Abstract
Background
Drug diversion by health care personnel poses a risk for serious patient harm. Public health identified 2 patients diagnosed with acute hepatitis C virus (HCV) infection who shared a common link with a hospital. Further investigation implicated a drug-diverting, HCV-infected surgical technician who was subsequently employed at an ambulatory surgical center.
Methods
Patients at the 2 facilities were offered testing for HCV infection if they were potentially exposed. Serum from the surgical technician and patients testing positive for HCV but without evidence of infection before their surgical procedure was further tested to determine HCV genotype and quasi-species sequences. Parenteral medication handling practices at the 2 facilities were evaluated.
Results
The 2 facilities notified 5970 patients of their possible exposure to HCV, 88% of whom were tested and had results reported to the state public health departments. Eighteen patients had HCV highly related to the surgical technician’s virus. The surgical technician gained unauthorized access to fentanyl owing to limitations in procedures for securing controlled substances.
Conclusions
Public health surveillance identified an outbreak of HCV infection due to an infected health care provider engaged in diversion of injectable narcotics. The investigation highlights the value of public health surveillance in identifying HCV outbreaks and uncovering a method of drug diversion and its impacts on patients.
Keywords: Drug theft in healthcare, Healthcare associated infections, Unsafe injection practice
Hepatitis C virus (HCV) transmission during health care procedures has been increasingly identified in the United States, with outbreaks occurring in a variety of health care settings.1,2 The majority of outbreaks have involved patient-to-patient transmission, largely though unsafe injection practices (eg, reuse of syringes); however, transmission from HCV-infected health care personnel to patients from diversion of injectable narcotics has been documented as well.1,3–8 In these instances, diversion has involved some form of tampering with the injectable narcotic, exposing patients to a health care worker’s blood. As demonstrated by these outbreaks, drug diversion by health care personnel poses a serious threat to patient safety, potentially putting large numbers of patients at risk for acquiring infections.8,9
In late April 2009, Colorado state and local public health department officials conducted routine interviews10 with 2 patients newly diagnosed with acute HCV infection (index patients). Both patients denied traditional HCV infection risk behaviors or exposures, both had undergone a surgical procedure on consecutive days at the same hospital (facility A), and both had HCV genotype 1b infection. Public health officials initiated an investigation to (1) determine whether these patients acquired their infections at the facility, (2) identify the mode of transmission, (3) determine whether other patients were infected, and (4) prevent additional infections.
METHODS
Review of facility A records and identification of the infected surgical technician
Following interviews with the 2 index patients, state public health officials contacted facility A to request these patients’ medical records, a list of all patients who had undergone surgical procedures during the 6 days before the surgery date for the first index patient, and a list of personnel assigned to the index patients’ surgical procedures. At this time, facility A management indicated that they had recently dismissed a surgical technician owing to suspicion of narcotic drug diversion. In addition, facility A’s records indicated that the technician had tested positive for HCV antibodies (anti-HCV) on pre-employment screening.
Interviews with the surgical technician
This technician was contacted by a state public health official, who learned that the technician began working at an ambulatory surgical center in Colorado (facility B) following dismissal from facility A. Public health officials conducted interviews with the surgical technician to determine dates and locations of employment, whether narcotics diversion had occurred, and relevant details of the diversion. Public health officials advised the technician to refrain from providing any patient care while the investigation was pending and received consent to obtain a blood sample for HCV, hepatitis B virus (HBV), and human immunodeficiency virus (HIV) testing.
Case finding and case definitions
Following the interview with the surgical technician, public health officials requested an expanded list of patients representing persons who had undergone surgery during the time that the surgical technician performed clinical duties at facility A or B (October 21, 2008, to April 22, 2009, for facility A and May 4, 2009, to July 1, 2009, for facility B).
In conjunction with public health officials, facilities A and B contacted patients by letter and/or phone to advise them of their potential exposure to HCV.11,12 Patients were offered free testing for HCV infection and serum alanine aminotransferase (ALT) levels through a contracted laboratory (as outlined below). Patients who tested positive for HCV infection were referred for follow-up care and medical management.
