Abstract
BACKGROUND
We investigated an outbreak of fungal infections of the central nervous system that occurred among patients who received epidural or paraspinal glucocorticoid injections of preservative-free methylprednisolone acetate prepared by a single compounding pharmacy.
METHODS
Case patients were defined as patients with fungal meningitis, posterior circulation stroke, spinal osteomyelitis, or epidural abscess that developed after epidural or paraspinal glucocorticoid injections. Clinical and procedure data were abstracted. A cohort analysis was performed.
RESULTS
The median age of the 66 case patients was 69 years (range, 23 to 91). The median time from the last epidural glucocorticoid injection to symptom onset was 18 days (range, 0 to 56). Patients presented with meningitis alone (73%), the cauda equina syndrome or focal infection (15%), or posterior circulation stroke with or without meningitis (12%). Symptoms and signs included headache (in 73% of the patients), new or worsening back pain (in 50%), neurologic symptoms (in 48%), nausea (in 39%), and stiff neck (in 29%). The median cerebrospinal fluid white-cell count on the first lumbar puncture among patients who presented with meningitis, with or without stroke or focal infection, was 648 per cubic millimeter (range, 6 to 10,140), with 78% granulocytes (range, 0 to 97); the protein level was 114 mg per deciliter (range, 29 to 440); and the glucose concentration was 44 mg per deciliter (range, 12 to 121) (2.5 mmol per liter [range, 0.7 to 6.7]). A total of 22 patients had laboratory confirmation of Exserohilum rostratum infection (21 patients) or Aspergillus fumigatus infection (1 patient). The risk of infection increased with exposure to lot 06292012@26, older vials, higher doses, multiple procedures, and translaminar approach to epidural glucocorticoid injection. Voriconazole was used to treat 61 patients (92%); 35 patients (53%) were also treated with liposomal amphotericin B. Eight patients (12%) died, seven of whom had stroke.
CONCLUSIONS
We describe an outbreak of fungal meningitis after epidural or paraspinal glucocorticoid injection with methylprednisolone from a single compounding pharmacy. Rapid recognition of illness and prompt initiation of therapy are important to prevent complications. (Funded by the Tennessee Department of Health and the Centers for Disease Control and Prevention.)
More than 500,000 epidural gluco-corticoid injections are administered in the United States each year in the Medicare population alone.1 Complications after epidural glucocorticoid injections are rare; when complications do occur, the most common are epidural abscesses and meningitis due to bacterial pathogens, with the complications frequently occurring in immunosuppressed persons.2–6 Infectious disease outbreaks associated with epidural glucocorticoid injections have rarely been reported.3,5,7
Fungal infections of the central nervous system are also uncommon and typically occur in immunosuppressed persons. Outbreaks of fungal meningitis after epidural or spinal injection are extremely rare; an outbreak of Exophiala dermatitidis meningitis in 2002 associated with contaminated methylprednisolone acetate prepared at a compounding pharmacy affected five patients.3 An outbreak of Aspergillus fumigatus meningitis associated with contaminated needles used for epidural anesthesia after the Indian Ocean tsunami affected five patients.8,9 Exserohilum species are environmental fungi common in grass and soil but have rarely been identified as human pathogens.10–13
We report preliminary results from Tennessee of an ongoing multistate investigation of fungal infections associated with preservative-free methylprednisolone acetate produced at a single compounding pharmacy.
METHODS
SURVEILLANCE
The Tennessee Department of Health (TDH) conducts ongoing surveillance for health care–associated infections, including outbreaks. In response to a report of a single patient in whom aspergillus meningitis developed after a recent epidural injection, active surveillance for additional case patients was performed. Hospitals, laboratories, and medical providers performing such procedures were asked to report to the TDH all possible cases of sterile-site fungal infections after epidural injections. Pharmacy records with information on the manufacture and distribution of the implicated product were obtained, and all patients reported by medical facilities as having received potentially contaminated product were actively contacted.
CASE PATIENTS
Case patients were defined as persons who had fungal meningitis or nonbacterial and nonviral meningitis of subacute onset, posterior circulation stroke when no cerebrospinal fluid was obtained (with no other obvious cause of stroke such as dissection of vertebral artery or cardioembolic source), or spinal or paraspinal osteomyelitis or epidural abscess at the site of injection, after an epidural or paraspinal glucocorticoid injection that was administered after May 21, 2012, in Tennessee.14 Cerebrospinal fluid, isolates, and tissue obtained from clinical specimens were sent to the Centers for Disease Control and Prevention (CDC) for identification of the pathogen with the use of polymerase chain reaction (PCR) amplification of fungal DNA and genomic sequencing.15 (PCR for fungal detection is a research test. The test has not been cleared or approved by the Food and Drug Administration [FDA]. The performance characteristics have not been established. The results of this test should not be used for the diagnosis, treatment, or assessment of patient health or management.)
