Abstract
Importance
Intermittent preventive therapy with sulfadoxine-pyrimethamine to control malaria during pregnancy is used in 37 countries in sub-Saharan Africa, and 31 of those countries use the standard 2-dose regimen. However, 2 doses may not provide protection during the last 4 to 10 weeks of pregnancy, a pivotal period for fetal weight gain.
Objective
To perform a systematic review and meta-analysis of trials to determine whether regimens containing 3 or more doses of sulfadoxine-pyrimethamine for intermittent preventive therapy during pregnancy are associated with a higher birth weight or lower risk of low birth weight (LBW) (<2500 g) than standard 2-dose regimens.
Data Sources and Study Selection
ISI Web of Knowledge, EMBASE, SCOPUS, PubMed, LILACS, the Malaria in Pregnancy Library, Cochrane CENTRAL, and trial registries from their inception to December 2012, without language restriction. Eligible studies included randomized and quasi-randomized trials of intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine monotherapy.
Data Extraction
Data were independently abstracted by 2 investigators. Relative risk (RR), mean differences, and 95% CIs were calculated with random-effects models.
Results
Of 241 screened studies, 7 trials of 6281 pregnancies were included. The median birth weight in the 2-dose group was 2870 g (range, 2722–3239 g) and on average 56 g higher (95% CI, 29–83 g; I2=0%) in the ≥3-dose group. Three or more doses were associated with fewer LBW births (RR,0.80; 95% CI, 0.69–0.94; I2=0%), with a median LBW risk per 1000 women in the 2-dose group (assumed control group risk) of 167 per 1000 vs 134 per 1000 in the ≥3-dose group (absolute risk reduction, 33 per 1000 [95% CI, 10–52]; number needed to treat=31). The association was consistent across a wide range of sulfadoxine-pyrimethamine resistance (0% to 96% dihydropteroate-synthase K540E mutations). There was no evidence of small-study bias. The ≥3-dose group had less placental malaria (RR,0.51; 95% CI, 0.38–0.68; I2=0%, in 6 trials, 63 vs 32 per 1000; absolute risk reduction,31 per 1000 [95% CI, 20–39]). In primigravid plus secundigravid women, the risk of moderate to severe maternal anemia was lower in the ≥3-dose group (RR,0.60; 95% CI, 0.36–0.99; I2=20%; in 6 trials, 36 vs 22 per 1000; absolute risk reduction,14 per 1000 [95% CI, 0.4–23]). There were no differences in rates of serious adverse events.
Conclusions and Relevance
Among pregnant women in sub-Saharan Africa, intermittent preventive therapy with 3 or more doses of sulfadoxine-pyrimethamine was associated with a higher birth weight and lower risk of LBW than the standard 2-dose regimens. These data provide support for the new WHO recommendations to provide at least 3 doses of intermittent preventive therapy during pregnancy at each scheduled antenatal care visit in the second and third trimester.
In Areas Of Stable Malaria Transmission in sub-Saharan Africa, Plasmodium falciparum infection in pregnant women is associated with maternal anemia and low birth weight (LBW) (<2500 g),1–3 especially among primigravida and secundigravida and human immunodeficiency virus (HIV)–infected women.1 The World Health Organization (WHO) recommended intermittent preventive therapy during pregnancy, consisting of at least 2 full treatment doses of sulfadoxine-pyrimethamine for HIV-negative women and at least 3 doses for HIV-positive women not receiving cotrimoxazole, administered presumptively in the second and third trimesters at least 1 month apart.4,5 Each dose suppresses or clears any existing asymptomatic infections from the placenta and provides up to 6 weeks of posttreatment prophylaxis.4,6 Although the standard 2-dose regimen provides at most 12 weeks of prophylaxis,6 it has been shown to be effective in reducing LBW7–13 and was adopted by 31 of 37 endemic countries in Africa with a policy for intermittent preventive therapy during pregnancy; the remaining countries use a 3-dose or monthly regimen.14 Nevertheless, reinfections are common with the 2-dose regimen, especially among women who complete their last dose early in the third trimester.8,9 A previous meta-analysis7 of 3 trials confirmed that additional doses of sulfadoxine-pyrimethamine may add benefit over 2 doses among HIV-infected primigravida plus secundigravida (G1–G2 women), but there was insufficient evidence on HIV-negative women or intermittent preventive therapy during pregnancy when used in combination with insecticide-treated nets. Furthermore, increasing sulfadoxine-pyrimethamine resistance, which results in a progressive decrease of the duration of the prophylactic effect,6 may also require more frequent dosing.7
The objective of this analysis was to evaluate whether 3 or more doses of intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine are associated with higher birth weight or a lower risk of LBW than the current standard 2-dose regimen and to examine whether this is moderated by sulfadoxine-pyrimethamine resistance, HIV status, gravidity, or use of insecticide-treated nets.
METHODS
Eligibility Criteria
Study inclusion criteria, outcomes, and methods for the analysis were prespecified in the protocol. Studies had to be quasi-randomized or randomized controlled trials conducted with pregnant women living in sub-Saharan Africa, comparing the standard 2-dose regimen with sulfadoxine-pyrimethamine with a regimen of intermittent preventive therapy during pregnancy consisting of 3 doses or monthly dosing. Studies or study groups that combined sulfadoxine-pyrimethamine with other antimalarial drugs, such as artemisinin derivatives or azithromycin, or other interventions, such as screening for malaria, were excluded. Use of mosquito nets was not an exclusion criterion. Trial inclusion was unrestricted by gravida group, HIV status, and type of outcomes reported.
Study Selection
Studies were identified by searching PubMed, SCOPUS, ISI Web of Knowledge, EMBASE, LILACS, Cochrane CENTRAL, the Malaria in Pregnancy Library,15 WHO’s International Clinical Trials Registry Platform, and the Cochrane Central Register of Controlled Trials from their inception to December 11, 2012, without language restrictions; scanning reference lists of articles; and consultation with experts in the field (see eFigure 1 and eMethods, available at http://www.jama.com). For trial selection, 2 authors (K.K. and A.M.v.E.) independently screened and assessed trials for eligibility and final inclusion in the analysis in a standardized manner. Disagreement between reviewers was resolved through consensus after discussion and consultation with the senior author (F.O.t.K.).
Data Collection and Analysis
Data extraction was conducted independently by 2 unblinded investigators (K.K. and A.M.v.E.) using pre-tested standardized data extraction forms. Authors of primary studies were contacted for missing information or if reported data did not fit the required format. For each study, the following information was extracted: first author, publication year, year of study start and end, study design, randomization procedures, inclusion criteria (eg, any restrictions by gravidity, age, or HIV status), insecticide-treated net or bed net use, folate supplementation and dosage, local malaria transmission, details of study groups, number of women enrolled, and outcomes assessed, including adverse events overall and stratified by subgroup. The Cochrane Collaboration’s tool for assessing the risk of bias16 was used to determine the quality of included trials as low (high risk of bias), high (low risk of bias), or unclear. Uncertainties were resolved by consensus and by contacting the corresponding authors.17
Time- and location-matched data on molecular resistance to sulfadoxine-pyrimethamine were obtained from published articles, as described previously,18 and through correspondence with the authors of the trials. The prevalence of the K540E mutation in the dihydropteroate synthase (DHPS) gene was used as a proxy for the prevalence of the combined dihydrofolate reductase DHFR (N51I, C59R, and S108N)/DHPS (A437G, K540E) quintuple genotype that is strongly associated with treatment failure of sulfadoxine-pyrimethamine.19
Synthesis
The primary outcome measures were LBW and mean birth weight. Secondary outcomes included maternal hemoglobin level, maternal anemia (hemoglobin level <11 g/dL) and moderate to severe anemia (defined by the individual trials as hemoglobin level <6, 7, or 8 g/dL) at term or delivery, maternal malaria infection (peripheral blood) at delivery, placental malaria infection (all species), preterm delivery (<37 weeks’ gestation), spontaneous miscarriage, stillbirth, and neonatal death (death within 0–27 days in live-born infants). All analyses were stratified a priori by HIV status and gravidity status (G1–G2 vs ≥G3 pregnancies [multigravida]), with the aim to provide independent subgroup estimates and overall estimates of the pooled data.
