Abstract
Objectives
Age-at-menopause and leukocyte telomere length (LTL) are both associated with biologic aging. Therefore, it would be reasonable to hypothesize that LTL may also serve as a marker for reproductive aging as shorter LTL may be associated with earlier age-at-menopause.
Methods
We analyzed data from 799 post-menopausal (ages 41–85) participants in the National Health and Nutrition Examination Survey (1999–2002), a nationally representative sample of U.S. women.
Results
Controlling for behavioral, socio-demographic, and health-related determinants of menopause, we found that among non-Hispanic white women, an increase of one standard deviation in LTL was associated with a 0.43 year higher reported age-at-menopause. Among Mexican–Americans, an increase of one standard deviation in LTL was associated with a 1.56 year earlier menopause. There was no significant association between LTL and age-at-menopause among non-Hispanic black women.
Conclusions
Our main finding is evidence of a strong interaction by race/ethnicity in the association between LTL and age-at-menopause. This evidence does not support the hypothesis that shorter LTL is a predictor of earlier age-at-menopause, as the magnitude and direction of the associations between LTL and age-at-menopause varied across racial/ethnic groups.
Keywords: Menopause, Telomeres, Reproductive aging
1. Introduction
Age-at-menopause can be viewed as an index of biologic aging [1,2], the evidence for this assertion is found in the association between early reproductive senescence and onset of age-related morbidities [3–7] as well as early mortality [8–12]. Biologic aging is, in essence, cellular functional decline that leads to tissue dysfunction and eventually mortality; and leukocyte telomere length (LTL) is a mechanism of this functional decline [13,14]. By capping the ends of chromosomes, telomeres protect against chromosomal degradation and breakdown. However, telomeres’ protective function declines as they shorten with each round of cell division; likelihood of cellular senescence and apoptosis increases as the average telomere length decreases [13]. Given that both age-at-menopause and LTL are associated with biologic aging it would be reasonable to hypothesize that LTL may also serve as a marker for reproductive aging as shorter LTL may be associated with earlier age-at-menopause. Examination of the link between LTL and age- at-menopause, independent of behavioral, socio-demographic, and health-related determinants of menopause, can potentially also provide a biologic explanation for the differing rates of reproductive aging observed among women.
To date, three studies have specifically focused on the link between LTL and age-at-menopause. Among a Turkish convenience sample (n = 37) of healthy women aged 50, crude bivariate analyses revealed menstruating women to have longer LTL than menopausal women [15]. Two other studies, one of Caucasian post-menopausal women at risk of cognitive decline (n = 53; age: 49–69), and the other of Caucasian women participating in the Cardiovascular Health Study (n = 486; age: 64–80+), found a positive association between LTL and age-at-menopause [16,17]. However, only the latter study controlled for confounders of this association. Two case-control studies of Caucasian cancer patients, both nested within Nurses’ Health Study, included age-at-menopause as a confounder, neither study found an association between LTL and age-at-menopause [18,19].
We addressed some limitations of existing studies, specifically their relatively small sample sizes and exclusive reliance on Caucasian populations, by examining the link between LTL and age-at-menopause among a diverse sample of postmenopausal women drawn from the National Health and Nutrition Examination Survey (NHANES: 1999–2002), a large, nationally representative, sample of the civilian, non-institutionalized US population. We also examined whether the association between LTL and age-at-menopause is consistent among different racial-ethnic groups. This inquiry was motivated by a report of such effect modification by Gray et al. [17] (which led them to focus their investigation on Caucasian women) and by evidence of racial/ethnic differences in rates of telomere shortening with age [15], with reports of both longer [14,20–22] and shorter [23,24], LTL among historically disadvantaged populations.
2. Methods
2.1. Study population
Data were from 1999 to 2002 National Health and Nutrition Examination Survey (NHANES), a cross-sectional survey of the civilian, non-institutionalized US population (0–85 years of age) conducted continuously in 2-year survey cycles [25]. The NHANES uses a multistage probability sampling design, with some subgroups oversampled (e.g., low-income white persons). Unweighted response rates for the total examined female sample range from 77% to 80% for the survey cycles covering 1999–2002, the only NHANES cycles for which telomere data are available [26].
The analytic sample was drawn from the 2129 women, ages 30–85 years who completed the mobile examination component (MEC) and had LTL data available, who were not pregnant or lactating, and responded negatively to the question “Have you had regular periods in the past 12 months?” Women with a history of hysterectomy, unilateral/bilateral oophorectomy, or who reported their menopause status as attributable to other medical conditions or treatments, or reported chronic oligomenorrhea/amenorrhea were also excluded from our analytic sample (n = 1059), but reserved for a subsequent sensitivity analyses. Of the eligible sample of 1070 potentially postmenopausal women, 254 were excluded due to missing data for age-at-menopause or information about medical/surgical history. Additionally, women who reported to have undergone menopause before age 30 or after age 60 were excluded from the analytic sample (n = 17), consistent with prior studies that had used these thresholds to rule out implausible values [17], leaving a final analytic sample of 799 (75%) women ages 41–85 years (Supplemental Fig. 1).