For patients meeting the case definition, detailed information was obtained from medical chart reviews and interviews with the patients, their families, and physicians. Data were abstracted with the use of a standardized form that included information on demographic characteristics, symptoms, results of laboratory tests, treatment, and outcomes. For all patients who had received an epidural or paraspinal glucocorticoid injection at one of the three clinics that had received methylprednisolone from the same compounding pharmacy, information on patient characteristics, the type and date of the procedure, the personnel involved, the supplies and equipment used, and the medications administered was also collected. Since medication lot numbers were not recorded in patient clinic records, clinic protocols and invoices were evaluated to determine the probable lot used for each procedure. We determined the lots for each procedure by working back from the remaining vials in the clinic and using data collected on the volume used during each procedure. Lot assignment had to be estimated for our calculations and therefore was not considered authoritative.
STATISTICAL ANALYSIS
A cohort analysis was performed of all patients who had undergone epidural or paraspinal glucocorticoid injection procedures at a single clinic (Clinic A) since July 1, 2012, to assess for risk factors for infection. We performed analyses on both the patient and procedure level, since many patients had undergone multiple procedures. We excluded patients whose case status was under investigation. We stratified exposures according to the assigned medication lot and the vial age (defined as the number of days from lot production to injection). Patient age was analyzed as a dichotomous variable on the basis of the median age of 61 years. We evaluated the risk of infection by developing a logistic-regression model that included the age and sex of the patient, the cumulative dose of methylprednisolone according to the vial age (in 15-day increments) and lot, the procedure approach (translaminar vs. other), and the use or nonuse of contrast material. This model excluded procedures that were performed on days on which two different lots could have been used.
The data were analyzed with the use of SAS software, version 9.3. Fisher’s exact test or the Mantel–Haenszel chi-square statistic was used for categorical variables, and Student’s t-test or Wilcoxon rank-sum test was used for continuous variables. All data were analyzed as of October 19, 2012. This investigation was considered to be a public health response and was not considered to be research that was subject to approval by an institutional review board or that required written informed consent from patients.
RESULTS
INITIAL INVESTIGATION
On September 18, 2012, an astute clinician reported to the TDH a case of A. fumigatus meningitis in an immunocompetent adult after an epidural glucocorticoid injection at Clinic A; this report prompted an epidemiologic investigation.16 Two days later, active surveillance identified two additional cases of meningitis of unknown cause in hospitalized immunocompetent adults who had also recently received an epidural glucocorticoid injection at Clinic A; the CDC was notified of the TDH investigation. On-site review of Clinic A, which had closed voluntarily, revealed no obvious source of environmental contamination, such as recent construction or water damage, or relevant lapses in sterile technique. By September 25, a total of eight potential case patients (including the index patient) at Clinic A were identified; the patients had undergone epidural glucocorticoid injections on separate days and different times of the day.
Multiple common products had been used for all the patients. These included a commercially available epidural procedure tray, povidone–iodine, lidocaine, and single-dose vials containing 80 mg per milliliter of preservative-free methylprednisolone acetate from the New England Compounding Center (NECC, Framingham, MA). As specified in the package instructions, all products were stored at room temperature. The TDH contacted the Massachusetts Department of Health on September 24 to express concern and to obtain a distribution list of facilities that had received methylprednisolone from NECC in order to assist with enhanced case finding. On September 25, the TDH, in collaboration with the Massachusetts Department of Public Health, the Massachusetts Board of Registration in Pharmacy, and the CDC, contacted the compounding pharmacy, requested a list of facilities that had received methylprednisolone, and determined that the pharmacy had not received any reports of adverse events. The FDA was also notified about the ongoing public health investigation.
On September 26, 2012, NECC, in consultation with the Massachusetts Board of Registration in Pharmacy, issued a voluntary recall of the three lots of methylprednisolone that had been associated with case-patient exposure (05212012@68, 06292012@26, and 08102012@51); vials from these lots had been distributed to 76 facilities in 23 states. An analysis of Clinic A data identified no important clinic-related risk factors for infection (e.g., with respect to the day and time of the procedure, the room in which the procedure was performed, or the provider) but did indicate a relationship between increased exposure to methylprednisolone from this pharmacy and the likelihood of becoming a case patient. Active surveillance was conducted in the two additional clinics in Tennessee that had received medication from at least one of these three lots. The TDH activated emergency operations with the use of a statewide incident command structure and initiated a large-scale investigation, including personal contact and tracking of exposed patients. On October 4, 2012, the FDA announced that on microscopic examination, the agency had detected fungal contamination of unopened vials of methylprednisolone (lot 08102012@51) that had been collected from NECC.17
DESCRIPTION OF CASE PATIENTS
In the three clinics in Tennessee that had received methylprednisolone from NECC, 1009 patients had received epidural or paraspinal glucocorticoid injections with methylprednisolone from one or more of the three recalled lots. A total of 66 of these patients (7%) met the case definition through October 19, 2012. In the index patient, culturing of the cerebrospinal fluid yielded A. fumigatus; in 21 patients Exserohilum rostratum was identified from cultures of cerebrospinal fluid, tissue, or abscess fluid (6 patients) or was detected by means of PCR in cerebrospinal fluid (14 patients) or tissue (1 patient). Although all the patients had received epidural or paraspinal glucocorticoid injections at one of three clinics in Tennessee, case patients presented in one of seven states, and 2 case patients were outside the United States, when symptoms developed.
