Abstract
Background
The establishment of the bisexual fertile fish hybrid lineage including the allodiploid and allotetraploid hybrids, from interspecific hybridization of red crucian carp (Carassius auratus red var. 2n = 100, 2n = AA) (♀) × common carp (Cyprinus carpio L. 2n = 100, 2n = BB) (♂), provided a good platform to investigate genetic relationship between the parents and their hybrid progenies.
Results
The chromosomal inheritance of diploid and allotetraploid hybrid progenies in successive generations, was studied by applying 5S rDNA fluorescence in situ hybridization. Signals of 5S rDNA distinguished the chromosomal constitution of common carp (B-genome) from red crucian carp (A-genome), in which two strong signals were observed on the first submetacentric chromosome, while no major signal was found in common carp. After fish hybridization, one strong signal of 5S rDNA was detected in the same locus on the chromosome of diploid hybrids. As expected, two strong signals were observed in 4nF3 tetraploid hybrids offspring and it is worth mentioning that two strong signals were detected in a separating bivalent of a primary spermatocyte in 4nF3. Furthermore, the mitosis of heterozygous chromosomes was shown normal and stable with blastular tissue histological studies.
Conclusions
We revealed that 5S rDNA signal can be applied to discern A-genome from B-genome, and that 5S rDNA bearing chromosomes can be stably passed down in successive generations. Our work provided a significant method in fish breeding and this is important for studies in fish evolutionary biology.
Keywords: Interspecific hybridization, Heterozygous chromosomes, FISH, 5S rDNA
Background
In general, interspecific hybridization and polyploidy in plants were potent evolutionary mechanisms [1, 2]. Recently, new genetic evidence suggested that hybrid speciation was more common in plants and animals than we thought, and it has played a very constructive part in animal evolution [3, 4]. As fertile hybrids, the integrity and inheritance of heterozygous genome was a focused issue, which concerned to mitotic and meiotic stability of hybrid offspring and formation of fertile progenies. Now the inheritance rule of heterozygous genome had been widely studied in hybrid plants, while as there was a limit to the material, related research only focused on such a few hybrid vertebrates as fish and frog [5, 6].
Through selecting and breeding for more than 20 years, the bisexual fertile allotetraploid hybrid fish (abbreviated as AT) (4n = 200) has been acquired, which resulted from fertilization of unreduced eggs and sperm produced by hybrids of red crucian carp (Carassius auratus red var. 2n = 100) (♀) (abbreviated as RCC) × common carp (Cyprinus carpio L. 2n = 100) (♂) (abbreviated as CC). It provided a unique material for exploring the inheritance rule of heterozygous chromosomes in hybrid lineage [7–10]. The production procedure of AT was as follows: RCC females were mated with CC males to produce F1 fish (abbreviated as 2nF1) which were then mated with each other to produce F2 fish (abbreviated as 2nF2). The males and females of diploid F2 hybrids could generate unreduced diploid eggs and diploid sperm, respectively, which were fertilized to form the allotetraploid hybrids in F3 fish (abbreviated as 4nF3). Fertile tetraploid female and male F3 fish were found to generate diploid eggs and sperm, respectively. By self-breeding of F3, the tetraploid F4 fish were produced. Until now, the F3–F24 AT has been formed through successive breeding. Our previous study has indicated that the AT has inherited large amount of genetic material from their original parents with some variations [11–15]. However, the chromosomes constitution and inheritance pattern of hybrid lineage were still unknown. Identification of all genome chromosomes was crucial to understanding the genome constitution and inheritance rule of the hybrid genome. It has been widely studied in hybrid plant, using by fluorescence in situ hybridization (abbreviated as FISH), and genomic in situ hybridization (abbreviated as GISH) [16–18]. However, due to the lack of chromosome-specific molecular probes, the current reported studies were few in hybrid fish related to chromosomal localization.
The 5S rDNA in higher eukaryotes was organized in tandem repeat units that consisted of highly conserved 120 bp transcribing sequence and variable non-transcribed spacers (abbreviated as NTS) [19]. Variations in NTS, related to insertions-deletions, minirepeats and pseudo genes, were often species specific and have successfully been served as markers in evolutionary studies [20–22]. In addition, due to the numerous copies of repeated sequences, their chromosomal localization was easily detected by FISH. Analysis of 5S rDNA sequences and chromosomal localization of them were an effective method of genetic diversity monitoring, which were widely used to explore the phylogeny relationship among closer species and polyploidy origin [23–25]. Masaru and Hideo [26] also found that the 5S rDNA could be candidates for phylogenetic molecular markers for the crucian carp.
