Highlight
An olive β-glucosidase expressed heterologously in tobacco cells results in an active enzyme, a constituent of the two-component defence system comprising a β-glucosidase that activates oleuropein into a toxic cross-linking agent.
Keywords: β-Glucosidase, heterologous expression, oleuropein, olive development, plant defence, protein cross-linking.
Abstract
Oleuropein, the major secoiridoid compound in olive, is involved in a sophisticated two-component defence system comprising a β-glucosidase enzyme that activates oleuropein into a toxic glutaraldehyde-like structure. Although oleuropein deglycosylation studies have been monitored extensively, an oleuropein β-glucosidase gene has not been characterized as yet. Here, we report the isolation of OeGLU cDNA from olive encoding a β-glucosidase belonging to the defence-related group of terpenoid-specific glucosidases. In planta recombinant protein expression assays showed that OeGLU deglycosylated and activated oleuropein into a strong protein cross-linker. Homology and docking modelling predicted that OeGLU has a characteristic (β/α)8 TIM barrel conformation and a typical construction of a pocket-shaped substrate recognition domain composed of conserved amino acids supporting the β-glucosidase activity and non-conserved residues associated with aglycon specificity. Transcriptional analysis in various olive organs revealed that the gene was developmentally regulated, with its transcript levels coinciding well with the spatiotemporal patterns of oleuropein degradation and aglycon accumulation in drupes. OeGLU upregulation in young organs reflects its prominent role in oleuropein-mediated defence system. High gene expression during drupe maturation implies an additional role in olive secondary metabolism, through the degradation of oleuropein and reutilization of hydrolysis products.
Introduction
Sessile organisms like plants produce a wide variety of secondary metabolites as a general strategy of chemical defence to tolerate biotic and abiotic stress. Although diverse plants contain various common secondary metabolites, the olive (Olea europaea L.) contains a number of unusual secoiridoids commonly known as oleosides that are unique to Oleaceae. They comprise phenolic conjugates characterized by an exocyclic 8,9-olefinic functional group (Obied et al., 2008). The secoiridoids are an important subclass of iridoids that serve as intermediates in the terpene metabolic pathway for the production of indole alkaloids. The most significant secoiridoids in olive are oleuropein, an ester of elenolic acid with 3,4-dihydroxyphenylethanol (hydroxytyrosol), and its direct precursor, ligstroside (El Riachy et al., 2011a ). Oleuropein is responsible for the bitter taste and browning of green olives during harvest and processing. Secoiridoids possess a wide range of pharmaceutical activities. Oleuropein, in particular, possesses powerful antioxidant and anti-inflammatory properties and may prevent neurodegenerative diseases, along with many other beneficial effects to human health attributed to oleuropein aglycon and its derivatives as the main polyphenols found in extra virgin olive oil (Bulotta et al., 2013).
The mechanism of the oleuropein defence system against herbivore, insect, or pathogen attack has been described in privet (Ligustrum obtusifolium). This potent secoiridoid is stored in the vacuoles or cytosol of the privet leaf cell in a glycosylated and thus inactive form, physically separated from the endogenous β-glucosidase (Konno et al., 1999). Upon tissue destruction, cellular compartmentalization is abolished and oleuropein is hydrolysed and activated by β-glucosidase to an unstable aglycon moiety that is converted into a glutaraldehyde-like structure with strong protein-denaturing/protein-cross-linking properties (Lo Scalzo et al., 1994; Konno et al., 1999). A similar two-component chemical defence system has been evolved in Apocynaceae (Catharanthus roseus and Rauvolfia serpentine) or Plantago species. Substrate-specific β-glucosidases convert compounds chemically related to oleuropein, such as monoterpene indole alkaloids or iridoid glucosides, into unstable aglycons with protein cross-linking potential and cytotoxic effects (Guirimand et al., 2010; Pankoke et al., 2013).
Oleuropein has been detected in various vegetative organs of the olive tree, like leaves, bark, and roots, but it is highly abundant in young drupes where it may reach up to 14% of dry weight (Amiot et al., 1986; Ortega-García and Peragón, 2010). Oleuropein catabolism and biotransformation routes during olive fruit growth and maturation are still not fully resolved. An accepted pathway involves its conversion into demethyloleuropein and/or elenolic acid by a concerted action of specific esterases (Amiot et al., 1989). Concomitantly, another route recruits specific β-glucosidase activity that leads into an extensive hydrolysis of oleuropein (Fig. 1). This pathway seems to be critical in the activation of the oleuropein-mediated chemical defence mechanism (Briante et al., 2002; Ryan et al., 2003). The substantial rise in oleuropein aglycon levels during drupe maturation is most likely the result of oleuropein hydrolysis (Gutierrez-Rosales et al., 2010, 2012).
Fig. 1.
A simplified scheme of the oleuropein biosynthesis and degradation pathway (Obied et al., 2008).
Plants contain a number of β-glucosidases (β-d-glucoside glucohydrolases, EC 3.2.1.21) of diverse functions, including chemical defence response, activation of plant growth regulators, and lignification or cell-wall catabolism (Dietz et al., 2000; Kristoffersen et al., 2000; Lee et al., 2006). These functions are furnished by different enzymes often showing high substrate specificity. This is accomplished by the recognition and discrimination of the aglycon moiety of the substrate (Czjzek et al., 2000; Verdoucq et al., 2003, 2004). Olive, like other plants, contains a number of different β-glucosidases as recent proteomic and transcriptomic studies have shown (Wang et al., 2009; Alagna et al., 2012; Corrado et al., 2012; Bianco et al., 2013). Romero-Segura et al. (2009) reported the purification of a β-glucosidase enzyme from mature olive fruit that was able to hydrolyse olive glucosides and exhibited high substrate specificity to oleuropein. Despite the biological and technological importance of an oleuropein-hydrolysing β-glucosidase from olive, the respective gene has remained unknown.
Here, we present the isolation and functional characterization of OeGLU cDNA from olive encoding a β-glucosidase enzyme able to both deglycosylate and activate oleuropein into a potent protein cross-linking agent. In silico protein structural analysis revealed that OeGLU shares conserved motifs with other β-glucosidases of the GH1 family. These motifs include the pocket-shaped active site with conserved and non-conserved amino acid residues indicative of its general glucosidase activity and high substrate specificity, respectively. Transcriptional analysis in drupes and other reproductive or vegetative organs revealed that gene expression is developmentally regulated, coinciding well with patterns of oleuropein accumulation/degradation.
Materials and methods
cDNA cloning of OeGLU and heterologous expression of OeGLU in Escherichia coli cells
Total RNA was isolated from olive young leaves (O. europaea cv. Koroneiki) using a phenol/chloroform procedure. After multiple sequence alignment of various plant defence-related β-glucosidases, two highly conservative amino acid motifs were detected, VT(L/I)FHWD and IY(I/V)TENG, and degenerate primers (F1 and R1) were designed to amplify a cDNA fragment. For 5′-rapid amplification of cDNA ends (RACE), the first-strand cDNA was primed with the reverse specific primer SP1, dA-tailed, and amplified using the nested primers TD1 and TD2 in combination with oligo(dT)17. For 3′-RACE, the first-strand cDNA was primed with the oligo(dT)17 primer and amplified using in reverse the specific forward primer 3SP1. The final full-length cDNA of the gene (termed OeGLU) was amplified using the primers 5F and 3R, respectively.
The coding sequence of OeGLU was amplified with primers EXPF1 and EXPR, which introduced SacI and HindIII restriction sites. The construct was cloned in pET28a and the recombinant protein was expressed in E. coli strain BL21(DE3). The primers are shown in Supplementary Table S1 at JXB online.