2.2. Measures
Age-at-menopause was defined based on the question “About how old were you when you had your last menstrual period?” with responses in years. Telomere length assay is detailed elsewhere [14]. Briefly, DNA was extracted from whole blood and stored at −80°. LTL was assayed using the quantitative polymerase chain reaction method to measure telomere length relative to standard reference DNA (T/S ratio) [16]. The single-copy gene used as a control to normalize input DNA was human beta-globin. Each sample was assayed 3 times on 3 different days. The samples were assayed on duplicate wells, resulting in 6 data points. Sample plates were assayed in groups of 3 plates, and no 2 plates were grouped together more than once. Each assay plate contained 96 control wells with 8 control DNA samples. Assay runs with 8 or more invalid control wells were excluded from further analysis (<1% of runs). Control DNA values were used to normalize between-run variability. Runs with more than 4 control DNA values falling outside 2.5 standard deviations from the mean for all assay runs were excluded from further analysis (<6% of runs). For each sample, any potential outliers were identified and excluded from the calculations (<2% of samples). The mean and standard deviation of the T/S ratio were then calculated normally. The interassay coefficient of variation was 6.5%. Finally, it is noteworthy that observed LTL length can vary across laboratories and types of assays. While these values tend to be highly correlated, they can have different means [27].
Demographic characteristics were collected during the NHANES household interview. All models included age (as a linear variable) and race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican–American and other). However, because the group of participants identified as belonging to “other” races/ethnicities is small and heterogeneous, results are not presented for this group, though they were included in the analytic sample. We also controlled for key determinants of age-at-menopause and LTL: reported lifetime smoking (never, former, current [28,29]; body mass index (BMI) [29,30], and household income to poverty ratio as a proxy for individual-level SES. Additional covariates were included as prior work had reported associations with age-at-menopause [17,31]: gravidity (nulligravid or not), marital status (single/never married, married/cohabitating, divorced/widowed/separated), and reported history of cardiovascular disease (i.e., coronary heart disease, stroke, congestive heart failure, angina, or heart attack) [17]; or cancer. Finally, to control for the lapsed time between occurrence of menopause and when blood samples were drawn, age-at-interview, which was when the blood was drawn, was also included as a linear variable. Missing data on these covariates further reduced the sample to 635 women for fully adjusted models (Table 1).
Table 1.
Socio-demographic characteristics of post-menopausal US women (NHANES: 1999–2002).
| Post-menopausal women (n = 799) | Women with surgical/medical menopause (n = 739) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | n | Mean (%) | (95% CI) [SE] | n | Mean (%) | 95% CI | P |
| Age | 799 | 63.45 | (62.48–64.42) | 739 | 60.42 | (59.02–61.82) | <0.001 |
| Age at reported menopause (overall) | 799 | 48.88 | (48.59–49.18) | 739 | 40.58 | (39.92–41.24) | <0.001 |
| Non-Hispanic White | 453 | 49.0 | [SE: 0.2] | n/a | |||
| Non-Hispanic Black | 108 | 49.1 | [SE: 0.5] | n/a | |||
| Mexican–American | 172 | 47.9 | [SE: 0.4] | n/a | |||
| Mean telomere length (overall) | 799 | 0.95 | (0.92–0.99) | 739 | 0.99 | (0.95–1.02) | 0.024 |
| LTL: Non-Hispanic White | 453 | 0.95 | (0.91–0.98) | 432 | 0.98 | (0.94–1.01) | |
| LTL: Non-Hispanic Black | 108 | 1.01a | (0.96–1.06) | 135 | 1.06a | (1.00–1.12) | |
| LTL: Mexican–American | 172 | 0.95 | (0.89–1.00) | 130 | 0.91a | (0.85–0.97) | |
| Race/ethnicity | 799 | 739 | <0.001 | ||||
| Non-Hispanic White | 453 | 78.78% | (73.76–83.80%) | 432 | 81.40% | (76.49–86.31%) | |
| Non-Hispanic Black | 108 | 6.85% | (4.51–9.20%) | 135 | 9.18% | (6.10–12.26%) | |
| Mexican–American | 172 | 3.54% | (2.20–4.89%) | 130 | 2.89% | (1.61–4.17%) | |
| Other | 66 | 10.82% | (5.60–16.05%) | 42 | 6.53% | (2.38–10.68%) | |
| Income-to-poverty ratio | 699 | 677 | |||||
| <100% FPT | 116 | 13.49% | (10.05–16.93%) | 104 | 12.98% | (10.06–15.90%) | 0.527 |
| 100–199% FPT | 195 | 21.52% | (17.54–25.50%) | 189 | 24.07% | (20.49–27.66%) | |
| 200–399% FPT | 185 | 27.75% | (23.83–31.68%) | 204 | 29.03% | (23.91–34.14%) | |
| ≥400% FPT | 203 | 37.23% | (31.65–42.82%) | 180 | 33.92% | (28.31–39.53%) | |