We used both random-effects (primary method) and fixed-effects models to calculate the summary relative risks (RRs) for dichotomous outcomes (Mantel-Haenszel) or differences in means for continuous outcomes (inverse variance) and we prespecified that any heterogeneity would be investigated by subgroup analysis. To provide estimates of absolute risk and effect, values for the assumed control-group risk in 2-dose recipients and the corresponding intervention-group risk and 95% CI in ≥3-dose recipients were computed as assumed control-group risk = median risk (expressed per 1000 women) across the included trials in the 2-dose group; corresponding intervention-group risk=assumed control-group risk ×RR (95% CI), where the RR was taken from random-effects models.20 The absolute risk reduction was calculated as the assumed control-group risk×(1−RR) and expressed per 1000 women. Similar methods were used with the lower and upper CI of the RR to obtain the 95% CI of the absolute risk reduction. The number needed to treat (NNT) for LBW (the primary end point) was computed as NNT = 1/(assumed control-group risk×[1−RR]).20 For the continuous end points, the observed median birth weight or hemoglobin concentration in the 2-dose group was reported as the assumed control-group median. The corresponding value in ≥3-dose recipients was expressed as the corresponding intervention-group median and 95% CI, which were computed as the assumed control-group median+mean difference (95% CI).
Heterogeneity was quantified with the I2 statistic and χ2 test.21 The Deeks and Higgens method was used to test for heterogeneity between the different summary estimates across subgroups.22 Publication and small-study bias was assessed by visual inspection of funnel plots and the Harbord test. To evaluate the change in pooled summary estimates for the RR with addition of new evidence, we created cumulative meta-analysis plots.23 Prespecified sensitivity analysis for the primary outcomes was performed by excluding all studies that were scored as low quality for allocation concealment or other sources of bias.16 Further sensitivity analysis was conducted to test the effect of each study on the pooled estimates and heterogeneity by removing one study at a time from the meta-analysis. We used P <.05 to indicate statistical significance (2-sided tests). Data were analyzed with Review Manager version 5.2, GradePro version 3.6, and Stata version 12.
RESULTS
Studies and Outcomes
A total of 241 studies were screened, and 7 trials including a total of 6281 pregnancies were included (eFigure 1),9,12,24–29 one of which was unpublished29 (Table 1). Authors of all primary studies provided further unpublished information where available. Five trials compared monthly sulfadoxine-pyrimethamine against the standard 2-dose regimen and the remaining 2 compared 3- vs 2-dose intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine.9,28 Sulfadoxine-pyrimethamine intake was supervised in all trials.
Table 1.
Characteristics of Included Trials
| Source | Parise et al12 | Filler et al24 | Hamer et al25 | Luntamo et al26 | Valea et al28 | Diakite et al9 | MacArthur et al29 |
|---|---|---|---|---|---|---|---|
| Country | Kenya | Malawi | Zambia | Malawi | Burkina Faso | Mali | Tanzania |
|
| |||||||
| Year published | 1998 | 2006 | 2007 | 2010 | 2010 | 2011 | Unpublisheda |
|
| |||||||
| Study, years | 1994–1996 | 2002–2005 | 2003–2004 | 2003–2006 | 2006–2008 | 2006–2008 | 2003–2006 |
|
| |||||||
| Gravidity | G1–G2 | G1–G2 | All | All | All | All | G1–G2 |
|
| |||||||
| No. of women | |||||||
| ≥3-Dose group | 661 | 351 | 224 | 441 | 656 | 413 | 400 |
|
| |||||||
| 2-Dose group | 680 | 347 | 232 | 436 | 640 | 401 | 399 |
|
| |||||||
| Total (G1–G2) | 1341 (1341) | 698 (698) | 456 (251) | 877 (381) | 1296 (536) | 814 (339) | 799 (799) |
|
| |||||||
| Intervention regimen | Monthly | Monthly | Monthly | Monthly | 3 doseb | 3 dose | Monthly |
|
| |||||||
| No. of doses in ≥3 group, median (range) | 3 (1–5) | 5 (1–5) | 4 (1–6) | 4 (1–6) | 2 (1–3)b | 3 (1–3) | 3 (1–5) |
|
| |||||||
| No. of ANC visits by dose, median (range) | Designed to be equalc | Designed to be equalc | Designed to be equalc | ||||
|
| |||||||
| ≥3-Dose group | 4 (1–9) | 4 (1–7) | 3 (1–6) | 4 (1–6) | |||
|
| |||||||
| 2-Dose group | 4 (1–9) | 4 (1–6) | 3 (1–6) | 3 (1–7) | |||
|
| |||||||
| HIV status | Positive + negative | Positive + negative | Positive only | Positive + negatived | Alle | Alle | Positive + negativef |
|
| |||||||
| Malaria transmissiong | Holoendemic | Holoendemic | Holoendemich | Holoendemic | Hyperendemic | Hyperendemic | Holoendemic |
|
| |||||||
| Entomologic inoculation rate/yi | 60–300 | 18–27 | NA | NA | NA | NA | 367 |
|
| |||||||
| SP resistance, No. (% DHPS K540E)j | 77 (14)30 | 76 (96)31 | 24 (46)32 | 88 (86)33 | 80 (0)34 | 9 (0)9 | 120 (46)35 |
|
| |||||||
| Folic acid dose, mg/d | 5 | 0.5 | 5 | 0.25 | 0.4 | 0.4 | 0.4 |
|
| |||||||
| Insecticide-treated net coverage, No. (%) | 148 (11) | 105 (15) | 114 (25) | 530 (60) | 40 (14)36 | 138 (17) | 296 (37) |
|
| |||||||
| Random sequence generation | Not random | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate |
|
| |||||||
| Sequence allocation | By day of visit | Inadequate | Adequate | Adequate | Adequate | Adequate | Adequate |
|
| |||||||
| Open label/placebo-controlled | Open label | Open label | Placebo | Open label | Open label | Open label | Open label |
|
| |||||||
| Assessor blinding birth weight | No | No | Yes | Yes | No | Yes | Yes |
|
| |||||||
| Loss to follow-up, No. (%) | 478 (36) | 143 (22) | 68 (15) | 86 (10) | 259 (20) | 73 (9) | 56 (7) |
Abbreviations: ANC, antenatal clinic; DHPS, dihydropteroate synthase; G1–G2, first and second pregnancies; HIV, human immunodeficiency virus; NA, not available.
All information was provided by 2 of the coauthors (A.M., J.R.M.).
Drug administration was provided as directly observed therapy in the home environment. However, because of logistic reasons, only 149 of the women (23%) in the 3-dose group received the third sulfadoxine-pyrimethamine dose and only 261 (41%) in the 2-dose group received a second sulfadoxine-pyrimethamine dose.
Actual number of visits not reported, but the studies were designed to have identical antenatal care schedules in both groups.
The HIV-negative group includes 81 women (41 in the ≥3-dose group) with unknown/undetermined HIV status.
HIV screening and testing not conducted, but HIV prevalence in the general ANC population was 1.0% and 1.3% in the study sites in Burkina Faso28 and Mali,9 respectively.
HIV screening and testing conducted, but HIV results were not available.
Holoendemic: malaria transmission occurs all year long; hyperendemic: intense but with periods of no malaria transmission during the dry season.
Transmission during the study period was reported to be lower than usual, described as “mild malaria transmission.”
The entomologic inoculation rate is a measure of malaria transmission intensity and is the number of infectious bites per person per unit of time (usually expressed per year). It is the product of the biting rate and the sporozoite rate.
Sulfadoxine-pyrimethamine resistance data matched for time and location (≤100 km) and defined as the proportion of symptomatic children younger than 5 or 12 years carrying DHPS K540E mutations for sulfadoxine-pyrimethamine resistance, except for the studies by Diakite et al9 in Mali and Lin et al33 in Malawi, which were based on samples from women attending antenatal care before receiving their first dose of sulfadoxine-pyrimethamine. The No. represents the total number of samples tested in the matched study (denominator).
