Abstract
Purpose
Progesterone receptors are expressed in approximately 70% of meningiomas. Mifepristone is an oral antiprogestational agent reported to have modest activity in a phase II study. This multicenter, prospective, randomized, placebo-controlled phase III trial conducted by SWOG was planned to define the role of mifepristone in the treatment of unresectable meningioma.
Patients and Methods
Eligible patients were randomly assigned to receive either mifepristone or placebo for 2 years unless disease progressed. Patients who were stable or responding to protocol therapy after 2 years had the option to continue with the same blinded therapy. Serial follow-up allowed assessment of efficacy and toxicity. Time to treatment failure and overall survival were ascertained for all randomly assigned patients. On progression, patients receiving placebo could cross over and receive active drug.
Results
Among 164 eligible patients, 80 were randomly assigned to mifepristone and 84 to placebo. Twenty-four patients (30%) were able to complete 2 years of mifepristone without disease progression, adverse effects, or other reasons for discontinuation. Twenty-eight patients (33%) in the placebo arm completed the 2-year study. There was no statistical difference between the arms in terms of failure-free or overall survival.
Conclusion
Long-term administration of mifepristone was well tolerated but had no impact on patients with unresectable meningioma.
INTRODUCTION
Meningiomas are the most common of all neurologic tumors, accounting for 35.8% of all brain tumors and representing 53.8% of nonmalignant tumors.1 Approximately 70% to 80% of meningiomas are benign (WHO grade 1), 20% to 30% are atypical or borderline (grade 2), and 1% to 2% are malignant (grade 3).2 For the latter, 2-, 5-, and 10-year survival rates are 76%, 65.4%, and 57.2%, respectively.1 Approach to the majority of patient cases is observation, but a small subset can involve invasive or symptomatic disease. These patients are managed by surgical resection or radiosurgery; there are no systemic curative therapies.3 Symptomatic or recurrent meningiomas that are not operable may be lethal. There is epidemiologic evidence of association between meningioma, pregnancy, and breast cancer, suggesting hormonal regulation of tumor growth.4,5
Progesterone receptors are expressed in approximately 70% of meningiomas.4 Although the nature and function of these receptors might be different than those expressed in breast cancer,6 the presence of progesterone receptors might provide a potential therapeutic target for growth inhibition of meningiomas. This concept is supported by pilot studies of effective meningioma inhibition by blockade of the progesterone receptor7,8
Mifepristone (17β-hydroxy-11β-[4-dimethylaminophenyl]-17α-[prop-l-ynyl]estra-4,9-dien-3-one; also called RU 486) is a synthetic competitive inhibitor of the progesterone receptor and, to a lesser degree, of the glucocorticoid receptor.9 The mechanism of action of mifepristone leads to irreversible inhibition of the transcriptional activity of the progesterone receptor complex, through an alteration of its conformation causing modifications of DNA signaling through promoter interference, at concentrations much lower than progesterone.10 Mifepristone was originally developed as an abortifacient and has also been investigated in treatment of Cushing's syndrome,11–13 endometriosis,14,15 endometrial cancer,16 uterine leiomyoma,15,17 breast cancer,18,19 and depression.20 Good tolerability and feasibility of long-term use were reported in a recent phase II study of patients with unresectable meningioma, with eight of 28 patients achieving minor remission.21
We report the long-term results of a multicenter, prospective, randomized, placebo-controlled phase III trial conducted by SWOG to determine the role of mifepristone in treating unresectable growing meningioma. The objective of this study was to compare daily oral mifepristone versus placebo with respect to failure-free survival (FFS) in patients with unresectable meningioma. Secondary objectives were to assess overall survival (OS) and tolerance of long-term use of mifepristone.
PATIENTS AND METHODS
Patient Eligibility
Patients age ≥ 18 years with histologically confirmed primary, recurrent, or residual unresectable meningioma were eligible if they had measurable or evaluable disease by computed tomography or magnetic resonance imaging, received radiotherapy for the disease at least 1 year before study enrollment (unless radiotherapy was either inappropriate because of tumor location or declined by patient), documented evidence of disease recurrence or progression within 2 years of random assignment, and performance status of 0 to 2. Adequate hematologic, renal, and hepatic functions were required. Patients were ineligible if they had adrenal insufficiency requiring corticosteroid replacement therapy, known allergy to mifepristone, any additive or ablative modulation of sex hormones or glucocorticoid pathway (excluding stable corticosteroids for cerebral edema), received prior cytotoxic chemotherapy or prior mifepristone for meningioma, other prior or concurrent malignancy within the preceding 5 years (except for surgically treated squamous or basal cell skin cancer or cervical cancer in situ), or meningiomatosis or malignant meningioma. Patients agreed to use a nonhormonal contraceptive method or abstinence during and for 3 months after study therapy. Women who were pregnant or lactating were excluded. Tissue blocks were requested to be submitted if available for assessment of estrogen and progesterone receptors.
The study was approved by the institutional review boards of participating SWOG member sites, and all patients provided informed written consent in accordance with institutional and federal guidelines. The study followed ethical guidelines for placebo-controlled trials, as described in the Declaration of Helsinki. The study was registered with Clinical Trials.gov.
Treatment Plan
Patients were randomly assigned to either oral mifepristone 200 mg daily or placebo for 2 years (Fig 1). The dose of 200 mg was chosen for its antiprogesterone activity and its minimal antiglucocorticoid activity.22 Patients carried a warning card to alert medical personnel that the investigational treatment could cause subclinical adrenal insufficiency and to recommend administration of exogenous glucocorticoids in case of emergency. After 2 years, patients with stable or responding disease had the option to continue with the same blinded therapy without breaking the randomization code. The randomization code was broken on disease progression. Patients who experienced progression with placebo had the option to cross over and begin daily open-label mifepristone at 200 mg. When disease progressed during mifepristone, patients were taken off study treatment and observed for survival.
Fig 1.