| Marital Status | 755 | 697 | 0.025 | ||||
| Married/cohabitating | 383 | 56.82% | (52.42–61.22%) | 387 | 61.85% | (57.76–65.95%) | |
| Single/never married | 336 | 37.60% | (32.58–42.62%) | 287 | 35.63% | (31.68–39.58%) | |
| Divorced/separated/widowed | 36 | 5.58% | (3.24–7.92%) | 23 | 2.52% | (0.96–4.07%) | |
| Body mass index | 769 | 28.46 | (27.9–29.03) | 717 | 28.79 | (28.19–29.38) | 0.42 |
| Nulliparous | 799 | 8.57% | (0.0549–0.1165) | 739 | 5.04% | (0.0336–0.0673) | 0.092 |
| Smoking | 797 | 737 | 0.366 | ||||
| Never | 471 | 55.57% | (50.92–60.21%) | 432 | 54.43% | (49.34–59.53%) | |
| Former | 217 | 27.93% | (23.48–32.38%) | 190 | 25.54% | (21.87–29.21%) | |
| Current | 109 | 16.51% | (12.78–20.23%) | 115 | 20.03% | (15.00–25.06%) | |
Note: statistically significant differences between the natural menopause and surgical/medical menopause group denoted in bold, p < 0.05 as assessed by a Wald test (continuous covariates) or Pearson χ2 test (categorical covariates). 95% CIs were obtained from linear regression models for continuous covariates and from survey-weighted proportions for categorical covariates, with variance estimates obtained using Taylor series linearization and appropriate survey procedures to account for the sample weights and design of NHANES.
Abbreviations: LTL, leukocyte telomere length; FPT, federal poverty threshold.
Indicates statistically significant difference from non-Hispanic white; p < 0.05 as assessed by a Wald test. LTL was not adjusted for age in these comparisons.
2.3. Analyses
We fit a series of linear regression models to estimate the association between LTL (independent variable) and age-at-menopause (dependent variable) and corresponding 95% confidence intervals (CIs). LTL was log-transformed for normality, as it was positively skewed. The first model included only age and race/ethnicity in addition to LTL. To explore potential differences by race/ethnicity in the associations between LTL and age-at-menopause, the second model added an interaction term between LTL and race/ethnicity, as there is evidence that LTL may differ by race/ethnicity [14,17,20,21,23,24]. The third model included all socio-demographic and health-related covariates noted above.
A sensitivity analysis examined LTL in relation to age-at-menopause among women who reported surgical or medical menopause; this analysis was conducted as a falsification test as age-at-menopause among this sample should be unrelated to LTL. MEC sample weights were used to account for differential probabilities of selection, non response and non-coverage; and survey procedures in Stata 12.1 SE were used to account for the complex survey design of NHANES. Standard errors and Wald 95% CIs were estimated using Taylor series linearization, with p < 0.05 indicating statistical significance.
Finally, sensitivity analyses replicated the model building process described above, but utilized multiply imputed data sets for age-at-menopause for the sample of 1070 post-menopausal women (and 844 women with medical/surgical menopause) to mitigate the impact of missing data. These analyses are fully described in Supplemental Appendix.
3. Results
Descriptive characteristics of postmenopausal women in the U.S. appear in Table 1. For comparison, characteristics of women meeting eligibility criteria but reporting menopause attributable to surgery or medical reasons (e.g., hysterectomy, oophorectomy) are also presented. Women were, on average, 63 years old (95% CI: 62.48–64.42 years), significantly older than the comparable sample of women who had a history of surgical or medically attributable menopause (60.42 years, 95% CI: 59.02–61.82). The mean age-at-menopause was 48.88 years (95% CI: 48.59–48.18); again, significantly older than women in the surgical/medical menopause group (40.58, 95% CI: 39.92–41.24). Most women were non-Hispanic white (79%) with 7% non-Hispanic black and 4% Mexican–American. Mexican–American women were younger, on average, than white women (p < 0.01). They also reported younger age-at-menopause (p = 0.03), and had a shorter time between their current attained age and their reported-age-atmenopause than white women (p < 0.01). There were no significant differences between the various age-related variables comparing Mexican–American women and non-Hispanic black women. Finally, the mean LTL (adjusted for age) was 1.11 (95% CI: 1.02–1.19) for non-Hispanic white women, 1.15 (95% CI: 1.08–1.23) for non-Hispanic black women, and 1.08 (95% CI: 1.01–1.14) for Mexican–American women; these latter values were not statistically different from that of non-Hispanic white women.