The median age of the patients was 69 years (range 23 to 91), and 71% were women (Table 1). A total of 124 procedures were performed in the 66 case patients between July 3, 2012, and September 26, 2012: 110 were lumbar epidural injections, 12 were cervical epidural injections, 1 was a sacroiliac-joint injection, and 1 was recorded as “other.” The median time from the last injection to symptom onset was 18 days (range 0 to 56). All the patients presented with one of three primary syndromes: 47 (71%) had meningitis alone, 11 (17%) had the cauda equina syndrome or focal infection near the injection site (4 of whom had documented epidural abscess), with or without meningitis, and 8 (12%) had posterior circulation stroke (with or without meningitis). Symptoms on admission included headache in 48 patients (73%), with 20 of these (43%) reporting severe headache. Other symptoms included new or worsening back pain in 33 patients (50%), nausea in 26 (39%), and stiff neck in 19 (29%).
Table 1.
Demographic Characteristics of the Patients and Signs and Symptoms at First Admission.
| Variable | Case Patients with Stroke (N = 13) | Case Patients without Stroke (N = 53) | P Value* | All Case Patients (N = 66) |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age — yr | 0.08 | |||
| Median | 72 | 67 | 69 | |
| Range | 56–91 | 23–90 | 23–91 | |
| Female sex — no. (%) | 11 (85) | 36 (68) | 0.32 | 47 (71) |
| White race — no. (%)† | 11 (85) | 42 (79) | 1.00 | 53 (80) |
| Signs and symptoms at first admission — no. (%) | ||||
| Fever‡ | 3 (23) | 20 (38) | 0.52 | 23 (35) |
| Headache | 9 (69) | 39 (74) | 0.74 | 48 (73) |
| Nausea | 7 (54) | 19 (36) | 0.34 | 26 (39) |
| Vomiting | 4 (31) | 12 (23) | 0.72 | 16 (24) |
| Slurred speech | 2 (15) | 0 | 0.04 | 2 (3) |
| Confusion | 2 (15) | 1 (2) | 0.10 | 3 (5) |
| Stiff neck | 5 (38) | 14 (26) | 0.50 | 19 (29) |
| New or worsening neck pain | 2 (15) | 10 (19) | 1.00 | 12 (18) |
| New or worsening back pain | 4 (31) | 29 (55) | 0.21 | 33 (50) |
| Numbness in lower extremities | 0 | 6 (11) | 0.58 | 6 (9) |
| Vertigo | 5 (38) | 6 (11) | 0.03 | 11 (17) |
| New fall or increased falling | 6 (46) | 3 (6) | 0.002 | 9 (14) |
| Self-reported hand or leg weakness | 2 (15) | 3 (6) | 0.25 | 5 (8) |
| Sensitivity to light | 0 | 6 (11) | 0.59 | 6 (9) |
| Meningeal signs | 2 (15) | 8 (15) | 1.00 | 10 (15) |
| Altered mental status | 3 (23) | 2 (4) | 0.05 | 5 (8) |
| Limb weakness on examination | 6 (46) | 3 (6) | 0.002 | 9 (14) |
| Facial droop | 2 (15) | 1 (2) | 0.10 | 3 (5) |
The P values are for the comparison between patients with stroke and those who did not present with stroke or in whom stroke did not develop.
Race was determined from information in the medical record.
The presence of fever was self-reported.
Other neurologic symptoms were seen in 32 patients (48%) and included vertigo in 11 patients (17%) and new or increasing falls in 9 (14%). Ten patients (15%) had symptoms suggestive of the cauda equina syndrome: new urinary incontinence or urinary retention in 6 (9%), gluteal or radiating leg pain in 3 (5%), and saddle anesthesia in 3 (5%). Signs on admission included fever (temperature >38°C) in 4 patients (6%), nuchal rigidity in 10 (15%), and altered mental status in 5 (8%). Results of a detailed neurologic examination were not recorded for many patients.
Lumbar puncture was performed within 24 hours after hospital admission in 50 patients (76%). The median time from the last epidural glucocorticoid injection to the lumbar puncture was 25 days (range, 18 to 36). The median cerebrospinal fluid white-cell count on the first lumbar puncture among patients who presented with meningitis, with or without stroke or focal infection, was 648 per cubic millimeter (range, 6 to 10,140), with 78% granulocytes (range, 0 to 97); the protein level was 114 mg per deciliter (range, 29 to 440); and the glucose concentration was 44 mg per deciliter (range, 12 to 121) (2.5 mmol per liter [range, 0.7 to 6.7]) (Table 2). The results from the first lumber puncture among patients in whom meningitis developed at any time (until October 19) are shown in Table 2. Initial imaging results identified abnormalities possibly related to fungal infection in 8 of 27 patients (30%) who underwent magnetic resonance imaging (MRI) of the head and in 16 of 35 (46%) who underwent MRI of the spine. Abnormalities included findings consistent with arachnoiditis, neuritis, epidural abscess, psoas or paraspinal muscle abscess, ventriculitis, enhancement of the meninges, and subarachnoid hemorrhage or infarcts involving the thalamus, pons, midbrain, or cerebellum.
