Abstract
Background
The main purpose of the present study is to evaluate whether treatment with long-acting human glucagon-like peptide-1 liraglutide was associated with an improvement of excessive daytime sleepiness (EDS) in obese subjects with type-2 diabetes.
Methods
This single-centre retrospective study included 158 obese (body mass index [BMI] ≥ 30 kg/m2) adult subjects with type-2 diabetes who were initiated with liraglutide treatment at least 3 months before study inclusion. Data of the Epworth Sleepiness Scale (ESS), anthropometric parameters, glucose-control and metabolic parameters were collected at liraglutide initiation (baseline) and at months 1 and 3 after liraglutide initiation.
Results
Significant reductions in ESS score were achieved at months 1 (−1.3 ± 2.8, p < 0.001) and 3 (−1.5 ± 3.0, p < 0.001) after liraglutide introduction. After 3 months of treatment with liraglutide, significant changes in body weight (p < 0.001), BMI (p < 0.001), waist (p < 0.001) and neck circumferences (p < 0.005), HbA1c (p < 0.001), mean blood glucose (p < 0.001), fasting plasma glucose (p < 0.001), triglycerides (p < 0.01) and total cholesterol (p < 0.001) were achieved.
Conclusions
After 3 months of treatment with liraglutide a significant reduction in EDS was observed in obese subjects with type-2 diabetes. Besides this, significant changes in body weight and metabolic parameters of diabetes control were also accomplished. Further investigation is required to determine whether liraglutide could improve other abnormal sleep patterns and obstructive sleep apnoea.
Keywords: Excessive daytime sleepiness, Abnormal sleep patterns, Obstructive sleep apnoea, Type 2 diabetes, Obesity, Liraglutide, Glucagon-like peptide-1
Background
Abnormal sleep patterns (ASPs), characterized by short or long durations of sleep and excessive daytime sleepiness (EDS), have a considerable impact on an individual’s health [1]. Obstructive sleep apnea (OSA) is the most common form of ASP, characterized by the repetitive complete or partial collapse of the upper airway during sleep [2]. There is a growing body of literature describing the association between ASPs and the subsequent development of type-2 diabetes, even in non-obese individuals [3–7]. EDS is more prevalent in subjects with diabetes and in subjects with insulin resistance, a well-known prediabetic condition [8, 9]. Additionally, OSA has also been shown to impact on glycemic control among diabetic patients, independently of obesity [10]. Recently, a study concluded that impaired sleep quality and daytime sleepiness are associated with decreased diabetes self-management in adults with type 2 diabetes [11]. EDS has been also related to severe hypoglycemia in subjects with type 2 diabetes in the Edinburgh type 2 diabetes study [12]. Quality of life and life expectancy could be affected by this condition in this population.
In a number of clinical studies, bariatric surgery-induced weight loss in obese subjects was associated with a dramatic improvement in subjective sleepiness, as well as other associated comorbidities [13–15]. EDS was also reduced with a weight reduction program, including dietary counselling and behavioral change support [16].
Liraglutide, a long-acting human glucagon-like peptide-1 receptor agonist (GLP-1ra) [17], is indicated for the treatment of type 2 diabetes. Liraglutide exerts its potent glucose-lowering effect through different mechanisms that lead to clinically significant reductions in glycated haemoglobin (HbA1c) and body weight [18]. A reduction of insulin resistance has been proved in patients with type 2 diabetes treated with liraglutide [19].
Lower body weight, decreased insulin resistance and an improvement in glycaemic control could ameliorate the perceived quality of life of subjects with type 2 diabetes and, to some extent, to relieve EDS.
Therefore, all the above prompted us to evaluate whether treatment with GLP-1ra liraglutide was associated with an improvement of EDS in obese subjects with type-2 diabetes in routine clinical practice setting.
Methods
This retrospective observational study was carried out at the Hospital General de Segovia (Segovia, Spain) in accordance with the Declaration of Helsinki, including all amendments. It was approved on February 4th 2012 by the Clinical Research and Ethics Commission of the Hospital General de Segovia (Study code SEE-DIA-2012-11). All participants provided written informed consent to use their data prior to the study.
