Abstract
Background
Malaria is an important cause of illness and death in people living in many parts of the world, especially sub‐Saharan Africa. Long‐lasting insecticide treated bed nets (LLINs) and indoor residual spraying (IRS) reduce malaria transmission by targeting the adult mosquito vector and are key components of malaria control programmes. However, mosquito numbers may also be reduced by larval source management (LSM), which targets mosquito larvae as they mature in aquatic habitats. This is conducted by permanently or temporarily reducing the availability of larval habitats (habitat modification and habitat manipulation), or by adding substances to standing water that either kill or inhibit the development of larvae (larviciding).
Objectives
To evaluate the effectiveness of mosquito LSM for preventing malaria.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; EMBASE; CABS Abstracts; and LILACS up to 24 October 2012. We handsearched the Tropical Diseases Bulletin from 1900 to 2010, the archives of the World Health Organization (up to 11 February 2011), and the literature database of the Armed Forces Pest Management Board (up to 2 March 2011). We also contacted colleagues in the field for relevant articles.
Selection criteria
We included cluster randomized controlled trials (cluster‐RCTs), controlled before‐and‐after trials with at least one year of baseline data, and randomized cross‐over trials that compared LSM with no LSM for malaria control. We excluded trials that evaluated biological control of anopheline mosquitoes with larvivorous fish.
Data collection and analysis
At least two authors assessed each trial for eligibility. We extracted data and at least two authors independently determined the risk of bias in the included studies. We resolved all disagreements through discussion with a third author. We analyzed the data using Review Manager 5 software.
Main results
We included 13 studies; four cluster‐RCTs, eight controlled before‐and‐after trials, and one randomized cross‐over trial. The included studies evaluated habitat modification (one study), habitat modification with larviciding (two studies), habitat manipulation (one study), habitat manipulation plus larviciding (two studies), or larviciding alone (seven studies) in a wide variety of habitats and countries.
Malaria incidence
In two cluster‐RCTs undertaken in Sri Lanka, larviciding of abandoned mines, streams, irrigation ditches, and rice paddies reduced malaria incidence by around three‐quarters compared to the control (RR 0.26, 95% CI 0.22 to 0.31, 20,124 participants, two trials, moderate quality evidence). In three controlled before‐and‐after trials in urban and rural India and rural Kenya, results were inconsistent (98,233 participants, three trials, very low quality evidence). In one trial in urban India, the removal of domestic water containers together with weekly larviciding of canals and stagnant pools reduced malaria incidence by three quarters. In one trial in rural India and one trial in rural Kenya, malaria incidence was higher at baseline in intervention areas than in controls. However dam construction in India, and larviciding of streams and swamps in Kenya, reduced malaria incidence to levels similar to the control areas. In one additional randomized cross‐over trial in the flood plains of the Gambia River, where larval habitats were extensive and ill‐defined, larviciding by ground teams did not result in a statistically significant reduction in malaria incidence (2039 participants, one trial).
Parasite prevalence
In one cluster‐RCT from Sri Lanka, larviciding reduced parasite prevalence by almost 90% (RR 0.11, 95% CI 0.05 to 0.22, 2963 participants, one trial, moderate quality evidence). In five controlled before‐and‐after trials in Greece, India, the Philippines, and Tanzania, LSM resulted in an average reduction in parasite prevalence of around two‐thirds (RR 0.32, 95% CI 0.19 to 0.55, 8041 participants, five trials, moderate quality evidence). The interventions in these five trials included dam construction to reduce larval habitats, flushing of streams, removal of domestic water containers, and larviciding. In the randomized cross‐over trial in the flood plains of the Gambia River, larviciding by ground teams did not significantly reduce parasite prevalence (2039 participants, one trial).
Authors' conclusions
In Africa and Asia, LSM is another policy option, alongside LLINs and IRS, for reducing malaria morbidity in both urban and rural areas where a sufficient proportion of larval habitats can be targeted. Further research is needed to evaluate whether LSM is appropriate or feasible in parts of rural Africa where larval habitats are more extensive.
Keywords: Animals, Humans, Culicidae, Disease Vectors, Disease Reservoirs, Disease Reservoirs/parasitology, Ecosystem, Insecticides, Larva, Malaria, Malaria/prevention & control, Mosquito Control, Mosquito Control/methods, Randomized Controlled Trials as Topic, Randomized Controlled Trials as Topic/methods
Mosquito larval source management for controlling malaria
What is larval source management and how might it work?
Malaria is an infectious disease transmitted from person to person by mosquitoes, and the main interventions insecticide treated bed‐nets and indoor residual spraying reduce malaria infection by targeting adult mosquitoes. Larval source management (LSM) also aims to reduce malaria but instead targets immature mosquitoes, which are found in standing water, before they develop into flying adults. This is done by permanently removing standing water, for example by draining or filling land; making temporary changes to mosquito habitats to disrupt breeding, for example by clearing drains to make the water flow; or by adding chemicals, biological larvicides, or natural predators to standing water to kill larvae.
What does the research show?
We examined all the published and unpublished research up to 24 October 2012, and included 13 studies in this review.
Where larval habitats are not too extensive and a sufficient proportion of these habitats can be targeted, LSM probably reduces the number of people that will develop malaria (moderate quality evidence), and probably reduces the proportion of the population infected with the malaria parasite at any one time (moderate quality evidence).
LSM was shown to be effective in Sri Lanka, India, the Philippines, Greece, Kenya, and Tanzania, where interventions included adding larvicide to abandoned mine pits, streams, irrigation ditches and rice paddies where mosquitos breed, and building dams, flushing streams, and removing water containers from around people’s homes.
In one study from The Gambia where mosquitos were breeding in large swamps and rice paddies, spraying swamps with larvicide using ground teams did not show any benefit.
Summary of findings
Summary of findings for the main comparison.
LSM for controlling malaria
| LSM for controlling malaria | ||||||
| Patient or population: People living in malaria endemic areas Settings: Urban or rural settings in Africa, Asia and Europe Intervention: LSM | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | LSM | |||||
| Malaria incidence | 65 per 1000 | 17 per 1000 (14 to 20) | Rate Ratio 0.26 (0.22 to 0.31) | 20124 (2 cluster‐RCTs) | ⊕⊕⊕⊝ moderate1,2,3,4 | The 95% CI may be falsely narrow as trials did not adjust for cluster design. |
| 232 per 1000 | 118 per 1000 (42 to 334) | Rate Ratio 0.51 (0.18 to 1.44) | 98233 (3 controlled before and after studies) | ⊕⊝⊝⊝ very low5,6,7,8 | ||
| Parasite prevalence | 44 per 1000 | 5 per 1000 (2 to 10) | Risk Ratio 0.11 (0.05 to 0.22) | 2963 (1 cluster‐RCT) | ⊕⊕⊕⊝ moderate4,9,10 | The 95% CI may be falsely narrow as the trial did not adjust for cluster design. |
| 157 per 1000 | 50 per 1000 (30 to 86) | Risk Ratio 0.32 (0.19 to 0.55) | 8041 (5 controlled before and after studies) | ⊕⊕⊕⊝ moderate11,12,13,14,15 | ||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 for serious risk of bias: Both studies were described as randomized but did not adequately describe a process to reduce the risk of selection bias. 2 No serious inconsistency: There was no statistical heterogeneity. 3 No serious indirectness: Both studies were conducted in rural Sri Lanka. The primary vectors were An. culicifacies and An. subpictus and the primary mosquito larval habitats were river bed pools, streams irrigation ditches and rice paddies (Yapabandara 2004 LKA), and abandoned gem mine pits (Yapabandara 2001 LKA). The intervention was larviciding with pyriproxyfen approximately every six months. Generalization of this result to wider settings is supported by the findings from the non‐randomized studies. 4 No serious imprecision: Although these studies did not adjust for the cluster design, a sensitivity analysis adjusting this result for the cluster design suggested the result is likely to be both statistically significant and clinically important. 5 Downgraded by 1 for risk of bias: In two of these studies, there were important baseline differences in malaria incidence between groups. The incidence was higher in the intervention group pre‐intervention and reduced to similar levels as the control group post‐intervention. 6 Not downgraded for inconsistency: There was heterogeneity in this result which can be explained by baseline differences in two of the studies. However, there was a reduction in malaria incidence in the intervention groups in all three studies. 7 No serious indirectness: Sharma 2008 IND was conducted in rural India where the primary vectors were An. fluviatilis and An. culicifacies, the main larval habitats of which were streams, stagnant pools, ditches and irrigation channels. A dam was constructed across the stream, reducing the number of larval habitats in the intervention village. Fillinger 2009 KEN was conducted in highland villages in rural Kenya, where the major vectors were An. gambiae and An. funestus. The primary larval habitats were small streams and papyrus swamps, which were treated weekly with Bs for six months and then Bti for 13 months. Samnotra 1980 IND was conducted in a desert fringe area of urban India where the primary vectors were An. culicifacies and An. stephensi, the main larval habitats of which were containers, wells, canals and rainwater pools and drains. Larviciding with pirimiphos‐methyl was conducted weekly for 15 months. 8 Downgraded by 1 for imprecision: The overall effect is not statistically significant but is difficult to interpret due to the baseline differences. 9 Downgraded by 1 for serious risk of bias: This study was described as randomized but did not adequately describe a process to reduce the risk of selection bias. 10 No serious indirectness: This single study was conducted in rural Sri Lanka where the primary larval habitats were abandoned gem mine pits and the findings may not be easily generalized elsewhere. However generalization of this result to wider settings is supported by the findings from the non‐randomized studies. 11 No serious risk of bias: the risk of bias inherent in these non‐randomized studies is already accounted for in the initial downgrading to 'low quality evidence'. 12 No serious inconsistency: All five studies showed a large benefit with LSM. The smallest effect was a 40% reduction in malaria prevalence which is still considered clinically important. 13 No serious indirectness: These five studies were in conducted in urban and rural settings in Greece, Tanzania, India and the Philippines. Mosquito larval habitats ranged from man‐made habitats, containers and wells to rainwater pools, irrigation channels, ditches and streams, and interventions included dam construction, flushing of streams, straightening or lining of streams, drainage of marshland and larviciding. 14 No serious imprecision: All studies showed clinically important and statistically significant effects. 15 Upgraded by 1 as the effects seen were large. The two studies with smaller effects (Sharma 2008 IND; Fillinger 2009 KEN) had baseline differences which would lead to an underestimation of the true effect.
Background
Description of the condition
Malaria is the most common vector‐borne disease in the world, caused by Plasmodium spp. parasites which are transmitted by adult anopheline mosquitoes. In 2010, the number of deaths due to malaria was estimated to be between 655,000 (WHO 2011) and 1.24 million (Murray 2012). Most deaths occur in children aged less than five years old in sub‐Saharan Africa (WHO 2011).
Malaria is both a disease of poverty (Chima 2008; Teklehaimanot 2008), and an impediment to socioeconomic development (Gallup 2001). Acute malaria episodes and chronic disease reduce labour productivity, increase absenteeism from work, and cause premature mortality. At the macroeconomic level, there are broader costs stemming from the effect of malaria on tourism, trade, and foreign investment. The total cost to sub‐Saharan Africa has been estimated at around US$12 billion annually (approximately 5.8% of the total sub‐Saharan Africa gross domestic product) (Sachs 2001).
The Global Malaria Action Plan (GMAP) currently advocates four primary strategies to decrease malaria morbidity and mortality: 1) population coverage with long‐lasting insecticidal nets (LLINs), 2) indoor residual spraying (IRS), 3) prompt effective case management, and 4) intermittent preventive treatment during pregnancy (IPTp) (RBM 2008). Two of these strategies, LLINs and IRS, are methods of vector control that are highly effective in reducing malaria transmission by indoor host‐seeking mosquitoes (Lengeler 2004; Pluess 2010).
Description of the intervention
Mosquito larval source management (LSM) is the management of water bodies that are potential larval habitats to prevent the development of immature mosquitoes into adults (Kitron 1989; Bockarie 1999; Killeen 2002a; Walker 2007; Fillinger and Lindsay 2011).
Mosquitoes undergo complete metamorphosis and their immature stages develop in standing water in a range of different habitats. Some anopheline species breed predominately in water storage containers (for example, Anopheles stephensi), while other species breed in a wide variety of water bodies (for example, An. gambiae). The abundance of adult mosquitoes is dependent on: the number, quality, and size of potential habitats; their distance from humans and other blood meal sources; the density of larval stages in the habitats; and various other environmental factors such as temperature, rainfall patterns, soil types, and human behaviour (Muirhead‐Thomson 1951; Holstein 1954; Gillies 1988; Rozendaal 1997). Depending on the vector species, the eco‐epidemiological setting, and climatic conditions, mosquito larval habitats can be either stable or dynamic (with new habitats forming after rainfall or due to human activity, but disappearing during dry periods).
LSM can be classified as: (1) habitat modification; (2) habitat manipulation; (3) biological control; or (4) larviciding (Rozendaal 1997). (1) Habitat modification is a permanent change of land and water. It includes landscaping; drainage of surface water; land reclamation and filling; and coverage of large water storage containers (for example, wells) with mosquito‐proof lids and permanent slabs, or complete coverage of water surfaces with a material that is impenetrable to mosquitoes (for example, expanded polystyrene beads). (2) Habitat manipulation is a recurrent activity and includes water‐level manipulation, flushing of streams, drain clearance, shading, or exposing habitats to the sun depending on the ecology of the vector. (3) Biological control of mosquitoes is the introduction of natural enemies of mosquitoes into aquatic habitats, for example predatory fish or invertebrates, parasites, or other disease‐causing organisms. The most common approach used for malaria control is the introduction of larvivorous fish (fish that eat mosquito larvae and pupae) into larval habitats. This topic will be covered by a separate Cochrane review (Burkot 2009). (4) Larviciding is the regular application of biological or chemical insecticides to larval habitats to control mosquitoes. Currently available insecticides have different modes of action. They include surface films such as mineral oils and alcohol‐based surface products that suffocate larvae and pupae; synthetic organic chemicals such as organophosphates (for example, temephos and pirimiphos‐methyl) that interfere with the nervous system of larvae; microbials such as Bacillus thuringiensis israeliensis (Bti) and Bacillus sphaericus (Bs) that kill only larvae since their toxins have to be ingested and lead to starvation; and insect‐growth regulators (such as pyriproxyfen, methoprene and diflubenzuron) that interfere with insect metamorphoses and prevent adult emergence from the pupae stage. Historically, Paris Green (copper acetoarsenite), an arsenic‐based compound that is toxic to larvae, was extensively used for anopheline larval control (Soper 1943; Shousha 1948; Rozendaal 1997; WHO 2005; WHO 2006a).
How the intervention might work
LSM aims to reduce malaria transmission by targeting the immature stages (larvae and pupae) of the anopheline mosquito, to reduce the number of mosquitoes that reach adulthood. In this way, LSM may reduce transmission of Plasmodium spp. parasites by adult mosquitoes and reduce malaria prevalence and morbidity (Figure 1).
Figure 1.
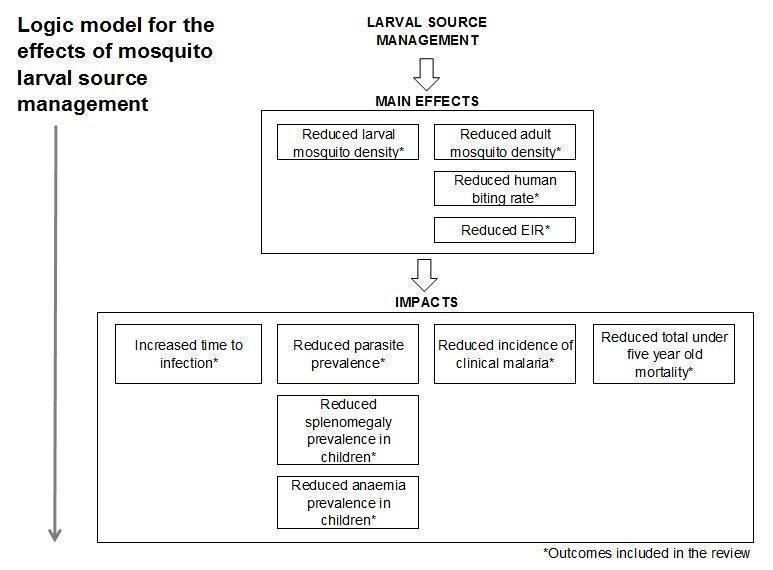
Logic model for the effects of mosquito LSM on malaria
Malaria transmission intensity is determined by the frequency with which malaria vectors bite humans (the human biting rate) and the proportion of vector mosquitoes with sporozoites in their salivary glands (the sporozoite rate). The product of these values is the entomological inoculation rate (EIR), which is the number of infectious bites received by an individual annually or seasonally. In general, the larger the mosquito population, the higher the human biting rate (unless protective measures against mosquito bites are in place) and the higher the EIR. The proportion of the human population with malaria parasites in their blood (parasite prevalence) is related linearly to the log value of the EIR. Parasite prevalence is unlikely to fall unless the EIR is less than one infectious bite per person per year (Beier 1999,Smith 2005). The relationship between EIR and the incidence of clinical malaria is mediated by reduced transmission efficiency at high levels of transmission intensity (Smith 2010), with incidence increasing with EIR before peaking at moderate transmission levels (Ghani 2009). Use of interventions that reduce adult vector populations will reduce the EIR (assuming that all other factors remain the same) (Smith 2007).
Vectorial capacity represents the efficiency of the malaria vector (the expected number of humans infected per day per infected human, assuming perfect transmission efficiency). This concept was formalized mathematically in the Ross‐MacDonald model (Macdonald 1957; Smith 2004; Smith 2007), which demonstrated that reducing the daily survival rate of adult mosquitoes produces the greatest reductions in transmission. As a result, malaria vector control has largely focused on the use of IRS and LLINs, which reduce adult survivorship. However, the Ross‐Macdonald model does not explicitly consider larval populations (Smith 2013). In practice, mosquitoes may avoid insecticides on walls or nets by feeding outdoors, or earlier in the night, and by resting outdoors (Molineaux 1980; Najera 2001). Only a small proportion of the vector population may be exposed to a fatal dose of insecticide, whilst the majority of the vector population remains unaffected. LSM targets both indoor and outdoor vectors (for example, An. arabiensis) and less anthropophilic secondary vectors that sustain transmission despite high coverage using LLIN, or IRS, or both.
Mosquito larvae are highly susceptible to vector control measures because they are confined to their aquatic habitat and, unlike adults, cannot develop behavioural resistance to avoid interventions (Charlwood 1987; Yohannes 2005; Geissbühler 2007). LSM might aid malaria control by targeting immature mosquitoes either without insecticide or using insecticides that have a different mode of action than those used for adult control. The elimination of larval habitats (through habitat modification) can provide long‐term and cost‐effective solutions because once a larval habitat is removed it cannot produce any flying and biting mosquitoes (Utzinger 2001; Keiser 2005; Castro 2009). In many settings, a large proportion of potential larval habitats are man‐made (Fillinger 2004; Minakawa 2005; Mutuku 2006a; Mwangangi 2007) and could be readily removed. Where habitats have a domestic or economic function (Utzinger 2001; Utzinger 2002; Mutuku 2006a), larviciding or biological control might be appropriate.
Why it is important to do this review
Prior to the advent of IRS with the insecticide dichlorodiphenyltrichloroethane (DDT), LSM was the primary method of malaria control. The Tennessee Valley Authority, which played a key role in the control of malaria in the south‐eastern United States, relied primarily on environmental management to reduce mosquito larval habitats (Gartrell 1954) and the construction of the Panama Canal was made possible through malaria and dengue fever control by engineering that eliminated mosquito larval habitats (Dehne 1955). Brazil eliminated An. gambiae by 1940, following its introduction in the late 1920s, using the chemical larvicide Paris Green (Soper 1943; Killeen 2002b). Egypt eliminated An. gambiae in 1945 using the same strategy, following its introduction in the early 1940s (Shousha 1948). LSM has since contributed to elimination efforts elsewhere (Soper 1943; Shousha 1948; Watson 1953; Russell 1955; Kitron 1989; Utzinger 2001; Killeen 2002b; Keiser 2005).
Today, vector control programmes are being encouraged to adopt Integrated Vector Management (IVM) strategies for the control of malaria and other vector borne diseases. In IVM, multiple tools are recommended to increase the efficacy and cost‐effectiveness of control efforts and to reduce dependence on insecticides (WHO 2008). LSM might have the capacity to supplement primary vector control measures (LLINs and IRS) since it targets outdoor biting and resting vectors and less anthropophilic vectors that sustain transmission, despite high coverage of LLINs, or IRS, or both. Resistance to all four classes of insecticides available for IRS (of which only one can be used on LLINs), and evidence of behavioural resistance (such as earlier evening biting) in areas with high IRS and LLIN coverage (Yohannes 2005; Geissbühler 2007; Bayoh 2010; Govella 2010) may undermine LLIN and IRS programmes. Continued reliance on these interventions may exacerbate the problem (N'Guessan 2007; Ranson 2011). Complementary methods of vector control, such as LSM, may therefore be increasingly necessary.
Currently, a number of malaria‐endemic countries in sub‐Saharan Africa and elsewhere are running or planning LSM programmes (Killeen 2002b; Utzinger 2002; Fillinger 2003; Gu 2005; Keiser 2005; Yohannes 2005; Chen 2006; Fillinger 2006; Mutuku 2006a; Shililu 2007; Walker 2007; Fillinger 2008; Geissbühler 2009). However, there is a lack of consensus on how effectively LSM reduces clinical and entomological outcomes. This is partly because few rigorously evaluated studies exist because cluster‐RCTs (cRCTs) with sufficient clusters are difficult to perform with this type of environmental intervention. Since the impact of LSM may be mediated by environmental factors, such as the vector species and type of larval habitats, there has also been debate over where and when LSM might be appropriate (Fillinger and Lindsay 2011). Discussions have also focused on how LSM can be operationalized and evaluated because some types of LSM, such as larviciding, need to be well managed, supervised, and require substantial involvement of local labour, similar to the organization of IRS programmes (Killeen 2006; Mukabana 2006; Mutuku 2006a; Fillinger 2008).
The GMAP states that in areas where malaria transmission is low to moderate, and seasonal or focal, targeted LSM may be appropriate in addition to LLINs, or IRS, or both. However, the plan encourages more operational research into LSM application in various settings (RBM 2008). More recently, the World Health Organization (WHO) published a position statement on the role of larviciding for malaria control in sub‐Saharan Africa, giving interim recommendations whilst urging caution due to gaps in the evidence (WHO 2012). Given the lack of consensus on the role of LSM in malaria control, it is timely to review the evidence for its impact on clinical and entomological outcomes, and to identify in which settings and under what conditions LSM is appropriate.
Objectives
To compare mosquito LSM (excluding biological control with larvivorous fish) for malaria control, applied either alone or in combination with other malaria control interventions, with no LSM.