Table 2.
Cerebrospinal Fluid Findings from the First Lumbar Puncture among Patients with Meningitis.*
| Finding | Patients in Whom Meningitis Developed by October 19, 2012 (N = 59) | Patients Who Presented with Meningitis with or without Stroke or Focal Infection (N = 57) |
|---|---|---|
| median (range) | ||
| White-cell count (cells/mm3) | 534 (4–10,140) | 648 (6–10,140) |
| Granulocytes (%) | 76 (0–97) | 78 (0–97) |
| Protein (mg/dl) | 114 (29–440) | 114 (29–440) |
| Glucose (mg/dl) | 45 (12–121) | 44 (12–121) |
Meningitis developed in some patients later, and the original findings of cerebrospinal fluid testing were within normal limits. A cerebrospinal fluid white-cell count of greater than 5 per cubic millimeter was considered to be indicative of pleocytosis and together with headache, fever, or neck stiffness was considered to indicate meningitis. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
The 13 patients who had a stroke did not differ significantly from those who did not have a stroke with respect to the site of injection (lumbar, cervical, sacroiliac-joint, or other), the time from symptom onset to lumbar puncture (7 days [range, 0 to 43] and 8 days [range, 0 to 39], respectively; P = 0.76), or the time from symptom onset to initiation of intravenous antifungal therapy (12 days [range, 0 to 44] and 10.5 days [range, 0 to 39], respectively; P = 0.65). Eight of these 13 patients initially presented with posterior circulation stroke; of these, 4 had onset of symptoms less than 48 hours before admission. The remaining 5 patients had a posterior circulation stroke during hospitalization; none of these 5 patients had received antifungal therapy on admission. These 5 patients presented with meningitis early during the outbreak before a fungal cause was clearly established. We compared these 5 patients with the 46 patients who did not have a stroke at admission and who received antifungal therapy. There was no significant difference with respect to the median time from onset of symptoms to the initiation of intravenous antifungal therapy (12 days [range, 10 to 26] and 10.5 days [range, 0 to 39], respectively; P = 0.33); however there was a significant difference in the median time from admission to initiation of intravenous antifungal therapy (6 days [range, 3 to 23] as compared with 1 day [range, 0 to 31]; P = 0.006). Three patients presented with posterior circulation strokes early in the outbreak and did not undergo a lumbar puncture before death to confirm meningitis. No alternate explanation for stroke in this vascular territory was found (e.g., no cardioembolic source or evidence of dissection of vertebral artery).
A total of eight patients (12%) died. Seven of the eight deaths (88%) occurred in patients who had a stroke. The other patient who died initially presented after 2 weeks of having nonspecific symptoms that developed into radicular pain in a saddle distribution, headache, and fever. Imaging revealed extradural and intradural abscesses; the results of a lumbar puncture showed meningitis. He was given liposomal amphotericin B and voriconazole, with resulting improvement in cerebrospinal fluid variables but paroxysmal atrial fibrillation developed, and he had a fatal arrest.
Voriconazole was initially administered in 61 of the patients (92%); 35 of those patients (57%) were also treated with liposomal amphotericin B. The median time from symptom onset to initiation of voriconazole therapy was 10 days (range, 0 to 44), and the median time from symptom onset to initiation of amphotericin B therapy was 14 days (range, 0 to 44). Serum drug levels were tested in 29 of the patients who received voriconazole (48%). The median time from initiation of voriconazole therapy to the first test for serum voriconazole level was 8 days (range, 3 to 21). Initial levels were greater than 2 μg per milliliter in 24 patients (83%); of these, 7 (29%) had levels higher than 6 μg per milliliter. Treatment with liposomal amphotericin B was discontinued in 34 patients (97%) after a median of 4 days (range, 1 to 25), primarily because of renal toxic effects. In these patients, the glomerular filtration rate decreased from baseline by a median of 31% (with the change ranging from 28% to −88%).
ASSESSMENT OF RISK FACTORS FOR INFECTION
Clinic A used 1663 vials of methylprednisolone from the three recalled lots during the outbreak period (83% of the vials they had received). Clinic B used 189 vials (86%), and Clinic C used 211 vials (70%). For the cohort analysis, we abstracted data on 817 patients who underwent a total of 1335 procedures at Clinic A between July 1, 2012, and September 20, 2012. The procedures included 779 translaminar epidural glucocorticoid injections (23 thoracic, 146 cervical, and 610 lumbar), 368 transforaminal epidural glucocorticoid injections, 132 caudal injections, 31 facet-joint injections, 17 sacroiliac-joint injections, and 8 other injections. A patient-level cohort analysis revealed that all the patients had received methylprednisolone; no cases of fungal infection occurred among 124 persons who underwent procedures without methylprednisolone.