Study subjects
The study population comprised obese (BMI ≥ 30 kg/m2) adult subjects with a documented diagnosis of type 2 diabetes who were initiated on liraglutide (Victoza®, Novo Nordisk, Bagsvaerd, Denmark) treatment at least 3 months before study inclusion. A pre-existing clinical diagnosis of OSA was not deemed necessary for inclusion. No patient was treated with continuous positive airway pressure (CPAP) during this study period. Subjects were excluded if they had undergone bariatric surgery or presented weight loss > 5 % within the previous 6 months. Patients with cognitive impairment were also excluded.
Measurements
Following routine clinical practice, anthropometric parameters, glycaemic control and metabolic measurements, and the Epworth Sleepiness Scale (ESS) score data were retrospectively obtained from medical records at three time points: at liraglutide initiation (baseline) and at months 1 and 3 after liraglutide initiation.
Anthropometric parameters
Height (m) and weight (kg) were recorded. Body mass index (BMI) was calculated as weight/height squared (kg/m2). Waist circumference was measured at umbilical level in standing positions with a non-stretchable tape (cm). Also, body composition measurements were determined by bioelectrical impedance.
Glucose control measurements and metabolic parameters
Glucose control measurements included HbA1c, mean blood glucose (MBG) from self-monitored blood glucose profiles and fasting plasma glucose (FPG). The metabolic parameters included the collection of high-density-lipoprotein cholesterol (HDL), low-density-lipoprotein cholesterol (LDL), total cholesterol (TC) and triglycerides (TG) levels.
Epworth Sleepiness Scale
The ESS score was used to measure the clinical impact of EDS. It is defined as a simple and self-administered questionnaire which provides a measurement of the subject’s general level of sleep propensity and likelihood of dozing off in eight different real-life situations. All items are rated on a 4-point Likert scale, 0 = would never doze, 1 = slight chance of dozing, 2 = moderate chance of dozing, 3 = high chance of dozing. Scores vary from 0 to 24, with higher scores indicating higher levels of sleepiness [20]. At present, there are no universally adapted cut-off ranges for the ESS, but Drager et al. determined the sensitivity and specificity of ESS in predicting OSA (defined as ESS score >10) as 49 % and 80 %, respectively [21]. According to domain experts, we considered a 1-point reduction in ESS score to be clinically significant [22].
Statistical considerations
The values are expressed as mean ± standard deviation (SD) for quantitative variables and as percentages for categorical variables. Means were compared with T-test and the correlation analyses were assessed with the Pearson/Spearman correlation coefficient. The significance level was determined at p < 0.05 and all statistical analyses were carried out with the statistical package SPSS v.18.0 (SPSS, Inc., Chicago, IL).
Results
Subjects characteristics
Between March 2012 and June 2013, a total of 163 subjects were included. Four subjects were excluded due to screening failure (n = 2) and incomplete data (n = 2), and another subject did not meet all the selection criteria. Thus, the evaluable population comprised 158 subjects, whose clinical and metabolic parameters are listed in Table 1. At baseline, mean ESS score was 5.9 ± 4.5 and 26 (17.4 %) subjects reached an ESS > 10.
Table 1.
Baseline characteristics (N = 158)
| Characteristics | Value |
|---|---|
| Age (years), mean ± SD | 58.2 ± 11.5 |
| Gender, n (%) | |
| Male | 78 (49.4) |
| Female | 80 (50.6) |
| Duration of type 2 diabetes (years), mean ± SD | 11.0 ± 7.2 |
| BMI (kg/m2), mean ± SD | 38.1 ± 6.6 |
| Waist perimeter (cm), mean ± SD | 119.2 ± 13.7 |
| Body fat (%), mean ± SD | 40.6 ± 7.7 |
| HbA1c (mmol/mol), mean ± SD | 68 ± 16 |
| MBG (mmol/L), mean ± SD | 10.8 ± 2.4 |
| FPG (mmol/L), mean ± SD | 10.1 ± 3.6 |
| HDL (mmol/L), mean ± SD | 1.1 ± 0.3 |
| LDL (mmol/L), mean ± SD | 2.7 ± 1.0 |
| TC (mmol/L), mean ± SD | 4.8 ± 1.3 |
BMI body mass index, FPG fasting plasma glucose, HbA1c haemoglobin A1c, HDL high-density-lipoprotein cholesterol, LDL low-density-lipoprotein cholesterol, MBG mean blood glucose, SD standard deviation, TC total cholesterol
Subjects were initiated with liraglutide treatment injected subcutaneously from the starting dose of 0.6 mg/day and were increased over one week to 1.2 mg/day by the attending physicians.