Agrobacterium infiltration-mediated gene expression in tobacco
The full-length OeGLU cDNA was amplified using bGlu-For and bGlu-Rev primers (Supplementary Table S1), containing the strong Kozak consensus sequence ccaccAUG (Kozak, 1984) and the FLAG epitope, respectively, and cloned into the pGPTV-HPT binary vector (Becker et al., 1992), carrying the 35S cauliflower mosaic virus promoter. The resulting vector, pGPTV/35S-OeGLU-FLAG (Supplementary Fig. S1 at JXB online), was introduced into Agrobacterium tumefaciens strain C58C1 RifR. Agrobacterium-transformed cells were harvested and resuspended in infiltration medium (10mM 2-N-morpholino-ethanesulfonic acid pH 5.6, 10mM MgCl2, 150 μM acetosyringone). The cell suspension was mixed with a second Agrobacterium suspension carrying the pBIN61/35S-p19 expression vector to avoid genome silencing (Voinnet et al., 2003). The mixture was infiltrated into the abaxial side of 2- to 4-week-old Nicotiana benthamiana leaves. Crude protein was extracted from a total of eight leaf disks of either infiltrated or untreated leaves from different plants in triplicate. Leaf discs were homogenized in ice-cold extraction buffer (100mM Tris/HCl, pH 8.8, 100mM EDTA, 1mM PMSF, 100mM KCL, 10mM Na2SO3, 100mM glycine), centrifuged, and the soluble protein was quantified by a Bradford assay. SDS-PAGE was performed on 10% polyacrylamide gels with 10 μg per lane of the extracts followed by Coomassie Brilliant Blue (CBB) staining or western blotting. Western blot analysis was performed using rabbit anti-FLAG as a primary antibody (Santa Cruz Biotechnology) and horseradish peroxidase-conjugated goat anti-rabbit IgG as a secondary antibody (Santa Cruz Biotechnology) according to standard protocols.
β-Glucosidase activity assay and functional analysis
The hydrolysis of oleuropein was measured following incubation of 20 μg of total protein in a hydrolysis reaction medium (100 μl), containing 150mM sodium acetate buffer (pH 5.5), 5mM or 50 μΜ commercial oleuropein, 50 μM luteolin (Extrasynthese, Genay, France), 50 μM rutin (Sigma, London, UK) and 0.05% (w/v) bovine serum albumin (BSA), at 37 °C. Aliquots of 15 μl were transferred at regular time intervals (0, 10, 30, and 60min) into new tubes and the reaction was stopped with the addition of 1vol. of methanol. The sample was transferred to a new tube containing 870 μl of water/methanol (40:60, v/v). The amount of intact oleuropein (i.e. not hydrolysed) in the sample was measured by high-performance liquid chromatography (HPLC) performed on a Jasco system (Tokyo, Japan) equipped with a PU-2089 pump, a UV-2070 detector and a Discovery HS C18 column (5 μm, 24cm, 4.6mm; Supelco). Samples were run at a flow rate of 0.5ml min–1 and detected at 254nm. The injected volume was 20 μl, and the mobile phase consisted of eluent A containing water/acetic acid (99:1, v/v) and methanol as eluent B. Chromatographic conditions started with an initial isocratic elution A:B (60:40) for 40min, followed by gradient elution to A:B (30:70) within 5min. Oleuropein was identified and quantified according to its retention time by comparison with external standards. The β-glucosidase activity of the enzyme fraction on oleuropein was assayed by measuring the amounts of glucose produced in the reaction solutions. From the reactions, 50 μl vols of glucose-containing fractions of each sample were collected at 30min and the glucose concentration was measured using a glucose assay kit (Boehringer Mannheim, Darmstadt, Germany).
The protein cross-linking activity of the oleuropein deglycosylation product(s) derived from the above hydrolysis reaction was assayed in vitro using an electrophoretic mobility shift assay (EMSA) essentially as described by Guirimand et al. (2010).
For the in-gel activity assay (zymography), crude protein extracts from tobacco plants (25 μg) infiltrated with either the pGPTV/35S-OeGLU-FLAG or with the pGPTV/35S (empty) construct, along with crude extract from olive mesocarp at 17 weeks after flowering (WAF) (2.5 μg) were subjected to 7% SDS-PAGE under semi-native conditions. The electrophoresis sample buffer was depleted of SDS and β-mercaptoethanol, and samples were not heat treated. After electrophoresis, the gel was equilibrated with at least 10 changes of 150mM sodium acetate buffer (pH 5.5). The zymogram was developed in 150mM sodium acetate buffer (pH 5.5) containing 1.5mM 4-methylumbelliferyl-β-d-glucopyranoside (MUGlc) for 10min. The fluorescent bands corresponding to β-glucosidase activity were photographed under UV light. The fluorescent bands were excised and incubated with oleuropein in hydrolysis reaction medium and the remaining oleuropein was measured by HPLC, in triplicate.
Southern and northern blotting and in situ hybridization analysis
For Southern blot analysis, genomic DNA was extracted from olive leaves (cv. Koroneiki), digested, and probed with a 32P-labelled cDNA corresponding to the open reading frame (ORF) or with a 645 nt fragment containing the 3′-untranslated region (UTR) of the gene (3′-end cDNA) under high-stringency conditions at 65 °C. Northern blot analysis was conducted using a 32P-labelled 3′-end cDNA probe. Total RNA was isolated from various organs/tissues as described previously (Banilas et al., 2011) and from trichomes that were carefully dissected out from the abaxial leaf side by surface scraping with razor blades. Samples of trichomes were examined under a light microscope to ensure no contamination from adjacent tissues. RNA in situ hybridization was performed essentially as described previously (Banilas et al., 2011) using the 3′-end cDNA as a probe. Sites of positive hybridization were detected as blue/violet regions by bright-field microscopy (Olympus BX50).
Phylogenetic analysis and three-dimensional modelling
Amino acid sequences of characterized plant GH1 β-glucosidases were retrieved from the CAZy database (http://www.cazy.org). Multiple alignment was performed using ClustalX 1.83 software (http://www.clustal.org) and graphically displayed (Supplementary Fig. S2 at JXB online) with the ESPript program (http://espript.ibcp.fr). A neighbour-joining tree was inferred from alignment of protein sequences (see also Supplementary Table S2 at JXB online) by using PHYLIP (Phylogeny Inference Package, version 3.65), with bootstrap values from 1000 replications. Image representation of the phylogenetic tree was produced with Dendroscope 3 (http://ab.inf.uni-tuebingen.de/software). The three-dimensional protein structure prediction of OeGLU (NCBI accession no. AAL93619) was generated ab initio with the Phyre2 application (http://www.sbg.bio.ic.ac.uk/phyre2) using the ‘intensive’ modelling mode. The resulting PDB file was submitted to the Molegro Virtual Docker software (Molegro ApS, Aarhus, Denmark) for molecular docking analysis with oleuropein as a ligand (PubChem CID: 5281544). Two- and three-dimensional images of the resulting structure were generated with LigPlot+ (http://www.ebi.ac.uk/thornton-srv/software/LigPlus) and PyMOL (http://www.pymol.org), respectively. Subcellular localization predictions were performed using cNLS Mapper (http://nls-mapper.iab.keio.ac.jp) or PSORT (http://psort.org).
Accession numbers
The full-length cDNA sequence of OeGLU can be found in the GenBank/EMBL/DDBJ databases under accession number AY083162. Accession numbers for the sequences used in the phylogenetic analysis can be found in Supplementary Table S3 at JXB online.
Results
Isolation of OeGLU cDNA encoding an olive β-glucosidase of the GH1 family
To isolate an oleuropein β-glucosidase cDNA from olive, a pair of degenerate primers was designed based on conserved amino acid sequences of various plant defence-related β-glucosidases of the GH1 family. Since olive leaves contain high levels of both oleuropein and β-glucosidase activity (Silva et al., 2006), we used RNA from leaves to amplify a partial cDNA of 850bp. The full-length cDNA of 1902bp contained an ORF of 1656bp encoding a polypeptide of 551 aa with a predicted molecular weight of 62.9kDa and a pI of 6.3. a BLAST search revealed that a genomic sequence from the Ayvalik cultivar was recently deposited in GenBank (accession no: KF623043.1) corresponding to the β-glucosidase cDNA (AY083162). This genomic sequence is 4398 nt and compared with OeGLU cDNA contains 10 exons and nine introns.