Colorado physicians and laboratories are required to report to the state or local health department any tests that indicate HCV infection.13,14 These reports are maintained within a state disease reporting system, from which the 2 index patients were identified for routine public health interviews. For this investigation, facilities A and B also reported positive and negative HCV infection test results to the state health departments for individuals who were tested through the contracted laboratory. The names and birth dates of these patients were matched to the state disease reporting system to identify patients already diagnosed with HCV infection and to determine whether HCV infection had been documented before the surgical procedure at facility A or B.
For all patients identified with HCV infection, public health officials assessed the patient’s medical history, previous hepatitis test results, risk factors for HCV infection, and date and time of surgical procedure through a review of the medical records from facility A or B and data captured in the disease reporting system.10 Patients were classified according to five case definitions developed for this investigation (Table 1).
Table 1.
Classification of cases and case definitions
| Case classification | Case definition |
|---|---|
| Confirmed case | A patient with no known positive HCV test result before the date of implicated surgical procedure at facility A or B, a positive HCV test result ≥2 weeks after the surgical procedure, and genotype 1b HCV infection, with HCV viral sequences highly related to those of the HCV-infected surgical technician |
| Possible case | A patient with no known diagnosis of HCV infection before the date of implicated surgical procedure at facility A or B, a positive HCV test result ≥2 weeks after the surgical procedure, and HCV genotyping and/or HCV viral sequencing could not be performed |
| HCV-infected–not related to surgical technician | A patient with a diagnosis of HCV infection before the date of surgical procedure at facility A or B, or HCV infection with a genotype not 1b, or genotype 1b HCV infection, with HCV viral sequences not highly related to those of the HCV-infected surgical technician |
| HCV-negative (not infected) | A patient with no known diagnosis of HCV infection before the date of implicated surgical procedure at facility A or B, and who was both anti-HCV negative and HCV RNA-negative when tested during the investigation. |
| HCV status unknown | A patient with no known diagnosis of HCV infection before the date of implicated surgical procedure at facility A or B, and who did not undergo HCV testing as part of the investigation. |
Commercial laboratory testing of patients for HCV
Public health officials recommended that patients undergo testing for serum ALT levels and for the presence of anti-HCV, with confirmatory testing by a recombinant immunoblot assay (RIBA) when necessary. For patients who underwent their first anti-HCV test within 6 weeks after the date of their surgical procedure, HCV RNA testing was also recommended. All patients found to have a positive anti-HCV or RIBA test result were tested for the presence of HCV RNA to identify ongoing infections. For those with detectable HCV RNA, HCV genotype testing was performed.
Molecular investigation of HCV
Serum specimens from patients identified to be HCV RNA-positive were forwarded to the Centers for Disease Control and Prevention (CDC), Division of Viral Hepatitis laboratory if they were HCV genotype 1b (the same as the surgical technician) or an unknown HCV genotype and if, based on review of the disease reporting system or patient interview, the patient was not known to have HCV infection before the surgical procedure. A specimen submitted from the surgical technician was also forwarded to the CDC. At the CDC, serum samples were tested for HCV RNA by polymerase chain reaction (PCR) using the AMPLICOR HCV Test, version 2.0 (Roche Molecular Systems, Branchburg, NJ), with a lower limit of detection of ~50 copies/mL. Then HCV genotype was determined using the VERSANT HCV Genotype 2.0 Assay (LiPA) (Siemens Healthcare Diagnostics, Tarrytown, NY). Subsequently, the subgenotype was determined from a 300-nucleotide NS5B coding region of the HCV genome.15,16
The genetic relatedness of virus from the surgical technician and patients was determined by analysis of HCV quasi-species by sequencing a segment amplified from the E1-hypervariable region 1 (HVR1) of the HCV genome (291 nucleotides in length), as described previously.12 The E1-HVR1 quasi-species sequences from the surgical technician and patient specimens were compared with each other, and also compared with the sequences of 5 randomly selected individuals with HCV genotype 1b infection from the Third National Health and Nutrition Examination Survey (NHANES III), a representative sample of the noninstitutionalized civilian population of the United States.17
Statistical analysis
The pairwise genetic distances of nucleotide quasi-species sequences were estimated with the DNADIST program in the PHYLIP package, version 3.68 (Joseph Felsenstein, University of Washington, Seattle, WA). Differences in the distributions of the HVR1 genetic distances were compared using the ANOVA program in SAS for Windows, version 9.3 (SAS Institute, Cary, NC). A P value <.05 was considered significant.