In a univariate analysis of patients in this cohort with known case status (including those who did not receive methylprednisolone) (Table 3), patients who had multiple procedures had an increased risk of a fungal infection (11.5% [41 of 355 patients] vs. 4.0% [17 of 425 patients]; relative risk, 2.9; 95% confidence interval [CI], 1.7 to 5.0). The attack rates were 5.0%, 8.4%, 13.7%, and 14.3% among those who had undergone one, two, three, and four procedures, respectively. Patients who had received at least one epidural glucocorticoid injection with the use of a translaminar approach were more likely to become case patients than were those who had never been treated with a translaminar approach (9.6% [47 of 488 patients] vs. 3.8% [11 of 291 patients]; relative risk, 2.5; 95% CI, 1.3 to 4.8). Women were nearly twice as likely to become case patients as were men (9.5% [41 of 431 patients] vs. 4.9% [17 of 346 patients]; relative risk, 1.9; 95% CI, 1.1 to 3.4). Patients older than 60 years of age were four times as likely to become case patients as were those 60 years of age or younger (11.8% [47 of 400 patients] vs. 2.9% [11 of 380 patients]; relative risk, 4.1; 95% CI, 2.1 to 7.7). There were no significant associations between infection and factors related to physicians, technicians, operating room, operating room time, time spent with physician or technician, time of day, day of the week, or cervical versus lumbar sites.
Table 3.
Univariate Patient-Level Analysis of Risk Factors among Clinic A Patients with Known Case Status.
| Risk Factor | Cases | Non-Cases | Relative Risk (95% CI) |
|---|---|---|---|
| no. of patients/total no. | |||
| Female sex | 41/431 | 17/346 | 1.9 (1.1–3.4) |
| Age >60 yr | 47/400 | 11/380 | 4.1 (2.1–7.7) |
| Translaminar epidural glucocorticoid injection | 47/488 | 11/291 | 2.5 (1.3–4.8) |
| Multiple procedures | 41/355 | 17/425 | 2.9 (1.7–5.0) |
| Methylprednisolone, lot 06292012@26, vial >50 days old | 29/149 | 6/190 | 6.2 (2.6–14.5) |
Among 656 persons who received methylprednisolone at Clinic A, infections developed in 58 (9%). The risk of disease among patients who had any exposure to the 06292012@26 lot was significantly greater than the risk among those who had exposure to the 05212012@68 lot or the 08102012@51 lot alone: infections developed in 51 of 424 patients (12%) who received methylprednisolone from the 06292012@26 lot as compared with 7 of 231 (3%) who had received injections from one of the other lots (relative risk, 4.0; 95% CI, 1.8 to 8.6). Figure 1 shows the attack rate on a procedure level, according to lot number, over time. Among patients receiving methylprednisolone from the 06292012@26 lot, exposure to only older vials (vial age >50 days) was associated with higher attack rates than was exposure to only newer vials, with infections developing in 29 of 149 patients exposed to methylprednisolone from older vials (19%) as compared with infections in 6 of 190 (3%) exposed to methylprednisolone from newer vials (relative risk, 6.2; 95% CI, 2.6 to 14.5). Among recipients of medication from the older vials from lot 06292012@26, the attack rates after injection of 40 to 80 mg, 120 to 160 mg, and more than 160 mg were 15% (11 of 73 patients), 19% (26 of 138), and 35% (8 of 23), respectively.
Figure 1. Number of Epidural and Paraspinal Glucocorticoid Injections and Attack Rate.
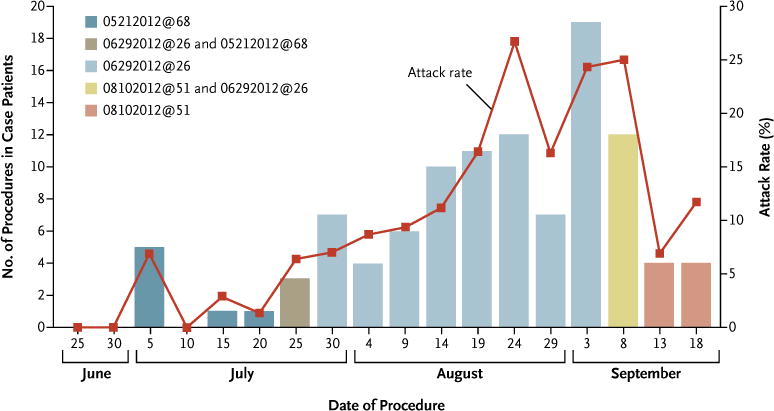
Shown are the number of epidural and paraspinal glucocorticoid injection procedures performed in case patients, as well as the attack rates among persons who received methylprednisolone acetate from the implicated lots during these procedures. Data are shown according to 5-day time periods.