The primary structure of the predicted protein contained the N-terminal signature sequence of β-glucosidases belonging to the GH1 family (PROSITE entry PS00653: F-x-[FYWM]-[GSTA]-x-[GSTA]-x-[GSTA](2)-[FYNH]-[NQ]-x-E-x-[GSTA], which is located between aa 42 and 56 (FvFGaAtASYQvEgA). In silico analysis predicted that OeGLU contains a putative nuclear localization signal at aa 542–550 (DRRKRLRGS).
A BLAST search showed that the predicted protein, termed OeGLU, shared the highest similarity (>65%) with raucaffricine- and strictosidine-β-glucosidases, which are involved in the biosynthesis of monoterpenoid indole alkaloids. Following amino acid sequence alignment with previously characterized plant GH1 β-glucosidases, a phylogenetic tree was reconstructed (Fig. 2). The neighbour-joining tree clustered the enzymes according to their function or specificity, i.e. implication in defence response, lignification, hormone deglycosylation, β-mannosidases, or myrosinases (thio-β-glucosidases). Within the defence-related clade of dicots, further subclustering was observed into four subclades according to the type of substrate, i.e. cyanogenic glucosides, isoflavonoid conjugates, alkaloid glucosides, and terpenoid glucosides. A simplified alignment matrix illustrating the similarities among selected sequences of the phylogenetic tree was created by using a single member of each defence dicot clade or subclade (see Supplementary Table S2). Although OeGLU shared a higher similarity with Rauvolfia serpentina raucaffricine β-glucosidase (RsRAU) than with cardenolide-specific 16′-O-glucohydrolase (DlGLU) from Digitalis lanata, the higher gap character shared with DlGLU (Supplementary Table S2) placed the predicted β-glucosidase from olive within the defence-related subclade of enzymes hydrolysing terpenoid glucosides. Based on the phylogenetic tree, the alkaloid- and terpenoid-hydrolysing β-glucosidases might share a common ancestral gene and are rather separated from the cyanogenic glucoside and isoflavonoid conjugate subclades (Fig. 2).
Fig. 2.
Phylogenetic tree of plant GH1 β-glucosidases retrieved from the CAZy database (see Supplementary Table S3). The enzymes were clustered according to their functions or to the group of their specific substrates as indicated. Olive OeGLU (underlined) was placed within the defence-related subclade of enzymes hydrolysing terpenoid glucosides. Bootstrap reproducibility values (1000 permutations) are shown on internal branches. Bar, 0.1 amino acid substitutions per site.
OeGLU encodes an oleuropein-hydrolysing β-glucosidase
To assess the potential role of the OeGLU-encoded protein in oleuropein deglycosylation, the cDNA was cloned in E. coli BL21 cells using the pET28a expression system. The recombinant protein was clearly expressed, but the protein was detected only in the insoluble fraction. Despite numerous attempts to increase the ratio of the recombinant protein in the soluble fraction by applying various growth temperatures, isopropyl β-d-1-thiogalactopyranoside concentrations, or adjustments of osmolytes, no enzyme activity was detected in either soluble or insoluble fractions (Supplementary Fig. S3 at JXB online). Therefore, an in planta transient transformation regime was followed using tobacco (N. benthamiana), since any conformational competence important for olive β-glucosidase enzymatic activity is likely to be better attained within a plant cell. Moreover, as the tobacco plant does not contain oleuropein, any interference from a respective β-glucosidase was expected to be minimal.
Tobacco leaves were infiltrated with A. tumefaciens carrying the pGPTV/35S-OeGLU-FLAG (OeGLU expression construct). The pGPTV/35S vector (empty vector) was used as a negative control to monitor residual β-glucosidase activity of tobacco cells. Expression of the OeGLU construct in infiltrated leaves was optimum at 6 d post-infiltration (Supplementary Fig. S4 at JXB online). A single protein band of ~63kDa was identified exclusively in the extracts of leaves infiltrated with A. tumefaciens carrying the OeGLU transgene (Fig. 3B, C), while a distinct band was not detectable in Coomassie stained gels indicating a low level of expression compared with the total tobacco leaf proteins (Fig. 3B).
Fig. 3.

Transient expression of the OeGLU gene in N. benthamiana leaves and β-glucosidase activity assay. (A) A combined chromatogram depicting oleuropein levels at 60min of β-glucosidase activity assay (black line), oleuropein spike (green line), or using empty vector as control (red line). The reaction with crude protein extract from leaves infiltrated with Agrobacterium containing the pGPTV/35S-OeGLU-FLAG vector (Expr) is indicated in black, while those with the empty pGPTV/35S vector (Empty) and the non-infiltrated leaves (Untr) are depicted in red and green, respectively. The concentration of oleuropein after 0, 10, 30, and 60min of reaction, expressed as percentages of the blank reaction (without crude protein), is shown in the inset. (B) SDS-PAGE of the crude protein extracted from Expr (lane 1), Empty (lane 2) and Untr (lane 3) tobacco leaves. Molecular mass markers (lane M) are given in kDa. (C) Western blot analysis with an anti-FLAG antibody showing an ~63kDa band only in the Expr sample (lane 1). (D) Protein-denaturing/cross-linking activities on BSA as assayed by SDS/PAGE. The degree of denaturation and cross-linking is indicated by the disappearance of the main BSA band and the appearance of fuzzy bands in the upper parts of the separating gel. Lane 1, Expr; Lane 2, Empty; lane 3, Untr; lane 4, blank (no leaf protein extracts). Molecular mass markers (lane M) are given in kDa.
The recombinant OeGLU protein was extracted to perform enzymatic reactions using oleuropein as a substrate. Untreated tobacco leaf extracts showed negligible enzyme activity, and oleuropein levels remained unchanged after 1h of reaction (Fig. 3A). This showed that tobacco leaves do not possess endogenous β-glucosidase activity to hydrolyse oleuropein. In contrast, HLPC analysis of the protein extracts of leaves infiltrated with the OeGLU construct revealed that oleuropein was hydrolysed in a time-dependent manner. As early as 10min after the start of the reaction, more than 70% of oleuropein was hydrolysed. Oleuropein levels decreased substantially after 30min and became undetectable after 60min, showing that the OeGLU encodes an active β-glucosidase with specificity towards oleuropein (Fig. 3A). Taken together, these results showed that the oleuropein-hydrolysing β-glucosidase activity was due to the recombinant enzyme and not to endogenous enzyme activity or activity induced by agroinfiltration.
To verify whether β-glucosidase activity is specific to oleuropein, we assayed the enzyme fraction of the heterologous expressed OeGLU in tobacco cells on oleuropein and other β-glucosides (Table 1). The enzyme fraction derived from fresh tobacco leaves showed negligible activity on the two other substrates compared with oleuropein. With p-nitrophenyl-β-glucopyranoside, an artificial substrate often used in assaying β-glucosidase activity, as a substrate, this enzyme fraction had minimum activity, as found by others (Konno et al., 1999; Romero-Segura et al., 2009).
Table 1.
Percentage of β-glucosidase activity of the enzyme encoded by the heterologously expressed OeGLU gene in tobacco leaves
| Substrate | Relative β-glucosidase activity (%)a |
|---|---|
| Oleuropein | 100b |
| Rutin | 1.8±0.1 |
| Luteolin | 7.9±3.6 |
a Mean±SD (n=3).
b Oleuropein β-glucosidase enzyme activity was set arbitrary to 100%. Reactions: 1h at 37 °C and 50 μM substrate concentration.
To validate the β-glucosidase activity, we examined the degree of deglycosylation under the assay conditions by assessing the amounts of glucose produced after the reactions (Table 2). The enzyme fraction derived from tobacco leaves expressing OeGLU deglycosylated 95.3±5.4% (mean±SD) of oleuropein while tobacco leaves infiltrated with empty vector showed negligible deglycosylation. These data indicated that the enzyme encoded by OeGLU has a strong and substrate-specific β-glucosidase activity on oleuropein.
Table 2.