In the current study, based on our establishment of 2nF1, 2nF2, 4nF3 and 4nF22 hybrid lineage of RCC × CC, a comparative analysis of 5S rDNA fragments among all samples were carried by PCR and related sequences were screened out as probes to determine the chromosomal localization of the 5S rDNA for 2nF1, 2nF2, and 4nF3 fish by FISH, which elucidated the inheritance rules of heterozygous chromosomes in course of polyploid hybrid fish origin and propagation. Furthermore, cytological observation of early embryos in blastulastage was used to determine the mitotic stability in hybrid offspring.
Methods
Ethics statement
All experiments were approved by Animal Care Committee of Hunan Normal University and followed guidelines statement of the Administration of Affairs Concerning Animal Experimentation of China. All samples are raised in natural ponds and all dissections are performed under sodium pentobarbital anesthesia, and all efforts are made to minimize suffering.
Fishes and genomic DNA samples
Specimens of RCC (2n = 100), CC (2n = 100), 2nF1 (2n = 100), 2nF2 (2n = 100), 4nF3 (4n = 200) and 4nF22 (4n = 200) were obtained from the Engineering Center of Polyploid Fish Breeding of National Education Ministry located at Hunan Normal University. Total genomic DNA was isolated from the peripheral blood cells according to the standard phenol-chloroform extraction procedures described by Sambrook et al. [27] with minor modifications.
PCR amplification, cloning and sequencing
One pair of primers (5’-TATGCCCGATCTCGTCTGATC-3’ and 5’- CAGGTTGGTATGGCCGTAAGC-3’) [26] was synthesized to amplify the 5S rDNA repeats directly from genomic DNA by PCR. The genomic DNA of RCC, CC, 2nF1, 2nF2, 4nF3 and 4nF22 were used as a template for subsequent PCR (2 to 10 individuals for each of the 6 samples). The PCR cycling conditions were: 5 min at 94 °C, 30 cycles of denaturation at 94 °C for 30s, annealing at 56 °C for 30s, and extension 72 °C for 1 min, ending with 10 min of extension at 72 °C. The PCR products were analyzed in 1.2 % agarose gels stained by ethidium bromide, purified by Gel Ex- traction Kit (Sangon), cloned into the pMD18-T vector (Takara), and transferred into E. coli DH5α. Then the positive clones were sequenced by Sangon. The sequences were analyzed by ClustalW2.
Chromosome preparation
The kidney cells in RCC, CC, 2nF1, 2nF2 and 4nF3 were used for the chromosome observation at the metaphase of mitosis and the testis cells in 4nF3 were used to observe the process of meiosis. The concanvalin A was injected into the abdominal cavity of the samples for one to three times at the dosage of 2-8 μg/g. The interval time was 12–24 h. Two to six hours prior to harvest, colchicines (2-4 μg/g) were used to arrest the chromosome at the metaphase. All the kidney tissue in the above samples was ground in 0.8 % NaCl. The hypotonic treatment was accomplished with 0.075 mol/L KCl for 40–60 min, followed by fixation in 3:1 methanol-acetic acid (three changes). The cells were spread on clean slides.
Probe preparation of the 5S rDNA sequence and fluorescence in situ hybridization
FISH is used to assess chromosomal location of 5S rDNA of RCC, CC, 2nF1, 2nF2, and 4nF3 fish. The hybridization was performed according to the method described by Masaru and Hideo with minor modifications [26]. In brief, the purified PCR product of related 5S rDNA fragments of RCC labeled with Dig-11-dUTP by PCR DIG Probe Synthesis Kit (Roche, Germany) were used as probes. After pretreatment with 2 × SSC for 30 min, 70 %, and 100 % ethanol for 5 min each, the slides with chromosome metaphase spreads of all samples were denatured in 70 % deionized formamide /2 × SSC for 2 min at 75 °C, dehydrate in a 70 % (−20 °C, to avoid DNA renaturation) and 100 % ethanol series for 5 min each, and then air-dry. The probe was prepared by adding labeled DNA with 20 × SSC, deionized formamide and 50 % dextran sulphate and denaturing in boiling water for 5 min. Hybridizations were allowed to proceed under a sealed cover slip in a moist chamber at 37 °C overnight. The next day the slides were washed; twice for 15 min in 2 × SSC with 50 % formamide and then in 2 × SSC and 1 × SSC for 5 min each. After a series of post-hybridization washing were performed, the spectrum signals were achieved with 10ul of 1ug ml−1 FITC-conjugated anti-digoxigenin antibody from sheep (Roche, Germany). Then the slides were washed three times for 5 min each in TNT (formulas of eluate: 0.1 M Tris–HCl, 0.15 M NaCL,0.05 % Tween 20). After slides were placed in 10ul anti-fade solution containing 0.5 μg/ml of 4, 6-diamidino-2-phenylindole (abbreviated as DAPI), they were viewed using a Leica inverted DMIRE2 microscope image system (Leica, Germany). Images were captured with CW4000 FISH software (Leica, Germany). Good-quality metaphase spreads were photographed and used for analysis of karyotypes.