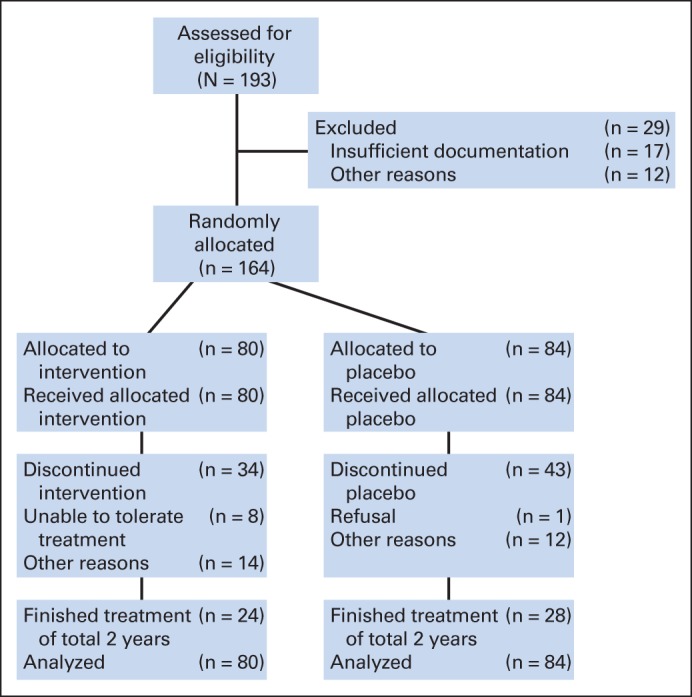
CONSORT diagram of patients participating in this trial.
Patient Follow-Up and Evaluation
Patients were assessed monthly with complete physical, gynecologic, and neurologic examinations during the first year and every 3 months during the next year. All eligible patients were assessed for toxicities using National Cancer Institute Common Toxicity Criteria (version 2.0). Hematologic, renal, and hepatic functions were tested every 3 months and thyroid and adrenal functions every 6 months. Tumor restaging, using the same technique as at baseline, and automated visual field examination (if vision abnormalities were noted at baseline) were repeated every 6 months. Pill count was performed at each visit. Patients who continued therapy past 2 years were observed on the same schedule, with imaging performed annually.
Criteria for Response
Complete response (CR) was defined as complete disappearance of tumor on computed tomography or magnetic resonance imaging scan. Partial response (PR) was defined as ≥ 50% reduction of the sum of the products of the perpendicular diameters of measurable lesions with no significant neurologic deterioration. For CR or PR, response was confirmed with subsequent examination 4 weeks after first documentation of response. Progressive disease was defined as one of the following: > 25% increase or an increase of 10 cm2 (whichever was smaller) in the sum of products of perpendicular diameters of measurable lesions over the smallest sum observed, reappearance of any lesion that had disappeared, clear worsening of any evaluable disease, or significant neurologic deterioration. Stable disease was defined as anything else.
Statistical Considerations
At the time of random assignment, patients were stratified by sex and menopausal status (male v premenopausal female v postmenopausal female), prior radiotherapy or none, and disease status (documented progressive or recurrent disease v new diagnosis).
The primary end point of this study was FFS. OS was also recorded with additional follow-up. The study design called for a 4-year accrual period and 2-year follow-up. Assuming 200 eligible patients and median FFS in the placebo group of 12 months, the study had 83% power to detect a 50% improvement (hazard ratio [HR], 1.5) in favor of mifepristone at the 0.045 level. One formal interim analysis was planned after 75% of accrual was completed, testing superiority of mifepristone at the 0.01 level (overall level for study, 0.05), with consideration for stopping early in the case of positive results.
OS was defined as the date from registration until date of death resulting from any cause. Patients still known to be alive at the time of analysis were censored at the last known date of contact. FFS was defined as the date from registration to the first date of documented progression, significant neurologic deterioration, discontinuation of treatment for any reason, or death resulting from any cause. Patients still known to be alive without treatment failure were considered to be censored at their last follow-up time. OS and FFS estimates and curves were generated using the Kaplan-Meier method. Comparisons between arms for OS and FFS were analyzed using the Cox proportional hazards model, adjusting for the variables used in stratification at time of random assignment.
RESULTS
Patient Characteristics
A total of 193 patients were enrolled between 1992 and 1998. In April 1996, the formal interim analysis was presented to the data and safety monitoring committee, and the trial continued. Of 193 patients, 29 were not eligible (Fig 1) and were excluded from these analyses. Characteristics of eligible patients are listed in Table 1. Treatment and placebo arms were well balanced with regard to baseline characteristics: age, sex, race, menopausal status, prior radiotherapy, histology, and disease status. A majority of patients were female and postmenopausal. Progesterone receptor status was determined for 85 eligible patients based on submitted specimens assessed centrally. Of these, 88% had overexpression of the progesterone receptor and 5% had overexpression of the estrogen receptor by immunohistochemistry.
Table 1.
Patient Demographic and Clinical Characteristics (N = 164)
| Characteristic | Mifepristone (n = 80) | Placebo (n = 84) | P |
|---|---|---|---|
| Age, years | .27 | ||
| Median | 60.6 | 53.2 | |
| Range | 30.7-79.6 | 20.6-87.1 | |
| Sex | .89 | ||
| Male | 23 | 25 | |
| Female | 57 | 59 | |
| Menopausal status | .36 | ||
| Premenopausal | 14 | 19 | |
| Postmenopausal | 43 | 40 | |
| Race | .90 | ||
| White | 68 | 68 | |
| Black | 10 | 14 | |
| Asian | 1 | 1 | |
| Unknown | 1 | 1 | |
| Prior radiotherapy | .75 | ||
| Yes | 22 | 25 | |
| No | 58 | 59 | |
| Disease status | .54 | ||
| Progressive or recurrent | 65 | 65 | |
| Newly diagnosed | 15 | 19 | |
| Baseline disease status | .78 | ||
| Measurable | 55 | 56 | |
| Nonmeasurable | 25 | 28 | |
| Histology | .88 | ||
| Atypical meningioma | 8 | 9 | |
| Meningioma NOS | 72 | 75 | |
| Progesterone status | 42 | 43 | .48 |
| Positive | 36 | 39 | |
| Negative | 6 | 4 | |
| No/insufficient sample | 38 | 41 |
Abbreviation: NOS, not otherwise specified.