Three trials in Kenya and Malawi involved both HIV-infected and uninfected women,12,24,26 and 1 trial in Zambia involved HIV-infected women only.25 In 3 other trials, the HIV status was unknown,9,28,29 2 of which were from areas with very low HIV prevalence among pregnant women (1% in Burkina Faso and 1.3% in Mali)9,28; results were therefore pooled with those of the HIV-negative women. The third trial from Tanzania29 was conducted in an area with high HIV prevalence and analyzed as a separate “HIV status unknown” stratum. Two of the 7 trials were considered of low quality (eFigure 2), including a trial in Burkina Faso, in which two-thirds of participants did not receive the intended regimen.28 The other study was a quasi-randomized trial12 conducted before the introduction of the Consolidated Standards of Reporting Trials (CONSORT) guidelines for clinical trials37 (Table 1).
Primary Outcomes: Birth Weight
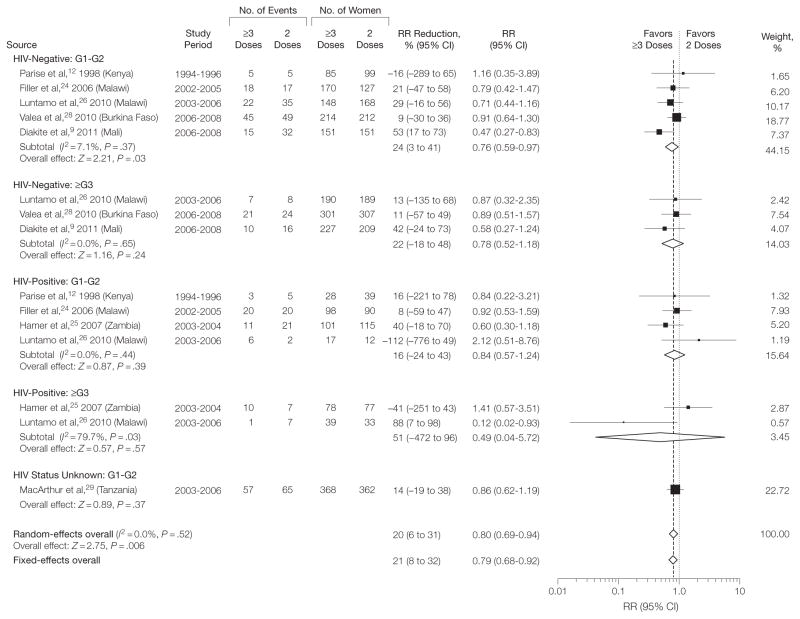
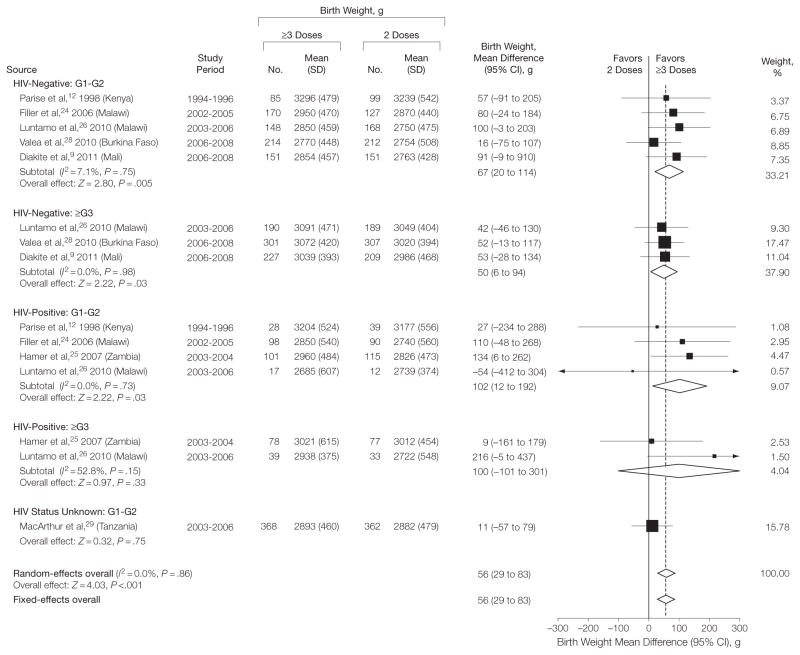
Women in the ≥3-dose group had fewer infants with LBW (random-effects model RR=0.80; 95% CI, 0.69–0.94; P =.006; I2=0%) (Figure 1), corresponding to an RR reduction (RR reduction = 100% =[1 −RR]) of 20% (95% CI, 6–31). The absolute risk reduction was 33 per 1000 women (95% CI, 10–52), from a median risk of 167 per 1000 in the 2-dose group (assumed control-group risk) to 134 per 1000 in the ≥3-dose recipients (NNT=31). The median birth weight in the 2-dose group was 2870 g (range, 2722–3239 g) and on average 56 g (95% CI, 29–83 g) higher in the ≥3-dose group (Figure 2, Table 2). Analyses by gravida and HIV subgroup showed that the mean difference in birth weight was statistically significant in HIV-negative women (random-effects mean difference=58 g; 95% CI, 26–90 g), HIV-positive women (mean difference=97 g; 95% CI, 22–172) (Table 2), G1–G2 women (mean difference=57 g; 95% CI, 22–93 g) (eTable 1), and multigravida (mean difference=53 g; 95% CI, 11–95 g) (eTable 2) (between-subgroup difference, I2=0%; P =.53) (Figure 2). The RR estimates for LBW, however, were significant only in HIV-negative women (RR = 0.77 [95% CI, 0.63–0.94] [Table 2]; assumed control-group risk=106 per 1000; absolute risk reduction=24 per 1000 [95% CI, 6–39]; NNT = 42) and G1–G2 women (RR=0.80 [95% CI, 0.68–0.95] [eTable 1]; assumed control-group risk=181 per 1000; absolute risk reduction=36 per 1000 [95% CI, 9–58]; NNT=28) but not in HIV-positive women (RR=0.86 [95% CI, 0.53–1.39] [Table 2]; assumed control-group risk = 175 per 1000; absolute risk reduction=24 per 1000 [95% CI, −68 to 82]; NNT=42) or multigravida (RR = 0.79 [95% CI, 0.49–1.27] [eTable 2]; assumed control-group risk=78 per 1000; absolute risk reduction=16 per 1000 [95% CI, −21 to 40]; NNT = 63). The difference in the RR estimates between the subgroups was not significant (between-subgroup difference I2= 0%; P = .96) (Figure 1). The results of fixed-effects models overall and by gravidity or HIV groups were mostly identical or very similar (eTable 3).
Figure 1.
Meta-analysis of the Risk of Low Birth Weight in Trials Comparing the Standard 2-Dose vs 3 or More Doses of Intermittent Preventive Therapy During Pregnancy With Sulfadoxine-Pyrimethamine
G1–G2 indicates first and second pregnancies; ≥G3, 2 or more previous pregnancies; HIV, human immunodeficiency virus; RR, relative risk. P values after the I2 statistics represent the χ2 test for heterogeneity. Dersimonian-Laird method used to calculate random-effects models; Mantel-Haenszel for fixed-effects models. Weights are from random-effects analysis. Data marker sizes indicate the weight applied to each study with random-effects meta-analysis. Test for subgroup differences: , P= .96, l2= 0.0%.
Figure 2.
Meta-analysis of Mean Birth Weight in 7 Trials Comparing the Standard 2-Dose vs 3 or More Doses of Intermittent Preventive Therapy During Pregnancy With Sulfadoxine-Pyrimethamine
G1–G2 indicates first and second pregnancies; ≥G3, 2 or more previous pregnancies; HIV, human immunodeficiency virus status. P values after the I2 statistics represent the χ2 test for heterogeneity. Dersimonian-Laird method used for random-effects models; inverse-variance method used in the fixed-effects models. Weights are from random-effects analysis. Data marker sizes indicate the weight applied to each study with random-effects meta-analysis. Test for subgroup differences: , P= .53, l2= 0.0%.
Table 2.