Facility A and B onsite evaluations
Public health officials conducted an onsite evaluation and review of infection control practices at facilities A and B. Surgical procedures at both facilities were observed, and selected personnel were interviewed to ascertain storage, preparation, and waste procedures for parenteral medication, with a focus on controlled substances (eg, fentanyl).
Human Subjects Review
The activities involved in this investigation constituted a response to an emerging public health problem to prevent and control the spread of HCV infection. As such, it was not subject to review by a Institutional Review Board.
RESULTS
Laboratory testing and interview with the surgical technician
The implicated surgical technician admitted to the theft of injectable fentanyl while working at both facility A and facility B. Testing of the blood specimen obtained from the surgical technician revealed the presence of HCV genotype 1b infection (the same genotype as the 2 index case patients), negativity for HIV infection, and vaccine-induced immunity to HBV infection.
The surgical technician described removing predrawn syringes of fentanyl from unattended anesthesia carts and replacing them with syringes that the technician had previously taken from a cart, used, and refilled with saline solution. The technician then left the area, self-injected the fentanyl, and refilled the syringes with saline solution in anticipation of a future syringe swap. The technician reported engaging in this practice during assigned procedures, as well as for procedures to which she had not been assigned.
Before being employed at facility A in Colorado, the technician had worked at hospitals in Texas and New York. The Colorado Department of Public Health and Environment informed the New York State Department of Health and the Texas Department of State Health Services of the investigation underway in Colorado and of the technician’s locations of previous employment. The New York facility in which the technician previously worked also notified approximately 2800 patients of their possible exposure to HCV.18
Case finding
Two additional patients with newly diagnosed HCV infection were identified through cross-matching of the state disease reporting system and a list of patients who had undergone surgery within the 6 days before the first index patient.
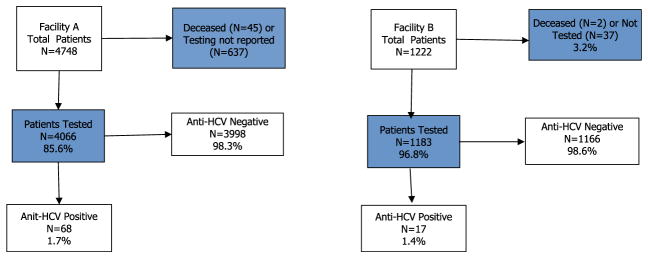
During the surgical technician’s employment at facilities A and B, 5970 patients underwent a surgical procedure and were subsequently recommended to be tested for HCV infection. Among the 4066 patients tested from facility A, 68 (1.7%) were found to have past or present HCV infection (ie, were anti-HCV positive), and among the 1183 patients tested from facility B, 17 (1.4%) had past or present HCV infection (Fig 1).
Fig. 1.
Outcome of recommended HCV testing for patients who underwent surgery during the technician’s employment at facilities A and B. Shaded boxes use total patients as the denominator for percentage; other boxes use patients tested as the denominator for percentage.
