC in `Delicious’ spur tissues: changes with temperature and time. Journal of the American Society for Horticultural Science 116, 846–850. [Google Scholar]
- Dal Cin V, Barbaro E, Danesin M, Murayama H, Velasco R, Ramina A. 2009. Fruitlet abscission: A cDNA-AFLP approach to study genes differentially expressed during shedding of immature fruits reveals the involvement of a putative auxin hydrogen symporter in apple (Malus domestica L. Borkh). Gene 442, 26–36. [DOI] [PubMed] [Google Scholar]
- Dal Cin VD, Boschetti A, Dorigoni A, Ramina A. 2007. Benzylaminopurine application on two different apple cultivars (Malus domestica) displays new and unexpected fruitlet abscission features. Annals of Botany 99, 1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Cin VD, Danesin M, Boschetti A, Dorigoni A, Ramina A. 2005. Ethylene biosynthesis and perception in apple fruitlet abscission (Malus domestica L. Borck). Journal of Experimental Botany 56, 2995–3005. [DOI] [PubMed] [Google Scholar]
- del Campillo E, Bennett AB. 1996. Pedicel breakstrength and cellulase gene expression during tomato flower abscission. Plant Physiology 111, 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanalakshmi R, Prasad TG, Udayakumar M. 2003. Is auxin a diffusible signal mediating abscission of recessive sinks? Plant Science 164, 689–696. [Google Scholar]
- Drazeta L, Lang A, Cappellini C, Hall AJ, Volz RK, Jameson PE. 2004. Vessel differentiation in the pedicel of apple and the effects of auxin transport inhibition. Physiologia Plantarum 120, 162–170. [DOI] [PubMed] [Google Scholar]
- Duchêne E, Jaegli N, Salber R, Gaudillère JP. 2003. Effects of ripening conditions on the following season’s growth and yield components for Pinot noir and Gewurztraminer grapevines (Vitis vinifera L.) in a controlled environment. Journal International des Sciences de la Vigne et du Vin 37, 39–49. [Google Scholar]
- Ebadi A, Coombe BG, May P. 1995. Fruit-set on small Chardonnay and Shiraz vines grown under varying temperature regimes between budburst and flowering. Australian Journal of Grape and Wine Research 1, 3–10. [Google Scholar]
- Ebadi A, May P, Coombe BG. 1996. Effect of short-term temperature and shading on fruit-set, seed and berry development in model vines of V. vinifera, cvs Chardonnay and Shiraz. Australian Journal of Grape and Wine Research 2, 1–8. [Google Scholar]
- Eccher G, Botton A, Dimauro M, Boschetti A, Ruperti B, Ramina A. 2013. Early induction of apple fruitlet abscission is characterized by an increase of both isoprene emission and abscisic acid content. Plant Physiology 161, 1952–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einset JW, Cheng A, Elhag H. 1980. Citrus tissue culture: regulation of stylar abscission in excised pistils. Canadian Journal of Botany 58, 1257–1261. [Google Scholar]
- Ellis CM, Nagpal P, Young JC, Hagen G, Guilfoyle TJ, Reed JW. 2005. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana . Development 132, 4563–4574. [DOI] [PubMed] [Google Scholar]
- Else MA, Stankiewicz-Davies AP, Crisp CM, Atkinson CJ. 2004. The role of polar auxin transport through pedicels of Prunus avium L. in relation to fruit development and retention. Journal of Experimental Botany 55, 2099–2109. [DOI] [PubMed] [Google Scholar]
- Estornell LH, Agustí J, Merelo P, Talón M, Tadeo FR. 2013. Elucidating mechanisms underlying organ abscission. Plant Science 199, 48–60. [DOI] [PubMed] [Google Scholar]
- Ferree DC, McArtney SJ, Scurlock DM. 2001. Influence of irradiance and period of exposure on fruit set of French-American hybrid grapes. Journal of the American Society for Horticultural Science 126, 283–290. [Google Scholar]
- García-Tejero I, Romero-Vicente R, Jiménez-Bocanegra JA, Martínez-García G, Durán-Zuazo VH, Muriel-Fernández JL. 2010. Response of citrus trees to deficit irrigation during different phenological periods in relation to yield, fruit quality, and water productivity. Agricultural Water Management 97, 689–699. [Google Scholar]