Changes of ESS score, anthropometric data, glucose-control measurements and metabolic parameters
The evolution of body weight, HbA1c and ESS score from baseline to months 1 and 3 are depicted in Fig. 1. Pairwise comparisons showed that the ESS score significantly decreased from baseline to month 1 (6.3 ± 4.6 vs 4.9 ± 3.9; p < 0.001) and from baseline to month 3 (5.7 ± 4.4 vs 4.2 ± 3.6; p < 0.001). After three months of treatment with liraglutide, significant reductions were achieved in the means of body weight (102.8 ± 17.9 kg vs 98.4 ± 16.9 kg, p < 0.001), BMI (39.6 ± 6.8 kg/m2vs 37.9 ± 6.4 kg/m2; p < 0.001), waist circumference (122.1 ± 14.0 cm vs 118.9 ± 13.3 cm; p < 0.001) and neck circumference (42.2 ± 3.3 cm vs 40.8 ± 3.3 cm; p < 0.005), while the body fat percentage hardly changed (42.1 ± 7.6 % vs 41.2 ± 8.1 %; p = 0.173). Comparison of glucose-control measurements and metabolic parameters between baseline and month 3 are presented in Table 2.
Fig. 1.
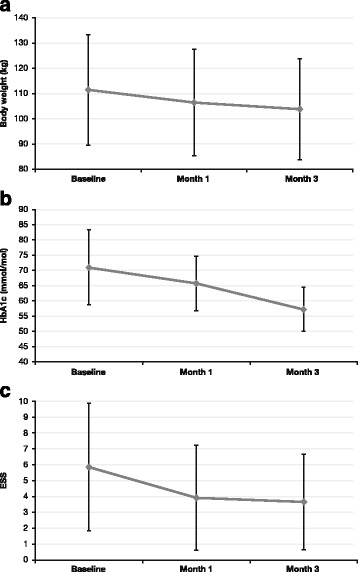
Evolution of body weight (a), HbA1c (b) and ESS score (c)
Table 2.
Pairwise comparison of glucose-control measurements and metabolic parameters after 3 months of treatment with liraglutide
| Baseline (mean ± SD) | Month 3 (mean ± SD) | p-value | |
|---|---|---|---|
| HbA1c (mmol/mol) | 68 ± 16 | 56 ± 14 | <0.001 |
| MBG (mmol/L) | 10.8 ± 2.4 | 9.1 ± 2.0 | <0.001 |
| FPG (mmol/L) | 10.7 ± 4.1 | 7.9 ± 2.1 | <0.001 |
| TG (mmol/L) | 2.7 ± 3.7 | 1.7 ± 1.2 | <0.01 |
| HDL (mmol/L) | 1.0 ± 0.2 | 0.9 ± 0.2 | NS |
| LDL (mmol/L) | 3.0 ± 1.1 | 2.6 ± 0.6 | NS |
| TC (mmol/L) | 5.1 ± 1.2 | 4.3 ± 0.7 | <0.001 |
FPG fasting plasma glucose, HbA1c haemoglobin A1c, HDL high-density-lipoprotein cholesterol, LDL low-density-lipoprotein cholesterol, MBG mean blood glucose, NS not significant, SD standard deviation, TC total cholesterol, TG triglycerides
The relative changes in HbA1c and body weight, HbA1c and ESS, as well as in body weight and ESS from baseline to month 3 after liraglutide initiation are presented as scatter plots in Fig. 2a, b and c, respectively. Overall, the majority of the study subjects were located in the lower left quadrant, i.e. HbA1c, body weight, and ESS score decreased for these individuals.
Fig. 2.