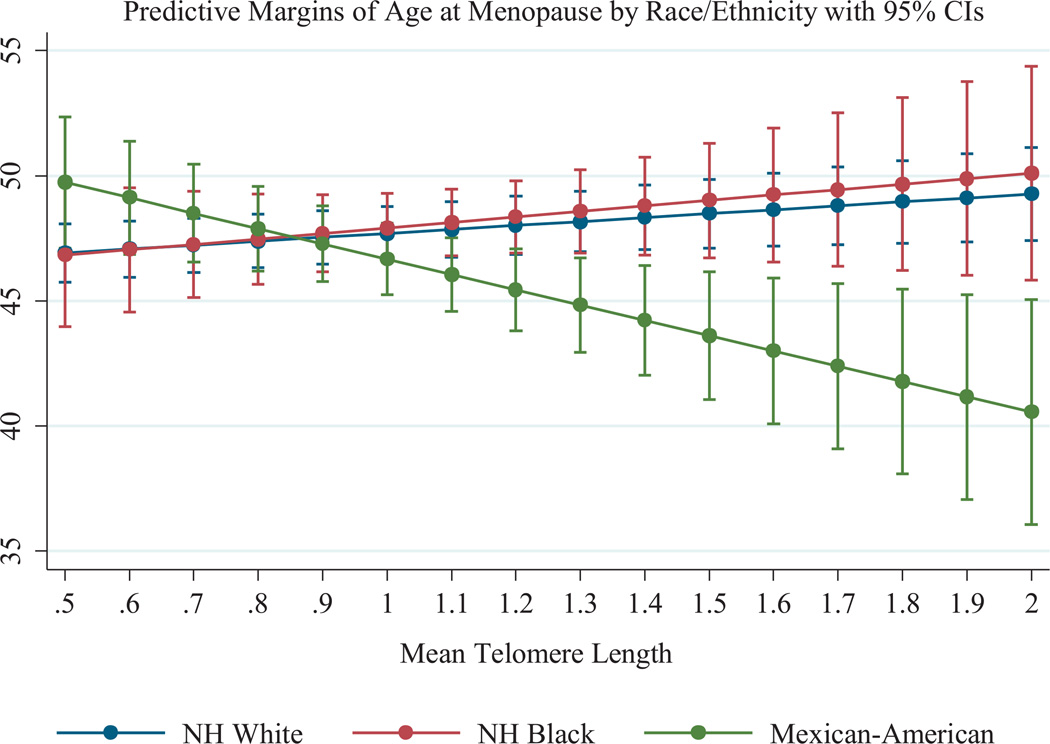
Results of the linear regression models are presented in Table 2. Model 1 included only LTL, age, and race/ethnicity. None of these covariates were significantly associated with ageat- menopause. Model 2 added an interaction term between race/ethnicity and LTL. In this model, significant differences were observed by race/ethnicity in the associations between LTL and age-at-menopause. For non-Hispanic white women, longer LTL was positively associated with age-at-menopause (b = 1.72, 95% CI: 0.04–3.39). For Mexican–American women, the linear combination of the coefficients for the main and interaction effects was calculated and indicated that longer LTL was negatively associated with age-at-menopause (b = −7.71, 95% CI: −11.49 to −3.93). For non- Hispanic black women, the linear combination of terms suggested that there was no association between LTL and age-at-menopause (b = −0.19, 95% CI: −3.56 to 3.18). The differences by race/ethnicity in the associations between LTL and age-at-menopause are presented in Fig. 1.
Table 2.
Model results examining LTL in relation to age-at-menopause, by race/ethnicity, among post-menopausal US women in NHANES, 1999–2002.