- Gaspar TH, Kevers C, Faivre-Rampant O, Crèvecoeur M, Penel CL, Greppin H, Dommes J. 2003. Changing concepts in plant hormone action. In Vitro Cellular and Developmental Biology – Plant 39, 85–106. [Google Scholar]
- George AP, Nissen RJ. 1988. The effects of temperature, vapour pressure deficit and soil moisture stress on growth, flowering and fruit set of custard apple (Annona cherimola × Annona squamosa) ‘African Pride’. Scientia Horticulturae 34, 183–191. [Google Scholar]
- Goldschmidt EE, Koch KE. 1996. Citrus. In: Zaminski E, Schaffer AA. Photoassimilate distribution in plants and crops: source-sink relations, New York: Marcel Dekker, 797–823. [Google Scholar]
- Goldschmidt EE, Monselise SP. 1977. Physiological assumptions toward the development of a Citrus fruiting model. Proceedings of the International Society of Citriculture 2, 668–672. [Google Scholar]
- Gomes de MA, Campostrini E, Leal NR, Viana AP, Ferraz TM, Siqueira L, do N, Rosa RCC, Netto AT, Nuñez-Vázquez M, Zullo MAT. 2006. Brassinosteroid analogue effects on the yield of yellow passion fruit plants (Passiflora edulis f. flavicarpa). Scientia Horticulturae 110, 235–240. [Google Scholar]
- Gómez-Cadenas A, Mehouachi J, Tadeo FR, Primo-Millo E, Talon M. 2000. Hormonal regulation of fruitlet abscission induced by carbohydrate shortage in citrus. Planta 210, 636–643. [DOI] [PubMed] [Google Scholar]
- Gómez-Cadenas A, Tadeo FR, Primo-Millo E, Talon M. 1998. Involvement of abscisic acid and ethylene in the responses of citrus seedlings to salt shock. Physiologia Plantarum 103, 475–484. [Google Scholar]
- Gómez-Cadenas A, Tadeo FR, Talon M, Primo-Millo E. 1996. Leaf abscission induced by ethylene in water-stressed intact seedlings of Cleopatra mandarin requires previous abscisic acid accumulation in roots. Plant Physiology 112, 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Carranza ZH, Elliott KA, Roberts JA. 2007. Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana . Journal of Experimental Botany 58, 3719–3730. [DOI] [PubMed] [Google Scholar]
- González-Carranza ZH, Lozoya-Gloria E, Roberts JA. 1998. Recent developments in abscission: shedding light on the shedding process. Trends in Plant Science 3, 10–14. [Google Scholar]
- González-Carranza ZH, Whitelaw CA, Swarup R, Roberts JA. 2002. Temporal and spatial expression of a polygalacturonase during leaf and flower abscission in Oilseed Rape and Arabidopsis . Plant Physiology 128, 534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren R. 1993. Anatomical, physiological, and hormonal aspects of abscission in citrus. Horticultural Reviews 15, 145–182. [Google Scholar]
- Greer DH, Weston C. 2010. Heat stress affects flowering, berry growth, sugar accumulation and photosynthesis of Vitis vinifera cv. Semillon grapevines grown in a controlled environment. Functional Plant Biology 37, 206–214. [Google Scholar]
- Guinn G. 1982. Fruit age and changes in abscisic acid content, ethylene production, and abscission rate of cotton fruits. Plant Physiology 69, 349–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinn G, Dunlap JR, Brummett DL. 1990. Influence of water deficits on the abscisic acid and indole-3-acetic acid contents of cotton flower buds and flowers. Plant Physiology 93, 1117–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunes NT. 2006. Frost hardiness of some Turkish apricot cultivars during the bloom period. HortScience 41, 310–312. [Google Scholar]
- Haeseler CW, Fleming HK. 1967. Response of Concord grapevines to various controlled day-temperatures. The Pennsylvania State University Bulletin 739, 16. [Google Scholar]
- Hayat S, Ahamad A, Mobin M, Hussain A, Fariduddin Q. 2000. Photosynthetic rate, growth and yield of mustard plants sprayed with 28-homo-brassinolide. Photosynthetica 38, 469–471. [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. 2007. Warm temperatures at bloom reduce fruit set in sweet cherry. Journal of Applied Botany and Food Quality 81, 158–164. [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. 2008. Global warming and plant sexual reproduction. Trends in Plant Science 14, 30–36. [DOI] [PubMed] [Google Scholar]