Methods
Criteria for considering studies for this review
Types of studies
We included:
RCTs for which the unit of randomization was the cluster, provided that:
Intervention and control groups were comparable in terms of ecological baseline characteristics and access to antimalarial interventions, including rainfall, vector species, biting habits, and population, types of vector larval habitats, transmission intensity, transmission season, implementation of other malaria control or monitoring interventions. We did not include the study if characteristics were not reported.
-
Controlled before‐and‐after trials for which the unit of allocation was the cluster, provided that:
Intervention and control groups were comparable in terms of ecological baseline characteristics and access to antimalarial interventions, including rainfall, vector species, biting habits, and population, types of vector larval habitats, transmission intensity, transmission season, implementation of other malaria control or monitoring interventions. We did not include the study if characteristics were not reported.
In non‐randomized trials, there was at least one year or one transmission season of baseline data to demonstrate comparability.
-
Randomized cross‐over trials for which the unit of randomization was the cluster, provided that:
The intervention was restricted to larviciding only. We excluded the study if the intervention included habitat modification or manipulation, which are likely to be more permanent.
There was a washout period at least as long as that expected for complete disappearance of the larvicide in question, based on reported longevity of the larvicide, and for larval and adult densities to return to normal.
We excluded studies if:
The intervention was applied for less than one year in trials with perennial (year‐round) transmission (as reported by the study authors); or less than one transmission season (defined as the period from the onset of rains until one month afterwards) in trials with seasonal transmission (as reported by the study authors).
None of the outcomes of interest specified in this review were reported.
The follow‐up periods for the intervention and control periods were not identical.
Types of participants
Children and adults living in rural and urban malaria‐endemic areas.
Types of interventions
Intervention
We included interventions that aimed to reduce the emergence of adult vectors from aquatic habitats, including combinations of the following methods:
Habitat modification: a permanent change of land and water including landscaping; drainage of surface water; land reclamation and filling; and coverage of large water storage containers (for example, wells) with mosquito‐proof lids and permanent slabs, or complete coverage of water surfaces with a material that is impenetrable to mosquitoes (such as expanded polystyrene beads).
Habitat manipulation: a recurrent activity, such as water‐level manipulation, flushing, drain clearance, shading, or exposing habitats to the sun depending on the ecology of the vector.
Larviciding: the regular application of biological or chemical insecticides to water bodies to control mosquitoes, for example surface films such as mineral oils and alcohol‐based surface products; synthetic organic chemicals such as organochlorines and organophosphates; microbials; insect‐growth regulators; and copper acetoarsenite (Paris Green).
Biological control (excluding larvivorous fish): the introduction of natural enemies into aquatic habitats, for example predatory invertebrates, parasites or other disease‐causing organisms.
We excluded the following interventions:
Plant products, because formulations have not been standardized and studies are thus not comparable.
Larvivorous fish, as this is being covered in a separate Cochrane review (Burkot 2009), unless both intervention and control areas were equally treated with larvivorous fish as part of a combination of malaria interventions.
Interventions that did not target larval habitats, such as removal of vegetation around homes.
Control
No LSM intervention.
Additional interventions (co‐interventions)
We included studies that described more than one intervention, in which LSM was used in combination with another intervention, providing that the additional interventions were comparable across groups.
Types of outcome measures
Primary outcomes
Incidence of malaria: diagnostically confirmed by rapid diagnostic test or microscopy.
Parasite prevalence: diagnostically confirmed by rapid diagnostic test or microscopy.
Secondary outcomes
Splenomegaly prevalence in children.
Anaemia prevalence in children.
Time to infection.
Total mortality of children aged under five years.
EIR: the estimated number of bites by infectious mosquitoes per person per unit time (measured directly using human baits or indirectly using light traps, knock‐down catches, baited huts, or other methods of biting rate determination).
-
Adult mosquito density: measured by a technique previously shown to be appropriate for the vector:
Human biting rate: number of mosquitoes per person per time period, measured directly using human baits, or indirectly using light traps, knock‐down catches, baited huts, or other methods of biting rate determination.
Density measures other than human biting rate: number of mosquitoes per person or catch, measured using light traps, knock‐down catches, baited huts, or other methods of adult vector density determination.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; CABS Abstracts and LILACS (May 10, 2013). We handsearched the US Armed Forces Pest Management Board Defense Pest Management Literature Retrieval System (search completed March 2, 2011) and the Tropical Diseases Bulletin from 1900 to 2010 (search completed March 2, 2011) using the terms: malaria AND mosquito control.
Searching other resources
Organizations
We handsearched the archives of the WHO using the terms: malaria AND mosquito control. These archives included WHO Technical Documents pre c1983; the catalogue of the material of the WHO (stored in WHO archives in microform) from 1946 to 1950 and 1950 to 1955; the catalogue of the material of the WHO (stored as centralized files) pre 1991; and the archives of the Parasitology Collection of the Communicable Diseases Documentation Centre at the WHO Headquarters from 1911 to date (search completed February 11, 2011).
Researchers
We contacted heads of malaria control and prominent researchers in countries with active or former programmes using LSM and requested access to both published and unpublished manuscripts describing controlled trials. We made these requests between July 8, 2011 and December 16, 2011.
Data collection and analysis
Selection of studies
SL and JT independently screened the electronic search results for potentially relevant studies. We attempted to retrieve the full articles for all studies identified by either SL or JT. Both LT and JT independently screened the handsearch results for potentially relevant studies. JT, LT, and KB assessed eligibility using an eligibility form. Two authors (JT, LT, or KB) assessed each article independently, and we resolved any disagreements through discussions with the third author. If any disagreement remained, SL or JG made a final judgment. Native speakers evaluated the foreign language studies in consultation with one of the authors. We checked study reports to ensure that multiple publications from the same study were included only once. We listed excluded studies and the reasons for their exclusion in the ‘Characteristics of excluded studies’ section.
Data extraction and management
LT and KB independently extracted data from the study reports into a pre‐designed data extraction form. LT and KB resolved any disagreement through discussion with each another and then with JT. JT reviewed all data extraction. We attempted to collect unreported data by directly contacting study authors. Where results were reported for multiple time points or for multiple areas, we extracted each result and synthesized the data as outlined in the 'Data synthesis' section.
Data extraction for cluster‐RCTs
For trials randomized using clusters, we extracted the number of clusters in the trial, the average size of clusters, and the unit of randomization (for example, household or community). Where possible, we documented the statistical methods used to analyze the trial. We examined the methods for adjustments for clustering or other covariates. We recorded estimates of the intra‐cluster correlation (ICC) coefficient for each outcome when they were reported. We contacted authors to request missing information.
Where results were not adjusted for clustering, for count data (incidence of clinical malaria) we extracted the number of events in the treatment and control group and the total person time at risk in each group. For dichotomous outcomes (parasite or splenomegaly prevalence), we extracted the number of participants that experienced the event and the number of participants in each treatment group. For continuous outcomes (the entomological outcomes), we extracted arithmetic or geometric means, standard deviations or standard errors, and the number of participants in each treatment group.
Data extraction for controlled before‐and‐after trials
For controlled before‐and‐after trials, we extracted the same information as for cluster‐RCTs that had not been adjusted for clustering. We extracted details regarding the study design methods. When studies adjusted for covariates in the analyses and reported an adjusted measure of effect, we extracted the measure of effect and its standard error. We recorded the variable or variables used for adjustment.
Data extraction for randomized cross‐over trials
For randomized cross‐over trials, we extracted the same information as for controlled before‐and‐after trials.
Assessment of risk of bias in included studies
JT and JG independently assessed the risk of bias for each selected study using the Effective Practice and Organisation of Care (EPOC) risk of bias assessment form (Cochrane 2009). We modified this form to encompass the needs of our study designs. We resolved any discrepancies between the two assessments through discussion with a third co‐author. We assigned a judgement of unclear, high, or low risk of bias for each component of each study, as outlined in Table 8. We presented the results in a risk of bias summary and figure.
Table 1.
Assessment of risk of bias
| Risk of bias component | Low | High | Unclear |
| Sequence generation | Random component in the sequence generation process is described. | Non‐random method is used. | No or unclear information reported. |
| Allocation concealment | Patients and investigators could not foresee assignment. | Patients and investigators could foresee assignment. | No or unclear information reported. |
| Blinding (performance) | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | No evidence of performance bias due to knowledge of the allocated interventions by participants and personnel during the study | No or unclear information reported. |
| Blinding (detection) | Primary outcomes assessed blinded. | Primary outcomes not assessed blinded. | No or unclear information reported. |
| Incomplete outcome data | No or low missing data, reason for missing data is unlikely to be related to the true outcome, or missing data is balanced across groups. | High missing data, reason for missing data is likely to be related to the true outcome, or missing data is unbalanced across groups. | No or unclear information reported. |
| Selective outcome reporting | All pre‐specified outcomes are reported (expected or see protocol). | Not all pre‐specified outcomes are reported; or additional outcomes reported. | No or unclear information reported. |
| Recruitment bias | No change in size or number of clusters after randomization. | Possible change in size or number of clusters after randomization. | No or unclear information reported. |
| Baseline characteristics | If baseline characteristics of the study and control areas are reported and similar. | If there are differences between control and intervention areas. | No or unclear information reported. |
| Contamination | it is unlikely that the control group received the intervention. | It is likely that the control group received the intervention. | No or unclear information reported. |
|
Incorrect analysis (Randomized studies only) |
Randomized studies: clustering taken into account in analysis. | Randomized studies: clustering not taken into account in analysis. | Randomized studies: No or unclear information reported. |
| Other biases (confounding) | Non‐randomized studies: no evidence of confounding (selection bias) | Non‐randomized studies: evidence of confounding (selection bias) | Non‐randomized studies: no or unclear information reported. |
Measures of treatment effect
For count data (malaria incidence), we presented rate ratios. For dichotomous outcomes (parasite or splenomegaly prevalence), we presented the risk ratio. We summarized continuous outcomes by arithmetic mean values and we reported the percent reduction. We presented all results with 95% CIs.
Unit of analysis issues
When the analyses did not adjust for clustering, we contacted trial authors to ask for estimates of ICC. When these were unavailable, we conducted a sensitivity analysis imputing a range of values (from 0.01 to 0.1) for the ICC. For rate and prevalence estimates, we multiplied the standard errors of the estimates (from an analysis ignoring clustering) by the square root of the design effect, where the design effect was calculated as DEff = 1 + (m ‐ 1)*ICC and m = the average cluster size.
Dealing with missing data
Due to the nature of the study designs, trials did not follow‐up individual patients and we do not know the number of missing patients. We extracted data as reported in the studies.
Data synthesis
We calculated the outcome measure (for example, parasite prevalence) separately for each year, month, or survey and we took an unweighted average to aggregate data from multiple years, months, surveys, or sites. We compared data from the follow‐up period (for both control and intervention areas) for the same portion of the year to take into account seasonality where baseline data were available only for portion of a year. For data collected from multiple cross‐sectional surveys, we used data during or immediately after a transmission season, rather than during a dry season or at the beginning of a transmission season. Where longitudinal data were presented separately for the transmission and non‐transmission season, we used the data for the transmission season. For studies where no events were observed in one or both arms, we added a fixed value (0.5) to all cells of study results tables.
Clinical data
For cluster‐RCTs and controlled before‐and‐after trials, we stratified the data by intervention: (1) habitat modification alone; (2) habitat modification with larviciding; (3) habitat manipulation alone; (4) habitat manipulation with larviciding; (5) larviciding alone; or (6) any LSM. We then stratified by outcome: (1) incidence of malaria; (2) parasite prevalence; or (3) splenomegaly prevalence. Finally, we stratified the data by study design: (1) cluster‐RCTs; or (2) controlled before‐and‐after trials. Although the interventions used in these trials were highly variable, we justified pooling of data across interventions in the final analysis as all trials shared the common aim to reduce mosquito numbers. In this respect, we judged the interventions as appropriately different as they were designed to suit the local vector biology and larval habitats.
We presented the data as forest plots. We used fixed effect meta‐analysis where we did not detect significant heterogeneity, and random‐effects meta‐analysis where we found significant heterogeneity. We conducted the analyses using Review Manager (RevMan). For randomized cross‐over trials, where each cluster acted as its own control and there were no baseline data, we presented the data in tables. For count data, we calculated rate ratios for each zone so we could compare control and treatment years. For dichotomous outcomes, we calculated risk ratios for each zone so we could compare control and treatment years.
Entomological data
We could not analyze the entomological data using the same methods as for the clinical data because we did not identify a sufficient number of trials. For cluster‐RCTs and controlled before‐and‐after trials, we presented the data in tables. We presented one table for each outcome: (1) EIR; (2) adult mosquito density (human biting rate); and (3) adult mosquito density (measures other than human biting rate). Within each table, we stratified the data by intervention and then by study design. We presented data from non‐randomized cross‐over trials in a separate table.
For all studies in which data were available at baseline and post‐intervention for at least one control and one intervention site, we adopted a 'difference in differences' (or ratio of ratios for a multiplicative model) approach to estimate the percent reduction in the outcome due to the intervention. We estimated the effect of the intervention (RR) by using the formula (q1/q0)/(p1/p0), where q1 and q0 are, respectively, the entomological indicators (EIR, mean density, or biting rate) observed in the intervention and control areas post‐intervention respectively and p1 and p0 are the corresponding baseline estimates of these entomological indicators. We calculated the percentage reduction in entomological indicators as 100 x (1 ‐ RR). We calculated the 95% CIs for log(RR) using the delta method. We then back‐transformed these intervals (we took the anti‐log of the lower and upper bounds) to obtain CIs for RR.
The difference in differences estimate assumes that: 1) changes over time are similar for the control and intervention sites; and 2) time and intervention effects combine multiplicatively. Estimates will be biased if there is a change that is unrelated to the intervention that does not occur equally across both areas. Estimates would be more robust if they were based on data from multiple control and intervention sites and analysed as in a cluster‐RCT (such as, accounting for correlated outcomes in the same cluster).
For studies in which data were only available post‐intervention for one control and one intervention site, we calculated the percent reduction in the outcome in the treatment group, as compared to the control group, by the formula 100x(1‐(q1/q0)). We did not calculate the 95% CIs.
Where data were available from multiple control or intervention sites, we took the average values of the outcome measures (EIR, mean density, or biting rate) and we gave equal weight to all sites. We averaged the data from multiple time points within a year or transmission season, either pre‐ or post‐intervention, in a similar manner.
Sensitivity analysis
Where we combined numerous trials in meta‐analysis, we planned to conduct a sensitivity analysis including only trials with low risk of bias to investigate the robustness of the results. However, since all included studies were at variable risk of bias, we had an insufficient number of trials at low risk of bias and therefore we did not conduct this analysis.
Quality of evidence
We assessed the quality of evidence across each outcome measure using the GRADE approach. We used a quality rating across studies that had four levels: high, moderate, low, or very low. We initially categorized RCTs as high quality but we could downgrade each trial after we assessed five criteria: risk of bias, consistency, directness, imprecision, and publication bias. Similarly, we initially categorized observational studies as low quality and we downgraded trials by these same criteria. However, in exceptional circumstances, we upgraded trials by three further criteria: large effect size, all plausible confounders would act to reduce the effect size, and evidence of a dose‐response effect (Guyatt 2008).
Results
Description of studies
Results of the search
We identified 2687 studies through the electronic search, and a further 195 from other sources (handsearching and contacting researchers in the field). We removed duplicates and screened all abstracts for possible inclusion. Of these, 520 unique studies were identified for full text screening (Figure 2).
Figure 2.
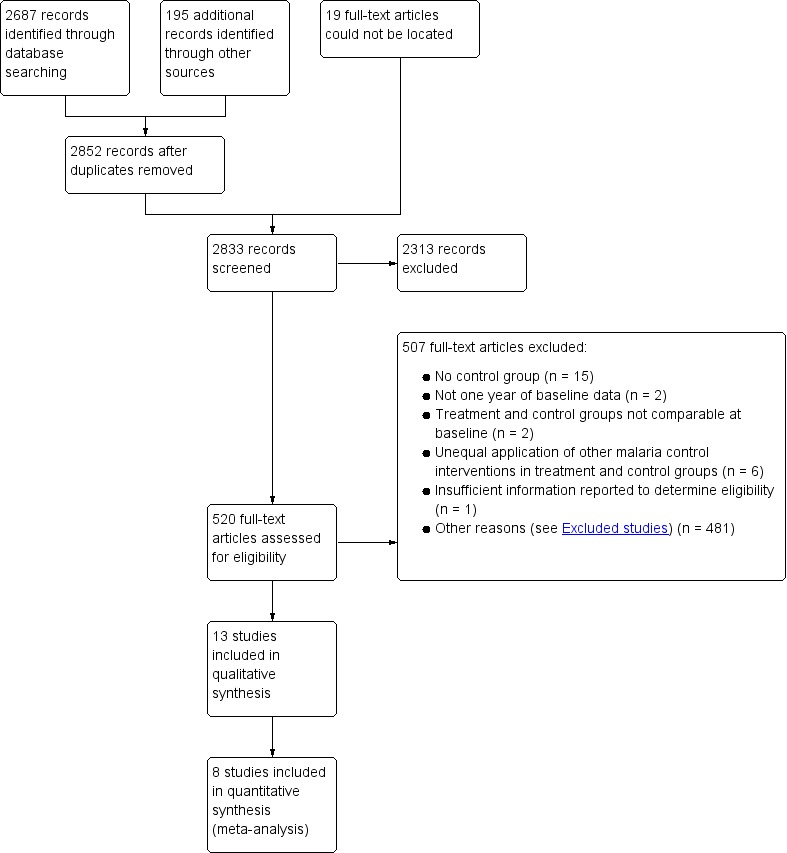
Study flow diagram.
Included studies
Thirteen studies met the inclusion criteria, and these are described in the Characteristics of included studies tables, and Table 9.
Table 2.
Summary of interventions and eco‐epidemiological settings
| Intervention | Study ID | Study design | Details of the intervention | Who was responsible for LSM? | Ecosystem | Primary vectors (primary larval habitats) | Malaria transmission intensity |
| Habitat modification alone | Sharma 2008 IND | Controlled before‐and‐after | Dam construction | Community, government | Forest; rural | An. fluviatilis (streams), An. culicifacies (stagnant pools, ditches, irrigation channels) | Moderate |
|
Habitat modification with larviciding |
Shililu 2007 ERI | Cluster‐RCT | Land filling and grading; drainage; larviciding with synthetic organic compounds and microbials | Study staff, community | Desert fringe, highland and lowland; rural | An. arabiensis (stream bed pools, canals, drainage channels, wells, communal water supply points) | Not stated |
| Balfour 1936 GRC | Controlled before‐and‐after | Straightening, deepening and lining of natural streams; drainage; larviciding with Paris Green | Government | Coastal; urban and rural | An. elutus; An. superpictus (primarily man‐made habitats) | Low to moderate | |
| Habitat manipulation alone | Santiago 1960 PHL | Controlled before‐and‐after | Controlling water levels and stream flushing | Coastal; urban | An. minimus flavirostris (streams fed by a lake) | High | |
|
Habitat manipulation with larviciding |
Castro 2009 TZA | Controlled before‐and‐after | Clearing of aquatic vegetation and debris; larviciding with microbials | Study staff, community, government | Coastal; urban | An. gambiae, An. funestus (drains) | Low to moderate |
| Samnotra 1980 IND | Controlled before‐and‐after | Removal of 'domestic' larval habitats; Larviciding with synthetic organic compounds | Study staff, community | Desert fringe; urban | An. culicifacies, An. stephensi (containers, wells, rainwater pools, canals, stagnant pools in drains) | Low | |
|
Larviciding alone |
Coulibaly 2011 MLI | Cluster‐RCT | Larviciding with microbials | Study staff, community | Savannah; rural | An. gambiae (brick pits, ponds, tyre prints) | High |
| Yapabandara 2001 LKA | Cluster‐RCT | Larviciding with insect growth regulators | Study staff, community | Forest; rural | An. culicifacies, An. subpictus Grassi. (abandoned gem mine pits) | Moderate to high | |
| Yapabandara 2004 LKA | Cluster‐RCT | Larviciding with insect growth regulators | Study staff | 'Dry zone'; rural | An. culifacies, An. subpictus (river bed pools, streams, irrigation ditches (dry season); rice paddies (rainy season)) | Moderate | |
| Fillinger 2008 TZA | Controlled before‐and‐after | Larviciding with microbials | Study staff, community | Coastal; urban | An. gambiae s.s., An. arabiensis (man‐made habitats exposed to sunlight) | Low to moderate | |
| Fillinger 2009 KEN | Controlled before‐and‐after | Larviciding with microbials | Study staff | Highland; rural | An. gambiae s.l.,An. funestus s.l. (small streams, papyrus swamps) | Moderate | |
| Geissbühler 2009 TZA | Controlled before‐and‐after | Larviciding with microbials | Study staff, community | Coastal; urban | An. gambiae s.l. (man‐made habitats exposed to sunlight) | Low to moderate | |
| Majambere 2010 GMB | Randomized cross‐over | Larviciding with microbials | Study staff, community | Savannah; rural | An. gambiae (flood plains, rice paddy fields) | High |
Four studies were cluster‐RCTs (Yapabandara 2001 LKA; Yapabandara 2004 LKA; Shililu 2007 ERI; Coulibaly 2011 MLI), eight studies were controlled before‐and‐after trials (Balfour 1936 GRC; Santiago 1960 PHL; Samnotra 1980 IND; Fillinger 2008 TZA; Sharma 2008 IND; Castro 2009 TZA; Fillinger 2009 KEN; Geissbühler 2009 TZA) and one study was a randomized cross‐over trial (Majambere 2010 GMB). None of the randomized studies made adjustments for clustering.
Seven studies were conducted in sub‐Saharan Africa (urban Tanzania, rural Mali, rural Kenya, rural Gambia, and rural Eritrea), five studies in Asia (rural India, urban India, urban Philippines, and rural Sri Lanka), and one study in Europe (urban and rural Greece).
The studies targeted a variety of habitat types including both discrete habitats (such as drains, ditches, pits, ponds, and containers), and extensive habitats (such as rice paddies, swamps, and river flood plains).
The studies conducted in Africa targeted the major vectors An. arabiensis (the larval habitats of which were predominantly stream bed pools, canals, drainage channels, and wells in these studies), An. gambiae (drains and other man‐made urban habitats, small streams and swamps, brick pits, ponds, tyre prints, flood plains, rice paddies, and other habitats associated with agriculture), and An. funestus (drains and other man‐made urban habitats, small streams, and swamps). In Asia, the main vectors targeted were An. fluviatilis (streams), An. culicifacies (stagnant pools, ditches, irrigation channels, containers, wells, abandoned mine pits, and rice paddies), An. stephensi (containers, wells, rainwater pools, and canals), An. minimus flavirostris (streams), and An. subpictus (river bed pools, streams, irrigation ditches, and rice paddies). The study conducted in Europe targeted An. elutus and An. superpictus (man‐made habitats).