β-Glucosidase activity of the enzyme derived from the heterologously expressed OeGLU gene in tobacco leaves
| Reaction | Deglycosylation (%)a |
|---|---|
| Oleuropein+leaf extracts without OeGLU | 7.8±6.3 |
| Oleuropein+leaf extracts with OeGLU | 95.3±5.4 |
a Mean±SD (n=3).
OeGLU enzymatically activates oleuropein into a strong protein cross-linking agent
It is worth noting that the aglycon products of oleuropein or strictosidine hydrolysis have not been detected by HPLC analysis (Konno et al., 1999; Guirimand et al., 2010). The hydrolysis of oleuropein by the privet tree enzyme has been reported to lead to the production of a reactive agent possessing strong protein cross-linking activity (Konno et al., 1999). To test whether the hydrolysis of oleuropein by OeGLU was able to promote in vitro a similar protein cross-linking activity via generation of an active aglycon product, we applied an EMSA using BSA as a reference protein substrate. EMSA analysis of both negative controls, i.e. protein extracts from non-infiltrated leaves or from leaves infiltrated with the empty vector, displayed an intact BSA band with a typical 66kDa migration pattern (Fig. 3D). In contrast, EMSA analysis of samples derived from the enzymatic reaction in the presence of extracted recombinant OeGLU and BSA showed the formation of high-molecular-weight aggregates of BSA that were barely resolved in the separation gel. These results confirmed that the recombinant OeGLU is able to promote strong protein cross-linking activity upon oleuropein hydrolysis.
Protein structural analysis reveals common features of OeGLU with functionally related β-glucosidases
Given that OeGLU is phylogenetically similar to β-glucosidases for terpenoid or alkaloid glucosides, we examined whether they shared common sequence motifs and/or protein structure. Amino acid sequence alignment of OeGLU with well-characterized β-glucosidases of these groups revealed highly conserved sequence elements. OeGLU contains the characteristic TLNEP (aa 199–203) and YITENG (aa 430–435) motifs of the GH1 β-glucosidases (Supplementary Fig. S5 at JXB online) participating in glycosylation/deglycosylation steps (Wang et al., 1995; Wiesmann et al., 1995). The putative three-dimensional structure of OeGLU (Fig. 4) was superimposed with the resolved structures of Rauvolfia serpentina raucaffricine β-glucosidase (RsRAU; Xia et al., 2011) and strictosidine β-glucosidase (RsSTR; Barleben et al., 2007), and shared an identical core (β/α)8 TIM barrel fold (Supplementary Fig. S6 at JXB online).
Fig. 4.
Overall putative architecture of OeGLU docked with oleuropein. (A) The three-dimensional structure resembles the (β/α)8 TIM barrel fold, characteristic of the GH1 family. The α-helices and β-sheets of the barrel are shown in cyan and magenta, respectively. The extra strands, helices, and connecting loops are shown in light brown. The N and C termini are marked. (B) Zoomed view of (A) showing the proposed active site residues in stick form (yellow) docked with oleuropein (red). (C) Three-dimensional structure of the 14 residues forming the binding pocket of OeGLU docked with oleuropein (green). (D) Two-dimensional schematic diagram of (C).
Docking of the oleuropein in the active site of OeGLU (Fig. 4) showed that it binds to the enzyme pocket with high affinity. The free energy change (ΔG) of the structure shown in Fig. 4C was found to be –7.56 kcal mol–1. The docking model revealed that 14 aa are implicated in the pocket. The amino acid residues His156, Glu 202, Trp482, Glu489, and Trp490 of OeGLU, located within the pocket-shaped active site, are most likely involved in glucose binding and are located at similar positions compared with the RsRAU and RsSTR enzymes. However, the amino acid residues Trp204, Ser205, Gln209, Phe216, Cys291, and Phe498 of OeGLU that are located within the pocket-shaped active site that potentially recognizes the aglycon part and are thus implicated in substrate specificity showed considerable variability, compared with the RsRAU and RsSTR enzymes, except for Met315, Tyr363, and Trp405 (Fig. 4 and Supplementary Fig. S5). Taken together, the specificity of the aglycon binding within the pocket of OeGLU is determined by non-conserved amino acid residues.
OeGLU cDNA is encoded by a single-copy gene
The genomic DNA from olive was digested with EcoRI, EcoRI/HindIII, or BamHI and separated on an agarose gel. Figure 5 shows the autoradiogram of a Southern blot hybridization under high-stringency conditions using a probe corresponding either to the full coding sequence (ORF) or to the 3′-end cDNA. Single major hybridization bands were detected with both probes, while the BamHI digest probed with the entire ORF revealed a strong and a weak hybridizing band. Given that both probes possess neither EcoRI nor HindIII restriction sites, while the ORF contains two BamHI recognition sites, these results suggested that the OeGLU cDNA is encoded by a single-copy gene.
Fig. 5.

Southern blot analysis to determine the OeGLU gene copy number in the olive genome. Genomic DNA (cv. Koroneiki) was digested with BamHI (B), EcoRI (E), EcoRI/HindIII (E/H), or HindIII (H) and probed with the full-length cDNA (A) or the 3′-UTR of OeGLU (B). Numbers indicate molecular size markers in kb.
The OeGLU gene is developmentally regulated in olive drupes
The pattern of OeGLU gene expression in different olive fruit tissues during drupe development was analysed. In very young drupes (5 WAF) the expression of OeGLU was at its highest levels, similar to those detected in mesocarp at 22 WAF (Fig. 6Α). Thereafter, transcripts started to decline but remained at relatively high levels till 11 WAF. There was no expression detected in embryos between the early (13 WAF) and late (22 WAF) torpedo stages. Similarly, no transcripts were detected in the respective endosperms, except for a weak signal at 13 WAF. To examine whether the gene upregulation detected in the very young drupes could be attributed to seed tissues and/or mesocarps, in situ hybridization analysis was conducted. As shown in Fig. 7, OeGLU was expressed mostly in the developing seed coat tissues and the perisperm, while prominent staining was also observed in the mesocarp of immature drupes (7 WAF). In contrast, no signal was apparent in the respective endosperm.
Fig. 6.
Northern blot analysis of OeGLU transcripts. (A) Total RNA was extracted from intact drupes, embryos, endosperms, and mesocarps at different developmental stages. Drupe lanes: 5, 7, 9, and 11 WAF; embryo lanes: 13 (early torpedo stage), 16 (early-mid-torpedo), 19 (mid-late torpedo), and 22 (late torpedo) WAF; endosperm lanes: 13, 16, 19, and 22 WAF; mesocarp lanes: 13, 16, 19, 22, 25, and 28 WAF. L, young olive leaves. (B) Accumulation of transcripts in mature leaves (lane 1), young leaves (lane 2), leaf trichomes (lane 3), and the respective leaves without trichomes (lane 4). Hybridization was performed using the 3′-UTR of the gene as a probe. Equivalent amounts of RNA were loaded onto gels and stained with ethidium bromide to evaluate equal loading in each lane (lower panels).
Fig. 7.
Localization of OeGLU transcripts in young drupes at 7 WAF. Longitudinal sections were processed with digoxygenin-labelled antisense RNA probes of OeGLU (B, C). For negative controls, labelled RNA sense probes were used (A). OeGLU expression was detected in mesocarp (me), perisperm (pe), and integument (in) tissues, while no expression was detected in the endosperm (en). Sites of positive hybridization signals are shown as blue/violet regions. Bar, 300 μm.
A different gene expression pattern was observed in the developing mesocarps from 13 WAF until veraison (28 WAF), when the colour of drupes turned from green to black. At the green maturation stage (19 WAF), the OeGLU transcripts started to accumulate, reaching maximal expression at 22 WAF (Fig. 6A). Thereafter, transcripts declined, although gene expression remained at relatively high levels.
Spatiotemporal OeGLU expression in vegetative organs and floral buds
Oleuropein has been detected in leaves, roots, shoots, and buds (Amiot et al., 1986; Malik and Bradford, 2006; Ortega-García and Peragón, 2010). The northern blot analysis showed quite prominent levels of OeGLU expression in young leaves, comparable to those detected in the mesocarps at 13 or at 19–25 WAF (Fig. 6A). The mRNA accumulation in young (expanding) leaves was higher than that detected in mature (fully expanded) leaves. Notably, no OeGLU expression was detected in leaf trichomes (Fig. 6B). In situ hybridization analysis in young leaves revealed that the gene was expressed preferentially in spongy cells and the vascular bundles (Fig. 8H, I).