Cytological observations of early embryos
After 6 h after fertilization, the F3 embryos in blastulastage were fixed in Smith solution for 4 to12 h, and then are washed by alcohol for 2 to 3 times. The paraffin-embedded sections were cut at 6 μm and stained with Harris hematoxylin and eosin. The structure of zygote was observed under a light microscope and photographed with Pixera Pro 600 ES.
Results
Analysis of 5S rDNA fragments
The electrophoretic band pattern of DNA fragments amplified with the primers of 5 s rDNA was distinctive between RCC and CC. RCC exhibited two major DNA bands (approximately 200 bp and 340 bp) and some repeated ladder-like bands, CC exhibited other two major DNA bands (approximately 200 bp and 400 bp) and some repeated ladder-like bands as well. As their hybrid offspring, 2nF1 and 4n F22 exhibited three major DNA bands (approximately 200 bp, 340 bp and 400 bp) and some repeated ladder-like bands (Fig. 1). After ligation of sized DNA fragments and transformation, a total number of 100 clones were sequenced to examine the different patterns of 5SrDNA, including 30 clones from 5 RCC individuals, 20 clones from 5 CC individuals, 20 clones from 5 2 nF1 individuals and 30 clones from 5 4 nF22 individuals. consensus sequences of the 5 s rDNA repeat units were obtained. All of the typical sequences were deposited at GenBank under the accession numbers (KM359661- KM359679) (Table 1).
Fig. 1.
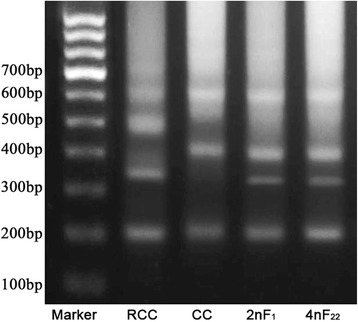
DNA bands amplify from RCC, CC and their hybrid offspring. Marker: DNA ladder markers (100 bp increments); RCC: two DNA bands (200, 340 bp) and some repeated ladder-like bands; CC: two DNA bands (200, 400 bp) and some repeated ladder-like bands; 2nF1 and 4nF22: three DNA bands (200, 340 and 400 bp) and some repeated ladder-like bands
Table 1.
Size (bp), type, and GenBank accession number for each of the 5SrDNA repeat units (coding sequence plus NTS) found in RCC, CC, 2nF1, and 4nF22
| Species | Length(bp) | Accession no. | ||
|---|---|---|---|---|
| 5 s repeat | Coding sequence | NTS | ||
| RCC | 201 | 119 | 82 = 0*138 + 82RCC-a | KM359661 |
| 202 | 120 | 82 = 0*138 + 82RCC-b | KM359662 | |
| 340 | 121 | 219 = 1*137 + 82 | KM359663 | |
| 339 | 121 | 218 = 1*136 + 82 | KM359664 | |
| 477 | 121 | 356 = 2*137 + 82 | KM359665 | |
| 613 | 120 | 493 = 3*137 + 82 | KM359666 | |
| CC | 202 | 120 | 82 = 0*138 + 82CC-a | KM359667 |
| 204 | 121 | 83 = 0*138 + 83CC-b | KM359668 | |
| 2nF1 | 203 | 121 | 82 = 0*138 + 82 | KM359671 |
| 201 | 120 | 82 = 0*138 + 82 | KM359672 | |
| 341 | 120 | 219 = 1*137 + 82 | KM359673 | |
| 339 | 120 | 219 = 1*137 + 82 | KM359674 | |
| 4nF22 | 201 | 119 | 82 = 0*138 + 82 | KM359675 |
| 202 | 120 | 82 = 0*138 + 82 | KM359676 | |
| 338 | 119 | 219 = 1*137 + 82 | KM359677 | |
| 477 | 120 | 357 = 2*138 + 82 | KM359678 | |
| 639 | 122 | 504 = 3*137 + 93 | KM359679 | |
RCC-a, RCC-b, CC-a, CC-b: indicate the different approximately 82 bp-units. The alignment data of them was showed in Table 2
Nucleotide sequencing and BlastN sequences corresponded to 5 s rDNA repeat units. All units consist of a 120 bp coding region and a variable NTS. In RCC, four kinds of fragments of 5 s rDNA (202 bp, 340 bp, 477 bp and 613 bp) were characterized by different lengths of NTS. In CC, there was only one kind of fragment of 5 s rDNA (202 bp) with 120 bp coding region and one 82 bp NTS unit. The 400 bp fragment from amplified DNA bands was just two repeats of 202 bp unit. In 2nF1 and 4nF22, like their original parents, two kinds of fragments of 5 s rDNA (approximately 200 bp and 340 bp) both contained similar coding region and NTS (Table 1).