One study conducted habitat modification alone (Sharma 2008 IND), two studies conducted habitat modification with larviciding (Balfour 1936 GRC; Shililu 2007 ERI), one study conducted habitat manipulation alone (Santiago 1960 PHL), two studies conducted habitat manipulation with larviciding (Samnotra 1980 IND; Castro 2009 TZA) and seven studies conducted larviciding alone (Yapabandara 2001 LKA; Yapabandara 2004 LKA; Fillinger 2008 TZA; Fillinger 2009 KEN; Geissbühler 2009 TZA; Majambere 2010 GMB; Coulibaly 2011 MLI).
LSM was not conducted by the community alone in any of the included studies. In seven studies, study staff conducted LSM in conjunction with specifically trained and employed members of the local community (Samnotra 1980 IND; Yapabandara 2001 LKA; Shililu 2007 ERI; Fillinger 2008 TZA; Geissbühler 2009 TZA; Majambere 2010 GMB; Coulibaly 2011 MLI). In one study, LSM was co‐ordinated by study staff but actively conducted by specially trained and paid members of the local community, with local government support (Castro 2009 TZA). In one study, the government conducted LSM in conjunction with members of the local community (Sharma 2008 IND). In two studies, local (Balfour 1936 GRC) and foreign (Santiago 1960 PHL) government staff conducted LSM, and in two studies, study staff alone conducted LSM (Yapabandara 2004 LKA; Fillinger 2009 KEN).
Of the studies that recorded clinical outcomes, these were measured in children aged between six months to 10 years (Fillinger 2009 KEN; Majambere 2010 GMB), two years to 10 years (Santiago 1960 PHL), 0 years to five years (Geissbühler 2009 TZA) and of "school age" (Balfour 1936 GRC). Five studies recorded clinical outcomes in all age groups (Castro 2009 TZA; Samnotra 1980 IND; Sharma 2008 IND; Yapabandara 2001 LKA; Yapabandara 2004 LKA).
Excluded studies
We excluded 45 studies for the following reasons (see Characteristics of excluded studies table):
Lack of control group (15 studies).
Lack of one year of baseline data (two studies).
Lack of baseline comparability between intervention and control areas (two studies).
Uneven application of other malaria control interventions between intervention and control arms (for example, weekly active surveillance and treatment, chemoprophylaxis, indoor residual spraying) (six studies).
Unable to locate full‐text article (19 studies).
Insufficient information reported to determine eligibility (one study)
We also excluded 481 studies for one or more of the following reasons (not included in the Characteristics of excluded studies):
Did not study LSM as described in our methods.
Did not report outcomes in either adult mosquitoes, human malaria or both.
Did not have at least one year or one transmission season of data following the beginning of the intervention.
Malaria control programme description in which LSM was one of many interventions.
Review or opinion article.
Studies awaiting classification
We identified one potentially eligible study that did not report sufficient data to make a judgement about eligibility, and is therefore awaiting classification (Kinde‐Gazard 2012).
Risk of bias in included studies
We have given a summary of our judgement of risks of bias in included studies in Figure 3. We listed individual risk of bias assessments in the Characteristics of included studies section.
Figure 3.
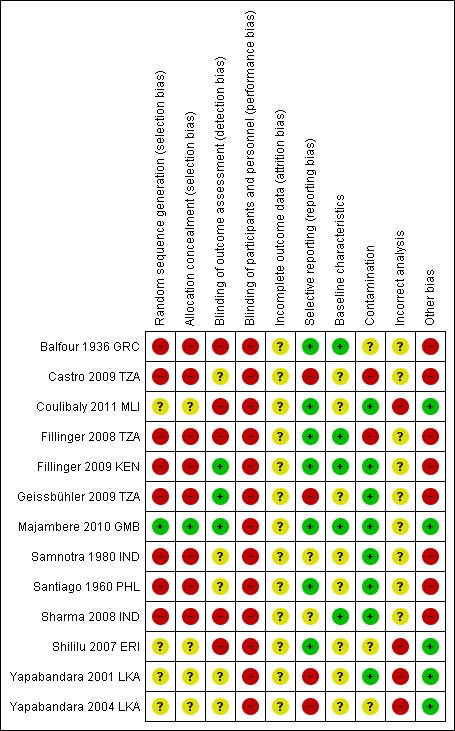
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
+ low risk of bias; ‐ high risk of bias; ? unclear risk of bias.
Allocation
We judged all four cluster‐RCTs at an unclear risk of selection bias due to an inadequate description of the method of randomization and allocation concealment (Coulibaly 2011 MLI; Shililu 2007 ERI; Yapabandara 2001 LKA; Yapabandara 2004 LKA). We judged the eight controlled before‐and‐after studies at high risk of selection bias due to the non‐randomized design (Balfour 1936 GRC; Castro 2009 TZA; Fillinger 2008 TZA; Fillinger 2009 KEN; Geissbühler 2009 TZA; Samnotra 1980 IND; Santiago 1960 PHL; Sharma 2008 IND). We considered the cross‐over trial to be at low risk of bias as each arm functioned as its own control (Majambere 2010 GMB).
Blinding
Due to the nature of the intervention, blinding of the implementers and the recipients was not possible, and we therefore classified all trials at high risk of performance bias.
Two cluster‐RCTs only reported entomological data. As it would have been impossible to blind the data collectors, we classified these trials at high risk of bias (Coulibaly 2011 MLI; Shililu 2007 ERI). We judged two cluster‐RCTs reporting clinical outcomes at unclear risk of detection bias (Yapabandara 2001 LKA; Yapabandara 2004 LKA). Two of the controlled before‐and‐after studies blinded the microscopists to allocation and we considered these trials at low risk of detection bias (Fillinger 2009 KEN; Geissbühler 2009 TZA). Three trials did not blind the microscopists to allocation and we considered these trials at high risk of detection bias (Balfour 1936 GRC; Fillinger 2008 TZA; Sharma 2008 IND). In three trials it was unclear if microscopists were blinded to allocation (Samnotra 1980 IND; Sharma 2008 IND; Castro 2009 TZA). The cross‐over trial again blinded microscopists to allocation and we judged this trial at low risk of bias (Majambere 2010 GMB).
Incomplete outcome data
One cluster‐RCT reported the loss of two clusters during the second year of the study (Coulibaly 2011 MLI). The remaining studies did not report losses to follow‐up. We judged all trials to be at unclear risk of attrition bias.
Selective reporting
We judged two cluster‐RCTs at high risk of reporting bias as they had evidence of selective reporting for entomological outcomes. The authors described several methods of data collection but they did not report all (Yapabandara 2001 LKA; Yapabandara 2004 LKA). We deemed two controlled before‐and‐after trials at high risk of selective reporting as they collected data on the whole population but only reported data on children (Castro 2009 TZA; Geissbühler 2009 TZA).
Baseline characteristics
We considered the cluster‐RCTs at unclear risk of bias because they did not clearly report baseline characteristics. We considered four of the controlled before‐and‐after studies at low risk of bias (Balfour 1936 GRC; Fillinger 2008 TZA; Sharma 2008 IND; Fillinger 2009 KEN) and four to be at unclear risk of bias (Santiago 1960 PHL; Samnotra 1980 IND; Castro 2009 TZA; Geissbühler 2009 TZA).
Contamination
We judged two cluster‐RCTs at low risk of bias (Yapabandara 2001 LKA; Coulibaly 2011 MLI) and two trials at unclear risk of bias (Yapabandara 2004 LKA; Shililu 2007 ERI). We judged five controlled before‐and‐after trials at low risk of bias (Santiago 1960 PHL; Samnotra 1980 IND; Sharma 2008 IND; Fillinger 2009 KEN; Geissbühler 2009 TZA), two trials at high risk (Castro 2009 TZA; Fillinger 2008 TZA) and one trial at unclear risk of bias (Balfour 1936 GRC).
Incorrect analysis
We judged the four cluster‐RCTs at high risk of bias because they did not adjust for clustering (Yapabandara 2001 LKA; Yapabandara 2004 LKA; Shililu 2007 ERI; Coulibaly 2011 MLI).
Other potential sources of bias
We considered the eight controlled before‐and‐after studies at high risk of confounding due to the study design (Balfour 1936 GRC; Santiago 1960 PHL; Samnotra 1980 IND; Fillinger 2008 TZA; Sharma 2008 IND; Castro 2009 TZA; Fillinger 2009 KEN; Geissbühler 2009 TZA ).
Effects of interventions
See: Table 1
Comparison 1. Habitat modification alone versus control
One controlled before‐and‐after study, conducted in a rural, forested area of India, compared dam construction in one village with no intervention in two control villages (Sharma 2008 IND). The primary vector An. culicifacies was found breeding mainly in streams, stagnant pools, ditches, and irrigation channels. IRS was conducted annually in all villages.
Malaria incidence: At baseline, the incidence of malaria was twice as high in the treatment village than in the controls (Rate ratio 2.29, 95% CI 1.76 to 2.97, one study, 570 participants, Analysis 1.1). Following dam construction, the incidence of malaria in the treatment villages was reduced to similar levels as the control villages. In the treatment villages the incidence of malaria decreased from 638 to 262 cases per 1000 person years (one study, 570 participants, Analysis 1.1).
Analysis 1.1.
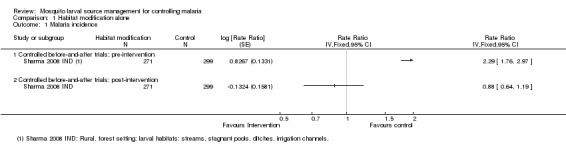
Comparison 1 Habitat modification alone, Outcome 1 Malaria incidence.
Parasite prevalence: At baseline, parasite prevalence did not significantly differ between treatment and control villages (one study, 570 participants, Analysis 1.2). Following dam construction, parasite prevalence significantly decreased in the treatment village compared to the controls (Risk ratio 0.23, 95% CI 0.12 to 0.43; one study, 570 participants, Analysis 1.2). Parasite prevalence in the treatment village decreased from 16% to 4%.
Analysis 1.2.
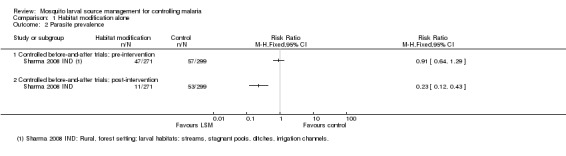
Comparison 1 Habitat modification alone, Outcome 2 Parasite prevalence.
Comparison 2. Habitat modification with larviciding versus control
One cluster‐RCT and one controlled before‐and‐after study conducted habitat modification with larviciding. The cluster‐RCT, conducted in lowland and highland rural desert fringe areas of Eritrea, compared land filling and grading, drainage, and larviciding with Bti and temephos with no intervention. The primary vector An. arabiensis was mainly found breeding in stream bed pools, canals, drainage channels, and wells (Shililu 2007 ERI).
The controlled before‐and‐after trial, conducted in urban and rural Greece, compared straightening, deepening and lining of streams, drainage and larviciding with Paris Green with no intervention. The main larval habitats of the major vectors An. elutus and An. superpictus were man‐made habitats (Balfour 1936 GRC). Balfour 1936 GRC reported five years of post‐intervention data (1931‐1935) (Table 10) but only data for 1931 was included for the post‐intervention period.
Table 3.
Summary of original data for Balfour 1936 GRC
|
Outcome |
Group |
Parasite or splenomegaly prevalence (total positive/total examined) |
|||||
| Pre‐intervention | Post‐intervention | ||||||
| 1930 | 1931 | 1932 | 1933 | 1934 | 1935 | ||
| Parasite prevalence |
Control | 9.1% (59/650) |
23.9% (164/685) |
15.0% (104/692) |
21.9% (147/670) |
10.0% (69/690) |
18.0% (123/682) |
| Treatment | 4.0% (43/1087) |
6.0% (51/853) |
9.0% (75/837) |
4.0% (33/830) |
1.0% (8/834) |
1.6% (13/827) |
|
| Splenomegaly prevalence |
Control | 46.0% (299/650) |
56.9% (390/685) |
43.1% (298/692) |
44.0% (295/670) |
35.9% (248/690) |
40.0% (273/682) |
| Treatment | 26.5% (288/1087) |
23.4% (200/853) |
18.0% (151/837) |
13.0% (108/830) |
12.0% (100/834) |
7.0% (58/827) |
|
Parasite prevalence: In the controlled before‐and‐after study, parasite prevalence was lower at baseline in the treatment group (4%) than in the control group (9%) (Risk ratio 0.44, 95% CI 0.30 to 0.64; one study, 1737 participants, Analysis 2.1). Post‐intervention, parasite prevalence remained low in the treatment group (6%) but increased substantially in the control group (24%) (Risk ratio 0.25, 95% CI 0.19 to 0.34; one study, 1538 participants, Analysis 2.1).
Analysis 2.1.
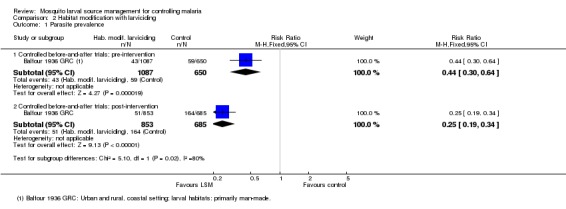
Comparison 2 Habitat modification with larviciding, Outcome 1 Parasite prevalence.
Splenomegaly prevalence: In the controlled before‐and‐after study, splenomegaly prevalence was again lower at baseline in the treatment group (27%) than in the control group (46%) (Risk ratio 0.58, 95% CI 0.51 to 0.66; one study, 1737 participants, Analysis 2.2). Post‐intervention, splenomegaly prevalence decreased slightly in the treatment group (24%) and increased in the control group (57%) (Risk ratio 0.41, 95% CI 0.36 to 0.47; one study, 1538 participants, Analysis 2.2).
Analysis 2.2.
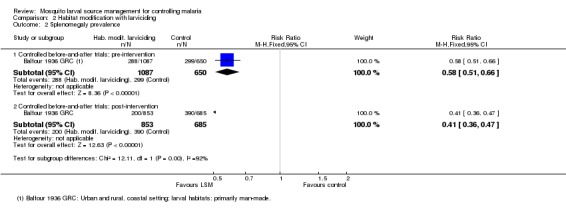
Comparison 2 Habitat modification with larviciding, Outcome 2 Splenomegaly prevalence.
Adult mosquito density (measures other than human biting rate): The cluster‐RCT only collected data on adult mosquito density (Shililu 2007 ERI) and did not report baseline data. Post‐intervention the adult mosquito density decreased by 15.2% in the treatment group but the trial authors did not report if this reduction was statistically significant (Table 11).
Table 4.
Entomological data: Adult mosquito density (density measures other than human biting rate)
| Intervention | Study ID | Study design | Mean adult mosquito density (95% CI) |
Percent reduction (95% CI)1 |
Notes | |||
| Pre‐intervention | Post‐intervention | |||||||
| Control | Treatment | Control | Treatment | |||||
| Habitat modification with larviciding | Shililu 2007 ERI | Cluster‐RCT | ‐ | ‐ | 4.99 | 4.23 | 15.2 | Mean number of female adult anophelines per night (light traps) |
| Habitat manipulation alone | Santiago 1960 PHL | Controlled before‐and‐after trial | 0.15 | 0.20 | 0.17 | 0.02 | 91.2 | Mean number of adult anophelines per catching station (human‐baited traps) |
| Habitat manipulation with larviciding | Samnotra 1980 IND | Controlled before‐and‐after trial | 222 | 702 | 696 | 213 | 90.3 | Mean number of adult anophelines per catching station (resting catch) |
| Larviciding alone | Coulibaly 2011 MLI (2009 data) | Cluster‐RCT | ‐ | ‐ | 2.27 | 1.49 | 34.4 | ‐ |
| Coulibaly 2011 MLI(2010 data) | Cluster‐RCT | ‐ | ‐ | 6.03 | 3.75 | 37.8 | ‐ | |
| Yapabandara 2001 LKA | Cluster‐RCT | 16.88 | 27.63 | 22.13 | 3.38 | 90.7 | Mean number of adult anophelines per man per night (partial night human landing catches) (An. culicifacies) | |
| Yapabandara 2001 LKA2 | Cluster‐RCT | ‐ | ‐ | ‐ | ‐ | ‐ | Mean number of adult anophelines per man per night (all night human landing catches) (An. culicifacies) | |
| Yapabandara 2004 LKA | Cluster‐RCT | 6.64 | 9.11 | 8.75 | 1.44 | 88.0 | Mean resting density of adult anophelines (cattle baited huts) (An. culicifacies) | |
| Fillinger 2009 KEN | Controlled before‐and‐after trial | 3.69 (2.25 to 6.06) | 3.49 (2.49 to 4.88) | 0.60 (0.45 to 0.79) | 0.08 (0.06 to 0.13) | 85.9 (68.3 to 93.7) |
Mean number adult anophelines per house (pyrethrum spray catch) | |
1 Where pre‐ and post‐intervention data are reported: percent reduction is calculated by difference in differences method (see Methods); Where post‐intervention data only are reported: percent reduction is calculated as: 1 ‐ (mean density in treatment group/mean density in control group).
2 Paper states "Percentage change An. culicifacies density in treatment group before and after intervention was ‐58% (95% CI ‐ 84% to + 5%)".
Comparison 3. Habitat manipulation alone versus control
One controlled before‐and‐after study, conducted in an urban area of the Philippines, compared the construction of siphons for stream flushing in five areas of a town with no intervention in three areas (Santiago 1960 PHL). The main larval habitat of the primary vector An. minimus flavirostris was lake‐fed streams. Two years of baseline data were reported (1952‐1953), but we only included data from 1953 in the analysis. Data were presented for each of the five treatment and three control areas for total number of participants examined and total number of participants with parasitaemia or splenomegaly. We summed these data across areas and calculated a combined parasite and splenomegaly prevalence individually for treatment and control areas.
Parasite prevalence: In this study, parasite prevalence did not differ significantly at baseline between groups (one study, 847 participants, Analysis 3.1). Post‐intervention, parasite prevalence was decreased significantly in the treatment village compared to the controls (Risk ratio 0.02, 95% CI 0.00 to 0.15; one study, 846 participants, Analysis 3.1), and decreased from 5.1% to 0.1% in the treatment village.
Analysis 3.1.
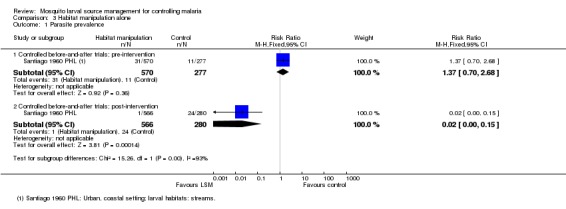
Comparison 3 Habitat manipulation alone, Outcome 1 Parasite prevalence.
Splenomegaly prevalence: At baseline, splenomegaly prevalence was lower in the treatment group than the control group (Risk ratio 0.51, 95% CI 0.31 to 0.85; one study, 832 participants, Analysis 3.2). Post‐intervention, there was a substantial reduction in splenomegaly prevalence in the treatment group compared to the control group (Risk ratio 0.02, 95% CI 0.00 to 0.17; one study, 846 participants, Analysis 3.2).
Analysis 3.2.
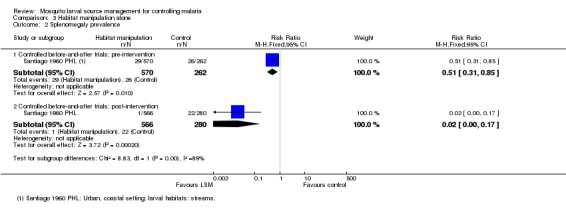
Comparison 3 Habitat manipulation alone, Outcome 2 Splenomegaly prevalence.
Adult mosquito density (measures other than human biting rate): Controlling for baseline differences, adult mosquito density decreased by 91% in the treatment group compared to the control group (Table 11). The trial authors did not report the statistical significance of this result.
Comparison 4. Habitat manipulation with larviciding versus control
Two controlled before‐and‐after trials conducted habitat manipulation with larviciding. One study, conducted in urban Tanzania (Dar es Salaam), compared clearance of vegetation and debris from drains in one site and larviciding with microbials in another site with a control site with no intervention. The primary vectors An. gambiae and An. funestus were mainly found breeding in man‐made habitats, including drains (Castro 2009 TZA). The second study, conducted in an urban, desert fringe area of India, encouraged households to eliminate domestic larval habitats alongside larviciding with pirimiphos‐methyl conducted by study staff. The main larval habitats of the primary vectors An. culicifacies and An. stephensi were containers, wells, and rainwater pools (Samnotra 1980 IND).
Malaria incidence: In one controlled before‐and‐after trial, baseline incidence did not significantly differ between treatment (64 cases per 1000 person years) and control groups (56 cases per 1000 person years) (97000 participants, one trial, Analysis 4.1). Post‐intervention, the incidence was significantly lower in the treatment group (57 cases per 1000 person years) compared to controls (240 cases per 1000 person years at risk) (Rate ratio 0.24, 95% CI 0.22 to 0.25; one study, 97,000 participants, Analysis 4.1), due to a large increase in incidence in the control areas.
Analysis 4.1.
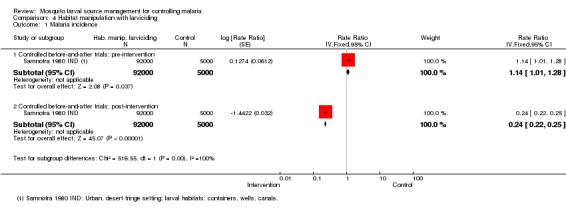
Comparison 4 Habitat manipulation with larviciding, Outcome 1 Malaria incidence.
Parasite prevalence: While both studies collected data on parasite prevalence, only Samnotra 1980 IND reported the necessary data for inclusion in Analysis 4.2. Baseline parasite prevalence did not differ significantly between treatment and control groups (1887 participants, one study, Analysis 4.2). Post‐intervention, parasite prevalence was significantly reduced in the treatment group compared to the control (Risk ratio 0.54, 95% CI 0.45 to 0.65; one study, 2713 participants, Analysis 4.2). Castro 2009 TZA did not report parasite prevalence in both treatment and control groups pre‐ and post‐intervention, and therefore we could not include this trial in the analysis. The study reported a significant reduction in the odds of malaria infection in the post‐intervention period compared to baseline in sites with habitat manipulation (drain clearance) (Odds ratio 0.23, 95% CI 0.14 to 0.38), with a greater effect observed when adjusted for age, rainfall, bed net use, and a short period of larviciding in addition to habitat manipulation (Odds ratio 0.12, 95% CI 0.05 to 0.3). The study also reported that post‐intervention, the risk of infection was significantly higher in the habitat manipulation site compared to the control (Odds ratio 1.7, 95% CI 1.1 to 2.4) when adjusted for age, rainfall, bed net use, and a short period of larviciding in addition to habitat manipulation. However, post‐intervention, parasite prevalence did not differ significantly between larviciding and control sites (Castro 2009 TZA).