Random-Effects Meta-analysis of Trials Comparing the Standard 2-Dose vs ≥3 Doses of Sulfadoxine-Pyrimethamine for Intermittent Preventive Therapy During Pregnancy by HIV Status
| No. of Studies | 2 Doses
|
≥3 Doses
|
Random-Effects Model
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Events | Total No. | ACR per 1000 or ACM (Range)a | No. Events | Total No. | CIR per 1000 or CIM (95% CI)a | Relative Risk (95% CI)b | ARR per 1000 or Mean Difference (95% CI)c | P Valueb | I2, % | ||
| Primary End Points | |||||||||||
| Low birth weight | |||||||||||
| HIV+ | 4 | 62 | 366 | 175 (91–222) | 51 | 361 | 151 (93–243) | 0.86 (0.53–1.39) | 24 (−68 to 82) | .54 | 33 |
|
| |||||||||||
| HIV− | 5 | 186 | 1462 | 106 (42–231) | 143 | 1486 | 82 (67–100) | 0.77 (0.63–0.94) | 24 (6 to 39) | .01 | 0 |
|
| |||||||||||
| Unknown | 1 | 65 | 362 | 180d | 57 | 368 | 155 (112–214) | 0.86 (0.62–1.19) | 25 (−34 to 68) | .37 | d |
|
| |||||||||||
| Overall | 7 | 313 | 2190 | 167 (42–231) | 251 | 2215 | 134 (115–157) | 0.80 (0.69–0.94) | 33 (10 to 52) | .006 | 0 |
|
| |||||||||||
| Birth weight, g | |||||||||||
| HIV+ | 4 | 366 | 2783 (2722–3177) | 361 | 2880 (2805–2955) | 97 (22 to 172) | .01 | 0 | |||
| HIV− | 5 | 1462 | 2928 (2750–3239) | 1486 | 2986 (2954–3018) | 58 (26 to 90) | <.001 | 0 | |||
|
| |||||||||||
| Unknown | 1 | 362 | 2882d | 368 | 2893 (2825–2961) | 11 (−57 to 79) | .75 | d | |||
|
| |||||||||||
| Overall | 7 | 2190 | 2870 (2722–3239) | 2215 | 2926 (2899–2953) | 56 (29 to 83) | <.001 | 0 | |||
|
| |||||||||||
| Secondary End Points | |||||||||||
| Maternal hemoglobin, g/dL | |||||||||||
| HIV+ | 4 | 349 | 11.0 (9.7–11.4) | 327 | 11.1 (10.9–11.4) | 0.11 (−0.15 to 0.37) | .40 | 0 | |||
|
| |||||||||||
| HIV− | 5 | 1395 | 10.8 (10.2–11.6) | 1461 | 11.0 (10.8–11.1) | 0.15 (0.04 to 0.26) | .009 | 0 | |||
|
| |||||||||||
| Unknown | 1 | 344 | 11.1d | 340 | 11.1 (10.8–11.4) | 0 (−0.31 to 0.31) | 1 | d | |||
|
| |||||||||||
| Overall | 7 | 2088 | 10.9 (9.7–11.6) | 2128 | 11.0 (10.9–11.1) | 0.13 (0.03 to 0.22) | .009 | 0 | |||
|
| |||||||||||
| Maternal anemia, <11 g/dL | |||||||||||
| HIV+ | 4 | 214 | 349 | 582 (333–795) | 190 | 327 | 559 (506–623) | 0.96 (0.87–1.07) | 23 (−41 to 76) | .51 | 0 |
|
| |||||||||||
| HIV− | 5 | 665 | 1395 | 473 (269–660) | 682 | 1461 | 459 (426–492) | 0.97 (0.90–1.04) | 14 (−19 to 47) | .37 | 0 |
|
| |||||||||||
| Unknown | 1 | 175 | 344 | 509d | 152 | 340 | 448 (382–524) | 0.88 (0.75–1.03) | 61 (−15 to 127) | .11 | d |
|
| |||||||||||
| Overall | 7 | 1054 | 2088 | 509 (269–795) | 1024 | 2128 | 484 (458–514) | 0.95 (0.90–1.01) | 25 (−5 to 51) | .10 | 0 |
|
| |||||||||||
| Moderate/severe maternal anemia (<8, 7, or 6 g/dL) | |||||||||||
| HIV+ | 2 | 7 | 124 | 0 (0–65) | 3 | 135 | 0 (0–0) | 0.60 (0.06–5.85) | 0 (0 to 0) | .66 | 48 |
|
| |||||||||||
| HIV− | 4 | 38 | 1296 | 38 (9–63) | 27 | 1376 | 27 (14–52) | 0.70 (0.36–1.36) | 11 (−14 to 24) | .29 | 34 |
|
| |||||||||||
| Unknown | 2e | 25 | 776 | 32 (30–35) | 21 | 771 | 27 (15–48) | 0.85 (0.48–1.50) | 5 (−16 to 17) | .57 | 0 |
|
| |||||||||||
| Overall | 6 | 70 | 2196 | 34 (0–65) | 51 | 2282 | 25 (16–38) | 0.73 (0.48–1.11) | 9 (−4 to 18) | .14 | 15 |
|
| |||||||||||
| Maternal parasitemia | |||||||||||
| HIV+ | 4 | 51 | 338 | 112 (0–359) | 13 | 328 | 29 (17–52) | 0.26 (0.15–0.46) | 83 (60 to 95) | <.001 | 0 |
|
| |||||||||||
| HIV− | 5 | 265 | 1407 | 104 (31–350) | 234 | 1445 | 89 (77–105) | 0.86 (0.74–1.01) | 15 (−1 to 27) | .06 | 0 |
|
| |||||||||||
| Unknown | 1 | 7 | 351 | 20d | 2 | 349 | 6 (1–27) | 0.29 (0.06–1.37) | 14 (−7 to 19) | .12 | d |
|
| |||||||||||
| Overall | 7 | 323 | 2096 | 92 (0–359) | 249 | 2122 | 63 (48–82) | 0.68 (0.52–0.89) | 29 (10 to 44) | .005 | 47 |
|
| |||||||||||
| Placental malaria | |||||||||||
| HIV+ | 4 | 39 | 338 | 102 (0–256) | 14 | 320 | 39 (21–70) | 0.38 (0.21–0.69) | 63 (32 to 81) | .001 | 0 |
|
| |||||||||||
| HIV− | 4 | 82 | 753 | 67 (0–201) | 47 | 782 | 38 (26–55) | 0.57 (0.39–0.82) | 29 (12 to 41) | .003 | 9 |
|
| |||||||||||
| Unknown | 1 | 7 | 345 | 20d | 4 | 344 | 11 (3–39) | 0.57 (0.17–1.94) | 9 (−19 to 17) | .37 | d |
|
| |||||||||||
| Overall | 6 | 128 | 1436 | 63 (0–256) | 65 | 1446 | 32 (24–43) | 0.51 (0.38–0.68) | 31 (20 to 39) | <.001 | 0 |
|
| |||||||||||
| Secondary End Points | |||||||||||
| Preterm delivery | |||||||||||
| HIV+ | 3 | 130 | 331 | 306 (46–655) | 113 | 340 | 278 (211–370) | 0.91 (0.69–1.21) | 28 (−64 to 95) | .51 | 32 |
|
| |||||||||||
| HIV− | 4 | 209 | 1479 | 107 (16–248) | 191 | 1554 | 93 (72–122) | 0.87 (0.67–1.14) | 14 (−15 to 35) | .32 | 41 |
|
| |||||||||||
| Unknown | 2e | 51 | 769 | 61 (21–102) | 66 | 777 | 78 (55–111) | 1.28 (0.90–1.82) | −17 (−50 to 6) | .17 | 1 |
|
| |||||||||||
| Overall | 7 | 390 | 2579 | 122 (16–655) | 370 | 2671 | 116 (98–137) | 0.95 (0.80–1.12) | 6 (−15 to 24) | .52 | 35 |
|
| |||||||||||
| Miscarriage | |||||||||||
| HIV+ | 2 | 3 | 147 | 0 (0–30) | 5 | 171 | 0 (0–0) | 1.54 (0.38–6.28) | 0 (0 to 0) | .55 | d |
|
| |||||||||||
| HIV− | 4 | 19 | 1515 | 0 (0–29) | 28 | 1587 | 0 (0–0) | 1.31 (0.64–2.70) | 0 (0 to 0) | .46 | 20 |
|
| |||||||||||
| Unknown | 2e | 5 | 809 | 6 (0–12) | 9 | 809 | 11 (4–32) | 1.80 (0.61–5.34) | −5 (−26 to 2) | .29 | d |
|
| |||||||||||
| Overall | 6 | 27 | 2471 | 0 (0–30) | 42 | 2567 | 0 (0–0) | 1.43 (0.88–2.33) | 0 (0 to 0) | .15 | 0 |
|
| |||||||||||
| Stillbirth | |||||||||||
| HIV+ | 3 | 11 | 352 | 40 (0–56) | 8 | 362 | 27 (11–70) | 0.68 (0.27–1.74) | 13 (−30 to 29) | .43 | 0 |
|
| |||||||||||
| HIV− | 4 | 44 | 1515 | 30 (15–53) | 60 | 1587 | 40 (27–59) | 1.33 (0.90–1.95) | −10 (−29 to 3) | .15 | 0 |
|
| |||||||||||
| Unknown | 2e | 24 | 809 | 30 (25–34) | 24 | 809 | 29 (13–68) | 0.97 (0.42–2.27) | 1 (−38 to 17) | .95 | 54 |
|
| |||||||||||
| Overall | 7 | 79 | 2676 | 30 (0–56) | 92 | 2758 | 34 (26–46) | 1.14 (0.85–1.55) | −4 (−16 to 4) | .38 | 0 |
|
| |||||||||||
| Neonatal deathf | |||||||||||
| HIV+ | 2 | 10 | 137 | 77 (29–167) | 6 | 160 | 39 (14–112) | 0.51 (0.18–1.45) | 38 (−35 to 63) | .21 | 0 |
|
| |||||||||||
| HIV− | 4 | 25 | 1472 | 19 (8–31) | 32 | 1549 | 23 (13–39) | 1.19 (0.69–2.05) | −4 (−20 to 6) | .54 | 0 |
|
| |||||||||||
| Unknown | 2e | 14 | 796 | 18 (14–22) | 7 | 800 | 8 (2–33) | 0.47 (0.12–1.84) | 10 (−15 to 16) | .28 | 37 |
|
| |||||||||||
| Overall | 6 | 49 | 2405 | 21 (8–167) | 45 | 2509 | 18 (12–28) | 0.88 (0.57–1.35) | 3 (−7 to 9) | .55 | 0 |
Abbreviations: ACM, assumed control-group median; ACR, assumed control-group risk; ARR, absolute risk reduction (risk difference); CIM, corresponding intervention-group median; CIR, corresponding intervention-group risk; HIV, human immunodeficiency virus.