| Model 1; age; LTL and race/ethnicity | Model 2 race/ethnicity × LTL interaction | Model 3 fully adjusted | Sensitivity analysis: surgical/medical menopause | |||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Coefficient (95% CI) | P | Coefficient (95% CI) | P | Coefficient (95% CI) | P | Coefficient (95% CI) | P |
| Log-transformed LTL | 1.08 (−0.46 to 2.62) | 0.163 | 1.72 (0.04–3.39)* | 0.045 | 1.84 (0.31–3.52) | 0.026 | 1.22 (−1.87 to 4.31) | 0.427 |
| Race/ethnicity | ||||||||
| Non-Hispanic White | Reference | Reference | Reference | Reference | ||||
| Non-Hispanic Black | 0.10 (−0.98 to 1.17) | 0.855 | 0.10 (−1.03 to 1.07) | 0.970 | 0.25 (−1.07 to 1.56) | 0.729 | 0.10 (−1.12 to 1.32) | 0.868 |
| Mexican-American | −1.01 (−2.05 to 0.04) | 0.059 | −1.63 (−2.57 to −0.69)*** | 0.001 | −1.14 (−2.54 to −0.26) | 0.066 | −0.05 (−1.14 to 1.04) | 0.929 |
| LTL × race/ethnicity | ||||||||
| Non-Hispanic white | Reference | Reference | Reference | |||||
| Non-Hispanic black | – | −1.92 (−5.16 to 1.31) | 0.233 | 0.65 (−4.57 to 5.87) | 0.829 | −1.97 (−9.33 to 5.38) | 0.588 | |
| Mexican–American | – | −7.79 (−11.52 to −4.07)*** | <0.001 | −7.80 (−12.03 to −3.56)*** | 0.001 | −2.79 (−9.30 to 3.76) | 0.393 | |
| Age | 0.03 (−0.02 to 0.08) | 0.258 | 0.03 (−0.02 to 0.08) 0.260 | 0.08 (0.02–0.14)** | 0.014 | 0.25 (0.20–0.30)*** | <0.001 | |
| Income-to-poverty ratio | – | – | 0.36 (0.57–−0.65)* | 0.017 | 0.31 (−0.12 to 0.74) | 0.153 | ||
| Marital status | ||||||||
| Married/cohabitating | Reference | Reference | ||||||
| Single/never married | – | – | −0.99 (−2.04 to 0.07) | 0.080 | −1.03 (−2.45 to 0.40) | 0.152 | ||
| Divorced/separated/widowed | – | – | −0.92 (−3.28 to 1.45) | 0.579 | 1.36 (−1.78 to 4.50) | 0.383 | ||
| Body mass index | – | – | 0.09 (0.02–0.16)* | 0.012 | 0.02 (−0.10 to 0.13) | 0.767 | ||
| Nulliparous | – | – | −1.31 (−2.91 to 0.30)* | 0.038 | −1.64 (−4.36 to 1.07) | 0.226 | ||
| Smoking | ||||||||
| Never | Reference | Rreference | ||||||
| Former | – | – | 0.21 (−0.91 to 1.32) | 0.600 | −0.22 (−1.65 to 1.20) | 0.752 | ||
| Current | – | – | −0.11 (−1.63 to 1.40) | 0.892 | −0.43 (−1.94 to 1.07) | 0.560 | ||
Note: statistically significant associations denoted by *p < 0.05,
p < 0.01,
p < 0.001.
Fig. 1.
Associations between LTL and age-at-menopause by race/ethnicity, estimated from fully adjusted models with interaction terms between race/ethnicity and LTL.
These patterns were robust to the inclusion of several additional socio-demographic characteristics and potential confounders (Model 3). In fully adjusted models, LTL was positively associated with age-at-menopause for non-Hispanic white women (b = 1.84, 95% CI: 0.23–3.44), while there was a negative association for Mexican–American women (b = −7.17, 95% CI: −11.44 to −2.90), and no statistically significant association among non- Hispanic black women (b = 2.61, 95% CI: −2.43 to 7.64). Results were also the same for models controlling for a history of CVD or cancer, however those results are not shown as those two covariates were not associated with age-at-menopause.
Models examining the association between LTL and age-at-menopause among women with surgically or medically induced menopause (e.g., hysterectomy, oophorectomy, or other conditions or treatments leading to amenorrhea) were run as a falsification test, as there is no expectation that LTL would be related to age-at-menopause in this group. Indeed, there was no significant association between LTL and age-at-menopause for any racial/ethnic subgroup among women with surgical or medically induced menopause (Table 2).
In sensitivity analyses using the multiply imputed data for ageat- menopause, results were similar to the complete-case analysis presented above. The complete results of our sensitivity analyses are presented in Supplemental Table 1.
4. Discussion
We conducted the first study of the association between LTL and age-at-menopause among a nationally representative sample of postmenopausal US women. This study’s main finding is evidence of a strong interaction by race/ethnicity in the association between LTL and age-at-menopause. Among non-Hispanic white women, an increase of one standard deviation in LTL was associated with a 0.43 year higher reported age-at-menopause, independent of a variety of potential determinants of menopause. In contrast, among Mexican–American women an increase of one standard deviation in LTL was associated with a 1.56 year earlier menopause. While non-Hispanic black women evinced a positive association between LTL and age-at-menopause, similar to that of white women, this association was not statistically significant. We attribute this to inadequate statistical power due to the small sample size of postmenopausal non-Hispanic black women and the relatively limited variation in LTL among this sample.