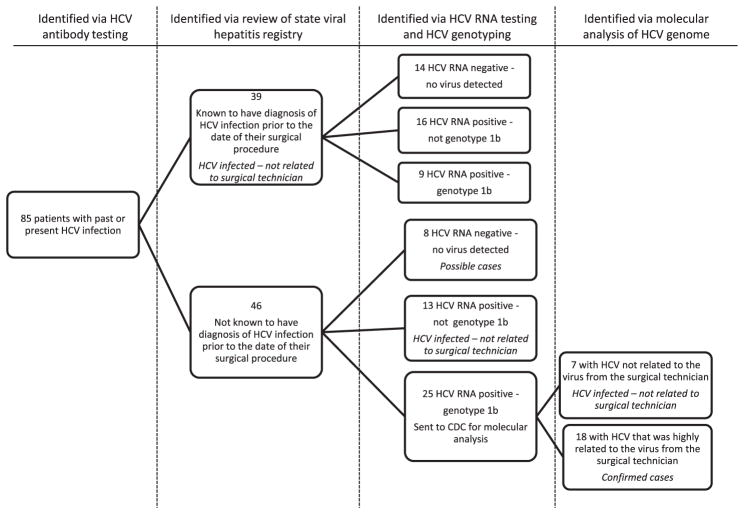
The 85 patients identified with past or present HCV infection (68 from facility A and 17 from facility B) underwent HCV RNA testing and, if positive, HCV genotyping (Fig 2). Information from the state’s disease reporting systems and patient interviews indicated that 39 patients had a history of HCV infection before their surgical procedure. Among the other 46 patients, 13 had a HCV genotype other than 1b and were categorized as HCV-infected but not related to the technician, and 8 were HCV antibody-positive but RNA-negative and were classified as possible cases.
Fig. 2.
Epidemiologic findings and case classification of patients from facilities A and B found to have past or present HCV infection.
Molecular investigation
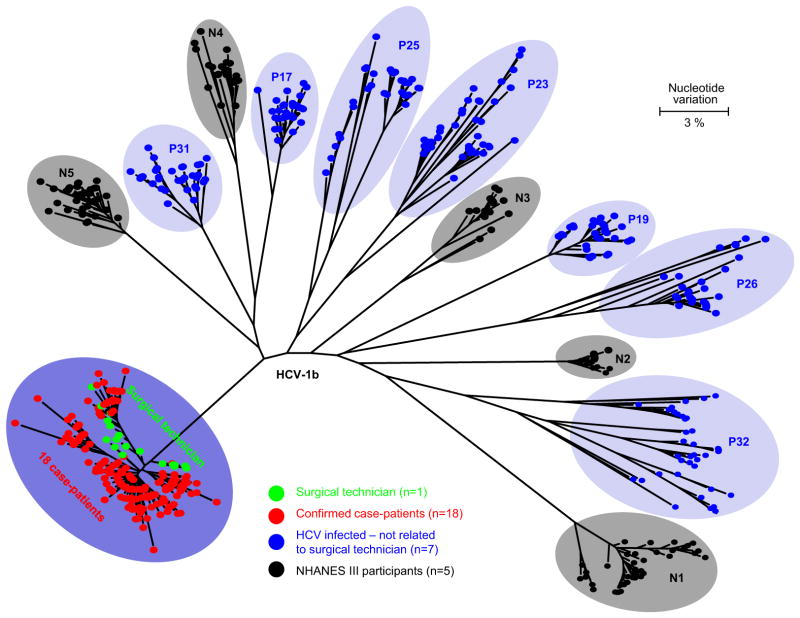
Serum from the remaining 25 HCV RNA-positive patients and the surgical technician were evaluated by quasi-species analysis at the CDC (Fig 2). The HVR1 quasi-species obtained from the surgical technician and 18 of the 25 HCV-infected patients formed a single distinct phylogenetic cluster (Fig 3), and these 18 patients were classified as confirmed cases. This cluster was significantly different from other genotype 1b quasi-species clusters identified from the other 7 patients and the 5 NHANES III participants (P < .001). The maximum nucleotide identity among 263 unique E1-HVR1 quasi-species sequences obtained from the surgical technician and the 18 patients ranged from 98.28% to 100%, whereas it ranged from 87.29% to 92.78% when these sequences were compared with those obtained from NHANES III participants and from 86.25% to 90.03% when compared with sequences from 7 unrelated HCV-infected patients.