Fig. 8.
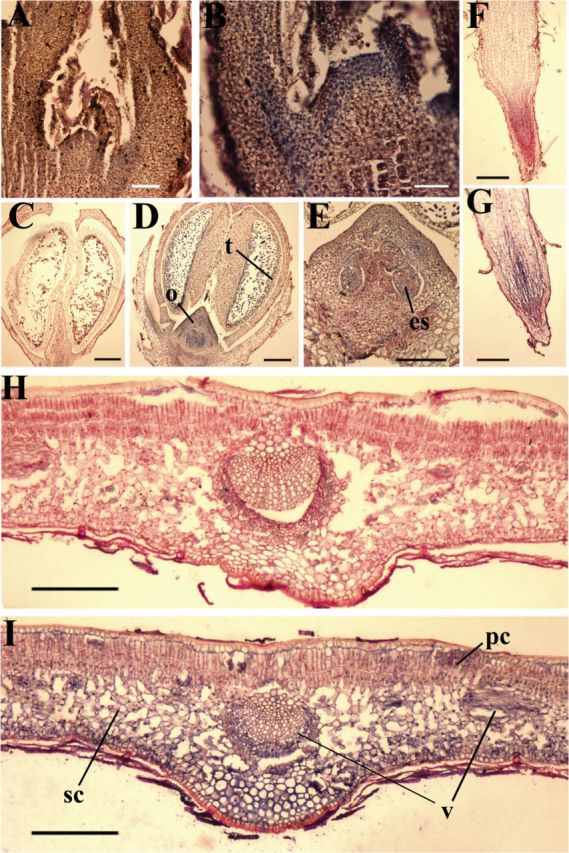
In situ localization of OeGLU transcripts in shoot apical meristems (A, B), developing buds of 3.0mm (C–E), root tips (F, G), and expanding leaves (H, I). Longitudinal sections were processed for in situ hybridization with digoxygenin-labelled antisense RNA of OeGLU (B, D, E, G, and I). For negative controls, sense RNA of OeGLU was digoxygenin labelled and probed in the respective tissues (A, C, F, and H). Sites of positive hybridization signals are shown as blue/violet regions. es, Embryo sac; o, ovary; pc, palisade cells; sc, spongy cells; t, tapetum; v, vascular bundles. Bars, 250 μm.
Expression was also evident in shoot (Fig. 8A, B) and root (Fig. 8F, G) meristematic cells, and in the vascular cambium tissue of roots. In addition, in situ hybridization analysis revealed gene expression in different tissues of floral buds (about 3.0mm) but mostly in the developing ovaries (Fig. 8C–E) and in the tapetal cells of the anther (Fig. 8D). These results showed that expression of the OeGLU gene in different vegetative organs and floral buds was associated with active growth and proliferating tissues. These tissues may be more vulnerable to common pests and thus may have a greater demand for the oleuropein-mediated defence mechanism.
Transgene OeGLU enzyme has a similar pattern to that from olive mesocarp
The zymogram clearly showed that the pattern among the proteins extracted from olive mesocarp and tobacco plants expressing OeGLU was similar (Fig. 9). It is interesting to note that about 10 times more protein from transgene tobacco leaves was required to reproduce a signal with similar intensity to that of olive drupe. The excised band from transgene tobacco leaves was able to hydrolyse oleuropein at comparable levels to that from olive mesocarp (Fig. 9). The molecular weight of the fluorescent band was estimated to be higher than 180kDa. Since the size of the monomer is ~63kDa, it is plausible that the native conformation of the enzyme is a multimer.
Fig. 9.
Comparison of β-glucosidase activity between O. europaea and N. benthamiana. Proteins were extracted from olive mesocarp (lane 1, 2.5 μg) or tobacco plants infiltrated with empty vector (lane 2, 25 μg) or expressing OeGLU (lane 3, 25 μg) and subjected to 7% SDS-PAGE. (A) β-Glucosidase zymogram using MUGlc as substrate. (B) CBB staining. (C) Relative activity of the excised fluorescent bands from (A) after incubation with oleuropein. Olive mesocarp activity was set to 100%. Molecular mass markers (lane M) are given in kDa. The two horizontal lines on the left of (A) define the gel area that was excised and used for reaction studies.
Discussion
Plants have evolved a plethora of chemical defence mechanisms against biotic stress by employing secondary metabolites. The glycosidic defence compounds along with their hydrolytic enzymes often form a dual-partner defence system (Pankoke et al., 2013). In order to prevent toxicity to host cells, glycosides are usually stored apart from hydrolysing enzymes until tissue damage disrupts cell compartmentalization. Then, the enzyme activates the substrate by converting it into a deterrent or highly toxic metabolite. Characteristic examples include the activation by specific glucosidases of strictosidine in Apocynaceae (Guirimand et al., 2010), glucosinolates in Brassicaceae (Halkier and Gershenzon, 2006; Winde and Wittstock, 2011), and iridoid glycosides in Plantago spp. (Pankoke et al., 2013). In the case of oleuropein in leaves of the privet tree, a β-glucosidase enzyme converts oleuropein into a highly potent protein-denaturant agent with the strongest protein cross-linking activity ever reported in plant systems (Konno et al., 1999). Yet, there are few relevant studies on the secoiridoid-activating β-glucosidases from other plant species.
Recently, much attention has been paid to oleuropein as being the most significant secoiridoid in olive. Apart from its predominant role in chemical defence, it is a valuable biophenol that shapes the organoleptic characters of olive fruit and virgin olive oil (Esti et al., 1998; Brenes et al., 1999; Servili and Montedoro, 2002; Morelló et al., 2004). Oleuropein aglycon and its derivatives exhibit several beneficial effects on human health, showing strong antioxidant, anti-inflammatory, and antiviral properties (Omar, 2010; Bulotta et al., 2013). Although the routes of oleuropein metabolism in olives are not fully resolved and data from analytical studies are often controversial, it has been widely accepted that a highly specific β-glucosidase is crucial in oleuropein degradation/modification (Briante et al., 2002; Ryan et al., 2003; Gutierrez-Rosales et al., 2010, 2012). A respective gene (GenBank accession no. KF623043.1) corresponding to the OeGlu cDNA has been isolated from olive. Here, we presented the isolation of a β-glucosidase cDNA from olive (OeGLU) and the functional characterization of its encoded protein that deglycosylates and activates oleuropein by the generation of derivatives with strong protein cross-linking potential.
Conformational similarities of OeGLU with defence-related GH1-type β-glucosidases
Phylogenetic analysis revealed that OeGLU belongs to the defence-related subclade of GH1 β-glucosidase enzymes that hydrolyse glucosides of the monoterpenoid indole alkaloids (MIA) family (strictosidine, raucaffricine), isoquinoline alkaloids (ipecoside), and steroidal glucosides (cardenolide). OeGLU most likely diverged along with the cardenolide-specific 16′-O-glucohydrolase (DlGLU) of D. lanata from a common ancestral gene. Sequence alignment of representative members from this subclade revealed that OeGLU contains all the strictly conserved motifs and amino acid residues involved in the recognition and hydrolysis of the glucose moiety from the substrate. However, low conservation was detected in amino acid residues such as Trp204, Ser205, Gln209, Phe216, Cys291, and Phe498 predicted to compose the site that binds the aglycon group of oleuropein, suggesting that the enzymes RsRAU, RsSTR, and OeGLU probably possess high substrate specificity due to recognition of the aglycon moiety (Barleben et al., 2007; Xia et al., 2011). Consistent with this, raucaffricine- and strictosidine-specific β-glucosidases (EC 3.2.1.125 and EC 3.2.1.105, respectively) are often unable to act on closely related glucosides (Hemscheidt and Zenk, 1980; Schübel et al., 1986), as with most GH1 enzymes whose highly specific functions are determined by the aglycon moiety of their natural substrates (Hösel and Conn, 1982; Seshadri et al., 2009).