ACR represents the observed median risk (range) (expressed per 1000 women) across the trials in the 2-dose group (the range is only provided to illustrate low- and high-risk populations, whereas the median risk is illustrative of a population with a moderate risk); the CIR (and 95% CI) is based on the assumed risk in ≥3 dose recipients, computed as ACR×RR (95% CI).20 For the 2 continuous end points, the ACM represents the median birth weight or hemoglobin concentration in the 2-dose arm. The CIM values were computed as the ACM + mean difference (95% CI).
Effect size, 95% CIs, and P values for the overall effect (last rows) and for each HIV-status subgroup were obtained from random-effects models and are adjusted for gravidity group (all estimates [G1–G2, ≥G3]) and HIV status (for last rows representing the overall effect) by using the independent subgroups as the unit of analysis.
The ARR was calculated as the ACR×(1 − RR) and expressed per 1000 women.
Range or heterogeneity cannot be estimated because the data contain only a single trial in the subgroup or no events occurred in 1 of the 2 included studies.12
Results for the study by Parise et al12 in Kenya were not reported by HIV status for these end points.
Death of a live-born infant within the first 28 days of life. One study assessed early neonatal death only (death within 7 days of life).29
There was no evidence for publication bias after visual inspection of funnel plots or with the Harbord modified test for small-study effects (P =.72) (eFigure 3). Cumulative meta-analysis, ordered by publication date, showed that a significant association with LBW emerged with the addition of new evidence from trials reported since 2010 (eFigures 4 and 5). Sensitivity analysis showed that after removal of both low-quality studies,12,28 the point estimates for LBW and mean birth weight were RR=0.76 (95% CI, 0.61–0.93), I2=16%; and mean difference=62 g (95% CI, 29–95 g), I2=0%. Removal of any individual trial also had relatively little effect and pooled results remained statistically significant at P <.05 for all 7 analyses with fixed-effects models and at P =.06 with random-effects models (eFigures 6 and 7).
Secondary Outcomes
The median maternal hemoglobin level at term in the 2-dose group was 10.9 g/dL (range, 9.7–11.6 g/dL), and this was on average 0.13 g/dL higher (95% CI, 0.03–0.22 g/dL) in the ≥3-dose group (Table 2, eFigure 8). This group had a lower risk of moderate to severe maternal anemia, but this was evident only in G1–G2 women (RR=0.60 [95% CI, 0.36–0.99]; I2=20%) (eTable 1), not overall (RR = 0.73 [95% CI, 0.48–1.11]; I2=15%) (Table 2 and eFigure 9). Women in the ≥3-dose group were approximately half as likely to have placental malaria (6 studies) compared with those in the 2-dose group, regardless of HIV status (RR=0.51 [95% CI, 0.38–0.68]; I2=0%) (Table 2, eFigure 10), but this was evident only in G1–G2 women (RR = 0.50 [95% CI, 0.35–0.70]; I2=0%) (eTable 1), not in multigravida (RR = 0.71 [95% CI, 0.26–1.95]; I2=21%) (eTable 2). Similarly, ≥ 3 doses were associated with less peripheral (maternal) malaria (RR=0.68 [95% CI, 0.52–0.89]; I2=47%) (Table 2), but this was evident in G1–G2 women only (RR=0.54 [95% CI, 0.37–0.80]; I2= 56%) (eTable 1), not in multigravida (RR = 0.97 [95% CI, 0.75–1.24]; I2= 0%) (eTable 2). No difference in preterm delivery was detected (RR = 0.95 [95% CI, 0.80–1.12]; I2= 35%) or in the number of stillbirths (RR=1.14 [95% CI, 0.85–1.55]; I2=0%), miscarriages (RR=1.43 [95% CI, 0.88–2.33]; I2= 0%), or neonatal deaths (RR = 0.88 [95% CI, 0.57–1.35]; I2=0%) (Table 2).
Stratified Analysis for LBW and Mean Birth Weight
There was no clear correlation between resistance level and the strength of the association between treatment regimen and LBW or mean birth weight; the point estimates were similar in areas with less than 50% DHPS-K540E mutations (5 trials) and areas with 50% or more DHPS-K540E (2 trials) (eFigures 11 and 12). There was also no evidence that intensity of malaria transmission or the median number of sulfadoxine-pyrimethamine doses in the ≥3-dose group modified the association (P >.17 for all tests for subgroup differences). There was no clear difference in the association between the dose group and the risk of LBW or mean birth weight in the 2 trials that used high-dose folate supplementation (5 mg/d)12,25 (which has since been contraindicated) vs the standard dose (0.25–0.5 mg/d). Three studies reported results stratified by insecticide-treated net use9,26,29; the associations with LBW and mean birth weight were statistically significant in the nonusers only. There was no evidence for an association with LBW in insecticide-treated net users (eFigures 11 and 12).
Adverse Events
The risks of neonatal icterus and congenital malformation were comparable between the groups, as were the number of adverse events in the mother. One study reported a case of Stevens-Johnson syndrome, which occurred in the 3 or more dose group, 3 weeks after the first dose (Table 3).25
Table 3.