Clinical Outcome
Eighty eligible patients were assigned to the mifepristone arm and 84 to the placebo arm. Twenty-four patients assigned to mifepristone (30%) received at least 2 years of treatment on study. Thirty-four patients discontinued treatment for disease progression or relapse, eight for intolerable adverse effects, and 14 for various reasons (Table 2). Seventy-one patients receiving mifepristone were assessable for response. One patient (1.4%) had a confirmed PR. One additional patient receiving mifepristone had a shrinkage that was not confirmed with a second assessment. Forty-four patients receiving mifepristone (55%) had stable disease and 25 (31%) had increasing disease as best response. Of the 84 patients assigned to the placebo arm, 28 (33%) received at least 2 years of protocol treatment as planned. Forty-three patients discontinued protocol therapy because of disease progression or death, and 12 were taken off study for various reasons (Table 2). Of 73 assessable patients, one patient (1%) had a confirmed PR, 44 (52%) had stable disease, and 28 (33%) had progressive disease.
Table 2.
Treatment Summary
| Treatment | Total (N = 164) | Mifepristone (n = 80) | Placebo (n = 84) |
|---|---|---|---|
| Treatment completed as planned at 2 years | 52 | 24 | 28 |
| Reason for stopping treatment | |||
| Adverse events | 8 | 8 | 0 |
| Refusal (unrelated to adverse events) | 1 | 0 | 1 |
| Progression, relapse, or death | 77 | 34 | 43 |
| Other (not protocol specified) | 26 | 14 | 12 |
| Total | 112 | 56 | 56 |
Median FFS for the placebo arm was 11 months (95% CI, 6 to 18 months); for the mifepristone arm, it was 10 months (95% CI, 7 to 13 months). There was no evidence of superior FFS in the mifepristone arm (two-sided P = .90 [adjusted for sex/menopausal status, prior radiotherapy, or disease status]). FFS was nearly the same in the placebo arm, with an estimated mifepristone to placebo HR of 1.02 (95% CI, 0.72 to 1.48; Fig 2). OS was also not significantly better in the mifepristone arm (estimated mifepristone to placebo HR, 1.05; 95% CI, 0.69 to 1.59; two-sided P = .84 [adjusted for the same stratification factors]; Fig 3).
Fig 2.
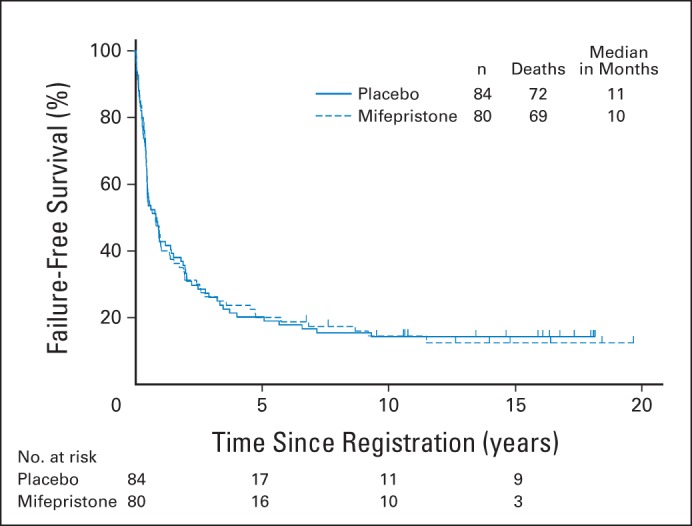
Failure-free survival (FFS) was analyzed using Kaplan-Meier methodology to compare intervention versus placebo. Median FFS: placebo, 11 months (95% CI, 6 to 18 months); mifepristone, 10 months (95% CI, 7 to 13 months).
Fig 3.
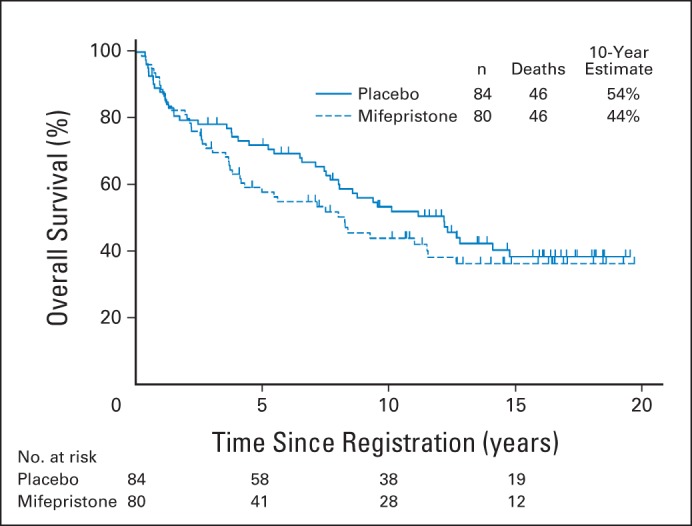
Overall survival (OS) was analyzed using Kaplan-Meier methodology to compare intervention versus placebo. Median OS: placebo, 12 years; mifepristone, 8 years.
Fifty patients who experienced progression with placebo registered for the cross-over phase of the trial. Forty-one patients were eligible and started treatment. Of the 41 patients, six completed the study as planned; 15 patients stopped open-label mifepristone because of disease progression, and no responses were noted.