Fig. 3.
Phylogenetic tree of the E1-HRV1 genomic region from intra-host HCV variants sampled from 25 case patients, 1 surgical technician, and 5 randomly selected NHANES III participants.
Evaluation of medication handling at facilities A and B
Facilities A and B used an automated medication dispensing machine and a locked cabinet, respectively, to secure controlled substances before dispensing. Access codes or keys were available only to authorized personnel, which did not include technicians. In facility A, each operating room had its own lockable anesthesia cart that remained in the room. The carts were maintained and filled by anesthesia personnel. Only anesthesia personnel and the charge nurses had keys to access these carts. At facility B, the nursing director or clinical manager stocked the anesthesia carts with a quantity of controlled substances sufficient for each day’s scheduled cases. Each anesthesiologist was responsible for his or her own cart, with an individual key, and that cart traveled with the anesthesiologist to each operating room.
At both facilities, anesthesia personnel stated that they often prepared medications, including controlled substances, in advance of patient arrival in the operating room, while another case was in progress. To aid in correct identification, medication syringes were labeled with preprinted and color-coded medication labels that were present on the top of the anesthesia cart. They were not labeled with the patient’s name or date/time of preparation. At both facilities, surgical technicians were frequently alone in the operating room while they were preparing the room for the next case. Anesthesia personnel at one of the facilities stated that there were times when controlled substances would be left on or in unlocked anesthesia carts when anesthesia personnel were not present in the room. Some personnel indicated that they believed these controlled substances were secure, even if they were left unlocked, when technicians or other personnel were present. At the other facility, personnel reported that anesthesia carts were always locked when anesthesia personnel were not in the room; however, at the conclusion of 1 surgical case observed by public health investigators, an anesthesia provider failed to lock the cart, which contained fentanyl, before exiting the room.
All 18 confirmed case patients underwent their procedure at facility A, and 13 (72%) underwent their procedure within a 5-week period during the technician’s 6 months of employment at this facility. Medical record reviews identified that all 18 patients underwent a procedure on a day when the technician was working, but the technician was documented to be an assigned member of the surgical team for only 10 of the 18 procedures (56%). All 18 patients had documented receipt of fentanyl during their procedure.
DISCUSSION
This investigation identified a large outbreak of HCV infection associated with drug diversion by an HCV-infected surgical technician, who was able to gain unauthorized access to syringes filled with fentanyl. Patients were identified in the community through the public health disease reporting system and a public notification. The ensuing investigation indicated that the threat posed by the drug diversion extended beyond the procedures to which the surgical technician had been assigned.
After pleading guilty to charges of tampering with a consumer product and illegally obtaining a controlled substance, the surgical technician was sentenced to 30 years in prison.19,30 Reports of HCV transmission due to drug diversion have been relatively rare in the United States, but include recent HCV infection outbreaks in Florida and New Hampshire.1–7 The most recent outbreak, initially identified in New Hampshire, involved a traveling radiology technician who infected at least 45 patients at 4 facilities in 3 states.7
Although HCV transmission attributed to drug diversion is seemingly rare, drug diversion by health care personnel is not uncommon.2–8,19,20 Drug diversion by health care personnel has broad public health implications, potentially putting large numbers of patients at risk for bloodborne6–8,18–20,24 and other infections.21–23 Consequently, when drug diversion of injectable medication by health care personnel is suspected or identified, it is imperative that health care management engage public health officials to evaluate the potential threat to patient safety, including the risk for transmission of infections.20
The surgical technician was able to access fentanyl because of limitations in procedures for securing controlled substances. This incident demonstrates the importance of adhering to requirements from the Drug Enforcement Administration (DEA) and the Centers for Medicare & Medicaid Services regarding security of controlled substances.25,26,31 A publication from the American Society of Anesthesiologists highlighted these requirements, including sample language for a hospital policy on anesthesia medication security.27 They emphasized that all controlled substances (eg, schedule II, III, IV medications) should be kept under lock and key at all times unless they are under the direct control of the anesthesiologist or other clinician involved in the immediate administration of the drug. As demonstrated by this outbreak and other recent outbreaks, the presence of other health care personnel does not guarantee the security of controlled substances.6,7 Key access should be limited only to those authorized individuals who stock or administer the medication. In addition, preparing medications as close as possible to the time of administration and properly labeling predrawn medications to include the patient’s name may make it more challenging for health care personnel to swap or tamper with medications.