Analysis 4.2.
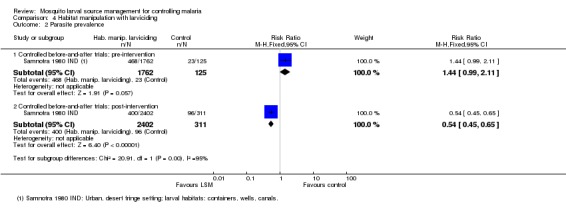
Comparison 4 Habitat manipulation with larviciding, Outcome 2 Parasite prevalence.
Adult mosquito density (measures other than human biting rate): Controlling for baseline differences, in one study adult mosquito density in the treatment group fell by 90% compared to the control group (Samnotra 1980 IND, Table 11). The trial authors did not report the statistical significance of this result.
Comparison 5. Larviciding alone versus control
Three cluster‐RCTs, one randomized cross‐over study, and three controlled before‐and‐after studies evaluated larviciding alone.
Two cluster‐RCTs were conducted in rural Sri Lanka, where larvicide (pyriproxyfen) was applied to larval habitats two to three times over a one year period. The main larval habitats of the primary vectors An. culicifacies and An. subpictus were abandoned gem mine pits (Yapabandara 2001 LKA) and river bed pools, streams, irrigation ditches, and rice paddies (Yapabandara 2004 LKA). The third RCT was conducted in Mali and reported entomological data only (Coulibaly 2011 MLI). Larvicide (Bti and Bs) was applied to larval habitats every one to two weeks for 18 months. The main larval habitats of the primary vector An. gambiae were brick pits, ponds, and tyre prints.
The controlled before‐and‐after studies were conducted in urban Tanzania (Fillinger 2008 TZA; Geissbühler 2009 TZA), and rural Kenya (Fillinger 2009 KEN). In Tanzania, Bti was applied weekly to open, sunlit habitats and Bs was applied every three months to closed habitats. The main larval habitats of the primary malaria vectors An. gambiae and An. funestus included man‐made habitats associated with agriculture (rice paddies, sweet potato ridges, irrigation channels, and garden wells), construction and city drains, and natural pools and swamps associated with streams and high ground water level. In Kenya, a controlled before‐and‐after study compared weekly larviciding with Bti and Bs together with LLINs, with LLINs alone. The main larval habitats of the primary vectors An. gambiae and An. funestus were man‐made drains, borrow pits, and swampy areas with low vegetation close to natural streams.
A randomized cross‐over study was conducted in The Gambia, where larviciding with Bti and Bs was carried out weekly. The main larval habitats of the primary vector An. gambiae were extensive, largely inaccessible flood plains and rice paddies (Majambere 2010 GMB).
Fillinger 2009 KEN reported baseline data for two long rainy seasons (April to June 2004; April to June 2005) and one short rainy season (November 2004 to January 2005). The trial authors reported post‐intervention data for one long rainy season (April to June 2006) and two short rainy seasons (November 2005 to January 2006; November 2006 to January 2007). To allow comparability, we included data for one long and one short rainy season in the analysis for baseline and post‐intervention periods. We included April to June 2005 and November 2004 to January 2005 in the baseline and April to June 2006 and November 2006 to January 2007 in the post‐intervention data.
Malaria incidence: In the two cluster‐RCTs from Sri Lanka, malaria incidence was comparable at baseline between the two groups (19981 participants, two studies, Analysis 5.1), and significantly reduced in the intervention group post‐intervention (Rate ratio 0.26, 95% CI 0.22 to 0.31; 20124 participants, two studies, Analysis 5.1). The authors of these studies did not adjust the results for the effects of clustering, so we conducted a sensitivity analysis to assess the robustness of this result. The reduction in malaria incidence remained statistically significant even with a conservative ICC statistic of 0.1 (Rate ratio 0.25, 95% CI 0.06 to 0.98, Analysis 5.2).
Analysis 5.1.
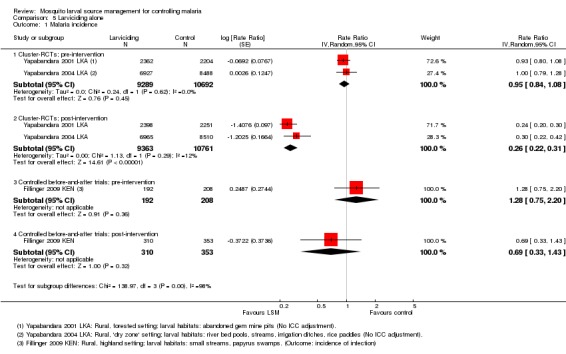
Comparison 5 Larviciding alone, Outcome 1 Malaria incidence.
Analysis 5.2.
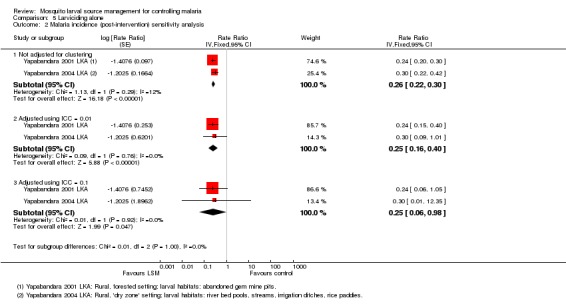
Comparison 5 Larviciding alone, Outcome 2 Malaria incidence (post‐intervention) sensitivity analysis.
In the before‐and‐after study from Kenya, the incidence of new parasitaemia was higher in the treatment group at baseline. However the difference was not significant (400 participants, one study, Analysis 5.1). Post‐intervention, the incidence of new infections decreased in the treatment group compared to the control, but the difference was not statistically significant (Risk ratio 0.69, 95% CI 0.33 to 1.43, 663 participants, one study, Analysis 5.1).
Due to its cross‐over design, we could not include the randomized cross‐over study in the meta‐analysis (Majambere 2010 GMB), and have presented the data separately (Table 12). Each of the four zones acted its own control. When we compared the intervention period with the non‐intervention period for each zone, the effect of larviciding was inconsistent. Indeed, incidence appeared to decrease in all four zones between the first and second years of the study, regardless of the intervention. We found that this finding was consistent with the entomological data, which indicated that adult mosquito density and EIR decreased slightly across all zones between the two years (Table 13).
Table 5.
Summary of additional results for Majambere 2010 GMB (clinical data)
| Outcome | Zone | Incidence or prevalence |
Rate or Risk Ratio |
|||
|
Control year (2006) |
Treatment year (2007) |
Treatment year (2006) |
Control year (2007) |
|||
| Malaria incidence1 | 1 | ‐ | ‐ | 70.9 (58.8 to 85.6) |
7.2 (4.3 to 11.9) |
9.85 (4.58 to 21.19) |
| 2 | 30.3 (23.1 to 39.7) |
17.0 (12.4 to 23.5) |
‐ | ‐ | 0.56 (0.31 to 1.02) |
|
| 3 | ‐ | ‐ | 44.1 (35.2 to 55.2) |
27.2 (20.9 to 35.4) |
1.62 (1.01 to 2.61) |
|
| 4 | 29.1 (22.1 to 38.4) |
24.7 (18.8 to 32.3) |
‐ | ‐ | 0.85 (0.50 to 1.45) |
|
| Parasite prevalence2 | 1 | ‐ | ‐ | 41.0% (163/398) |
20.7% (95/458) |
1.97 (1.59 to 2.45) |
| 2 | 12.2% (54/443) |
8.2% (39/474) |
‐ | ‐ | 0.67 (0.46 to 1.00) |
|
| 3 | ‐ | ‐ | 12.8% (57/447) |
10.4% (47/452) |
1.23 (0.85 to 1.76) |
|
| 4 | 10.5% (45/430) |
22.3% (105/472) |
‐ | ‐ | 2.13 (1.54 to 2.94) |
|
| Splenomegaly prevalence3 | 1 | ‐ | ‐ | 12.0% (47/393) |
7.7% (35/456) |
1.56 (1.03 to 2.36) |
| 2 | 5.9% (26/442) |
6.2% (12/471) |
‐ | ‐ | 0.43 (0.22 to 0.85) |
|
| 3 | ‐ | ‐ | 6.5% (29/447) |
2.6% (12/455) |
2.46 (1.27 to 4.76) |
|
| 4 | 5.8% (25/434) |
3.8% (18/471) |
‐ | ‐ | 0.66 (0.37 to 1.20) |
|
1 Total cases (95% CI) per 100 person years at risk; rate ratio.
2 Parasite prevalence (total positive / total examined); risk ratio.
3 Splenomegaly prevalence (total positive / total examined); risk ratio.
Table 6.
Summary of additional results for Majambere 2010 GMB (entomological data)
| Outcome | Zone | Density or rate |
Percent reduction across all zones (95% CI) 3 |
||||
|
Pre‐intervention year (2005) |
Post‐intervention | ||||||
| Control year (2006) | Treatment year (2007) | Treatment year (2006) | Control year (2007) | ||||
| Adult mosquito density (measures other than human biting rate) 1 | 1 | 3 (0 to 7) | ‐ | ‐ | 1 (0 to 3) | 2 (0 to 5) | 11.3 (‐217.6 to 75.2) |
| 2 | 19 (4 to 44) | 13 (6 to 26) | 13 (4 to 26) | ‐ | ‐ | ||
| 3 | 24 (6 to 78) | ‐ | ‐ | 12 (4 to 31) | 34 (10 to 69) | ||
| 4 | 11 (3 to 26) | 3 (1 to 11) | 9 (2 to 26) | ‐ | ‐ | ||
| EIR 2 | 1 | 8.80 | ‐ | ‐ | 0.00 | 2.24 | 17.6 (‐376.1 to 85.7) |
| 2 | 8.29 | 0.00 | 2.32 | ‐ | ‐ | ||
| 3 | 16.55 | ‐ | ‐ | 5.82 | 17.00 | ||
| 4 | 6.13 | 3.13 | 3.91 | ‐ | ‐ | ||
1 Median female An. gambiae / trap / night (interquartile range).
2 Seasonal EIR.
3 Overall percent reduction calculated using difference in differences method (see Data synthesis).
Parasite prevalence: In the cluster‐RCT (Yapabandara 2001 LKA), baseline prevalence did not significantly differ between treatment and control groups (3351 participants, one study, Analysis 5.3), and prevalence decreased significantly in the treatment group post‐intervention (Risk ratio 0.11, 95% CI 0.05 to 0.22, 2963 participants, one study, Analysis 5.3). In the sensitivity analysis, this reduction in parasite prevalence remained statistically significant with an ICC statistic of 0.01 (Rate ratio 0.13, 95% CI 0.03 to 0.56, Analysis 5.4), but became non‐significant with the conservative ICC statistic of 0.1 (Analysis 5.4).
Analysis 5.3.
Comparison 5 Larviciding alone, Outcome 3 Parasite prevalence.
Analysis 5.4.
Comparison 5 Larviciding alone, Outcome 4 Parasite prevalence (post‐intervention) sensitivity analysis.
In the cross‐over trial (which we excluded from the meta‐analysis because of the cross‐over design), we did not identify a consistent effect of larviciding on parasite prevalence across the four zones (Majambere 2010 GMB; Table 12). In the controlled before‐and‐after study, baseline prevalence was higher in the treatment group than the control group (Risk ratio 1.29, 95% CI 1.04 to 1.59, 2439 participants, one study, Analysis 5.3) and was significantly lower in the treatment group than the control group post‐intervention (Risk ratio 0.60, 95% CI 0.42 to 0.87, 2374 participants, one study, Analysis 5.3).
Splenomegaly prevalence: In the cross‐over trial, as with incidence and parasite prevalence, we did not identify a consistent effect of larviciding on splenomegaly prevalence across the four zones (Majambere 2010 GMB; Table 12).
EIR: In one cluster‐RCT and three controlled before‐and‐after studies, the percent reduction in EIR ranged from 21% to 73% (Table 14). However, due to unreported data, we could neither calculate CIs nor take into account baseline density for all studies. We did not identify any reduction in EIR in the randomized cross‐over study (Majambere 2010 GMB; Table 13).
Table 7.
Entomological data: EIR
| Intervention | Study ID | Study design | EIR (95% CI) |
Percent reduction (95% CI)1 |
Notes | |||
| Pre‐intervention | Post‐intervention | |||||||
| Control | Treatment | Control | Treatment | |||||
| Larviciding alone | Coulibaly 2011 MLI(2009 data) | Cluster‐RCT | ‐ | ‐ | 0.00 | 0.18 | Not estimable | Monthly EIR |
| Coulibaly 2011 MLI(2010 data) | Cluster‐RCT | ‐ | ‐ | 2.92 | 0.45 | 84.6 | Monthly EIR | |
| Fillinger 2008 TZA | Controlled before‐and‐after trial | 1.05 (0.68 to 1.65) |
0.81 (0.58 to 1.15) |
1.06 (0.64 to 1.77) |
0.56 (0.43 to 0.77) |
31.5 (‐59.4 to 70.6) |
Annual EIR (An. gambiae) | |
| Fillinger 2009 KEN | Controlled before‐and‐after trial | 11.98 (7.39 to 19.40) |
10.30 (7.20 to 14.95) |
1.68 (1.16 to 2.42) |
0.39 (0.19 to 0.79) |
73.0 (22.0 to 90.7) |
Annual EIR | |
| Geissbühler 2009 TZA | Controlled before‐and‐after trial | 1.44 (1.14 to 1.81) |
1.18 (0.80 to 1.73) |
1.24 (0.97 to 1.57) |
0.80 (0.60 to 1.06) |
21.3 (‐42.3 to 56.4) | Annual EIR | |
1Where pre‐ and post‐intervention data are reported, percent reduction was calculated by difference in differences method (see Methods). Where post‐intervention data only were reported, percent reduction was calculated as: 1 ‐ (mean density in treatment group/mean density in control group).
Adult mosquito density (human biting rate): The percent reduction in density ranged from 31% and 73% in one cluster‐RCT (Coulibaly 2011 MLI) and two controlled before‐and‐after studies (Fillinger 2008 TZA; Fillinger 2009 KEN; Table 15). However, we could not calculate CIs or take into account baseline density for all of these studies.
Table 8.
Entomological data: Adult mosquito density (human biting rate)
| Intervention | Study ID | Study design | Human biting rate (95% CI) |
Percent reduction (95% CI)1 |
Notes | |||
| Pre‐intervention | Post‐intervention | |||||||
| Control | Treatment | Control | Treatment | |||||
| Larviciding alone | Coulibaly 2011 MLI(2009 data) | Cluster‐RCT | ‐ | ‐ | 16.40 | 8.37 | 49.0 | Mean number of bites per person per month |
| Coulibaly 2011 MLI (2010 data) | Cluster‐RCT | ‐ | ‐ | 41.40 | 22.43 | 45.8 | Mean number of bites per person per month | |
| Fillinger 2008 TZA | Controlled before‐and‐after trial | 0.93 (0.60 to1.46) | 0.72 (0.51 to 1.02) | 0.94 (0.57 to 1.56) | 0.50 (0.38 to 0.68) |
31.3 (‐59.2 to 70.4) |
Mean number of bites per person per year (An. gambiae) | |
| Fillinger 2009 KEN | Controlled before‐and‐after trial | 0.45 (0.28 to 0.73) | 0.39 (0.27 to 0.56) | 0.06 (0.04 to 0.09) | 0.014 (0.006 to 0.028) | 73.1 (20.3 to 90.9) |
Mean number of blood fed female anophelines per person per sampling date | |
1 Where pre‐ and post‐intervention data were reported, percent reduction was calculated by difference in differences method (see Methods). Where post‐intervention data only were reported, percent reduction was calculated as: 1 ‐ (mean density in treatment group/mean density in control group).
Adult mosquito density (density measures other than human biting rate): The percent reduction in density ranged from 34% to 91% in three cluster‐RCTs (Yapabandara 2001 LKA; Yapabandara 2004 LKA; Coulibaly 2011 MLI) and one controlled before‐and after trial (Fillinger 2009 KEN; Table 11). However, we could not calculate CIs for these studies and we could only account for differences at baseline in some studies. In one study there was no reduction in adult mosquito density in the treatment group compared to the control group (Majambere 2010 GMB; Table 13).
Comparison 6. Any LSM versus control
Malaria incidence: In two cluster‐RCTs, LSM reduced malaria incidence by 74% in the treatment group compared to the control (Rate ratio 0.26, 95% CI 0.22 to 0.31; 20124 participants, two trials, Analysis 6.1, Figure 4). The interventions and settings of these two trials were similar therefore there was little heterogeneity between trials (I2 = 12%).
Analysis 6.1.
Comparison 6 Larval source management versus control, Outcome 1 Malaria incidence.
Figure 4.
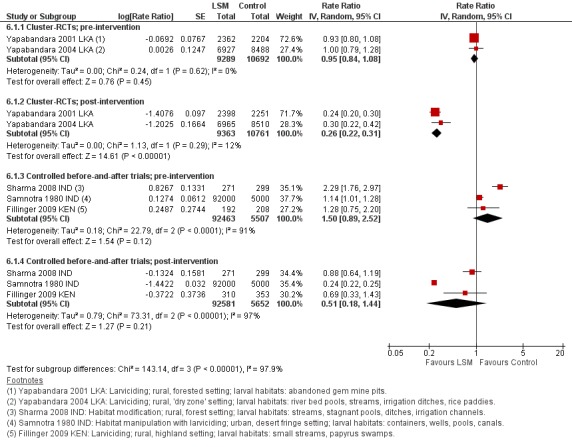
Forest plot of comparison: 6 LSM versus control, outcome: 6.1 Malaria incidence.
In three controlled before‐and‐after trials, malaria incidence was not consistently reduced (98233 participants, three trials, Analysis 6.1), with variation in results (I2 = 97%) possibly arising from significantly higher baseline incidence in the intervention areas compared to the controls in two trials. In both of these trials, LSM reduced malaria incidence in the intervention arm to levels similar to the control arm. As there were too few studies, we could not adequately investigate other potential causes of this heterogeneity. In one randomized cross‐over trial, which we could not present in this analysis, incidence was not significantly reduced (Table 12).
Parasite prevalence: In one cluster‐RCT, LSM reduced parasite prevalence by 89% in the intervention group compared to the control (Risk ratio 0.11, 95% CI 0.05 to 0.22; 2963 participants, one trial, Analysis 6.2, Figure 5). In five controlled before‐and‐after trials, parasite prevalence was reduced by around two‐thirds in the treatment groups compared to the controls (Risk ratio 0.32, 95% CI 0.19 to 0.55; 8041 participants, five trials, Analysis 6.2). In one randomized cross‐over trial, parasite prevalence was not significantly reduced (Table 12). Statistical heterogeneity between these trials was high (I2 = 89%), however this related to the magnitude rather than the direction of the effect. We could not investigate the potential causes of this heterogeneity as there were too few studies. In the single randomized cross‐over trial, parasite prevalence was not significantly reduced (Table 12).
Analysis 6.2.
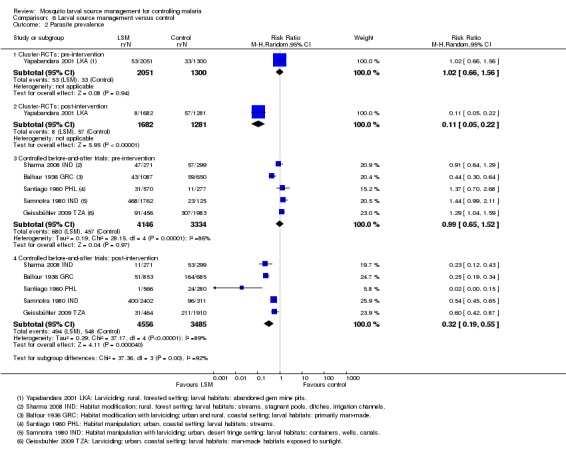
Comparison 6 Larval source management versus control, Outcome 2 Parasite prevalence.
Figure 5.
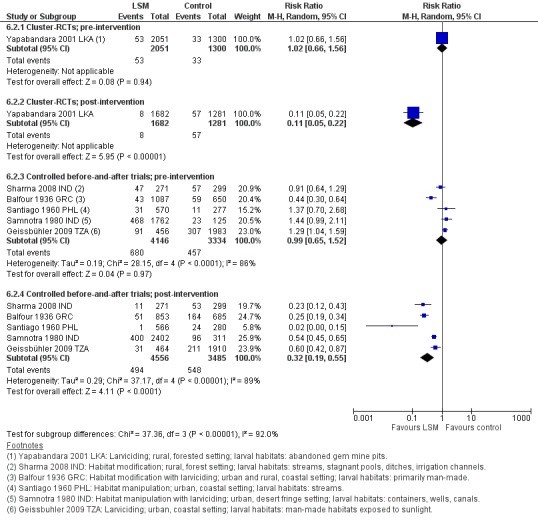
Forest plot of comparison: 6. LSM versus control, outcome: 6.2 Parasite prevalence.
Splenomegaly prevalence: In two controlled before‐and‐after trials, cluster‐RCTs, splenomegaly prevalence was 43% lower in the treatment group compared to the control (Risk ratio 0.57, 95% CI 0.50 to 0.65; 2569 participants, two trials, Analysis 6.3). In two controlled before‐and‐after trials, splenomegaly prevalence was not significantly reduced (2384 participants, two trials, Analysis 6.3). In one randomized cross‐over trial, splenomegaly prevalence was not significantly reduced (Table 12).
Analysis 6.3.
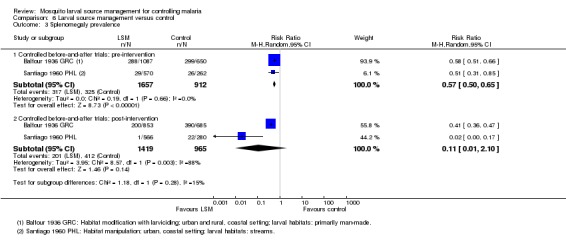
Comparison 6 Larval source management versus control, Outcome 3 Splenomegaly prevalence.
EIR: In four studies the percent reduction in EIR ranged from 21% to 84.6% (Table 14). However, we could not calculate CIs or take into account baseline density for one of these studies due to unreported data. In one study EIR increased in the control group from 0.00 to 2.92 in the second year of the intervention (Coulibaly 2011 MLI). In one study there was no reduction in EIR (Majambere 2010 GMB; Table 13).
Adult mosquito density (human biting rate): The percent reduction in density ranged from 31% and 73% in three studies (Table 15). We were not able to calculate CIs or take into account baseline density in two studies due to unreported data.