Summary of Adverse Events in Women and Neonates After Intermittent Preventive Therapy During Pregnancy With ≥3 Doses vs 2 Doses of Sulfadoxine-Pyrimethamine During Pregnancy
| Source | Sulfadoxine-Pyrimethamine Treatment
|
No./No. (%)
|
Severe Skin Reactions | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Neonatal Icterus
|
Congenital Abnormalities
|
Maternal Drug Reaction
|
|||||||
| No. of Courses | No. of Women | ≥3 Doses | 2 Doses | ≥3 Doses | 2 Doses | ≥3 Doses | 2 Doses | ||
| Parise et al,12 1998 | 2276 | 1086a | 60/431 (14) | 69/432 (15) | Not reported | 7/661 (1.4) | 14/680 (2.3) | None observedb | |
|
| |||||||||
| Filler et al,24 2006 | 1734 | 641a | 0.4%c | Not reported | <1%c | None observed | |||
|
| |||||||||
| Hamer et al,25 2007 | 1039 | 456 | 1/189 (0.5) | 0/198 (0) | Not reported | 1.13 (0.56 to 2.18)d | 1 Case reported in the monthly groupe | ||
|
| |||||||||
| Luntamo et al,26 2010 | 2603 | 877 | Not reported | 3/443 (0.7) | 4/439 (0.9) | Not reported | Not reported | ||
|
| |||||||||
| Valea et al,28 2010 | 2213 | 1296 | Not reported | Not reported | Not reported | Not reported | |||
|
| |||||||||
| Diakite et al,9 2011 | 1997 | 814 | 11/400 (2.7) | 10/383 (2.5) | 1/400 (0.3) | 3/383 (0.8) | 0/413 (0) | 0/401 (0) | None observed |
|
| |||||||||
| McArthur et al29 | 1692 | 799 | 14/272 (5.1) | 21/290 (7.2) | 5/383 (1.3) | 7/384 (1.8) | 23/399 (5.7)f | 28/400 (6.7)f | None observed |
|
| |||||||||
| Relative risk (95% CI) | 0.87 (0.66 to 1.14) | 0.65 (0.28 to 1.50) | 0.73 (0.46 to 1.15) | ||||||
|
| |||||||||
| I2 (95% CI), % | 0 (0 to 61) | 0 (0 to 53) | 0 (0 to 0) | ||||||
|
| |||||||||
| P value for heterogeneity | .76 | .80 | .38 | ||||||
Reported only for women followed up prospectively.
In 193 treatment episodes in 94 HIV-positive women and 502 treatment episodes in 230 HIV-negative women. Cases were assessed during the study but not observed by investigators, but 2 of 94 HIV-positive (2%) and 0 of 230 HIV-negative women had sulfadoxine-pyrimethamine withheld due to adverse drug reactions (mild rash or oral lesions).
Reported only for all groups pooled, but no statistical difference was observed between treatment groups.
Numerator and denominators were not reported.
The case of Stevens-Johnson syndrome reported in the monthly arm occurred 3 weeks after the first dose of sulfadoxine-pyrimethamine.
Maternal drug reactions collected from the first dose (enrollment) to the last dose, including diarrhea, rash, weakness, seizures, sleepiness, and difficulty walking.
COMMENT
This meta-analysis of 7 trials demonstrated that regimens of intermittent preventive therapy during pregnancy consisting of ≥3 doses of sulfadoxine-pyrimethamine were well tolerated and, compared with the standard 2-dose regimen, were associated with higher mean birth weight, less LBW, and less placental and maternal malaria at delivery. The ≥3-dose regimen was also associated with slightly higher mean maternal hemoglobin levels at term overall, but a significant association with moderate to severe maternal anemia was observed only in G1–G2 women. The associations with birth weight were consistent across trials despite variations in study design, malaria endemicity, and the degree of sulfadoxine-pyrimethamine resistance. Although the number of trials was limited, there was no suggestion of publication or other small-study bias. There was also no suggestion that the results were affected by the weight of a single influential study. Two of the trials were classified as low quality, but sensitivity analysis indicated that their effect on the overall pooled estimate for LBW was minor. The consistency of these findings across the trials suggests the results are generalizable.
Although the summary point estimates of the association with mean birth weight were modest (56-g difference overall and 67 g among HIV-negative G1–G2 women), these were associated with clinically relevant changes in the risk of LBW, particularly among HIV-negative G1–G2 women (RR reduction=25%) (eTable 1). These estimates were comparable to that reported in previous studies for 2-dose intermittent preventive therapy during pregnancy relative to none (mean difference=79 g; RR reduction=29%) and for insecticide-treated nets alone (mean difference=55 g; RR reduction=23%).7,38 The magnitude of the observed association is remarkable, given that approximately 28% of women were protected by insecticide-treated nets in these 7 trials and considering that the control group benefited from protection of the 2-dose intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine. The association mainly reflects an association with fetal growth, rather than with preterm delivery, and indicates that more complete protection in the second and third trimesters, including the last 6 to 10 weeks of pregnancy, may be pivotal for fetal growth. This result is consistent with observations in healthy pregnancies, which show that of the total fetal weight gain, 28% and 55% of it occurs during the last 6 and 10 weeks of pregnancy, respectively.39
Although the lack of heterogeneity across the sulfadoxine-pyrimethamine resistance range is encouraging, it does not imply that sulfadoxine-pyrimethamine efficacy is unaffected at higher levels of resistance. A possible explanation is that the extra doses compensate for any reductions in efficacy of the 2-dose regimen resulting from a progressive decrease of the duration of posttreatment prophylaxis.
The association with placental infections is an expected outcome because the 3 or more dose group received their last dose on average 1 month closer to delivery and is likely to reflect clearance of existing infections near term and prevention of new infections by the extra period of prophylaxis. However, the association with mean birth weight among multigravida was unexpected because most multigravida in endemic countries have acquired a pregnancy-specific protective immunity during exposures in previous pregnancies. Overall, the evidence for a beneficial association in multigravida was weak, and the finding in this study may therefore reflect a chance observation (eg, because of multiple comparisons) or mechanisms other than the prevention of malaria. Although the point estimates for LBW (RR reduction 21%) and placental malaria (RR reduction 29%) were in the same direction as those observed in primigravida and secundigravida, none were statistically significant and there was no suggestion that ≥3 doses were associated with less maternal malaria or moderate to severe anemia. On the other hand, the lack of significant association with LBW may reflect lack of power because only 4 of the 7 studies included multigravida.
Our meta-analysis has some limitations. First, although all trials were designed to standardize the number of visits and antenatal care (eg, hematinic supplementation) between the 2 groups, in one trial in Tanzania the women in the ≥3-dose group had on average 1 extra visit compared with the 2-dose group and thus potentially better antenatal care.29 However, exclusion of this study in the sensitivity analysis did not change the conclusion (eFigures 6 and 7). Second, only 1 of the 7 trials was placebo controlled, which may have biased the results and affected some outcomes because of lack of expectations in a 2-dose group or differential behaviors across intervention groups. We did not use blinding in the selection, evaluation, and data abstraction phases, and because the authors were familiar with all included studies, this could have introduced bias.40 Third, none of the trials were conducted in regions where additional DHFR 164L or DHPS 581G mutations are prevalent, as reported from parts of Rwanda, Uganda, and northern Tanzania, conferring the highest level of sulfadoxine-pyrimethamine resistance.18,41–43 Last, only 3 trials reported results stratified by insecticide-treated net use, limiting our evaluation of the potential modifying role of insecticide-treated nets. In this smaller subgroup of studies, significant associations with LBW and mean birth weight were observed among the non-users of insecticide-treated nets only, consistent with results of previous evaluations of 2-dose intermittent preventive therapy during pregnancy against placebo.15,44,45
Only 1 serious cutaneous reaction was reported in the current meta-analysis involving 13 554 sulfadoxine-pyrimethamine treatments among 6281 pregnancies, and this occurred in an HIV-positive woman 3 weeks after she received her first dose of sulfadoxine-pyrimethamine for intermittent preventive therapy during pregnancy.25 We found no indication that more frequent dosing (ie, resulting in doses administered closer to delivery) was associated with increased risk of neonatal jaundice, the main safety signal of interest in neonates. Sulfonamides have the potential to displace unconjugated bilirubin from albumin, which could increase a newborn’s risk of kernicterus if received near delivery. Our observations, combined with the evidence reviewed by Peters et al44 from the experience with sulfonamides for rheumatic fever prophylaxis, urinary tract infections, and congenital toxoplasmosis (which involve higher doses and prolonged use of sulfadoxine-pyrimethamine), suggest that concerns regarding kernicterus should not restrict the use of monthly sulfadoxine-pyrimethamine for intermittent preventive therapy during pregnancy. There was no indication that ≥3-dose regimens increased or reduced the risk of stillbirth or neonatal death. The risk of spontaneous miscarriages in G1–G2 women was higher among the 3-dose group (RR=1.78, P =.046 with fixed-effects models and RR = 1.75, P = .06 with random-effects models). These miscarriages, however, were not associated with the third dose because in 3 of the 4 trials that contributed 80% of the study weight, they occurred before 28 weeks of gestation when the third dose had not yet been provided.9,24,28 In the fourth trial, the risk of miscarriage was 2.0% with a monthly regimen, higher than the 1.1% in the 2-dose group but similar to the 2.3% in a third control group consisting of women randomized to passive case detection only instead of intermittent preventive therapy during pregnancy.12
Since the strategic framework for the control of malaria in pregnancy in sub-Saharan Africa was first developed, at least 3 doses of sulfadoxine-pyrimethamine for intermittent preventive therapy during pregnancy has been recommended by WHO for HIV-infected women or for all women in high-HIV-prevalence areas (>10%) where screening for HIV is not conducted. Some countries, such as Cameroon,45 Ghana, Zambia, and Zimbabwe, selected 3 doses of sulfadoxine-pyrimethamine in their policy for all pregnant women, but most other countries, including many high-HIV-prevalence countries, implemented the 2-dose regimen and use cotrimoxazole for HIV-infected women.14 However, more recently other countries, including Kenya and Malawi, implemented a monthly regimen among HIV-negative women mainly because of concerns about sulfadoxine-pyrimethamine resistance and for pragmatic reasons to minimize the risk for missed opportunities to deliver a second dose46 and to achieve better alignment with WHO’s focused antenatal care schedule (a goal-oriented antenatal care approach consisting of 4 visits providing essential evidence-based interventions). In southern Malawi, this has resulted in a marked increase in the uptake of 2 or more doses of sulfadoxine-pyrimethamine.47
Our cumulative meta-analysis showed that, with the accumulation of results from the 4 most recent trials reported since 2010, evidence has emerged that 3-dose or monthly sulfadoxine-pyrimethamine for intermittent preventive therapy during pregnancy was associated with a higher birth weight and lower risk of LBW than the standard 2-dose regimens among pregnant women in sub-Saharan Africa. These data provide support for the new WHO recommendation that intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine be provided at each scheduled focused antenatal-care visit in the second and third trimesters in all settings in which intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine is recommended.48 Future research should focus on how best to implement the updated WHO guidelines for intermittent preventive therapy during pregnancy with sulfadoxine-pyrimethamine48 and specifically their integration with focused antenatal care. Continued monitoring of the association between population-level sulfadoxine-pyrimethamine resistance and the effectiveness of intermittent preventive therapy during pregnancy is required.