- Herrero M. 2003. Male and female synchrony and the regulation of mating in flowering plants. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences 358, 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero M, Hormaza J. 1996. Pistil strategies controlling pollen tube growth. Sexual Plant Reproduction 9, 343–347. [Google Scholar]
- Higuchi H, Utsunomiya N, Sakuratani T. 1998. High temperature effects on cherimoya fruit set, growth and development under greenhouse conditions. Scientia Horticulturae 77, 23–31. [Google Scholar]
- Hilt C, Bessis R. 2003. Abscission of grapevine fruitlets in relation to ethylene biosynthesis. Vitis 42, 1–4. [Google Scholar]
- Hodges HF, Reddy KR, McKinnon JM, Reddy VR. 1993. Temperature effects on cotton. Mississippi Agricultural and Forestry Experiment Station Bulletin 990. [Google Scholar]
- Iglesias DJ, Cercós M, Colmenero-Flores JM, et al. 2007. Physiology of citrus fruiting. Brazilian Journal of Plant Physiology 19, 333–362. [Google Scholar]
- Iglesias DJ, Tadeo FR, Primo-Millo E, Talon M. 2003. Fruit set dependence on carbohydrate availability in citrus trees. Tree Physiology 23, 199–204. [DOI] [PubMed] [Google Scholar]
- Iglesias DJ, Tadeo FR, Primo-Millo E, Talon M. 2006. Carbohydrate and ethylene levels related to fruitlet drop through abscission zone A in citrus. Trees 20, 348–355. [Google Scholar]
- IPCC. 2013. Climate Change 2013: The Physical Science Basis - Summary for Policymakers. Working Group I Contribution to the IPCC Fifth Assessment Report. Cambridge University Press. [Google Scholar]
- Iwahori S, Tominaga S, Higuchi S. 1990. Retardation of abscission of citrus leaf and fruitlet explants by brassinolide. Plant Growth Regulation 9, 119–125. [Google Scholar]
- Iwanami H, Moriya-Tanaka Y, Honda C, Wada M, Moriya S, Okada K, Haji T, Abe K. 2012. Relationships among apple fruit abscission, source strength, and cultivar. Scientia Horticulturae 146, 39–44. [Google Scholar]
- Khezri M, Talaie A, Javanshah A, Hadavi F. 2010. Effect of exogenous application of free polyamines on physiological disorders and yield of ‘Kaleh-Ghoochi’ pistachio shoots (Pistacia vera L.). Scientia Horticulturae 125, 270–276. [Google Scholar]
- Kliewer WM. 1977. Effect of high temperatures during the bloom-set period on fruit-set, ovule fertility, and berry growth of several grape cultivars. American Journal of Enology and Viticulture 28, 215–222. [Google Scholar]
- Kuang JF, Wu JY, Zhong HY, Li CQ, Chen JY, Lu WJ, Li JG. 2012. Carbohydrate stress affecting fruitlet abscission and expression of genes related to auxin signal transduction pathway in litchi. International Journal of Molecular Sciences 13, 16084–16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashbrook CC, Cai S. 2008. Cell wall remodeling in Arabidopsis stamen abscission zones: Temporal aspects of control inferred from transcriptional profiling. Plant Signaling and Behavior 3, 733–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavee S, Nashef M, Wodner M, Harshemesh H. 1990. The effect of complementary irrigation added to old olive trees (Olea europaea L.) cv. Souri on fruit characteristics, yield and oil production. Advances in Horticultural Science 4, 135–138. [Google Scholar]
- Ledesma NA, Nakata M, Sugiyama N. 2008. Effect of high temperature stress on the reproductive growth of strawberry cvs. ‘Nyoho’ and ‘Toyonoka’. Scientia Horticulturae 116, 186–193. [Google Scholar]
- Liao HL, Chen H, Chung KR. 2006. Plant hormone inhibitors for reducing postblomm fruit drop (PFD) of citrus. Proceedings of the Florida State Horticultural Society 119, 78–81. [Google Scholar]
- Lipe JA, Morgan PW. 1973. Ethylene, a regulator of young fruit abscission. Plant Physiology 51, 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Delfine S. 2000. Emission of isoprene from salt-stressed Eucalyptus globulus leaves. Plant Physiology 123, 1605–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahouachi J, Iglesias DJ, Agustí M, Talon M. 2009. Delay of early fruitlet abscission by branch girdling in citrus coincides with previous increases in carbohydrate and gibberellin concentrations. Plant Growth Regulation 58, 15–23. [Google Scholar]