Adult mosquito density (measures other than human biting rate): The percent reduction in density ranged from 15% to 91% in seven studies (Table 11). However, we could not calculate CIs or take into account baseline density for all studies due to unreported data. In one study there was no reduction in adult mosquito density (Majambere 2010 GMB; Table 13).
We did not identify any trials that reported total under five year old mortality, time to infection, or prevalence of anaemia in children.
Discussion
Summary of main results
We included four cluster‐RCTs, eight controlled before‐and‐after trials, and one randomized cross‐over trial in this review.
Malaria incidence
In two cluster‐RCTs in Sri Lanka, larviciding of abandoned mines, streams, irrigation ditches, and rice paddies reduced malaria incidence by around three‐quarters compared to controls (moderate quality evidence). In three controlled before‐and‐after trials in urban and rural India and rural Kenya, results were inconsistent (very low quality evidence). In one trial in urban India, the removal of domestic water containers together with weekly larviciding of canals and stagnant pools reduced malaria incidence by three quarters. In one trial in rural India and one trial in rural Kenya, malaria incidence was higher at baseline in intervention areas than in controls. However dam construction in India, and larviciding of streams and swamps in Kenya, reduced malaria incidence to levels similar to the control areas. In one additional randomized cross‐over trial in the flood plains of the Gambia River, where larval habitats were extensive and ill‐defined, larviciding by ground teams did not result in a statistically significant reduction in malaria incidence .
Parasite prevalence
In one cluster‐RCT in Sri Lanka, larviciding reduced parasite prevalence by almost 90% (moderate quality evidence). In five controlled before‐and‐after trials in Greece, India, the Philippines, and Tanzania, LSM resulted in an average reduction in parasite prevalence of around two‐thirds (moderate quality evidence). The interventions in these five trials included dam construction to reduce larval habitats, flushing of streams, removal of domestic water containers, and larviciding. In the randomized cross‐over trial in the flood plains of the Gambia River, larviciding by ground teams did not significantly reduce parasite prevalence.
Overall completeness and applicability of evidence
Effectiveness of LSM
Despite numerous historical reports on LSM programmes and examples of its effectiveness, such as the eradication of An. gambiae in Brazil and Wadi Haifa, Egypt (Soper 1943; Najera 2001), few trials have been conducted to rigorously evaluate the intervention and of these, very few are randomized studies. Our review therefore included non‐randomized studies with adequate controls and baseline data. There is a lack of negative results among the non‐randomized trials and it is possible that we were unable to access studies with negative results due to publication bias. Trials were likely to have been conducted in environments in which experienced entomologists considered success likely. Thus the eligible studies may not reflect the likely impact of LSM in every habitat, but those in which it was deemed appropriate. Due to the small number of eligible studies, we were unable to construct funnel plots and assess the risk of publication bias or other sources of bias, such as poor study quality leading to artificially inflated effects in the smaller studies, selective outcome or analysis reporting, or chance. Also, we were unable to retrieve 19 full‐text articles which may have introduced some bias.
However the included trials demonstrate that in carefully selected circumstances in various Asian and African settings, LSM can contribute to a reduction in incidence of clinical malaria, parasite prevalence, and splenomegaly prevalence. Our analysis was stratified by intervention type, and although each group contained only a small number of studies, the effect of LSM was relatively consistent suggesting that LSM can be effective when tailored appropriately to local ecology and infrastructure.
Feasibility of LSM
It is probable that LSM could be effective in most settings where adequate coverage of larval habitats can be achieved. What will change across settings therefore, is the feasibility and cost of achieving adequate coverage, which will depend on the number, type and ease of access of larval habitats, and the resources available. The included studies demonstrated large effects in Asia where larval habitats were relatively discrete and often man‐made (for example, drainage ditches, pits, water storage containers, old mine pits, and irrigation channels), and also where larval habitats were more extensive, including rice paddies. All three included trials of LSM in urban Africa were conducted in Dar es Salaam, Tanzania, and demonstrated the protective effect of larviciding (and habitat manipulation) in this setting. In rural Africa, a significant reduction in clinical and entomological outcomes was observed in rural, highland Kenya, where larval habitats were confined to valley floors. In rural, lowland, savannah in Mali, a reduction in EIR, human biting rates, and other measures of adult mosquito density was observed. However, it is not known if the reductions were statistically significant or if human clinical outcomes were assessed. In rural, highland and lowland, desert fringe areas of rural Eritrea, a reduction in adult mosquito density was observed. All of these studies demonstrate the potential impact of LSM in urban and rural Africa where habitats might be numerous but are relatively discrete and accessible.
In the flood plains of the Gambia River, where larval habitats were very numerous and ill‐defined, hand and knapsack sprayer application of microbials by a ground team of 64 men was not associated with a reduction in malaria incidence, parasite prevalence, or splenomegaly. Clinical outcomes decreased in all zones over the two years of the study regardless of the intervention; an observation consistent with the entomological data. This study was conducted in an area where larval habitats in marshland stretched for several kilometres along the river, often two kilometres wide (Bogh 2003; Majambere 2008), making it difficult to cover the entire area with larvicides. Moreover, in this part of the country mosquitoes can fly long distances, often over two kilometres (Bogh 2007), making it likely that mosquitoes from non‐intervention areas entered the study zones treated with larvicide. This area is not typical of rural sub‐Saharan Africa where larval habitats are typically less extensive. We conclude that ground application of larvicide to areas of extensive flooding, such as the flood plains of major rivers or large‐scale rice irrigation projects, is not effective at reducing malaria transmission. Programmes including aerial spraying or large environmental management associated with the river and its flood plains may be able to address this limitation and could be evaluated.
The logistical feasibility of LSM is also affected by the type of intervention planned. In this review, we assessed larviciding, habitat manipulation, and habitat modification. While in practice these interventions may often be combined, each type of LSM is appropriate for different environmental conditions and has very different requirements. The majority of included trials carried out larviciding, which requires regular treatment of the majority of habitats within a target area. It is therefore labour intensive and needs a rigorous management system for application, surveillance, and evaluation. The type and quality of the larvicide product used is also an important consideration. Habitat manipulation may require regular maintenance but it would rarely require its own programme and management system. It may be integrated into ongoing activities, for example those of the ministries of public works or agriculture. Habitat modification is a more permanent approach and may be a one‐time expense suited to specific settings, potentially those ill‐suited to larviciding.
LSM should not be misconstrued as an intervention that can be set up and managed by the local community alone. Similar to IRS, it is an intervention that requires an intensive and carefully co‐ordinated effort and the effort required to conduct LSM in the included studies was great. It is salient to note that LSM was not conducted by the local community alone in any of the included studies. Moreover, where members of the community were involved, they were actively trained, employed, and managed by study staff or the government. In general, the relative contributions of the community and ‘professionals’ were not well quantified. These measures of ‘coverage’ need to be taken into account and quantified in future studies.
Quality of the evidence
We appraised the quality of the evidence using the GRADE approach.
The two cluster‐RCTs that reported clinical outcomes provide moderate quality evidence that larviciding, when applied appropriately, can have a large impact on the incidence and prevalence of malaria (Table 1). We downgraded this evidence from high because we had risk of bias concerns. Although they are described as randomized, neither study adequately described how intervention and control areas were selected. Since both studies were conducted in Sri Lanka, we considered downgrading the evidence further under 'directness' as the result could be considered poorly applicable to other settings. However, the evidence from the non‐randomized trials from a wider variety of countries and eco‐epidemiological settings indicates that where adult mosquito numbers are reduced, LSM will probably have important effects on malaria incidence and prevalence. The randomized trials did not adjust for the effects of clustering, therefore the 95% CIs presented are likely to be misleadingly narrow. However, our sensitivity analysis suggest that the results will probably remain statistically significant once clustering is taken into account and so we did not downgrade the evidence further. Moderate quality evidence implies that we can have reasonable confidence in these estimates of effect.
Potential biases in the review process
In most of the included trials, LSM demonstrated a major positive impact. LSM, chemoprophylaxis, and disease surveillance, were staples of many malaria control programmes between 1910 and 1940, prior to the DDT IRS era. LSM was reintroduced into some malaria control programmes with the advent of insecticide resistance. Many of the articles we reviewed were programme reports from the first half of the twentieth century when controlled trials were rare. Thus, we were not able to contact many of the authors. Our requests for unpublished studies were largely unfruitful, but it is possible that there exists a body of unpublished negative evidence with LSM. Some historical programme reports suggested that LSM was not particularly effective in some areas, especially in comparison to IRS with DDT (Mandekos 1948), but we did not include these trials as they did not meet the inclusion criteria. However, we were not able to locate many negative LSM studies and this is likely to be a significant source of bias in the review.
Agreements and disagreements with other studies or reviews
Peer‐reviewed literature
This is the first Cochrane review of LSM. In general, our findings concur with the conclusions of other major LSM reviews.
Takken 1990 described the notable success of LSM for malaria control in Indonesia before the advent of DDT and its relevance for malaria control today, especially in the light of insecticide and drugs resistance. Lindsay 2004 highlighted the potential role of LSM in integrated vector management in the East Asia and Pacific region. Both narrative reviews concur with the findings of our review because we found that LSM was effective at reducing malaria transmission in various Asian settings: urban India (Samnotra 1980 IND), urban Philippines (Santiago 1960 PHL), rural, forested and irrigated India (Sharma 2008 IND), and rural Sri Lanka (Yapabandara 2001 LKA; Yapabandara 2004 LKA).
Keiser 2005 conducted the first systematic review of the effect of environmental management on malaria and included studies where the intervention was predominantly or exclusively environmental management and the outcome was incidence of clinical malaria, parasite prevalence, splenomegaly prevalence, or mortality rates. The authors excluded studies with entomological outcomes only or studies assessing the effect of LLINs. Overall, they included 40 studies, of which 85% (n = 34) were conducted before the era of the Global Malaria Eradication Campaign (1955 to 1969). They conducted a meta‐analysis of sixteen trials of habitat manipulation and modification, with a reduction in risk of 88.0% (95% CI 81.7% to 92.1%) (of which the clinical malaria outcome being assessed was unclear). Our review was more systematic in its inclusion criteria and search strategy and we therefore included different studies.
Based on the premise that the environment mediates the effect of LSM, Keiser 2005 assigned studies to four eco‐epidemiological settings: (1) malaria of deep forests, forest fringe, and hills; (2) rural malaria attributable to irrigation and large dams; (3) rural malaria attributable to wetlands, rivers, streams, coasts, and non‐agricultural man‐made water habitats; and (4) urban and peri‐urban malaria. The review concluded that"malaria control programmes that emphasise environmental management are highly effective in reducing morbidity and mortality". The authors did not conduct any subgroup analyses to assess whether the effect differed across the four defined settings. We judge the quality of the data in the Keiser 2005 review to be poor, due in part to the inclusion of uncontrolled before‐and‐after studies. Our review concurs generally with the conclusions of Keiser 2005 but presents stronger evidence.
Walker 2007 highlighted that malaria control programmes in Africa have focused on targeting adult vectors and that renewed interest in LSM has been stimulated by concerns over insecticide resistance, rising costs of IRS, environmental impacts of interventions, and the move towards IVM. This review suggested that the use of LSM has been discouraged in sub‐Saharan Africa due to the paucity of information on larval ecology and the ability of the major vector An. gambiae to breed in a variety of habitats. The authors reviewed large‐scale field trials of LSM conducted in Africa between 1992 and 2007, which were described as limited in number. The review concluded that in particular settings where larval habitats are man‐made or limited in number, such as in urban areas, LSM can be an effective intervention against malaria. In some rural settings, LSM might supplement LLINs or IRS, particularly during the dry season. LSM has minimal risk of environmental contamination or exposure of humans to pesticides. Our findings support the conclusions of Walker 2007. We similarly provide evidence that LSM is effective in select settings in sub‐Saharan Africa, both rural and urban, where larval habitats are discrete and accessible.
Fillinger and Lindsay 2011 proposed that LSM will work best and be most cost‐effective in areas where larval habitats are either seasonal, relatively few, where well‐defined habitats are accessible by ground crews, or in cooler parts of the tropics where larval development is prolonged. The review authors suggest that these conditions occur frequently, and thus this method can be an effective tool for malaria control in selected eco‐epidemiological conditions, such as areas of low to medium transmission intensity, areas of focal transmission, or epidemic prone areas. Such conditions are common in urban environments, desert fringe communities, highland settlements, and rural areas with high population densities. The review states that LSM is not a strategy for country wide application and should not be the primary tool selected in areas of intensive transmission. Nevertheless, LSM has the potential to be integrated into control programmes after LLINs or IRS have reduced transmission to moderate or low levels of transmission. Therefore LSM should be considered in the consolidation phase of control and elimination programmes where it can be targeted in space and time. LSM may also be required for managing insecticide resistance and when outdoor transmission contributes substantially to overall transmission. Our review supports the finding that LSM can be effective in highland, urban, and desert fringe areas of Africa, and that ground application of larvicides may not be appropriate in areas with extensive flooding (such as the flood plains and paddy fields along the Gambia River).
Worrall and Fillinger 2011 recently concluded that the costs per person protected by LSM compares favourably with IRS and LLINs, especially in areas with moderate and focal malaria transmission where mosquito larval habitats are accessible and well defined. However, more data on the epidemiological impact of LSM is required to gauge the cost effectiveness of LSM. In such settings, it may be pragmatic to integrate LSM into existing control programmes. In our review we did not assess the cost‐effectiveness or the overall cost of LSM.
WHO recommendations
In 2006, WHO made recommendations on the role of LSM based on its suitability in different eco‐epidemiological settings (WHO 2006b). More recently, WHO recommendations specifically for larviciding state that "further evidence is needed of the value of larviciding as a routine and large‐scale operation in both urban and rural areas" (WHO 2012). While this review concurs with aspects of the WHO position statement, in particular that more evidence is needed before definitive recommendations can be made regarding the appropriate use of LSM, there are several differences. The WHO position statement makes a comparison between the ratio of larval habitats to people in urban areas (low) and rural areas (high). We caution against such an urban‐rural distinction since in some rural areas in Africa and elsewhere larval habitats may be equally limited in number, easily mapped, and accessed. While WHO does not generally recommend larviciding in rural sub‐Saharan Africa unless particular circumstances limit larval habitats, this review provides evidence that larviciding in rural Africa may reduce malaria transmission, for example in rural Mali (Coulibaly 2011 MLI), rural Eritrea (Shililu 2007 ERI), and rural Kenya (Fillinger 2009 KEN). WHO recommends that "larviciding should be considered for malaria control (with or without other interventions) only in areas where the larval habitats are few, fixed and findable" (WHO 2012). While the extent to which larval habitats are 'findable' may be important, this review found that larviciding may be effective where larval habitats are not necessarily few or fixed (Shililu 2007 ERI; Fillinger 2008 TZA; Castro 2009 TZA; Fillinger 2009 KEN; Geissbühler 2009 TZA; Coulibaly 2011 MLI).
Authors' conclusions
In Africa and Asia, LSM (when conducted in the manner and with the level of effort as in these trials) could be considered as another policy option alongside LLINs or IRS, or both, for reducing malaria morbidity in both urban and rural areas where a sufficient proportion of larval habitats can be targeted. Further large‐scale studies are required to assess LSM effectiveness in rural areas of Africa where larval habitats are extensive. If applied in appropriate locations with the required management and funding, LSM is likely to reduce malaria morbidity. Given the paucity of data regarding efficacy in many settings, LSM should be implemented with rigorous on‐going surveillance of both entomological indicators and of human disease indicators to determine whether it is having the desired impact. This would also improve understanding of the potential benefit of LSM in addition to other vector control interventions, such as LLINs or IRS, or both.
Further cluster‐RCTs of LSM in rural areas of Africa where larval habitats are extensive, although difficult and expensive to conduct, will improve the quality of the evidence. Research into the role of LSM (both larviciding and habitat modification and manipulation) in supplementing control measures that target adult vectors, in controlling malaria where insecticide resistance and outdoor vector biting are problematic, in targeting hotspots of transmission, and in malaria elimination programmes will be informative. Funding is needed to support this important research.
Acknowledgements
The academic editor for this review was Dr Patricia Graves. The editorial base for the Cochrane Infectious Diseases Group is funded by UKaid from the UK government for the benefit of developing countries. Steve Lindsay received partial funding for this work from the Research and Policy for Infectious Disease Dynamics (RAPIDD) Program of the Science and Technology Directory, Department of Homeland Security, and Fogarty International Center, National Institutes of Health. Ulrike Fillinger is supported through a National Institute of Health (NIH) Grant (grant number R01 AI082537). Lucy Tusting received a Fellowship from the Cochrane Infectious Diseases Group.
Achuyt Bhattarai, Andre Machado De Siqueira, Andrea Thoumi, Carlotta Modestini, Claudia Vera Garcia, Elizabeth Tissinge, Francesca Solmi, Gabriel Ponce de Leon, Junko Kiriya, Liam Crosby, Lucy Haurisa, Mariana De Niz Hidalgo, Marta Buysana, Marta Maia, Maryna Braga, Sara Carrillo de Albornoz, Yulia Iossifova, and Tapan Bhattacharyya assisted with the translation of foreign language papers. The London School of Hygiene and Tropical Medicine Library and Archives and the Centers for Disease Control and Prevention Library Services helped with article retrieval. Paul Garner, Sarah Donegan and Anne‐Marie Stephani (UK Cochrane Infectious Diseases Group) provided guidance on data abstraction, analysis and write‐up and comments on the manuscript. Tomas Allen, Carole Modis and Marie Sarah Villemin Partow (WHO Library and Archives, Geneva) and Christianne Esparza (UK Cochrane Infectious Diseases Group) assisted with literature retrieval. The Roll Back Malaria Larval Source Management Work Stream provided helpful input.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Appendices
Appendix 1. Methods of the review: detailed search strategies
| Search set | CIDG SR1 | CENTRAL | MEDLINE | EMBASE | LILACS | CABS Abstracts |
| 1 | Mosquito* | Malaria [Mesh] | Malaria [Mesh] | Malaria [Emtree] | Mosquito* | Mosquito* |
| 2 | Anopheles | Anopheles {Mesh] | Anopheles ti, ab, Mesh | Anopheles ti, ab, Emtree | Anopheles | Anopheles |
| 3 | 1 or 2 | Mosquito* ti, ab | Mosquito* ti, ab | Mosquito* ti, ab | 1 or 2 | 1 or 2 |
| 4 | malaria | 2 or 3 | 2 or 3 | 2 or 3 | malaria | malaria |
| 5 | 3 and 4 | 1 and 4 | 1 and 4 | 1 and 4 | 3 and 4 | 3 and 4 |
| 6 | control | Mosquito control [Mesh] | Mosquito control [Mesh] | Mosquito control ti, ab | control | control |
| 7 | Larvicid* | Larvicid* ti, ab | Larvicid* ti, ab | Larvicid* ti, ab | Larvicid* | Larvicid* |
| 8 | Manag* | Larval control ti, ab | Larval control ti, ab | Larval control ti, ab | Manag* | Manag* |
| 9 | 6 or 7 or 8 | 6 or 7 or 8 | Bacillus thuringiensis ti, ab | Bacillus thuringiensis ti, ab | 6 or 7 or 8 | Bacillus thuringiensis |
| 10 | 5 and 9 | 5 and 9 | Bacillus sphericus ti, ab | Bacillus sphericus ti, ab | 5 and 9 | Bacillus sphericus |
| 11 | Paris green ti, ab, sn | Paris green ti, ab | Paris green | |||
| 12 | Temefos ti, ab, sn | Temefos ti, ab | Temefos | |||
| 13 | Pyriproxyfen ti, ab | Pyriproxyfen ti, ab | Pyriproxyfen | |||
| 14 | pirimiphos‐methyl ti, ab | pirimiphos‐methyl ti, ab | pirimiphos‐methyl | |||
| 15 | Juvenile hormones [mesh] | Insect growth regulator* ti, ab | Insect growth regulator* | |||
| 16 | Insect growth regulator* ti, ab | Environmental management ti, ab, Emtree | Environmental management | |||
| 17 | Environmental management ti, ab | Habitat modification ti, ab | Habitat modification | |||
| 18 | Habitat modification ti, ab | Biological pest control [Emtree] | Biological pest control | |||
| 19 | Pest Control, Biological [Mesh] | 6‐18/OR | 6‐18/OR | |||
| 20 | 6‐19/or | 5 and 19 | 5 and 19 | |||
| 21 | 5 and 20 |
1Cochrane Infectious Diseases Group Specialized Register
Data and analyses
Comparison 1.
Habitat modification alone
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Malaria incidence | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Controlled before‐and‐after trials; pre‐intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Controlled before‐and‐after trials; post‐intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Parasite prevalence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Controlled before‐and‐after trials; pre‐intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Controlled before‐and‐after trials; post‐intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2.
Habitat modification with larviciding
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasite prevalence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Controlled before‐and‐after trials; pre‐intervention | 1 | 1737 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.64] |
| 1.2 Controlled before‐and‐after trials; post‐intervention | 1 | 1538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.19, 0.34] |
| 2 Splenomegaly prevalence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Controlled before‐and‐after trials; pre‐intervention | 1 | 1737 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.51, 0.66] |
| 2.2 Controlled before‐and‐after trials; post‐intervention | 1 | 1538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.36, 0.47] |
Comparison 3.
Habitat manipulation alone
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasite prevalence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Controlled before‐and‐after trials; pre‐intervention | 1 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.70, 2.68] |
| 1.2 Controlled before‐and‐after trials; post‐intervention | 1 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.15] |
| 2 Splenomegaly prevalence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Controlled before‐and‐after trials; pre‐intervention | 1 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.31, 0.85] |
| 2.2 Controlled before‐and‐after trials; post‐intervention | 1 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.17] |
Comparison 4.
Habitat manipulation with larviciding
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Malaria incidence | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Controlled before‐and‐after trials; pre‐intervention | 1 | 97000 | Rate Ratio (Fixed, 95% CI) | 1.14 [1.01, 1.28] |
| 1.2 Controlled before‐and‐after trials; post‐intervention | 1 | 97000 | Rate Ratio (Fixed, 95% CI) | 0.24 [0.22, 0.25] |
| 2 Parasite prevalence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Controlled before‐and‐after trials; pre‐intervention | 1 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.99, 2.11] |
| 2.2 Controlled before‐and‐after trials; post‐intervention | 1 | 2713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.45, 0.65] |
Comparison 5.