We performed a multivariate analysis confined to patients who received methylprednisolone with a known case status and excluded time periods of potential lot overlap. This analysis confirmed the following risk factors: age older than 60 years (adjusted odds ratio, 4.01; 95% CI, 1.95 to 8.24); female sex (adjusted odds ratio, 2.56; 95% CI, 1.29 to 5.12); and cumulative dose of 06292012@26 lot injected 45 to 60 days and more than 60 days after production, in 40-mg increments (adjusted odds ratio, 1.29; 95% CI, 1.02 to 1.63 and adjusted odds ratio 1.65; 95% CI, 1.29 to 2.11, respectively). Two factors improved the fit of the model, but were not significant: a translaminar approach (adjusted odds ratio, 2.01; 95% CI, 0.96 to 4.23) and the use of contrast material (adjusted odds ratio, 0.23; 95% CI, 0.05 to 1.14).
DISCUSSION
The case cluster described here is part of the ongoing multistate outbreak of fungal infections associated with epidural, paraspinal, and peripheral-joint glucocorticoid injections. On October 18, the CDC and FDA announced that E. rostratum had been identified in unopened vials of methylprednisolone from the 08102012@51 lot; exserohilum was also subsequently identified in the 06292012@26 lot.16 The outbreak is ongoing and involves multiple states14; morbidity and mortality have been high. Rapid recognition and evaluation of infections after a patient’s exposure to implicated methylprednisolone are critical, and appropriate therapy should be initiated promptly.
We found a strong association between the age of the methylprednisolone vials and the rate of infection in one clinic. One possible explanation for this observation is that the level of contamination in the vials may have increased over time, with subsequent higher fungal burdens present in older vials. Injectable, preservative-free glucocorticoid preparations have been shown to be suitable media to support or increase the growth of pathogenic fungi, including A. fumigatus.3,18 We also describe the increased risk of infection associated with increasing amounts of methylprednisolone administered. This may reflect exposure to an increasing amount of contaminant with increased volume of methylprednisolone administered. In addition, because the medication came in 80-mg vials, multiple injections or single injections with a dose of more than 80 mg increased the likelihood of exposure to at least one contaminated vial.
Among the most striking features of this outbreak are the high prevalence and anatomical location of strokes. Epidural glucocorticoid injections can lead to localized infection, and fungal pathogens can invade the dura, leading to meningitis and, in some patients, invasion of the posterior circulation vasculature leading to stroke, hemorrhage, or both.16 Stroke was seen more commonly early in the outbreak, with four patients presenting with stroke less than 48 hours after the onset of any symptoms. The incidence of stroke declined as diagnostic testing became more prevalent and aggressive and patients were identified earlier in their clinical course; stroke did not develop in any patients in this report in whom therapeutic doses of antifungal medications were instituted within 48 hours after the initial presentation.
In this series, the mortality associated with untreated A. fumigatus and E. rostratum meningitis was very high; all eight deaths in our series occurred in persons who received delayed, minimal, or no treatment. We found that the initial presenting symptoms were frequently mild and nonspecific and often difficult to distinguish from the chronic symptoms for which the epidural or paraspinal glucocorticoid injection was originally administered. Lumbar puncture performed promptly at the first suspicion of clinical illness allowed early identification of infection and prompt initiation of therapy.
Exserohilum species are environmental fungi that are common in grass and soil but have rarely been identified as human pathogens.10–13 Although uncertainty exists about the appropriate treatment of exserohilum infections,12,19 treatment recommendations have been developed by the CDC in response to this outbreak. These recommendations include treatment with voriconazole (at an initial dose of 6 mg per kilogram of body weight every 12 hours). The addition of liposomal amphotericin B can be considered in patients who present with severe disease or whose conditions deteriorate or do not improve with voriconazole alone.14,20 Voriconazole can cause substantial side effects, including hepatotoxic effects, rash, central nervous system toxic effects including visual disturbances and hallucinations, prolongation of the corrected QT interval, and drug interactions.21–24 Serum voriconazole levels can be assessed as soon as the fifth day of treatment, with a suggested therapeutic range of 2 to 5 μg per milliliter.14 The use of liposomal amphotericin B was associated with decreasing renal function and early cessation of therapy in all but one of the patients in this series. Additional studies are needed to assess the best possible treatment.
There are several limitations of this investigation. First, the pathogen was laboratory-confirmed in only 22 patients at the time of the analysis. PCR assay was helpful in identifying the pathogen in several patients; however, the sensitivity of a fungal PCR assay is unknown. Second, there was a potential for misclassification of exposure to specific lots of medication. Third, because case patients continue to be identified, the overall estimates of risk and risk by lot may change over time. Fourth, we do not have long-term outcome data for many of the patients in this series, data that will be important in developing more definitive treatment recommendations. Fifth, our attack rates represent cumulative risk at the time of last injection; as the time from injection increases, the current risk of an infection decreases dramatically. Sixth, our analysis was conducted on data available as of October 19, 2012, and the clinical status of patients, including complications, continues to evolve. Finally, the results of the investigation in Tennessee may not be generalizable to other states because of differences in lot exposure and procedure types.