- Malik AU, Agrez V, Singh Z. 2003. Fruitlet abscission of mango in relation to ethylene. Journal of Horticultural Science and Biotechnology 78, 458–462. [Google Scholar]
- Malik AU, Singh Z. 2003. Abscission of mango fruitlets as influenced by biosynthesis of polyamines. Journal of Horticultural Science and Biotechnology 78, 721–727. [Google Scholar]
- Malik AU, Singh Z. 2006. Improved fruit retention, yield and fruit quality in mango with exogenous application of polyamines. Scientia Horticulturae 110, 167–174. [Google Scholar]
- Marcelis LFM, Heuvelink E, Baan Hofman-Eijer LR, Den Bakker J, Xue LB. 2004. Flower and fruit abortion in sweet pepper in relation to source and sink strength. Journal of Experimental Botany 55, 2261–2268. [DOI] [PubMed] [Google Scholar]
- McArtney SJ. 2002. Ethylene evolution from detached apple spurs in response to chemical thinners. HortScience 37, 662–665. [Google Scholar]
- Meir S, Hunter DA, Chen JC, Halaly V, Reid MS. 2006. Molecular changes occurring during acquisition of abscission competence following auxin depletion in Mirabilis jalapa . Plant Physiology 141, 1604–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir S, Philosoph-Hadas S, Sundaresan S, Selvaraj KSV, Burd S, Ophir R, Kochanek B, Reid MS, Jiang CZ, Lers A. 2010. Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiology 154, 1929–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesejo C, Rosito S, Reig C, Martínez-Fuentes A, Agustí M. 2012. Synthetic auxin 3,5,6-TPA provokes Citrus clementina (Hort. ex Tan) fruitlet abscission by reducing photosynthate availability. Journal of Plant Growth Regulation 31, 186–194. [Google Scholar]
- Michelakis N. 1989. Yield response of table and oil olive varieties to different water use levels under drip irrigation. International Symposium on Olive Growing 286, 271–274. [Google Scholar]
- Osborne DJ. 1989. Abscission. Critical Reviews in Plant Sciences 8, 103–129. [Google Scholar]
- Peleg Z, Blumwald E. 2011. Hormone balance and abiotic stress tolerance in crop plants. Current Opinion in Plant Biology 14, 290–295. [DOI] [PubMed] [Google Scholar]
- Peng G, Wu J, Lu W, Li J. 2013. A polygalacturonase gene clustered into clade E involved in lychee fruitlet abscission. Scientia Horticulturae 150, 244–250. [Google Scholar]
- Peres NA, MacKenzie SJ, Peever TL, Timmer LW. 2008. Postbloom fruit drop of citrus and key lime anthracnose are caused by distinct phylogenetic lineages of Colletotrichum acutatum . Phytopathology 98, 345–352. [DOI] [PubMed] [Google Scholar]
- Petoukhov V, Semenov VA. 2010. A link between reduced Barents-Kara sea ice and cold winter extremes over northern continents. Journal of Geophysical Research: Atmospheres 115, D21. [Google Scholar]
- Preiss J. 1982. Regulation of the biosynthesis and degradation of starch. Annual Review of Plant Physiology 33, 431–454. [Google Scholar]
- Rasori A, Ruperti B, Bonghi C, Tonutti P, Ramina A. 2002. Characterization of two putative ethylene receptor genes expressed during peach fruit development and abscission. Journal of Experimental Botany 53, 2333–2339. [DOI] [PubMed] [Google Scholar]
- Reddy KR, Hodges HF, Reddy VR. 1992. Temperature effects on cotton fruit retention. Agronomy Journal 84, 26–30. [Google Scholar]
- Reddy VR, Baker DN, Hodges HF. 1991. Temperature effects on cotton canopy growth, photosynthesis, and respiration. Agronomy Journal 83, 699–704. [Google Scholar]
- Reid MS. 1985. Ethylene and abscission. HortScience 20, 45–50. [Google Scholar]
- Rodrigo J, Herrero M. 2002. Effects of pre-blossom temperatures on flower development and fruit set in apricot. Scientia Horticulturae 92, 125–135. [Google Scholar]
- Ruperti B, Bonghi C, Rasori A, Ramina A, Tonutti P. 2001. Characterization and expression of two members of the peach 1-aminocyclopropane-1-carboxylate oxidase gene family. Physiologia Plantarum 111, 336–344. [DOI] [PubMed] [Google Scholar]