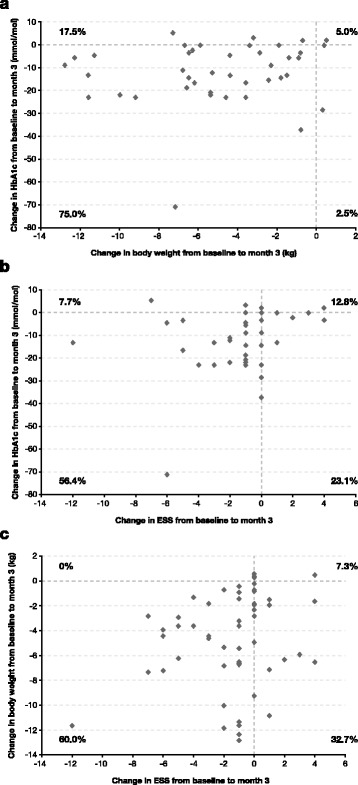
Scatter plots of changes in HbA1c and body weight (a), HbA1c and ESS (b) and body weight and ESS (c) from baseline to month 3 of treatment with liraglutide
Correlation of ESS score with the analytical parameters and the anthropometric measures
Baseline ESS score was negatively correlated with baseline body weight, BMI, waist perimeter, percentage of body fat, and HDL levels (Table 3). However, the only variable associated with changes in ESS score was ‘reductions in body weight’ (r = 0.269, p < 0.05). The rest of the parameters did not achieve statistical significance.
Table 3.
Correlations between baseline parameters and ESS score
| Baseline ESS score | ||
|---|---|---|
| r | p-value | |
| Body weight | −0.179 | <0.05 |
| BMI | −0.179 | <0.05 |
| Waist circumference | −0.175 | <0.05 |
| Body fat | −0.188 | <0.05 |
| HDL | −0.224 | <0.05 |
BMI body mass index, ESS Epworth Sleepiness Scale, HDL high-density-lipoprotein cholesterol
Discussion
To the best of our knowledge, this is the first study showing an association of liraglutide-induced body weight reduction and an improvement of EDS in obese subjects with type 2 diabetes in routine clinical practice. Recent scientific contributions are suggesting that ASPs, which includes EDS, are not only related to impaired health-related quality of life, but also influence the microvascular complications of diabetes (i.e. diabetic neuropathy) [23].
Within three months of therapy with liraglutide, significant reductions were achieved not only in body weight and BMI, but also in waist and neck circumferences. Concurrently, ESS score was reduced following one month of follow-up and was maintained after three months.
In our study, baseline anthropometric measures of obesity were negatively correlated with baseline ESS score. Some previous studies showed that the association between diabetes or insulin resistance and EDS was independent of obesity [8, 9]. As an example, Dixon et al. found that anthropometric measures explained only 3 % of ESS score variance in 1055 subjects presenting for obesity surgery [8]. Although our study population comprised a sample of very obese (mean BMI, 38.1 kg/m2) subjects with type 2 diabetes, due to the reimbursement policy for liraglutide prescription in Spain, it is possible that obesity in this particular group of patients was not determinant for baseline EDS. Despite changes in ESS score were shown to correlate positively with the reduction in body weight, no significant relation was achieved with the change in total body fat mass. This finding is consistent with earlier studies showing similar reductions in ESS score with surgical or non-pharmacological interventions reducing body weight [13–16].
Additionally to the direct effect of weight loss in EDS, a positive impact on perceived wellbeing or mood could be implicated in this improvement, because EDS was associated with poor energy and symptoms of depression in previous studies [7].
Liu et al. showed that EDS diagnosed with ESS, was prevalent amongst the insulin-resistant subgroup of obese individuals in comparison to their weight-matched insulin-sensitive counterpart [24]. We measured two surrogate markers of insulin resistance: HDL-cholesterol levels and waist circumference. Baseline ESS was correlated with low HDL-cholesterol levels in our sample. Regarding anthropometric markers of insulin resistance, previous studies confirmed a relationship between EDS and greater waist circumference [25, 26]. The present study showed that liraglutide effectively reduced waist circumference, finally accompanied by a significant decrease in the mean ESS score.