Acknowledgments
Funding/Support: This review was funded by a grant from the Centers for Disease Control and Prevention for salary support through a cooperative agreement between the Division of Parasitic Diseases and Malaria (Centers for Disease Control and Prevention, USA) and the Malaria Epidemiology Unit of the Child and Reproductive Health Group, Liverpool School of Tropical Medicine (LSTM), held by Dr ter Kuile. Dr Ashorn and Dr Luntamo were supported by grants from the Academy of Finland and the Finnish Cultural Foundation. Dr Kayentao was supported by a PhD training grant from the European and Developing Countries Clinical Trials Partnership, The Hague, the Netherlands. Drs ter Kuile and van Eijk are partly funded by the Malaria in Pregnancy Consortium, which is funded through a grant from the Bill & Melinda Gates Foundation to the LSTM. Dr Doumbo reports receiving support from National Institute of Allergy and Infectious Diseases (NIH).
Role of the Sponsor: The funding institutions had no role in the protocol development; the design and conduct of the review; data collection, analysis, and interpretation; or preparation, review, or approval of the manuscript.
Footnotes
Online-Only Material: eMethods, eReferences, eTables 1 through 3, and eFigures 1 through 12 are available at http://www.jama.com.
Author Contributions: Drs Kayentao and ter Kuile had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kayentao, Garner, ter Kuile.
Acquisition of data: Kayentao, Garner, van Eijk, Naidoo, Roper, Mulokozi, MacArthur, Luntamo, Ashorn, Doumbo, ter Kuile.
Analysis and interpretation of data: Kayentao, Garner, van Eijk.
Drafting of the manuscript: Kayentao, Garner, ter Kuile.
Critical revision of the manuscript for important intellectual content: Kayentao, Garner, van Eijk, Naidoo, Roper, Mulokozi, MacArthur, Luntamo, Ashorn, Doumbo, ter Kuile.
Statistical analysis: Kayentao, Garner, ter Kuile.
Obtained funding: MacArthur, ter Kuile.
Administrative, technical, or material support: van Eijk, MacArthur, Doumbo, ter Kuile.
Study supervision: Garner, ter Kuile.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr ter Kuile reports receiving reimbursement for meeting presentation expenses from Pfizer. Drs Luntamo and Ashorn report institutional support from Pfizer in the form of active drug and placebo for a trial of preterm delivery.
Additional Contributions: We thank Vittoria Lutje, PhD, information specialist, Cochrane Infectious Diseases Group, LSTM, for helping with the search strategy; Alison Reynolds, MA, and Helen Wong, BA, Child and Reproductive Health Group, LSTM, for invaluable administrative support; the authors of the other studies who provided results in addition to the published data to allow stratification of the results by gravidity and HIV groups (Monica Parise, MD, and Scott Filler, MD, Centers for Disease Control and Prevention, Atlanta, Georgia; Davidson Hamer, MD, Boston University Schools of Public Health and Medicine; Innocent Valea, MD, and Halidou Tinto, PharmD, PhD, Laboratory of Parasitology and Entomology, Centre Muraz, Bobo-Dioulasso, and Institut de Recherche en Sciences de la santé, Direction Régionale de l’Ouest, Bobo-Dioulasso, Burkina Faso; and Umberto d’ Alessandro, MD, PhD, Medical Research Council, Gambia Unit, Banjul, The Gambia, and the Institute of Tropical Medicine, Antwerp, Belgium); Brian Greenwood, MD, London School of Hygiene and Tropical Medicine, and Stephen Rogerson, FRACP, PhD, Department of Medicine, University of Melbourne, for useful comments on the manuscript; and Sarah Donegan, MSc, Cochrane Infectious Disease Group, Liverpool School of Tropical Medicine, for statistical advice. No one received financial compensation for his or her contributions.
References
- 1.Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7(2):93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 2.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1–2 Suppl):28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Malaria and HIV Interactions and Their Implications for Public Health Policy: Report of a Technical Consultation Geneva, Switzerland, 23–25 June 2004. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 4.World Health Organization. A Strategic Framework for Malaria Prevention and Control During Pregnancy in the African Region. Brazzaville, Africa: World Health Organization: Regional Office for Africa; 2004. AFR/MAL/04/01. [Google Scholar]
- 5.World Health Organization. Recommendations on the Use of Sulfadoxine-Pyrimethamine (SP) for Intermittent Preventive Treatment During Pregnancy (IPT) in Areas of Moderate to High Resistance to SP in the African Region; October 2005. 2006 http://www.who.int/malaria/publications/atoz/who_sp_statement.pdf.