- Sagee O, Erner Y. 1991. Gibberellins and abscisic acid contents during flowering and fruit set of ‘Shamouti’orange. Scientia Horticulturae 48, 29–39. [Google Scholar]
- Santner A, Estelle M. 2009. Recent advances and emerging trends in plant hormone signalling. Nature 459, 1071–1078. [DOI] [PubMed] [Google Scholar]
- Sauer M, Robert S, Kleine-Vehn J. 2013. Auxin: simply complicated. Journal of Experimental Botany 64, 2565–2577. [DOI] [PubMed] [Google Scholar]
- Schneider GW. 1975. 14C-sucrose translocation in apple. Journal of the American Society for Horticultural Science 100, 22–24. [Google Scholar]
- Schröder M, Link H, Bangerth KF. 2013. Correlative polar auxin transport to explain the thinning mode of action of benzyladenine on apple. Scientia Horticulturae 153, 84–92. [Google Scholar]
- Sedgley M. 1977. Physiology of pollination and fruit set and possibilities of manipulation. In: Proceedings of the Avocado Research Workshop. South Queensland, Australia, 59–65. [Google Scholar]
- Sedgley M, Annells CM. 1981. Flowering and fruit-set response to temperature in the avocado cultivar ‘Hass’. Scientia Horticulturae 14, 27–33. [Google Scholar]
- Simons RK. 1969. Tissue response of young developing apple fruits to freeze injury. Journal of the American Society for Horticultural Science 94, 376–382. [Google Scholar]
- Sipes DL, Einset JW. 1982. Role of ethylene in stimulating stylar abscission in pistil explants of lemons. Physiologia Plantarum 56, 6–10. [Google Scholar]
- Sipes DL, Einset JW. 1983. Cytokinin stimulation of abscission in lemon pistil explants. Journal of Plant Growth Regulation 2, 73–80. [Google Scholar]
- Smékalová V, Doskočilová A, Komis G, Šamaj J. 2013. Crosstalk between secondary messengers, hormones and MAPK modules during abiotic stress signalling in plants. Biotechnology Advances 32, 2–11. [DOI] [PubMed] [Google Scholar]
- Smith OE. 1969. Changes in abscission-accelerating substances with development of cotton fruit. The New Phytologist 68, 313–322. [Google Scholar]
- Smithyman RP, Howell GS, Miller DP. 1998. The use of competition for carbohydrates among vegetative and reproductive sinks to reduce fruit set and botrytis bunch rot in Seyval blanc grapevines. American Journal of Enology and Viticulture 49, 163–170. [Google Scholar]
- Stern RA, Stern D, Harpaz M, Gazit S. 2000. Applications of 2,4,5-TP, 3,5,6-TPA, and combinations there of increase lychee fruit size and yield. Horticultural Science 35, 661–664. [Google Scholar]
- Stopar M. 1998. Apple fruitlet thinning and photosynthate supply. Journal of Horticultural Science and Biotechnology 73, 461–466. [Google Scholar]
- Stösser R, Anvari S. 1982. On the senescence of ovules in cherries. Scientia Horticulturae 16, 29–38. [Google Scholar]
- Sun N, Adachi F, Kadowaki M, Nakatsuka A, Esumi T, Itamura H. 2010. Effects of photosynthate transport and water flow to young fruit on fruit drop via ethylene synthesis in Persimmon (Diospyros kaki Thunb.). Journal of the Japanese Society for Horticultural Science 79, 340–347. [Google Scholar]
- Talon M, Tadeo FR, Ben-Cheikh W, Gómez-Cadenas A, Mehouachi J, Perez-Botella J, Primo-Millo E. 1997. Hormonal regulation of fruit set and abscission in citrus: classical concepts and new evidence. VIII International Symposium on Plant Bioregulation in Fruit Production 463, 209–218. [Google Scholar]
- Taylor JE, Whitelaw CA. 2001. Signals in abscission. New Phytologist 151, 323–340. [Google Scholar]
- Tripathi SK, Sane AP, Nath P, Tuteja N. 2008. Organ abscission in plants: Understanding the process through transgenic approaches. In: Rivera-Dominguez M, Troncoso-Rojas R, Tiznado-Hernandez ME. A transgenic approach in plant biochemistry and physiology, Research Signpost, 155–180. [Google Scholar]
- Tromp J, Borsboom O. 1994. The effect of autumn and spring temperature on fruit set and on the effective pollination period in apple and pear. Scientia Horticulturae 60, 23–30. [Google Scholar]