Safety Profile
Most common adverse events are listed in Table 3. There were more adverse effects with mifepristone; most were mild (Table 3; Appendix Table A1, online only). In the placebo arm, 24 patients (29%) reported grade 3 adverse events, and one patient (1%) reported grade 4. With mifepristone, 31 (39%) and six patients (8%) experienced grade 3 or 4 adverse events (χ2 P = .03), respectively. There were no grade 5 events related to treatment. Thirty-six of the 41 patients who crossed over were assessed for toxicities; 11 (31%) and two (6%) experienced grade 3 or 4 adverse events with mifepristone, respectively.
Table 3.
Major Adverse Events
| Adverse Event | No. (%) |
|
|---|---|---|
| Mifepristone (n = 80) | Placebo (n = 84) | |
| Grade 3 | 31 (39) | 24 (29) |
| Grade 4 | 6 (8) | 1 (6) |
| Neurologic* | ||
| Headache | 36 (45) | 35 (42) |
| Weakness (motor neuropathy) | 23 (29) | 14 (17) |
| Dizziness | 23 (29) | 20 (24) |
| Ataxia | 21 (26) | 17 (20) |
| Mood or consciousness change | 19 (24) | 16 (19) |
| Pain | 16 (20) | 11 (13) |
| Other* | ||
| Fatigue | 60 (75) | 46 (55) |
| Hot flashes | 31 (39) | 22 (26) |
| Nausea | 25 (31) | 21 (25) |
| Alopecia | 22 (28) | 9 (11) |
| Menses change | 14 (18) | 12 (14) |
| Gynecomastia | 13 (16) | 9 (11) |
Grade 1 to 4 in ≥ 15% of patients.
Grade 3 and 4 events likely related to mifepristone (and not seen in patients receiving placebo) included infection (n = 4), cardiac ischemia (n = 3), thrombosis (n = 2), and epistaxis (n = 1). Endocrine-related events were mildly increased with mifepristone and consisted of alopecia, gynecomastia, hot flashes, and vaginal bleeding. Nausea, vomiting, and fatigue were also reported more commonly in patients receiving mifepristone.
DISCUSSION
The diagnosis of meningioma is usually incidental or results from a constellation of neurologic symptoms. Several studies have reported the natural history of meningioma.23–27 In the largest study of 273 tumors in 244 patients managed conservatively, linear growth was seen in 44% of the tumors and volumetric growth seen in 73% of the patient cases within 4 years.25 Patient characteristics in our study were comparable to Central Brain Tumor Registry of the United States (CBTRUS) data: higher female to male ratio, advanced age, and majority white. The CBTRUS is the largest database collected from the SEER program and the National Program of Cancer Registries.28,29
This randomized study was an attempt to use the biologic evidence of a hormonal environment to treat meningioma. Epidemiologic and observational studies have suggested a role for hormonal modulation in the development and progression of meningioma.4,30–33 A previous phase II study suggested good tolerability and modest clinical improvement21 and served as the basis for this prospective, randomized phase III trial. In the phase II study of 28 patients with unresectable meningioma,21 eight patients achieved minor responses, with maximal reduction in tumor area of 10%. Most responders were men or premenopausal women. The results of our randomized trial failed to confirm the efficacy of progesterone modulation by oral mifepristone in stabilizing unresectable meningioma.
The strenghts of our study are the prospective randomized design and multiple participating institutions. It is also the only phase III trial to our knowledge to have been conducted in this patient population. Progesterone receptor status at study entry did not predict for response, because most meningiomas express the progesterone receptor, and patients treated with the progesterone antagonist mifepristone did not fare better than patients receiving placebo. Progesterone receptors are expressed predominantly in benign meningiomas with low proliferation indices.34 Eligibility for our phase III trial required progressive or refractory tumors, which are most likely associated with higher grade. Although the lack of efficacy could have been the result of the loss of progesterone receptor expression in aggressive meningiomas with increased proliferation index and histologic grade,35 our data do not support the hypothesis, because 88% of patients had progesterone receptor expression in the meningioma. There are at least three isoforms of progesterone receptor in meningioma.6 Progesterone anticancer activity in hormonally sensitive tumors usually occurs through downregulation of the estrogen receptor, which is present in < 5% of meningiomas. The progesterone receptor signaling pathway in meningioma does not seem functional, whether related to a lack of estrogen receptor expression, a biologically different progesterone receptor isoform ratio, or an alternative signaling pathway that does not interfere with cell growth. Thus, it is not effectively inhibited by a competitive antagonist,36 and hormonal modulation may not be the driving force of meningioma progression.
Many small studies of systemic biologic manipulation have recently been performed, rarely on solid mechanistic ground. Most molecules might seem to stabilize disease, but none of them provide survival benefits (Table 4). A few studies have demonstrated that vascular endothelial growth factor A is secreted by meningiomas.43 Its expression is associated with meningioma vascularity, causing increased tumor size, peritumoral brain parenchyma vascularization, and vascular permeability.43–46 The use of bevacizumab has been tested in clinical studies for the treatment of meningioma, with disappointing results (Table 4).41,42,47
Table 4.
Summary of Current Clinical Trials (targeted therapy) for Meningioma
| Drug | Mechanism | Total No. of patients | Meningioma Grade | No. of Patients | PFS |
Median OS (months) | ORR (%) | |
|---|---|---|---|---|---|---|---|---|
| Median (months) | 6 Months (%) | |||||||
| Imatinib37 | PDGFR inhibitor | 22 | I | 12 | 3 | 45 | — | SD, 47; no CR or PR |
| II/III | 10 | 2 | 0 | — | ||||
| Erlotinib or gefitinib38 | EGFR inhibitor | 25 | I | 8 | 2.25 | 25 | 13 | SD, 32; no objective responses |
| II/III | 17 | 4 | 29 | 33 | ||||
| Imatinib plus hydroxyurea39 | PDGFR-positive cytotoxic | 21 | I | 8 | 13.9 | 87.5 | 66.0 | SD, 38 |
| II/III | 13 | 5.3 | 46.2 | 20.9 | ||||
| Sunitinib40 | TKI | 36 | II/III | 36 | 5.2 | 42 | 24.6 | — |
| Bevacizumab ± chemotherapy41,42 | VEGF-A inhibitor | 29 | I | 5 | 12.2 | 80 | — | SD, 79 |
| II/III | 24 | 15.8-26 | 43.8-87.5 | — | ||||
Abbreviations: CR, complete response; EGFR, epidermal growth factor receptor; ORR, overall response rate; OS, overall survival; PDGFR, platelet-derived growth factor receptor; PFS, progression-free survival; PR, partial response; SD, stable disease; TKI, tyrosine kinase inhibitor; VEGF-A, vascular endothelial growth factor A.