After being dismissed from facility A, the surgical technician was quickly able to gain employment at a second facility within the same state. Two conditions contributed to this. First, reference checks from a current employer were not required. Second, unlike some types of health care personnel, such as physicians and nurses, surgical technicians were not required to be licensed in Colorado. Without professional licensing or registration, states have limited authority and fewer options to respond to complaints or pursue disciplinary action. Nonetheless, there are actions that can and should be taken if a health care provider is suspected of diverting controlled substances. These include external reporting to local law enforcement and the DEA.25 The DEA requires that registrants notify them of “the theft or significant loss of any controlled substance within one business day of discovery of such a loss or theft”.21 Furthermore, Colorado Board of Health regulations require health entities to notify the health department of “any occurrence in which drugs intended for use by patients or residents are diverted to use by other persons”.28 These actions can help prevent criminal activity and patient harm from occurring elsewhere.
Following this investigation, several state-level policy changes were pursued. State public health officials created an enhanced protocol for responding to diversion reports involving an injectable drug. Although such reporting had been required previously, disease control personnel now receive and review these reports, matching the names of suspected perpetrators to the communicable disease and HIV disease reporting systems. Facility investigators ask additional questions to discern possible patient infection risks. Health department officials review each case to determine whether additional follow-up and patient notification are warranted. Since 2009, Colorado public health officials have reviewed 20–40 reports per month to identify occurrences of injectable drug diversion, investigated 4 instances of drug diversion, and conducted 2 additional patient notifications.
State statutes have been revised to require registration of surgical technicians in Colorado.29 In addition, surgical technicians in the state are now required to provide written notice to the Department of Regulatory Agencies of any civil, criminal, or administrative actions taken against them within 30 days. Employers are required to check the database of registrants before allowing a surgical technician to work and to report disciplinary actions related to individual surgical technicians. In response to a request by an employer, the legislature also clarified that it is not unlawful “for an employer, when acting in good faith, to disclose information known about any involvement in drug diversion, drug tampering, patient abuse, violation of drug or alcohol policies, or crimes of violence…committed by a registrant who is an employee or former employee of the responding employer”.29 Given the state-to-state differences in health care personnel licensing and regulations, it is likely that identifying health care personnel with a past history of drug diversion will remain challenging, as has been recently illustrated.2,7,26
This report is subject to several limitations. The actual number of patients who acquired HCV infection from the surgical technician may be greater than the 18 confirmed case patients reported here. Public health officials did not receive test results for 674 individuals (11.4% of living patients) in the facility A and B cohort, and whether these patients were not tested or tested by a noncontracted laboratory is unclear. Further, sufficient information to conduct an epidemiologic analysis to identify any specific risk factors (eg, surgery-related factors) that facilitated HCV transmission to certain patients but not in others was not collected. Finally, it was not possible to explain why transmission was confirmed only among patients at facility A.
These findings highlight the value of robust public health surveillance for HCV infection and underscore the need for improved safeguards for controlled medications in health care settings. This outbreak likely would not have been detected without surveillance for acute HCV infection and tracking of positive HCV test results through the state disease reporting system.14 Through timely and comprehensive investigation of individual case reports of acute HCV infection, public health officials identified 2 patients who had undergone surgical procedures at the same hospital within days of each other. This finding prompted wider investigation and subsequent notification of almost 6000 patients who were potentially exposed to HCV during the receipt of surgical procedures at 2 health care facilities in Colorado. Mounting this large public health investigation required extensive resources and collaboration of federal, state, and local public health agencies, as well as the 2 health care facilities involved and their contracted laboratories. This outbreak led to changes in Colorado statutes aimed at reducing patient harms and infection risks associated with narcotic diversion and tampering, which may serve as a model for other states.