Oleuropein hydrolysis and activation
We therefore asked whether OeGLU could hydrolyse and efficiently activate oleuropein, in accordance with the proposed oleuropein/β-glucosidase dual defence system (Konno et al., 1999). OeGLU activity was detected only after using Agrobacterium-mediated transient expression in tobacco leaves. Our efforts to detect this activity in bacteria were unsuccessful. This was most likely due to the fact that the defence-related β-glucosidases may require post-translational modification for activity. Lack of post-translational modification was also suggested to be the cause for the absence of activity of three recombinant β-glucosidases from Arabidopsis expressed in either E. coli or yeast cells (Ahn et al., 2010).
Expression of the OeGLU cDNA in tobacco leaves yielded a single protein band of the expected size. Protein extracts from leaf cells expressing the OeGLU cDNA had the ability to hydrolyse oleuropein, as depicted from the time-dependent decrease in oleuropein levels. The heterologously expressed cDNA showed strong specificity towards oleuropein when compared with the other substrates used in this study (Table 1) and a prominent deglycosylase activity. Interestingly, tobacco leaf protein extracts were unable to hydrolyse oleuropein, demonstrating that a substrate-specific β-glucosidase is required to furnish this enzymatic activity, as suggested previously (Gutierrez-Rosales et al., 2010). The high specificity of the cloned enzyme towards oleuropein was also supported by the fact that very low amounts of the recombinant enzyme, visible only in western blots, were adequate to observe activity.
The zymogram showed a similar pattern providing a direct comparison between the olive mesocarp β-glucosidase and that extracted from tobacco plants expressing OeGLU. The estimated size of the native enzyme implied that OeGLU is a multimer. This result is in agreement with the closely related strictosidine-specific β-glucosidase (Geerlings et al., 2000). Interestingly, the excised bands that had comparable intensity after staining with MUGlc also showed close hydrolysis activity towards oleuropein, indicating that the transiently expressed OeGLU is responsible for the major activity against oleuropein detected in the olive mesocarp.
The cloned enzyme seems to fulfil the necessary criteria for a defence-related β-glucosidase, i.e. to hydrolyse oleuropein to its aglycon form able to crosslink and inactivate proteins. The results from EMSA revealed high-molecular-weight aggregates of BSA as the products of strong protein-denaturing/cross-linking activity derived from oleuropein deglycosylation by the expressed enzyme. Supporting this, in recent proteomics and transcriptomics studies, increased accumulation of the present β-glucosidase was recorded in olive fruits infested by the fruit fly Bactrocera oleae (Corrado et al., 2012), as well as during the colonization process of olive roots by Pseudomonas fluorescens (Schilirò et al., 2012). At early stages of mesocarp development, the high oleuropein content with the concomitant increase in β-glucosidase could serve as a defence mechanism against pest species such as insects. During drupe ripening, factors such as the release of volatile components and oleuropein degradation products such as oleuropein aglycon, elenolic acids, and hydroxytyrosol eventually become attractive to animal vectors, which serve to disperse the kernels. Degradation products of oleuropein causing leakage of cytoplasmic constituents such as glutamate potassium and phosphate from bacteria cells prevent their growth (Beuchat, 2007).
The entire olive genome sequence is still unknown, yet olive trees like other plants probably contains many β-glucosidases of diverse as well as similar functions and substrate specificities. Indeed, in both olive leaves and fruit mesocarp, a number of β-glucosidase isoforms have been detected (Mazzuca et al., 2006; Wang et al., 2009). Thus, although Southern blot analysis showed that OeGLU cDNA is encoded by a single-copy gene in the olive genome, it should be noted that hybridization was conducted under high-stringency conditions and therefore the occurrence of other isozymes hydrolysing oleuropein cannot be excluded. In relation to this, a closely related gene from Catharanthus roseus that specifically deglycosylates strictosidine was found to be present as a single copy (Geerlings et al., 2000).
Transcriptional regulation of OeGLU is critical for enzyme synthesis
OeGLU gene expression varied with the developmental stage of the fruit and cell/tissue type. While transcripts were detected at fruit set, in situ hybridization showed that the gene was already expressed in ovary and anther tissues of the developing floral buds. In accordance with this, olive buds are known to be rich in oleuropein, the levels of which are developmentally regulated (Malik and Bradford, 2006).
Oleuropein is highly abundant in immature olive drupes, but the total content may vary among cultivars and different agronomic practices, and with climate (Briante et al., 2002; Malik and Bradford, 2006; Gutierrez-Rosales et al., 2012). Its concentration gradually declines during drupe development or may remain constant until the beginning of green maturation. Thereafter, it sharply falls to very low levels as the olive turns black (Bianco et al., 1993; El Riachy et al., 2011b ). Here, we showed that, at the very early stages of seed development, OeGLU was only expressed in the developing seed coat tissues and the perisperm. Transcripts were undetectable in both embryo and endosperm. It is therefore most likely that the decrease in transcript levels detected during the growth of immature drupes is attributed to the absence of gene expression in the developing seed tissues.
In mesocarp, an opposite trend was observed. OeGLU transcripts started to increase after stone lignification, peaked at about green maturation (22 WAF), and declined thereafter, but remained at relatively high levels until the onset of black ripening (28 WAF). Interestingly, in a previous comparative transcriptome analysis, the present β-glucosidase was found to be the second most abundant transcript at the last stage of olive ripening (Parra et al., 2013). Briante et al. (2002) showed that β-glucosidase activity in pulp extracts increased considerably during the green maturation stage. In a more recent study, Gutierrez-Rosales et al. (2012) confirmed that β-glucosidase enzyme activity exhibited a clear developmental regulation. This pattern coincides perfectly with the developmental OeGLU expression profile detected here, suggesting that a major step in regulating the synthesis of the β-glucosidase might be at the transcriptional level. However, a decompartmentalization-mediated process could stimulate the reactivity of the enzyme towards its substrate.
Besides olive fruits, oleuropein is known to be highly abundant in leaves and also to accumulate in stem and root tissues, although at lower levels than in leaf (De Nino et al., 1997; Silva et al., 2006; Ortega-García and Peragón, 2010). OeGLU was highly expressed in leaves and also in the apical shoot meristems and root tips. Actively growing plant parts are most vulnerable to herbivores in addition to pathogens. This is why chemical defence compounds like β-glucosides and their hydrolytic enzymes, β-glucosidases, occur in abundance in such plant parts. In leaves, oleuropein content declines with age (Laguerre et al., 2009). Accordingly, transcript accumulation of OeGLU was more intense in developing leaves than in mature leaves. Notably, no gene expression was detected in leaf trichomes. To the best of our knowledge, there are no reports of oleuropein being detected in olive leaf trichomes.
In conclusion, the present results indicate a defence role of OeGLU in young organs and meristematic tissues as well as in mature tissues by oleuropein activation into a potent protein cross-linking agent during a decompartmentalized process caused by pests. However, the well-documented massive hydrolysis of oleuropein upon fruit ripening along with the concomitant gene upregulation suggest a critical catabolic role of OeGLU in secondary metabolism during these stages. It is likely that the massive cell apoptosis and decompartmentalization in olive mesocarp during ripening (Matteucci et al., 2011) facilitates the enzyme–substrate encounter. Oleuropein hydrolysis products are highly valuable for reutilization in the biosynthesis of metabolites related to ripening of drupes, such as deglycosylated secoiridoids and free glucose for the synthesis of anthocyanins, and oleuropein aglycon for shaping the antioxidant profile of olive oil. In addition, it is plausible that, as seed maturation completes and massive oil accumulation starts, the mighty OeGLU/oleuropein weapon should progressively get depleted as it becomes obsolete; otherwise, it would prevent animals from feeding on drupes, a crucial process in olive seed dispersal.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Schematic representation of the pGPTV/35S-OeGLU-FLAG construct.
Supplementary Fig. S2. Sequence alignment of GH1 proteins used for reconstruction of the phylogenetic tree.