A better understanding of the biologic mechanisms driving meningioma behavior should lead to evidence-based studies. Genomic profiles of meningiomas have recently been published. The tumor suppressor NF2 is known to be disrupted in approximately half of all meningiomas.48 Frequent mutations of protein kinase B (PKB, also called AKT1) and smoothened frizzled family receptor (SMO) were seen in patients who did not have NF2 mutations.49 Genomic sequencing of a larger set of 50 meningiomas confirmed these results and, in addition to AKT1 and SMO mutations, identified mutations in the tumor necrosis factor receptor associated factor 7 (TRAF7), a gene encoding a proapoptotic protein not previously implicated in cancer.50 Recently, a small-molecule inhibitor of the p21-activated kinase, a validated downstream effector of NF2, showed antitumor activity in NF2-associated schwannoma.51 One may speculate that this molecule may also have antitumor activity in NF2-associated meningioma. In NF2 wild-type patient cases, targeting the AKT/mammalian target of rapamycin– or SMO-associated hedgehog pathway is a good hypothesis.
In conclusion, the study was the first and only randomized phase III trial to our knowledge to investigate a systemic treatment for meningioma. Despite the presence of progesterone receptors in most meningiomas, mifepristone fails to control the disease. Future studies based on molecular and genetic characteristics will be important to define systemic therapies that have efficacy in treating recurrent, progressive meningioma.
Supplementary Material
Appendix
Accruing institutions for SWOG S9005 study: University of Mississippi Medical Center, University of Southern California School of Medicine, University of California at Los Angeles, Temple University, University of California at Davis, University of Arizona Medical Center, University of Arkansas for Medical Sciences, Loyola University Stritch School of Medicine, Northwest National Cancer Institute (NCI) Community Oncology Research Program (NCORP), Pacific Cancer Research Consortium NCORP, MD Anderson Cancer Center, Atlanta Regional Community Clinical Oncology Program (CCOP), Boston Medical Center, Kansas City NCORP, Oregon Health and Science University, University of Kentucky Medical Center, Columbia University, University of New Mexico, University of Oklahoma Health Science Center, University of Texas Southwestern Medical Center, University of Texas Health Science Center at San Antonio, University of South Alabama Cancer Center Minority-Based CCOP, University of Texas Medical Branch at Galveston, Upstate Carolina CCOP, Brooke Army Medical Center, Cleveland Clinic Foundation, Bay Area Tumor Institute, Cancer Research for the Ozarks NCORP, Kings County CCOP, Louisiana State University Medical Center, NCORP of the Carolinas (Greenville Health System), Santa Rosa Memorial Hospital Regional CCOP, Scott and White Clinic CCOP, St Louis–Cape Girardeau CCOP, Sutter Cancer Research Consortium, University of Colorado Cancer Center, University of Michigan Medical Center, University of Utah Medical Center, University of Vermont, Wayne State University Medical Center, Wichita NCI Community Oncology Research, Henry Ford Hospital, and Eastern Cooperative Oncology Group/American College of Radiology Imaging Network.
Table A1.
All Adverse Events
| Adverse Event | Mifepristone (n = 80) |
Placebo (n = 84) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade |
Grade |
|||||||||
| Unknown | ≤ 2 | 3 | 4 | 5 | Unknown | ≤ 2 | 3 | 4 | 5 | |
| Anorexia | 2 | 78 | 0 | 0 | 0 | 4 | 80 | 0 | 0 | 0 |
| Endocrine, other | 1 | 78 | 1 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Eye, other | 1 | 79 | 0 | 0 | 0 | 0 | 83 | 1 | 0 | 0 |
| Seizures | 1 | 78 | 1 | 0 | 0 | 0 | 83 | 1 | 0 | 0 |
| Abdominal pain or cramping | 0 | 79 | 1 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Alkaline phosphatase increase | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Alopecia | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Anemia | 0 | 79 | 1 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Anxiety or agitation | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Arthralgia | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Ataxia (incoordination) | 0 | 75 | 5 | 0 | 0 | 0 | 81 | 3 | 0 | 0 |
| Bilirubin increase | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Blurred vision | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Bone pain | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Cardiac ischemia or infarction | 0 | 78 | 0 | 2 | 0 | 1 | 83 | 0 | 0 | 0 |
| Confusion | 0 | 77 | 3 | 0 | 0 | 0 | 81 | 2 | 1 | 0 |
| Conjunctivitis | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Constipation or bowel obstruction | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Cough | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Creatinine increase | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Depression | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Diarrhea without colostomy | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Dizziness or light headedness | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Dizziness or vertigo, NOS | 0 | 78 | 1 | 1 | 0 | 1 | 80 | 3 | 0 | 0 |
| Double vision | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Dry eyes | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Dry skin | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Dyspepsia or heartburn | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Dyspnea | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Ear, other | 0 | 80 | 0 | 0 | 0 | 0 | 83 | 1 | 0 | 0 |
| Edema | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Epistaxis | 0 | 79 | 0 | 1 | 0 | 0 | 84 | 0 | 0 | 0 |
| Erythema, rash, eruption, or desquamation, NOS | 0 | 79 | 1 | 0 | 0 | 0 | 82 | 2 | 0 | 0 |
| Erythema multiforme or blistering | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Fatigue, malaise, or lethargy | 0 | 76 | 4 | 0 | 0 | 1 | 77 | 6 | 0 | 0 |