In this context, an important perspective to consider is whether sleep/wake patterns could be partly modulated by food intake, especially with a high-fat dietary intake. Findings from several studies conducted in animal models demonstrated that high-fat diet and weight gain were associated with impaired sleep patterns, with more sleep time during the animals’ subjective day [27–29]; Wells et al. explored in a small group of adult volunteers (n = 16) the effects of a meal on objective and subjective measures of daytime sleepiness, showing that regular ingestion of fat-rich meals and significant excess of nutrients can predict EDS and poor quality of nocturnal sleep [30]. Clinical data showed reduced food intake, enhanced satiety and subsequent reductions in body weight were achieved upon the administration of GLP-1ra in patients with type 2 diabetes [31] and non-diabetic adults [32]. Interestingly, in a small study conducted with 20 obese and type-2 diabetic Japanese subjects, short-term treatment with liraglutide effectively reduced visceral fat adiposity, appetite and the urge for fat intake [33]. Collectively, we could contemplate that GLP-1ra liraglutide could improve EDS not only as a result of weight reduction, but also by decreasing appetite and reducing fat intake.
Our study participants were obese and had type-2 diabetes. After three months of treatment with liraglutide 75 % of subjects resulted in the reduction of both HbA1c and body weight, 17.5 % of patients revealed weight reduction but deterioration of HbA1c, while 2.6 % of subjects presented improvement in HbA1c levels but weight gain (Fig. 2a). These results entail that GLP-1ra liraglutide exerts its glucose-lowering action and improvement of body weight through different mechanisms. Regulatory agencies and international guidelines, such as the National Institute for Health and Care Excellence (NICE guidelines) [34] for the management of type 2 diabetes recommend to promote not only HbA1c reduction, as an indicator of metabolic improvement, but also weight loss (even at a lesser degree) as definition of “responders” to a GLP-1ra treatment for type 2 diabetes. Therefore, long-acting GLP-1ra liraglutide therapy appears to be a promising new agent for the management of ASPs in obese subjects with type 2 diabetes, especially when weight loss is a major concern.
Some limitations of this study need to be highlighted when interpreting the results. It should be noted that data from sleep studies (i.e. polysomnography) are lacking. The reason behind is that the study was aimed to reflect merely routine clinical practice, and no interventions or new procedures other than physicians’ daily routine were conducted. However, in light of all the promising results obtained, further prospective clinical studies should be conducted addressing this clinical endpoint. It is also worth acknowledging that the present study is an observational study, but not a randomized clinical trial. Although conducting observational studies are of great relevance to learn about the conditions derived from the routine clinical practice setting, they do not provide strong evidence. Furthermore, other factors in a free living environment might impact on the results, such as diet or exercise. Finally, it can be ascertained from the lack of a comparator group and the retrospective nature of the study design that biases could have been introduced when collecting data.
Conclusions
In conclusion, three months of treatment with liraglutide effectively reduce body weight and waist circumference and ameliorates the glycaemic control measurements and lipid parameters. Concurrently, an improvement in mean ESS score was also achieved. Nevertheless, further research is needed to confirm and expand these preliminary results.
Acknowledgements
The authors gratefully acknowledge financial support of Fundación de la Sociedad Española de Endocrinología y Nutrición through the eDM-11 grant. Medical writing support was provided by Ana López-Ballesteros and Antonio Torres-Ruiz at Dynamic S.L., during the preparation of this paper. Responsibility for opinions, conclusions and interpretation of data lies entirely with the authors.
Abbreviations
- ASP
Abnormal sleep patterns
- BMI
Body mass index
- EDS
Excessive daytime sleepiness
- ESS
Epworth sleepiness scale
- FPG
Fasting plasma glucose
- GLP-1
Glucagon-like peptide-1
- HbA1c
Glycated haemoglobin
- HDL
High-density-lipoprotein cholesterol
- LDL
Low-density-lipoprotein cholesterol
- MBG
Mean blood glucose
- OSA
Obstructive sleep apnea
- SD
Standard deviation
- TC
Total cholesterol
- TG
Triglycerides
Footnotes
Competing interests
F. Gomez-Peralta has participated in the Advisory Panel for Sanofi-aventis and Novo Nordisk; he has also participated as Research Support for Sanofi-aventis, Novo Nordisk, Boehringer Ingelheim, and Lilly; and has received honoraria for speaking for Sanofi-aventis, Novo Nordisk, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, and Lilly. C. Abreu has participated as Research Support for Sanofi-aventis, Novo Nordisk, Boehringer Ingelheim, and Lilly; and has received honoraria for speaking from Sanofi-aventis, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals, AstraZeneca, and Bristol-Myers Squibb. J.C. Castro has participated as Research Support for Sanofi-aventis, Novo Nordisk, Boehringer Ingelheim, and Lilly. E. Alcarria has participated as Research Support for Sanofi-aventis, Novo Nordisk, Boehringer Ingelheim, and Lilly. M. Cruz-Bravo has participated as Research Support for Sanofi-aventis, Novo Nordisk, Boehringer Ingelheim, and Lilly. M.J. Llorente has participated as Research Support for Sanofi-aventis, Novo Nordisk, Boehringer Ingelheim, and Lilly. C. Albornos has participated as Research Support for Sanofi-aventis, Novo Nordisk, Boehringer Ingelheim, and Lilly. C. Moreno has participated as Research Support for Sanofi-aventis, Novo Nordisk, Boehringer Ingelheim, and Lilly. M. Cepeda: None. F. Almodóvar has participated as Research Support for Sanofi-aventis, Novo Nordisk; and has received honoraria for speaking for Novo Nordisk and Lilly.