Supplementary Fig. S3. SDS-PAGE analysis of the recombinant OeGLU cloned in pET28a and expressed in E. coli strain BL21(DE3).
Supplementary Fig. S4. SDS-PAGE and Western blot analysis of the infiltrated N. benthamiana leaves with Agrobacterium containing the pGPTV/35S-OeGLU-FLAG vector at different days post-infiltration.
Supplementary Fig. S5. Structure-based alignment of OeGLU with selected GH1 β-glucosidases.
Supplementary Fig. S6. Comparison of the putative 3D structure of OeGLU with two solved 3D structures of GH1 enzymes.
Supplementary Table S1. List of primers used in this study.
Supplementary Table S2. The alignment matrix of OeGLU with selected sequences from the phylogenetic tree.
Supplementary Table S3. Protein sequences used to reconstruct the phylogenetic tree.
Acknowledgements
We fulfilled this work in memory of Fotis Gazis, a beloved friend and colleague. This work was partially funded by GSRT ARISTEIA to PH, by the Republic of Cyprus (Research Promotion Foundation ; Call 2009–2010, YGEIA/TROPHI/0609/03) and the European Regional Development Fund of EU. KK was supported by the Greek State Scholarships Foundation (IKY).
Glossary
Abbreviations:
- BSA
bovine serum albumin
- EMSA
electrophoretic mobility shift assay
- HPLC
high-performance liquid chromatography
- ORF
open reading frame
- RACE
rapid amplification of cDNA ends
- UTR
untranslated region
- WAF
weeks after flowering.
References
- Ahn YO, Shimizu B, Sakata K, Gantulga D, Zhou Z, Bevan DR, Esen A. 2010. Scopolin-hydrolyzing β-glucosidases in roots of Arabidopsis. Plant and Cell Physiology 51, 132–143. [DOI] [PubMed] [Google Scholar]
- Alagna F, Mariotti R, Panara F, et al. 2012. Olive phenolic compounds: metabolic and transcriptional profiling during fruit development. BMC Plant Biology 12, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiot MJ, Fleuriet A, Macheix JJ. 1986. Importance and evolution of phenolic compounds in olive during growth and maturation. Journal of Agricultural and Food Chemistry 34, 823–826. [Google Scholar]
- Amiot MJ, Fleuriet A, Macheix JJ. 1989. Accumulation of oleuropein derivatives during olive maturation. Phytochemistry 28, 67–69. [Google Scholar]
- Banilas G, Karampelias M, Makariti I, Kourti A, Hatzopoulos P. 2011. The olive DGAT2 gene is developmentally regulated and shares overlapping but distinct expression patterns with DGAT1 . Journal of Experimental Botany 62, 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barleben L, Panjikar S, Ruppert M, Koepke J, Stockigt J. 2007. Molecular architecture of strictosidine glucosidase: the gateway to the biosynthesis of the monoterpenoid indole alkaloid family. Plant Cell 19, 2886–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Kemper E, Schell J, Masterson R. 1992. New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Molecular Biology 20, 1195–1197. [DOI] [PubMed] [Google Scholar]
- Beuchat LR. 2007. Control of foodborne pathogens and spoilage microorganisms by naturally occurring antimicrobials. In: Wilson CL, ed. Microbial food contamination , 2nd edn Boca Raton, FL: CRC Press, 319–346. [Google Scholar]
- Bianco A, Scalzo RL, Scarpati ML. 1993. Isolation of cornoside from Olea europaea and its transformation into halleridone. Phytochemistry 32, 455–457. [Google Scholar]
- Bianco L, Alagna F, Baldoni L, Finnie C, Svensson B, Perrotta G. 2013. Proteome regulation during Olea europaea fruit development. PLoS One 8, e53563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenes M, García A, García P, Rios JJ, Garrido A. 1999. Phenolic compounds in spanish olive oils. Journal of Agricultural and Food Chemistry 47, 3535–3540. [DOI] [PubMed] [Google Scholar]
- Briante R, Patumi M, Limongelli S, Febbraio F, Vaccaro C, Di Salle A, La Cara F, Nucci R. 2002. Changes in phenolic and enzymatic activities content during fruit ripening in two Italian cultivars of Olea europaea L. Plant Science 162, 791–798. [Google Scholar]
- Bulotta S, Oliverio M, Russo D, Procopio A. 2013. Biological activity of oleuropein and its derivatives. In: Ramawat KG, Mérillon JM, eds. Natural products . Berlin/Heidelberg: Springer, 3605–3638. [Google Scholar]
- Corrado G, Alagna F, Rocco M, Renzone G, Varricchio P, Coppola V, Coppola M, Garonna A, Baldoni L, Scaloni A. 2012. Molecular interactions between the olive and the fruit fly Bactrocera oleae . BMC Plant Biology 12, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czjzek M, Cicek M, Zamboni V, Bevan DR, Henrissat B, Esen A. 2000. The mechanism of substrate (aglycone) specificity in β-glucosidases is revealed by crystal structures of mutant maize β-glucosidase-DIMBOA,-DIMBOAGlc, and -dhurrin complexes. Proceedings of the National Academy of Sciences, USA 97, 13555–13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nino A, Lombardo N, Perri E, Procopio A, Raffaelli A, Sindona G. 1997. Direct identification of phenolic glucosides from olive leaf extracts by atmospheric pressure ionization tandem mass spectrometry. Journal of Mass Spectrometry 32, 533–541. [Google Scholar]
- Dietz KJ, Sauter A, Wichert K, Messdaghi D, Hartung W. 2000. Extracellular β‐glucosidase activity in barley involved in the hydrolysis of ABA glucose conjugate in leaves. Journal of Experimental Botany 51, 937–944. [PubMed] [Google Scholar]
- El Riachy M, Priego-Capote F, León L, Rallo L, de Castro L, Dolores M. 2011. a . Hydrophilic antioxidants of virgin olive oil. Part 1: Hydrophilic phenols: a key factor for virgin olive oil quality. European Journal of Lipid Science and Technology 113, 678–691. [Google Scholar]
- El Riachy M, Priego-Capote F, León L, Rallo L, de Castro L, Dolores M. 2011. b . Hydrophilic antioxidants of virgin olive oil. Part 2: Biosynthesis and biotransformation of phenolic compounds in virgin olive oil as affected by agronomic and processing factors. European Journal of Lipid Science and Technology 113, 692–707. [Google Scholar]
- Esti M, Cinquanta L, La Notte E. 1998. Phenolic compounds in different olive varieties. Journal of Agricultural and Food Chemistry 46, 32–35. [DOI] [PubMed] [Google Scholar]
- Geerlings A, Ibanez M, Memelink J, van der Heijden R, Verpoorte R. 2000. Molecular cloning and analysis of strictosidine β- d -glucosidase, an enzyme in terpenoid indole alkaloid biosynthesis in Catharanthus roseus . Journal of Biological Chemistry 275, 3051–3056. [DOI] [PubMed] [Google Scholar]
- Guirimand G, Courdavault V, Lanoue A, Mahroug S, Guihur A, Blanc N, Giglioli-Guivarc’h N, St-Pierre B, Burlat V. 2010. Strictosidine activation in Apocynaceae: towards a “nuclear time bomb”? BMC Plant Biology 10, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Rosales F, Romero MP, Casanovas M, Motilva MJ, Mínguez-Mosquera MI. 2010. Metabolites involved in oleuropein accumulation and degradation in fruits of Olea europaea L.: Hojiblanca and Arbequina varieties. Journal of Agricultural and Food Chemistry 58, 12924–12933. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Rosales F, Romero MP, Casanovas M, Motilva MJ, Mínguez-Mosquera MI. 2012. β-Glucosidase involvement in the formation and transformation of oleuropein during the growth and development of olive fruits (Olea europaea L. cv. Arbequina) grown under different farming practices. Journal of Agricultural and Food Chemistry 60, 4348–4358. [DOI] [PubMed] [Google Scholar]
- Halkier BA, Gershenzon J. 2006. Biology and biochemistry of glucosinolates. Annual Review of Plant Biology 57, 303–333. [DOI] [PubMed] [Google Scholar]