The NTS sequence of 5S rDNA also showed an extensive variation between RCC and CC. In RCC, different-sized 5S rDNA units contained one 120 bp coding region and one 82 bp repeat unit, which were spaced at regular interval with zero, one, two, or three 138 bp repeat units (Interposed Region: IPR), respectively showing 202 bp, 340 bp, 477 bp and 613 bp 5S rDNA repeat units. While in CC, only around 200 bp-sized 5S rDNA units consisting of one 120 bp coding region and one monomeric 82 bp repeat unit were identified. The similarity of 82 bp-unit among different clones of RCC was 95.1 % and the similarity of 82 bp-unit among different clones of CC was 91.5 %, while the 82 bp-unit of RCC was different from 82 bp-unit of CC with lower similarity of 52.4 %-68.3 % (Table 2). Sequence alignments showed high similarity of 340 bp units between RCC, 2nF1 and 4nF22, even in gibel carp, Carassius auratus(abbreviated as Cag) [25] (Fig. 2 and Table 3), so the 340 bp-fragments were screened out as a probe to assess chromosomal location of 5S rDNA of RCC, CC, 2nF1, 2nF2, and 4nF3 fish.
Table 2.
Nucleotide similarities of 82 bp-units of 5S rDNA sequence between RCC and CC
| Sequence name | RCC-a | RCC-b | CC-a | CC-b |
|---|---|---|---|---|
| RCC-a | - | 95.1 % | 68.3 % | 53.7 % |
| RCC-b | - | - | 68.3 % | 52.4 % |
| CC-a | - | - | - | 91.5 % |
| CC-b | - | - | - | - |
Fig. 2.

Sequence alignments of 340 bp-fragments of RCC, Cag, 2nF1 and 4nF22. The 120 bp coding regions were included in the boxes. The sequence data of RCC, Cag, 2nF1 and 4nF22 were available from GenBank under accession number KM359663, DQ659260, KM359673, KM359677
Table 3.
Nucleotide similarities of 340 bp- fragments among RCC, Cag, 2nF1 and 4nF22
| sequence name | Cag-340 | 2nF1-340 | 4nF22-340 |
|---|---|---|---|
| RCC-340 | 97.1 % | 94.4 % | 97.3 % |
| Cag-340 | - | 95.0 % | 97.9 % |
| 2nF1-340 | - | - | 95.6 % |
5S rDNA localization
The diploid RCC, CC, 2nF1, and 2nF2 samples showed 100 chromosomes which were classified into 22 metacentric (abbreviated as M), 34 submetacentric (abbreviated as SM), 22 subtelelocentric (abbreviated as ST) and 22 telocentric (abbreviated as T) chromosomes. The tetraploid 4nF3 samples revealed 200 chromosomes, which were classified into 44 metacentric (M), 68 submetacentric (SM), 44 subtelelocentric (ST) and 44 telocentric(T) chromosomes [7, 28]. No difference was observed between the females and the males.
Two signals of 340 bp-fragment were clearly detected in the first SM chromosomes pair of RCC, with a few weak signals being detected in other chromosomes (Fig. 3a, b), while the CC fish has no major signal. As to their hybrid lineage offspring, one strong signal was found in the first SM chromosome of 2nF1 and 2nF2 fish (Fig. 3c, d) and two strong signals were detected in the same chromosomes of AT (Fig. 3e, f), also with a few weak signals being detected in other chromosomes.
Fig. 3.