To better understand our paradoxical findings among Mexican–American women, we considered confounding by factors more prominent in this population that presumably includes a larger proportion of immigrants than the two other populations we have examined. However, inclusion of variables related to immigration status (e.g., whether the participant was born in the US) did not change our findings. Confounding as an explanation for this finding is further discounted (but not completely negated) by the fact that the negative association between LTL and age-at-menopause does not appear among Mexican–American women with surgical/medical menopause.
We also considered that some Mexican–American women may have responded to items on the reproductive history questionnaire such that they were included in our analytic sample, but were not truly postmenopausal (i.e., they may have had oligomenorrhea/amenorrhea but did not report it as such). However, results did not change in models that included only women whose predominant language was English (data not shown). It is possible that the language of interview may have influenced the responses, but that item was not available in the public use data file, and presumably reported predominant language would be highly related to language of interview. Moreover, the percentage of our analytic sample identifying as Mexican–American was similar to that of all women 40 years and older in NHANES (3.5% in our analytic sample vs. 4.3% overall). If pre-menopausal Mexican–American women were inadvertently included in our analytic sample, it might be expected that the proportion of Mexican–American women in our analytic sample would be higher than for the overall NHANES sample of women over 40, but this was not the case.
Our findings of a positive association between LTL and age-at-menopause among white women is consistent with earlier reports [15–17] and is supportive of the view that LTL is a predictor of reproductive aging among this subpopulation. However, this notion is negated by evidence of a negative association among Mexican-American women. Gray et al. [17] also reported distinct associations between age-at-menopause and LTL by race and ethnicity. Among their sample, longer LTL predicted later age-at-menopause among white women but earlier age-at-menopause among nonwhite women. Gray et al. attributed their findings to chance or potential selection bias. However, there is an accumulating body of paradoxical findings related to LTL. In particular, reports of longer LTL among historically disadvantaged populations have been made with some regularity [14,20,21] but not without reports to the contrary [23,24]. Our findings add to the body of paradoxical findings related to LTL.
Our findings are best considered in light of the study’s strengths and weaknesses. Strengths of this study include it being the first study conducted among a large, multi-ethnic, sample of women representative of non-institutionalized US population. We have controlled for a larger number of covariates than has been possible to date and conducted sensitivity and post-hoc analyses to confirm our paradoxical findings. Our study’s weakness, emanating from its cross-sectional design, is the time span between mean age-at-menopause (49 years) and mean age at which blood samples were drawn (63 years). While a reasonable approach might be to limit our analyses to a sample of women who were interviewed within 10 years after menopause, we lack statistical power to do so. However, we have addressed this issue by including age at interview, which was when blood was drawn, as a covariate in the regression models. Furthermore, because LTL is at least moderately heritable, we reason that LTL measured after menopause is likely a useful marker of LTL prior to menopause [17]. It is also noteworthy that observed telomere length can vary across extraction methods and types of assays. However, our results are comparable to results from other studies using NHANES data and are likely to be similar to other PCR-derived measures of telomere length if they used the same methods outlined here and detailed elsewhere [27].
In sum, results presented here did not support the hypothesis that shorter LTL is a predictor of earlier age-at-menopause, as the magnitude and direction of the associations between LTL and age-at-menopause varied across racial/ethnic groups. Consequently, our findings of null or inverse associations for some racial/ethnic subgroups are also inconsistent with the notion that LTL is a biologic determinant of reproductive aging.
It has been noted that introduction of molecular data to epidemiologic studies has added, rather than reduced, complexity in the analysis of the intertwined effects of social history and biologic factors on health [32]. Various subpopulations often occupy different positions within the social hierarchy with concomitant distinctions, such as chronicity of exposure to stress, which have differential effects on health, even at the cellular level [33]. In the context of reports of longer LTL among African Americans or inverse associations between age-at-menopause and LTL among Mexican–Americans, as was illustrated in this analysis, it becomes particularly difficult to disentangle innate biologic differences from differential social exposures throughout the life course. Further longitudinal analyses may help clarify the trajectories of biologic differences and the consequences of social exposures across racial/ethnic subpopulations.
Supplementary Material
Acknowledgments
Funding
The authors have received no funding for this article.
Appendix
Multiple imputation methods and results
To examine and mitigate the impact of missing data, age-at-menopause was multiply imputed (with values constrained to fall between 30 and 60 years, or current attained age if younger than 60) for the sample of 1070 potentially postmenopausal women with missing age-at-menopause data. The multiple imputation models used truncated regression to predict age-at-menopause based on current age, race/ethnicity and estimated the imputed values separately for the subsample of women reporting surgical or medical menopause (i.e., a history of hysterectomy, oophorectomy, or other medical conditions or treatments leading to oligomenorrhea/ amenorrhea).