Larviciding alone
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Malaria incidence | 3 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Cluster‐RCTs; pre‐intervention | 2 | 19981 | Rate Ratio (Random, 95% CI) | 0.95 [0.84, 1.08] |
| 1.2 Cluster‐RCTs; post‐intervention | 2 | 20124 | Rate Ratio (Random, 95% CI) | 0.26 [0.22, 0.31] |
| 1.3 Controlled before‐and‐after trials; pre‐intervention | 1 | 400 | Rate Ratio (Random, 95% CI) | 1.28 [0.75, 2.20] |
| 1.4 Controlled before‐and‐after trials; post‐intervention | 1 | 663 | Rate Ratio (Random, 95% CI) | 0.69 [0.33, 1.43] |
| 2 Malaria incidence (post‐intervention) sensitivity analysis | 2 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Not adjusted for clustering | 2 | Rate Ratio (Fixed, 95% CI) | 0.26 [0.22, 0.30] | |
| 2.2 Adjusted using ICC = 0.01 | 2 | Rate Ratio (Fixed, 95% CI) | 0.25 [0.16, 0.40] | |
| 2.3 Adjusted using ICC = 0.1 | 2 | Rate Ratio (Fixed, 95% CI) | 0.25 [0.06, 0.98] | |
| 3 Parasite prevalence | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Cluster‐RCTs; pre‐intervention | 1 | 3351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.66, 1.56] |
| 3.2 Cluster‐RCTs; post‐intervention | 1 | 2963 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.05, 0.22] |
| 3.3 Controlled before‐and‐after trials; pre‐intervention | 1 | 2439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.04, 1.59] |
| 3.4 Controlled before‐and‐after trials; post‐intervention | 1 | 2374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.42, 0.87] |
| 4 Parasite prevalence (post‐intervention) sensitivity analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Not adjusted for clustering | 1 | 2963 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.05, 0.22] |
| 4.2 Adjusted using ICC = 0.01 | 1 | 631 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.56] |
| 4.3 Adjusted using ICC = 0.1 | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.14] |
Comparison 6.
Larval source management versus control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Malaria incidence | 5 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Cluster‐RCTs; pre‐intervention | 2 | 19981 | Rate Ratio (Random, 95% CI) | 0.95 [0.84, 1.08] |
| 1.2 Cluster‐RCTs; post‐intervention | 2 | 20124 | Rate Ratio (Random, 95% CI) | 0.26 [0.22, 0.31] |
| 1.3 Controlled before‐and‐after trials; pre‐intervention | 3 | 97970 | Rate Ratio (Random, 95% CI) | 1.50 [0.89, 2.52] |
| 1.4 Controlled before‐and‐after trials; post‐intervention | 3 | 98233 | Rate Ratio (Random, 95% CI) | 0.51 [0.18, 1.44] |
| 2 Parasite prevalence | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Cluster‐RCTs; pre‐intervention | 1 | 3351 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.66, 1.56] |
| 2.2 Cluster‐RCTs; post‐intervention | 1 | 2963 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.05, 0.22] |
| 2.3 Controlled before‐and‐after trials; pre‐intervention | 5 | 7480 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.65, 1.52] |
| 2.4 Controlled before‐and‐after trials; post‐intervention | 5 | 8041 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.19, 0.55] |
| 3 Splenomegaly prevalence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Controlled before‐and‐after trials; pre‐intervention | 2 | 2569 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.50, 0.65] |
| 3.2 Controlled before‐and‐after trials; post‐intervention | 2 | 2384 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.10] |
What's new
| Date | Event | Description |
|---|---|---|
| 26 January 2016 | Amended | The summary of findings table was amended for clarity. |
Differences between protocol and review
Types of studies
We planned to include uncontrolled interrupted time series and before‐and‐after trials in which LSM was the only intervention introduced during the study period. However, we found these trials were too susceptible to bias introduced by confounding factors, such as natural fluctuations in vector populations and climate.
Conference proceedings
We intended to search the conference proceedings of the MIM Pan‐African Malaria Conferences, the American Society of Tropical Medicine and Hygiene, the American Mosquito Control Association and the Society for Vector Ecology for relevant abstracts. However, we did not do this.
Data extraction for cluster‐RCTs
Where results were adjusted for clustering, we planned to extract a point estimate and report the 95% confidence interval (CI). However, none of the RCTs we included adjusted for clustering.
Assessment of heterogeneity
To assess heterogeneity, we planned to inspect the forest plots and to implement the I2 statistic with the following definitions of heterogeneity: heterogeneity might not be important (0% to 40%); moderate heterogeneity (30% to 60%); substantial heterogeneity (50% to 90%); or considerable heterogeneity (75% to 100%). We planned to use P = 0.1 as the threshold for statistical significance. However, we did not identify a sufficient number of studies (10 trials or more).
Subgroup analysis and investigation of heterogeneity
Where trials were combined in meta‐analysis, we planned to conduct subgroup analyses to investigate heterogeneity in the effect of LSM across eco‐epidemiological settings. However we did not identify a sufficient number of trials.
Assessment of reporting biases
We planned to construct funnel plots to look for evidence of publication bias but we did not identify a sufficient number of trials (10 trials or more).
Changes to author list
We added Lucy Tusting, Kimberley Bonner, Christian Bottomley and David Sinclair as authors. Robert Newman left the author team.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
Trial design: Controlled before‐and‐after trial Type of cluster: Town and rural areas Cluster size: Population of towns: 1700; 1130; 830; 32,200; 31,550 individuals Number of clusters in each arm: Intervention arm: two; control arm: three Adjusted for clustering? No |
|
| Participants |
Age: School children Sex: Any Co‐morbidities and pregnancy: Any Primary outcome sample size (Parasite prevalence): 210, 112, 97, 853, 650 participants per survey Secondary outcome sample size (Splenomegaly prevalence): 210, 112, 97, 853, 650 participants per survey |
|
| Interventions |
Intervention: Habitat modification with larviciding Details of the intervention: Habitat modification: Drainage and reclamation of marshland, straightening of rivers and construction of embankments Larviciding: Larval habitats were treated with Paris Green (dosage not stated) Frequency of application: Not stated Duration of intervention period: 60 months Who was responsible for LSM? The government Co‐interventions: Case management: treatment with quinine (coverage not stated) Co‐interventions equal in each arm? Not stated |
|
| Outcomes |
1. Parasite prevalence (measured with yearly cross‐sectional surveys) 2. Splenomegaly prevalence (measured with yearly cross‐sectional surveys) |
|
| Notes |
Continent: Europe Country: Greece Ecosystem: Coastal Urban or rural: Urban and rural Extensive or localized larval habitats: Localized Primary larval habitats: Primarily man‐made habitats Transmission intensity: Low to moderate Transmission season(s): May to October Primary and secondary vector:An. elutus, An. superpictus Primary malaria parasite:P. falciparum, P. vivax Source of funding: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly chosen. |
| Allocation concealment (selection bias) | High risk | Not randomly chosen. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Impossible to blind evaluators to intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reporting ceased from one clinic. Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting complete. |
| Baseline characteristics | Low risk | Baseline characteristics reported. |
| Contamination | Unclear risk | Not stated how far apart the towns were. |
| Incorrect analysis | Unclear risk | Cluster adjustment not applicable. |
| Other bias | High risk | High risk of confounding. |
| Methods |
Trial design: Controlled before‐and‐after trial Type of cluster: Area of city (area around large drain) Cluster size: Unclear Number of clusters in each arm: Intervention arm: four; control arm: two Adjusted for clustering? No |
|
| Participants |
Age: Any Sex: Any Co‐morbidities and pregnancy: Any Primary outcome sample size (Parasite prevalence): 1162, 1513, 1991, 1793, 1711, 900 participants in the surveys |
|
| Interventions |
Intervention: Habitat manipulation with larviciding Details of the intervention: Habitat manipulation: Drains in the city were cleared to increase the water flow and to reduce flooding in the rainy season. Minor repairs such as slab replacement were conducted Larviciding: In half the intervention neighbourhoods, larval habitats were treated with larvicide by the Urban Malaria Control Progam (details not given) Frequency of application: Not stated Duration of intervention period: Not stated Who was responsible for LSM? Drain clearance was initially conducted by a contractor with 90% of the workforce local. Intensive education of the local community led to community‐led maintenance of drains. Larviciding was organized by the Urban Malaria Control Program. Co‐interventions: None. However ITNs are used in the study area (coverage not stated). Co‐interventions equal in each arm? Not stated |
|
| Outcomes | 1. Parasite prevalence (measured with six cross‐sectional surveys (one every two months) | |
| Notes |
Continent: Africa Country: Tanzania Ecosystem: Coastal Urban or rural: Urban Extensive or localized larval habitats: Localized Primary larval habitats: Drains Transmission intensity: Low to moderate Transmission season(s): March to June, October to December Primary and secondary vector:An. gambiae, An. funestus Primary malaria parasite:P. falciparum Source of funding: Japan International Cooperation Agency |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Environmental management sites purposefully chosen according to stated criteria. |
| Allocation concealment (selection bias) | High risk | Sites purposefully selected. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Parasite prevalence assessed by blinded reading of blood slides collected from randomly selected participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No way to blind participants and personnel to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | Outcomes reported as per methods, however little detail pertaining to the data is reported. |
| Baseline characteristics | Unclear risk | Stated to be similar, but not specified. |
| Contamination | High risk | In one EM cluster, drain not maintained; distances of clusters from one another not reported. |
| Incorrect analysis | Unclear risk | Cluster adjustment not applicable. |
| Other bias | High risk | High risk of confounding. |
| Methods |
Trial design: Cluster‐RCT Type of cluster: Village Cluster size: Not stated Number of clusters in each arm: Three Adjusted for clustering? No |
|
| Participants |
Age: n/a Sex: n/a Co‐morbidities and pregnancy: n/a Primary outcome sample size (EIR): 12 sentinel houses per village Secondary outcome sample size (Adult mosquito density (measures other than human biting rate)): 12 sentinel houses per village |
|
| Interventions |
Intervention: Larviciding Details of the intervention: Larviciding: Larval habitats were treated with Bti (Vectobac®, applied at 400g/ha using a sprayer) and Bs (VectoLex®, dosage not stated) Frequency of application: Larviciding with Bti: weekly; larviciding with Bs: every two weeks Duration of intervention period: 18 months Who was responsible for LSM? Malaria Research and Training Center staff and selected members of the community were trained to conduct larviciding. The local community was educated about the importance of larviciding. Co‐interventions: IRS: two rounds of district‐wide were conducted, covering all study villages in July to August 2008 and June to July 2009 (coverage not stated) Co‐interventions equal in each arm? Not stated |
|
| Outcomes |
1. EIR (measured with monthly pyrethrum spray collections in sentinel houses) 2. Adult mosquito density (measured with monthly pyrethrum spray collections in sentinel houses) |
|
| Notes |
Continent: Africa Country: Mali Ecosystem: Savannah Urban or rural: Rural Extensive or localized larval habitats: Localized Primary larval habitats: Brick pits, ponds, tyre prints Transmission intensity: High Transmission season(s): June to October Primary and secondary vector:An. gambiae Primary malaria parasite:P. falciparum Source of funding: Malaria Research and Training Center, University of Bamako; Research Triangle International; National Institues of Health; Centers for Disease Control; United States Agency for International Development; United States President's Malaria Initiative |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Villages randomly assigned; however method of randomization not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Impossible to blind entomologic data collection. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Stated outcomes reported. |
| Baseline characteristics | Unclear risk | Baseline characteristics not stated, though villages chosen from same health district. |
| Contamination | Low risk | Villages a sufficient distance apart. |
| Incorrect analysis | High risk | Not adjusted for clustering. |
| Other bias | Low risk | Low risk of confounding. |
| Methods |
Trial design: Controlled before‐and‐after trial Type of cluster: Area of city (ward) Cluster size: 0.96 to 15km2 Number of clusters in each arm: Intervention arm: three; control arm: 12 Adjusted for clustering? No |
|
| Participants |
Age: n/a Sex: n/a Co‐morbidities and pregnancy: n/a Primary outcome sample size (EIR): 67 sentinel sites |
|
| Interventions |
Intervention: Larviciding alone Details of the intervention: Larviciding: Open (light‐exposed) larval habitats were treated with Bti water‐dispersible granules (VectoBac®, applied at 0.04g/m2 using knapsack sprayers), Bs water‐dispersible granules (VectoLex®, applied at 0.2g/m2 using knapsack sprayers), Bti corn granule formulations (VectoBac®, applied at 1g/m2 by hand) and Bs corn granule formulations (VectoLex®, applied at 3g/m2 by hand). Closed habitats (the main larval habitat of Culex quinquefaciatus, a nuisance‐biting mosquito) were treated with Bs corn cob granules (VectoLex®, applied at 1g/m2 by hand). Frequency of application: Larviciding of open habitats: weekly; closed habitats: every three months. Duration of intervention period: 15 months Who was responsible for LSM? Open habitats were treated by modestly paid members of the community, Mosquito Contro CORPs, each of which was assigned to a specific area (mtaa). An additional team of CORPs was responsible for treating closed habitats. CORPs reported to the Ward Office. Co‐interventions: None. However ITNs were used in the study area (coverage not stated). Co‐interventions equal in each arm? Not stated |
|
| Outcomes |
1. EIR (measured with weekly CDC light trap catches and pyrethrum spray catches) 2. Adult mosquito density (human biting rate) (measured with weekly CDC light trap catches and pyrethrum spray catches) |
|
| Notes |
Continent: Africa Country: Tanzania Ecosystem: Coastal Urban or rural: Urban Extensive or localized larval habitats: Localized Primary larval habitats: Man‐made habitats exposed to sunlight Transmission intensity: Low to moderate Transmission season(s): March to June (primary), October to December (secondary) Primary and secondary vector:An. gambiae s.s., An. arabiensis Primary malaria parasite:P. falciparum Source of funding: Swiss Tropical Institute, the United States Agency for International Development (Environmental Health Project, Dar es Salaam Mission and the United States President's Malaria Initiative), the Bill and Melinda Gates Foundation, Valent BioSciences Corporation, Wellcome Trust. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly chosen. |
| Allocation concealment (selection bias) | High risk | Not randomly chosen. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Impossible to blind entomologic data collection. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Complete outcome reporting. |
| Baseline characteristics | Low risk | Baseline mosquito densities reported. |
| Contamination | High risk | Control and intervention clusters are adjacent. |
| Incorrect analysis | Unclear risk | Cluster adjustment not applicable. |
| Other bias | High risk | High risk of confounding. |
| Methods |
Trial design: Controlled before‐and‐after trial Type of cluster: Highland valley villages Cluster size: Between 107 and 214 individuals in each group (2‐4km sq) Number of clusters in each arm: Three Adjusted for clustering? No |
|
| Participants |
Age: 6 months to 10 years Sex: Any Co‐morbidities and pregnancy: Any Primary outcome sample size (Malaria incidence): 720 participants Secondary outcome sample size (EIR): 10 sentinel sites per valley |
|
| Interventions |
Intervention: Larviciding alone Details of the intervention: Larviciding: Larval habitats were treated with Bs water‐dispersible and corn granules (VectoLex®) during months one to six, then Bti water‐dispersible and corn granules (VectoBac®) during months seven to 19. Frequency of application: Weekly Duration of intervention period: 19 months Who was responsible for LSM? Study staff Co‐interventions: ITNs (coverage: intervention arm: 25% to 51%; non‐intervention arm: 24% to 51%). Co‐interventions equal in each arm? Yes |
|
| Outcomes |
1. Malaria incidence (measured by three cross‐sectional surveys in the pre‐intervention period, and three cross‐sectional surveys in the post‐intervention period, two months apart, using rapid malaria tests and microscopy) 2. EIR (measured by monthly indoor resting collection (pyrethrum spray collection) at sentinel sites) 3. Adult mosquito density (human biting rate) (measured by monthly indoor resting collection (pyrethrum spray collection) at sentinel sites) 4. Adult mosquito density (measures other than human biting rate (measured by monthly indoor resting collection (pyrethrum spray collection) at sentinel sites) |
|
| Notes |
Continent: Africa Country: Kenya Ecosystem: Highland Urban or rural: Rural Extensive or localized larval habitats: Localized and extensive Primary larval habitats: Small streams, papyrus swamps Transmission intensity: Moderate Transmission season(s): April to June, November to January Primary and secondary vector:An. gambiae s.l., An. funestus s.l. Primary malaria parasite:P. falciparum Source of funding: Environmental Health Project of the United States Agency for International Development |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly chosen. |
| Allocation concealment (selection bias) | High risk | Not randomly chosen. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Malaria incidence determined by blinded reading of blood smears. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Complete outcome reporting. |
| Baseline characteristics | Low risk | Baseline characteristics reported and similar. |
| Contamination | Low risk | Clusters at least 1 km apart. |
| Incorrect analysis | Unclear risk | Cluster adjustment not applicable. |
| Other bias | High risk | High risk of confounding. |
| Methods |
Trial design: Controlled before‐and‐after trial Type of cluster: Ward Cluster size: Total study population of 4761 Number of clusters in each arm: Intervention arm: three; control arm: 12 Adjusted for clustering? No |
|
| Participants |
Age: 0 to five years Sex: Any Co‐morbidities and pregnancy: Any Primary outcome sample size (Parasite prevalence): 4450 participants Secondary outcome sample size (EIR): 268 sentinel sites (4 sites in each of 67 mitaa) |
|
| Interventions |
Intervention: Larviciding Details of the intervention: Larviciding: Open (light‐exposed) larval habitats were treated with Bti water‐dispersible granules (VectoBac®, applied at 0.04g/m2 using knapsack sprayers) and Bti corn granules (VectoBac®, applied at 1 g/m2 by hand). Closed habitats (the main larval habitat of Culex quinquefaciatus, a nuisance‐biting mosquito) were treated with Bs corn cob granules (VectoLex®, applied at a dosage rate of 1 g/m2 by hand). Frequency of application: Larviciding of open habitats: weekly; closed habitats: every three months. Duration of intervention period: 12 months Who was responsible for LSM? Open habitats were treated by modestly paid members of the community, Mosquito Contro CORPs, each of which was assigned to a specific area (mtaa). An additional team of CORPs was responsible for treating closed habitats. CORPs reported to the Ward Office. Co‐interventions: None. However ITNs were used in the study area. Coverage: Non‐intervention area: 23.6% (year 1), 27.7% (year 2), 24.6% (year 3); Intervention area: 23.3% (year 1), 26.3% (year 2), 22.4% (year 3). Co‐interventions equal in each arm? Yes |
|
| Outcomes |
1. Parasite prevalence (measured with randomized, cluster‐sampled household surveys in May to September 2004, November to July 2004, September 2005 to May 2006, July 2006 to March 2007, with parasite prevalence determined by microscopy). 2. EIR (measured by (1) human landing catch for 45 minutes of each hour from 6pm to 6am, at sentinel sites every four weeks, and (2) laboratory analysis of specimens for sporozoites). |
|
| Notes |
Continent: Africa Country: Tanzania Ecosystem: Coastal Urban or rural: Urban Extensive or localized larval habitats: Localized Primary larval habitats: Man‐made habitats exposed to sunlight Transmission intensity: Low to moderate Transmission season(s): July to September Primary and secondary vector:An. gambiae s.l. Primary malaria parasite:P. falciparum Source of funding: Bill & Melinda Gates Foundation; Valent Biosciences Corporation; United States Centers for Disease Control and Prevention and United States Agency for International Development (Environmental Health Program, Dar es Salaam Mission and the President’s Malaria Initiative, all administered through Research Triangle International); Wellcome Trust. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly chosen. |
| Allocation concealment (selection bias) | High risk | Not randomly chosen. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Malaria prevalence determined by blinded reading of blood smears of randomly chosen individuals. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | All household members tested, but results presented only for children aged 0 to five years. |
| Baseline characteristics | Unclear risk | Baseline characteristics not specified. |
| Contamination | Low risk | Most of control clusters > 1 km from intervention clusters. |
| Incorrect analysis | Unclear risk | Cluster adjustment not applicable. |
| Other bias | High risk | High risk of confounding. |
| Methods |
Trial design: Randomized cross‐over trial Type of cluster: Area of land (zone) Cluster size: Each zone was 12 x 8 km and was subdivided into three parallel 4 km wide bands perpendicular to the river. Study villages were recruited from the central band of each zone. Number of clusters in each arm: Two Adjusted for clustering? Yes, included as random effects. |
|
| Participants |
Age: Six months to 10 years Sex: Any Co‐morbidities and pregnancy: Any Primary outcome sample size (Malaria incidence): Zone 1: 496; Zone 2: 508; Zone 3: 525; Zone 4: 510 Secondary outcome sample size (EIR): 15 traps per zone, divided between the villages with one to three sentinel houses per village proportional to village size |
|
| Interventions |
Intervention: Larviciding alone Larviciding: Larval habitats in areas of low vegetation coverage were treated with Bti water‐dispersible granules (VectoBac® AM65‐52, applied at 0.2kg/hectare using knapsack compression sprayers). Less accessible larval habitats in areas of high vegetation coverage were treated with Bti corn granules (VectoBac® AM65‐52, applied at 5.0kg/hectare by hand from buckets or using motorized knapsack granule blowers). Frequency of application: Weekly Duration of intervention period: June to November 2006 (6 months), May to November 2007 (7 months) Who was responsible for LSM? Field applicators were recruited from local communities and trained for one month before larviciding. Applicators were supervised by one field supervisor in each of the four study zones Co‐interventions: None. However ITNs were used in the study area (coverage: Zone 1: 27.6% (2006), 37.2% (2007); Zone 2: 6.1% (2006), 81.4% (2007); Zone 3: 38.3% (2006), 71.2% (2007); Zone 4: 34.3% (2006), 70.4% (2007). Co‐interventions equal in each arm? Yes |
|
| Outcomes |
1. Malaria incidence (measured with passive case detection by study nurses and government village health workers) 2. Parasite prevalence (measured with two cross‐sectional surveys per year, one before and one after the main transmission season) 3. Splenomegaly prevalence (measured with two cross‐sectional surveys per year, one before and one after the main transmission season) 4. EIR (measured using CDC light traps at 60 sentinel sites every two weeks). 5. Adult mosquito density (measures other than human biting rate) (measured using CDC light traps at 60 sentinel sites every two weeks). |
|
| Notes |
Continent: Africa Country: The Gambia Ecosystem: Savannah Urban or rural: Rural Extensive or localized larval habitats: Extensive Primary larval habitats: Flood plains, rice paddy fields Transmission intensity: High Transmission season(s): July to October Primary and secondary vector:An. gambiae Primary malaria parasite:P. falciparum Source of funding: National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each area served as its own control. |
| Allocation concealment (selection bias) | Low risk | Each area served as its own control. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collectors blinded to intervention status. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers or inhabitants to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcomes all reported as specified. |
| Baseline characteristics | Low risk | Each area served as its own control. |
| Contamination | Low risk | Clusters bordered by 4 km zones. |
| Incorrect analysis | Unclear risk | Cluster adjustment not applicable. |
| Other bias | Low risk | Low risk of confounding. |
| Methods |
Trial design: Controlled before‐and‐after trial Type of cluster: Town Cluster size: Intervention arm 92,000 individuals; control arm 5000 individuals Number of clusters in each arm: One Adjusted for clustering? n/a |
|
| Participants |
Age: Any Sex: Any Co‐morbidities and pregnancy: Any Primary outcome sample size (Malaria incidence): Intervention arm: 92,000; control arm: 5000 Secondary outcome sample size (Adult mosquito density (measures other than human biting rate)): 80 sentinel sites |