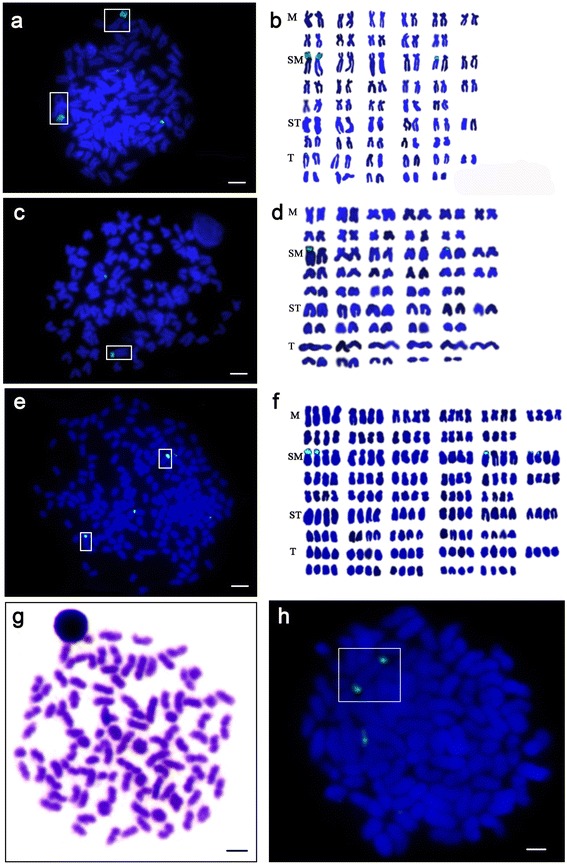
Mitotic metaphase spreads and karyotypes of RCC, 2 nF2 and 4 nF3 fish with FISH signals of 5S rDNA. (a) Two strong and a few weak signals were detected in RCC fish; the boxes indicated two major signals of 5S rDNA (green). (b) The karyotype of RCC fish revealed two strong signals located on the first pair of larger-sized SM chromosomes. (c) One strong signal and a few weak signals in 2nF2 fish (green),the box indicated the one major signal of 5S rDNA (green). (d) The karyotype of 2nF2 fish show one strong signal on the first group of larger-sized SM chromosomes. (e) The FISH analysis of 4nF3 fish exhibited the double signal feature of two strong and a few weak signals (green), the boxes indicated the two major signals (green). (f ) The karyotype of 4nF3 fish showed two strong signals on the first group of larger-sized SM chromosomes. All metaphase chromosomes were counterstained with DAPI and appear blue. (g) The chromosome pairing in Meiosis I of spermatocytes in 4nF3 showed 100 bivalents under light microscope; (h) Two signals of 5S rDNA located on a separating bivalent. M: metacentric; SM: submetacentric; ST subtelelocentric; T: telocentric. (a-f) Bar = 3 μm; (g,h) Bar = 5 μm
Chromosomal localization of 340 bp-fragment in metaphase of meiosis spreads in primary spermatocytes of 4nF3 indicated that two strong signals being located on a separating bivalent (Fig. 3g, h).
Cytological observation of early embryos in blastulastage
Cytological observation of mitosis in F3 early embryo cells showed that F3 performed normal and stable mitosis, with normal distribution of chromosomes and structure of spindle body during metaphase (Fig. 4a), anaphase (Fig. 4b), and telophase (Fig. 4c) of mitosis, not being affected by the genetic attributes of heterozygous genome.
Fig. 4.
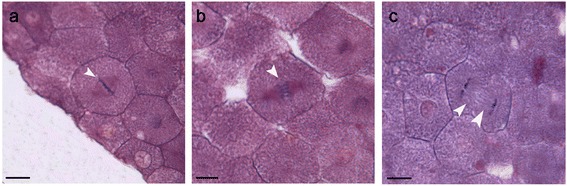
Cytological observations of early embryos in blastula stage. (a) Early embryo cells in metaphase of mitosis; (b) Early embryo cells in anaphase of mitosis; (c) Early embryo cells in telophase of mitosis; the arrowheads showed the normal distribution of chromosomes and structure of spindle body. Bar = 10 μm
Discussion
Different 5 s rDNA classes were reported in several mammals and fish species, and generally two different type of 5 s rDNA units have been characterized by distinct NTS types and base substitution [29, 30]. In our work, the construction of different-sized 5S rDNA units were further elucidated as containing one 120 bp coding region and one 82 bp repeat unit, which were spaced at regular interval with zero, one, two, or three 138 bp-IPR repeat units (Table 1), this result revealed the assembly rule of NTS in RCC, which enriched the current data of molecular organization analysis of 5 s rDNA.
Modern cytogenetic analyses, such as FISH and GISH have been widely applied for studies in many processes of chromosome evolution, including structural rearrangements, as well as extensive studies on phylogenetic and genomic relationships [31]. In this work, chromosome inheritance of hybrid lineage progenies of RCC × CC were investigated by detecting the signal of 340 bp fragments of 5 s rDNA on chromosomes in successive generations, i.e. RCC, CC, 2nF1, 2nF2, and 4nF3 fish. The 340 bp fragments was highly conserved between RCC and their hybrid lineage progenies, but not in CC genome, which made the 340 bp fragments in RCC a suitable indecator to monitor chromosomal inheritance in RCC, CC and their hybrid progeny, as it was recently showed in other hybrid fish [32, 33]. Besides that, our results revealed that the chromosomal localization of the 340 bp fragments of RCC could serve as a suitable genetic marker to distinguish the chromosomal constitution of CC from RCC, as two strong detected signals on the first pair of larger-sized SM chromosomes were observed, but not in CC chromosomes. The other weak FISH signals were also 5S rDNA locus with less copy of repeat units.