Pharmaceutical compounding refers to the combining, mixing, or altering of ingredients of a drug by a licensed pharmacist to produce a drug that is tailored to an individual patient’s medical needs, on the basis of a valid prescription from a licensed medical practitioner. There are few reliable data on the prevalence of compounding, but it has been estimated that 0.25% to more than 2% of dispensed prescriptions in the United States are compounded drugs.14 Under certain conditions, compounding may serve an important public health benefit by providing access to medications tailored to the needs of individual patients when a commercially available product is unavailable; however, compounded drugs are not approved by the FDA and should not be confused with generic drugs. Unlike brand name and generic drugs, all of which must be approved by FDA before marketing, compounded drugs are not reviewed and approved by the FDA; therefore, their safety, efficacy, quality, and conformance with federal manufacturing standards have not been established. The current outbreak is only the most recent example of deaths and serious adverse events associated with drugs made by a compounding pharmacy.3,5,25–28 The regulatory authority of the FDA over compounding pharmacies is different and more limited than is its authority over pharmaceutical manufacturers; states license pharmacies and have primary responsibility for the oversight of the day-to-day operations of compounding pharmacies.
An aggressive public health response to a single report of an unusual infection resulted in the identification of a multistate outbreak of fungal infections and the rapid recall of the implicated product involved. The investigation of this outbreak in Tennessee required a close and collaborative approach between the public health system and the medical community. Maintaining a strong public health infrastructure is critical to ensuring that there is capacity to investigate such outbreaks quickly and effectively.
Supplementary Material
Acknowledgments
Supported by the Tennessee Department of Health, by an appointment to the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists and funded by the Centers for Disease Control and Prevention Cooperative Agreement 5U38HM000414-5, and by the Prevention and Public Health Fund 2012: Epidemiology and Laboratory Capacity for Infectious Diseases (ELC) Building and Strengthening Epidemiology, Laboratory and Health Information Systems Capacity in State and Local Health Departments Cooperative Agreement 3U5OCI000929-02S5; the Epidemiology and Laboratory Capacity for Infectious Diseases-Program Components Cooperative Agreement 1U5OCK000211-01; and the Emerging Infections Program Cooperative Agreement 1U5OCK000198-01 (all, Centers for Disease Control and Prevention).
We thank the staff of the involved clinics who cooperated in all aspects of the investigation and the clinicians, laboratory staff, infection preventionists, and all personnel involved in the outbreak response, including the Tennessee Department of Health, Infectious Diseases Pathology Branch, Division of High Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, and the Massachusetts Department of Health and Hygiene and Board of Registration in Pharmacy.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Carrino JA, Morrison WB, Parker L, Schweitzer ME, Levin DC, Sunshine JH. Spinal injection procedures: volume, provider distribution, and reimbursement in the U.S. Medicare population from 1993 to 1999. Radiology. 2002;225:723–9. doi: 10.1148/radiol.2253011401. [DOI] [PubMed] [Google Scholar]
- 2.Kolbe AB, McKinney AM, Kendi AT, Misselt D. Aspergillus meningitis and discitis from low-back procedures in an immunocompetent patient. Acta Radiol. 2007;48:687–9. doi: 10.1080/02841850701342153. [DOI] [PubMed] [Google Scholar]
- 3.Exophiala infection from contaminated injectable steroids prepared by a compounding pharmacy — United States, July–November 2002. MMWR Morb Mortal Wkly Rep. 2002;51:1109–12. [PubMed] [Google Scholar]
- 4.Hooten WM, Kinney MO, Huntoon MA. Epidural abscess and meningitis after epidural corticosteroid injection. Mayo Clin Proc. 2004;79:682–6. doi: 10.4065/79.5.682. [DOI] [PubMed] [Google Scholar]
- 5.Civen R, Vugia DJ, Alexander R, et al. Outbreak of Serratia marcescens infections following injection of betamethasone compounded at a community pharmacy. Clin Infect Dis. 2006;43:831–7. doi: 10.1086/507336. [DOI] [PubMed] [Google Scholar]
- 6.Huang RC, Shapiro GS, Lim M, Sandhu HS, Lutz GE, Herzog RJ. Cervical epidural abscess after epidural steroid injection. Spine (Phila PA 1976) 2004;29(1):E7–E9. doi: 10.1097/01.BRS.0000106764.40001.84. [DOI] [PubMed] [Google Scholar]
- 7.Invasive Staphylococcus aureus infections associated with pain injections and reuse of single-dose vials — Arizona and Delaware 2012. MMWR Morb Mortal Wkly Rep. 2012;61:501–4. [PubMed] [Google Scholar]