|
| Interventions |
Intervention: Habitat manipulation with larviciding Details of the intervention: Habitat manipulation: attempts to persuade householders to remove domestic water storage containers made with limited success Larviciding: Larval habitats (excluding stored domestic water) were treated with pirimiphos‐methyl (applied at 12.5g active ingredient/ha, with knapsack sprayers) Frequency of application: Weekly Duration of intervention period: 15 months Who was responsible for LSM? Study staff were responsible for larviciding. Attempts were made to persuade the local community to conduct habitat modification Co‐interventions: Case management (active case detection): presumptive treatment of all fever cases with chloroquine (coverage not stated) Co‐interventions equal in each arm? Yes |
|
| Outcomes |
1. Malaria incidence (measured with continuous community surveillance) 2. Parasite prevalence (measured with community surveys) 3. Adult mosquito density (measures other than human biting rate): (measured with weekly indoor resting collections using an aspirator, at sentinel sites. 16 of 80 sentinel sites visited each week day) |
|
| Notes |
Continent: Asia Country: India Ecosystem: Desert fringe Urban or rural: Urban Extensive or localized larval habitats: Localized Primary larval habitats: Containers, wells, rainwater pools, canals, stagnant pools in drains Transmission intensity: Low Transmission season(s): May to September Primary and secondary vector:An. culicifacies, An. stephensi Primary malaria parasite:P. falciparum Source of funding: Haryana State Health Authorities; Alkali and Chemical Corporation of India Ltd; ICI Plant Protection Division |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly chosen. |
| Allocation concealment (selection bias) | High risk | Not randomly chosen. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given as to blinding of those seeing patients and reading blood slides. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers or inhabitants to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not specified. |
| Baseline characteristics | Unclear risk | Baseline characteristics not stated; intervention town much larger than control town. |
| Contamination | Low risk | 8 km between control and intervention towns. |
| Incorrect analysis | Unclear risk | Cluster adjustment not applicable. |
| Other bias | High risk | High risk of confounding. |
| Methods |
Trial design: Controlled before‐and‐after trial Type of cluster: Area of town (barrio) Cluster size: 25,545 people (intervention cluster) Number of clusters in each arm: One Adjusted for clustering? No |
|
| Participants |
Age: Two to 10 years Sex: Any Co‐morbidities and pregnancy: Any Primary outcome sample size (Parasite prevalence): Intervention arm: 500; control arm: 200 Secondary outcome sample size (Adult mosquito density (measures other than human biting rate)): Not stated |
|
| Interventions |
Intervention: Habitat manipulation alone Details of the intervention: Habitat manipulation: automatic siphons were constructed over two streams which were the main larval habitats. Water was flushed to control larvae over distances of 1073m and 2897m downstream, respectively. Existing siphons were repaired. Frequency of application: Constant Duration of intervention period: 12 months Who was responsible for LSM? United Stated Public Health Service Co‐interventions: None Co‐interventions equal in each arm? n/a |
|
| Outcomes |
1. Parasite prevalence (measured with community‐based cross‐sectional surveys) 2. Splenomegaly prevalence (measured with community‐based cross‐sectional surveys) 3. Adult mosquito density (measures other than human biting rate) (sampled with human baited traps and carabao baited traps every two weeks) |
|
| Notes |
Continent: Asia Country: Philippines Ecosystem: Coastal Urban or rural: Urban Extensive or localized larval habitats: Localized Primary larval habitats: Streams fed by a lake Transmission intensity: High Transmission season(s): Not stated Primary and secondary vector:An. minimus flavirostris Primary malaria parasite:P. falciparum Source of funding: Malaria Eradication Project, San Pablo City |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly chosen. |
| Allocation concealment (selection bias) | High risk | Not randomly chosen. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Sampling method for periodic surveys not stated, though reportedly surveyed 50% to 80% of children per year. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers or inhabitants to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified. |
| Baseline characteristics | Unclear risk | Clusters in same town, but no baseline characteristics specified. Only 6 months of pre‐treatment entomological data were collected. |
| Contamination | Low risk | Clusters 8 km apart. |
| Incorrect analysis | Unclear risk | Cluster adjustment not applicable. |
| Other bias | High risk | High risk of confounding. |
| Methods |
Trial design: Controlled before‐and‐after trial Type of cluster: Village Cluster size: Intervention arm: 271 individual; control arms: 143 and 156 individuals Number of clusters in each arm: Intervention arm: one; control arm: two Adjusted for clustering? No |
|
| Participants |
Age: Any Sex: Any Co‐morbidities and pregnancy: Any Primary outcome sample size (Malaria incidence): Total study population: 570 Secondary outcome sample size (Parasite prevalence): 40% households sampled in each of the three clusters (combined total population 570) |
|
| Interventions |
Intervention: Habitat modification alone Details of the intervention: Habitat modification: Construction of a small concrete dam 25m x 4m across the stream in the village to provide water for irrigation reduced the number of larval habitats in the village. Frequency of application: n/a Duration of intervention period: 23 months Who was responsible for LSM? The district administration constructed the dam at the request of the village panchayat (governing body) Co‐interventions: None. However indoor residual spraying was conducted annually with DDT and a synthetic pyrethroid (coverage: 60% to 80%). Co‐interventions equal in each arm? Yes |
|
| Outcomes |
1. Malaria incidence (measured with weekly longitudinal surveillance and continuous passive case detection) 2. Parasite prevalence (measured with three cross‐sectional surveys per year) |
|
| Notes |
Continent: Asia Country: India Ecosystem: Forest Urban or rural: Rural Extensive or localized larval habitats: Localized Primary larval habitats: Streams (An. fluviatilis), stagnant pools, ditches, irrigation channels (An. culicifacies) Transmission intensity: Moderate Transmission season(s): October to December Primary and secondary vector:An. fluviatilis, An. culifacies Primary malaria parasite:P. falciparum Source of funding: Indian Council of Medical Research; Ministry of Health and Family Welfare, Government of India |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly chosen. |
| Allocation concealment (selection bias) | High risk | Not randomly chosen. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Surveillance personnel not blinded to intervention status. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers or inhabitants to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported as specified. |
| Baseline characteristics | Low risk | Baseline incidences reported and similar. |
| Contamination | Low risk | Control and intervention villages 30 km apart. |
| Incorrect analysis | Unclear risk | Cluster adjustment not applicable. |
| Other bias | High risk | High risk of confounding. |
| Methods |
Trial design: Cluster‐RCT Type of cluster: Village Cluster size: Not stated. Number of clusters in each arm: Four Adjusted for clustering? No |
|
| Participants |
Age: n/a Sex: n/a Co‐morbidities and pregnancy: n/a Primary outcome sample size (Adult mosquito density (measures other than human biting rate)): 12 light traps per study village Secondary outcome sample size: n/a |
|
| Interventions |
Intervention: Habitat modification with larviciding Details of the intervention: Habitat modification: Filling or drainage of rain pools, puddles at water supply points and stream bed pools Larviciding: Larval habitats which could not be eliminated by habitat modification were treated in rotation with Bti granules (VectoBac®, applied at 11.2kg/ha using a granular spreader), Bs corn granules (VectoLex®, applied at 22.4kg/ha using a granular spreader) and temephos (Abate®, applied at 112 ml/ha using a liquid sprayer) Frequency of application: Weekly Duration of intervention period: 24 months Who was responsible for LSM? Study staff; local community Co‐interventions: None. However ITNs and IRS were conducted as part of the national malaria control programme (coverage not stated). Co‐interventions equal in each arm? Not stated |
|
| Outcomes | 1. Adult mosquito density (measures other than human biting rate) (measured using CDC light traps from dusk to dawn (12 hours) 2 days per week for 24 months) | |
| Notes |
Continent: Africa Country: Eritrea Ecosystem: Desert fringe, highland and lowland Urban or rural: Rural Extensive or localized larval habitats: Localized Primary larval habitats: Stream bed pools, canals, drainage channels, wells, communal water supply points Transmission intensity: Not stated Transmission season(s): Short period of transmission coinciding with short rainy season Primary and secondary vector:An. arabiensis Primary malaria parasite:P. falciparum Source of funding: United States Agency for International Development, Environmental Health Project, International Center of Insect Physiology and Ecology, National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Clusters randomly assigned; however method of randomization not stated. |
| Allocation concealment (selection bias) | Unclear risk | One village randomly selected in each zone; however method of randomization not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Surveillance personnel not blinded to intervention status. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers or inhabitants to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified. |
| Baseline characteristics | Unclear risk | Pairs of villages selected to be similar but baseline characteristics not reported. |
| Contamination | Unclear risk | Distance of villages from one another not stated. |
| Incorrect analysis | High risk | Not adjusted for clustering. |
| Other bias | Low risk | Low risk of confounding. |
| Methods |
Trial design: Cluster‐RCT Type of cluster: Village Cluster size: Four villages of <500 people, four villages of 600‐1100 people Number of clusters in each arm: Four Adjusted for clustering? No |
|
| Participants |
Age: Any Sex: Any Co‐morbidities and pregnancy: Not stated Primary outcome sample size (Malaria incidence): 4566 (pre‐intervention); 4659 (post‐intervention) Secondary outcome sample size (Parasite prevalence): 3351 |
|
| Interventions |
Intervention: Larviciding Details of the intervention: Larviciding: Gem pits and riverbed and stream pools were treated with pyriproxyfen S‐31183 granules (Adeal® 0.5%, applied at 2g/m3). Frequency of application: December 1994, June to July 1995, November 1995 Duration of intervention period: 12 months Who was responsible for LSM? Study staff Co‐interventions: Case management following whole community survey (coverage comprehensive). Co‐interventions equal in each arm? Yes |
|
| Outcomes |
1. Malaria incidence (measured by passive case detection) 2. Parasite prevalence (measured by cross‐sectional surveys (two in pre‐intervention year, two in post‐intervention year) 3. Adult mosquito density (measures other than human biting rate) (measured by window exit trap collection, pyrethrum spray sheet, indoor human landing catch, cattle‐baited hut collection, cattle‐baited net trap collection at sentinel sites) |
|
| Notes |
Continent: Asia Country: Sri Lanka Ecosystem: Forest Urban or rural: Rural Extensive or localized larval habitats: Localized Primary larval habitats: Abandoned gem mine pits Transmission intensity: Moderate to high Transmission season(s): October to December Primary and secondary vector:An. culicifacies, An. subpictus Grassi Primary malaria parasite:P. vivax Source of funding: Sumitomo Corporation, United Nations Development Program, World Bank, WHO |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, though method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Parasite prevalence determined by blinded reading of blood slides, but incidence in local clinics and blinding impossible. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers or inhabitants to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | Several methods of collection of entomologic data described, not all reported. |
| Baseline characteristics | Unclear risk | Characteristics not reported, but stratification and randomization were performed based on baseline data. Baseline data for 12 months pre‐treatment is presented. |
| Contamination | Low risk | At least 1.5 km between villages. |
| Incorrect analysis | High risk | Not adjusted for clustering. |
| Other bias | Low risk | Low risk of confounding. |
| Methods |
Trial design: Cluster‐RCT Type of cluster: Village Cluster size: Each of the 12 villages was defined as a circle of 1.5km radius centred on a stream or irrigation canal Number of clusters in each arm: Six Adjusted for clustering? No |
|
| Participants |
Age: Any Sex: Any Co‐morbidities and pregnancy: Any Primary outcome sample size (Malaria incidence): 15415 individuals Secondary outcome sample size (Adult mosquito density (measures other than human biting rate)): Not stated |
|
| Interventions |
Intervention: Larviciding alone Details of the intervention: Larviciding: Riverbed pools, streams, irrigation ditches, quarry pits and agricultural wells were treated with pyriproxyfen S‐31183 0.5% granules (Sumilarv®, applied at 2g/m3 using a spoon). Frequency of application: Two rounds of larviciding were conducted: July 2001 and December 2001. Duration of intervention period: 12 months Who was responsible for LSM? Study staff Co‐interventions: Larvivorous fish: Poecillia reticulata were added to drinking water wells. IRS was conducted as part of the national malaria control programme during November and June each year (coverage not stated). Co‐interventions equal in each arm? Yes |
|
| Outcomes |
1. Malaria incidence (measured by passive case detection at two field clinics and two clinics at outpatient departments at a hospital and dispensary) 2. Adult mosquito density (measures other than human biting rate) (measured using cattle‐baited huts at sentinel sites) |
|
| Notes |
Continent: Asia Country: Sri Lanka Ecosystem: 'Dry zone' Urban or rural: Rural Extensive or localized larval habitats: Localized and extensive Primary larval habitats: River bed pools, streams, irrigation ditches (dry season); rice paddies (rainy season) Transmission intensity: Moderate Transmission season(s): January to March Primary and secondary vector:An. culifacies, An. subpictus Primary malaria parasite:P. vivax Source of funding: United Nations Development Program, World Bank, World Health Organization Special Program for Research and Training in Tropical Diseases |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, though method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Parasite prevalence determined by blinded reading of blood slides, but incidence measured at local clinics and blinding impossible. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind implementers or inhabitants to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Individual patients not followed up therefore not possible to measure percentage loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | Several methods of collection of entomologic data described, not all reported. |
| Baseline characteristics | Unclear risk | Characteristics not reported, but stratification and randomization performed based on baseline data. |
| Contamination | Unclear risk | Distance of villages from one another not specified. |
| Incorrect analysis | High risk | Not adjusted for clustering. |
| Other bias | Low risk | Low risk of confounding. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anon (a) | We could not obtain the full‐text article. |
| Anon (b) | We could not obtain the full‐text article. |
| Anon (c) | We could not obtain the full‐text article. |
| Anon (d) | We could not obtain the full‐text article. |
| Baduilin 1931 | We could not obtain the full‐text article. |
| Barbazan 1998 | No control. |
| Berti 1946 | We could not obtain the full‐text article. |
| Bini 1925 | We could not obtain the full‐text article. |
| Booker 1936 | We could not obtain the full‐text article. |
| Castro 2000 | No control. |
| Castro 2002 | No control. |
| Cross 1933 | No control. |
| Curry 1935 | We could not obtain the full‐text article. |
| Davis 1928 | We could not obtain the full‐text article. |
| Dryenski 1936 | Study did not have one year of baseline data. |
| Dua 1991 | Uneven application of other malaria control interventions between control and intervention areas: weekly active surveillance and treatment of fever cases in intervention area, but not in controls. |
| Dua 1997 | Uneven application of other malaria control interventions between control and intervention areas: weekly active surveillance and treatment of fever cases in intervention area, but not in controls. |
| Elmendorff 1948 | No control. |
| Essed 1932 | No control. |
| Fillinger 2006 | No control. |
| Gallus 1970 | We could not obtain the full‐text article. |
| Gammans 1926 | We could not obtain the full‐text article. |
| Gladney 1968 | No control. |
| Guelmino 1928 | We could not obtain the full‐text article. |
| Hackett 1925 | We could not obtain the full‐text article. |
| Ivorro Canno 1975 | Uneven application of other malaria control interventions between control and intervention areas: chloroquine chemoprophylaxis applied in intervention village and not in control village. |
| Kinde‐Gazard 2012 | Insufficient information reported to determine eligibility. |
| Kumar 1998 | No control. |
| Lee 2010 | No control. |
| Martini 1931 | We could not obtain the full‐text article. |
| Mulligan 1982 | No control. |
| Murray 1984 | No control. |
| Okan 1949 | We could not obtain the full‐text article. |
| Rodriguez Ocana 2003 | We could not obtain the full‐text article. |
| Rojas 1987 | Uneven application of other malaria control interventions between control and intervention areas: indoor residual spraying with DDT every six to 10 months used in intervention area, but not in control. |
| Sharma 1989 | Uneven application of other malaria control interventions between control and intervention areas: weekly active surveillance and treatment in intervention area, as well as extensive use of larvivorous fish; control villages changed multiple times over the life of the study, compromising comparability. |
| Singh 1984 | No control. |
| Singh 1989 | Uneven application of other malaria control interventions between control and intervention areas: weekly active surveillance and treatment in intervention area, compared to bimonthly in control; DDT indoor residual spraying in control villages. |
| Stratman‐Thomas 1937 | We could not obtain the full‐text article. |
| Symes 1931 | Larval habitats differed between control and intervention sites at baseline. |
| Vittal 1982 | No control. |
| Williamson 1934 | We could not obtain the full‐text article. |
| Xu 1992 | No control. |
| Yasuoka 2006 | Study did not have one year of baseline data. |
| Yohannes 2005 | Larval habitats differed between control and intervention sites at baseline. |
Contributions of authors
Lucy Tusting assisted with article retrieval, reviewed search results, extracted and analyzed the data, and prepared the review. Julie Thwing coordinated protocol preparation, assisted with article retrieval, reviewed search results, extracted the data and assisted with preparing the review. Kimberly Bonner reviewed search results and extracted data. Christian Bottomley analyzed the data. David Sinclair analyzed the data and prepared the review. Ulrike Fillinger assisted with writing the protocol and edited the final version of the manuscript. John Gimnig assisted with protocol preparation and assisted with article retrieval, eligibility assessment and risk of bias assessment, and edited the final version of the manuscript. Steve Lindsay was involved in the conception of this review, assisted with writing the protocol, article retrieval, eligibility assessment, data abstraction, and prepared the review.
Sources of support
Internal sources
-
Achuyt Bhattarai, Andre Machado De Siqueira, Andrea Thoumi, Carlotta Modestini, Claudia Vera Garcia, Elizabeth Tissinge, Francesca Solmi, Gabriel Ponce de Leon, Junko Kiriya, Liam Crosby, Lucy Haurisa, Mariana De Niz Hidalgo, Marta Buysana, Marta Maia, Maryna Braga, Sara Carrillo de Albornoz, Tapan Bhattacharyya, UK.
Translation of foreign language papers
London School of Hygiene and Tropical Medicine Library and Archives, UK.
Centers for Disease Control and Prevention Library Services, USA.
External sources
-
Paul Garner, Sarah Donegan, Anne‐Marie Stephani, UK.
Cochrane Infectious Diseases Group (guidance on data abstraction, analysis and write‐up; comments on the manuscript)
-
Tomas Allen, Carole Modis and Marie Sarah Villemin Partow, Switzerland.
WHO Library and Archives, Geneva (retrieval of literature)
-
Christianne Esparza, UK.
Cochrane Infectious Diseases Group (retrieval of literature)
Roll Back Malaria Larval Source Management Work Stream, Switzerland.
Declarations of interest
Ulrike Fillinger, John Gimnig and Steve Lindsay have been the primary investigators and authors of studies that were reviewed. They did not review their own studies. Ulrike Fillinger, Steve Lindsay and Lucy Tusting have received financial support from Valent BioSciences Corporation, USA, a manufacturer of microbial larvicides. Valent BioSciences Corporation had no involvement in the data analysis or preparation of the final report. We have no other interests to disclose.
Unchanged
References
References to studies included in this review
- Balfour MC. Some features of malaria in Greece and experience with its control. Rivista di Malariologia 1936;15:114‐131. [Google Scholar]
- Castro MC, Tsuruta A, Kanamori S, Kannady K, Mkude S. Community‐based environmental management for malaria control: evidence from a small‐scale intervention in Dar es Salaam, Tanzania. Malaria Journal 2009;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly MB, Pharm D. Integrated vector management: Impact of the combination of larval control and Indoor Residual Spraying on Anopheles gambiae density and vector capacity for human malaria. Unpublished data2011.
- Fillinger U, Kannady K, William G, Vanek MJ, Dongus S, Nyika D, et al. A tool box for operational mosquito larval control: preliminary results and early lessons from the Urban Malaria Control Programme in Dar es Salaam, Tanzania. Malaria Journal 2008;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger U, Ndenga B, Githeko A, Lindsay SW. Integrated malaria vector control with microbial larvicides and insecticide‐treated nets in western Kenya: a controlled trial. Bulletin of the World Health Organization 2009;87(9):655‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissbühler Y, Kannady K, Chaki PP, Emidi B, Govella NJ, Mayagaya V, Kiama M, Mtasiwa D, Mshinda H, Lindsay SW, Tanner M, Fillinger U, Castro MC, Killeen GFet al. Microbial larvicide application by a large‐scale, community‐based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PLoS One 2009;4(3):e5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majambere S, Pinder M, Fillinger U, Ameh D, Conway DJ, Green C, et al. Is mosquito larval source management appropriate for reducing malaria in areas of extensive flooding in The Gambia? A cross‐over intervention trial. American Journal of Tropical Medicine and Hygiene 2010;82(2):176‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samnotra KG, Kumar P. Field evaluation of pirimiphos methyl as a mosquito larvicide in an urban area of India as part of the National Malaria Eradication Programme. Mosquito News 1980;40:257‐63. [Google Scholar]
- Santiago D. Malaria control by automatic flushing of streams. Philippine Journal Science 1960;88:373‐95. [Google Scholar]
- Sharma SK, Tyagi PK, Upadhyay AK, Haque MA, Adak T, Dash AP. Building small dams can decrease malaria: a comparative study from Sundargarh District, Orissa, India. Acta Tropica 2008;107(2):174‐8. [DOI] [PubMed] [Google Scholar]
- Shililu J, Mbogo C, Ghebremeskel T, Githure J, Novak R. Mosquito larval habitats in a semiarid ecosystem in Eritrea: impact of larval habitat management on Anopheles arabiensis population. American Journal of Tropical Medicine and Hygiene 2007;76(1):103‐10. [PubMed] [Google Scholar]
- Yapabandara AM, Curtis CF, Wickramasinghe MB, Fernando WP. Control of malaria vectors with the insect growth regulator pyriproxyfen in a gem‐mining area in Sri Lanka. Acta Tropica 2001;80(3):265‐76. [DOI] [PubMed] [Google Scholar]
- Yapabandara AM, Curtis CF. Control of vectors and incidence of malaria in an irrigated settlement scheme in Sri Lanka by using the insect growth regulator pyriproxyfen. Journal of the American Mosquito Control Association 2004;20(4):395‐400. [PubMed] [Google Scholar]
References to studies excluded from this review
- Anonymous. Papers on mosquito control. Mosquito News 1944;4(3):65‐101. [Google Scholar]
- Anonymous. First conference of the U.S.S.R. for aeroplane use in malaria control. Meditsinskaya Parazitologiya i Parazitarnye Bolezni. 1932; Vol. 1:2. [Google Scholar]
- Anonymous. Report of the Bureau of Malaria Control 1926‐27. Rep Comm Hlth Porto Rico 1929:62‐95. [Google Scholar]
- Anonymous. A review of the control of malaria in Palestine (1918‐1941). A Review of the Control of Malaria in Palestine 1941;40. [Google Scholar]
- Buduilin VG, Andreeva VV. Paris Green as a larvicide in the control of malaria. Tropicheskaya Meditsina i Veterinariya 1931;9(9):464‐9. [Google Scholar]
- Barbazan P, Baldet T, Darriet F, Escaffre H, Djoda DH, Hougard JM. Impact of treatments with Bacillus sphaericus on Anopheles populations and the transmission of malaria in Maroua, a large city in a savannah region of Cameroon. Journal of the American Mosquito Control Association 1998;14(1):33‐9. [PubMed] [Google Scholar]
- Berti AL, Montesinos M. Rice‐growing in connection with malaria: the problem in Venezuela. Cuad Verde Com Ejecut. 3rd Annual Conference Interamerican Agriculture. 1946:33. [Google Scholar]
- Bini G. Report on the anti‐malarial work at Fiumicino. Relazione sulla lotta antimalarica di Fiumicino1925:93.