Sensitivity analyses were conducted using the 10 multiply imputed data sets. Models presented in the main paper were replicated using the multiply imputed data sets for age-at-menopause for the sample of 1070 post-menopausal women (and 844 women with medical/surgical menopause) to mitigate the impact of missing data. Models using the 10 multiply imputed data sets were fit using the mi commands in Stata 12.1 SE as well as the survey procedures.
Results for models using the multiply imputed data sets were largely similar to those using the complete-case analysis. Although the association between LTL and age-at-menopause was no longer statistically significant among non-Hispanic white women (b = 1.42; 95% CI: −0.18 to 3.01, p = 0.079) in the fully adjusted multiply imputed model, the association was in the same direction and of generally similar magnitude compared to the complete-case analysis. Additionally, this association remained significant in the multiply imputed model with only the race/ethnicity by LTL interaction term. All other patterns remained the same as in the main analysis. There was a statistically significant interaction between race/ethnicity and LTL, such that Mexican–American women were different than non-Hispanic white women (p = 0.008); the linear combination of terms from this model suggested that the association between LTL and age-at-menopause among Mexican–American women remained negative and significant (b = −5.54; 95% CI: −10.04 to −1.04, p = 0.018). Similar to findings from the main analysis, the linear combination of terms from the model suggested that there was no significant association between LTL and age-at-menopause among non-Hispanic black women (b = 1.78; 95% CI: −2.15 to 5.71, p = 0.360). Finally, there were no associations between LTL and age-at-menopause among any racial/ethnic group in the falsification test models among women with surgical/medical menopause using the multiply imputed data (n = 844).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the National Center for Health Statistics, Centers for Disease Control and Prevention.
Author contribution
Edmond D. Shenassa conceived the study, wrote introduction and conclusion sections, contributed to the development of the analytic plan and writing of results and methods sections.
Lauren M. Rossen developed the analytic plan, wrote the methods and results sections, contributed to the writing of introduction and discussion sections.
Ethics
We analyzed publicly available de-identified data. IRB approval was not required for this work.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.maturitas.2015.07.009
References
- 1.Sievert LL. Menopause as a measure of population health: an overview. Am. J. Hum. Biol. 2001;13:429–433. doi: 10.1002/ajhb.1075. [DOI] [PubMed] [Google Scholar]
- 2.Sowers MF. The menopause transition and the aging process: a population perspective. [accessed 11.01.15];Aging (Milano) 2000 12(2):85–92. doi: 10.1007/BF03339895. http://www.ncbi.nlm.nih.gov/pubmed/10902050. [DOI] [PubMed] [Google Scholar]
- 3.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65(2):161–166. doi: 10.1016/j.maturitas.2009.08.003. http://dx.doi.org/10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okeke T, Anyaehie U, Ezenyeaku C. Premature menopause. Ann. Med. Health Sci. Res. 2013;3(1):90–95. doi: 10.4103/2141-9248.109458. http://dx.doi.org/10.4103/2141-9248.109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand JS, Van der Schouw YT, Onland-Moret NC. Age at menopause, reproductive life span, and type 2 diabetes risk. Diabetes Care. 2013;36:1012–1019. doi: 10.2337/dc12-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke the framingham heart study. Stroke. 2009;40:1044–1049. doi: 10.1161/STROKEAHA.108.542993. http://dx.doi.org/10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parashar S, Reid KJ, Spertus JA, Shaw LJ, Vaccarino V. Early menopause predicts angina after myocardial infarction. Menopause. 2010;17:938–945. doi: 10.1097/gme.0b013e3181e41f54. http://dx.doi.org/10.1097/gme.0b013e3181e41f54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snowdon DA, Kane RL, Beeson WL, et al. Is early natural menopause a biologic marker of health and aging. Am. J. Public Health. 1989;79(6):709–714. doi: 10.2105/ajph.79.6.709. http://dx.doi.org/10.2105/AJPH.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann. Epidemiol. 1998;8:229–235. doi: 10.1016/s1047-2797(97)00207-x. [DOI] [PubMed] [Google Scholar]