| Flu-like symptoms, other | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| GI, other | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Gastritis or ulcer, NOS | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Gynecomastia | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Headache | 0 | 78 | 2 | 0 | 0 | 0 | 77 | 7 | 0 | 0 |
| Hot flashes | 0 | 77 | 3 | 0 | 0 | 0 | 82 | 2 | 0 | 0 |
| Hyperglycemia | 0 | 79 | 1 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Hyperkalemia | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Hypertension | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Hyponatremia | 0 | 80 | 0 | 0 | 0 | 0 | 83 | 1 | 0 | 0 |
| Hypothyroidism | 0 | 80 | 0 | 0 | 0 | 1 | 83 | 0 | 0 | 0 |
| Incontinence | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Infection without grade 3 to 4 neutropenia | 0 | 78 | 2 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Inner ear, hearing loss | 0 | 77 | 3 | 0 | 0 | 0 | 83 | 1 | 0 | 0 |
| Insomnia | 0 | 79 | 1 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Leukopenia | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Libido loss | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Memory loss | 0 | 79 | 1 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Menses changes | 0 | 71 | 9 | 0 | 0 | 1 | 78 | 5 | 0 | 0 |
| Metabolic, other | 0 | 80 | 0 | 0 | 0 | 0 | 83 | 1 | 0 | 0 |
| Middle ear, hearing loss or otitis | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Mood or consciousness change, NOS | 0 | 78 | 1 | 1 | 0 | 0 | 82 | 2 | 0 | 0 |
| Muscle weakness (not neurologic) | 0 | 79 | 1 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Myalgia or arthralgia, NOS | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Nausea | 0 | 78 | 2 | 0 | 0 | 0 | 83 | 1 | 0 | 0 |
| Neurologic, other | 0 | 78 | 2 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Neutropenia or granulocytopenia | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Night blindness | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Nystagmus | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Pain, other | 0 | 80 | 0 | 0 | 0 | 0 | 83 | 1 | 0 | 0 |
| Personality or behavioral change | 0 | 79 | 1 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Pruritus | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Pyramidal tract dysfunction | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Rash or desquamation | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Rectal bleeding or hematochezia | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Respiratory infect without neutropenia | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| AST increase | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Sensory neuropathy | 0 | 79 | 1 | 0 | 0 | 0 | 82 | 2 | 0 | 0 |
| Skin, other | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Somnolence or consciousness loss | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Speech impairment | 0 | 78 | 2 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Sweating | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Taste disturbance | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Thrombosis or embolism | 0 | 79 | 0 | 1 | 0 | 0 | 84 | 0 | 0 | 0 |
| Toxicity of unknown category | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Tremor | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Urinary frequency or urgency | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Urinary retention | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Urinary tract infection without neutropenia | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Vaginal bleeding | 0 | 78 | 2 | 0 | 0 | 1 | 83 | 0 | 0 | 0 |
| Vision, NOS | 0 | 78 | 2 | 0 | 0 | 0 | 83 | 1 | 0 | 0 |
| Vision, flashing or floaters | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Vomiting | 0 | 79 | 1 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Weakness (motor neuropathy) | 0 | 76 | 4 | 0 | 0 | 0 | 80 | 4 | 0 | 0 |
| Weight gain | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Weight loss | 0 | 80 | 0 | 0 | 0 | 0 | 84 | 0 | 0 | 0 |
| Maximum-grade any adverse event | 0 | 43 | 31 | 6 | 0 | 0 | 59 | 24 | 1 | 0 |
Abbreviation: NOS, not otherwise specified.
Footnotes
Listen to the podcast by Dr Maher at www.jco.org.podcasts
Supported in part by Public Health Service Cooperative Agreement Grants No. CA180888, CA180819, CA180801, CA180818, CA180858, CA180830, CA180846, CA180834, CA180835, CA180820, CA02115, CA73590, CA20319, CA35431, CA46282, CA13612, CA35176, CA45560, CA35119, CA35192, CA45461, CA58882, and CA67663 from the National Cancer Institute.
Presented in part at the 37th Annual Meeting of the American Society of Clinical Oncology, San Francisco, CA, May 12-15, 2001.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Steven Grunberg
Administrative support: Steven Grunberg, Anne Schott
Provision of study materials or patients: Steven Grunberg, Andy E. Sherrod, Jamshid Ahmadi, Jeannette J. Townsend, Lynn G. Feun, Christy A. Russell, Fairooz F. Kabbinavar, Keith J. Stelzer
Collection and assembly of data: Yongli Ji, Cathryn Rankin, Steven Grunberg, Andy E. Sherrod, Jamshid Ahmadi, Jeannette J. Townsend, Lynn G. Feun, Christy A. Russell, Fairooz F. Kabbinavar, Keith J. Stelzer, Claire Verschraegen
Data analysis and interpretation: Yongli Ji, Cathryn Rankin, Steven Grunberg, Ruth K. Fredericks, Fairooz F. Kabbinavar, Anne Schott, Claire Verschraegen
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Double-Blind Phase III Randomized Trial of the Antiprogestin Agent Mifepristone in the Treatment of Unresectable Meningioma: SWOG S9005
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Yongli Ji
Employment: Shire (I)
Stock or Other Ownership: Shire (I)
Cathryn Rankin
Employment: Pathology Associates Medical Laboratories (I)
Steven Grunberg
No relationship to disclose
Andy E. Sherrod
Stock or Other Ownership: Abbott Laboratories, Abbvie, Amgen, Becton, Dickinson, Eli Lilly, pfizer, Johnson and Johnson, Invacare
Jamshid Ahmadi
No relationship to disclose
Jeannette J. Townsend
No relationship to disclose
Lynn G. Feun
No relationship to disclose
Ruth K. Fredericks
No relationship to disclose