Authors’ contributions
All authors read and approved the final manuscript; their individual contribution was as follows: FGP: conception and design of the study, acquisition, analysis and interpretation of data, drafting the article and revising it critically, approval of final article; CA: acquisition of data, analysis and interpretation of data, revising the article critically, approval of final article; JCC: acquisition of data, revising the article critically, approval of final article; EA: acquisition of data, revising the article critically, approval of final article; MC-B: acquisition of data, revising the article critically, approval of final article; MJG-LL: acquisition of data, revising the article critically, approval of final article; CA: acquisition of data, revising the article critically, approval of final article; CM: acquisition of data, revising the article critically, approval of final article; MC: acquisition of data, revising the article critically, approval of final article; FA: acquisition of data; revising the article critically, approval of final article.
References
- 1.Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28(1):113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 2.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–76. [PMC free article] [PubMed] [Google Scholar]
- 3.Resnick HE, Redline S, Shahar E, Gilpin A, Newman A, Walter R, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26(3):702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27(1):282–3. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165(8):863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 6.Tuomilehto H, Peltonen M, Partinen M, Seppa J, Saaristo T, Korpi-Hyovalti E, et al. Sleep duration is associated with an increased risk for the prevalence of type 2 diabetes in middle-aged women - The FIN-D2D survey. Sleep Med. 2008;9(3):221–7. doi: 10.1016/j.sleep.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Chaput JP, Despres JP, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50(11):2298–304. doi: 10.1007/s00125-007-0786-x. [DOI] [PubMed] [Google Scholar]
- 8.Dixon JB, Dixon ME, Anderson ML, Schachter L, O’brien PE. Daytime sleepiness in the obese: not as simple as obstructive sleep apnea. Obesity (Silver Spring) 2007;15(10):2504–11. doi: 10.1038/oby.2007.297. [DOI] [PubMed] [Google Scholar]
- 9.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 10.Kim NH, Cho NH, Yun CH, Lee SK, Yoon DW, Cho HJ, et al. Association of obstructive sleep apnea and glucose metabolism in subjects with or without obesity. Diabetes Care. 2013;36(12):3909–15. doi: 10.2337/dc13-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chasens ER, Korytkowski M, Sereika SM, Burke LE. Effect of poor sleep quality and excessive daytime sleepiness on factors associated with diabetes self-management. Diabetes Educ. 2013;39(1):74–82. doi: 10.1177/0145721712467683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inkster B, Riha RL, Van LL, Williamson R, McLachlan S, Frier BM, et al. Association between excessive daytime sleepiness and severe hypoglycemia in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2013;36(12):4157–9. doi: 10.2337/dc13-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritscher LG, Mottin CC, Canani S, Chatkin JM. Obesity and obstructive sleep apnea-hypopnea syndrome: the impact of bariatric surgery. Obes Surg. 2007;17(1):95–9. doi: 10.1007/s11695-007-9012-7. [DOI] [PubMed] [Google Scholar]
- 14.Holty JE, Parimi N, Ballesteros M, Blackwell T, Cirangle PT, Jossart GH, et al. Does surgically induced weight loss improve daytime sleepiness? Obes Surg. 2011;21(10):1535–45. doi: 10.1007/s11695-010-0213-0. [DOI] [PubMed] [Google Scholar]
- 15.Varela JE, Hinojosa MW, Nguyen NT. Resolution of obstructive sleep apnea after laparoscopic gastric bypass. Obes Surg. 2007;17(10):1279–82. doi: 10.1007/s11695-007-9228-6. [DOI] [PubMed] [Google Scholar]