Acknowledgments
We thank the following individuals for their assistance: Bernadette Albanese, El Paso County Department of Public Health and Environment; Stephanie Stark, Denver Public Health; Tracy Greene-Montfort, Assay Development and Diagnostic Reference Laboratory, Division of Viral Hepatitis, Centers for Disease Control and Prevention; and Sumathi Ramachandran, Molecular Epidemiology Laboratory, Division of Viral Hepatitis, Centers for Disease Control and Prevention.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of interest: None to report.
References
- 1.Williams IT, Perz JF, Bell BP. Viral hepatitis transmission in ambulatory health care settings. Clin Infect Dis. 2004;38:1592–8. doi: 10.1086/420935. [DOI] [PubMed] [Google Scholar]
- 2.Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital health care–associated hepatitis B and C virus transmission: United States, 1998–2008. Ann Intern Med. 2009;150:33–9. doi: 10.7326/0003-4819-150-1-200901060-00007. [DOI] [PubMed] [Google Scholar]
- 3.Sehulster L, Taylor J, Hendricks K, VanEgdom M, Whitely S, Manning S. Hepatitis C outbreak linked to narcotic tampering in an ambulatory surgical center. 1997 Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC: American Society for Microbiology Press; 1997. p. 293. [Google Scholar]
- 4.Williams IT, Perz JF, Bell BP. Hepatitis C virus transmission from healthcare workers to patients in the US [abstract] J Clin Virol. 2006;36(Suppl 2):S43–4. [Google Scholar]
- 5.Roche WF., Jr Former army nurse gets 41 months for hepatitis outbreak. [Accessed January 4, 2010];Pittsburgh Tribune Review. 2009 Dec 9; Available from: http://www.pittsburghlive.com/x/pittsburghtrib/news/s_655777.html#.
- 6.Hellinger WC, Bacalis LP, Kay RS, Thompson ND, Xia GL, Lin Y, et al. Health care–associated hepatitis C virus infections attributed to narcotic diversion. Ann Intern Med. 2012;156:477–82. doi: 10.7326/0003-4819-156-7-201204030-00002. [DOI] [PubMed] [Google Scholar]
- 7.US Attorney’s Office, District of New Hampshire. [Accessed December 10, 2013];Press release: Former employee of Exeter Hospital pleads guilty to charges related to multi-state hepatitis C outbreak. Available from: http://www.justice.gov/usao/nh/press/2013/Kwiatkowski.html.
- 8.Schaefer MK, Perz JF. Outbreaks of infections associated with drug diversion by US health care personnel. Mayo Clin Proc. 2014;89:878–87. doi: 10.1016/j.mayocp.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. [Accessed October 27, 2014];Risks of Healthcare-associated Infections from Drug Diversion. Available from: http://www.cdc.gov/injectionsafety/drugdiversion/index.html.
- 10.Centers for Disease Control and Prevention. [Accessed December 27, 2012];Guidelines for viral hepatitis surveillance and case management. Available from: http://www.cdc.gov/hepatitis/Statistics/SurveillanceGuidelines.htm.
- 11.Patel PR, Srinivasan A, Perz JF. Developing a broader approach to management of infection control breaches in health care settings. Am J Infect Control. 2008;36:685–90. doi: 10.1016/j.ajic.2008.04.255. [DOI] [PubMed] [Google Scholar]
- 12.Guh A, Thompson ND, Schaefer MK, Patel PR, Perz JF. Patient notification for bloodborne pathogen testing due to unsafe injection practices in US healthcare settings, 2001–2011. Med Care. 2012;50:785–91. doi: 10.1097/MLR.0b013e31825517d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powers and Duties of the Department of Public Health and Environment. C.R.S. § 25-1.5-102 (2014).