- Booker CG. Annual Report of the South African Railways and Harbours Organisation. Report of the Department of Public Health of South Africa 1936;193:5‐36. [Google Scholar]
- Blanco Castro SD, Martinez Arias A, Cano Velasquez OR, Tello Granados R, Mendoza I. Introduction of Bacillus sphaericus strain‐2362 (GRISELESF) for biological control of malaria vectors in Guatemala. Revista Cubana de Medicina Tropical 2000;52(1):37‐43. [PubMed] [Google Scholar]
- Castro SD, Colombi E, Flores LN, Canales D. Biolarvicide Bacillus sphaericus‐2362(GRISELESF) for the control of malaria in a health area of the Republic of Honduras. Revista Cubana de Medicina Tropical 2002;54(2):134‐41. [PubMed] [Google Scholar]
- Cross D. Some theoretical and experimental observations on malarial prevention on estates. Malayan Medical Journal 1933;8:261‐74. [Google Scholar]
- Curry DP. Report of Assistant Chief Health Officer. Report of the Health Department of Panama Canal 1935;12‐16. [Google Scholar]
- Davis NC, Rickard ER. Plan of work against urban malaria in the north of Argentina. Bol Inst Clin quirur 4th Reun Soc Argen Patol Region Norte. 1928; Vol. 4:119‐30. [Google Scholar]
- Dryenski K. The influence of intermittent and regular irrigation of the rice field upon malaria and rice production. Mitteilungen der Bulgarischen Entomologischen Gesellschaft 1936;11:11‐24. [Google Scholar]
- Dua VK, Sharma SK, Sharma VP. Bioenvironmental control of malaria at the Indian Drugs and Pharmaceuticals Ltd., Rishikesh, Uttah Pradesh. Indian Journal of Malariology 1991;28(4):227‐35. [PubMed] [Google Scholar]
- Dua VK, Sharma SK, Srivastava A, Sharma VP. Bioenvironmental control of industrial malaria at Bharat Heavy Electricals Ltd., Hardwar, India: Results of a nine‐year study (1987‐95). Journal of the American Mosquito Control Association 1997;13(3):278‐85. [PubMed] [Google Scholar]
- Elmendorf JE Jr. Second and supplementary report on field experiments to demonstrate effectiveness of various methods of malaria control. American Journal of Tropical Medicine and Hygiene 1948;28(3):425‐36. [DOI] [PubMed] [Google Scholar]
- Essed WFR. Successful malaria control at Banjoewangi, a typical example of species sanitation as conceived by Swellengrebel. Mededelingen van den Dienst der Volksgezondheid in Nederlandsch‐Indie 1932;21:41‐50. [Google Scholar]
- Fillinger U, Lindsay SW. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Tropical Medicine and International Health 2006;11(11):1629‐42. [DOI] [PubMed] [Google Scholar]
- Gallus MI. Organisation, function and activity of the regional centre for malaria. Organization, function and activity of the regional centre for malaria and insect control (Centre Régionale Antimalarico e Anti‐Insetti (C.R.A.I).. Cagliari, Sardinia.: CRAI, 1970:126. [Google Scholar]
- Gammans LD. Anti‐malaria work at Port Dickson. Malayan Medical Journal 1926;1(2):24‐8. [Google Scholar]
- Gladney WJ, Turner EC. Mosquito control on Smith Mountain Reservoir by pumped storage water level management. Mosquito News 1968;28:606‐18. [Google Scholar]
- Guelmino G. On larvicide powders. Glasnik Tzentr Khig Zavoda 1928;3(1‐6):42‐6. [Google Scholar]
- Hackett LW. The importance and uses of Paris Green (Copper Aceto Arsenite) as an Anopheles larvicide. Staz Sperim Per La Lotto1925:15.
- Ivorro Cano V. Trial of Abate larvicide for malaria control on the island of Grande Comore, Comoros Archipelago [Essai du larvicide abate dans la lutte contre le paludisme dans l'ile de Grand Comore, Archipel des Comores]. WHO Archives 1975;IRD MPD 2:11. [Google Scholar]
- Kinde‐Gazard D, Baglo T. Assessment of microbial larvicide spraying with Bacillus thuringiensis israelensis, for the prevention of malaria. Médecine et maladies infectieuses 2012;42(3):114–8. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sharma VP, Sumodan PK, Thavaselvam D. Field trials of biolarvicide Bacillus thuringiensis var. israelensis strain 164 and the larvivorous fish Aplocheilus blocki againstAnopheles stephensi for malaria control in Goa, India. Journal of the American Mosquito Control Association 1998;14(4):457‐62. [PubMed] [Google Scholar]
- Lee VJ, Ow S, Heah H, Tan MY, Lam P, Lam‐Phua SG, et al. Elimination of malaria risk through integrated combination strategies in a tropical military training island. American Journal of Tropical Medicine and Hygiene 2010;82(6):1024‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini E. Dusting, especially with Paris Green, in malaria control. Zeitschrift fur Desinfektion 1931;23(4):151‐66. [Google Scholar]
- Mulligan FS, Schaefer CH. A physical barrier for controlling mosquitoes which breed in urban storm drains. Mosquito News 1982;42:360‐5. [Google Scholar]
- Murray WC, Knutson H. Airplane dusting with Paris Green for control of Anopheles quadrimaculatus say in water‐chestnut covered areas of the Potomac River during 1943. Public Health Reports 1944;59(18):573‐83. 19315972 [Google Scholar]
- Okan S. Malaria control in Turkey. Sagl Sosy Yard Bakanl Yayin1949:67.
- Rodriguez Ocana E. The fight against malaria in Spain in the international context. Enfermedades Emergentes 2003;5(1):41‐52. [Google Scholar]
- Rojas W, Northup J, Gallo O, Motoya AE, Motoya F, Restrepo M, et al. Reduction of malaria prevalence after introduction of Romanomermis culicivorax (Mermithidae: Nematoda) in larval Anopheles habitats in Colombia. Bulletin of the World Health Organization 1987;65(3):331‐7. [PMC free article] [PubMed] [Google Scholar]
- Sharma VP, Sharma RC. Community based bioenvironmental control of malaria in Kheda District, Gujurat, India. Journal of the American Mosquito Control Association 1989;5(4):514‐21. [PubMed] [Google Scholar]
- Singh R, Dahiya BS. The assessment of larvicidal impact on malaria and malaria vector Anopheles culicifacies (Diptera: Culicidae) in Gurgaron urban. Entomology 1984;9:173‐6. [Google Scholar]
- Singh N, Sharma VP, Mishra AK, Singh OP. Bio‐environmental control of malaria in a tribal area of Mandla District, Madhya Pradesh, India. Indian Journal of Malariology 1989;26(2):103‐20. [PubMed] [Google Scholar]
- Stratman‐Thomas WK, Barber WK, Carter JC. Extract of the Report of the work of the International Health Division of the Rockefeller Foundation in Cyprus, 1936. Annu Med Sanit Rep Cyprus1936:44‐54.
- Symes CB. Observations on Anophelines and malaria in Kitale, with notes on experimental control with Paris Green. Kenya East African Medical Journal 1931;8:256‐67. [Google Scholar]
- Vittal M, Bhate MR, Joshi VS, Deshpande LB. Study of larviciding as a supplementary malaria control measure in a rural area of Maharashtra. Indian Journal of Public Health 1982;26(3):155‐62. [PubMed] [Google Scholar]
- Williamson KB. The control of rural malaria. M.A.H.A. Magazine 1934;4(1‐2):224‐8. [Google Scholar]
- Xu BZ, Becker N, Xianqi X, Ludwig HW. Microbial control of malaria vectors in Hubei Province, People’s Republic of China. Bulletin of the Society of Vector Ecology 1992;17:140‐9. [Google Scholar]
- Yasuoka J, Levins R, Mangione TW, Spielman A. Community‐based rice ecosystem management for suppressing vector anophelines in Sri Lanka. Transactions of the Royal Society of Tropical Medicine and Hygiene 2006;100(11):995‐1006. [DOI] [PubMed] [Google Scholar]
- Yohannes M, Haile M, Ghebreyseus TA, Witten KH, Getachew A, Byass P, et al. Can source reduction of mosquito larval habitat reduce malaria transmission in Tigray, Ethiopia?. Tropical Medicine and International Health 2005;10(12):1274‐85. [DOI] [PubMed] [Google Scholar]
Additional references
- Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide‐treated bed nets in western Nyanza Province, Kenya. Malaria Journal 2010;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JC, Killeen GF, Githure JI. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. American Journal of Tropical Medicine and Hygiene 1999;61(1):109‐13. [DOI] [PubMed] [Google Scholar]
- Bockarie MJ, Gbakima AA, Barnish G. It all began with Ronald Ross: 100 years of malaria research and control in Sierra Leone (1899‐1999). Annals of Tropical Medicine and Parasitology 1999;93(3):213‐4. [DOI] [PubMed] [Google Scholar]
- Bogh C, Clarke S, Jawara M, Thomas CJ, Lindsay SW. Localized breeding of the Anopheles gambiae complex (Diptera: Culicidae) along the River Gambia, West Africa. Bulletin of Entomological Research 2003;93(4):279‐87. [DOI] [PubMed] [Google Scholar]
- Bøgh C, Lindsay SW, Clarke SE, Dean A, Jawara M, Pinder M, et al. High spatial resolution mapping of malaria transmission risk in the Gambia, west Africa, using LANDSAT TM satellite imagery. American Journal of Tropical Medicine and Hygiene 2007;76(5):875‐81. [PubMed] [Google Scholar]
- Burkot T, Abdel‐Hameed Adeel AA, Pyke GH, Beach R, Wirtz RA, Garner P. Larvivorous fish for malaria prevention. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008090] [DOI] [Google Scholar]
- Castro MC, Tsuruta A, Kanamori S, Kannady K, Mkude S. Community‐based environmental management for malaria control: evidence from a small‐scale intervention in Dar es Salaam, Tanzania. Malaria Journal 2009;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood JD, Graves PM. The effect of permethrin‐impregnated bednets on a population of Anopheles farauti in coastal Papua New Guinea. Medical and Veterinary Entomology 1987;1(3):319‐27. [DOI] [PubMed] [Google Scholar]
- Chen H, Fillinger U, Yan G. Oviposition behavior of female Anopheles gambiae in western Kenya inferred from microsatellite markers. American Journal of Tropical Medicine and Hygiene 2006;75(2):246‐50. [PubMed] [Google Scholar]
- Chima RI, Goodman CA, Mills A. The economic impact of malaria in Africa: a critical review of the evidence. Health Policy 2008;63(1):17‐36. [DOI] [PubMed] [Google Scholar]
- Cochrane Effective Practice and Organisation of Care Group. Risk of bias guidelines. EPOC Author Resources2009; Vol. http://epoc.cochrane.org/.
- Dehné EJ. Fifty years of malaria control in the Panama area. American Journal of Tropical Medicine and Hygiene 1955;4(5):800‐11. [PubMed] [Google Scholar]
- Fillinger U, Knols BG, Becker N. Efficacy and efficiency of new Bacillus thuringiensis var israelensis and Bacillus sphaericus formulations against Afrotropical anophelines in Western Kenya. Tropical Medicine and International Health 2003;8(1):37‐47. [DOI] [PubMed] [Google Scholar]
- Fillinger U, Sonye G, Killeen GF, Knols BG, Becker N. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: operational observations from a rural town in western Kenya. Tropical Medicine and International Health 2004;9(12):1274‐89. [DOI] [PubMed] [Google Scholar]
- Fillinger U, Kannady K, William G, Vanek MJ, Dongus S, Nyika D, et al. A tool box for operational mosquito larval control: preliminary results and early lessons from the Urban Malaria Control Programme in Dar es Salaam, Tanzania. Malaria Journal 2008;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger U, Lindsay SW. Larval source management for malaria control in Africa: myths and reality. Malaria Journal 2011;10:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup JL, Sachs JD. The economic burden of malaria. American Journal of Tropical Medicine and Hygiene 2001;64(1‐2 Suppl):85‐96. [DOI] [PubMed] [Google Scholar]
- Gartrell FE, Ludvik GF. The role of insecticides in the TVA malaria control program. American Journal of Tropical Medicine and Hygiene 1954;3(5):817‐20. [DOI] [PubMed] [Google Scholar]
- Geissbühler Y, Chaki P, Emidi B, Govella NJ, Shirima R, Mayagaya V, et al. Interdependence of domestic malaria prevention measures and mosquito‐human interactions in urban Dar es Salaam, Tanzania. Malaria Journal 2007;6:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissbühler Y, Kannady K, Chaki PP, Emidi B, Govella NJ, Mayagaya V, et al. Microbial larvicide application by a large‐scale, community‐based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PloS ONE 2009;4(3):e5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani AC, Sutherland CJ, Riley EM, Drakeley CJ, Griffin JT, Gosling RD, et al. Loss of population levels of immunity to malaria as a result of exposure‐reducing interventions: consequences for interpretation of disease trends. PLoS ONE 2009;4(2):e4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies MT. Anopheline mosquitoes: vector behaviour and bionomics. In: W. H. Wernsdorfer, McGregor I editor(s). Malaria: principles and practice of malariology. 1. Edinburgh, U.K.: Churchill Livingstone, 1988:453‐85. [Google Scholar]
- Govella NJ, Okumu FO, Killeen GF. Insecticide‐treated nets can reduce malaria transmission by mosquitoes which feed outdoors. American Journal of Tropical Medicine and Hygiene 2010;82(3):415‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Novak RJ. Habitat‐based modelling of impacts of mosquito larval interventions on entomological inoculation rates, incidence, and prevalence of malaria. American Journal of Tropical Medicine and Hygiene 2005;73(3):546‐52. [PubMed] [Google Scholar]
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
- Holstein MH. Biology of Anopheles gambiae: Research in French West Africa. Vol. 9, Geneva: World Health Organization, 1954:172. [Google Scholar]
- Keiser J, Singer BH, Utzinger J. Reducing the burden of malaria in different eco‐epidemiological settings with environmental management: a systematic review. Lancet Infectious Diseases 2005;5(11):695‐708. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Fillinger U, Kiche I, Gouagna LC, Knols BG. Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa?. Lancet Infectious Diseases 2002;2(10):618‐27. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Fillinger U, Knols BG. Advantages of larval control for African malaria vectors: low mobility and behavioural responsiveness of immature mosquito stages allow high effective coverage. Malaria Journal 2002;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen GF, Tanner M, Mukabana WR, Kalongolela MS, Kannady K, Lindsay SW, et al. Habitat targeting for controlling aquatic stages of malaria vectors in Africa. American Journal of Tropical Medicine and Hygiene 2006;74(4):517‐8. [PubMed] [Google Scholar]
- Kitron U, Spielman A. Suppression of transmission of malaria through source reduction: anti‐anopheline measures applied in Israel, the United States and Italy. Reviews of Infectious Diseases 1989;11(3):391‐406. [DOI] [PubMed] [Google Scholar]
- Lengeler C. Insecticide‐treated bednets and curtains for preventing malaria. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD000363.pub2] [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Kirby M, Baris E, Bos R. Environmental management for malaria control in the East Asia and Pacific (EAP) Region. Available at http://www.who.int/water_sanitation_health/publications/whowbmalariacontrol.pdf. Geneva, 2004.
- Macdonald G. The epidemiology and control of malaria. London: Oxford University Press, 1957. [Google Scholar]
- Majambere S, Fillinger U, Sayer DR, Green C, Lindsay SW. Spatial distribution of mosquito larvae and the potential for targeted larval control in The Gambia. American Journal of Tropical Medicine and Hygiene 2008;79(1):19‐27. [PubMed] [Google Scholar]
- Mandekos AG, Damkas C, Zaphiropoulos M. Old and new methods of controlling malaria in Greek Macedonia. American Journal of Tropical Medicine and Hygiene 1948;28(1):35‐41. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Munga S, Atieli F, Mushinzimana E, Zhou G, Githeko AK, et al. Spatial distribution of anopheline larval habitats in Western Kenyan highlands: effects of land cover types and topography. American Journal of Tropical Medicine and Hygiene 2005;73(1):157‐65. [PubMed] [Google Scholar]
- Molineaux L, Grammiccia G. The Garki Project: research on the epidemiology and control of malaria in the Sudan savanna of West Africa. Geneva: WHO, 1980. [Google Scholar]
- Muirhead‐Thomson RC. Mosquito behaviour in relation to malaria transmission and control in the tropics. London: Edward Arnold & Co, 1951. [Google Scholar]
- Mukabana WR, Kannady K, Kiama GM, Ijumba JN, Mathenge EM, Kiche I, et al. Ecologists can enable communities to implement malaria vector control in Africa. Malaria Journal 2006;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 2012;379(9814):413‐31. [DOI] [PubMed] [Google Scholar]
- Mutuku FM, Alaii JA, Bayoh MN, Gimnig JE, Vulule JM, Walker ED, et al. Distribution, description, and local knowledge of larval habitats of Anopheles gambiae s.l. in a village in western Kenya. American Journal of Tropical Medicine and Hygiene 2006;74(1):44‐53. [PubMed] [Google Scholar]
- Mwangangi JM, Mbogo CM, Muturi EJ, Nzovu JG, Githure JI, Yan G, et al. Spatial distribution and habitat characterisation of Anopheles larvae along the Kenyan coast. Journal of Vector Borne Diseases 2007;44(1):44‐51. [PMC free article] [PubMed] [Google Scholar]
- N'Guessan R, Corbel V, Akogbéto M, Rowland M. Reduced efficacy of insecticide‐treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerging Infectious Diseases 2007;13(2):199‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najera JA. Malaria control: achievements, problems and strategies. Parassitologia 2001;43(1‐2):1‐89. [PubMed] [Google Scholar]
- Nauen 2007. Insecticide resistance in disease vectors of public health importance. Pest Management Science 2007;63(7):623‐77. [DOI] [PubMed] [Google Scholar]
- Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD006657.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control?. Trends in Parasitology 2011;27(2):91‐8. [DOI] [PubMed] [Google Scholar]
- Roll Back Malaria. Global malaria action plan. Roll Back Malaria Partnership2008:1‐274.
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Rozendaal JA. Vector control: Methods for use by individuals and communities. Geneva: WHO, 1997. [Google Scholar]
- Russell PF. Man's mastery of malaria. London: Oxford University Press, 1955. [Google Scholar]
- Sachs JD. Macroeconomics and health: Investing in health for economic development. Geneva: World Health Organization, 2001. [Google Scholar]
- Shililu J, Mbogo C, Ghebremeskel T, Githure J, Novak R. Mosquito larval habitats in a semiarid ecosystem in Eritrea: impact of larval habitat management on Anopheles arabiensis population. American Journal of Tropical Medicine and Hygiene 2007;76(1):103‐10. [PubMed] [Google Scholar]
- Shousha AT. The eradication of Anopheles gambiae from Upper Egypt 1942‐1945. Bulletin of the World Health Organization 1948;1(2):309‐42. [PMC free article] [PubMed] [Google Scholar]
- Smith D, McKenzie FE. Static and dynamics of malaria infection in Anopheles mosquitoes. Malaria Journal 2004;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 2005;438(7067):492‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, McKenzie FE, Snow RW, Hay SI. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biology 2007;5(3):e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, DL, Drakeley CJ, Chiyaka C, Hay SI. A quantitative analysis of transmission efficiency versus intensity for malaria. Nature Communications 2010;1:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Perkins TA, Tusting LS, Scott TW, Lindsay SW. Mosquito population regulation and Larval Source Management in heterogeneous environments. PLoS One In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper FL, Wilson DB. Anopheles gambiae in Brazil: 1930 ‐ 1940. New York: The Rockefeller Foundation, 1943:1‐262. [Google Scholar]
- Takken, W, Snellen WB, Verhave JP. Environmental measures for malaria control in Indonesia: an historical review on species sanitation. Wageningen: Wageningen Agricultural University Papers, 1990:90‐97. [Google Scholar]
- Teklehaimanot A, Mejia P. Malaria and poverty. Annals of the New York Academy of Science 2008;1136:32‐7. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Tozan Y, Singer BH. Efficacy and cost‐effectiveness of environmental management for malaria control. Tropical Medicine and International Health 2001;6(9):677‐87. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Tozan Y, Doumani F, Singer BH. The economic payoffs of integrated malaria control in the Zambian copperbelt between 1930 and 1950. Tropical Medicine and International Health 2002;7(8):657‐77. [DOI] [PubMed] [Google Scholar]
- Walker K, Lynch M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Medical and Veterinary Entomology 2007;21(1):2‐21. [DOI] [PubMed] [Google Scholar]
- Watson M. African highway: the battle for health in Central Africa. London: John Murray, 1953. [Google Scholar]
- World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. Guidelines for laboratory and field testing of mosquito larvicides. Geneva: World Health Organization, 2005:1‐39. [Google Scholar]
- World Health Organization. Pesticides and their application for the control of vectors and pests of public health importance. 6th Edition. Geneva: World Health Organization, 2006:1‐114. [Google Scholar]
- World Health Organization. Malaria vector control and personal protection: report of a WHO study group. Geneva: World Health Organization, 2006. [PubMed] [Google Scholar]
- World Health Organization. WHO position statement on integrated vector management. Weekly Epidemiological Record 2008;83(20):177‐81. [PubMed] [Google Scholar]
- World Health Organization. World Malaria Report 2011. Geneva: World Health Organization, 2011. [Google Scholar]
- WHO. Interim position statement: The role of larviciding for malaria control in sub‐Saharan Africa. Geneva: World Health Organization, April 2012. [Google Scholar]
- Worrall, E, Fillinger U. Large‐scale use of mosquito larval source management for malaria control in Africa: a cost analysis. Malaria Journal 2011;10:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Thwing J, Fillinger U, Gimnig J, Newman R, Lindsay S. Mosquito larval source management for controlling malaria. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008923] [DOI] [PMC free article] [PubMed] [Google Scholar]