- 16.Nerfeldt P, Nilsson BY, Mayor L, Udden J, Friberg D. A two-year weight reduction program in obese sleep apnea patients. J Clin Sleep Med. 2010;6(5):479–86. [PMC free article] [PubMed] [Google Scholar]
- 17.Elbrond B, Jakobsen G, Larsen S, Agerso H, Jensen LB, Rolan P, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes Care. 2002;25(8):1398–404. doi: 10.2337/diacare.25.8.1398. [DOI] [PubMed] [Google Scholar]
- 18.Bode B. An overview of the pharmacokinetics, efficacy and safety of liraglutide. Diabetes Res Clin Pract. 2012;97(1):27–42. doi: 10.1016/j.diabres.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105(8):1135–9. doi: 10.1016/j.amjcard.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Balk EM, Moorthy D, Obadan NO, Patel K, Ip S, Chung M, Bannuru RR, Kitsios GD, Sen S, Iovin RC, Gaylor JM, and et al. Diagnosis and Treatment of Obstructive Sleep Apnea in Adults. Comparative Effectiveness Review No. 32. (Prepared by Tufts Evidence-based Practice Center under Contract No. 290-2007-10055-1). In: AHRQ. 2011. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0016344/pdf/PubMedHealth_PMH0016344.pdf. Accessed 17 Apr 2015. [PubMed]
- 23.Raman R, Gupta A, Venkatesh K, Kulothungan V, Sharma T. Abnormal sleep patterns in subjects with type II diabetes mellitus and its effect on diabetic microangiopathies: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS, report 20) Acta Diabetol. 2012;49(4):255–61. doi: 10.1007/s00592-010-0240-2. [DOI] [PubMed] [Google Scholar]
- 24.Liu A, Kushida CA, Reaven GM. Risk for obstructive sleep apnea in obese, nondiabetic adults varies with insulin resistance status. Sleep Breath. 2013;17(1):333–8. doi: 10.1007/s11325-012-0696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayley AC, Williams LJ, Kennedy GA, Berk M, Brennan SL, Pasco JA. Excessive daytime sleepiness and metabolic syndrome: a cross-sectional study. Metabolism. 2015;64(2):244–52. doi: 10.1016/j.metabol.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Renko AK, Hiltunen L, Laakso M, Rajala U, Keinanen-Kiukaanniemi S. The relationship of glucose tolerance to sleep disorders and daytime sleepiness. Diabetes Res Clin Pract. 2005;67(1):84–91. doi: 10.1016/j.diabres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Basterfield L, Lumley LK, Mathers JC. Wheel running in female C57BL/6J mice: impact of oestrus and dietary fat and effects on sleep and body mass. Int J Obes (Lond). 2009;33(2):212–8. [DOI] [PubMed]
- 28.Jenkins JB, Omori T, Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased in mice with obesity induced by high-fat food. Physiol Behav. 2006;87(2):255–62. doi: 10.1016/j.physbeh.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Panossian LA, Veasey SC. Daytime sleepiness in obesity: mechanisms beyond obstructive sleep apnea--a review. Sleep. 2012;35(5):605–15. doi: 10.5665/sleep.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells AS, Read NW, Idzikowski C, Jones J. Effects of meals on objective and subjective measures of daytime sleepiness. J Appl Physiol (1985) 1998;84(2):507–15. doi: 10.1152/jappl.1998.84.2.507. [DOI] [PubMed] [Google Scholar]
- 31.Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276(5 Pt 2):R1541–4. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- 32.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) 2014;38(6):784–93. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue K, Maeda N, Kashine S, Fujishima Y, Kozawa J, Hiuge-Shimizu A, et al. Short-term effects of liraglutide on visceral fat adiposity, appetite, and food preference: a pilot study of obese Japanese patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:109. doi: 10.1186/1475-2840-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The National Institute for Health and Care Excellence. Management of type-2 diabetes. 2009. https://www.nice.org.uk/guidance/ta203/resources/nice-recommendsliraglutide-for-type-2-diabetes-mellitus4. Accessed 17 Apr 2015. [PubMed]