- 6.White NJ. Intermittent presumptive treatment for malaria. PLoS Med. 2005;2(1):e3. doi: 10.1371/journal.pmed.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297(23):2603–2616. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 8.Kayentao K, Kodio M, Newman RD, et al. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J Infect Dis. 2005;191(1):109–116. doi: 10.1086/426400. [DOI] [PubMed] [Google Scholar]
- 9.Diakite OS, Kayentao K, Traoré BT, et al. Superiority of 3 over 2 doses of intermittent preventive treatment with sulfadoxine-pyrimethamine for the prevention of malaria during pregnancy in mali: a randomized controlled trial. Clin Infect Dis. 2011;53(3):215–223. doi: 10.1093/cid/cir374. [DOI] [PubMed] [Google Scholar]
- 10.Njagi JK, Magnussen P, Estambale B, Ouma J, Mugo B. Prevention of anaemia in pregnancy using insecticide-treated bednets and sulfadoxine-pyrimethamine in a highly malarious area of Kenya: a randomized controlled trial. Trans R Soc Trop Med Hyg. 2003;97(3):277–282. doi: 10.1016/s0035-9203(03)90141-6. [DOI] [PubMed] [Google Scholar]
- 11.Shulman CE, Dorman EK, Cutts F, et al. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomised placebo-controlled trial. Lancet. 1999;353(9153):632–636. doi: 10.1016/s0140-6736(98)07318-8. [DOI] [PubMed] [Google Scholar]
- 12.Parise ME, Ayisi JG, Nahlen BL, et al. Efficacy of sulfadoxine-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg. 1998;59(5):813–822. doi: 10.4269/ajtmh.1998.59.813. [DOI] [PubMed] [Google Scholar]
- 13.Mbaye A, Richardson K, Balajo B, et al. A randomized, placebo-controlled trial of intermittent preventive treatment with sulphadoxine-pyrimethamine in Gambian multigravidae. Trop Med Int Health. 2006;11(7):992–1002. doi: 10.1111/j.1365-3156.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 14.van Eijk AM, Hill J, Alegana VA, et al. Coverage of malaria protection in pregnant women in sub-Saharan Africa: a synthesis and analysis of national survey data. Lancet Infect Dis. 2011;11(3):190–207. doi: 10.1016/S1473-3099(10)70295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Eijk AM, Hill J, Povall S, Reynolds A, Wong H, Ter Kuile FO. The “Malaria in Pregnancy” library: a bibliometric review. Malar J. 2012;11:362. doi: 10.1186/1475-2875-11-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. Chichester, England: John Wiley & Sons; 2008. Updated September 2008. [Google Scholar]
- 18.Naidoo I, Roper C. Drug resistance maps to guide intermittent preventive treatment of malaria in African infants. Parasitology. 2011;138(12):1469–1479. doi: 10.1017/S0031182011000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picot S, Olliaro P, de Monbrison F, Bienvenu AL, Price RN, Ringwald P. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar J. 2009;8:89. doi: 10.1186/1475-2875-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schünemann HJ, Oxman AD, Higgins JPT, et al. Presenting results and “Summary of Findings” tables. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. Cochrane Collaboration, John Wiley & Sons; 2008. Updated September 2008. [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deeks JJ, Higgins JPT. Statistical Algorithms in Review Manager 5. Cochrane Collaboration; 2010. http://ims.cochrane.org/revman/documentation/Statistical-methods-in-RevMan-5.pdf. [Google Scholar]
- 23.Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306(24):2704–2714. doi: 10.1001/jama.2011.1880. [DOI] [PubMed] [Google Scholar]
- 24.Filler SJ, Kazembe P, Thigpen M, et al. Randomized trial of 2-dose versus monthly sulfadoxine-pyrimethamine intermittent preventive treatment for malaria in HIV-positive and HIV-negative pregnant women in Malawi. J Infect Dis. 2006;194(3):286–293. doi: 10.1086/505080. [DOI] [PubMed] [Google Scholar]
- 25.Hamer DH, Mwanakasale V, Macleod WB, et al. Two-dose versus monthly intermittent preventive treatment of malaria with sulfadoxine-pyrimethamine in HIV-seropositive pregnant Zambian women. J Infect Dis. 2007;196(11):1585–1594. doi: 10.1086/522142. [DOI] [PubMed] [Google Scholar]
- 26.Luntamo M, Kulmala T, Mbewe B, Cheung YB, Maleta K, Ashorn P. Effect of repeated treatment of pregnant women with sulfadoxine-pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. Am J Trop Med Hyg. 2010;83(6):1212–1220. doi: 10.4269/ajtmh.2010.10-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luntamo M, Rantala AM, Meshnick SR, et al. The effect of monthly sulfadoxine-pyrimethamine, alone or with azithromycin, on PCR-diagnosed malaria at delivery: a randomized controlled trial. PLoS One. 2012;7(7):e41123. doi: 10.1371/journal.pone.0041123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valea I, Tinto H, Drabo MK, et al. Intermittent preventive treatment of malaria with sulphadoxine-pyrimethamine during pregnancy in Burkina Faso. Malar J. 2010;9:324. doi: 10.1186/1475-2875-9-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacArthur JR, Kabanywanyi AM, Baja A, et al. Abstract 830: efficacy of intermittent treatment with sulfadoxine-pyrimethamine alone or sulfadoxine-pyrimethamine plus artesunate for prevention of placental malaria in Tanzania. Paper presented at: 56th Annual Meeting of the American Society of Tropical Medicine and Hygiene; November 4–8, 2007; Philadelphia, PA. [Google Scholar]
- 30.Iriemenam NC, Shah M, Gatei W, et al. Temporal trends of sulphadoxine-pyrimethamine (SP) drug-resistance molecular markers in Plasmodium falciparum parasites from pregnant women in western Kenya. Malar J. 2012;11(1):134. doi: 10.1186/1475-2875-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzinjalamala FK, Macheso A, Kublin JG, et al. Association between the pharmacokinetics and in vivo therapeutic efficacy of sulfadoxine-pyrimethamine in Malawian children. Antimicrob Agents Chemother. 2005;49(9):3601–3606. doi: 10.1128/AAC.49.9.3601-3606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce RJ, Pota H, Evehe MS, et al. Multiple origins and regional dispersal of resistant dhps in African Plasmodium falciparum malaria. PLoS Med. 2009;6(4):e1000055. doi: 10.1371/journal.pmed.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin JT, Mbewe B, Taylor SM, Luntamo M, Meshnick SR, Ashorn P. Increased prevalence of dhfr and dhps mutants at delivery in Malawian pregnant women receiving intermittent preventive treatment for malaria. Trop Med Int Health. 2012 doi: 10.1111/tmi.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dokomajilar C, Lankoande ZM, Dorsey G, Zongo I, Ouedraogo JB, Rosenthal PJ. Roles of specific Plasmodium falciparum mutations in resistance to amodiaquine and sulfadoxine-pyrimethamine in Burkina Faso. Am J Trop Med Hyg. 2006;75(1):162–165. [PubMed] [Google Scholar]
- 35.Mbugi EV, Mutayoba BM, Malisa AL, Balthazary ST, Nyambo TB, Mshinda H. Drug resistance to sulphadoxine-pyrimethamine in Plasmodium falciparum malaria in Mlimba, Tanzania. Malar J. 2006;5:94. doi: 10.1186/1475-2875-5-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coulibaly SO, Gies S, D’Alessandro U. Malaria burden among pregnant women living in the rural district of Boromo, Burkina Faso. Am J Trop Med Hyg. 2007;77(6 Suppl):56–60. [PubMed] [Google Scholar]
- 37.Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276(8):637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 38.Gamble C, Ekwaru PJ, Garner P, ter Kuile FO. Insecticide-treated nets for the prevention of malaria in pregnancy: a systematic review of randomised controlled trials. PLoS Med. 2007;4(3):e107. doi: 10.1371/journal.pmed.0040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikolajczyk RT, Zhang J, Betran AP, et al. A global reference for fetal-weight and birthweight percentiles. Lancet. 2011;377(9780):1855–1861. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- 40.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 41.Gesase S, Gosling RD, Hashim R, et al. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at Codon 581. PLoS One. 2009;4(2):e4569. doi: 10.1371/journal.pone.0004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrington WE, Mutabingwa TK, Muehlenbachs A, et al. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proc Natl Acad Sci U S A. 2009;106(22):9027–9032. doi: 10.1073/pnas.0901415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrington WE, Mutabingwa TK, Kabyemela E, Fried M, Duffy PE. Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance. Clin Infect Dis. 2011;53(3):224–230. doi: 10.1093/cid/cir376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters PJ, Thigpen MC, Parise ME, Newman RD. Safety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatment. Drug Saf. 2007;30(6):481–501. doi: 10.2165/00002018-200730060-00003. [DOI] [PubMed] [Google Scholar]
- 45.Leke RG, Taylor DW. The use of intermittent preventive treatment with sulfadoxine-pyrimethamine for preventing malaria in pregnant women. Clin Infect Dis. 2011;53(3):231–233. doi: 10.1093/cid/cir383. [DOI] [PubMed] [Google Scholar]
- 46.Gill CJ, Macleod WB, Mwanakasale V, et al. Inferiority of single-dose sulfadoxine-pyrimethamine intermittent preventive therapy for malaria during pregnancy among HIV-positive Zambian women. J Infect Dis. 2007;196(11):1577–1584. doi: 10.1086/522137. [DOI] [PubMed] [Google Scholar]
- 47.Kalilani L, Taylor S, Madanitsa M, et al. Waning effectiveness of intermittent preventive treatment in pregnancy (IPTp) with sulphadoxine-pyrimethamine (SP) in the presence of high SP resistance in Malawi. Abstract 1179. Paper presented at: 60th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Abstract Book; December 4–8, 2011; Philadelphia, PA. [Google Scholar]
- 48.World Health Organization; Global Malaia Program. Updated WHO Policy Recommendation (October 2012): Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP) Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]