Since there have been very few studies on chromosomal inherence behavior in fish hybridization, we looked into the 5S rDNA bearing chromosome in successive hybrid generation. The strong signal with same locus as observed in RCC were also found in 2nF1 and 2nF2 fish and the number of signal doubled in 4nF3 fish. Furthermore, two strong signals were also detected in a separating bivalent of a primary spermatocyte in 4nF3, which promised that two 5S rDNA sites would equally distributed to each gamete and made every gamete acquire one 5S rDNA bearing chromosome. Those results may suggest that 2nF1 and 2nF2 fish contained one 5S rDNA bearing chromosome from A-genome and one 5S rDNA bearing chromosome from B-genome, and 4nF3 fish possessed two 5S rDNA bearing chromosomes from A-genome and two 5S rDNA bearing chromosomes from B-genome. Additional studies was made in order to investigate the mitosis, results demonstrated that in hybridization the mitosis process maintained normal and stable, which demonstrated the compatibility of genome between RCC and CC, and promised the genetic stability of hybrid progeny. Based on the fish analysis and cytological observation, we speculate that chromosomes of RCC and CC may equally pass down to hybrid generation (Fig. 5).
Fig. 5.
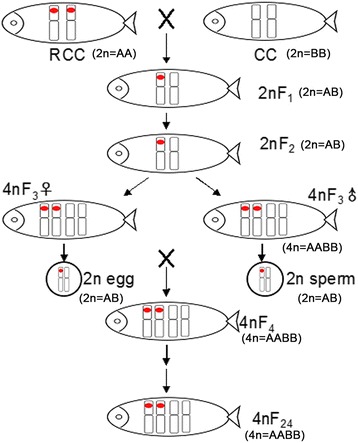
Schematic presentation of chromosome transmission in hybrid fish from RCC, CC to 2nF1, and to 2nF2, as well as to 4nF3 and 4nF24
The chromosomal rearrangements having occurred less frequently was a promising result supporting the development of stable allotetraploid hybrid lines, which were also proved in allohexaploid Brassica line [34] and the lineage of X.laevis [35]. However, the allotetraploid Festuca pratensis × Lolium perenne hybrid of three generations shared various rDNA loci profiles with chromosomal rearrangements [31], indicating a tendency of F. pratensis genome-like chromosomes to be less stable in hybrid of three generations. In our work, the strong FISH signals of 5S rDNA in RCC passed down stably in successive hybrid generation, which implied that chromosomal rearrangements have occurred less frequently in area of highly repeated sequence of 5S rDNA. Thus it can be seen that genomic contribution was different in different type of hybridized combinations, which will affect the genetic stability of hybrid progenies. Further studies were necessary to prove or reject this hypothesis as well as for deeper understanding of the mechanisms responsible for the fate of heterozygous chromosomes in hybrid lineage progenies.
Conclusions
Few studies focused on chromosomal inheritance in successive generations of hybrid fish. We executed the 5S rDNA FISH analysis in successive generations of diploid and allotetraploid hybrid progenies and revealed 5S rDNA signal can be applied to discern A-genome from B-genome, and that 5S rDNA bearing chromosomes respectively coming from A-genome and B-genome can be stably passed down in successive hybrid generations.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (30901100/31430088), the Cooperative Innovation Center of Engineering and New Products for Developmental Biology of Hunan Province (20134486), major international cooperation projects of the National Natural Science Foundation of China (31210103918), training Program of the Major Research Plan of the National Natural Science Foundation of China (91331105), the National High Technology Research and Development Program of China (2011AA100403), the National Key Basic Research Program of China (2012CB722305), The fund of education department of Hunan province (15A116), The fund of sci-tec innovation and entrepreneur for Hunan young talents, the Project Supported by Science and Technology Program of Hunan Province (No:2015JC3065; No:2014FJ3084) and the construct program of the key discipline in Hunan province and China.
Abbreviations
- AT
Allotetraploid hybrid fish
- RCC
Red crucian carp
- CC
Common carp
- 2nF1
The first generation of diploid hybrid fish of RCC × CC
- 2nF2
The second generation of diploid hybrid fish of RCC × CC
- 4nF3
The third generation of tetraploid hybrid fish of RCC × CC
- Cag
Gibel carp, Carassius auratus
- FISH
Fluorescence in situ h ybridization
- GISH
Genomic in situ hybridization
- NTS
Non-transcribed spacers
- IPR
Interposed region
- DAPI
4, 6-diamidino-2-phenylindole
- M
Metacentric
- SM
Submetacentric
- ST
Subtelelocentric
- T
Telocentric
Footnotes
Chun Zhang and Lihai Ye contributed equally to this work.
Competing interests
The authors declared no conflict of interest.
Authors’ contributions
CZ, LY and SL designed the study, carried out the analyses, performed the technical discussions, prepared and draft the manuscript. YC, JX and MT participated in data simulation and discussions. YW and YX were involved in the statistical analysis. All authors read and approved the final manuscript.
Contributor Information
Chun Zhang, Email: zhangchun421@163.com.
Lihai Ye, Email: haidoc@126.com.
Yiyi Chen, Email: 374660909@qq.com.
Jun Xiao, Email: cs5676@sina.com.cn.