Christy A. Russell
No relationship to disclose
Fairooz F. Kabbinavar
No relationship to disclose
Keith J. Stelzer
No relationship to disclose
Anne Schott
Research Funding: Dompe, Novartis
Patents, Royalties, Other Intellectual Property: Systems and methods for tissue imaging, 8, 185, 186, inventor, submitted April 2008; patent for use of diffusion magnetic resonance imaging technology to quantitate response to neoadjuvant breast cancer therapy
Claire Verschraegen
No relationship to disclose
REFERENCES
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16(suppl 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99:379–391. doi: 10.1007/s11060-010-0342-2. [DOI] [PubMed] [Google Scholar]
- 3.Sun SQ, Hawasli AH, Huang J, et al. An evidence-based treatment algorithm for the management of WHO Grade II and III meningiomas. Neurosurg Focus. 2015;38:E3. doi: 10.3171/2015.1.FOCUS14757. [DOI] [PubMed] [Google Scholar]
- 4.Wahab M, Al-Azzawi F. Meningioma and hormonal influences. Climacteric. 2003;6:285–292. [PubMed] [Google Scholar]
- 5.Blitshteyn S, Crook JE, Jaeckle KA. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26:279–282. doi: 10.1200/JCO.2007.14.2133. [DOI] [PubMed] [Google Scholar]
- 6.Verheijen FM, Sprong M, Jacobs HM, et al. Progesterone receptor isoform expression in human meningiomas. Eur J Cancer. 2001;37:1488–1495. doi: 10.1016/s0959-8049(01)00146-0. [DOI] [PubMed] [Google Scholar]
- 7.Grunberg SM, Daniels AM, Muensch H, et al. Correlation of meningioma hormone receptor status with hormone sensitivity in a tumor stem-cell assay. J Neurosurg. 1987;66:405–408. doi: 10.3171/jns.1987.66.3.0405. [DOI] [PubMed] [Google Scholar]
- 8.Maiuri F, Montagnani S, Gallicchio B, et al. Oestrogen and progesterone sensitivity in cultured meningioma cells. Neurol Res. 1989;11:9–13. doi: 10.1080/01616412.1989.11739853. [DOI] [PubMed] [Google Scholar]
- 9.Baulieu EE. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989;245:1351–1357. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- 10.Edwards DP, Leonhardt SA, Gass-Handel E. Novel mechanisms of progesterone antagonists and progesterone receptor. J Soc Gynecol Investig. 2000;7(suppl):S22–S24. doi: 10.1016/s1071-5576(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 11.Johanssen S, Allolio B. Mifepristone (RU 486) in Cushing's syndrome. Eur J Endocrinol. 2007;157:561–569. doi: 10.1530/EJE-07-0458. [DOI] [PubMed] [Google Scholar]
- 12.Beaufrère B, de Parscau L, Chatelain P, et al. RU 486 administration in a child with Cushing's syndrome. Lancet. 1987;2:217. doi: 10.1016/s0140-6736(87)90796-3. [DOI] [PubMed] [Google Scholar]
- 13.Nieman LK, Chrousos GP, Kellner C, et al. Successful treatment of Cushing's syndrome with the glucocorticoid antagonist RU 486. J Clin Endocrinol Metab. 1985;6:536–540. doi: 10.1210/jcem-61-3-536. [DOI] [PubMed] [Google Scholar]
- 14.Kettel LM, Murphy AA, Morales AJ, et al. Clinical efficacy of the antiprogesterone RU486 in the treatment of endometriosis and uterine fibroids. Hum Reprod. 1994;9(suppl 1):116–120. doi: 10.1093/humrep/9.suppl_1.116. [DOI] [PubMed] [Google Scholar]
- 15.Murphy AA, Castellano PZ. RU486: pharmacology and potential use in the treatment of endometriosis and leiomyomata uteri. Curr Opin Obstet Gynecol. 1994;6:269–278. [PubMed] [Google Scholar]
- 16.Fiscella J, Bonfiglio T, Winters P, et al. Distinguishing features of endometrial pathology after exposure to the progesterone receptor modulator mifepristone. Hum Pathol. 2011;42:947–953. doi: 10.1016/j.humpath.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy AA, Morales AJ, Kettel LM, et al. Regression of uterine leiomyomata to the antiprogesterone RU486: Dose-response effect. Fertil Steril. 1995;64:187–190. [PubMed] [Google Scholar]
- 18.Bakker GH, Setyono-Han B, Portengen H, et al. Treatment of breast cancer with different antiprogestins: Preclinical and clinical studies. J Steroid Biochem Mol Biol. 1990;37:789–794. doi: 10.1016/0960-0760(90)90421-g. [DOI] [PubMed] [Google Scholar]
- 19.Romieu G, Maudelonde T, Ulmann A, et al. The antiprogestin RU486 in advanced breast cancer: Preliminary clinical trial. Bull Cancer. 1987;74:455–461. [PubMed] [Google Scholar]
- 20.Gallagher P, Young AH. Mifepristone (RU-486) treatment for depression and psychosis: A review of the therapeutic implications. Neuropsychiatr Dis Treat. 2006;2:33–42. [PMC free article] [PubMed] [Google Scholar]
- 21.Grunberg SM, Weiss MH, Russell CA, et al. Long-term administration of mifepristone (RU486): Clinical tolerance during extended treatment of meningioma. Cancer Invest. 2006;24:727–733. doi: 10.1080/07357900601062339. [DOI] [PubMed] [Google Scholar]
- 22.Spitz IM, Grunberg SM, Chabbert-Buffet N, et al. Management of patients receiving long-term treatment with mifepristone. Fertil Steril. 2005;84:1719–1726. doi: 10.1016/j.fertnstert.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 23.Zeidman LA, Ankenbrandt WJ, Du H, et al. Growth rate of non-operated meningiomas. J Neurol. 2008;255:891–895. doi: 10.1007/s00415-008-0801-2. [DOI] [PubMed] [Google Scholar]
- 24.Olivero WC, Lister JR, Elwood PW. The natural history and growth rate of asymptomatic meningiomas: A review of 60 patients. J Neurosurg. 1995;83:222–224. doi: 10.3171/jns.1995.83.2.0222. [DOI] [PubMed] [Google Scholar]
- 25.Oya S, Kim SH, Sade B, et al. The natural history of intracranial meningiomas. J Neurosurg. 2011;114:1250–1256. doi: 10.3171/2010.12.JNS101623. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura M, Roser F, Michel J, et al. The natural history of incidental meningiomas. Neurosurgery. 2003;53:62–70. doi: 10.1227/01.neu.0000068730.76856.58. discussion 70-71. [DOI] [PubMed] [Google Scholar]
- 27.Niiro M, Yatsushiro K, Nakamura K, et al. Natural history of elderly patients with asymptomatic meningiomas. J Neurol Neurosurg Psychiatry. 2000;68:25–28. doi: 10.1136/jnnp.68.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Central Brain Tumor Registry of the United States. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2008. www.cbtrus.org/2012-NPCR-SEER/CBTRUS_Report_2004-2008_3-23-2012.pdf.