- 14.Rules and Regulations Pertaining to Epidemic and Communicable Disease Control. 6 Code Colo. Regs. 1009-1 (October 15, 2014).
- 15.Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, Irvine B, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74(Pt 11):2391–9. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran S, Xia GL, Ganova-Raeva LM, Nainan OV, Khudyakov Y. Endpoint limiting-dilution real-time PCR assay for evaluation of hepatitis C virus quasi-species in serum: performance under optimal and suboptimal conditions. J Virol Methods. 2008;151:217–24. doi: 10.1016/j.jviromet.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Massey JT, Waksberg J, Chu A, Maurer KR. Sample design: Third National Health and Nutrition Examination Survey. Vital Health Stat 2. 1992;113:1–35. [PubMed] [Google Scholar]
- 18.New York State Department of Health. [Accessed December 10, 2013];Press release: Westchester Co. hospital notifies patients of possible hep C exposure. Available from: http://www.health.ny.gov/press/releases/2009/2009-07-15_possible_hepatitis_c_exposure_warning.htm.
- 19.Brown J. 30-year term for surgical tech who swapped infected needles. [Accessed February 13, 2013];Denver Post. 2010 Feb 24; Available from: http://www.denverpost.com/news/ci_14463448?source=pkg#ixzz2Kn3xkmty.
- 20.Bell DM, McDonought JP, Ellison JS. Controlled drug misuse by Certified Registered Nurse Anestheists. AANA J. 1999;67:133–40. [PubMed] [Google Scholar]
- 21.Bosch X. Hepatitis C outbreak astounds Spain. Lancet. 1998;351:1415. [Google Scholar]
- 22.Ostrowsky BE, Whitener C, Bredenberg HK, Carson LA, Holt S, Hutwagner L, et al. Serratia marcescens bacteremia traced to an infused narcotic. N Engl J Med. 2002;346:1529–37. doi: 10.1056/NEJMoa012370. [DOI] [PubMed] [Google Scholar]
- 23.Behrens-Muller B, Conway J, Yoder J, Conover CS. Investigation and control of an outbreak of Achromobacter xylosoxidans bacterimia. Infect Control Hosp Epidemiol. 2012;33:180–4. doi: 10.1086/663710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berge KH, Dillon KR, Sikkink KM, Taylor TK, Lanier WL. Diversion of drugs within health care facilities, a multiple-victim crime: patterns of diversion, scope, consequences, detection, and prevention. Mayo Clin Proc. 2012;87:674–82. doi: 10.1016/j.mayocp.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Department of Justice, Drug Enforcement Administration, Office of Diversion Control. [Accessed December 20, 2013];Practitioner’s manual: an informational outline of the controlled substances act. Available from: http://www.deadiversion.usdoj.gov/pubs/manuals/pract/pract_manual012508.pdf.
- 26.Medicare and Medicaid Programs. Hospital conditions of participation: final rule. Fed Reg. 2006:68688–9. [PubMed] [Google Scholar]
- 27.“Statement on Security of Medications in the Operating Room” amended 2013. American Society of Anesthesiologists; [Accessed October 27, 2014.]. Available from: https://www.asahq.org/For-Members/Standards-Guidelines-and-Statements.aspx. [Google Scholar]
- 28.Standards for Hospitals and Health Facilities. General Licensure Standards, 6 Code Colo. Regs. 1011-1 Ch. 2 Section 3.2 (March 2,2014).
- 29.Professions and Occupations, Health Care. C.R.S. §12-43.2-102 (2014).
- 30.McPhee M. Former surgical tech reveals details of stolen drugs, hepatitic C outbreak. [Accessed February 13, 2013];Denver Post. 2010 Jan 17; Available from: http://www.denverpost.com/news/ci_14208687.
- 31.Perz JF, Thompson ND, Schaefer MK, Patel PR. US outbreak investigations highlight the need for safe injection practices and basic infection control. Clin Liver Dis. 2010;14:137–51. doi: 10.1016/j.cld.2009.11.004. [DOI] [PubMed] [Google Scholar]