Yanhong Wu, Email: 214314467@qq.com.
Min Tao, Email: minmindiu@126.com.
Yamei Xiao, Email: yameix@126.com.
Shaojun Liu, Phone: +86-0731-88873010, Email: lsj@hunnu.edu.cn.
References
- 1.Otto SP, Whitton J. Polyploid incidence and evolution. Annu Rev Genet. 2000;34:401–437. doi: 10.1146/annurev.genet.34.1.401. [DOI] [PubMed] [Google Scholar]
- 2.Leitch AR, Leitch IJ. Genomic plasticity and the diversity of polyploidy plants. Science. 2008;320:481–483. doi: 10.1126/science.1153585. [DOI] [PubMed] [Google Scholar]
- 3.Mallet J. Hybrid speciation. Nature. 2007;446:279–283. doi: 10.1038/nature05706. [DOI] [PubMed] [Google Scholar]
- 4.Van de PY, Maere S, Meyer A. The evolutionary significance of ancient genome duplications. Nat Rev Genet. 2009;10:725–732. doi: 10.1038/nrg2600. [DOI] [PubMed] [Google Scholar]
- 5.Choleva L, Janko K, De Gelas K, Bohlen J, Šlechtová V, Rábová M. Synthesis of clonality and polyploidy in vertebrate animals by hybridization between two sexual species. Evolution. 2012;66:2191–203. doi: 10.1111/j.1558-5646.2012.01589.x. [DOI] [PubMed] [Google Scholar]
- 6.Pruvost NBM, Hoffmann A, Reyer HU. Gamete production patterns, ploidy, and population genetics reveal evolutionary significant units in hybrid water frogs (Pelophylax esculentus) Ecol Evol. 2013;3:2933–2946. doi: 10.1002/ece3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu SJ, Liu Y, Zhou GJ, Zhang XJ, Luo C, Feng H, et al. The formation of tetraploid stocks of red crucian carp × common carp hybrids as an effect of interspecific hybridization. Aquaculture. 2001;192:171–186. doi: 10.1016/S0044-8486(00)00451-8. [DOI] [Google Scholar]
- 8.Liu SJ. Distant Hybridization Leads to Different Ploidy Fishes. Sci China Life Sci. 2010;53:416–425. doi: 10.1007/s11427-010-0057-9. [DOI] [PubMed] [Google Scholar]
- 9.Sun YD, Liu SJ, Zhang C, Li JZ, Huang WR, Zhang J, et al. The chromosome number and gonadal structure of F9-F11 allotetraploid crucian carp. Acta Genetica Sinica. 2003;30:414–418. [PubMed] [Google Scholar]
- 10.Liu Y, Zhou GJ. Cytologicalstudy on the gonadal development of F1 hybrids produced by crossing carassius auratus with cyprinus carpio. Acta Hydrobiological Sinica. 1986;10:101–111. [Google Scholar]
- 11.Li JZ, Lu SQ, Liu SJ, Liu ZH, Zhang XJ, Liu Y. RAPD analysis of genetic variation between the allotetraploid hybrid of red crucian carp × common carp and their original parents. J Fish China. 2003;27:403–408. [Google Scholar]
- 12.Li JZ, Liu SJ, Zhang XJ, Lu SQ, Liu Y. Microsatellite Marker analysis of genetic variation between the allotetraploid crucian carp and the original parents. Acta Genetica Sinica. 2005;32:378–383. [PubMed] [Google Scholar]
- 13.Liu LG, Yan JP, Liu SJ, Liu D, You CP, Zhong H, et al. Evolutionary analysis of allotetrapoid hybrids of red crucian carp common carp, based on ISSR, AFLP molecular markers and cloning of cyclins genes. Chinese Sci Bull. 2009;54:2849–2861. doi: 10.1007/s11434-009-0476-9. [DOI] [Google Scholar]
- 14.Chen L, Li W, Liu SJ, Tao M, Long Y, Duan W, et al. Novel genetic markers derived from the DNA fragments of Sox genes. Mol Cell Probe. 2009;23:157–165. doi: 10.1016/j.mcp.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Ye LH, Liu QZ, Peng LY, Liu W, Yi XG, et al. Rapid genomic DNA changes in allotetraploid fish hybrids. Heredity. 2015;114:601–609. doi: 10.1038/hdy.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leflon M, Eber F, Letanneur JC, Chelysheva L, Coriton O, Huteau V, et al. Pairing and recombination at meiosis of Brassica rapa(AA) × Brassica napus (AACC) hybrids. Theor Appl Genet. 2006;113:1467–1480. doi: 10.1007/s00122-006-0393-0. [DOI] [PubMed] [Google Scholar]
- 17.Kosmala A, Zwierzykowski Z, Gasior D, Rapacz M, Zwierzykowska E, Humphreys MW. GISH/FISH mapping of genes for freezing tolerance transferred from Festuca pratensis to Lolium multiflorum. Heredity. 2006;96:243–251. doi: 10.1038/sj.hdy.6800787. [DOI] [PubMed] [Google Scholar]