- Hemscheidt T, Zenk MH. 1980. Glucosidases involved in indole alkaloid biosynthesis of Catharanthus cell cultures. FEBS Letters 110, 187–191. [DOI] [PubMed] [Google Scholar]
- Hösel W, Conn EE. 1982. The aglycone specificity of plant β-glycosidases. Trends in Biochemical Sciences 7, 219–221. [Google Scholar]
- Konno K, Hirayama C, Yasui H, Nakamura M. 1999. Enzymatic activation of oleuropein: a protein crosslinker used as a chemical defence in the privet tree. Proceedings of the National Academy of Sciences, USA 96, 9159–9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. 1984. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Research 12, 857–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoffersen P, Brzobohaty B, Höhfeld I, Bako L, Melkonian M, Palme K. 2000. Developmental regulation of the maize Zm-p60.1 gene encoding a β-glucosidase located to plastids. Planta 210, 407–415. [DOI] [PubMed] [Google Scholar]
- Laguerre M, Lόpez Giraldo L, Piombo G, Figueroa-Espinoza M, Pina M, Benaissa M, Combe A, Rossignol Castera A, Lecomte J, Villeneuve P. 2009. Characterization of olive-leaf phenolics by ESI-MS and evaluation of their antioxidant capacities by the CAT assay. Journal of the American Oil Chemists’ Society 86, 1215–1225. [Google Scholar]
- Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I. 2006. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126, 1109–1120. [DOI] [PubMed] [Google Scholar]
- Lo Scalzo R, Scarpati M, Verzegnassi B, Vita G. 1994. Olea europaea chemicals repellent to Dacus oleae females. Journal of Chemical Ecology 20, 1813–1823. [DOI] [PubMed] [Google Scholar]
- Malik NS, Bradford JM. 2006. Changes in oleuropein levels during differentiation and development of floral buds in ‘Arbequina’ olives. Scientia Horticulturae 110, 274–278. [Google Scholar]
- Matteucci M, D’angeli S, Errico S, Lamanna R, Perrotta G, Altamura MM. 2011. Cold affects the transcription of fatty acid desaturases and oil quality in the fruit of Olea europaea L. genotypes with different cold hardiness. Journal of Experimental Botany 62, 3403–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuca S, Spadafora A, Innocenti AM. 2006. Cell and tissue localization of β-glucosidase during the ripening of olive fruit (Olea europaea) by in situ activity assay. Plant Science 171, 726–733. [Google Scholar]
- Morelló JR, Romero MP, Motilva MJ. 2004. Effect of the maturation process of the olive fruit on the phenolic fraction of drupes and oils from Arbequina, Farga, and Morrut cultivars. Journal of Agricultural and Food Chemistry 52, 6002–6009. [DOI] [PubMed] [Google Scholar]
- Obied HK, Prenzler PD, Ryan D, Servili M, Taticchi A, Esposto S, Robards K. 2008. Biosynthesis and biotransformations of phenol-conjugated oleosidic secoiridoids from Olea europaea L. Natural Product Reports 25, 1167–1179. [DOI] [PubMed] [Google Scholar]
- Omar SH. 2010. Oleuropein in olive and its pharmacological effects. Scientia Pharmaceutica 78, 133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-García F, Peragón J. 2010. HPLC analysis of oleuropein, hydroxytyrosol, and tyrosol in stems and roots of Olea europaea L. cv. Picual during ripening. Journal of the Science of Food and Agriculture 90, 2295–2300. [DOI] [PubMed] [Google Scholar]
- Pankoke H, Buschmann T, Müller C. 2013. Role of plant β-glucosidases in the dual defence system of iridoid glycosides and their hydrolyzing enzymes in Plantago lanceolata and Plantago major . Phytochemistry 94, 99–107. [DOI] [PubMed] [Google Scholar]
- Parra R, Paredes M, Sanchez-Calle I, Gomez-Jimenez M. 2013. Comparative transcriptional profiling analysis of olive ripe-fruit pericarp and abscission zone tissues shows expression differences and distinct patterns of transcriptional regulation. BMC Genomics 14, 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Segura C, Sanz C, Perez AG. 2009. Purification and characterization of an olive fruit β-glucosidase involved in the biosynthesis of virgin olive oil phenolics. Journal of Agricultural and Food Chemistry 57, 7983–7988. [DOI] [PubMed] [Google Scholar]
- Ryan D, Prenzler PD, Lavee S, Antolovich M, Robards K. 2003. Quantitative changes in phenolic content during physiological development of the olive (Olea europaea) cultivar Hardy’s Mammoth. Journal of Agricultural and Food Chemistry 51, 2532–2538. [DOI] [PubMed] [Google Scholar]
- Schilirò E, Ferrara M, Nigro F, Mercado-Blanco J. 2012. Genetic responses induced in olive roots upon colonization by the biocontrol endophytic bacterium Pseudomonas fluorescens PICF7. PLoS One 7, e48646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schübel H, Stöckigt J, Feicht R, Simon H. 1986. Partial purification and characterization of raucaffricine β‐ d ‐glucosidase from plant cell‐suspension cultures of Rauwolfia serpentina BENTH. Helvetica Chimica Acta 69, 538–547. [Google Scholar]
- Servili M, Montedoro G. 2002. Contribution of phenolic compounds to virgin olive oil quality. European Journal of Lipid Science and Technology 104, 602–613. [Google Scholar]
- Seshadri S, Akiyama T, Opassiri R, Kuaprasert B, Cairns JK. 2009. Structural and enzymatic characterization of Os3BGlu6, a rice β-glucosidase hydrolyzing hydrophobic glycosides and (1→3)-and (1→2)-linked disaccharides. Plant Physiology 151, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S, Gomes L, Leitão F, Coelho AV, Boas LV. 2006. Phenolic compounds and antioxidant activity of Olea europaea L. fruits and leaves. Food Science and Technology International 12, 385–395. [Google Scholar]
- Verdoucq L, Czjzek M, Moriniere J, Bevan DR, Esen A. 2003. Mutational and structural analysis of aglycone specificity in maize and sorghum β-glucosidases. Journal of Biological Chemistry , 278, 25055–25062. [DOI] [PubMed] [Google Scholar]
- Verdoucq L, Morinière J, Bevan DR, Esen A, Vasella A, Henrissat B, Czjze M. 2004. Structural determinants of substrate specificity in family 1 β-glucosidases: novel insights from the crystal structure of sorghum dhurrinase-1, a plant β-glucosidase with strict specificity, in complex with its natural substrate. Journal of Biological Chemistry 279, 31796–31803. [DOI] [PubMed] [Google Scholar]
- Voinnet O, Rivas S, Mestre P, Baulcombe D. 2003. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. The Plant Journal 33, 949–956. [DOI] [PubMed] [Google Scholar]
- Wang Q, Trimbur D, Graham R, Warren R, Withers S. 1995. Identification of the acid/base catalyst in Agrobacterium faecalis β-glucosidase by kinetic analysis of mutants. Biochemistry 34, 14554–14562. [DOI] [PubMed] [Google Scholar]
- Wang W, Li C, Hu X. 2009. Developmental expression of β-glucosidase in olive leaves. Biologia Plantarum 53, 138–140. [Google Scholar]
- Wiesmann C, Beste G, Hengstenberg W, Schulz GE. 1995. The three-dimensional structure of 6-phospho-β-galactosidase from Lactococcus lactis . Structure 3, 961–968. [DOI] [PubMed] [Google Scholar]
- Winde I, Wittstock U. 2011. Insect herbivore counteradaptations to the plant glucosinolate–myrosinase system. Phytochemistry 72, 1566–1575. [DOI] [PubMed] [Google Scholar]
- Xia L, Ruppert M, Wang M, Panjikar S, Lin H, Rajendran C, Barleben L, Stöckigt J. 2011. Structures of alkaloid biosynthetic glucosidases decode substrate specificity. ACS Chemical Biology 7, 226–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