- Trueman SJ. 2010. Benzyladenine delays immature fruit abscission but does not affect final fruit set or kernel size of Macadamia. African Journal of Agricultural Research 5, 1523–1530. [Google Scholar]
- Tudela D, Primo-Millo E. 1992. 1-Aminocyclopropane-1-carboxylic acid transported from roots to shoots promotes leaf abscission in Cleopatra Mandarin (Citrus reshni Hort. ex Tan.) seedlings rehydrated after water stress. Plant Physiology 100, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos MC, Castagnoli S. 2000. Leaf canopy structure and vine performance. American Journal of Enology and Viticulture 51, 390–396. [Google Scholar]
- Velikova V, Edreva A, Loreto F. 2004. Endogenous isoprene protects Phragmites australis leaves against singlet oxygen. Physiologia Plantarum 122, 219–225. [Google Scholar]
- Vernieri P, Tagliasacchi AM, Forino L, Lanfranchi A, Lorenzi R, Avanzi S. 1992. Abscisic acid levels and cell structure in single seed tissues of shedding affected fruits of Malus domestica Borkh. Journal of Plant Physiology 140, 699–706. [Google Scholar]
- Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laothawornkitkul J, Ryan A, Mullineaux PM, Nicholas Hewitt C. 2009. Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant, Cell and Environment 32, 520–531. [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1–14. [DOI] [PubMed] [Google Scholar]
- Weaver RJ, Pool RM. 1969. Effect of ethrel, abscisic acid, and a morphactin on flower and berry abscission and shoot growth in Vitis vinifera . Journal of the American Society for Horticultural Science 94, 474–478. [Google Scholar]
- Weinbaum SA, Simons RK. 1974. Histochemical appraisal of the relationship of seed abortion to chemical induction of apple fruit abscission following bloom. Journal of the American Society for Horticultural Science 99, 266–269. [Google Scholar]
- Williams RR, Flook VA. 1980. The mode of action of the hormone apple fruit-setting mixture PP 341B applied to Cox’s Orange Pippin. Journal of Horticultural Science 55, 275–277. [Google Scholar]
- Yuan R, Carbaugh DH. 2007. Effects of NAA, AVG, and 1-MCP on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Golden Supreme’ and ‘Golden Delicious’ apples. HortScience 42, 101–105. [Google Scholar]
- Yuan R, Greene DW. 2000. Benzyladenine as a chemical thinner for ‘McIntosh’ Apples. II. Effects of benzyladenine, bourse shoot tip removal, and leaf number on fruit retention. Journal of the American Society for Horticultural Science 125, 177–182. [Google Scholar]
- Yu TS, Lue WL, Wang SM, Chen J. 2000. Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation. Plant Physiology 12, 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacarias L, Talon M, Ben-Cheikh W, Lafuente MT, Primo-Millo E. 1995. Abscisic acid increases in non-growing and paclobutrazol-treated fruits of seedless mandarins. Physiologia Plantarum 95, 613–619. [Google Scholar]
- Zanchin A, Marcato C, Trainotti L, Casadoro G, Rascio N. 1995. Characterization of abscission zones in the flowers and fruits of peach [Prunus persica (L.) Batsch]. New Phytologist 129, 345–354. [DOI] [PubMed] [Google Scholar]
- Zhao D, Reddy KR, Kakani VG, Koti S, Gao W. 2005. Physiological causes of cotton fruit abscission under conditions of high temperature and enhanced ultraviolet-B radiation. Physiologia Plantarum 124, 189–199. [Google Scholar]
- Zhu H, Dardick CD, Beers EP, Callanhan AM, Xia R, Yuan R. 2011. Transcriptomics of shading-induced and NAA-induced abscission in apple (Malus domestica) reveals a shared pathway involving reduced photosynthesis, alterations in carbohydrate transport and signaling and hormone crosstalk. BMC Plant Biology 11, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Yuan R, Greene DW, Beers EP. 2010. Effects of 1-methylcyclopropene and naphthaleneacetic acid on fruit set and expression of genes related to ethylene biosynthesis and perception and cell wall degradation in apple. Journal of the American Society for Horticultural Science 135, 402–409. [Google Scholar]