- 18.Xiong ZY, Pires CJ. Karyotype and identification of all homoeologous chromosomes of allopolyploid brassica napus and its diploid progenitors. Genetics. 2011;187:37–49. doi: 10.1534/genetics.110.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long EB, David IB. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49:727–764. doi: 10.1146/annurev.bi.49.070180.003455. [DOI] [PubMed] [Google Scholar]
- 20.Martins C, Wasko AP. Orgnization and evolution of 5S ribosomal DNA in the fish genome. In: Williams CR, editor. Focus on Genome research. New York: Nova; 2004. pp. 289–318. [Google Scholar]
- 21.Qin QB, He WG, Liu SJ, Wang J, Xiao J, Liu Y. Analysis of 5S rDNA organization and variation in polyploid hybrids from crosses of different fish subfamilies. J Exp Zool (Mol Dev Evol) 2010;314:403–411. doi: 10.1002/jez.b.21346. [DOI] [PubMed] [Google Scholar]
- 22.He W, Qin QB, Liu SJ, Li TL, Wang J, Xiao J, et al. Organization and variation analysis of 5S rDNA in different ploidy-level hybrids of Red Crucian Carp × Topmouth Culter. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruth BP, Kent MR. Location of repetitive DNAs to zebrafish (Danio rerio) chromosomes by fluorescence in situ hybridization (FISH) Chromosome Res. 2000;8:27–35. doi: 10.1023/A:1009271017998. [DOI] [PubMed] [Google Scholar]
- 24.Singh M, Kumar R, Nagpure NS, Kushwaha B, Gond I, Lakra WS. Chromosomal localization of 18S and 5S rDNA using FISH in the genus Tor(Pisces, Cyprinidae) Genetica. 2009;137:245–252. doi: 10.1007/s10709-009-9367-x. [DOI] [PubMed] [Google Scholar]
- 25.Zhu HP, Ma DM, Gui JF. Triploid origin of the gibel carp as revealed by 5S rDNA localization and chromosome painting. Chromosome Res. 2006;14:767–776. doi: 10.1007/s10577-006-1083-0. [DOI] [PubMed] [Google Scholar]
- 26.Masaru M, Hideo F. Characterization of repetitive DNA sequences carrying 5S rDNA of the triploid ginbuna. Genes Genet Syst. 1998;73:9–20. doi: 10.1266/ggs.73.9. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964;52:197–201. [Google Scholar]
- 29.Martins C, Galetti PMJ. Two 5S Rdna arrays in Neotropical fish species: is it a general rule for fishes? Genetica. 2001;111:439–446. doi: 10.1023/A:1013799516717. [DOI] [PubMed] [Google Scholar]
- 30.Campo D, Machado-Schiaffino G, Horreo JL, Garcia-Vazquez E. Molecular Organization and Evolution of 5S rDNA in the Genus Merluccius and Their Phylogenetic Implications. J Mol Evol. 2009;68:208–216. doi: 10.1007/s00239-009-9207-8. [DOI] [PubMed] [Google Scholar]
- 31.Ksiazczyk T, Zwierzykowska E, Molik K, Taciak M, Kraiewski P, Zvierzykowski Z. Genome-dependent chromosome dynamics in three successive generations of the allotetraploid Festuca pratensis × Lolium perenne hybrid. Protoplasma. 2015;252:985–996. doi: 10.1007/s00709-014-0734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He WG, Xie LH, Li TL, Liu SJ, Xiao J, Hu J, et al. The formation of diploid and triploid hybrids of female grass carp × male blunt snout bream and their 5S rDNA analysis. BMC Genetics. 2013;14:110. doi: 10.1186/1471-2156-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin QB, Wang YD, Wang J, Dai J, Xiao J, Hu FZ, et al. The autotetraploid fish derived from hybridization of Carassius auratus red var. (Female) × Megalobrama amblycephala (Male) Biology of reproduction. 2014;91(4):93. doi: 10.1095/biolreprod.114.122283. [DOI] [PubMed] [Google Scholar]
- 34.Mason AS, Nelson MN, Takahira J, Cowling WA, Moreira Alves G, Chaudhuri A, et al. The fate of chromosomes and Alleles in an allohexaploid Brassica Population. Genetics. 2014;197:273–283. doi: 10.1534/genetics.113.159574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uno Y, Nishida C, Takagi C, Ueno N, Matsudal Y. Homoeologous chromosomes of Xenopus laevis are highly conserved after whole-genome duplication. Heredity. 2013;111(5):430–436. doi: 10.1038/hdy.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]