- 10.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am. J. Epidemiol. 2005;162:1089–1097. doi: 10.1093/aje/kwi324. http://dx.doi.org/10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen BK, Heuch I, Kvåle G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am. J. Epidemiol. 2003;157:923–929. doi: 10.1093/aje/kwg066. http://dx.doi.org/10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Rosenberg L, Wise LA, Boggs DA, Lavalley M, Palmer JR. Age at natural menopause in relation to all-cause and cause-specific mortality in a follow-up study of US black women. Maturitas. 2013;75:246–252. doi: 10.1016/j.maturitas.2013.04.003. http://dx.doi.org/10.1016/j.maturitas.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aubert G, Lansdorp PM. Telomeres and aging. Physiol. Rev. 2008;88(2):557–579. doi: 10.1152/physrev.00026.2007. http://dx.doi.org/10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 14.Needham BL, Adler N, Gregorich S, et al. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc. Sci. Med. 2013;85:1–8. doi: 10.1016/j.socscimed.2013.02.023. http://dx.doi.org/10.1016/j.socscimed.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aydos SE, Elhan AH, Tükün A. Is telomere length one of the determinants of reproductive life span. Arch. Gynecol. Obstet. 2005;272(2):113–116. doi: 10.1007/s00404-004-0690-2. http://dx.doi.org/10.1007/s00404-004-0690-2. [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Kroenke CH, Epel E. Greater endogenous estrogen exposure is associated with longer telomeres in postmenopausal women at risk for cognitive decline. Brain Res. 2011 doi: 10.1016/j.brainres.2010.10.033. http://dx.doi.org/10.1016/j.brainres.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray KE, Schiff MA, Fitzpatrick AL, Kimura M, Aviv A, Starra JR. Leukocyte telomere length and age-at-menopause. Epidemiology. 2014;25:139–146. doi: 10.1097/EDE.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Vivo I, Prescott J, Wong JYY, Kraft P, Hankinson SE, Hunter DJ. A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 2009;18(4):1152–1156. doi: 10.1158/1055-9965.EPI-08-0998. http://dx.doi.org/10.1158/1055-9965.EPI-08-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prescott J, McGrath M, Lee I-M, Buring JE, De Vivo I. Telomere length and genetic analyses in population-based studies of endometrial cancer risk. Cancer. 2010;116(18):4275–4282. doi: 10.1002/cncr.25328. http://dx.doi.org/10.1002/cncr.25328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aviv A, Chen W, Gardner JP, et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am. J. Epidemiol. 2009;169(3):323–329. doi: 10.1093/aje/kwn338. http://dx.doi.org/10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu H, Wang X, Gutin B, et al. Leukocyte telomere length in healthy Caucasian and African-American adolescents: relationships with race, sex, adiposity, adipokines, and physical activity. J. Pediatr. 2011;158(2):215–220. doi: 10.1016/j.jpeds.2010.08.007. http://dx.doi.org/10.1016/j.jpeds.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt SC, Chen W, Gardner JP, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7(4):451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diez Roux A, Ranjit N, Jenny N, Shea S, Cushman M, Fitzpatrick A. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell. 2009;8(3):251–257. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz Do TDUS. Black women experience stress-related accelerated biological aging? A novel theory and first population-based test of black-white differences in telomere length. Hum. Nat. 2010;21(1):19–38. doi: 10.1007/s12110-010-9078-0. http://dx.doi.org/10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics, Centers for disease control and prevention. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2:161. [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. National Health and Nutrition Examination Survey: NHANES response rates and CPS totals. http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm. [Google Scholar]
- 27.Centers for Disease Control and Prevention. Telomere Mean and Standard Deviation (Surplus). Natl Heal Nutr Exam Surv 1999–2000 Data Doc Codebook. [accessed 29.06.15];Freq. 2015 http://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/TELO_A.htm.
- 28.Parente RC, Faerstein E, Celeste RK, Werneck GL. The relationship between smoking and age at the menopause: a systematic review. Maturitas. 2008;61(4):287–298. doi: 10.1016/j.maturitas.2008.09.021. http://dx.doi.org/10.1016/j.maturitas.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Strandberg TE, Saijonmaa O, Tilvis RS, et al. Association of telomere length in older men with mortality and midlife body mass index and smoking. J. Gerontol. A: Biol. Sci. Med. Sci. 2011;66(7):815–820. doi: 10.1093/gerona/glr064. http://dx.doi.org/10.1093/gerona/glr064. [DOI] [PubMed] [Google Scholar]
- 30.Hardy R, Mishra GD, Kuh D. Body mass index trajectories and age-at-menopause in a British birth cohort. Maturitas. 2008;59:304–314. doi: 10.1016/j.maturitas.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am. J. Epidemiol. 2013;178(1):70–83. doi: 10.1093/aje/kws421. http://dx.doi.org/10.1093/aje/kws421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman JS, Cooper RS. Telomeres and race: what can we learn about human biology from health differentials. Aging Cell. 2008;7:448–450. doi: 10.1111/j.1474-9726.2008.00396.x. [DOI] [PubMed] [Google Scholar]
- 33.Shenassa ED. Society, physical health and modern epidemiology. Epidemiology. 2001;12(4):467–470. doi: 10.1097/00001648-200107000-00018. http://dx.doi.org/10.1097/00001648-200107000-00018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.