- 8.Gunaratne PS, Wijeyaratne CN, Chandrasiri P, et al. An outbreak of Aspergillus meningitis following spinal anaesthesia for caesarean section in Sri Lanka: a post-tsunami effect? Ceylon Med J. 2006;51:137–42. doi: 10.4038/cmj.v51i4.1142. [DOI] [PubMed] [Google Scholar]
- 9.Gunaratne PS, Wijeyaratne CN, Seneviratne HR. Aspergillus meningitis in Sri Lanka — a post-tsunami effect? N Engl J Med. 2007;356:754–6. doi: 10.1056/NEJMc062547. [DOI] [PubMed] [Google Scholar]
- 10.Lasala PR, Smith MB, McGinnis MR, Sackey K, Patel JA, Qiu S. Invasive Exserohilum sinusitis in a patient with aplastic anemia. Pediatr Infect Dis J. 2005;24:939–41. doi: 10.1097/01.inf.0000180988.96528.5c. [DOI] [PubMed] [Google Scholar]
- 11.McGinnis MR, Rinaldi MG, Winn RE. Emerging agents of phaeohyphomycosis: pathogenic species of Bipolaris and Exserohilum. J Clin Microbiol. 1986;24:250–9. doi: 10.1128/jcm.24.2.250-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler A, Yaniv I, Samra Z, et al. Exserohilum: an emerging human pathogen. Eur J Clin Microbiol Infect Dis. 2006;25:247–53. doi: 10.1007/s10096-006-0093-3. [Erratum, Eur J Microbiol Infect Dis 2006;25:254–6.] [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Singh SM. A case of cutaneous phaeohyphomycosis caused by Exserohilum rostratum, its in vitro sensitivity and review of literature. Mycopathologia. 1995;131:9–12. doi: 10.1007/BF01103898. [DOI] [PubMed] [Google Scholar]
- 14.Multistate outbreak of fungal infection associated with injection of methylprednisolone acetate solution from a single compounding pharmacy — United States, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:839–42. [PubMed] [Google Scholar]
- 15.Interpretive criteria for identification of bacteria and fungi by DNA target sequencing: approved guideline. Wayne, IN: Clinical and Laboratory Standards Institute; 2008. (CLSI document MM18-A) [Google Scholar]
- 16.Pettit AC, Kropski JA, Castilho JL, et al. The index case for the fungal meningitis outbreak in the United States. N Engl J Med. 2012;367:2119–25. doi: 10.1056/NEJMoa1212292. [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. FDA fungal meningitis updates, 10/5/12, 10/18/12. ( http://www.fda.gov/Drugs/DrugSafety/FungalMeningitis/default.htm)
- 18.Ng TT, Robson GD, Denning DW. Hydrocortisone-enhanced growth of Aspergillus spp.: implications for pathogenesis. Microbiology. 1994;140:2475–9. doi: 10.1099/13500872-140-9-2475. [DOI] [PubMed] [Google Scholar]
- 19.Lai CC, Tan CK, Huang YT, Shao PL, Hsueh PR. Current challenges in the management of invasive fungal infections. J Infect Chemother. 2008;14:77–85. doi: 10.1007/s10156-007-0595-7. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Interim treatment guidance for central nervous system (CNS) and parameningeal infections associated with injection of contaminated steroid products. ( http://www.cdc.gov/hai/outbreaks/clinicians/guidance_cns.html)
- 21.Scott LJ, Simpson D. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs. 2007;67:269–98. doi: 10.2165/00003495-200767020-00009. [DOI] [PubMed] [Google Scholar]
- 22.Muijsers RB, Goa KL, Scott LJ. Voriconazole: in the treatment of invasive aspergillosis. Drugs. 2002;62:2655–64. doi: 10.2165/00003495-200262180-00010. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs F, Selleslag D, Aoun M, Sonet A, Gadisseur A. An observational efficacy and safety analysis of the treatment of acute invasive aspergillosis using voriconazole. Eur J Clin Microbiol Infect Dis. 2012;31:1173–9. doi: 10.1007/s10096-011-1425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poluzzi E, Raschi E, Motola D, Moretti U, De PF. Antimicrobials and the risk of torsades de pointes: the contribution from data mining of the US FDA Adverse Event Reporting System. Drug Saf. 2010;33:303–14. doi: 10.2165/11531850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.McPherson TB, Fontane PE, Jackson KD, et al. Prevalence of compounding in independent community pharmacy practice. J Am Pharm Assoc. 2006;46:568–73. doi: 10.1331/1544-3191.46.5.568.mcpherson. [DOI] [PubMed] [Google Scholar]
- 26.Sunenshine RH, Tan ET, Terashita DM, et al. A multistate outbreak of Serratia marcescens bloodstream infection associated with contaminated intravenous magnesium sulfate from a compounding pharmacy. Clin Infect Dis. 2007;45:527–33. doi: 10.1086/520664. [DOI] [PubMed] [Google Scholar]
- 27.Jones TF, Feler CA, Simmons BP, et al. Neurologic complications including paralysis after a medication error involving implanted intrathecal catheters. Am J Med. 2002;112:31–6. doi: 10.1016/s0002-9343(01)01032-4. [DOI] [PubMed] [Google Scholar]
- 28.Frost BA, Kainer MA. Safe preparation and administration of intravitreal bevacizumab injections. N Engl J Med. 2011;365:2238. doi: 10.1056/NEJMc1105759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