- 30.Bickerstaff ER, Small JM, Guest IA. The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurol Neurosurg Psychiatry. 1958;21:89–91. doi: 10.1136/jnnp.21.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller RE. Breast cancer and meningioma. J Surg Oncol. 1986;31:182–183. doi: 10.1002/jso.2930310309. [DOI] [PubMed] [Google Scholar]
- 32.Roelvink NC, Kamphorst W, van Alphen HA, et al. Pregnancy-related primary brain and spinal tumors. Arch Neurol. 1987;44:209–215. doi: 10.1001/archneur.1987.00520140069020. [DOI] [PubMed] [Google Scholar]
- 33.Custer BS, Koepsell TD, Mueller BA. The association between breast carcinoma and meningioma in women. Cancer. 2002;94:1626–1635. doi: 10.1002/cncr.10410. [DOI] [PubMed] [Google Scholar]
- 34.Wolfsberger S, Doostkam S, Boecher-Schwarz HG, et al. Progesterone-receptor index in meningiomas: Correlation with clinico-pathological parameters and review of the literature. Neurosurg Rev. 2004;27:238–245. doi: 10.1007/s10143-004-0340-y. [DOI] [PubMed] [Google Scholar]
- 35.Fewings PE, Battersby RD, Timperley WR. Long-term follow up of progesterone receptor status in benign meningioma: A prognostic indicator of recurrence? J Neurosurg. 2000;92:401–405. doi: 10.3171/jns.2000.92.3.0401. [DOI] [PubMed] [Google Scholar]
- 36.Blankenstein MA, Verheijen FM, Jacobs JM, et al. Occurrence, regulation, and significance of progesterone receptors in human meningioma. Steroids. 2000;65:795–800. doi: 10.1016/s0039-128x(00)00193-8. [DOI] [PubMed] [Google Scholar]
- 37.Wen PY, Yung WK, Lamborn KR, et al. Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01-08) Neuro Oncol. 2009;11:853–860. doi: 10.1215/15228517-2009-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norden AD, Raizer JJ, Abrey LE, et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2010;96:211–217. doi: 10.1007/s11060-009-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reardon DA, Norden AD, Desjardins A, et al. Phase II study of Gleevec® plus hydroxyurea (HU) in adults with progressive or recurrent meningioma. J Neurooncol. 2012;106:409–415. doi: 10.1007/s11060-011-0687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaley TJ, Wen P, Schiff D, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17:116–121. doi: 10.1093/neuonc/nou148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lou E, Sumrall AL, Turner S, et al. Bevacizumab therapy for adults with recurrent/progressive meningioma: A retrospective series. J Neurooncol. 2012;109:63–70. doi: 10.1007/s11060-012-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nayak L, Iwamoto FM, Rudnick JD, et al. Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol. 2012;109:187–193. doi: 10.1007/s11060-012-0886-4. [DOI] [PubMed] [Google Scholar]
- 43.Goldman CK, Bharara S, Palmer CA, et al. Brain edema in meningiomas is associated with increased vascular endothelial growth factor expression. Neurosurgery. 1997;40:1269–1277. doi: 10.1097/00006123-199706000-00029. [DOI] [PubMed] [Google Scholar]
- 44.Samoto K, Ikezaki K, Ono M, et al. Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Res. 1995;55:1189–1193. [PubMed] [Google Scholar]
- 45.Pistolesi S, Boldrini L, Gisfredi S, et al. Angiogenesis in intracranial meningiomas: Immunohistochemical and molecular study. Neuropathol Appl Neurobiol. 2004;30:118–125. doi: 10.1046/j.0305-1846.2003.00516.x. [DOI] [PubMed] [Google Scholar]
- 46.Lamszus K, Lengler U, Schmidt NO, et al. Vascular endothelial growth factor, hepatocyte growth factor/scatter factor, basic fibroblast growth factor, and placenta growth factor in human meningiomas and their relation to angiogenesis and malignancy. Neurosurgery. 2000;46:938–947. doi: 10.1097/00006123-200004000-00033. discussion 947-948. [DOI] [PubMed] [Google Scholar]
- 47.Puchner MJ, Hans VH, Harati A, et al. Bevacizumab-induced regression of anaplastic meningioma. Ann Oncol. 2010;21:2445–2446. doi: 10.1093/annonc/mdq634. [DOI] [PubMed] [Google Scholar]
- 48.Choy W, Kim W, Nagasawa D, et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30:E6. doi: 10.3171/2011.2.FOCUS1116. [DOI] [PubMed] [Google Scholar]
- 49.Brastianos PK, Horowitz PM, Santagata S, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45:285–289. doi: 10.1038/ng.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark VE, Erson-Omay EZ, Serin A, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339:1077–1080. doi: 10.1126/science.1233009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Licciulli S, Maksimoska J, Zhou C, et al. FRAX597, a small molecule inhibitor of the p21-activated kinases, inhibits tumorigenesis of neurofibromatosis type 2 (NF2)-associated Schwannomas. J Biol Chem. 2013;288:29105–29114. doi: 10.1074/jbc.M113.510933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


